Submitted:
30 June 2023
Posted:
04 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
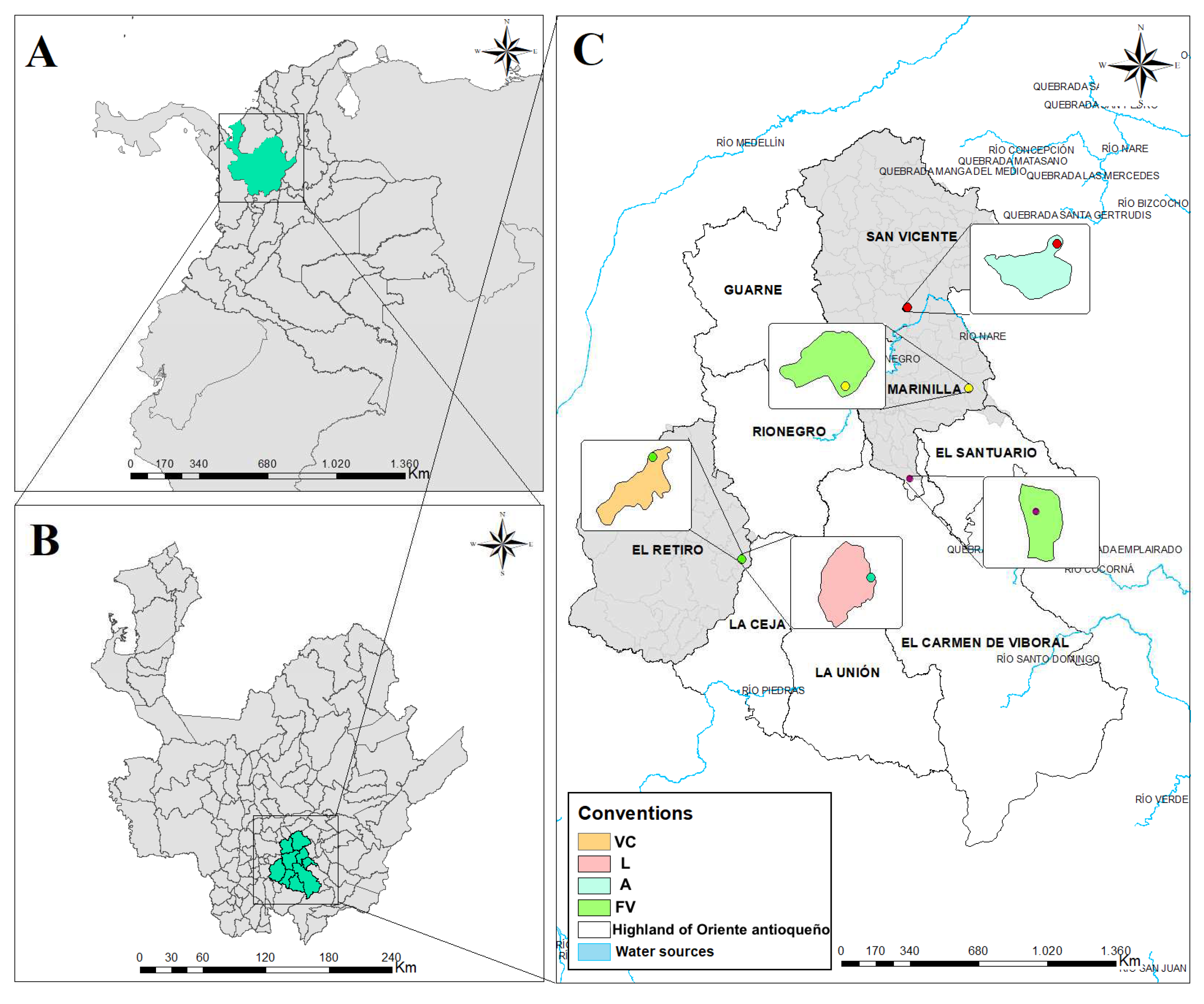
2.1. Study area
2.2. Sampling design
2.3. Data analysis
- Where:
- Cm= sampling coverage
- S= total number of species sampled
- pi= relative abundance of the i-th species
- m= sample size
- Where:
- Specificity = Nindij/Nindi
- Fidelity = Ntrapij/Ntrapj
- Nindij = the average number of individuals of species i in type j habitat
- Nindi= the sum of the average number of individuals of species i in all habitat types
- Ntrapij= the number of individuals in habitat j where species i is present
- Ntrapj = the total number of individuals in habitat j.
3. Results
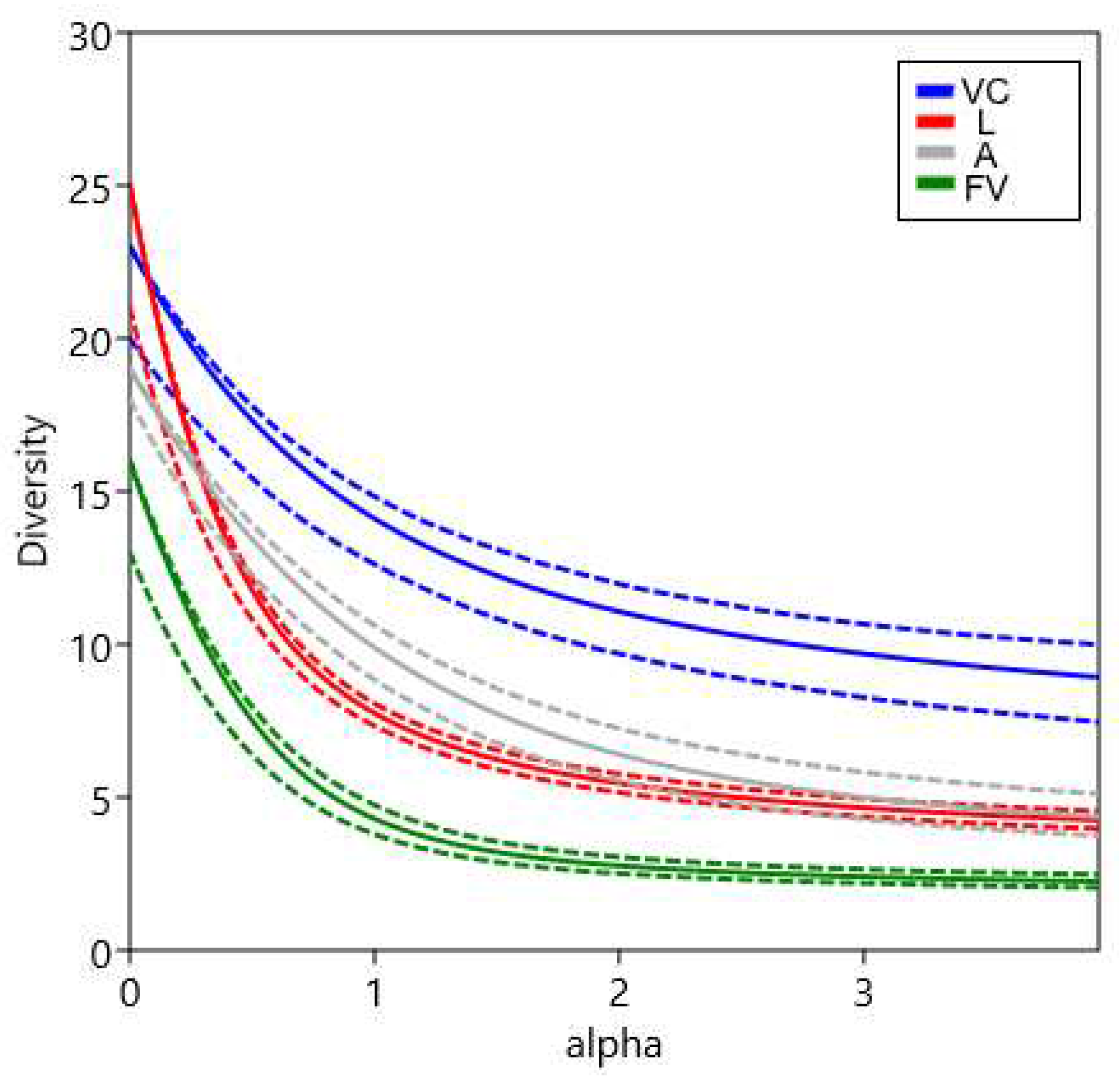
3.1. Species richness
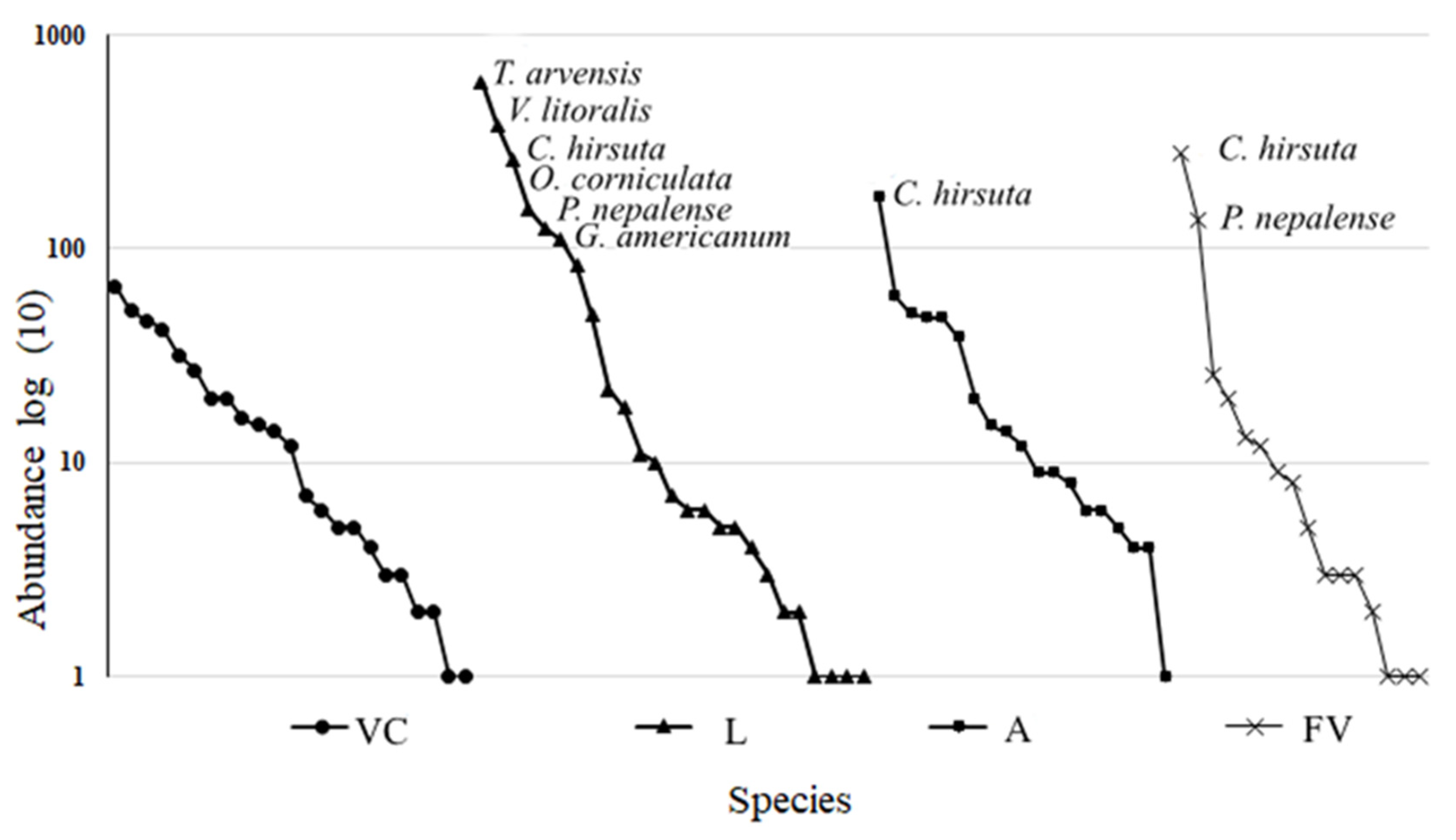
3.2. Species composition
3.2.1. Seed Banks composition
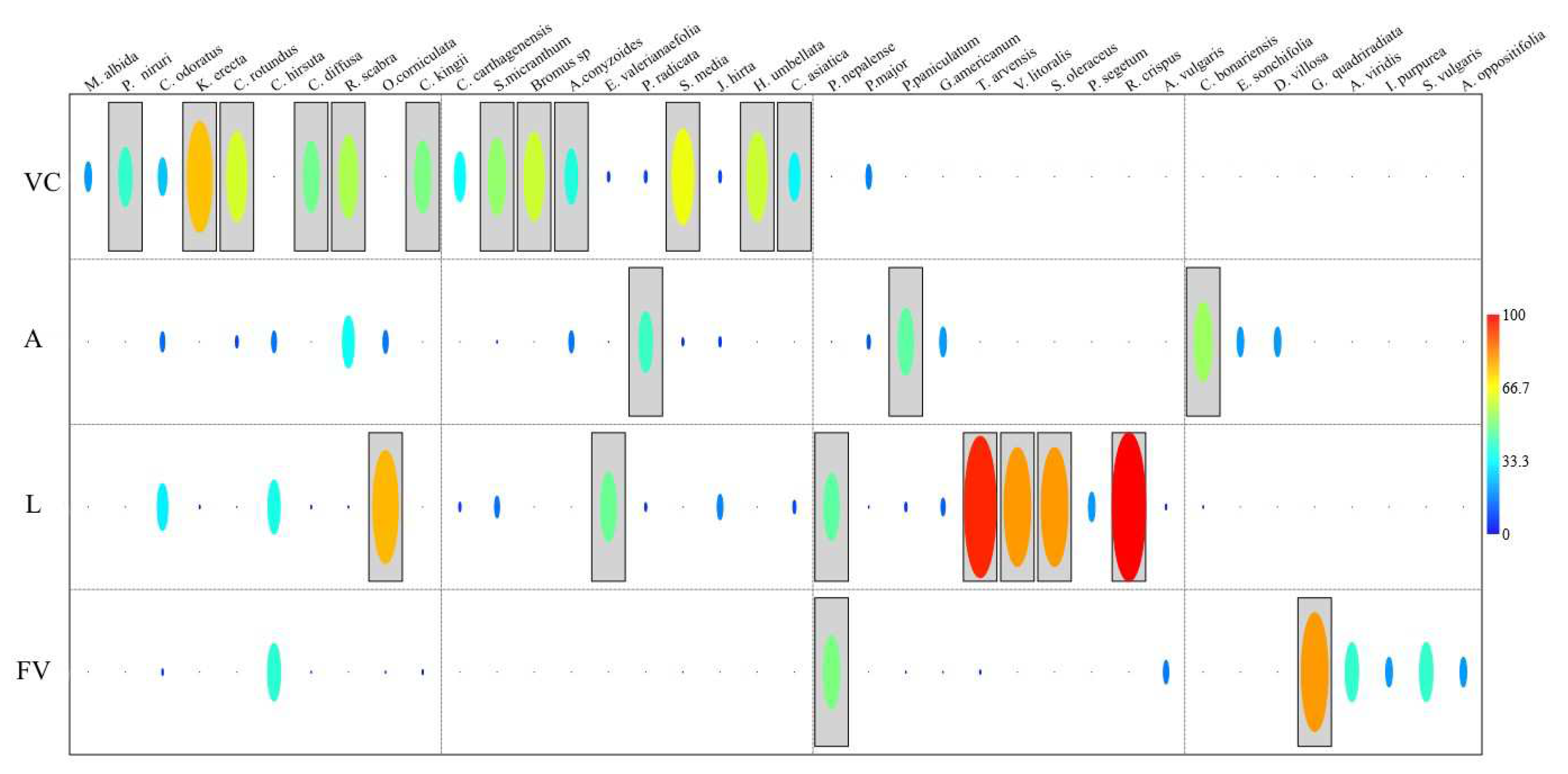
3.2.2. Composition of the seed banks and their surface vegetation
3.2.3. Indicator species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monaco, T.J.; Weller, S.G.; Ashton, F.M. Weed Science: Principles and Practices; 4th ed.; Wiley-Blackwell: New Jersey, United States, 2002; ISBN 978-0-471-37051-2. [Google Scholar]
- Buhler, D.D.; Kohler, K.A.; Thompson, R.L. Weed Seed Bank Dynamics During a Five-Year Crop Rotation. Weed Technol. 2001, 15, 170–176. [Google Scholar] [CrossRef]
- Baker, H. The Evolution of Weeds. Annu. Rev. Ecol. Syst. 1974, 5, 1–24. [Google Scholar] [CrossRef]
- Williams, C. Potential Valuable Ecological Functions of Nonindigenous Plants. In Assessment and Management of Plant Invasions; Luken, J.., Thieret, J.., Eds.; Springer-Verlag: New York, U.S.A, 1997; pp. 26–34. [Google Scholar]
- Jordan, N.; Vatovec, C. Agroecological Benefits from Weeds. In Weed Biology and Management; Inderjit, Ed.; Springer: Dordrecht, 2004; pp. 137–158. [Google Scholar]
- Gideon, K.M.; Samuel, O.A.; Joseph, S.; Marius, M.; Emmanuel, H. Effect of Linear View Approach of Weed Management in Agro-Ecosystem: A Review. African J. Agric. Res. 2021, 17, 238–246. [Google Scholar] [CrossRef]
- Zimbahl, R.L. Fundamentals of Weed Science; Project manager: Colorado, 2018; ISBN 978-0-12-811143-7. [Google Scholar]
- Thomas, A.G.; Derksen, D.A.; Blackshaw, R.E.; Van Acker, R.C.; Légère, A.; Watson, P.R.; Turnbull, G.C. A Multistudy Approach to Understanding Weed Population Shifts in Medium- to Long-Term Tillage Systems. Weed Sci. 2004, 52, 874–880. [Google Scholar] [CrossRef]
- Sheley, R.L.; James, J.; Rinella, M.-M.; Blumenthal, D.; DiTomaso, J. Invasive Plant Management on Anticipated Conservation Benefits: A Scientific Assessment 2011, 291–336.
- Nichols, V.; Verhulst, N.; Cox, R.; Govaerts, B. Weed Dynamics and Conservation Agriculture Principles: A Review. F. Crop. Res. 2015, 183, 56–68. [Google Scholar] [CrossRef]
- Buhler, D.D.; Mester, T.C.; Kohler, K.A. The Effect of Maize Residues and Tillage on Emergence of Setaria faberi, Abutilon theophrasti, Amaranthus retroflexus and Chenopodium album. Weed Res. 1996, 36, 153–165. [Google Scholar] [CrossRef]
- Farooq, M.; Flower, K.C.; Jabran, K.; Wahid, A.; Siddique, K.H.M. Crop Yield and Weed Management in Rainfed Conservation Agriculture. Soil Tillage Res. 2011, 117, 172–183. [Google Scholar] [CrossRef]
- Liebman, M.; Dyck, E. Crop Rotation and Intercropping Strategies for Weed Management. Ecol. Appl. 2011, 3, 92–122. [Google Scholar] [CrossRef]
- Travlos, I.S.; Cheimona, N.; Roussis, I.; Bilalis, D.J. Weed-Species Abundance and Diversity Indices in Relation to Tillage Systems and Fertilization. Front. Environ. Sci. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Raczka, M.F.; Mosblech, N.A.; Giosan, L.; Valencia, B.G.; Folcik, A.M.; Kingston, M.; Baskin, S.; Bush, M.B. A Human Role in Andean Megafaunal Extinction? Quat. Sci. Rev. 2019, 205, 154–165. [Google Scholar] [CrossRef]
- Correa Ayram, C.A.; Etter, A.; Díaz-Timoté, J.J.; Rodríguez Buriticá, S.; Ramírez, W.; Corzo, G. Spatiotemporal Evaluation of the Human Footprint in Colombia: Four Decades of Anthropic Impact in Highly Biodiverse Ecosystems. Ecol. Indic. 2020, 117, 1–24. [Google Scholar] [CrossRef]
- Plaza, G.; Quintana, D.; Aponte, L.; Chaves, B. Characterization of the Weed Community of a Rose Greenhouse Production System in the Bogota Plateau. Agron. Colomb. 2009, 27, 385–394. [Google Scholar]
- Moreno-Preciado, O.E.; Balaguera-Lopez, H.E. Characterization of the Weed Community and Its Diversity in a Statistical Modeling in Peach (Prunus Persica (L.) Batsch.) Orchard. Rev. U.D.C.A Actual. Divulg. Científica 2021, 24, 1–10. [Google Scholar] [CrossRef]
- Quijano-abril, M.A.; Castaño-López, M. de los Á.; Marín-Henao, D.; Sánchez-Gómez, D.; Rojas-Villa, J.M.; Sierra-Escobar, J. Functional Traits of the Invasive Species Thunbergia Alata (Acanthaceae) and Its Importance in the Adaptation to Andean Forests. Acta Bot. Mex. 2021, 128, 1–23. [Google Scholar] [CrossRef]
- Buhler, D.D.; Hartzler, R.G.; Forcella, F. Weed Seed Bank Dynamics: Implications to Weed Management. J. Crop Prod. 1998, 1, 145–168. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. The Role of Seed Ecology in Improving Weed Management Strategies in the Tropics. Adv. Agron. 2010, 105, 221–262. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeler, R.A.; Mittermeler, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Holdridge, L.R. Life Zone Ecology; 1967; ISBN B0007JDYF2.
- Cornare; Alianza Clima y Desarrollo; Fundación Natura; World Wildlife Fund Actividad Económica Actual En El Oriente Antioqueño y Perspectivas de Crecimiento Verde y Desarrollo Compatible Con El Clima - Anexo 4. 2016, 55.
- Cámara de comercio Oriente Antioqueño Concepto Economico Del Oriente Antioqueño 2018. Cámara Comer. del Oriente Antioqueño 2017.
- Ideam Leyenda Nacional de Coberturas de La Tierra. Metodología CORINE Land Cover Adaptada Para Colombia, Escala 1:100.000; 2010; Vol. TH-62-04-1; ISBN 9789588067292.
- Buhler, D.D.; Kohler, K.A.; Thompson, R.L. Weed Seed Bank Dynamics During a Five-Year Crop Rotation. Weed Technol. 2001, 15, 170–176. [Google Scholar] [CrossRef]
- Bigwood, D.W.; Inouye, D.W. Spatial Pattern Analysis of Seed Banks : An Improved Method and Optimized Sampling Author ( s ): Douglas W. Bigwood and David W. Inouye Published by : Ecological Society of America Stable URL : Http://Www.Jstor.Org/Stable/1940448. Ecology 1998, 69, 497–507. [Google Scholar] [CrossRef]
- Montenegro, A.; Ávila, Y.; Mendivelso, H.; Vargas, O. Potential of the Seed Bank in the Regeneration of Plant Communities at Jaboque Wetland, Bogotá, Colombia. Caldasia 2006, 28, 285–306. [Google Scholar]
- Idárraga Piedrahíta, Á.; Ortíz, R.del C. Callejas Posada, R.; Merello, M. Flora de Antioquia. Catálogo de Las Plantas Vasculares. Listado de Las Plantas Vasculares Del Departamento de Antioquia; Medellin, Colombia, 2011; ISBN 9789588709598.
- Lorena Gámez, A.; Rojas, L.M. Guía Ilustrada de Plantas Arvenses Del Centro Agropecuario Marengo (CAM); 2018; ISBN 978-958-783-393-5.
- Salazar Gutiérrez, L.F. Arvenses Frecuentes En El Cultivo de Café En Colombia; Cenicafé.; 2021; ISBN 9789588490489.
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Jost, L. Entropy and Diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.H.; Hsieh, T.C.; Inouye, B.D. Proposing a Resolution to Debates on Diversity Partitioning. Ecology 2012, 93, 2037–2051. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Jost, L. Coverage-Based Rarefaction and Extrapolation: Standardizing Samples by Completeness Rather than Size Author(s): Anne Chao and Lou Jost Source: Ecology 2012, 93, 2533–2547.
- Chao, A.; Chiu, C.H.; Hsieh, T.C.; Inouye, B.D. Proposing a Resolution to Debates on Diversity Partitioning. Ecology 2012, 93, 2037–2051. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Springer: Dordrecht, 1988. [Google Scholar]
- Jost, L. Partitioning Diversity into Independent Alpha Beta Concepts. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef]
- Baselga, A. Separating the Two Components of Abundance-Based Dissimilarity: Balanced Changes in Abundance vs. Abundance Gradients. Methods Ecol. Evol. 2013, 4, 552–557. [Google Scholar] [CrossRef]
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. DufreneLegendre1997_species Assemblages and Indicator Species_the Need for Flexible Asymmetrical Approach.PDF. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- Wilson, W.L.; Abernethy, V.J.; Murphy, K.J.; Adam, A.; Mccracken, D.I.; Downie, I.S.; Foster, G.N.; Furness, R.W.; Waterhouse, A.; Ribera, I. Prediction of Plant Diversity Response to Land-Use Change on Scottish Agricultural Land. Agric. Ecosyst. Environ. 2003, 94, 249–263. [Google Scholar] [CrossRef]
- Légère, A.; Stevenson, F.C.; Benoit, D.L. Diversity and Assembly of Weed Communities: Contrasting Responses across Cropping Systems. Weed Res. 2005, 45, 303–315. [Google Scholar] [CrossRef]
- Storkey, J.; Neve, P. What Good Is Weed Diversity? Weed Res. 2018, 58, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Barroso, J.; Miller, Z.J.; Lehnhoff, E.A.; Hatfield, P.G.; Menalled, F.D. Impacts of Cropping System and Management Practices on the Assembly of Weed Communities. Weed Res. 2015, 55, 426–435. [Google Scholar] [CrossRef]
- Mitja, D.; Miranda, I.S. Weed Community Dynamics in Two Pastures Grown after Clearing Brazilian Amazonian Rainforest. Weed Res. 2010, 50, 163–173. [Google Scholar] [CrossRef]
- Dorado, J.; Lo, C. The Effect of Tillage System and Use of a Paraplow on Weed Flora in a Semiarid Soil from Central Spain. Weed Res. 2006, 424–431. [Google Scholar] [CrossRef]
- Armengot, L.; José-maría, L. Weed Harrowing in Organically Grown Cereal Crops Avoids Yield Losses without Reducing Weed Diversity. Agron. Sustain. Dev. 2012, 33, 405–411. [Google Scholar] [CrossRef]
- Moreno-Preciado, O.E.; Balaguera-Lopez, H.E. Caracterización de La Comunidad de Malezas y Su Diversidad En Una Modelación Estadística En Un Cultivo de Duraznero (Prunus persica (L.) Batsch.). Rev. U.D.C.A Actual. Divulg. Científica 2021, 24, 1–10. [Google Scholar] [CrossRef]
- Shrestha, A.; Knezevic, S.Z.; Roy, R.C.; Ball-Coelho, B.R.; Swanton, C.J. Effect of Tillage, Cover Crop and Crop Rotation on the Composition of Weed Flora in a Sandy Soil. Weed Res. 2002, 42, 76–87. [Google Scholar] [CrossRef]
- Bàrberi, P.; Silvestri, N.; Bonari, E. Weed Communities of Winter Wheat as Influenced by Input Level and Rotation. Weed Res. 1997, 37, 301–313. [Google Scholar] [CrossRef]
- Swanton, C.J.; Clements, D.R.; Derksen, D.A. Weed Succession under Conservation Tillage: A Hierarchical Framework for Research and Management. Weed Technol. 1993, 7, 286–297. [Google Scholar] [CrossRef]
- Anderson, R.L. A Multi-Tactic Approach to Manage Weed Population Dynamics in Crop Rotations. Agron. J. 2005, 97, 1579–1583. [Google Scholar] [CrossRef]
- Buhler, D.D.; Stoltenberg, D.E.; Becker, R.L.; Gunsolus, J.L. Perennial Weed Populations After 14 Years of Variable Tillage and Cropping Practices. Weed Sci. 1994, 42, 205–209. [Google Scholar] [CrossRef]
- Cardina, J.; Herms, C.P.; Doohan, D.J. Crop Rotation and Tillage System Effects on Weed Seedbanks. Weed Sci. 2002, 50, 448–460. [Google Scholar] [CrossRef]
- Sosnoskie*, L.M.; Cardina, J.; Herms, C.P.; Kleinhenz, M. Weed Seedbank Community Composition in a 35-Year-Old Tillage and Rotation Experiment. HortScience 2019, 39, 845C–845. [Google Scholar] [CrossRef]
- Santín-Montanyá, M.I.; Martín-Lammerding, D.; Walter, I.; Zambrana, E.; Tenorio, J.L. Effects of Tillage, Crop Systems and Fertilization on Weed Abundance and Diversity in 4-Year Dry Land Winter Wheat. Eur. J. Agron. 2013, 48, 43–49. [Google Scholar] [CrossRef]
- Gruber, S.; Claupein, W. Effect of Tillage Intensity on Weed Infestation in Organic Farming. Soil Tillage Res. 2009, 105, 104–111. [Google Scholar] [CrossRef]
- Sharp, D. A Phytosociological Study of Weed Communities on the Southwestern Coastal Plain of North Carolina. Plant Ecol. 1976, 31, 103–119. [Google Scholar] [CrossRef]
- Tuesca, D.; Puricelli, E.; Papa, J.C. A Long-Term Study of Weed Flora Shifts in Different Tillage Systems. Weed Res. 2001, 41, 369–382. [Google Scholar] [CrossRef]
- Taa, A.; Tanner, D.; Bennie, A.T.P. Effects of Stubble Management, Tillage and Cropping Sequence on Wheat Production in the South-Eastern Highlands of Ethiopia. Soil Tillage Res. 2004, 76, 69–82. [Google Scholar] [CrossRef]
- Heijting, S.; Van Der Werf, W.; Kropff, M.J. Seed Dispersal by Forage Harvester and Rigid-Tine Cultivator in Maize. Weed Res. 2009, 49, 153–163. [Google Scholar] [CrossRef]
- Kudoh, H.; Nakayama, M.; Lihová, J.; Marhold, K. Does Invasion Involve Alternation of Germination Requirements? A Comparative Study between Native and Introduced Strains of an Annual Brassicaceae, Cardamine hirsuta. Ecol. Res. 2007, 22, 869–875. [Google Scholar] [CrossRef]
- Pedraza, M.; Plaza, G.A. Reconocimiento y Caracterización Ecológica de La Flora Arvense Asociada Al Cultivo de Uchuva. 2007, 25, 306–313.
- Yatsu, Y.; Kachi, N.; Kudoh, H. Ecological Distribution and Phenology of an Invasive Species, Cardamine Hirsuta L., and Its Native Counterpart, Cardamine Flexuosa With., in Central Japan. Plant Species Biol. 2003, 18, 35–42. [Google Scholar] [CrossRef]
- Robertson, K.R. The Oxalidaceae in the Southeastern United States. J. Arnold Arbor. 1975, 56, 223–239. [Google Scholar] [CrossRef]
- Holt, J.S. Factors Affecting Germination in Greenhouse-Produced Seeds of Oxalis Corniculata, a Perennial Weed. Am. J. Bot. 1987, 74, 429–436. [Google Scholar] [CrossRef]
- Gómez-Aristizábal, A.; Rivera-Posada, H. Descripción de Malezas En Las Plantaciones de Café. 1987, 481.
- Roberts, H.A.; Stokes, F.G. Studies on the Weeds of Vegetable Crops. VI. Seed Populations of Soil Under Commercial Cropping. J. Appl. Ecol. 1966, 3, 181. [Google Scholar] [CrossRef]
- Köllmann, P.; Waldhardt, R. Farming Intensity Affects Soil Seedbank Composition and Spontaneous Vegetation of Arable Weeds. Diversity 2022, 14. [Google Scholar] [CrossRef]
- Ludvíková, V.; Pavl, V.; Pavl, L.; Gaisler, J.; Hejcman, M. Sward-Height Patches under Intensive and Extensive Grazing Density in an Agrostis Capillaris Grassland. Folia Geobotanica 2015, 50, 219–228. [Google Scholar] [CrossRef]
- Gazoulis, I.; Kanatas, P.; Antonopoulos, N. Cultural Practices and Mechanical Weed Control for the Management of a Low-Diversity Weed Community in Spinach. Diversity 2021, 13. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Ecological Studies on Cyperus Difformis, Cyperus Iria and Fimbristylis Miliacea: Three Troublesome Annual Sedge Weeds of Rice. Ann. Appl. Biol. 2009, 155, 103–112. [Google Scholar] [CrossRef]
- Gaba, S.; Perronne, R.; Fried, G.; Gardarin, A.; Bretagnolle, F.; Biju-Duval, L.; Colbach, N.; Cordeau, S.; Fernández-Aparicio, M.; Gauvrit, C.; et al. Response and Effect Traits of Arable Weeds in Agro-Ecosystems: A Review of Current Knowledge. Weed Res. 2017, 57, 123–147. [Google Scholar] [CrossRef]
- Romero, A.; Chamorro, L.; Sans, F.X. Weed Diversity in Crop Edges and Inner Fields of Organic and Conventional Dryland Winter Cereal Crops in NE Spain. Agric. Ecosyst. Environ. 2008, 124, 97–104. [Google Scholar] [CrossRef]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape Perspectives on Agricultural Intensification and Biodiversity - Ecosystem Service Management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Marleen, M. Riemens, Roei M. W. Groeneveld, Martin J. J. Kropff, Lambertus A. P. Lotz, R.J.R.; Wijnand Sukkel, and R.Y. van der W. Linking Farmer Weed Management Behavior with Weed Pressure: More than Just Technology. Weed Sci. 2010, 58, 490–496. [Google Scholar] [CrossRef]
| Family | Species | VC | L | A | FV |
|---|---|---|---|---|---|
| Amaranthaceae | Amaranthus viridis L. | 3* | |||
|
Apiaceae Araliaceae |
Centella asiatica (L.) Urb | 7* | 6 | ||
| Hydrocotyle umbellata L. | 6* | ||||
| Asteraceae | Ageratum conyzoides L. | 20* | 12* | ||
| Erechtites valerianaefolia C.E.C. Fisch | 5* | 22* | 1* | ||
| Porcellites radicata (L.) Cass. | 15* | 7* | 48* | ||
| Jaegeria hirta (Lag.) Less. | 3* | 6* | 5* | ||
| Gnaphalium americanum Mill. | 110* | 60* | 9* | ||
| Sonchus oleraceus L. | 18* | ||||
| Artemisia vulgaris L. | 5 | 20* | |||
| Conyza bonariensis (L.) Cronquist | 1* | 9* | |||
| Emilia sonchifolia (L.) DC. | 6* | ||||
| Galinsoga quadriradiata Ruiz & Pav. | 13* | ||||
| Senecio vulgaris L. | 3* | ||||
| Acmella oppositifolia (Lam.) R.K. Jansen | 1* | ||||
| Brassicaceae | Cardamine hirsuta L. | 2 | 263 | 176 | 282 |
| Caryophyllaceae | Stellaria media (L.) Vill. | 46 | 1 | 8* | 2* |
| Drymaria villosa Schltdl. & Cham | 15* | ||||
| Commelinaceae | Commelina diffusa Burm. f. | 12* | 2* | 1* | |
| Convolvulaceae | Ipomoea purpurea (L.) Roth | 1* | |||
| Cyperaceae | Cyperus odoratus L. | 67 | 83 | 48* | 12* |
| Cyperus rotundus L. | 51 | 2 | 14* | ||
| Kyllinga erecta Schumach. | 42 | 3 | |||
| Fabaceae | Mimosa albida Humb. & Bonpl. ex Willd | 3* | |||
| Melastomataceae | Chaetogastra kingii (Wurdack) P.J.F. Guim. & Michelang. | 14* | 3* | ||
| Lythraceae | Cuphea carthagenensis (Jacq.) J.F. Macbr. | 20* | 4* | ||
| Iridaceae | Sisyrinchium micranthum Cav. | 27* | 10* | 4* | |
| Lamiaceae | Trixella arvensis (L.) Fourr. | 1 | 605* | 4* | 26* |
| Phyllanthaceae | Phyllanthus niruri L. | 2* | |||
| Rubiaceae | Richardia scabra L. | 32* | 5 | 20* | |
| Oxalidaceae | Oxalis corniculata L. | 1 | 152* | 39* | 8* |
| Poaceae | Bromus sp. L. | 16* | |||
| Paspalum paniculatum L. | 11* | 50* | 5* | ||
| Polygonaceae | Polygonum nepalense Meisn. | 4* | 125* | 9* | 136* |
| Polygonum segetum Kunth | 1* | ||||
| Rumex crispus L. | 49* | ||||
| Plantaginaceae | Plantago major L. | 5* | 1* | 6* | |
| Verbenaceae | Verbena litoralis Kunth | 380* |
| Diversity | VC | L | A | FV | Total | |
|---|---|---|---|---|---|---|
| Observed | q0 | 23 [18.89;27.11] | 25 [19.16;30.84] | 19 [17.59;20.41] | 16 [8.37;23.63] | 38 [32;43] |
| q1 | 14.11 [13.03;15.19] | 7.75 [7.37;8.13] | 9.89 [9.10;10.68] | 4.30 [3.84;4.76] | 13.40 [12.87;13.94] | |
| q2 | 11.07 [9.80;12.35] | 5.47 [5.16;5.77] | 6.40 [5.48;7.33] | 2.77 [2.50;3.04] | 8.58 [8.18;8.97] | |
| Estimated | q0 | 23.86 [18.42;29.30] | 27.53 [18.54;36.51] | 19.00 [17.78; 20.22] | 18.19 [8.52;27.86] | 40.19 [32.80;47.58] |
| q1 | 14.39 [13.28;15.49] | 7.79 [7.40;8.18] | 10.01 [9.13;10.88] | 4.35 [3.88;4.81] | 13.46 [12.92;14.00] | |
| q2 | 11.21 [9.89;12.54] | 5.47 [5.17;5.78] | 6.44 [5.55;7.33] | 2.77 [2.50;3.04] | 8.59 [8.19;8.98] | |
| Sample coverage | 0.995 | 0.998 | 1 | 0.994 | 0.9991 | |
| VC x L | VC x A | VC x FV | L x A | L x FV | A x FV | |
|---|---|---|---|---|---|---|
| β 0 | 0.29 | 0.33 | 0.58 | 0.27 | 0.51 | 0.54 |
| β 1 | 0.41 | 0.46 | 0.79 | 0.24 | 0.21 | 0.30 |
| β 2 | 0.84 | 0.60 | 0.91 | 0.43 | 0.41 | 0.15 |
| β.bray.bal (turnover) | |||||
|---|---|---|---|---|---|
| β.bray.gra (nesting) | VC | L | A | FV | |
| VC | 0 | 0.69 | 0.655 | 0.935 | |
| L | 0.2 | 0 | 0.299 | 0.133 | |
| CA | 0.048 | 0.389 | 0 | 0.571 | |
| FV | 0.008 | 0.487 | 0.003 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




