Submitted:
29 June 2023
Posted:
03 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. β-cell dysfunction and death
3. Insulin resistance
4. Obesity
5. Dysbiosis
6. Vascular complications
7. Polyphenols and advanced glycation.
8. Lipid metabolism
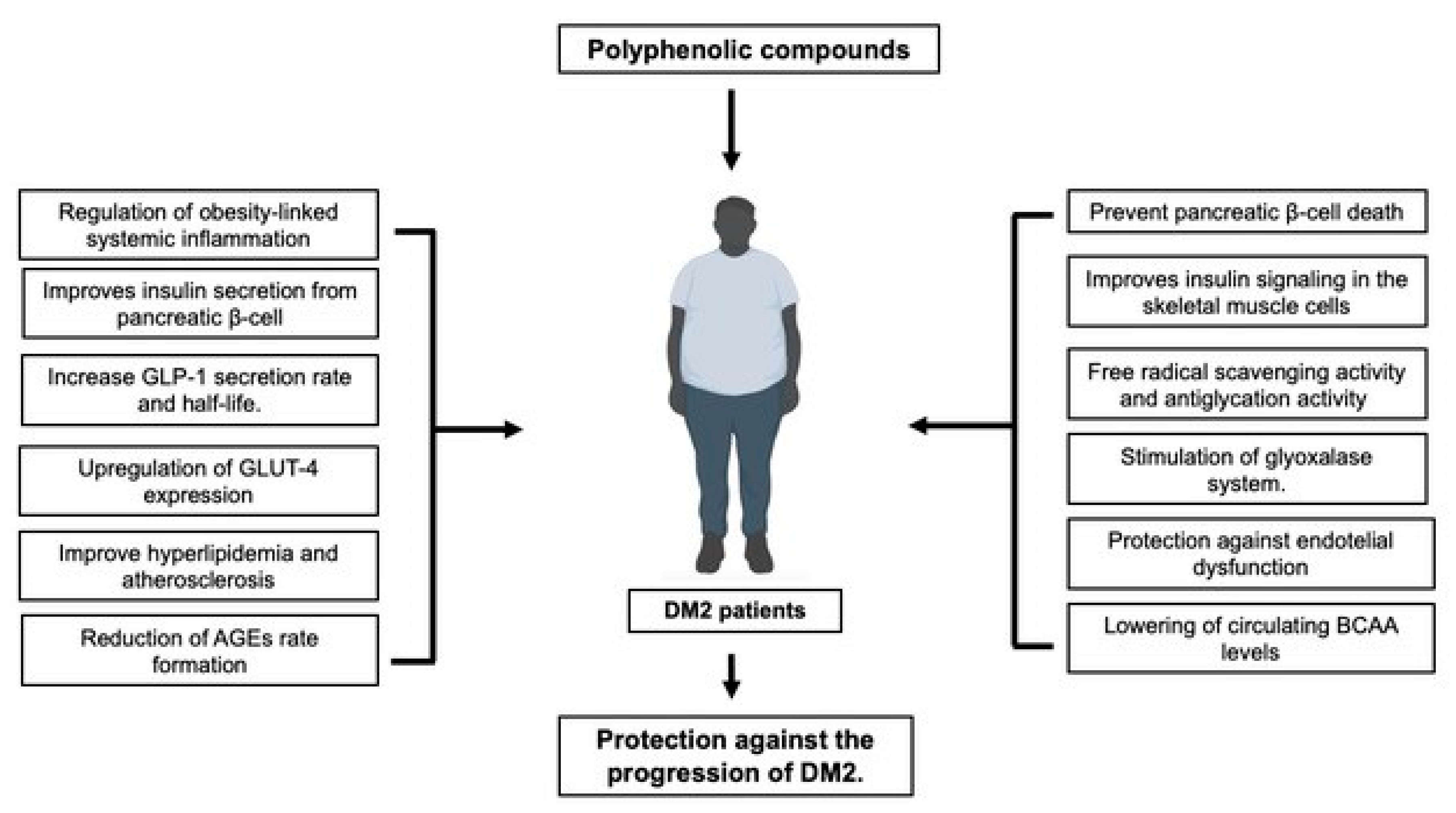
9. Conclusion remarks and future directions
References
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of type 2 diabetes – global burden of disease and forecasted trends. J. Epidemiol. Glob. Health. 2019, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Yan, LJ. Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. J. Diabetes Res. 2014, 2014, 137919. [Google Scholar] [CrossRef] [PubMed]
- Black, H.S. A synopsis of the associations of oxidative stress, ROS, and antioxidants with diabetes mellitus. Antioxidants 2022, 11, 2003. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A Review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Arigela, C.S.; Gan, S.H.; Salam, S.K.N.; Krishnan, K.T.; Rahman, N.A.; Jeffree, M.S. A Review on Oxidative Stress, Diabetic Complications, and the Roles of Honey Polyphenols. Oxid. Med. Cell. Longev. 2020, 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Andrés-Lacueva, C. Polyphenols and Health: Current State and Progress. J. Agric. Food Chem. 2012, 60, 8773–8775. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Cerf, M.E. Beta Cell Dysfunction and Insulin Resistance. Front. Endocrinol. 2013, 4. [Google Scholar] [CrossRef]
- White, M.G.; Shaw, J.A.M.; Taylor, R. Type 2 Diabetes: The Pathologic Basis of Reversible β-Cell Dysfunction. Diabetes Care 2016, 39, 2080–2088. [Google Scholar] [CrossRef]
- Ferrannini, E.; Mari, A. β-Cell Function in Type 2 Diabetes. Metabolism 2014, 63, 1217–1227. [Google Scholar] [CrossRef]
- Porte, D.; Kahn, S.E. Beta-Cell Dysfunction and Failure in Type 2 Diabetes: Potential Mechanisms. Diabetes 2001, 50, S160. [Google Scholar] [CrossRef]
- Cerf, M.E. Beta Cell Physiological Dynamics and Dysfunctional Transitions in Response to Islet Inflammation in Obesity and Diabetes. Metabolites 2020, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative Stress in the Pathophysiology of Type 2 Diabetes and Related Complications: Current Therapeutics Strategies and Future Perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Drews, G.; Krippeit-Drews, P.; Düfer, M. Oxidative Stress and Beta-Cell Dysfunction. Pflugers Arch. 2010, 460, 703–718. [Google Scholar] [CrossRef]
- Dludla, P.V.; Mabhida, S.E.; Ziqubu, K.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Hanser, S.; Basson, A.K.; Pheiffer, C.; Kengne, A.P. Pancreatic β-Cell Dysfunction in Type 2 Diabetes: Implications of Inflammation and Oxidative Stress. World J. Diabetes 2023, 14, 130–146. [Google Scholar] [CrossRef]
- Dinić, S.; Arambašić Jovanović, J.; Uskoković, A.; Mihailović, M.; Grdović, N.; Tolić, A.; Rajić, J.; Đorđević, M.; Vidaković, M. Oxidative Stress-Mediated Beta Cell Death and Dysfunction as a Target for Diabetes Management. Front. Endocrinol. 2022, 13, 1006376. [Google Scholar] [CrossRef]
- Eguchi, N.; Vaziri, N.D.; Dafoe, D.C.; Ichii, H. The Role of Oxidative Stress in Pancreatic β Cell Dysfunction in Diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef]
- Ma, Z.A. The Role of Peroxidation of Mitochondrial Membrane Phospholipids in Pancreatic β -Cell Failure. Curr. Diabetes Rev. 2012, 8, 69–75. [Google Scholar] [CrossRef]
- Rowley, T.J.; Bitner, B.F.; Ray, J.D.; Lathen, D.R.; Smithson, A.T.; Dallon, B.W.; Plowman, C.J.; Bikman, B.T.; Hansen, J.M.; Dorenkott, M.R.; et al. Monomeric Cocoa Catechins Enhance β-Cell Function by Increasing Mitochondrial Respiration. J. Nutr. Biochem. 2017, 49, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Vetterli, L.; Brun, T.; Giovannoni, L.; Bosco, D.; Maechler, P. Resveratrol Potentiates Glucose-Stimulated Insulin Secretion in INS-1E β-Cells and Human Islets through a SIRT1-Dependent Mechanism. J. Biol. Chem. 2011, 286, 6049–6060. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Moonsan, P.; Yibchok-anun, S. Insulin-Releasing Properties of a Series of Cinnamic Acid Derivatives in Vitro and in Vivo. J. Agric. Food Chem. 2008, 56, 7838–7844. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.; Dragunow, M.; Cooper, G.J.S. Fibrillogenic Amylin Evokes Islet β-Cell Apoptosis through Linked Activation of a Caspase Cascade and JNK1. J. Biol. Chem. 2003, 278, 52810–52819. [Google Scholar] [CrossRef] [PubMed]
- Kanatsuka, A.; Kou, S.; Makino, H. IAPP/Amylin and β-Cell Failure: Implication of the Risk Factors of Type 2 Diabetes. Diabetol. Int. 2018, 9, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, I.; Poppitt, S. Unfolding Novel Mechanisms of Polyphenol Flavonoids for Better Glycaemic Control: Targeting Pancreatic Islet Amyloid Polypeptide (IAPP). Nutrients 2017, 9, 788. [Google Scholar] [CrossRef] [PubMed]
- Mahboob, A.; Senevirathne, D.K.L.; Paul, P.; Nabi, F.; Khan, R.H.; Chaari, A. An Investigation into the Potential Action of Polyphenols against Human Islet Amyloid Polypeptide Aggregation in Type 2 Diabetes. Int. J. Biol. Macromol. 2023, 225, 318–350. [Google Scholar] [CrossRef]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin Receptor Signaling in Normal and Insulin-Resistant States. Cold Spring Harb. perspect. biol. 2014, 6, a009191. [Google Scholar] [CrossRef]
- Saltiel, A.R. Insulin Signaling in Health and Disease. J. Clin. Investig. 2021, 131, e142241. [Google Scholar] [CrossRef]
- Siddle, K. Signalling by Insulin and IGF Receptors: Supporting Acts and New Players. J. Mol. Endocrinol. 2011, 47, R1–R10. [Google Scholar] [CrossRef]
- Taylor, R. Insulin Resistance and Type 2 Diabetes. Diabetes 2012, 61, 778–779. [Google Scholar] [CrossRef]
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef]
- Hurrle, S.; Hsu, W.H. The Etiology of Oxidative Stress in Insulin Resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.-T.; Price, J.W.; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 Emission and Cellular Redox State Link Excess Fat Intake to Insulin Resistance in Both Rodents and Humans. J. Clin. Invest. 2009, 119, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, E.J.; Diamond-Stanic, M.K.; Marchionne, E.M. Oxidative Stress and the Etiology of Insulin Resistance and Type 2 Diabetes. Free Radic. Biol. Med. 2011, 51, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Sowers, J.R.; Nistala, R.; Gong, H.; Uptergrove, G.M.-E.; Clark, S.E.; Morris, E.M.; Szary, N.; Manrique, C.; Stump, C.S. Angiotensin II-Induced NADPH Oxidase Activation Impairs Insulin Signaling in Skeletal Muscle Cells. J. Biol. Chem. 2006, 281, 35137–35146. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Becattini, B. JNK at the Crossroad of Obesity, Insulin Resistance, and Cell Stress Response. Mol. Metab. 2017, 6, 174–184. [Google Scholar] [CrossRef]
- Mi, Y.; Zhang, W.; Tian, H.; Li, R.; Huang, S.; Li, X.; Qi, G.; Liu, X. EGCG Evokes Nrf2 Nuclear Translocation and Dampens PTP1B Expression to Ameliorate Metabolic Misalignment under Insulin Resistance Condition. Food Funct. 2018, 9, 1510–1523. [Google Scholar] [CrossRef]
- Vlavcheski, F.; Den Hartogh, D.J.; Giacca, A.; Tsiani, E. Amelioration of High-Insulin-Induced Skeletal Muscle Cell Insulin Resistance by Resveratrol Is Linked to Activation of AMPK and Restoration of GLUT4 Translocation. Nutrients 2020, 12, 914. [Google Scholar] [CrossRef]
- Zin, C.A.J.C.M.; Mohamed, W.M.I.W.; Khan, N.A.K.; Ishak, W.R.W. Effects of Fruit and Vegetable Polyphenols on the Glycemic Control and Metabolic Parameters in Type 2 Diabetes Mellitus: A Review. Prev. Nutr. Food Sci. 2022, 27, 257–264. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity and Its Impact on Metabolic Syndrome. J. Clin. Invest. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Keaney, J.F.; Larson, M.G.; Vasan, R.S.; Wilson, P.W.F.; Lipinska, I.; Corey, D.; Massaro, J.M.; Sutherland, P.; Vita, J.A.; Benjamin, E.J. Obesity and Systemic Oxidative Stress: Clinical Correlates of Oxidative Stress in The Framingham Study. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Skalicky, J.; Muzakova, V.; Kandar, R.; Meloun, M.; Rousar, T.; Palicka, V. Evaluation of Oxidative Stress and Inflammation in Obese Adults with Metabolic Syndrome. Clin. Chem. Lab. Med. 2008, 46. [Google Scholar] [CrossRef]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Skoumas, I.; Papademetriou, L.; Economou, M.; Stefanadis, C. The Implication of Obesity on Total Antioxidant Capacity in Apparently Healthy Men and Women: The ATTICA Study. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Savini, I.; Catani, M.; Evangelista, D.; Gasperi, V.; Avigliano, L. Obesity-Associated Oxidative Stress: Strategies Finalized to Improve Redox State. Int. J. Mol. Sci. 2013, 14, 10497–10538. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef]
- Aloo, S.-O.; Ofosu, F.K.; Kim, N.-H.; Kilonzi, S.M.; Oh, D.-H. Insights on Dietary Polyphenols as Agents against Metabolic Disorders: Obesity as a Target Disease. Antioxidants 2023, 12, 416. [Google Scholar] [CrossRef]
- Zamani-Garmsiri, F.; Emamgholipour, S.; Rahmani Fard, S.; Ghasempour, G.; Jahangard Ahvazi, R.; Meshkani, R. Polyphenols: Potential Anti-inflammatory Agents for Treatment of Metabolic Disorders. Phytother. Res. 2022, 36, 415–432. [Google Scholar] [CrossRef]
- Badshah, H.; Ullah, I.; Kim, S.E.; Kim, T.; Lee, H.Y.; Kim, M.O. Anthocyanins Attenuate Body Weight Gain via Modulating Neuropeptide Y and GABAB1 Receptor in Rats Hypothalamus. Neuropeptides 2013, 47, 347–353. [Google Scholar] [CrossRef]
- Buchholz, T.; Melzig, M.F. Polyphenolic Compounds as Pancreatic Lipase Inhibitors. Planta Med. 2015, 81, 771–783. [Google Scholar] [CrossRef]
- Kim, N.-H.; Jegal, J.; Kim, Y.N.; Heo, J.-D.; Rho, J.-R.; Yang, M.H.; Jeong, E.J. Chokeberry Extract and Its Active Polyphenols Suppress Adipogenesis in 3T3-L1 Adipocytes and Modulates Fat Accumulation and Insulin Resistance in Diet-Induced Obese Mice. Nutrients 2018, 10, 1734. [Google Scholar] [CrossRef]
- Rocha, A.; Bolin, A.P.; Cardoso, C.A.L.; Otton, R. Green Tea Extract Activates AMPK and Ameliorates White Adipose Tissue Metabolic Dysfunction Induced by Obesity. Eur. J. Nutr. 2016, 55, 2231–2244. [Google Scholar] [CrossRef]
- Lee, M.-S.; Shin, Y.; Jung, S.; Kim, Y. Effects of Epigallocatechin-3-Gallate on Thermogenesis and Mitochondrial Biogenesis in Brown Adipose Tissues of Diet-Induced Obese Mice. Food Nutr. Res. 2017, 61, 1325307. [Google Scholar] [CrossRef]
- Valli, V.; Heilmann, K.; Danesi, F.; Bordoni, A.; Gerhäuser, C. Modulation of Adipocyte Differentiation and Proadipogenic Gene Expression by Sulforaphane, Genistein, and Docosahexaenoic Acid as a First Step to Counteract Obesity. Oxid. Med. Cell. Longev. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Pejenaute, H.; Del Moral, R.; Boulet, N.; Hijona, E.; Andrade, F.; Villanueva-Millán, M.J.; Aguirre, L.; Arbones-Mainar, J.M. The Dietary Antioxidant Piceatannol Inhibits Adipogenesis of Human Adipose Mesenchymal Stem Cells and Limits Glucose Transport and Lipogenic Activities in Adipocytes. Int. J. Mol. Sci. 2018, 19, 2081. [Google Scholar] [CrossRef] [PubMed]
- Matijašić, M.; Meštrović, T.; Čipčić Paljetak, H.; Perić, M.; Barešić, A.; Verbanac, D. Gut Microbiota beyond Bacteria—Mycobiome, Virome, Archaeome, and Eukaryotic Parasites in IBD. Int. J. Mol. Sci. 2020, 21, 2668. [Google Scholar] [CrossRef]
- Hills, R.; Pontefract, B.; Mishcon, H.; Black, C.; Sutton, S.; Theberge, C. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLOS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Microbiota and Diabetes: An Evolving Relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-Chain Fatty Acids in Control of Body Weight and Insulin Sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet–Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Gosav, E.M.; Neculae, E.; Costea, C.F.; Ciocoiu, M.; Hurjui, L.L.; Tarniceriu, C.C.; Maranduca, M.A.; Lacatusu, C.M.; Floria, M.; et al. Role of Gut Microbiota on Onset and Progression of Microvascular Complications of Type 2 Diabetes (T2DM). Nutrients 2020, 12, 3719. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Grasset, E.; Puel, A.; Charpentier, J.; Collet, X.; Christensen, J.E.; Tercé, F.; Burcelin, R. A Specific Gut Microbiota Dysbiosis of Type 2 Diabetic Mice Induces GLP-1 Resistance through an Enteric NO-Dependent and Gut-Brain Axis Mechanism. Cell Metab. 2017, 25, 1075–1090.e5. [Google Scholar] [CrossRef] [PubMed]
- Koudoufio, M.; Desjardins, Y.; Feldman, F.; Spahis, S.; Delvin, E.; Levy, E. Insight into Polyphenol and Gut Microbiota Crosstalk: Are Their Metabolites the Key to Understand Protective Effects against Metabolic Disorders? Antioxidants 2020, 9, 982. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A Polyphenol-Rich Cranberry Extract Protects from Diet-Induced Obesity, Insulin Resistance and Intestinal Inflammation in Association with Increased Akkermansia Spp. Population in the Gut Microbiota of Mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef]
- Chen, K.; Gao, Z.; Ding, Q.; Tang, C.; Zhang, H.; Zhai, T.; Xie, W.; Jin, Z.; Zhao, L.; Liu, W. Effect of Natural Polyphenols in Chinese Herbal Medicine on Obesity and Diabetes: Interactions among Gut Microbiota, Metabolism, and Immunity. Front. Nutr. 2022, 9, 962720. [Google Scholar] [CrossRef]
- Hara, T.; Hirasawa, A.; Sun, Q.; Sadakane, K.; Itsubo, C.; Iga, T.; Adachi, T.; Koshimizu, T.; Hashimoto, T.; Asakawa, Y.; et al. Novel Selective Ligands for Free Fatty Acid Receptors GPR120 and GPR40. Naunyn-Schmiedeberg's Arch. Pharmacol. 2009, 380, 247–255. [Google Scholar] [CrossRef]
- Takikawa, M.; Kurimoto, Y.; Tsuda, T. Curcumin Stimulates Glucagon-like Peptide-1 Secretion in GLUTag Cells via Ca2+/Calmodulin-Dependent Kinase II Activation. Biochem. Biophys. Res. Commun. 2013, 435, 165–170. [Google Scholar] [CrossRef]
- Kato, M.; Nishikawa, S.; Ikehata, A.; Dochi, K.; Tani, T.; Takahashi, T.; Imaizumi, A.; Tsuda, T. Curcumin Improves Glucose Tolerance via Stimulation of Glucagon-like Peptide-1 Secretion. Mol. Nutr. Food Res. 2017, 61, 1600471. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Ali, M.B.; Akash, M.S.H. Genistein Enhances the Secretion of Glucagon-like Peptide-1 (GLP-1) via Downregulation of Inflammatory Responses. Biomed. Pharmacother. 2019, 112, 108670. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Johnson, M.H.; Lila, M.A.; Yousef, G.; De Mejia, E.G. Berry and Citrus Phenolic Compounds Inhibit Dipeptidyl Peptidase IV: Implications in Diabetes Management. Evid.-Based Complementary Altern. Med. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.C.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Flavanol Monomer-Induced Changes to the Human Faecal Microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef]
- Unno, T.; Sakuma, M.; Mitsuhashi, S. Effect of Dietary Supplementation of (-)-Epigallocatechin Gallate on Gut Microbiota and Biomarkers of Colonic Fermentation in Rats. J. Nutr. Sci. Vitaminol. 2014, 60, 213–219. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Doble, M. Synergistic Effect of Phytochemicals in Combination with Hypoglycemic Drugs on Glucose Uptake in Myotubes. Phytomedicine 2009, 16, 1119–1126. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Doble, M. Interaction of Phytochemicals with Hypoglycemic Drugs on Glucose Uptake in L6 Myotubes. Phytomedicine 2011, 18, 285–291. [Google Scholar] [CrossRef]
- Upadhyay, S.; Dixit, M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxid. Med. Cell. Longev. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human Gut Microbes Impact Host Serum Metabolome and Insulin Sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-Analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef]
- Bragg, F.; Kartsonaki, C.; Guo, Y.; Holmes, M.; Du, H.; Yu, C.; Pei, P.; Yang, L.; Jin, D.; Chen, Y.; et al. Circulating Metabolites and the Development of Type 2 Diabetes in Chinese Adults. Diabetes Care 2022, 45, 477–480. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Lapworth, A.L.; An, J.; Wang, L.; McGarrah, R.W.; Stevens, R.D.; Ilkayeva, O.; George, T.; Muehlbauer, M.J.; Bain, J.R.; et al. Branched-Chain Amino Acid Restriction in Zucker-Fatty Rats Improves Muscle Insulin Sensitivity by Enhancing Efficiency of Fatty Acid Oxidation and Acyl-Glycine Export. Mol. Metab. 2016, 5, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Bartova, S.; Madrid-Gambin, F.; Fernández, L.; Carayol, J.; Meugnier, E.; Segrestin, B.; Delage, P.; Vionnet, N.; Boizot, A.; Laville, M.; et al. Grape Polyphenols Decrease Circulating Branched Chain Amino Acids in Overfed Adults. Front. Nutr. 2022, 9, 998044. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.S.; Cuerrier, A.; Lamont, E.; Haddad, P.S.; Arnason, J.T.; Bennett, S.A.L.; Johns, T. Investigating Wild Berries as a Dietary Approach to Reducing the Formation of Advanced Glycation Endproducts: Chemical Correlates of In Vitro Antiglycation Activity. Plant Foods Hum. Nutr. 2014, 69, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Ramkissoon, J.; Mahomoodally, M.; Ahmed, N.; Subratty, A. Antioxidant and Anti–Glycation Activities Correlates with Phenolic Composition of Tropical Medicinal Herbs. Asian Pac. J. Trop. Med. 2013, 6, 561–569. [Google Scholar] [CrossRef]
- Coelho, O.G.L.; Ribeiro, P.V.M.; Alfenas, R.D.C.G. Can Grape Polyphenols Affect Glycation Markers? A Systematic Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 1208–1218. [Google Scholar] [CrossRef]
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Anti-Inflammatory Properties of a Pomegranate Extract and Its Metabolite Urolithin-A in a Colitis Rat Model and the Effect of Colon Inflammation on Phenolic Metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef]
- Nesto, RW. Correlation between cardiovascular disease and diabetes mellitus: current concepts. Am J Med. 2004, 116, 11–22. [Google Scholar] [CrossRef]
- Shi, Y.; Vanhoutte, P.M. Macro- and microvascular endothelial dysfunction in diabetes. J. Diabetes 2017, 9, 434–449. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liu, S.; Gao, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Diabetic Vascular Diseases: Molecular Mechanisms and Therapeutic Strategies. Signal Transduct. Target. Ther. 2023, 8, 152. [Google Scholar] [CrossRef]
- Giugliano, D.; Ceriello, A.; Paolisso, G. Oxidative Stress and Diabetic Vascular Complications. Diabetes Care 1996, 19, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Figueroa, H.; Re, L.; Morales, M.A. Oxidative Stress at the Vascular Wall. Mechanistic and Pharmacological Aspects. Arch. Med. Res. 2006, 37, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Mann, G.E. Vascular NAD(P)H Oxidase Activation in Diabetes: A Double-Edged Sword in Redox Signalling. Cardiovasc. Res. 2009, 82, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial Function and Dysfunction: Testing and Clinical Relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef]
- Boulanger, C. M. Endothelium. Arterioscler. Thromb. Vasc. Biol. 2016, 36, e26–e31. [Google Scholar] [CrossRef]
- Tousoulis, D.; Kampoli, A.-M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The Role of Nitric Oxide on Endothelial Function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Zou, M.-H.; Cohen, R.A.; Ullrich, V. Peroxynitrite and Vascular Endothelial Dysfunction in Diabetes Mellitus. Endothelium 2004, 11, 89–97. [Google Scholar] [CrossRef]
- Szabo, C. Role of Nitrosative Stress in the Pathogenesis of Diabetic Vascular Dysfunction. Br. J. Pharmacol. 2009, 156, 713–727. [Google Scholar] [CrossRef]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Martins, T.F.; Palomino, O.M.; Álvarez-Cilleros, D.; Martín, M.A.; Ramos, S.; Goya, L. Cocoa Flavanols Protect Human Endothelial Cells from Oxidative Stress. Plant Foods Hum. Nutr. 2020, 75, 161–168. [Google Scholar] [CrossRef]
- Zhou, H.; Fu, B.; Xu, B.; Mi, X.; Li, G.; Ma, C.; Xie, J.; Li, J.; Wang, Z. Rosmarinic Acid Alleviates the Endothelial Dysfunction Induced by Hydrogen Peroxide in Rat Aortic Rings via Activation of AMPK. Oxid. Med. Cell Longev. 2017, 2017, 7091904. [Google Scholar] [CrossRef]
- Serraino, I.; Dugo, L.; Dugo, P.; Mondello, L.; Mazzon, E.; Dugo, G.; Caputi, A.P.; Cuzzocrea, S. Protective Effects of Cyanidin-3-O-Glucoside from Blackberry Extract against Peroxynitrite-Induced Endothelial Dysfunction and Vascular Failure. Life Sci. 2003, 73, 1097–1114. [Google Scholar] [CrossRef] [PubMed]
- Spanier, G.; Xu, H.; Xia, N.; Tobias, S.; Deng, S.; Wojnowski, L.; Forstermann, U.; Li, H. Resveratrol Reduces Endothelial Oxidative Stress by Modulating the Gene Expression of Superoxide Dismutase 1 (SOD1), Glutathione Peroxidase 1 (GPx1) and NADPH Oxidase Subunit (Nox4). J. Physiol. Pharmacol. 2009, 60 (Suppl. S4), 111–116. [Google Scholar] [PubMed]
- Alvarez, S.; Zaobornyj, T.; Actis-Goretta, L.; Fraga, C.G.; Boveris, A. Polyphenols and Red Wine as Peroxynitrite Scavengers: A Chemiluminescent Assay. Ann. N. Y. Acad. Sci. 2002, 957, 271–273. [Google Scholar] [CrossRef]
- Liang, X.-X.; Wang, R.-Y.; Guo, Y.-Z.; Cheng, Z.; Lv, D.-Y.; Luo, M.-H.; He, A.; Luo, S.-X.; Xia, Y. Phosphorylation of Akt at Thr308 Regulates P-eNOS Ser1177 during Physiological Conditions. FEBS Open Bio 2021, 11, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Madeira, S.V.F.; Auger, C.; Anselm, E.; Chataigneau, M.; Chataigneau, T.; Soares de Moura, R.; Schini-Kerth, V.B. eNOS Activation Induced by a Polyphenol-Rich Grape Skin Extract in Porcine Coronary Arteries. J. Vasc. Res. 2009, 46, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, I.; Burton-Freeman, B.; Tissa Kappagoda, C. Mechanism of the Endothelium-Dependent Relaxation Evoked by a Grape Seed Extract. Clin. Sci. 2008, 114, 331–337. [Google Scholar] [CrossRef]
- Wallerath, T.; Poleo, D.; Li, H.; Förstermann, U. Red Wine Increases the Expression of Human Endothelial Nitric Oxide Synthase. J. Am. Coll. Cardiol. 2003, 41, 471–478. [Google Scholar] [CrossRef]
- Wallerath, T.; Li, H.; Gödtel-Ambrust, U.; Schwarz, P.M.; Förstermann, U. A Blend of Polyphenolic Compounds Explains the Stimulatory Effect of Red Wine on Human Endothelial NO Synthase. Nitric Oxide 2005, 12, 97–104. [Google Scholar] [CrossRef]
- Wallerath, T.; Deckert, G.; Ternes, T.; Anderson, H.; Li, H.; Witte, K.; Förstermann, U. Resveratrol, a Polyphenolic Phytoalexin Present in Red Wine, Enhances Expression and Activity of Endothelial Nitric Oxide Synthase. Circulation 2002, 106, 1652–1658. [Google Scholar] [CrossRef]
- Xia, N.; Strand, S.; Schlufter, F.; Siuda, D.; Reifenberg, G.; Kleinert, H.; Förstermann, U.; Li, H. Role of SIRT1 and FOXO Factors in eNOS Transcriptional Activation by Resveratrol. Nitric Oxide 2013, 32, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced Glycation End Products: Sparking the Development of Diabetic Vascular Injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M.; Vlassara, H.; Cerami, A. Nonenzymatic Glycosylation and the Pathogenesis of Diabetic Complications. Ann. Intern. Med. 1984, 101, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Cerami, A.; Vlassara, H.; Brownlee, M. Role of Advanced Glycosylation Products in Complications of Diabetes. Diabetes Care 1988, 11 Suppl 1, 73–79. [Google Scholar]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Peppa, M.; Uribarri, J.; Vlassara, H. Glucose, Advanced Glycation End Products, and Diabetes Complications: What Is New and What Works. Clin. Diabetes 2003, 21, 186–187. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Brings, S.; Fleming, T.; Freichel, M.; Muckenthaler, M.U.; Herzig, S.; Nawroth, P.P. Dicarbonyls and Advanced Glycation End-Products in the Development of Diabetic Complications and Targets for Intervention. Int. J. Mol. Sci. 2017, 18, 984. [Google Scholar] [CrossRef]
- Uribarri, J.; del Castillo, M.D.; de la Maza, M.P.; Filip, R.; Gugliucci, A.; Luevano-Contreras, C.; Macías-Cervantes, M.H.; Markowicz Bastos, D.H.; Medrano, A.; Menini, T.; et al. Dietary Advanced Glycation End Products and Their Role in Health and Disease. Adv. Nutr. 2015, 6, 461–473. [Google Scholar] [CrossRef]
- Gautieri, A.; Passini, F.S.; Silván, U.; Guizar-Sicairos, M.; Carimati, G.; Volpi, P.; Moretti, M.; Schoenhuber, H.; Redaelli, A.; Berli, M.; et al. Advanced Glycation End-Products: Mechanics of Aged Collagen from Molecule to Tissue. Matrix Biol. 2017, 59, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Añazco, C.; González, I.; Araya, P. Extracellular Matrix Glycation and Receptor for Advanced Glycation End-Products Activation: A Missing Piece in the Puzzle of the Association between Diabetes and Cancer. Carcinogenesis 2018, 39, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Delgado-López, F.; González, I.; Pérez-Castro, R.; Romero, J.; Rojas, I. The Receptor for Advanced Glycation End-Products: A Complex Signaling Scenario for a Promiscuous Receptor. Cell Signal. 2013, 25, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Hudson, B.I.; Lippman, M.E. Targeting RAGE Signaling in Inflammatory Disease. Annu. Rev. Med. 2018, 69, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxid. Med. Cell. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Wautier, M.P.; Chappey, O.; Corda, S.; Stern, D.M.; Schmidt, A.M.; Wautier, J.L. Activation of NADPH Oxidase by AGE Links Oxidant Stress to Altered Gene Expression via RAGE. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E685–694. [Google Scholar] [CrossRef]
- Yeh, W.-J.; Hsia, S.-M.; Lee, W.-H.; Wu, C.-H. Polyphenols with Antiglycation Activity and Mechanisms of Action: A Review of Recent Findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef]
- Khanam, A.; Ahmad, S.; Husain, A.; Rehman, S.; Farooqui, A.; Yusuf, M.A. Glycation and Antioxidants: Hand in the Glove of Antiglycation and Natural Antioxidants. Curr. Protein Pept. Sci. 2020, 21, 899–915. [Google Scholar] [CrossRef]
- González, I.; Morales, M.A.; Rojas, A. Polyphenols and AGEs/RAGE Axis. Trends and Challenges. Food Res. Int. 2020, 129, 108843. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary Polyphenols as Antidiabetic Agents: Advances and Opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Gugliucci, A.; Bastos, D.H.M.; Schulze, J.; Souza, M.F.F. Caffeic and Chlorogenic Acids in Ilex Paraguariensis Extracts Are the Main Inhibitors of AGE Generation by Methylglyoxal in Model Proteins. Fitoterapia 2009, 80, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Genaro-Mattos, T.C.; Maurício, Â.Q.; Rettori, D.; Alonso, A.; Hermes-Lima, M. Antioxidant Activity of Caffeic Acid against Iron-Induced Free Radical Generation—A Chemical Approach. PLOS ONE 2015, 10, e0129963. [Google Scholar] [CrossRef]
- Bhuiyan, M.N.I.; Mitsuhashi, S.; Sigetomi, K.; Ubukata, M. Quercetin Inhibits Advanced Glycation End Product Formation via Chelating Metal Ions, Trapping Methylglyoxal, and Trapping Reactive Oxygen Species. Biosci. Biotechnol. Biochem. 2017, 81, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yagiz, Y.; Buran, T.J.; Nunes, C.D.N.; Gu, L. Phytochemicals from Berries and Grapes Inhibited the Formation of Advanced Glycation End-products by Scavenging Reactive Carbonyls. Food Res. Int. 2011, 44, 2666–2673. [Google Scholar] [CrossRef]
- Thornalley, P.J. The Glyoxalase System: New Developments towards Functional Characterization of a Metabolic Pathway Fundamental to Biological Life. Biochem. J. 1990, 269, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, J.; Narayanasamy, P. Flavonoid Enhances the Glyoxalase Pathway in Cerebellar Neurons to Retain Cellular Functions. Sci. Rep. 2017, 7, 5126. [Google Scholar] [CrossRef] [PubMed]
- Buttari, B.; Profumo, E.; Facchiano, F.; Ozturk, E.I.; Segoni, L.; Saso, L.; Riganò, R. Resveratrol Prevents Dendritic Cell Maturation in Response to Advanced Glycation End Products. Oxid. Med. Cell. Longev. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Kanlaya, R.; Thongboonkerd, V. Molecular Mechanisms of Epigallocatechin-3-Gallate for Prevention of Chronic Kidney Disease and Renal Fibrosis: Preclinical Evidence. Curr. Dev. Nutr. 2019, 3, nzz101. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Chen, Q.; Liu, Y.; Wu, Z.; Pan, D.; Yan, N.; Liu, L. Cereal Polyphenols Inhibition Mechanisms on Advanced Glycation End Products and Regulation on Type 2 Diabetes. Crit. Rev. Food Sci. Nutr. 2023, 1–19. [Google Scholar] [CrossRef]
- Jonas, R.A.; Crabtree, T.R.; Jennings, R.S.; Marques, H.; Katz, R.J.; Chang, H.-J.; Stuijfzand, W.J.; van Rosendael, A.R.; Choi, J.H.; Doh, J.-H.; et al. Diabetes, Atherosclerosis, and Stenosis by AI. Diabetes Care 2023, 46, 416–424. [Google Scholar] [CrossRef]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New Insights into Oxidative Stress and Inflammation during Diabetes Mellitus-Accelerated Atherosclerosis. Redox Biol. 2019, 20, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Zhang, F.; Zhu, W.; Wu, J.; Liang, M. Copper in Diabetes Mellitus: A Meta-Analysis and Systematic Review of Plasma and Serum Studies. Biol. Trace Elem. Res. 2017, 177, 53–63. [Google Scholar] [CrossRef]
- Banerjee, J.; Mishra, N.; Damle, G.; Dhas, Y. Beyond LDL-c: The Importance of Serum Oxidized LDL in Predicting Risk for Type 2 Diabetes in the Middle-Aged Asian Indians. Diabetes Metab. Syndr. 2019, 13, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.S.; Brownlee, M. Molecular and Cellular Mechanisms of Cardiovascular Disorders in Diabetes. Circ. Res. 2016, 118, 1808–1829. [Google Scholar] [CrossRef]
- Islam, M.A.; Amin, M.N.; Siddiqui, S.A.; Hossain, M.P.; Sultana, F.; Kabir, M.R. Trans Fatty Acids and Lipid Profile: A Serious Risk Factor to Cardiovascular Disease, Cancer and Diabetes. Diabetes Metab. Syndr. 2019, 13, 1643–1647. [Google Scholar] [CrossRef]
- Heinloth, A.; Heermeier, K.; Raff, U.; Wanner, C.; Galle, J. Stimulation of NADPH Oxidase by Oxidized Low-Density Lipoprotein Induces Proliferation of Human Vascular Endothelial Cells. J. Am. Soc. Nephrol. 2000, 11, 1819–1825. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.Z.; Rabinovitch, P.S.; Tabas, I. Macrophage Mitochondrial Oxidative Stress Promotes Atherosclerosis and Nuclear Factor-κB-Mediated Inflammation in Macrophages. Circ. Res. 2014, 114, 421–433. [Google Scholar] [CrossRef]
- Feldman, F.; Koudoufio, M.; Desjardins, Y.; Spahis, S.; Delvin, E.; Levy, E. Efficacy of Polyphenols in the Management of Dyslipidemia: A Focus on Clinical Studies. Nutrients 2021, 13, 672. [Google Scholar] [CrossRef]
- Cayatte, A.J.; Rupin, A.; Oliver-Krasinski, J.; Maitland, K.; Sansilvestri-Morel, P.; Boussard, M.F.; Wierzbicki, M.; Verbeuren, T.J.; Cohen, R.A. S17834, a New Inhibitor of Cell Adhesion and Atherosclerosis That Targets Nadph Oxidase. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1577–1584. [Google Scholar] [CrossRef]
- Zang, M.; Xu, S.; Maitland-Toolan, K.A.; Zuccollo, A.; Hou, X.; Jiang, B.; Wierzbicki, M.; Verbeuren, T.J.; Cohen, R.A. Polyphenols Stimulate AMP-Activated Protein Kinase, Lower Lipids, and Inhibit Accelerated Atherosclerosis in Diabetic LDL Receptor-Deficient Mice. Diabetes 2006, 55, 2180–2191. [Google Scholar] [CrossRef] [PubMed]
- Frémont, L.; Belguendouz, L.; Delpal, S. Antioxidant Activity of Resveratrol and Alcohol-Free Wine Polyphenols Related to LDL Oxidation and Polyunsaturated Fatty Acids. Life Sci. 1999, 64, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Fuhrman, B. Wine Flavonoids Protect against LDL Oxidation and Atherosclerosis. Ann. N. Y. Acad. Sci. 2002, 957, 146–161. [Google Scholar] [CrossRef]
- Berrougui, H.; Grenier, G.; Loued, S.; Drouin, G.; Khalil, A. A New Insight into Resveratrol as an Atheroprotective Compound: Inhibition of Lipid Peroxidation and Enhancement of Cholesterol Efflux. Atherosclerosis 2009, 207, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Regnström, J.; Nilsson, J.; Tornvall, P.; Landou, C.; Hamsten, A. Susceptibility to Low-Density Lipoprotein Oxidation and Coronary Atherosclerosis in Man. Lancet 1992, 339, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, J.W. Oxidants and Antioxidants in the Pathogenesis of Atherosclerosis: Implications for the Oxidized Low Density Lipoprotein Hypothesis. Atherosclerosis 1998, 141, 1–15. [Google Scholar] [CrossRef]
- Li, J.; Zhong, Z.; Yuan, J.; Chen, X.; Huang, Z.; Wu, Z. Resveratrol Improves Endothelial Dysfunction and Attenuates Atherogenesis in Apolipoprotein E-Deficient Mice. J. Nutr. Biochem. 2019, 67, 63–71. [Google Scholar] [CrossRef]
- Yang, H.; Yang, T.; Heng, C.; Zhou, Y.; Jiang, Z.; Qian, X.; Du, L.; Mao, S.; Yin, X.; Lu, Q. Quercetin Improves Nonalcoholic Fatty Liver by Ameliorating Inflammation, Oxidative Stress, and Lipid Metabolism in Db/Db Mice. Phytother. Res. 2019, 33, 3140–3152. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and Metabolic Syndrome: A Review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




