1. Introduction
First introduced in adult colorectal surgery in the Netherlands some 40 years ago [
1], magnetic compression anastomosis (MCA) has lately been revisited under the term "Magnamosis", particularly in the context of pediatric minimal-invasive surgery (MIS) [
2,
3,
4,
5,
6].
Remarkably, the number of publications and presentations on the topic has been increasing over the past decade, especially in pediatric surgery. While this is indicative of a greater interest in the potential use of MCA, the more recent clinical applications are still limited to case presentations and small patient series [
4,
5,
6,
7,
8,
9,
10,
11,
12,
13].
Following the historical Dutch report [
1], a pilot project on MCA use in pediatric surgery, including pre-clinical animal experiments and clinical trials, was implemented at the Department of Pediatric Surgery, N.I. Pirogov Russian National Research Medical University the Moscow, N.F. Filatov Children’s Hospital, Moscow, starting from the 1980s and continuing well into the early 1990s. Most of these data, however, have not been published in the English literature.
The aim of this report is to reappraise our earlier and relatively extensive experience with MCA, and evaluate the results that may be pertinent to contemporary proposals for MCA advancement.
2. Materials and Methods
A retrospective review of the above-mentioned single-institution materials, dated from 1980 to 1995, was conducted on MCA in preclinical experiments on animal models and subsequent clinical trials. The originally designed MCA techniques for specific pediatric surgical and urological applications were assessed from historic records. The sources of the reviewed information included literature publications, summaries of PhD theses, certified patents documentation (all in Russian language) and some personal records of the authors.
Specifically, the analysis of the information focused on the aspects of safety and technical feasibility of MCA, considering the biological formation and biomechanical characteristics of the newly-formed anastomoses with a focus on potential prospective and innovative clinical applications.
The extrapolated information was used to propose ideas for MCA advancement in pediatric surgery in accord with recent reports on contemporary applications of MCA as identified in a literature search.
3. Results
3.1. General aspects and preclinical experiments
There were a total of 250 preclinical, experiemental MCA cases of different types in various locations of the digestive and urinary tracts, primarily performed in canine and rabbit models (
Table 1). All experiments were performed with the use of Samarium Cobalt (SaCo) magnets of variable geometrical shape, size and coercivity force.
Technical and biomechanical characteristics were evaluated in comparison with corresponding hand-sewn anastomoses as controls in animal models [
14,
15,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26].
Performing MCA was simple, effectively facilitating the formation of a sutureless anastomoses via an endoluminal aproach [
14,
16,
17,
18,
19,
20,
21,
22,
23,
24].
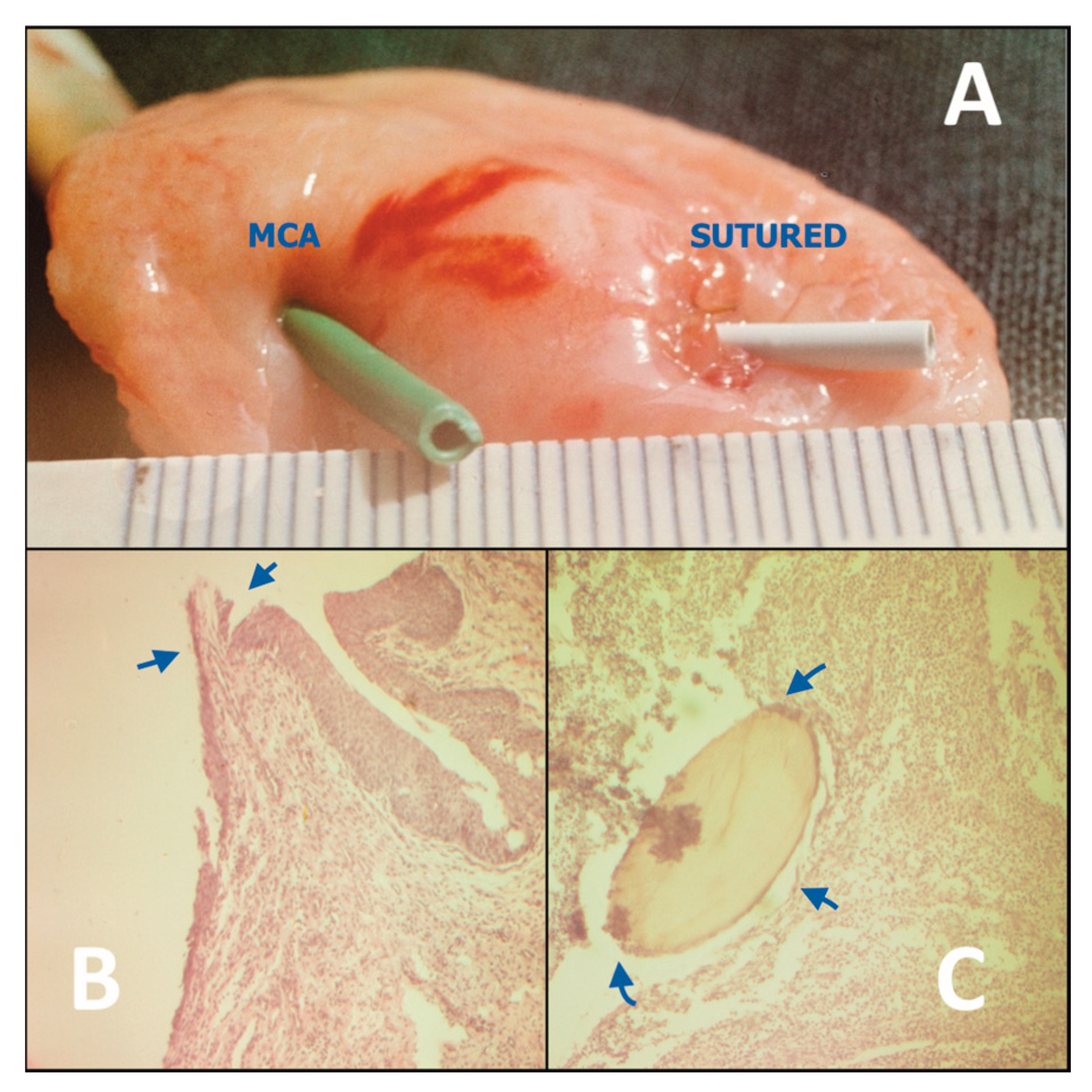
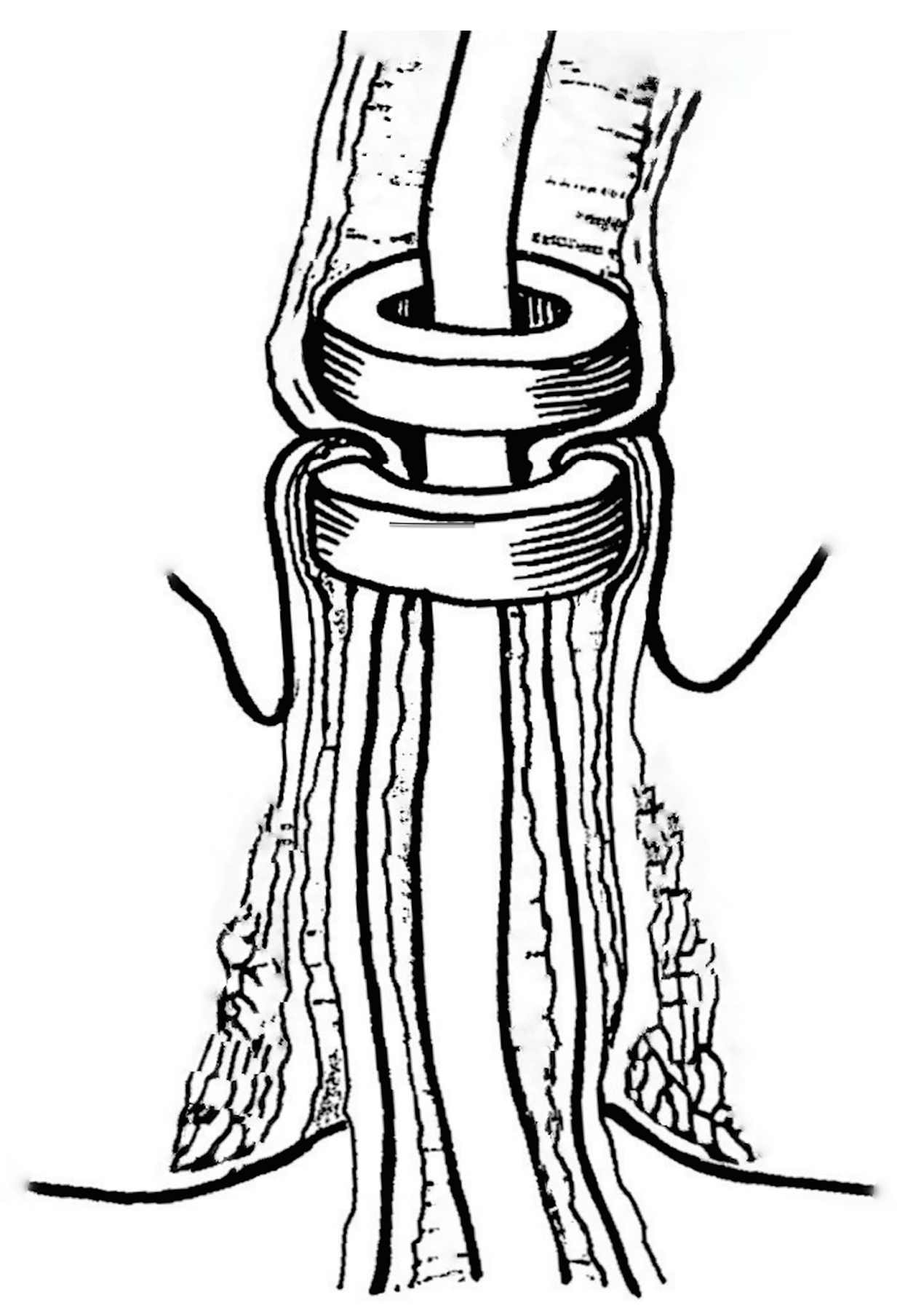
The results of biomechanical and histomorphological studies on all animal models showed that, at various postoperative intervals from day 1 to 1 month, the MCA in general had characteristically superior anastomotic properties compared to the hand-sewn controls (
Figure 1).
In particular, MCA demonstrated high tensile strength, most likely due to the healing by primary intention with no resultant leak or excessive scarring or strictures. [
17,
18,
19,
20,
21,
23,
24].
Tensile strength tests on the intestinal rabbit models at 1, 5, 7-10,14 and 21 postoperative days demonstrated bursting pressures that were 1.5 to 2-fold greater than after hand-sewn anastomoses in a control series (n= 35) [
16,
17].
Histological examinations of the MCA, at 1, 5, 7-10, 14, 21 and 30 days postoperatively confirmed seamless healing in both the gastrointestinal (n= 96) and urinary (n= 84) tract models. We also found primary epithelization within 7-10 days (
Figure 1B). In contrast, healing of hand-sewn analogues in all control groups was associated with ongoing suppurative inflammation with intramural micro-abscesses around the absorbable suture material (polyglactin or catgut), which were identifiable up to 1 month postoperatively, and resulted in fibrous tissue proliferation (
Figure 1C) [
16,
18,
19,
23].
In 7 cases of experimental colorectal MCA, anastomotic perforation and dehiscence was noted when using magnets with high coercivity force and small compression surfaces. A combination of these mechanical factors lead to focal tissue overload with subsequent tissue tearing and perforation [
16]. Therefore, a limitation for MCA was postulated as using strong magnets with minimally rounded edges and small compression surface.
In summary, the following factors were identified for successful MCA formation:
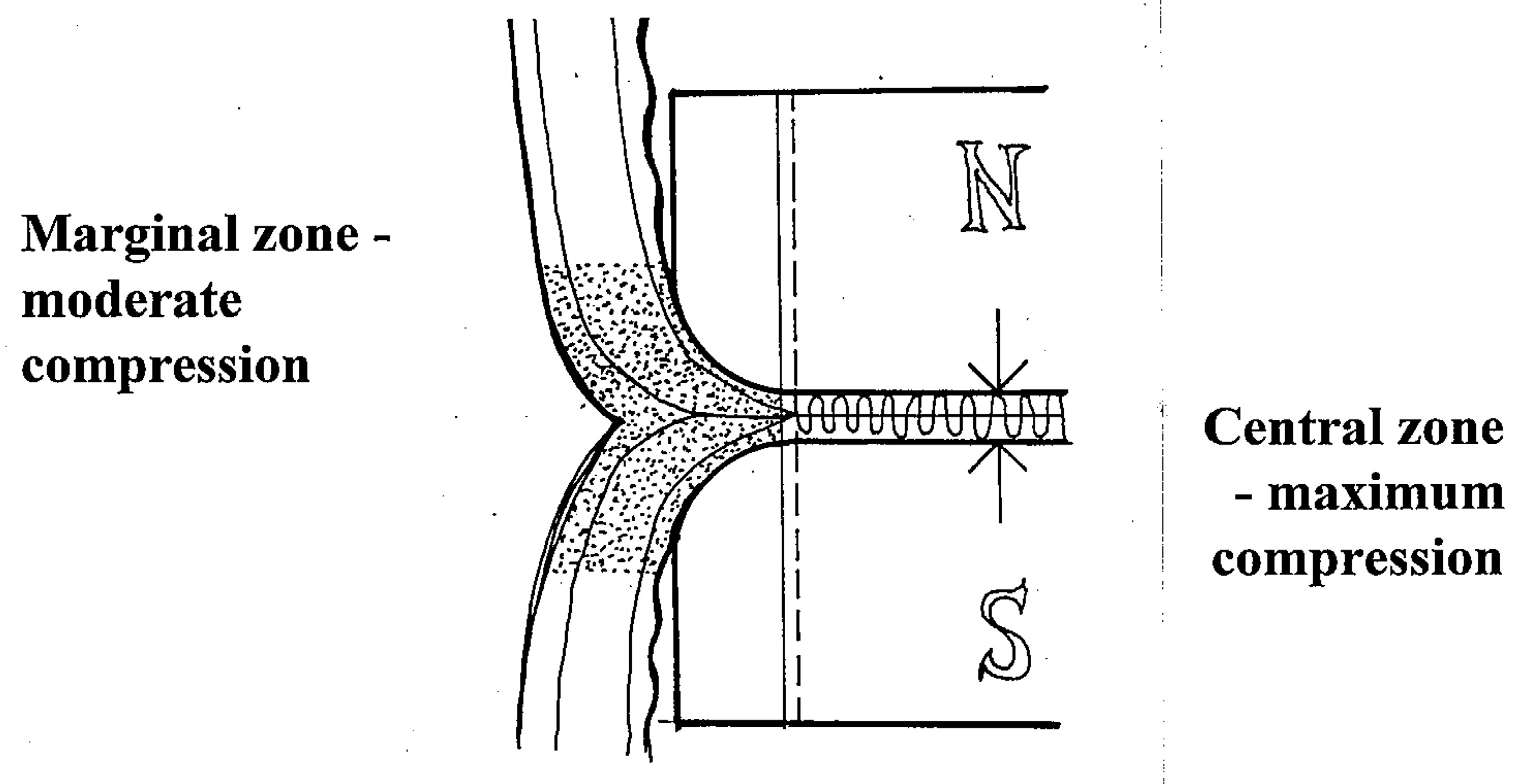
At the anastomotic line, epithelization and connective tissue matrix proliferation dynamically develop at the so called “zone of moderate compression”, where the compression forces decrease gradually towards the periphery. This is effectively modulated by rounded edges of magnetic compression surfaces. Flat magnets should not be used for MCA because they are associated with a higher degree of scarring and thus, stricture formation.
In the demarcated central zone, maximal compression is required to ensure reliable anastomotic coaptation, and to exponentially compress the subjected tissue to the point of dessiccation and necrosis, thus preventing suppurative necrosis with inflammation within the anastomosis itself, which may lead to inadequate anastomosis formation and increased proliferation of granulation tissue.
At the demarcation zone that constitues the inner anastomotic line, epithelization steadily progresses and bridges the rim of the thinned desiccated central tissue. The magnet thereby gradually become detached and pass distally within 7 to 10 days, thus completing the anastomotic healing by primary intention (
Figure 2) [
16,
18,
19,
23].
3.2. Review of Clinical trials
A review of the records revealed a total of 87 MCA procedures performed in 86 selected patients, 2 to 10 years of age. The MCA clinical applications involved using SaCo magnets of specific geometric configuration, size and compressing force, based on the preclinical experimental evaluations detailed above. Indications comprised various pathological conditions of the gastrointestinal and urinary tracts in pediatric surgical practice [
16,
17,
18,
19,
20,
21,
22,
25,
26,
27], detailed as follows.
3.2.1. Non-operative MCA esophageal recanalization
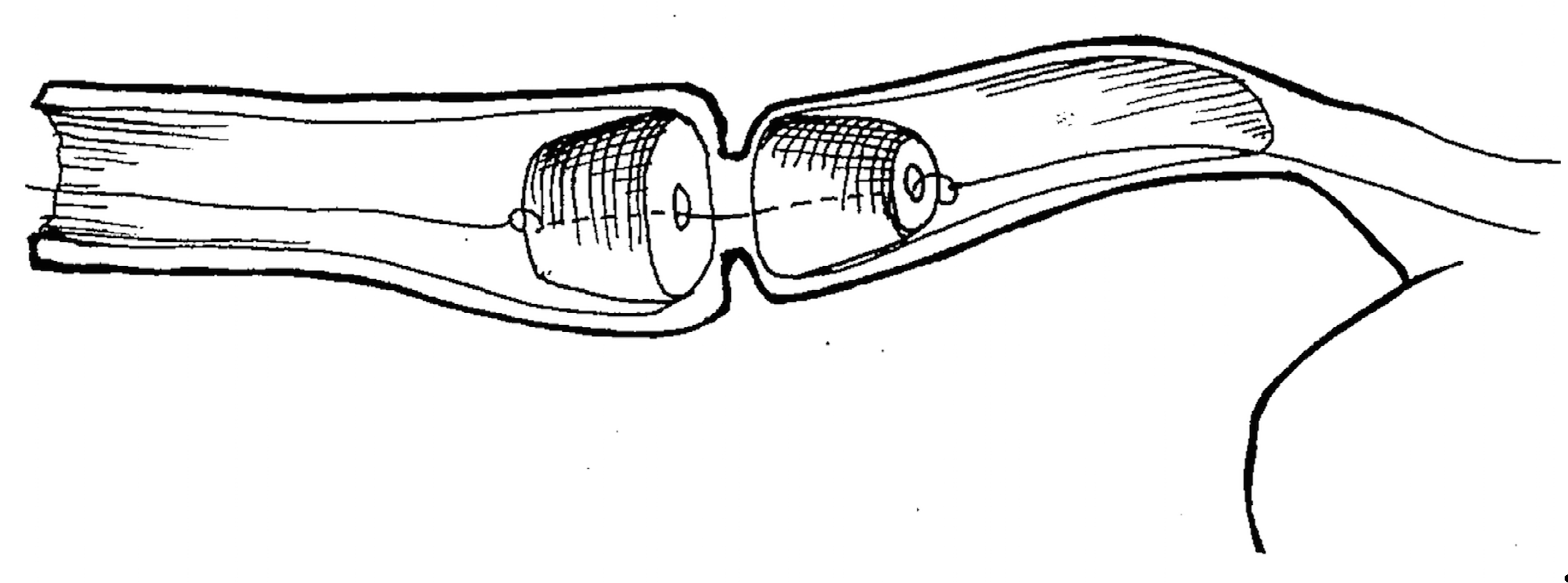
The original technique was developed to treat short-length esophageal strictures using magnetic cylinders by means of compressing and ablating the underlying scar tissue (
Figure 3). The technique was applied in fifteen patients (n=15) with esophageal strictures 3 to 15 mm in length [
14,
17,
20,
25,
26].
Under rigid esophagoscopy, specially-designed magnetic cylinders of 10, 12, or 14 mm diameter were deployed to the stricture site via the gastrostomy and perorally over a guidestring which was passed through the stricture narrowing after previous bougienage. Under fluoroscopy, the magnets were then brought together into coaptation.
The magnets were followed by daily repeat X-rays for the next 3 to 5 days. The resultant MCA formation usually occurred within 7 days, when the magnets detached from the anastomosis. They were subsequently removed under fluoroscopic control and endoscopic assistance by retracting them by the attached strings [
25,
26].
Complete esophageal recanalization was achieved in 9 cases, 5 of which were congeital membranous stenoses and 4 of which were postoperative anastomotic strictures. Adverse outcomes included esophageal perforation in one case and esophageal re-stenosis in 5. [
25,
26].
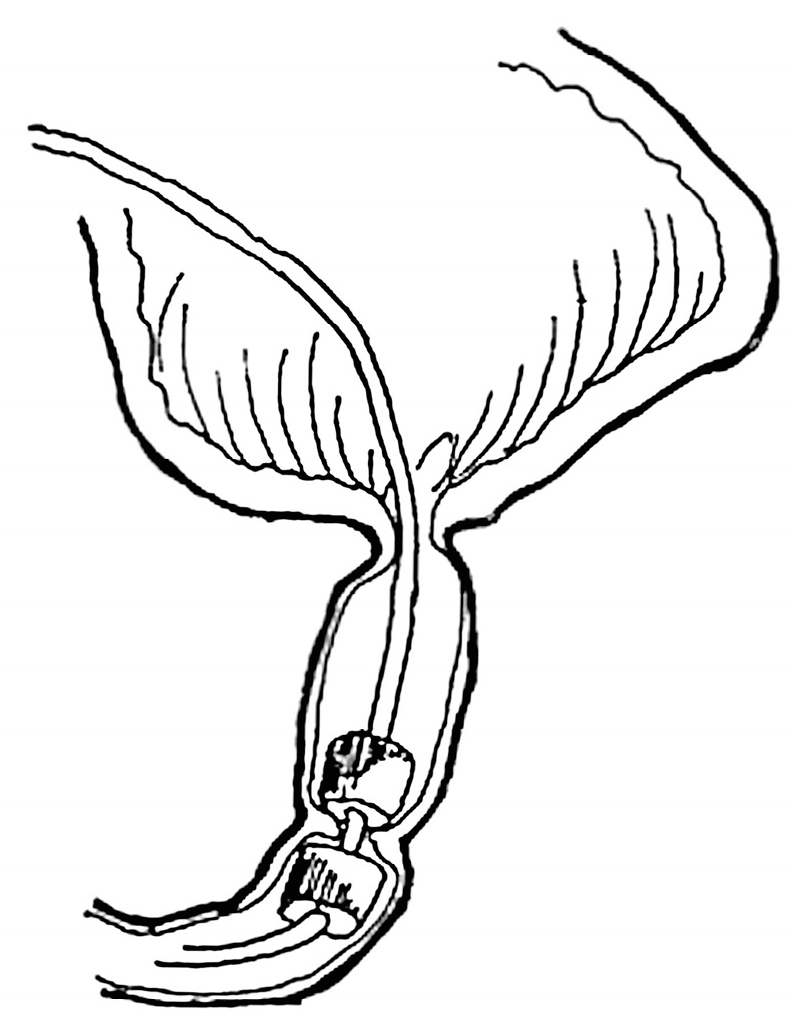
3.2.2. Non-operative MCA ileostomy undiversion
A technique for enterostomy undiversion was developed using flexible silicon-coated segmented block-shaped magnets in a side-to-side configuration (
Figure 4). The technique was used in forty-six patients with double barrel ileostomy when undiversion was clinically indicated [
20,
27].
Technically, the magnets were fashioned with attached strings for easy retrieval after successful MCA formation. They were manually placed into each stomal limb and brought to proper coaptation under fluoroscopic guidance. Subsequent MCA formation usually occurred between 7 and 10 days. [
20,
27].
Successsful MCA enterostomy undiversion was achieved in forty-four patients. There were no cases of leak, and the intestinal passage was restored in all patients. In 2 cases, magnet placement was aborted intraoperatively due to gross tissue interposition between the stomal limbs because it was deemed unsafe to bring magnets into the coaptation [
27].
3.2.3. Swenson type MCA-based pull-through for Hirschsprung disease
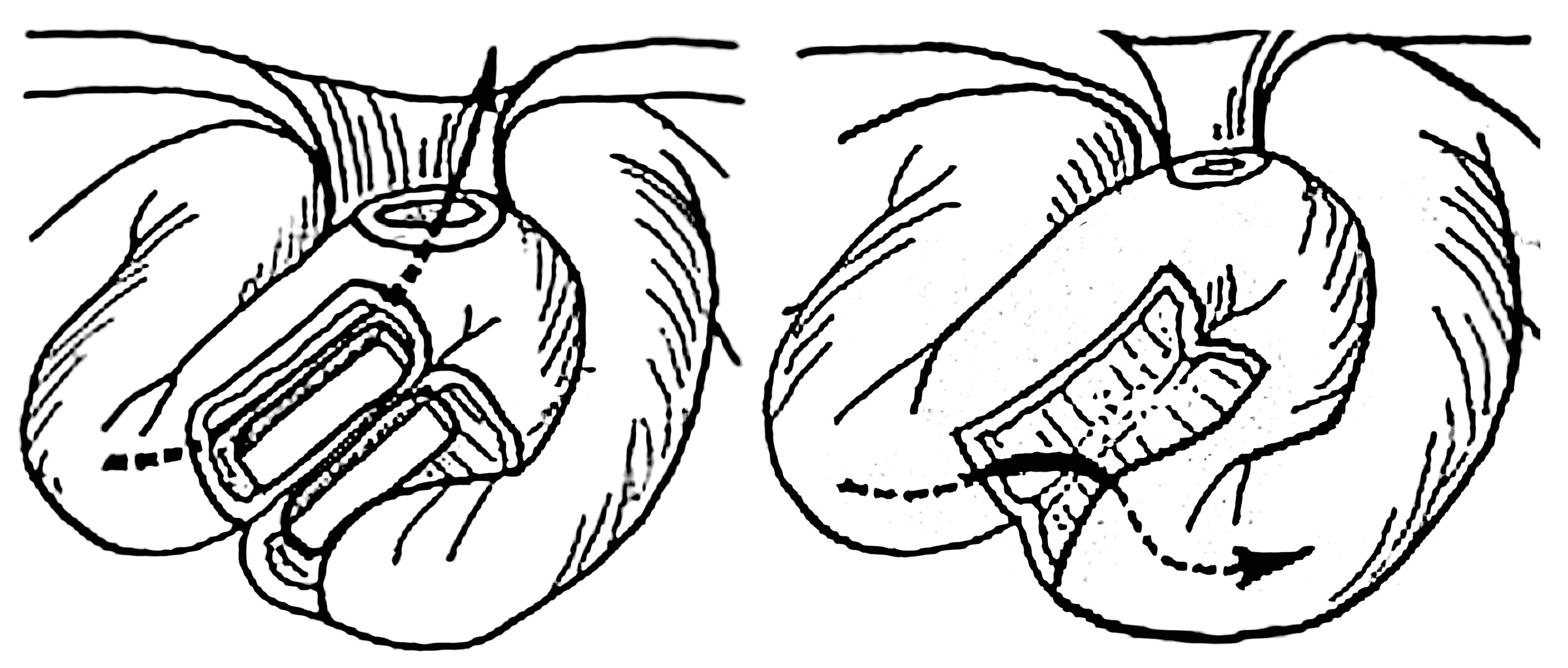
A modified MCA-based Swenson pull-through technique using flexible silicon-coated segmented magnetic rings of 20 to 40 mm diameter was designed to treat rectosigmoid Hirschsprung disease in 10 patients (
Figure 5) [
14,
16,
17,
20].
Technically, the procedure entailed an open approach via conventional laparotomy. The affected bowel segment was conventionally mobilized with deep pelvic dissection to the low rectal level. The proximal magnetic ring was delivered intraluminally through the anus to the upper limit of the intended resection, which was followed by eversion of the mobilized bowel portion transanally. The distal magnetic ring was then placed over the everted recto-sigmoidal cylinder. The magnetic rings were brought into coaptation at the level above the anal verge, thus creating an end-to-end colorectal MCA. A rectal tube was inserted intraluminally through the magnetic rings proximally to the anastomosis and the everted bowel cylinder was then excised.
Magnet coaptation was verified intraoperatively using fluoroscopy and monitored postoperatively by serial radiographs. The MCA usually formed within 8 to 12 days. Once the anastomosis had formed, the magnets detached and were manually removed through the anus [
14,
16,
17,
20].
An intact colorectal MCA with no leak or subsequent stenosis was achieved in six patients. Adverse outcomes included post-anastomotic stenosis in two patients (n= 2) with bulky-thickness bowel wall interposed between the magnetic rings [
17,
20].
3.2.4. Non-operative urethral recanalization
Small diameter magnetic cylinders were used to treat short length post-traumatic urethral strictures (
Figure 6). The technique was applied in five patients with short strictures that were less than 5 mm in length [
18,
20,
21,
22,
28].
The upper magnetic cylinder of 4 to 6mm diameter was delivered via rigid cystoscopy through a temporary cystostomy. The lower magnetic cylinder was delivered via cystoscopy through the meatus. Both magnets were introduced over a guide string that had been previously placed across the stricture. Again, fluoroscopy was used to verify good coaptation of the magnets.
Urethral MCA formation usually occured within7 to 10 days. Ater anastomosis formation, the magnets were removed by retracting them by the attached string using cystoscopy and fluoroscopic control. [
18,
21,
22,
28].
Complete restoration of urethral patency and normal voiding patters were achieved in four patients with membranous type strictures (n=4). Post-anastomotic restenosis was noted in one patient with a more extensive stricture of 5 mm in length [
18,
22].
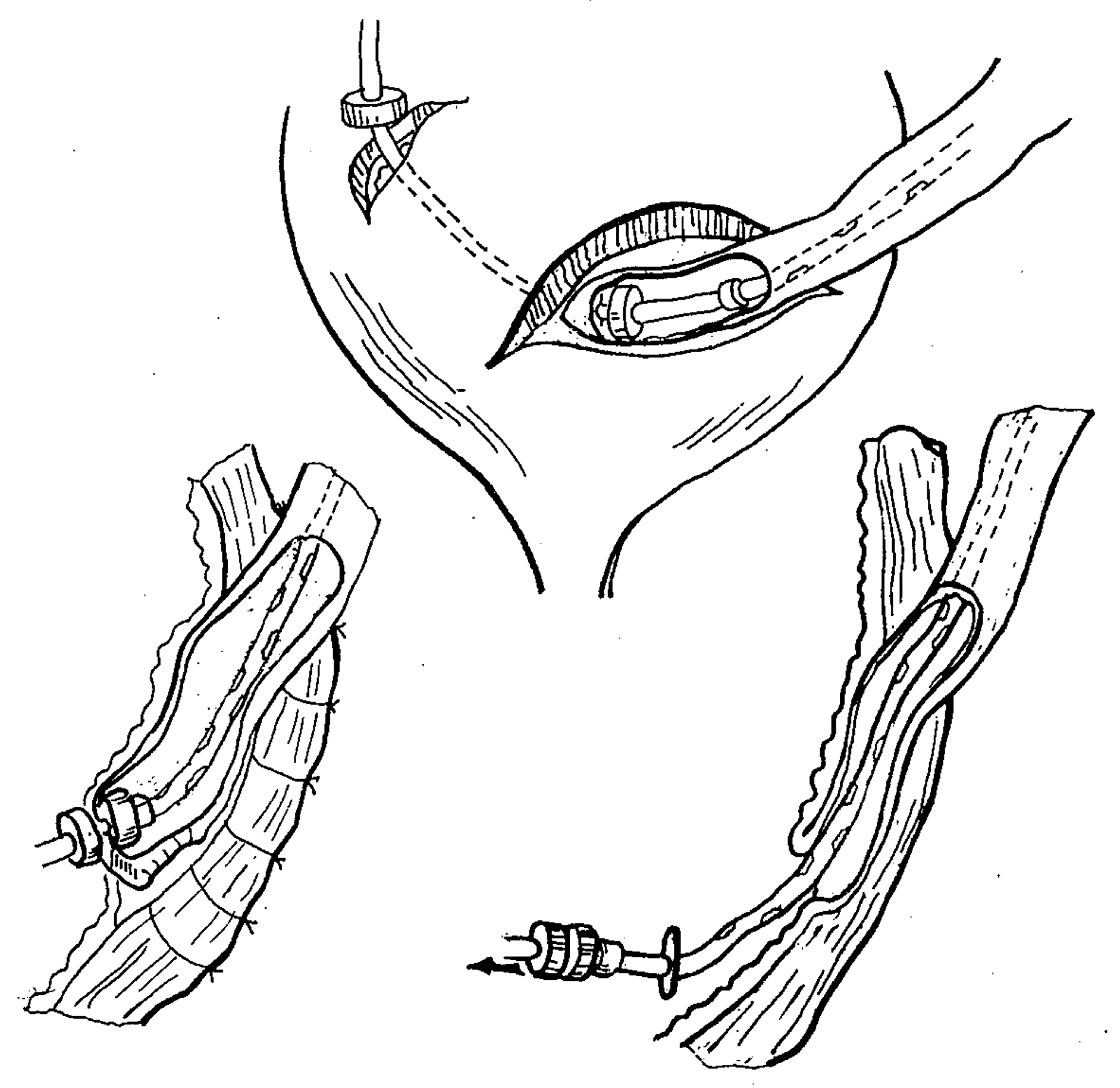
3.2.5. Extravesical ureterocystoneostomy
A technique of ureteric reimplantation using small magnetic rings of 4 to 5 mm diameter was designed to create an extravesical MCA between the ureter and urinary bladder (
Figure 7). It was employed on a total of 11 ureters in 10 patients with grade 3 to 4 vesicoureteral reflux (VUR) [
19,
20,
21,
29].
The procedure involved an open preperitoneal approach via a Pfannenstiel-type incision. The uretero-vesical segment was mobilized, the distal ureter was resected, and a Lich-Gregoir detrusorotomy was performed in conventional fashion. The ureterocystoneostomy was accomplished by placing the proximal magnetic ring over a stent into the lumen of the ureter. At the point of intended anastomosis, the distal tip of the stent (with the curl cut off) was brought into the bladder by using an attached stylet piercing through both the ureteric wall and the distal corner of the denuded submucosal surface of the bladder wall. The ureteric lumen was then closed with continuous absorbable suture. The distal magnetic ring was introduced into the bladder over the distal tip of the stent brought out via a mini-cystotomy, followed by a cystostomy tube placed over the stent. The magnets were then approximated, creating a side-to-side MCA between full-thickness ureteric wall and the submucosal layer of the bladder wall. Ureterocystoneostomy was completed by closing the tunnel in a Lich-Gregoir fashion using interrupted absorbable sutures.
MCA formation occurred within 7 to 10 days. The magnets detached from the anastomosis once it was formed and were removed via the cystostomy by retracting the stent and cystostomy tube [
19,
20,
21,
29].
The procedure was successfully completed in all 10 patients. No recurrent VUR or anastomotic strictures were noted in postoperative follow-up [
19,
20,
21].
3.3. Clinical success for MCA
A summary of clinical outcomes is presented in
Table 2, demonstrating that across all indications, MCA was successful in over 87% of cases [
17,
18,
19,
20,
21,
22,
25,
26,
27].
3.3. Recognized Adverse Effects associated with MCA
Adverse outcomes encountered in our experience are listed in table 2. In detail, they comprised:
Inability to safely perform ileostomy undiversion in two patients due to interposing of excessively thick tissue, so that the procedure was aborted due to safety concerns [
27];
Recurrent strictures after esophageal recanalization in five patients with corrosive strictures of irregular shape and more than 10 mm in length [
25,
26];
Recurrent strictures after urethral recanalization in a patient with a stricture approximately 5.0 mm in length (n= 1) [
18,
22];
Restenosis after Swenson colorectal MCA in two patients with a bulky bowel wall [
16,
17,
20] that impeded propper mating of the magnets.
The pathomechanism of postoperative restenosis or stenosis (esophageal and urethral, as well as colorectal) was thought to be related to a relatively insufficient magnetic compression force, which appeared to occur in circumstances of excessive distance between magnets due to disproportionately bulky tissue interposition. Conversely, esophageal perforation was possibly due to forceful compression applied to extensive strictured scar tissue and its inability to exhibit normal healthy tissue healing.
The above scenarios should be considered limitations for clinical MCA applications [
16,
17,
19,
23,
24,
25].
4. Discussion
Contrary to what many believe is a recent, novel development, MCA has previously been explored in both animal experiments and clinical trials in our institution in Moscow several decades ago. Our historic experience with MCA, reported for the first time in the English literature, dates as far back as the 1980s and early 1990s. Nonetheless, many of the issues regarding MCA and its challenges resonate with contemporary reports. Our experience spans a large spectrum of indications, ranging from pediatric upper gastrointestinal, colorectal, and urological applications.
Recently, other groups have been reviving MCA for the use in various pediatric surgical indications [
13], including esophageal atresia repair [
11,
12,
30] and for recanulation of the hypopharynx [
31]. Several experimental studies have been conducted in swine [
32,
33,
34,
35], rabbits [
35], and dogs [
36]. The studies confirm the above findings from decades ago and also seem to suggest that magnets can be used for creating the anastomosis, but that simultaneous approximation of pouches or structures under tension will lead to increased rates of anastomotic stricture [
12].
Given the retrospective review nature of our historic experience with MCA, we acknowledge that this report lacks a certain grade of evidenced-based robustness. The technical aspects of each individual study are described according to our laboratory notes, scientific reports in Russian language, and doctoral theses that resulted from the experiments and clinical studies. Nonetheless, for those interested, further details and copies of the original matirials are available from the corresponding authors upon specific request.
The first-hand information presented here accurately conveys our initial experience on the biological basis and applicability of MCA. To our knowledge, these are some of the first clinical applications reported, and constitute a pioneering effort to employ MCA in pediatric surgery. Particularly, the MCA experience described here were carried out in relatively large numbers, whereas many recent articles report much smaller case numbers of similar techniques.
Considering our earlier experience, we outline the rationale for the potential MCA application in gastro-intestinal and urinary tract surgery on the grounds of its superior biomechanical properties versus hand-sewn anastomosis and technical versatility allowing greater scope for creating anastomosis, especially, in settings of non-operative endoluminal approach and challenging surgical access in difficult anatomical areas.
We therefore hope that this information will provide stimulus for the current working groups on MCA to incorporate our findings and ideas for relevant future innovative development in pediatric surgery, in particular, in conjunction with a MIS approach. Combining MCA and MIS techniques create synergy and seem like a worthwhile future direction.
At this point, we propose that further research and development of MCA is undertaken to rationalize its formation, confirm efficacy for the described indications and explore new fields of application, particularly in pediatric surgery. Optimally, these endeavours should be conducted with interdisciplinary medical, bioscientific, and engineering input. Some of the work packages we propose are:
1. Designing and industrially manufacturing biocompatible and safe rare-earth magnetic devices for specific anastomotic indications with optimal parameters of compression, size, and specific geometrical shape for creating MCA in pediatric gastrointestinal and urinary structures.
2. Designing and industrially manufacturing an auxiliary magnetic driving device with computerized technology that would facilitate intraluminal magnet positioning and coaptation, thus ensuring efficient MCA creation while electronically monitoring and securing optimal tissue compression. This should be facilitated by measuring the distance and the resulting effective magnetic coercivity force and thereby detecting possible undue anastomotic tissue tension and/or bulky tissue interposition between magnets in real-time.
3. Push for further research into the development and standardization of MCA in conjuncture with pediatric MIS with a focus on the following procedures:
- -
Endoluminal recanalization in obstructive lesions of short-length of various pathogenesis and localization,
- -
Non- operative undiversion of intestinal stomas,
- -
Laparoscopic pull-through,
- -
Laparoscopic extravesical ureteric reimplantation,
- -
Laparoscopic biliary-digestive reconstruction,
- -
Laparoscopic duodenal atresia repair,
- -
Laparoscopi-assisted repair of certain anorectal malformations
- -
Thoracoscopic esophageal atresia repair with and without a fistula.
Our proposals for designing and industrially manufacturing magnets as a specific anastomosing device along with an auxiliary computerized magnetic driving device are intended to standardize MCA technical performance and to ensure its optimal biological formation while avoiding risks of the MCA limitations as identified in our past experience. We feel that this is paramount to ensure safe and effective MCA for neonatal and older pediatric patients. In summary, following our past experience with MCA, we believe that future research on MCA should be combined with pediatric MIS techniques that were not available when we performed our experiments and clinical studies.
5. Conclusions
This is the first comprehensive report of our historic MCA experience in the English language. The experience described in this report can be considered a true pioneering effort which unfortunately has not been as visible as it merrits so far. Our studies show that MCA should be considered a valid alternative to hand-sewn anastomoses in a wide spectrum of pediatric surgical problems, particularly for future MIS applications. Our report underlines the surgical versatility of MCA, particular for endoluminal applications using endoscopic delivery techniques. It also shows the importance of finding the optimal compression pressure and other biophysical properties, with the goal of producing high-quality anastomoses. Based on our findings, novel devices should be designed for specific indications, taking into consideration biologic healing of the particular tissues and the required magnetic force to form the anastomosis.
Author Contributions
Conceptualization, AK and OM; Methodology, AK; Formal Analysis, AK and VN; Validation, AK, VN and AR; Writing – original draft, AK; Writing - review and editing, OM and AK. All authors have read and agreed to the published version of the manuscript. All images are images and personal drawings by AK.
Funding
This research received no external funding.
Institutional Review Board Statement
In the initial/earlier studies, neither animal welfare nor clinical ethics board approval was mandated at that time in Russia. Notably, all clinical trials were undertaken in the best interests of patients to treat their respective diseases and lessen their suffering. The animal experiments were carried out with the purpose of advancing surgical knowledge in the interest of future patients.
Informed Consent Statement
Not applicable, given the observatory retrospective nature of this review of the initial/studies and the time gap. More than 30 years have passed since the studies were conducted.
Data Availability Statement
Data on which this publication is based are available from the corresponding authors OM and AM upon reasonable request.
Acknowledgments
We acknowledge our senior colleagues and former mentors Professors Geraskin V.I., Isakov Y.F., Stepanov E.A. and Sharipov N.A., for their leading role in initiating the MCA pilot projects, for their involvement, and for pioneering MCA applications in pediatric surgery by devising a number of original ideas.
Conflicts of Interest
All authors declare no conflict of interest.
References
- Jansen, A.; Keeman, J.N.; Davies, G.A.; Klopper, P.J. Early experiences with magnetic rings in resection of
the distal colon. Neth. J. Surg. 1980, 32, 20‐27. PMID: 7366876.
- Jamshidi, R.; Stephenson, J.T.; Clay, J. G.; et al. Magnamosis: magnetic compression anastomosis with comparison to suture and staple techniques. J. Pediatr. Surg. 2009, 44, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, K.D.; Douglas, G.; Pichakron, K.O.; et al. Magnamosis III: delivery of a magnetic compression anastomosis device using minimally invasive endoscopic techniques. J. Pediatr. Surg. 2012, 47, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.; Diana, M.; Leroy, J.; et al. Magnamosis IV: magnetic compression anastomosis for minimally invasive colorectal surgery. Endoscopy 2013, 45, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Dorman, R.M.; Vali, K.; Harmon, C.M.; et al. Repair of esophageal atresia with proximal fistula using endoscopic magnetic compression anastomosis (Magnamosis) after staged lengthening. Pediatr. Surg. Int. 2016, 32, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Toselli, L.; Martinez-Ferro, M.; Cervio, G.; et al. Magnetic Compression Anastomosis (Magnamosis) for Functional Undiversion of Ileostomy in Pediatric Patients. J. Laparoendosc Adv. Surg. Tech. 2017, 27, 1314–1317. [Google Scholar] [CrossRef] [PubMed]
- Woo, R.; Wong, C. M.; Trimble, Z.; et al. Magnetic compression stricturoplasty for treatment of refractory esophageal strictures in children: Technique and lessons learned. Surg. Innov. 2017, 24, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Lv, Y.; Fang, Y.; Luo, R.X.; et al. Magnetic compression for anastomosis in treating an infant born with long-gap oesophageal atresia: A case report. Medicine 2020, 99, e22472. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Li, Q.F.; Lv, Y.; et al. Magnetic compression anastomosis for rectal atresia following necrotizing enterocolitis: A case report. Medicine 2020, 99, e23613. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Y.; Lv, et al. Magnetic compression stricturoplasty in patients with severe stricture after
simultaneous esophageal atresia and duodenal obstruction repair: A case report. Exp. Ther. Med. 2022, 23,
93. [CrossRef]
- Muensterer OJ, Evans LL, Sterlin A.; et al. Novel device for endoluminal esophageal atresia repair: Firstin‐
human experience. Pediatrics 2021, 148, e2020049627. [CrossRef]
- Holler, A.S.; König, T.T.; Chen, C.; et al. Esophageal magnetic compression anastomosis in esophageal atresia repair: A PRISMA-compliant systematic review and comparison with a novel approach. Children 2022, 9, 1113. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.G.; Evans, L.L.; Johnson, S. M.; Woo, R.K. The evolving use of magnets in surgery: biomedical considerations and a review of their current applications. Bioengineering 2023, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Isakov, Y.F.; Gerasʹkin, V.I.; Vasilʹev, G.S.; Sharipov, N.A. [Using permanent magnets in pediatric surgery].
Klin. Khir. 1981, 6, 36‐39. PMID: 7024620.
- Geraskin, V.I.; Aleksandrov, V.M.; Kotlovsky, A.M.; et al. [Universal anastomosing device with the use of
magnets]. Patent for invention – Authors’ copyright No. 1025420, 1981, Moscow, USSR. Bulletin Inventions
and Discoveries, 1983.
- Sharipov, N.A. [Colorectal resections with the use of permanent magnets in children]. Thesis of [candidate of medical science]. N.I. Pirogov National Medical University, Moscow, USSR, 1982.
- Stepanov, E.A.; Vasilʹev, G.S.; Sharipov, N.A.; et al. [Use of permanent magnets in digestive tract surgery
in children]. Vestn. Akad. Med. Nauk SSSR 1984, 9, 6‐11. PMID: 6388182.
https://pubmed.ncbi.nlm.nih.gov/6388182/.
- Nikolaev, V.V. [Treatment of urethral strictures with the use of permanent magnets in children]. Thesis of [candidate of medical science]. N.I. Pirogov National Medical University, Moscow, USSR, 1987.
- Kotlovsky, A.M. [Ureteric reimplantation with the use of permanent magnets in children]. Thesis of [candidate of medical science] N.I. Pirogov National Medical University, Moscow, USSR, 1987.
- Stepanov, E.A.; Vasiliev, G.S.; Kotlovsky, A.M.; et al. [Magnet‐compression anastomosis in surgery of tubular
organs in children]. Pediatrics, Scientific publications of Academy Medical Science, Moscow, USSR, 1987, 40 ‐ 44.
- Kotlovsky, A.M.; Nikolaev, V.V.; Vrublevsky, S.G. [Magnet‐compression anastomosis in the urinary tract
in children]. The National collection of scientific publications of Academy of Medical Science, Moscow, USSR,
1989, 20‐24.
- Isakov, Iu.F.; Stepanov, E.A.; Erokhin, A.P.; et al. [Surgical treatment of urethral strictures in children].
Vestn. Khir. Im. I I Grek. 1989, 142, 61‐66. PMID: 2800174. https://pubmed.ncbi.nlm.nih.gov/2800174/.
- Lubashevskiĭ, V.T.; Shabanov, A.M.; Vasilʹev, G.S. [The inflammatory‐reparative processes in the
implantation of the ureter into the bladder by using the mechanical forces of permanent magnets]. Bull.
Exp. Biol. Med. 1993, 116, 550‐552. PMID: 8312560. https://pubmed.ncbi.nlm.nih.gov/8312560/.
- Nikolaev, V.V. Technical principles of urethral compression anastomoses. Bull. Exp. Biol. Med. 1998, 126, 855–858. [Google Scholar] [CrossRef]
- Stepanov, E.A.; Vasil’ev, G.S.; Nikolaev, V.V.; et al. [Treatment of short esophageal stricture in children].
Vestn. Ross. Akad.. Med Nauk. 1994, 3, 15‐19. PMID: 7516212. https://pubmed.ncbi.nlm.nih.gov/7516212/.
- Sharipov, N.A. [Treatment of esophageal strictures] Thesis of [doctor of medical science]. N.I. Pirogov National Medical University, Moscow, Russia, 1996.
- Stepanov, E.A.; Vasil’ev, G.S.; Nikolaev, V.V. [The treatment of intestinal fistulas in children by applying a
by‐pass anastomosis using magnetic devices]. Khirurgiia (Mosk) 1992, 11, 93‐95. PMID: 1294807.
https://pubmed.ncbi.nlm.nih.gov/1294807/.
- Stepanov, E.A.; Nikolaev, V.V.; Kotlovsky, A.M.; et al. [Method of correction of urethral strictures]. Patent for
Invention – Authors’ copyright No. 1361753, 1986, Moscow, USSR. Bulletin Inventions and Discoveries,
49,1987.
- Isakov, Y.F., Stepanov, E.A., Kotlovsky, A.M., et al [Method of ureterocystoneostomy]. Patent for Invention
– authors’ copyright No. 1277452, 1985, Moscow, USSR. Bulletin Inventions and Discoveries, 46, 1986.
- Evans, LL; Chen, CS; Muensterer, OJ; et al. The novel application of an emerging device for salvage of primary repair in high-risk complex esophageal atresia. Journal of Pediatric Surgery. 2022;57:810-818. [CrossRef] [PubMed]
- Mascagni, P; Tringali, A; Boškoski, I;, et al. Magnetic kissing for the endoscopic treatment of a complete
iatrogenic stenosis of the hypopharynx. Endoscopy. 2023:E499‐E500. DOI: 10.1055/a‐2029‐6340. PMID:
36894138; PMCID: PMC9998230.
- Muensterer, OJ; Sterlin, A; Oetzmann von Sochaczewski, C; et al. An experimental study on magnetic esophageal compression anastomosis in piglets. J Pediatr Surg. 2020;55:425-432. [CrossRef]
- Sterlin, A; Evans, L; Mahler, S; et al. An experimental study on long term outcomes after magnetic esophageal compression anastomosis in piglets. J Pediatr Surg. 2022;57:34-40. [CrossRef]
- Oetzmann von Sochaczewski, C; Lindner, A; Heimann, A; et al. Beyond Magnamosis: A Method to Test Sutureless Esophageal Anastomotic Devices in Living Swine by Creating an Esophageal Bypass Loop for Natural Oral Nutrition. J Laparoendosc Adv ASurg Tech A. 2019;29:852-855. [CrossRef]
- Hornok, Z; Kubiak, R; Csukas, D; et al. Esophageal Magnetic Anastomosis Device (EMAD) to simplify and improve outcome of thoracoscopic repair for esophageal atresia with tracheoesophageal fistula: A proof of concept study. J Pediatr Surg. 2022;S0022-3468(22)00631-5. [CrossRef] [PubMed]
- Xu, XH; Lv, Y; Liu, SQ; et al. Esophageal magnetic compression anastomosis in dogs. World Journal of
Gastroenterology. 2022;28:5313‐5323. DOI: 10.3748/wjg.v28.i36.5313. PMID: 36185631; PMCID:
PMC9521523.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).