Submitted:
29 June 2023
Posted:
04 July 2023
You are already at the latest version
Abstract
Keywords:
Introduction
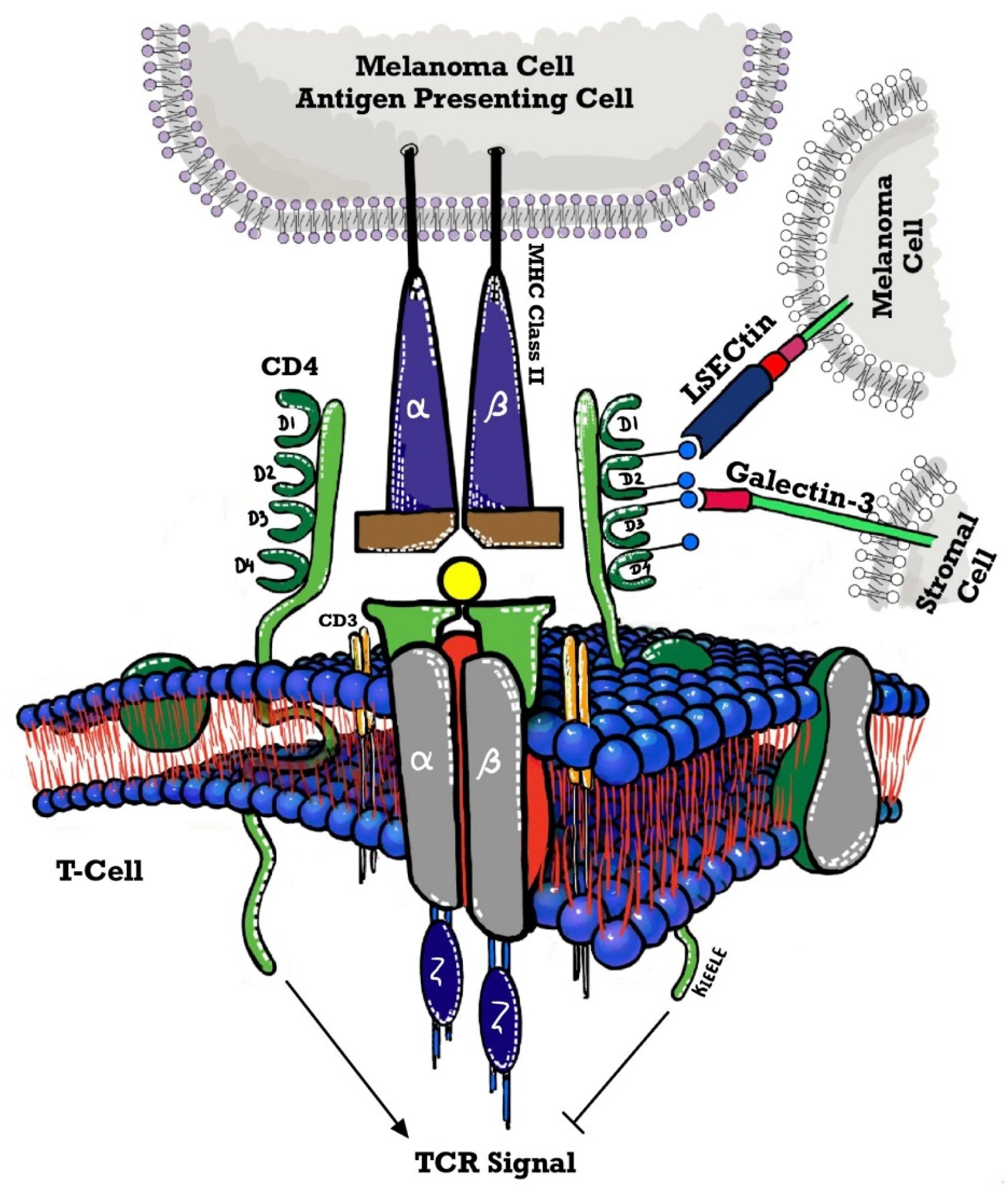
LAG-3 targeted therapy and its role in hematological malignancies
- 1.
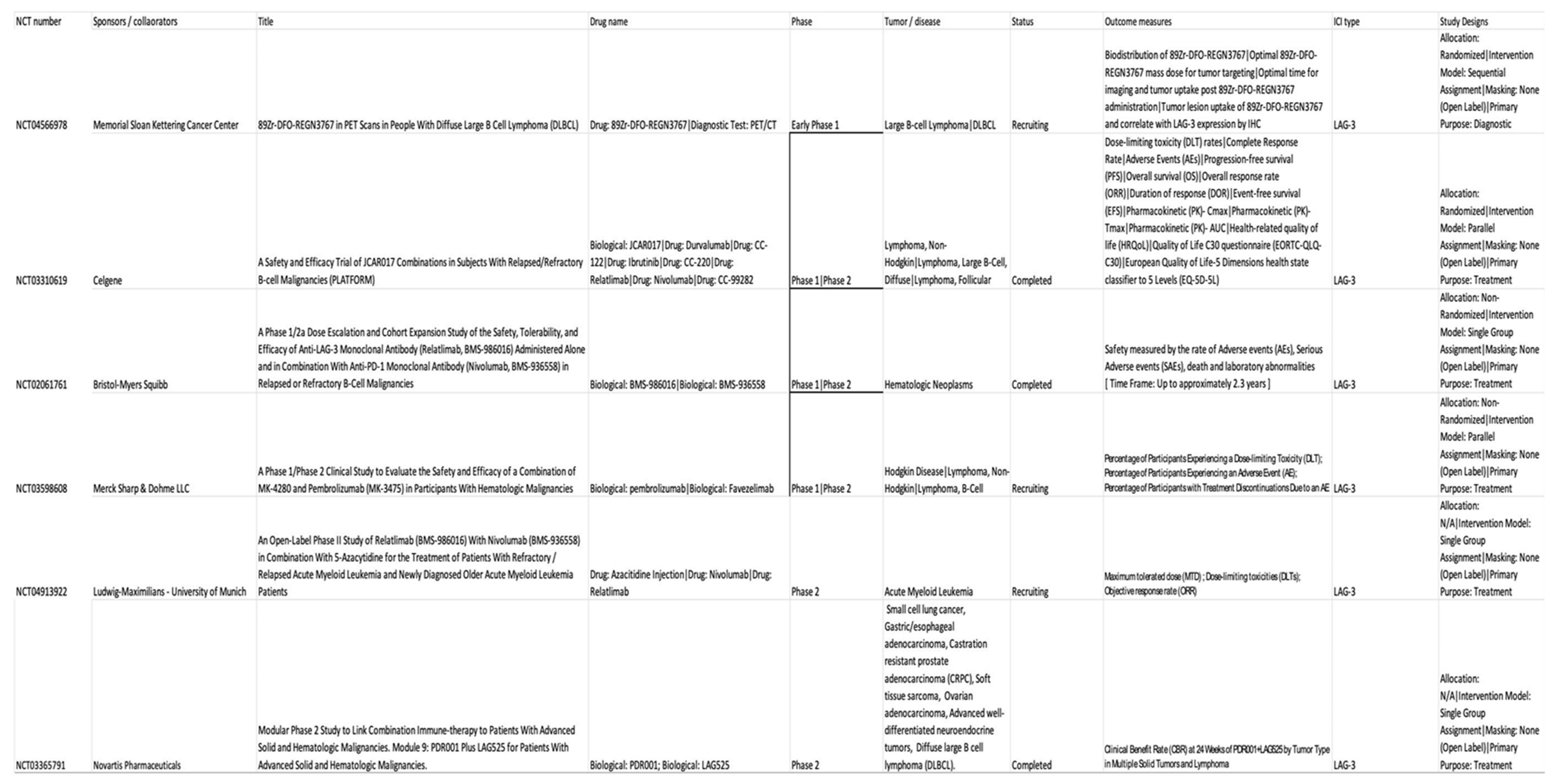
- Use of 89Zr-DFO-REGN3767 in PET Scans in people with diffuse large B Cell lymphoma (DLBCL) was the pilot study (NCT04566978) [32] undertaken at Memorial Sloan Kettering Hospital in 2022 with the main purpose of the study is looking at the way the body absorbs, distributes, and gets rid of 89Zr-DFO-REGN3767 [33]. 89Zr-DFO-REGN3767 is comprised of the anti-LAG-3 antibody, REGN3767 labeled with the positron-emitter zirconium-89 (89Zr) through the chelator-linker DFO and REGN3767 is an investigational monoclonal antibody that targets LAG-3 receptors. This study is a diagnostic research study determining the optimal time for imaging and tumor uptake post 89Zr-DFO-REGN3767 administration. However, it can help evaluate tumor uptake of 89Zr-DFO-REGN3767 and correlate with expression of LAG-3 by immunohistochemistry (IHC) in tumors that will be subsequently compared with other biomarkers of TME characterized in biopsies, such as IHC score (LAG-3 and/or other immune cell markers).
- 2.
- A safety and efficacy trial of JCAR017 (lisocabtagene maraleucel, also known as liso-cel) (a CD19-targeted chimeric antigen receptor CART-cell therapy) combinations in subjects with relapsed / refractory B-cell malignancies (PLATFORM) (NCT03310619) [34] was done. Relatlimab, BMS-986016 is an anti-LAG-3 fully human monoclonal IgG4-κ antibody that binds human LAG-3 with high affinity and inhibits its binding to MHC-II [35]. This trial was a global, open-label, multi-arm, parallel multi-cohort, multi-center, Phase 1/2 study to determine the safety, tolerability, pharmacokinetics, efficacy, and patient-reported quality of life of JCAR017 in combination with various agents including relatlimab, durvalumab, avadomide, iberdomide, ibrutinib, and nivolumab. The trial was completed, and the studied tumors were diffuse large B-cell lymphoma (DLBCL), non-Hodgkin lymphoma (NHL), and Follicular lymphoma (FL). The objective of the study during Phase 1 was to open different paths to test JCAR017 in combination with other agents in adult patients with R/R aggressive B-cell NHL. Different doses and schedules of JCAR017 were used in several arms and the combination agents were tested in several cohorts per arm. Phase 2 of the study involved the expansion of any dose level and schedule for any arm maintaining safety. All patients from Phase 1 and Phase 2 will then be followed for 24 months for adverse effects, survival, relapse, viral vector safety, and long term toxicity as per guidelines.
- 3.
- A similar trial was also designed with relatlimab by Bristol-Myers Squibb, NCT02061761 [36] administered alone or in combination with nivolumab to subjects with relapsed or refractory B-cell malignancies (relapsed or refractory Hodgkin lymphoma (HL) and relapsed or refractory DLBCL and to study its safety, tolerability, dose-limiting toxicities and maximum tolerated dose. The trial completed and studied hematological malignancies including chronic lymphocytic leukemia (CLL), HL, NHL, and Multiple Myeloma (MM). A detailed description of dose-related adverse events was studied and was +displayed in the result section of the trial.
- 4.
- Favezelimab (MK-4280) is another LAG-3 antibody that is studied in combination with pembrolizumab (MK-3475) in the clinical trial NCT03598608 [37] that was started in July 2018 to study and evaluate the safety and efficacy of these agents in hematologic malignancies. ). It included classical HL, DLBCL, and indolent HL. No results have been posted till the writing of this article. This study will also evaluate the safety and efficacy of pembrolizumab or favezelimab administered as monotherapy in participants with classical HL using a 1:1 randomized study design.
- 5.
- Relapsed or refractory acute myeloid leukemia (AML) and newly diagnosed older AML are included in the ClinicalTrials.gov Identifier: NCT04913922 [38] to study the combination of relatlimab with nivolumab and 5-azacytidine. No results have been posted yet.
Mechanism of action of LAG-3
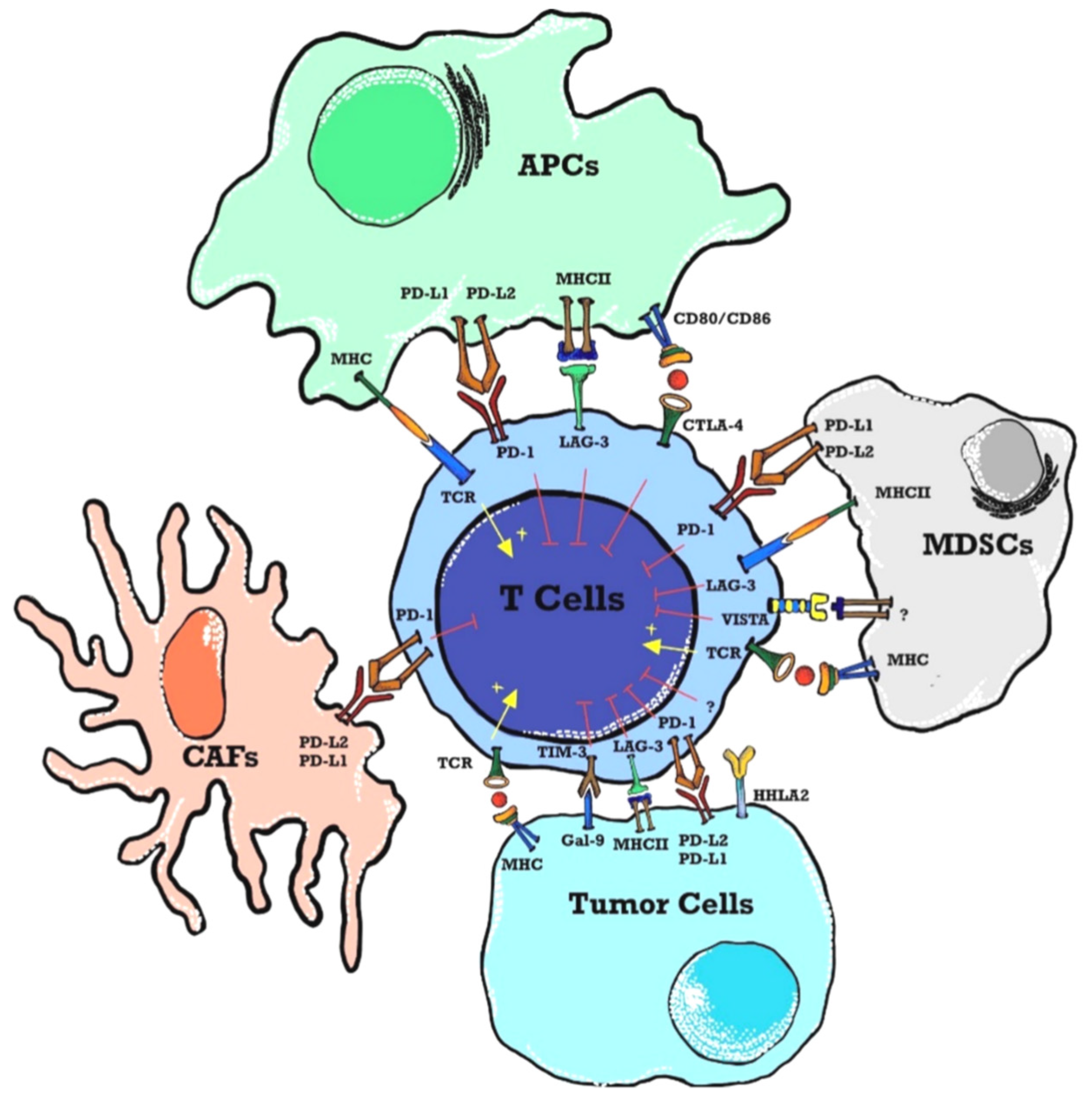
V-domain immunoglobulin suppressor of T cell activation (VISTA) targeted therapy and its role in hematological malignancies
Role of T cell immunoglobulin and mucin domain 3 (TIM-3) as next-generation ICI in hematological malignancies
Role of T cell immunoglobulin and immunoreceptor tyrosine-based inhibitory domain (TIGIT) as a target for next-generation ICI in hematological malignancies
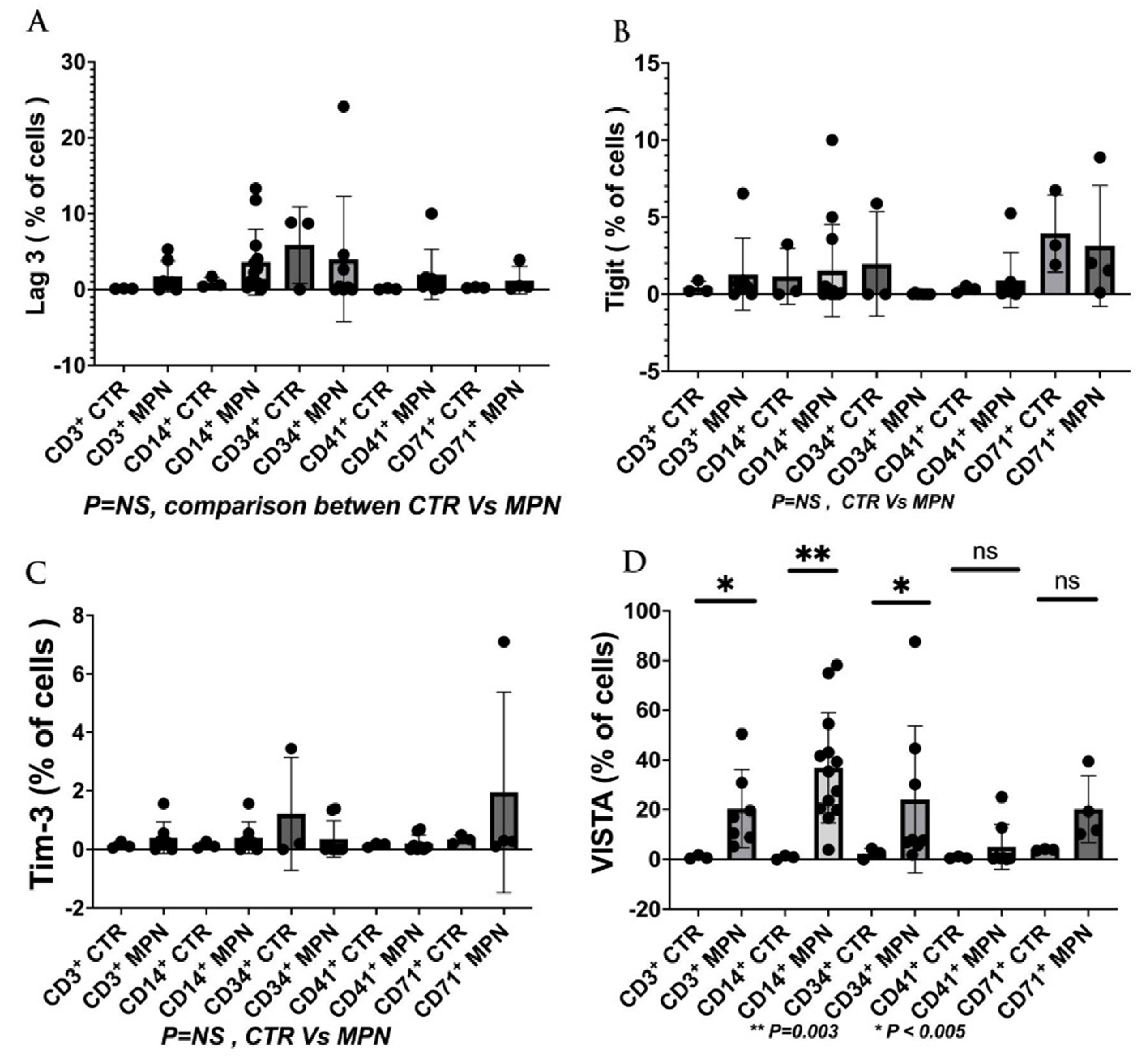
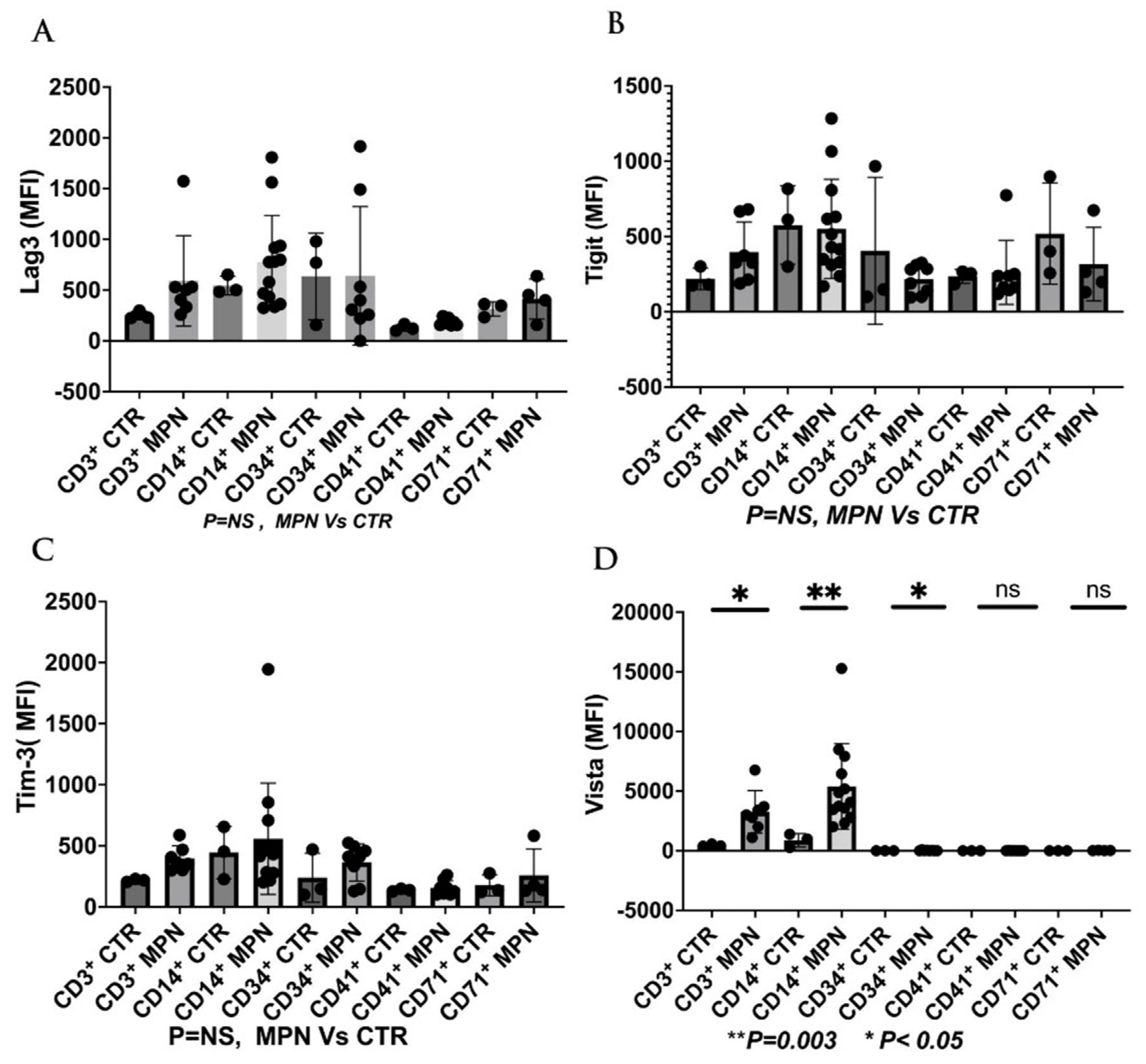
The current and future role of ICI in the management of MPN
Future directions and perspectives
References
- Passamonti F, Mora B, Maffioli M. New molecular genetics in the diagnosis and treatment of myeloproliferative neoplasms. Curr Opin Hematol. 2016;23(2):137-143. [CrossRef]
- Arber DA, Orazi A, Hasserjian RP, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140(11):1200-1228. [CrossRef]
- Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022;36(7):1703-1719. [CrossRef]
- Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017;129(6):667-679. [CrossRef]
- Fleischman AG, Aichberger KJ, Luty SB, et al. TNFα facilitates clonal expansion of JAK2V617F positive cells in myeloproliferative neoplasms. Blood. 2011;118(24):6392-6398. [CrossRef]
- James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144-1148. [CrossRef]
- Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. The Lancet. 2005;365(9464):1054-1061. [CrossRef]
- Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387-397. [CrossRef]
- Barosi G. An Immune Dysregulation in MPN. Curr Hematol Malig Rep. 2014;9(4):331-339. [CrossRef]
- Skov V, Larsen TS, Thomassen M, et al. Molecular profiling of peripheral blood cells from patients with polycythemia vera and related neoplasms: Identification of deregulated genes of significance for inflammation and immune surveillance. Leukemia Research. 2012;36(11):1387-1392. [CrossRef]
- Zhao W bo, Li Y, Liu X, Zhang L yan, Wang X. Involvement of CD4+CD25+ regulatory T cells in the pathogenesis of polycythaemia vera. Chin Med J (Engl). 2008;121(18):1781-1786.
- Skov V, Riley CH, Thomassen M, et al. Whole blood transcriptional profiling reveals significant down-regulation of human leukocyte antigen class I and II genes in essential thrombocythemia, polycythemia vera and myelofibrosis. Leukemia & Lymphoma. 2013;54(10):2269-2273. [CrossRef]
- Marty C, Lacout C, Droin N, et al. A role for reactive oxygen species in JAK2V617F myeloproliferative neoplasm progression. Leukemia. 2013;27(11):2187-2195. [CrossRef]
- Bjørn ME, Hasselbalch HC. The Role of Reactive Oxygen Species in Myelofibrosis and Related Neoplasms. Mediators of Inflammation. 2015;2015:e648090. [CrossRef]
- Wang JC, Kundra A, Andrei M, et al. Myeloid-derived suppressor cells in patients with myeloproliferative neoplasm. Leukemia Research. 2016;43:39-43. [CrossRef]
- Marzec M, Zhang Q, Goradia A, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad Sci U S A. 2008;105(52):20852-20857. [CrossRef]
- Abou Dalle I, Kantarjian H, Daver N, et al. Phase II study of single-agent nivolumab in patients with myelofibrosis. Ann Hematol. 2021;100(12):2957-2960. [CrossRef]
- Hobbs G, Cimen Bozkus C, Moshier E, et al. PD-1 inhibition in advanced myeloproliferative neoplasms. Blood Adv. 2021;5(23):5086-5097. [CrossRef]
- Long L, Zhang X, Chen F, et al. The promising immune checkpoint LAG-3: from tumor microenvironment to cancer immunotherapy. Genes Cancer. 2018;9(5-6):176-189. [CrossRef]
- Workman CJ, Dugger KJ, Vignali DAA. Cutting edge: molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J Immunol. 2002;169(10):5392-5395. [CrossRef]
- Saleh R, Toor SM, Sasidharan Nair V, Elkord E. Role of Epigenetic Modifications in Inhibitory Immune Checkpoints in Cancer Development and Progression. Front Immunol. 2020;11:1469. [CrossRef]
- Kouo T, Huang L, Pucsek AB, et al. Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells. Cancer Immunol Res. 2015;3(4):412-423. [CrossRef]
- Andreae S, Buisson S, Triebel F. MHC class II signal transduction in human dendritic cells induced by a natural ligand, the LAG-3 protein (CD223). Blood. 2003;102(6):2130-2137. [CrossRef]
- Wang J, Sanmamed MF, Datar I, et al. Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell. 2019;176(1-2):334-347.e12. [CrossRef]
- Zuazo M, Arasanz H, Fernández-Hinojal G, et al. Functional systemic CD4 immunity is required for clinical responses to PD-L1/PD-1 blockade therapy. EMBO Mol Med. 2019;11(7):e10293. [CrossRef]
- Lichtenegger FS, Rothe M, Schnorfeil FM, et al. Targeting LAG-3 and PD-1 to Enhance T Cell Activation by Antigen-Presenting Cells. Front Immunol. 2018;9:385. [CrossRef]
- Jing W, Gershan JA, Weber J, et al. Combined immune checkpoint protein blockade and low dose whole body irradiation as immunotherapy for myeloma. J Immunother Cancer. 2015;3(1):2. [CrossRef]
- Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107(17):7875-7880. [CrossRef]
- Chocarro L, Blanco E, Arasanz H, et al. Clinical landscape of LAG-3-targeted therapy. Immunooncol Technol. 2022;14:100079. [CrossRef]
- Bristol-Myers Squibb. A Phase 3, Randomized, Double-Blind Study of Adjuvant Immunotherapy With Nivolumab + Relatlimab Fixed-Dose Combination Versus Nivolumab Monotherapy After Complete Resection of Stage III-IV Melanoma. clinicaltrials.gov; 2023. Accessed March 9, 2023. https://clinicaltrials.gov/ct2/show/NCT05002569.
- Merck Sharp & Dohme LLC. A Phase 3 Study of MK-4280A (Coformulated Favezelimab [MK-4280] Plus Pembrolizumab [MK-3475]) Versus Standard of Care in Previously Treated Metastatic PD-L1 Positive Colorectal Cancer. clinicaltrials.gov; 2022. Accessed March 9, 2023. https://clinicaltrials.gov/ct2/show/NCT05064059.
- Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N Engl J Med. 2022;386(1):24-34. [CrossRef]
- Memorial Sloan Kettering Cancer Center. A Pilot Study of 89Zr-DFO-REGN3767 Anti LAG-3 Antibody Positron Emission Tomography in Patients With Relapsed/Refractory DLBCL. clinicaltrials.gov; 2022. Accessed March 12, 2023. https://clinicaltrials.gov/ct2/show/NCT04566978.
- Celgene. An Exploratory Phase 1/2 Trial To Evaluate The Safety And Efficacy Of JCAR017 Combinations In Subjects With Relapsed/Refractory B-Cell Malignancies (PLATFORM). clinicaltrials.gov; 2023. Accessed March 12, 2023. https://clinicaltrials.gov/ct2/show/NCT03310619.
- Albershardt TC, Parsons AJ, Reeves RS, et al. Therapeutic efficacy of PD1/PDL1 blockade in B16 melanoma is greatly enhanced by immunization with dendritic cell-targeting lentiviral vector and protein vaccine. Vaccine. 2020;38(17):3369-3377. [CrossRef]
- Bristol-Myers Squibb. A Phase 1/2a Dose Escalation and Cohort Expansion Study of the Safety, Tolerability, and Efficacy of Anti-LAG-3 Monoclonal Antibody (Relatlimab, BMS-986016) Administered Alone and in Combination With Anti-PD-1 Monoclonal Antibody (Nivolumab, BMS-936558) in Relapsed or Refractory B-Cell Malignancies. clinicaltrials.gov; 2022. Accessed March 12, 2023. https://clinicaltrials.gov/ct2/show/NCT02061761.
- Merck Sharp & Dohme LLC. A Phase 1/Phase 2 Clinical Study to Evaluate the Safety and Efficacy of a Combination of MK-4280 and Pembrolizumab (MK-3475) in Participants With Hematologic Malignancies. clinicaltrials.gov; 2022. Accessed March 13, 2023. https://clinicaltrials.gov/ct2/show/NCT03598608.
- MD VB. An Open-Label Phase II Study of Relatlimab (BMS-986016) With Nivolumab (BMS-936558) in Combination With 5-Azacytidine for the Treatment of Patients With Refractory/Relapsed Acute Myeloid Leukemia and Newly Diagnosed Older Acute Myeloid Leukemia Patients. clinicaltrials.gov; 2022. Accessed March 13, 2023. https://clinicaltrials.gov/ct2/show/NCT04913922.
- Triebel F, Jitsukawa S, Baixeras E, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171(5):1393-1405. [CrossRef]
- Wang JH, Meijers R, Xiong Y, et al. Crystal structure of the human CD4 N-terminal two-domain fragment complexed to a class II MHC molecule. Proc Natl Acad Sci U S A. 2001;98(19):10799-10804. [CrossRef]
- Huard B, Mastrangeli R, Prigent P, et al. Characterization of the major histocompatibility complex class II binding site on LAG-3 protein. Proc Natl Acad Sci U S A. 1997;94(11):5744-5749. [CrossRef]
- Hannier S, Triebel F. The MHC class II ligand lymphocyte activation gene-3 is co-distributed with CD8 and CD3-TCR molecules after their engagement by mAb or peptide-MHC class I complexes. Int Immunol. 1999;11(11):1745-1752. [CrossRef]
- Mastrangeli R, Micangeli E, Donini S. Cloning of murine LAG-3 by magnetic bead bound homologous probes and PCR (gene-capture PCR). Anal Biochem. 1996;241(1):93-102. [CrossRef]
- Andrews LP, Marciscano AE, Drake CG, Vignali DAA. LAG3 (CD223) as a Cancer Immunotherapy Target. Immunol Rev. 2017;276(1):80-96. [CrossRef]
- Ruiter DJ, Mattijssen V, Broecker EB, Ferrone S. MHC antigens in human melanomas. Semin Cancer Biol. 1991;2(1):35-45.
- Hemon P, Jean-Louis F, Ramgolam K, et al. MHC class II engagement by its ligand LAG-3 (CD223) contributes to melanoma resistance to apoptosis. J Immunol. 2011;186(9):5173-5183. [CrossRef]
- Donia M, Andersen R, Kjeldsen JW, et al. Aberrant Expression of MHC Class II in Melanoma Attracts Inflammatory Tumor-Specific CD4+ T- Cells, Which Dampen CD8+ T-cell Antitumor Reactivity. Cancer Res. 2015;75(18):3747-3759. [CrossRef]
- Dumic J, Dabelic S, Flögel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760(4):616-635. [CrossRef]
- Xu F, Liu J, Liu D, et al. LSECtin expressed on melanoma cells promotes tumor progression by inhibiting antitumor T-cell responses. Cancer Res. 2014;74(13):3418-3428. [CrossRef]
- Zarour HM. Reversing T-cell Dysfunction and Exhaustion in Cancer. Clin Cancer Res. 2016;22(8):1856-1864. [CrossRef]
- Workman CJ, Wang Y, El Kasmi KC, et al. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol. 2009;182(4):1885-1891. [CrossRef]
- Camisaschi C, De Filippo A, Beretta V, et al. Alternative activation of human plasmacytoid DCs in vitro and in melanoma lesions: involvement of LAG-3. J Invest Dermatol. 2014;134(7):1893-1902. [CrossRef]
- Huang B, Lei Z, Zhao J, et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007;252(1):86-92. [CrossRef]
- Li N, Wang Y, Forbes K, et al. Metalloproteases regulate T-cell proliferation and effector function via LAG-3. EMBO J. 2007;26(2):494-504. [CrossRef]
- Wang L, Rubinstein R, Lines JL, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208(3):577-592. [CrossRef]
- Borggrewe M, Grit C, Den Dunnen WFA, et al. VISTA expression by microglia decreases during inflammation and is differentially regulated in CNS diseases. Glia. 2018;66(12):2645-2658. [CrossRef]
- ElTanbouly MA, Croteau W, Noelle RJ, Lines JL. VISTA: a novel immunotherapy target for normalizing innate and adaptive immunity. Semin Immunol. 2019;42:101308. [CrossRef]
- Flies DB, Wang S, Xu H, Chen L. Cutting edge: A monoclonal antibody specific for the programmed death-1 homolog prevents graft-versus-host disease in mouse models. J Immunol. 2011;187(4):1537-1541. [CrossRef]
- Huang X, Zhang X, Li E, et al. VISTA: an immune regulatory protein checking tumor and immune cells in cancer immunotherapy. Journal of Hematology & Oncology. 2020;13(1):83. [CrossRef]
- ElTanbouly MA, Schaafsma E, Noelle RJ, Lines JL. VISTA: Coming of age as a multi-lineage immune checkpoint. Clin Exp Immunol. 2020;200(2):120-130. [CrossRef]
- Blando J, Sharma A, Higa MG, et al. Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer. Proc Natl Acad Sci U S A. 2019;116(5):1692-1697. [CrossRef]
- Rosenbaum SR, Knecht M, Mollaee M, et al. FOXD3 Regulates VISTA Expression in Melanoma. Cell Rep. 2020;30(2):510-524.e6. [CrossRef]
- Kuklinski LF, Yan S, Li Z, et al. VISTA expression on tumor-infiltrating inflammatory cells in primary cutaneous melanoma correlates with poor disease-specific survival. Cancer Immunol Immunother. 2018;67(7):1113-1121. [CrossRef]
- Wang L, Jia B, Claxton DF, et al. VISTA is highly expressed on MDSCs and mediates an inhibition of T cell response in patients with AML. Oncoimmunology. 2018;7(9):e1469594. [CrossRef]
- Aru B, Pehlivanoğlu C, Dal Z, Dereli-Çalışkan NN, Gürlü E, Yanıkkaya-Demirel G. A potential area of use for immune checkpoint inhibitors: Targeting bone marrow microenvironment in acute myeloid leukemia. Frontiers in Immunology. 2023;14. Accessed March 19, 2023. [CrossRef]
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253-268. [CrossRef]
- Lines JL, Sempere LF, Broughton T, Wang L, Noelle R. VISTA is a novel broad-spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol Res. 2014;2(6):510-517. [CrossRef]
- Le Mercier I, Chen W, Lines JL, et al. VISTA Regulates the Development of Protective Antitumor Immunity. Cancer Res. 2014;74(7):1933-1944. [CrossRef]
- Ru B, Wong CN, Tong Y, et al. TISIDB: an integrated repository portal for tumor-immune system interactions. Bioinformatics. 2019;35(20):4200-4202. [CrossRef]
- Loeser H, Kraemer M, Gebauer F, et al. The expression of the immune checkpoint regulator VISTA correlates with improved overall survival in pT1/2 tumor stages in esophageal adenocarcinoma. Oncoimmunology. 2019;8(5):e1581546. [CrossRef]
- Janssen Research & Development, LLC. An Open-Label, First-in-Human, Phase 1 Study of the Safety, Pharmacokinetics, and Pharmacodynamics of JNJ-61610588, a Fully Human IgG1 Kappa Anti-VISTA (V-Domain Ig Suppressor of T-Cell Activation) Monoclonal Antibody, in Subjects With Advanced Cancer. clinicaltrials.gov; 2018. Accessed March 16, 2023. https://clinicaltrials.gov/ct2/show/NCT02671955.
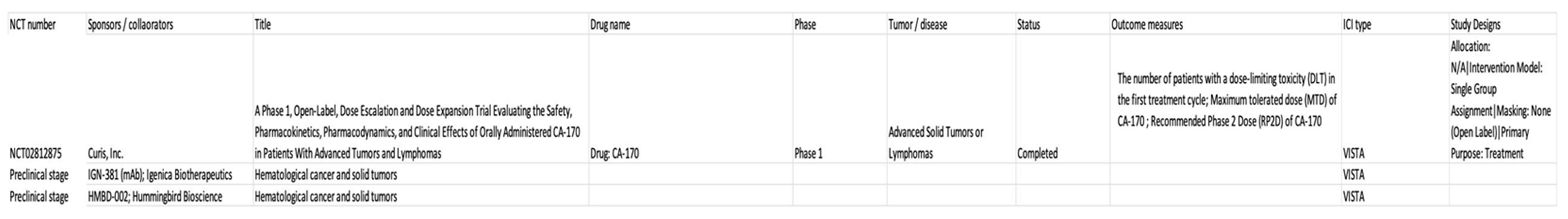
- Curis, Inc. A Phase 1, Open-Label, Dose Escalation and Dose Expansion Trial Evaluating the Safety, Pharmacokinetics, Pharmacodynamics, and Clinical Effects of Orally Administered CA-170 in Patients With Advanced Tumors and Lymphomas. clinicaltrials.gov; 2020. Accessed March 16, 2023. https://clinicaltrials.gov/ct2/show/NCT02812875.
- Wu C, Cao X, Zhang X. VISTA inhibitors in cancer immunotherapy: a short perspective on recent progresses. RSC Med Chem. 12(10):1672-1679. [CrossRef]
- Ingram PJ, Thakkar D, Boyd-Kirkup JD. Abstract 587: HMBD002, a novel neutralizing antibody targeting a specific epitope on the co-inhibitory immune checkpoint receptor VISTA, displays potent anti-tumor effects in pre-clinical models. Cancer Research. 2017;77(13_Supplement):587. [CrossRef]
- Gou R, Zhu L, Zheng M, et al. Annexin A8 can serve as potential prognostic biomarker and therapeutic target for ovarian cancer: based on the comprehensive analysis of Annexins. J Transl Med. 2019;17(1):275. [CrossRef]
- Monney L, Sabatos CA, Gaglia JL, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415(6871):536-541. [CrossRef]
- Ferris RL, Lu B, Kane LP. Too much of a good thing? Tim-3 and TCR signaling in T cell exhaustion. J Immunol. 2014;193(4):1525-1530. [CrossRef]
- Phong BL, Avery L, Sumpter TL, et al. Tim-3 enhances FcεRI-proximal signaling to modulate mast cell activation. J Exp Med. 2015;212(13):2289-2304. [CrossRef]
- Baitsch L, Baumgaertner P, Devêvre E, et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J Clin Invest. 2011;121(6):2350-2360. [CrossRef]
- Kikushige Y, Shima T, Takayanagi S ichiro, et al. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell. 2010;7(6):708-717. [CrossRef]
- Anderson AC, Anderson DE, Bregoli L, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318(5853):1141-1143. [CrossRef]
- Zhang Y, Ma CJ, Wang JM, et al. Tim-3 negatively regulates IL-12 expression by monocytes in HCV infection. PLoS One. 2011;6(5):e19664. [CrossRef]
- Maurya N, Gujar R, Gupta M, Yadav V, Verma S, Sen P. Immunoregulation of dendritic cells by the receptor T cell Ig and mucin protein-3 via Bruton’s tyrosine kinase and c-Src. J Immunol. 2014;193(7):3417-3425. [CrossRef]
- Su EW, Bi S, Kane LP. Galectin-9 regulates T helper cell function independently of Tim-3. Glycobiology. 2011;21(10):1258-1265. [CrossRef]
- Chiba S, Baghdadi M, Akiba H, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012;13(9):832-842. [CrossRef]
- DeKruyff RH, Bu X, Ballesteros A, et al. T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells. J Immunol. 2010;184(4):1918-1930. [CrossRef]
- Huang YH, Zhu C, Kondo Y, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517(7534):386-390. [CrossRef]
- Rangachari M, Zhu C, Sakuishi K, et al. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3–mediated cell death and exhaustion. Nat Med. 2012;18(9):1394-1400. [CrossRef]
- Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017;276(1):97-111. [CrossRef]
- Tao J lian, Li L juan, Fu R, et al. Elevated TIM3+ hematopoietic stem cells in untreated myelodysplastic syndrome displayed aberrant differentiation, overproliferation and decreased apoptosis. Leuk Res. 2014;38(6):714-721. [CrossRef]
- Tcvetkov N, Gusak A, Morozova E, et al. Immune checkpoints bone marrow expression as the predictor of clinical outcome in myelodysplastic syndrome. Leuk Res Rep. 2020;14:100215. [CrossRef]
- Asayama T, Tamura H, Ishibashi M, et al. Functional expression of Tim-3 on blasts and clinical impact of its ligand galectin-9 in myelodysplastic syndromes. Oncotarget. 2017;8(51):88904-88917. [CrossRef]
- Brück O, Blom S, Dufva O, et al. Immune cell contexture in the bone marrow tumor microenvironment impacts therapy response in CML. Leukemia. 2018;32(7):1643-1656. [CrossRef]
- Liu F, Zeng G, Zhou S, et al. Blocking Tim-3 or/and PD-1 reverses dysfunction of tumor-infiltrating lymphocytes in HBV-related hepatocellular carcinoma. Bull Cancer. 2018;105(5):493-501. [CrossRef]
- Schnell A, Bod L, Madi A, Kuchroo VK. The yin and yang of co-inhibitory receptors: toward anti-tumor immunity without autoimmunity. Cell Res. 2020;30(4):285-299. [CrossRef]
- Liu F, Liu Y, Chen Z. Tim-3 expression and its role in hepatocellular carcinoma. J Hematol Oncol. 2018;11(1):126. [CrossRef]
- Tan J, Huang S, Huang J, et al. Increasing Tim-3+CD244+, Tim-3+CD57+, and Tim-3+PD-1+ T cells in patients with acute myeloid leukemia. Asia Pac J Clin Oncol. 2020;16(3):137-141. [CrossRef]
- Hadadi L, Hafezi M, Amirzargar AA, Sharifian RA, Abediankenari S, Asgarian-Omran H. Dysregulated Expression of Tim-3 and NKp30 Receptors on NK Cells of Patients with Chronic Lymphocytic Leukemia. Oncol Res Treat. 2019;42(4):202-208. [CrossRef]
- Rezazadeh H, Astaneh M, Tehrani M, et al. Blockade of PD-1 and TIM-3 immune checkpoints fails to restore the function of exhausted CD8+ T cells in early clinical stages of chronic lymphocytic leukemia. Immunol Res. 2020;68(5):269-279. [CrossRef]
- Zeidan AM, Komrokji RS, Brunner AM. TIM-3 pathway dysregulation and targeting in cancer. Expert Rev Anticancer Ther. 2021;21(5):523-534. [CrossRef]
- Harding JJ, Moreno V, Bang YJ, et al. Blocking TIM-3 in Treatment-refractory Advanced Solid Tumors: A Phase Ia/b Study of LY3321367 with or without an Anti-PD-L1 Antibody. Clin Cancer Res. 2021;27(8):2168-2178. [CrossRef]
- Novartis Pharmaceuticals. Phase 1b, Multi-Arm, Open-Label Study of PDR001 and/or MBG453 in Combination With Decitabine in Patients With Acute Myeloid Leukemia or High Risk Myelodysplastic Syndrome. clinicaltrials.gov; 2023. Accessed May 12, 2023. https://clinicaltrials.gov/ct2/show/NCT03066648.
- Bewersdorf JP, Stahl M, Zeidan AM. Immune checkpoint-based therapy in myeloid malignancies: a promise yet to be fulfilled. Expert Review of Anticancer Therapy. 2019;19(5):393-404. [CrossRef]
- Yu X, Harden K, Gonzalez LC, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10(1):48-57. [CrossRef]
- Stanietsky N, Simic H, Arapovic J, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2009;106(42):17858-17863. [CrossRef]
- Harjunpää H, Guillerey C. TIGIT as an emerging immune checkpoint. Clin Exp Immunol. 2020;200(2):108-119. [CrossRef]
- Chan CJ, Andrews DM, Smyth MJ. Receptors that interact with nectin and nectin-like proteins in the immunosurveillance and immunotherapy of cancer. Curr Opin Immunol. 2012;24(2):246-251. [CrossRef]
- Boles KS, Vermi W, Facchetti F, et al. A novel molecular interaction for the adhesion of follicular CD4 T cells to follicular DC. Eur J Immunol. 2009;39(3):695-703. [CrossRef]
- Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56(5):855-865. [CrossRef]
- Eberlé F, Dubreuil P, Mattei MG, Devilard E, Lopez M. The human PRR2 gene, related to the human poliovirus receptor gene (PVR), is the true homolog of the murine MPH gene. Gene. 1995;159(2):267-272. [CrossRef]
- Reymond N, Borg JP, Lecocq E, et al. Human nectin3/PRR3: a novel member of the PVR/PRR/nectin family that interacts with afadin. Gene. 2000;255(2):347-355. [CrossRef]
- Johnston RJ, Comps-Agrar L, Hackney J, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26(6):923-937. [CrossRef]
- Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 axis regulates human T cell function. J Immunol. 2012;188(8):3869-3875. [CrossRef]
- Kong Y, Zhu L, Schell TD, et al. T-Cell Immunoglobulin and ITIM Domain (TIGIT) Associates with CD8+ T-Cell Exhaustion and Poor Clinical Outcome in AML Patients. Clin Cancer Res. 2016;22(12):3057-3066. [CrossRef]
- Chauvin JM, Pagliano O, Fourcade J, et al. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J Clin Invest. 2015;125(5):2046-2058. [CrossRef]
- Guillerey C, Harjunpää H, Carrié N, et al. TIGIT immune checkpoint blockade restores CD8+ T-cell immunity against multiple myeloma. Blood. 2018;132(16):1689-1694. [CrossRef]
- Stålhammar G, Seregard S, Grossniklaus HE. Expression of immune checkpoint receptors Indoleamine 2,3-dioxygenase and T cell Ig and ITIM domain in metastatic versus nonmetastatic choroidal melanoma. Cancer Med. 2019;8(6):2784-2792. [CrossRef]
- O’Brien SM, Klampatsa A, Thompson JC, et al. Function of Human Tumor-Infiltrating Lymphocytes in Early-Stage Non-Small Cell Lung Cancer. Cancer Immunol Res. 2019;7(6):896-909. [CrossRef]
- Kurtulus S, Sakuishi K, Ngiow SF, et al. TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Invest. 2015;125(11):4053-4062. [CrossRef]
- Degos C, Heinemann M, Barrou J, et al. Endometrial Tumor Microenvironment Alters Human NK Cell Recruitment, and Resident NK Cell Phenotype and Function. Front Immunol. 2019;10:877. [CrossRef]
- Josefsson SE, Huse K, Kolstad A, et al. T Cells Expressing Checkpoint Receptor TIGIT Are Enriched in Follicular Lymphoma Tumors and Characterized by Reversible Suppression of T-cell Receptor Signaling. Clin Cancer Res. 2018;24(4):870-881. [CrossRef]
- Fourcade J, Sun Z, Chauvin JM, et al. CD226 opposes TIGIT to disrupt Tregs in melanoma. JCI Insight. 2018;3(14):e121157, 121157. [CrossRef]
- Dixon KO, Schorer M, Nevin J, et al. Functional Anti-TIGIT Antibodies Regulate Development of Autoimmunity and Antitumor Immunity. J Immunol. 2018;200(8):3000-3007. [CrossRef]
- Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267-296. [CrossRef]
- Wu AA, Drake V, Huang HS, Chiu S, Zheng L. Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology. 2015;4(7):e1016700. [CrossRef]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. [CrossRef]
- Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563-580. [CrossRef]
- Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1-11. [CrossRef]
- Solomon BL, Garrido-Laguna I. TIGIT: a novel immunotherapy target moving from bench to bedside. Cancer Immunol Immunother. 2018;67(11):1659-1667. [CrossRef]
- Harjunpää H, Blake SJ, Ahern E, et al. Deficiency of host CD96 and PD-1 or TIGIT enhances tumor immunity without significantly compromising immune homeostasis. Oncoimmunology. 2018;7(7):e1445949. [CrossRef]
- Genentech, Inc. A Phase II, Randomized, Blinded, Placebo-Controlled Study of Tiragolumab, An Anti-TIGIT Antibody, In Combination With Atezolizumab In Chemotherapy-Naïve Patients With Locally Advanced Or Metastatic Non-Small Cell Lung Cancer. clinicaltrials.gov; 2023. Accessed March 16, 2023. https://clinicaltrials.gov/ct2/show/NCT03563716.
- Arcus Biosciences, Inc. A Phase 1 Study to Evaluate the Safety and Tolerability of AB154 Monotherapy and Combination Therapy in Participants With Advanced Malignancies. clinicaltrials.gov; 2023. Accessed March 16, 2023. https://clinicaltrials.gov/ct2/show/NCT03628677.
- Merck Sharp & Dohme LLC. A Phase 1 Trial of MK-7684 as Monotherapy and in Combination With Pembrolizumab in Subjects With Advanced Solid Tumors. clinicaltrials.gov; 2022. Accessed March 16, 2023. https://clinicaltrials.gov/ct2/show/NCT02964013.
- Bristol-Myers Squibb. Phase 1/2a First-In-Human Study of BMS-986207 Monoclonal Antibody Alone and in Combination With Nivolumab or With Nivolumab and Ipilimumab in Advanced Solid Tumors. clinicaltrials.gov; 2023. Accessed March 16, 2023. https://clinicaltrials.gov/ct2/show/NCT02913313.
- Astellas Pharma Global Development, Inc. A Phase 1b Study of ASP8374, an Immune Checkpoint Inhibitor, as a Single Agent and in Combination With Pembrolizumab in Subjects With Advanced Solid Tumors. clinicaltrials.gov; 2022. Accessed March 16, 2023. https://clinicaltrials.gov/ct2/show/NCT03260322.
- Astellas Pharma Inc. A Phase 1, Open Label Study of ASP8374, an Immune Checkpoint Inhibitor, in Japanese Patients With Advanced Solid Tumors. clinicaltrials.gov; 2021. Accessed March 16, 2023. https://clinicaltrials.gov/ct2/show/NCT03945253.
- Jin S, Zhang Y, Zhou F, Chen X, Sheng J, Zhang J. TIGIT: A promising target to overcome the barrier of immunotherapy in hematological malignancies. Frontiers in Oncology. 2022;12. Accessed May 14, 2023. [CrossRef]
- Catakovic K, Gassner FJ, Ratswohl C, et al. TIGIT expressing CD4+T cells represent a tumor-supportive T cell subset in chronic lymphocytic leukemia. Oncoimmunology. 2017;7(1):e1371399. [CrossRef]
- Minnie SA, Kuns RD, Gartlan KH, et al. Myeloma escape after stem cell transplantation is a consequence of T-cell exhaustion and is prevented by TIGIT blockade. Blood. 2018;132(16):1675-1688. [CrossRef]
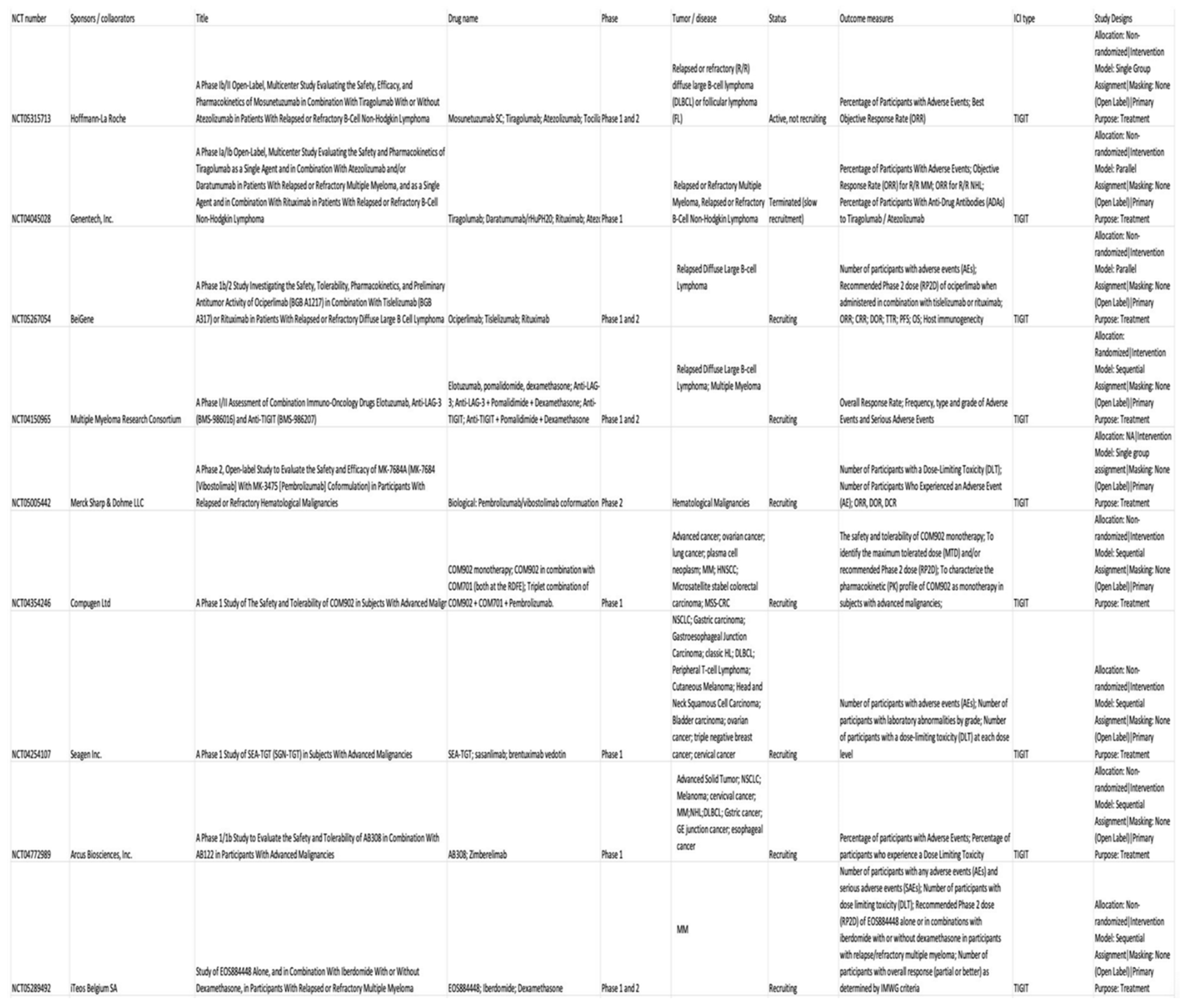
- Hoffmann-La Roche. A Phase Ib/II Open-Label, Multicenter Study Evaluating the Safety, Efficacy, and Pharmacokinetics of Mosunetuzumab in Combination With Tiragolumab With or Without Atezolizumab in Patients With Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma. clinicaltrials.gov; 2023. Accessed May 12, 2023. https://clinicaltrials.gov/ct2/show/NCT05315713.
- Genentech, Inc. A Phase Ia/Ib Open-Label, Multicenter Study Evaluating the Safety and Pharmacokinetics of Tiragolumab as a Single Agent and in Combination With Atezolizumab and/or Daratumumab in Patients With Relapsed or Refractory Multiple Myeloma, and as a Single Agent and in Combination With Rituximab in Patients With Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma. clinicaltrials.gov; 2023. Accessed May 12, 2023. https://clinicaltrials.gov/ct2/show/NCT04045028.
- BeiGene. A Phase 1b/2 Study Investigating the Safety, Tolerability, Pharmacokinetics, and Preliminary Antitumor Activity of Ociperlimab (BGB A1217) in Combination With Tislelizumab (BGB A317) or Rituximab in Patients With Relapsed or Refractory Diffuse Large B Cell Lymphoma. clinicaltrials.gov; 2022. Accessed May 12, 2023. https://clinicaltrials.gov/ct2/show/NCT05267054.
- Multiple Myeloma Research Consortium. A Phase I/II Assessment of Combination Immuno-Oncology Drugs Elotuzumab, Anti-LAG-3 (BMS-986016) and Anti-TIGIT (BMS-986207). clinicaltrials.gov; 2021. Accessed May 12, 2023. https://clinicaltrials.gov/ct2/show/NCT04150965.
- Merck Sharp & Dohme LLC. A Phase 2, Open-Label Study to Evaluate the Safety and Efficacy of MK-7684A (MK-7684 [Vibostolimab] With MK-3475 [Pembrolizumab] Coformulation) in Participants With Relapsed or Refractory Hematological Malignancies. clinicaltrials.gov; 2023. Accessed May 12, 2023. https://clinicaltrials.gov/ct2/show/NCT05005442.
- Compugen Ltd. A Phase 1 Study of The Safety and Tolerability of COM902 in Subjects With Advanced Malignancies. clinicaltrials.gov; 2023. Accessed May 12, 2023. https://clinicaltrials.gov/ct2/show/NCT04354246.
- Seagen Inc. A Phase 1 Study of SEA-TGT (SGN-TGT) in Subjects With Advanced Malignancies. clinicaltrials.gov; 2023. Accessed May 12, 2023. https://clinicaltrials.gov/ct2/show/NCT04254107.
- Arcus Biosciences, Inc. A Phase 1/1b Study to Evaluate the Safety and Tolerability of AB308 in Combination With AB122 in Participants With Advanced Malignancies. clinicaltrials.gov; 2023. Accessed May 12, 2023. https://clinicaltrials.gov/ct2/show/NCT04772989.
- iTeos Belgium SA. Study of EOS884448 Alone, and in Combination With Iberdomide With or Without Dexamethasone, in Participants With Relapsed or Refractory Multiple Myeloma. clinicaltrials.gov; 2022. Accessed May 12, 2023. https://clinicaltrials.gov/ct2/show/NCT05289492.
- Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29(6):761-770. [CrossRef]
- Masarova L, Bose P, Verstovsek S. The Rationale for Immunotherapy in Myeloproliferative Neoplasms. Curr Hematol Malig Rep. 2019;14(4):310-327. [CrossRef]
- Barbui T, Vannucchi AM, Buxhofer-Ausch V, et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J. 2015;5(11):e369. [CrossRef]
- Alvarez-Larrán A, Garrote M, Ferrer-Marín F, et al. Real-world analysis of main clinical outcomes in patients with polycythemia vera treated with ruxolitinib or best available therapy after developing resistance/intolerance to hydroxyurea. Cancer. 2022;128(13):2441-2448. [CrossRef]
- Wagner SM, Melchardt T, Greil R. Ropeginterferon alfa-2b for the treatment of patients with polycythemia vera. Drugs Today (Barc). 2020;56(3):195-202. [CrossRef]
- Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus Standard Therapy for the Treatment of Polycythemia Vera. New England Journal of Medicine. 2015;372(5):426-435. [CrossRef]
- Mascarenhas J, Hoffman R. A comprehensive review and analysis of the effect of ruxolitinib therapy on the survival of patients with myelofibrosis. Blood. 2013;121(24):4832-4837. [CrossRef]
- Kvasnicka HM, Thiele J, Bueso-Ramos CE, et al. Long-term effects of ruxolitinib versus best available therapy on bone marrow fibrosis in patients with myelofibrosis. J Hematol Oncol. 2018;11(1):42. [CrossRef]
- Vannucchi AM, Verstovsek S, Guglielmelli P, et al. Ruxolitinib reduces JAK2 p.V617F allele burden in patients with polycythemia vera enrolled in the RESPONSE study. Ann Hematol. 2017;96(7):1113-1120. [CrossRef]
- Deininger M, Radich J, Burn TC, Huber R, Paranagama D, Verstovsek S. The effect of long-term ruxolitinib treatment on JAK2p.V617F allele burden in patients with myelofibrosis. Blood. 2015;126(13):1551-1554. [CrossRef]
- Vannucchi AM, Kantarjian HM, Kiladjian JJ, et al. A pooled analysis of overall survival in COMFORT-I and COMFORT-II, 2 randomized phase III trials of ruxolitinib for the treatment of myelofibrosis. Haematologica. 2015;100(9):1139-1145. [CrossRef]
- Wang JC, Chen C, Kundra A, et al. Programmed Cell Death Receptor (PD-1) Ligand (PD-L1) expression in Philadelphia chromosome-negative myeloproliferative neoplasms. Leuk Res. 2019;79:52-59. [CrossRef]
- Fang W, Zhang J, Hong S, et al. EBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: Implications for oncotargeted therapy. Oncotarget. 2014;5(23):12189-12202. [CrossRef]
- Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. Dual Faces of IFNγ in Cancer Progression: A Role of PD-L1 Induction in the Determination of Pro- and Antitumor Immunity. Clinical Cancer Research. 2016;22(10):2329-2334. [CrossRef]
- Fujimura T, Ring S, Umansky V, Mahnke K, Enk AH. Regulatory T Cells Stimulate B7-H1 Expression in Myeloid-Derived Suppressor Cells in ret Melanomas. J Invest Dermatol. 2012;132(4):1239-1246. [CrossRef]
- Hou A, Hou K, Huang Q, Lei Y, Chen W. Targeting Myeloid-Derived Suppressor Cell, a Promising Strategy to Overcome Resistance to Immune Checkpoint Inhibitors. Frontiers in Immunology. 2020;11. Accessed March 28, 2023. [CrossRef]
- Weber R, Fleming V, Hu X, et al. Myeloid-Derived Suppressor Cells Hinder the Anti-Cancer Activity of Immune Checkpoint Inhibitors. Frontiers in Immunology. 2018;9. Accessed March 28, 2023. [CrossRef]
- Meyer C, Cagnon L, Costa-Nunes CM, et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother. 2014;63(3):247-257. [CrossRef]
- Martens A, Wistuba-Hamprecht K, Foppen MG, et al. Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Clinical Cancer Research. 2016;22(12):2908-2918. [CrossRef]
- Weide B, Martens A, Zelba H, et al. Myeloid-Derived Suppressor Cells Predict Survival of Patients with Advanced Melanoma: Comparison with Regulatory T Cells and NY-ESO-1- or Melan-A–Specific T Cells. Clinical Cancer Research. 2014;20(6):1601-1609. [CrossRef]
- Soda H, Ogawara D, Fukuda Y, et al. Dynamics of blood neutrophil-related indices during nivolumab treatment may be associated with response to salvage chemotherapy for non-small cell lung cancer: A hypothesis-generating study. Thoracic Cancer. 2019;10(2):341-346. [CrossRef]
- Feng J, Chen S, Li S, et al. The association between monocytic myeloid-derived suppressor cells levels and the anti-tumor efficacy of anti-PD-1 therapy in NSCLC patients. Translational Oncology. 2020;13(12):100865. [CrossRef]
- Northwestern University. A Phase 1, Single Arm, Single Center Pilot Study of Medi4736, an Anti-Pdl1 Therapy, for Patients With Myelofibrosis. clinicaltrials.gov; 2019. Accessed March 28, 2023. https://clinicaltrials.gov/ct2/show/NCT02871323.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





