1. Introduction
Primary brain tumors are a heterogeneous group of benign and malignant neoplasms, arising from the brain parenchyma and its surrounding structures. These tumors are an important cause of morbidity and mortality in both adults and children [
1,
2]. The overall pooled incidence rate of primary brain tumors was found to be 10.82 per 100,000 person-years [
3]. Glioblastoma multiforme (GBM) and malignant gliomas are the most common primary malignant brain tumors, in the USA with an annual incidence of 5.26 per 100,000 population or 17,000 new diagnoses per year, and are typically associated with a dismal prognosis and poor quality of life [
4]. In glioblastoma multiforme, an aggressive primary brain tumor, the usual therapies are weakly efficient. Even the most complex approach, debulking surgery followed by irradiation plus Temozolomide (TMZ) chemotherapy, increased median survival time to merely 14.6 months vs. 12.1 months with radiation therapy (RT) only. Moreover, progression is delayed by RT+TMZ by 6.9 months only [
5]. In recurrent gliomas, the progression-free survival, and the response to TMZ are much worse [
6].
Clinical complications (thromboembolic events, seizures, fluctuation of existing neurologic symptoms) are frequently seen in patients suffering from malignant gliomas. Unwanted effects due to corticosteroids and chemotherapeutic agents are also common and need to be managed properly. GBM is, despite improving therapeutic options, still very difficult to treat. Optimal management requires a multidisciplinary approach and knowledge of potential complications from both the disease and its treatment.
The possible role of naturally occurring deuterium (16.8 mmol/L in natural waters) was investigating in different studies, which revealed that deuterium depletion inhibited tumor cell growth
in vitro and caused tumor regression
in vivo [
7,
8,
9,
10,
11,
12] – appa-rently because of the significantly different chemical and physical properties of hydrogen (H) and deuterium (D) [
13,
14], and because changing D/H ratio exerts significant impact on cell physiology [
15,
16]. Numerous studies, conducted on different cell lines in culture media containing deuterium-depleted water (DDW), verified the determinative role of D in cell cycle regulation and tumor growth [
12,
17,
18,
19,
20]. D depletion influences protooncogenes and tumor suppressor genes. When experimental animals were given DDW to drink, induction of the expression of c-Myc, Ha-ras, and p53 genes by carcinogen exposure was significantly inhibited [
7]. Further, the apoptosis-triggering effect of DDW has been demonstrated both
in vitro [
17] and
in vivo [
21]. D as a natural cell growth regulator controls also mitochondrial oxidation-reduction balance [
22,
23,
24]. D depletion causes disbalance between production and neutralization of reactive oxygen species (ROS) in mitochondria, inducing oxidative stress which in turn induces apoptosis [
22].
The clinical outcome of D depletion was investigated in prospective, phase 2, double blind [
10] and retrospective human clinical studies [
10,
11,
25,
26]. For retrospective stu-dies, clinical data on the test results of the conventional therapies in patients consuming DDW were collected. Patients with early-stage breast cancer (n=158) achieved a median survival time (MST) of 217 months (18.1 years), and those with advanced disease (n=74), 52 months (4.3 years), compared to 17 months in patients not consuming DDW. Patients who took a single course of DDW (n=126) had an MST of 9 years whereas patients (n=53) who took at least two courses of DDW attained an MST of 24.4 years [
25]. In another study on patients with small cell and non-small cell lung cancers who consumed DDW, the MST was 25.9 months in male and 74.1 months in female patients – 2–4 times longer than it is generally observed in lung cancer patients [
11,
26].
It is known that median survival is longer in GBM patients who express the IDH1 (R132H) mutation compared to patients who express the wild allele [
27,
28]. IDH1-derived 2-hydroxyglutarate can facilitate degradation of Hif1-α and thus reduce the Warburg-effect through down-regulation of multiple genes in the glycolytic pathway [
29]. Further evidence of an inhibitory effect of the IDH1 mutation on glucose intake and glycolysis was obtained recently from PET analysis [
30].
Ketogenic diet has been used as complementary cancer therapy with high efficacy [
31]. A synergistic interaction between the effects of the IDH1 mutation and ketogenic metabolic therapy (KMT) could simultaneously down-regulate both the Warburg-effect and the Q-effect [
32] in the GBM neoplastic cell populations thus providing a novel mechanism contributing to the long-term survival of the patients [
33]. Ketogenic diet can facilitate delivery of small-molecule therapeutic drugs through the blood-brain barrier without toxicity. As GBM, like most malignant tumors, is dependent on fermentation for ATP synthesis and survival, simultaneous restriction of fermentable fuels, glucose, and glutamine, while elevating non-fermentable ketone bodies, offers a non-toxic therapeutic strategy for managing GBM [
34,
35,
36].
Based on the proved anticancer effect of DDW, we postulate that the beneficial impact of ketogenic diet in cancer treatment derives at least partly from its deuterium-depleting effect, since the mitochondria, when oxidizing fats instead of carbohydrates, produce metabolic water with as low as 118 ppm D level, due to the differences in D content of the various nutrients [
37].
In this paper, we present a retrospective study, based on hospital records of 55 GBM patients between 1994 and 2020. Our aim was to investigate how DDW consumption influenced the outcome of GBM in combination with conventional therapies. These were withheld but the patients’ daily water demand was fully covered with DDW. The primary endpoint was MST.
The data indicated that D depletion, when combined with existing conventional therapies, provides severalfold increase of MST.
2. Materials and Methods
2.1. Study Population
Patients suffering from GBM and receiving conventional forms of therapy plus follow-up examinations in different hospitals throughout Hungary were involved in this retrospective study. Altogether, 55 GBM patients started consuming DDW between April 1994 and October 2020. Data collection was closed in January 2021. Some of the patients drank DDW for a too short period (less than 4 months) or in an inappropriate way (e.g., alternating DDW with normal fluids or drinking DDW in insufficient D concentration). After excluding these subjects from the study, 33 patients remained for detailed evaluation. However, 2 of them started drinking DDW years after the diagnosis, so they had to be omitted not to cause an erroneous MST. At last, 21 males and 10 females were enrolled in the detailed retrospective evaluation.
2.2. Administration of DDW
The technology of DDW and the deuterium-depleted drinking water (Preventa) production for human consumption was detailed earlier [
11]. During the treatment, the patients covered their daily water demand by drinking 1.5 to 2 liters of Preventa DDW - with 85, 65, 45 and 25 ppm D concentration - instead of normal water, while their conventional therapy was also continued. Due to poor prognosis of GBM, DDW consumption in our cases typically started at 85 ppm D level, and was changed to 65, 45, and 25 ppm after every 1 to 3 months in order to maintain continuous depletion of deuterium. In cases where a longer progression-free interval was achieved, a 3 to 6 months break in DDW consumption could be introduced, followed by further DDW courses of 4 to 6 months lengths. Later, DDW application could be shortened to 3-4 months, and the breaks were prolonged accordingly. The overall duration of DDW consumption in the whole follow-up population was between 22 and 1,566 days.
The patients were continuously updated with, aware of, consented and provided access to all available information and publications regarding deuterium depletion.
2.3. Statistical Evaluation
The sole endpoint of the study was survival. Kaplan-Meier survival estimates were used, and log-rank test to compare groups. All statistical computations were performed by using software SPSS v25. The study was performed retrospectively, and all statistical results were declared significant with P < 0.05.
For correlation analysis, Pearson method was used. The calculations were performed by Adware Research Ltd. (Balatonfüred, Hungary).
3. Results
3.1. Evaluation of the Whole Study Population of 55 GBM Patients Consuming DDW
3.1.1. Characteristics of the Whole Study Population
All patients involved in the analysis had a positive diagnosis of GBM before starting DDW application. The tumor was present at start in all but two subjects who were tumor free thanks to a previous operation. All of the received conventional therapies including chemotherapeutical, irradiation, surgery, targeted therapy, or a combination of these. The time between diagnosis and begin of DDW consumption was varied.
3.1.2. Median Survival Time (MST) of the Whole Study Population
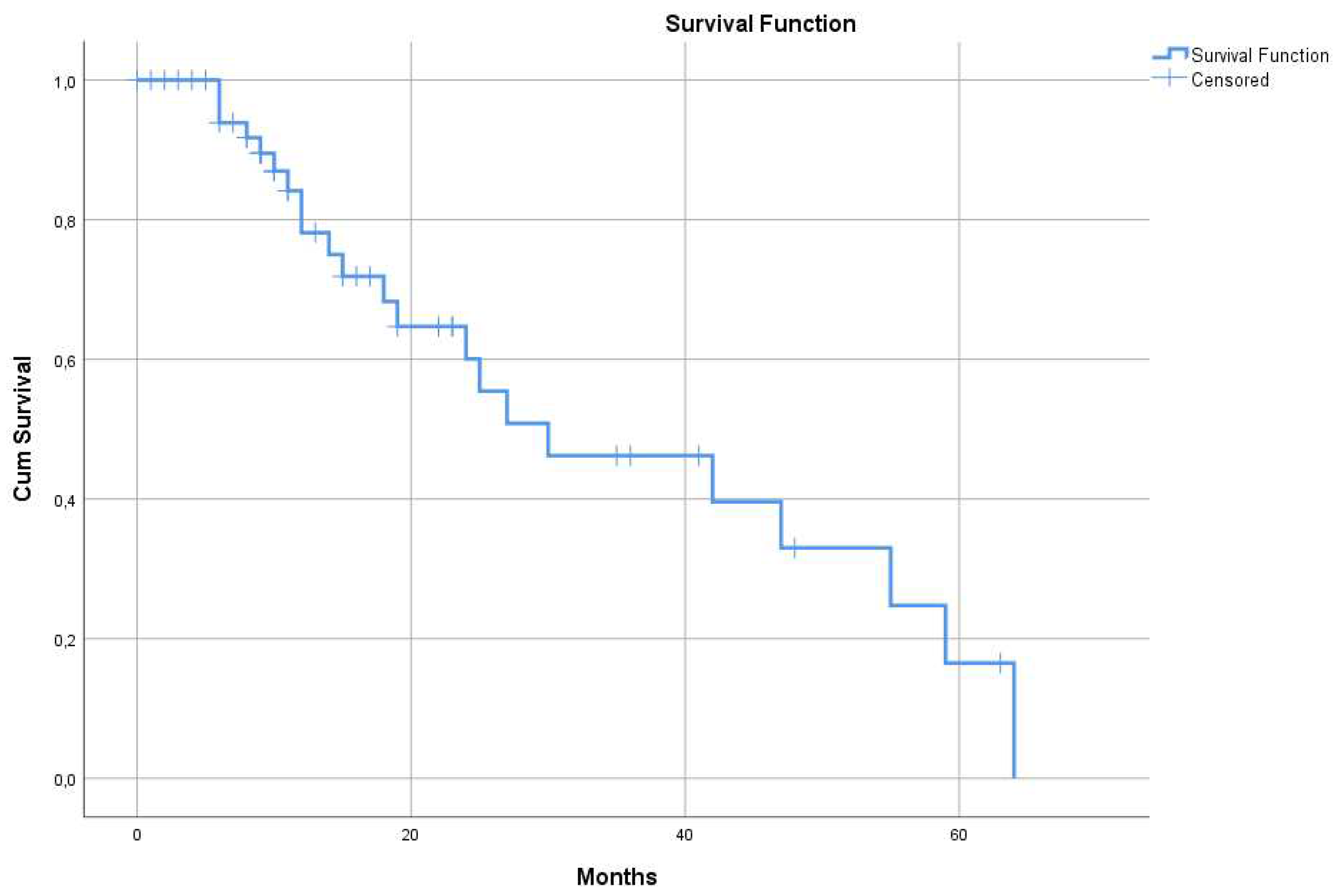
The 55 patients consuming DDW, including even those whose DDW consumption lasted only for 22-123 days, showed longer MST (95% CI: 9.4 – 50.5), 30 months, compared to historical control (12.1-14.4 months) (
Figure 1).
3.1.3. Differences of MSTs in the Whole Cohort by Gender
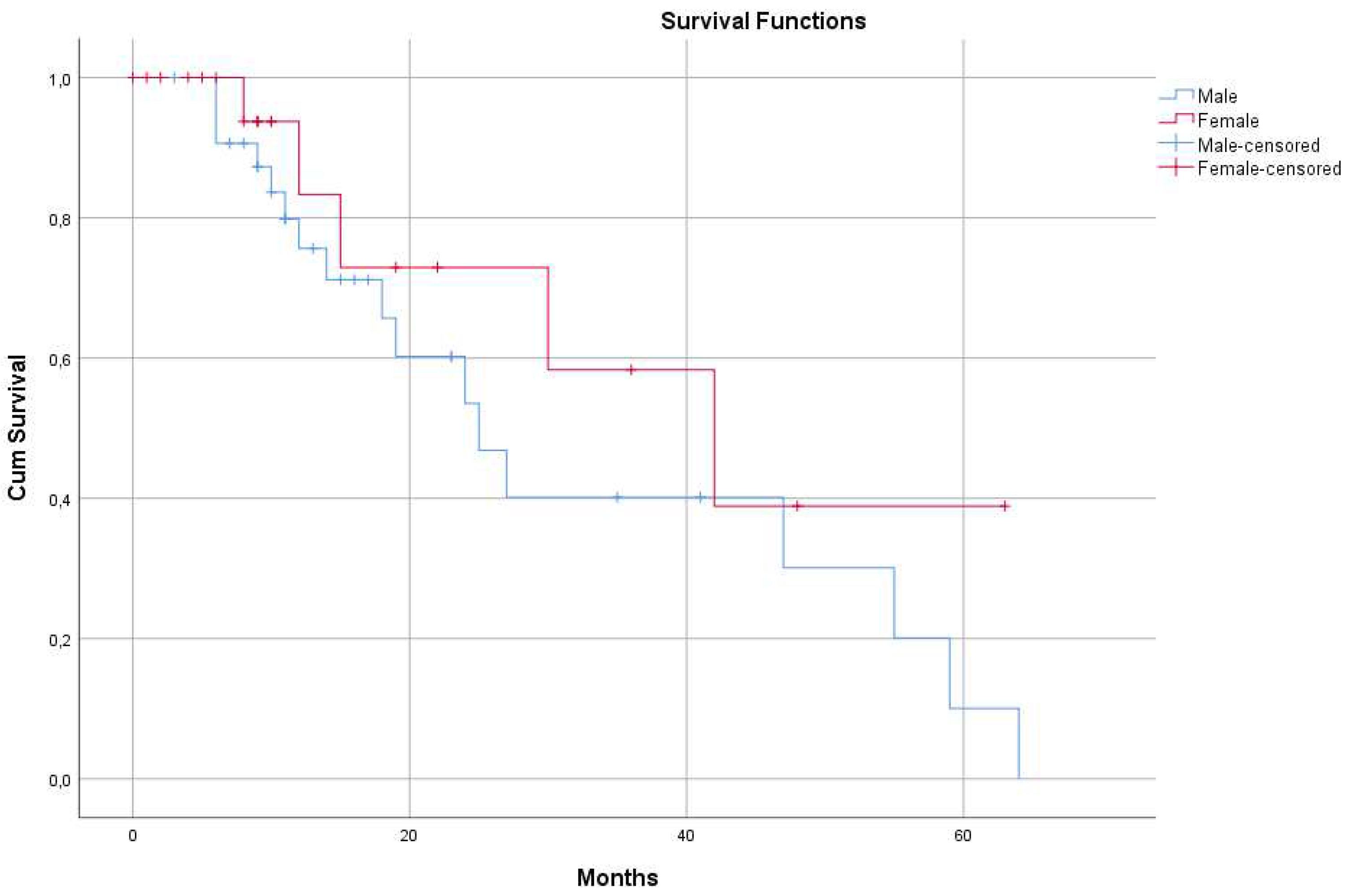
There was a massive (yet not significant, p=0.283) difference between the two genders in the calculated MST values. The median survival time was 25 months (95% CI: 15.9 – 34.0 months) in the male (33 patients) group and 42 months (95% CI: 18.3 – 65.6 months) in the female (22 patients) subgroup (
Figure 2).
3.1.4. Correlation between Survival Times and Duration of DDW Consumption in the Whole Study Population
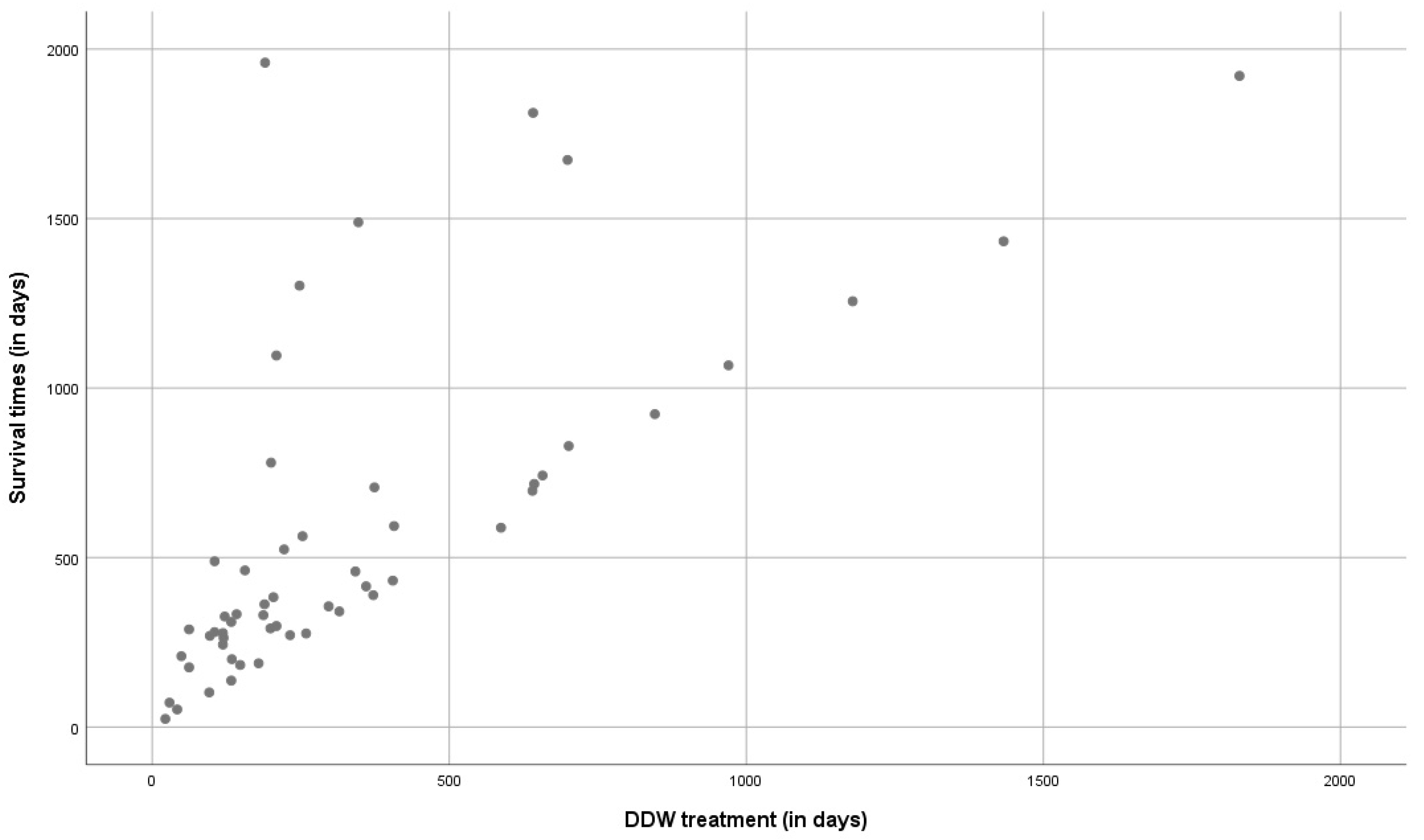
Pearson's correlation coefficient (r) was calculated to test to what extent the length of using DDW and survival time taken from the start of DDW consumption are correlated. An “r” value of 0.692 was obtained (well above 0.5 from which a strong correlation is assumed), the data thus support that longer DDW consumptions result in extended survival times (
Figure 3). The dots above the slope show that shorter duration of DDW consumption can also result in longer survival but may also indicate heterogeneity of the evaluated population regarding staging, size of tumor, and conventional treatment received.
3.1.5. MST in the Whole Study Population Stratified by Temozolomide Treatment
The majority of the patients had been recruited years before Temozolomide (TMZ) was registered, and access to TMZ remained limited for several years after registration. This gave an opportunity to evaluate survival in a TMZ naive and a TMZ treated subpopulation. MST in the former group (38 patients) was 27 months (95% CI: 18.8 – 35.1 months), and in the latter (17 patients), 42 months (95% CI: 14.6 – 69.3 months) with no statistically significant differences between the two (p=0.797). However, MST of both subpopulations was about 3-fold of historical control (
Table 1).
3.2. Detailed Evaluation of the Selected 31 GBM Patients
The patients started consuming DDW at different times (0-542 days, median 57 days) after diagnosis, and the length of DDW consumption varied between 133 and 1,566 days.
3.2.1. Median Survival Time of the Selected 31 GBM Patients
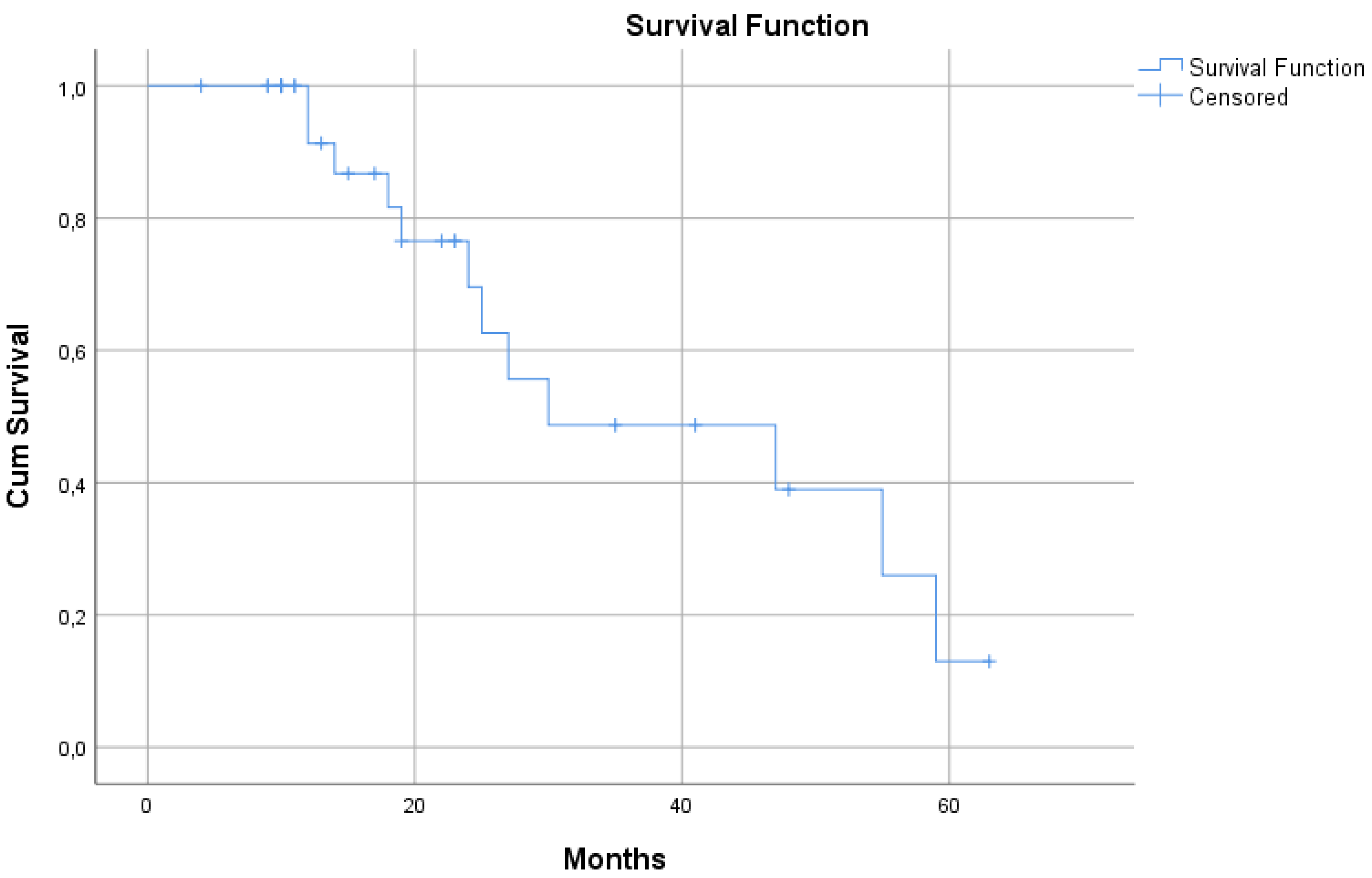
MST of the selected 31 patients who received DDW treatment along with conventional therapies was substantially longer vs. the historical control. Their median survival time was 30 months (95% CI: 0.5 – 59.4) (
Figure 4), suggesting that this subset of cases was representative for the whole set of 55 patients.
3.2.2. Calculation of MST in the Selected 31 GBM Patients Stratified by Relapse Status
The 31 cases were stratified into two categories: being in remission or having had relapse before commencing DDW consumption.
MST was 30 months in the subgroup of patients (20 subjects) who had relapse before DDW treatment, and 47 months in the subgroup of patients (10 subjects) who were in remission when DDW treatment started (and, accordingly, relapsed only later) (
Table 1). However, due to small sample size, this difference was not significant (p=0.246). One patient who had no relapse was excluded from this analysis.
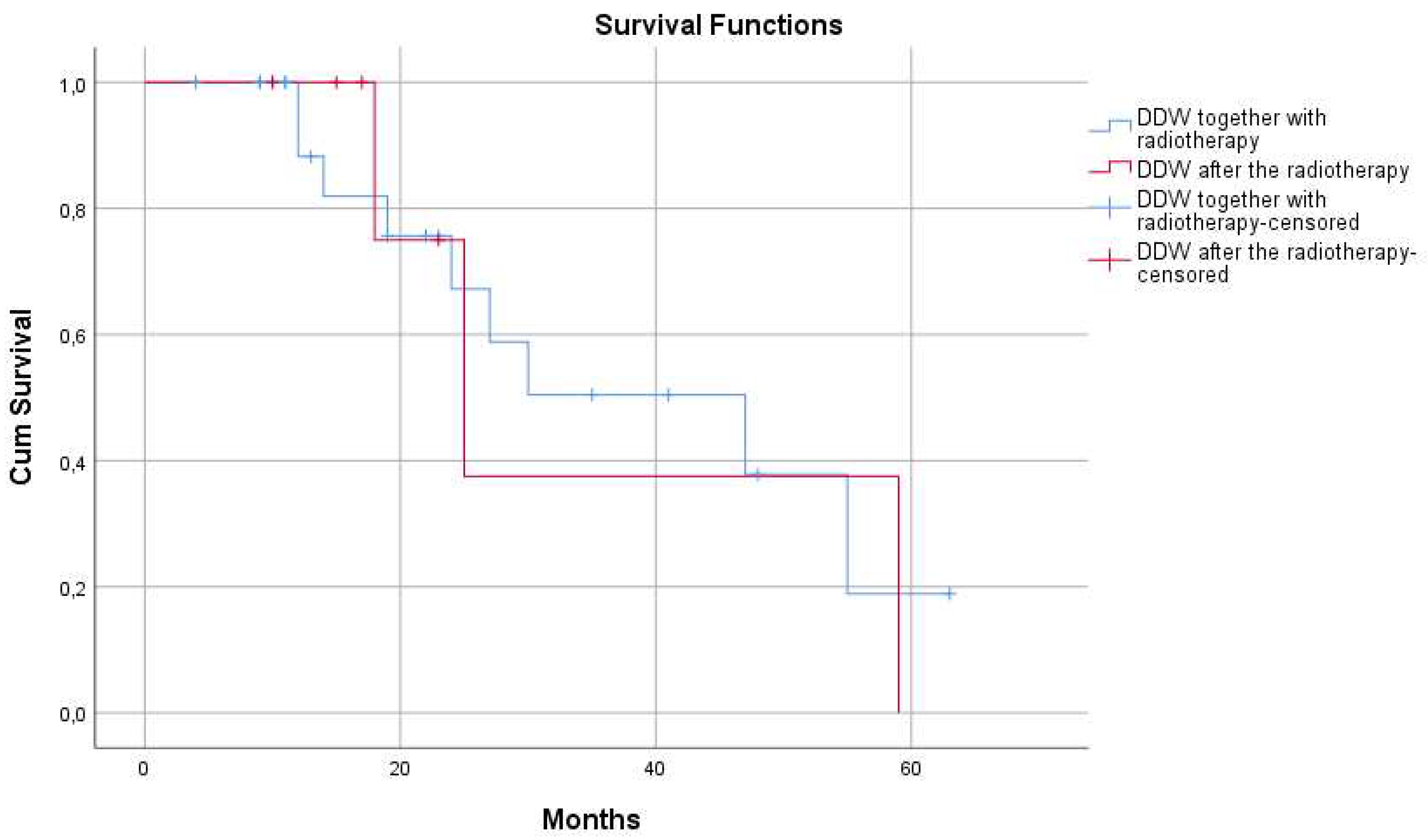
3.2.3. Calculation of MST in the Study Population Stratified by Radiotherapy
The subjects were stratified on the basis whether DDW treatment started together with radiotherapy (simultaneously or within 8 weeks after last radiation) or more than 8 weeks after radiotherapy was completed. MST was 47 months (95% CI: 18,8 - 75,1) in the subgroup of patients who started DDW treatment together with radiotherapy, and in the other group it was 25 months (95%CI: 14,5 - 35,4) (
Figure 5). However, sample size was too small to detect significant differences (p=0.942).
4. Discussion
It has been demonstrated in both
in vitro and
in vivo experiments that naturally occurring deuterium is a core factor in cell growth, and deuterium depletion induces apoptosis in tumor cells, resulting in partial or complete tumor regression [
9,
12,
17,
21].
The subjects involved in the study were dissimilar in their stage of disease when use of DDW started, in the delay between diagnosis and start of DDW consumption, and what conventional therapies they received (a typical situation when data are collected and ana-lyzed retrospectively). To extract maximum information on how efficient DDW applied in combination with conventional therapies, first the survival of all the 55 GBM patients involved was evaluated, then (after exclusion of those with too short DDW consumption or long survival without DDW) 31 of them were analyzed in detail. All results were summarized in
Table 1.
The duration of DDW consumption and length of survival showed positive correlation (r=0.692) which also supported that DDW in GBM patients had an antitumor effect indeed (
Figure 3).
Survival times observed in the present study were markedly longer compared to prospective clinical data of patients with primary GBM [
3,
4,
5,
6]; suggesting that D depletion and conventional treatments together can provide longer survival in advanced GBM than any targeted or combination therapy to date.
In the light of scientific evidence on the biological effect of deuterium depletion [
9,
12,
22,
38,
39] an important aim was to find the best combination of DDW and conventional therapies. The data in
Table 1 show that DDW consumption enhanced the efficacy of both TMZ and radiotherapy. The 47 months’ MST of patients being in remission at the start of DDW consumption is in line with our earlier data showing that DDW increased progression-free interval and/or prevented relapse [
7,
10,
11,
25]. One protocol option re-commended is to start DDW consumption after operation, continue it during radiotherapy by consuming DDW with 85 ppm D concentration, reduce D concentration to 65 ppm 2-3 weeks after the last radiation combining with TMZ treatment applied according to the Stupp protocol [
5] and after 1-3 months reduce the D concentration to 45 ppm and 25 ppm.
The substantial difference between MST of females (42.0 months) and males (25.0 months) may be due to the different expression of oncogenes in response to deuterium depletion, as it had been observed in female and male mice [
7]. Also in another human study, on non-small cell lung cancer, striking difference was found between the calculated MST (males, 41.2 months; females, 107.0 months; taken from the date of diagnosis) [
11].
Oral DDW treatment proved to be safe and innocuous, according to preclinical toxicology studies [
40] as well as to prospective and retrospective clinical trials [
8,
10,
25,
26].
In vitro growth inhibition of tumorous cell lines under deuterium-depleted conditions, and the clinically observed prolongation of progression-free interval and prevention of relapse in breast and lung cancer patients [
25,
26] were in agreement. Consuming 1.5-2 liters of DDW with 105 ppm D content day by day results in inner D level decrease of about 1 ppm per. Prolonged use of DDW with the same D level leads to equilibrium. For ongoing reduction of D concentration in the body fluids, a change to DDW of 20 ppm less D content is advisable every 2-3 months The massive effects of subnatural D concentration at cellular level, including induction of apoptosis [
9,
12,
17,
20,
41], and inhibition of tumor cell migration [
11], may both be of importance in MST lengthening and relapse prevention observed in tumorous patients ingesting DDW [
8,
10].
The changes of metabolic parameters in patients applying ketogenic diet, and the beneficial antitumor effects, are well-documented [
42,
43,
44]. The naturally low-D lipids in a ketogenic diet have significant inhibitory effect on tumor growth by preventing the cells from raising D/H ratio to the threshold.
We conclude that D-depletion offers additional benefits in addition to conventional therapies and can be integrated into standard treatment regimens for GBM. The best option for GBM patients is achieving improvement by combining DDW with conventional therapies. Application of DDW is recommended in all phases of the disease, before surgery, during radiotherapy, and in repeated DDW courses when remission has been achieved.
This retrospective study has its obvious limitations but may help with the assessment of the necessity and feasibility of prospective studies.
Author Contributions
Conceptualization, G.S., I.S.; Methodology, G.S.; Software, A.P.; Validation, G.S.; Formal Analysis, A.P.; Investigation, G.S., B.Zs.K., I.S.; Resources, G.S., I.S.; Data Curation, A.P.; Writing – Original Draft Preparation, G.S., B.Zs.K., I.S.; Writing – Review & Editing, A.P., I.S.; Visualization, B.Zs.K.; Supervision, G.S.; Funding Acquisition, G.S.
Funding
This work was sponsored by HYD LLC for Cancer Research and Drug Development.
Institutional Review Board Statement
Ethical approval to retrospective study is not applicable to this article
Informed Consent Statement
Verbal informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare that the research was conducted by HYD LLC for Cancer Research and Drug Development. HYD LLC has registered Vetera-DDW-25 deuterium-depleted medicinal anticancer drugs to treat household pets and commercialize Preventa deuterium-depleted drinking water for human consumption.
References
- Lacy, J.; Saadati, H.; Yu, J. Complications of brain tumors and their treatment. Hemat Oncol Clin North Am 2012, 26, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Jacques, G.; Cormac, O. Central Nervous System Tumors. Handb Clin Neurol 2013, 112, 931–958. [Google Scholar] [PubMed]
- DeRobles, P.; Fiest, K.M.; Frolkis, A.D.; Pringsheim, T.; Atta, C. DeRobles, P.; Fiest, K.M.; Frolkis, A.D.; Pringsheim, T.; Atta, C.; St. Germaine-Smith, C.; Day, L.; Lam, D.; Jette, N. The worldwide incidence and prevalence of primary brain tumors: a systematic review and meta-analysis. Neuro-Oncology 2015, 17, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; DeAngelis, L.M. Glioblastoma and other malignant gliomas: a clinical review. Jama 2013, 310, 1842–50. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; Van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; Curschmann, J.; Janzer, R.C.; Ludwin, S.K.; Gorlia, T.; Allgeier, A.; Lacombe, D.; Cairncross, J.G.; Eisenhauer, E.; Mirimanoff, R.O. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Eng. J. Med. 2015, 352, 352–996. [Google Scholar] [CrossRef]
- Young, W.K.; Albright, R.E.; Olson, J.; Frederics, R.; Fink, K.; Prados, M.D.; Brada, M.; Spence, A.; Hohl, R.J.; Shapiro, W.; Glantz, M.; Greenberg, H.; Selker, R.G.; Vick, N.A.; Rampling, R.; Friedman, H.; Phillips, P.; Bruner, J.; Yue, N.; Osoba, D.; Zaknoen, S.; Levin. V.A. A Phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first by relapse. Br. J. Cancer 2000, 83, 588–593.
- Gyöngyi, Z.; Somlyai, G. Deuterium depletion can decrease the expression of c-myc, Ha-ras and p53 gene in carcinogen-treated mice. In Vivo 2000, 14, 437–440. [Google Scholar]
- Krempels, K.; Somlyai, I.; Somlyai, G. A retrospective evaluation of the effects of deuterium depleted water consumption on four patients with brain metastases from lung cancer. Integrative Cancer Therapies 2008, 7, 172–181. [Google Scholar] [CrossRef]
- Cong, F.S.; Zhang, Y.R.; Sheng, H.C.; Ao, Z.H.; Zhang, S.Y. Deuterium-depleted water inhibits human lung carcinoma cell growth by apoptosis. Exp. Ther. Med. 2010, 1, 277–283. [Google Scholar] [CrossRef]
- Kovács, A.; Guller, I.; Krempels, K.; Somlyai, I.; Jánosi, I.; Gyöngyi, Z.; Szabó, I.; Ember, I.; Somlyai, G. Deuterium Depletion May Delay the Progression of Prostate Cancer. Journal of Cancer Therapy 2011, 2, 548–556. [Google Scholar] [CrossRef]
- Somlyai, G.; Kovács, B.Zs.; Somlyai, I.; Papp, A.; Nagy, L.I.; Puskás, L.G. Deuterium depletion inhibits lung cancer cell growth and migration in vitro and results in severalfold increase of median survival time of non-small cell lung cancer patients receiving conventional therapy. J. Cancer Res. Ther. 2021, 9, 12–19. [Google Scholar] [CrossRef]
- Somlyai, G.; Jancsó, G.; Jákli, G.; Vass, K.; Barna, B.; Lakics, V.; Gaál, T. Naturally occurring deuterium is essential for the normal growth rate of cells. FEBS Lett. 1993, 317, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.J.; Crespi, H.L. Isotope effects in biological systems. In Isotope Effects in Chemical Reactions, Collins, C.J.; Bowman, N.S., Ed.; Van Nostrand Reinhold: New York, USA, 1971; pp. 286–363. [Google Scholar]
- Jancsó, G. Isotope effects. In Handbook of Nuclear Chemistry, Vértes, A.; Nagy, S., Klencsár, Z., Eds.; Kluwer Academic Publishers: Dordrecht, Netherlands, 2003; pp. 85–116. [Google Scholar]
- Enright, J. Heavy water slows biological timing processes. Zeitschrift für vergleichende Physiologie 1971, 72, 1–16. [Google Scholar] [CrossRef]
- Harvey, E.N. Biological effects of heavy water. The Biological Bulletin 1934, 66, 91–96. [Google Scholar] [CrossRef]
- Somlyai, G.; Laskay, G.; Berkényi, T.; Jákli, Gy.; Jancsó, G. Naturally occurring deuterium may have a central role in cell signaling. In Synthesis and Applications of Isotopically Labelled Compounds, Heys, J.R.; Melillo, D., Ed.; John Wiley and Sons Ltd: New York, USA, 1988; pp. 137–141. [Google Scholar]
- Yavari, K.; Kooshesh, L. Deuterium Depleted Water Inhibits the Proliferation of Human MCF7 Breast Cancer Cell Lines by Inducing Cell Cycle Arrest. Nutrition and Cancer 2019, 71, 1019–1029. [Google Scholar] [CrossRef]
- Syroeshkin, A.; Levitskaya, O.; Uspenskaya, E.; Pleteneva, T.; Romaykina, D.; Ermakova, D. Deuterium Depleted Water as an Adjuvant in Treatment of Cancer. Sys. Rev. Pharm. 2019, 10, 112–117. [Google Scholar] [CrossRef]
- Zlatskiy, I.A.; Zlatska, A.V.; Antipova, N.V.; Syroeshkin, A.V. Effect of deuterium on the morpho-functional characteristics of normal and cancer cells in vitro. Trace Elements and Electrolytes 2018, 35, 211–214. [Google Scholar] [CrossRef]
- Somlyai, G.; Laskay, G.; Berkényi, T.; Galbács, Z.; Galbács, G.; Kiss, S.A.; Jákli, Gy.; Jancsó, G. The biological effects of deuterium-depleted water a possible new tool in cancer therapy. Journal of Oncology 1998, 30, 91–94. [Google Scholar]
- Zhang, X.; Gaetani, M.; Chernobrovkin, A.; Zubarev, R.A. Anticancer Effect of Deuterium Depleted Water – Redox Disbalance Leads to Oxidative Stress. Mol Cell Proteomics 2019, 18, 2373–2387. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zubarev, R.A. Slight deuterium enrichment in water acts as an antioxidant: is deuterium a cell growth regulator? Molecular & Cellular Proteomics 2020, 19, 1790–1804. [Google Scholar] [CrossRef]
- Boros, L.G.; D'Agostino, D.P.; Katz, H.E.; Roth, J.P.; Meuillet, E.J.; Somlyai, G. Submolecular regulation of cell transformation by deuterium depleting water exchange reactions in the tricarboxylic acid substrate cycle. Medical Hypothesis 2016, 87, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Krempels, K.; Somlyai, I.; Gyöngyi, Z.; Ember, I.; Balog, K.; Abonyi, O.; Somlyai, G. A retrospective study of survival in breast cancer patients undergoing deuterium depletion in addition to conventional therapies. Journal of Cancer Research & Therapy 2013, 1, 194–200. [Google Scholar] [CrossRef]
- Gyöngyi, Z.; Budán, F.; Szabó, I.; Ember, I.; Kiss, I.; Krempels, K.; Somlyai, I.; Somlyai, G. Deuterium Depleted Water Effects on Survival of Lung Cancer Patients and Expression of Kras, Bcl2, and Myc Genes in Mouse Lung. Nutrition and Cancer 2003, 65, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; J Riggins, G.; Friedman, J.; Friedman, A.; Reardon, D.; Herndon, J.; Kinzler, K.W.; Velculescu, V.E.; Vogelstein, B.; Bigner, D.D. IDH1 DD. and IDH2 mutations in gliomas. N Engl J Med 2009, 360, 765–73. [Google Scholar] [CrossRef]
- Lv, S.; Teugels, E.; Sadones, J.; Quartier, E.; Huylebrouck, M.; Du Four, S.; Le Mercier, M.; De Witte, O.; Salmon, I.; Michotte, A.; De Grève, J.; Neyns, B. Correlation between IDH1 gene mutation status and survival of patients treated for recurrent glioma. Anticancer Res 2011, 31, 4457–63. [Google Scholar] [CrossRef] [PubMed]
- Chesnelong, C.; Chaumeil, M.M.; Blough, M.D.; Al-Najjar, M.; Stechishin, O.D.; Chan, J.A.; O Pieper, R.; Ronen, S.M.; Weiss, S.; Luchman, H.A.; Cairncross. J.G. Lactate dehydrogenase. A silencing in IDH mutant gliomas. Neuro Oncol 2014, 16, 686–95. [CrossRef]
- Liu, F.M.; Gao, Y.F.; Kong, Y.; Guan, Y.; Zhang, J.; Li, S.H.; Ye, D.; Wen, W.; Zuo, C.; Hua, W. The diagnostic value of lower glucose consumption for IDH1 mutated gliomas on FDG-PET. BMC Cancer 2021, 21, 83. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Flores, R.E.; Poff, A.M.; D’Agostino, D.P. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis 2014, 35, 515–527. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Mukherjee, P.; Iyikesici, M.S.; Slocum, A.; Kalamian, M.; Spinosa, JP.; Chinopoulos, C. Consideration of Ketogenic Metabolic Therapy as a Complementary or Alternative Approach for Managing Breast Cancer. Frontiers in Nutrition 2020, 7. [CrossRef]
- Seyfried, T.N.; Shivane, A.G.; Kalamian, M.; Maroon, J.C.; Mukherjee, P.; Zuccoli, G. Ketogenic Metabolic Therapy, Without Chemo or Radiation, for the Long-Term Management of IDH1-Mutant Glioblastoma: An 80-Month Follow-Up Case Report. Frontiers in Nutrition 2012, 8. [Google Scholar] [CrossRef]
- Mukherjee, P.; Augur, Z.M.; Li, M.; Hill, C.; Greenwood, B.; Domin, M.A.; Kondakci, G.; Narain, N.R.; Kiebish, M.A.; Bronson, R.T.; Arishmendi-Morillo, G.; Chinopoulos, C.; Seyfried, T.N. Therapeutic benefit of combining calorie-restricted ketogenic diet and glutamine targeting in late-stage experimental glioblastoma. Commun Biol 2019, 2, 200. [Google Scholar] [CrossRef]
- Denny, C.A.; Heinecke, K.A.; Kim, Y.P.; Baek, R.C.; Loh, K.S.; Butters, T.D.; Bronson, R.T.; Platt, F.M.; Seyfried, T.N. Restricted ketogenic diet enhances the therapeutic action of N-butyldeoxynojirimycin towards brain GM2 accumulation in adult Sandhoff disease mice. J Neurochem 2010, 113, 1525–1535. [Google Scholar] [CrossRef]
- Altinoz, M.A.; Ozpinar, A.; Seyfried, T.N. Caprylic (Octanoic) Acid as a potential fatty acid chemotherapeutic for glioblastoma. Prostagl Leukot Essent Fatty Acids 2020, 159, 102142. [Google Scholar] [CrossRef]
- Basov, A.A.; Bykov, I.M.; Baryshev, M.G.; Dzhimak, S.S.; Bykov, M.I. Determination of deuterium concentration in foods and influence of water with modified isotopic composition on oxidation parameters and heavy hydrogen isotopes content in experimental animals. Vopr Pitan 2014, 83, 43–50. [Google Scholar] [PubMed]
- Kamal, Y.; Lida, K. Deuterium Depleted Water Inhibits the Proliferation of Human MCF7 Breast Cancer Cell Lines by Inducing Cell Cycle Arrest. Nutrition and Cancer 2019, 71, 1019–1029. [Google Scholar] [CrossRef]
- Siniak, I.E.; Turusov, V.S.; Grigoriev, A.I.; Zaridze, D.G.; Gaidadymov, V.B.; Gus'kova, E.I.; Antoshina, E.E.; Gor'kova,T.G.; Trukhanova, L.S. Consideration of the Deuterium-Free Water Supply to an Expedition to Mars. Aerospace and Environmental Medicine 2003, 37, 60.
- Török, G.; Csík, M.; Pintér, A. Effects of Different Deuterium Concentrations of the Media on the Bacterial Growth and Mutagenesis. Egészségtudomány/Health Science 2000, 44, 331. [Google Scholar]
- Somlyai, G.; Molnár, M.; Laskay, G.; Szabó, M.; Berkényi, T.; Guller, I.; Kovács, A. Biological significance of naturally occurring deuterium: the antitumor effect of deuterium depletion. Orv. Hetil. 2010, 151, 1455. [Google Scholar] [CrossRef]
- Cuezva, J.M.; Chen, G.; Alonso, A.M.; Isidoro, A.; Misek, D.E.; Hanash, S.M.; Beer, D.G. The bioenergetic signature of lung adenocarcinomas is a molecular marker of cancer diagnosis and prognosis. Carcinogenesis 2004, 25, 1157–63. [Google Scholar] [CrossRef]
- Zhou, W.; Mukherjee, P.; Kiebish, M.A.; Markis, W.T.; Mantis, J.G.; Seyfried, T.N. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond) 2007, 4, 5. [Google Scholar] [CrossRef]
- Khodadadi, S.; Sobhani, N.; Mirshekar, S.; Ghiasvand, R.; Pourmasoumi, M.; Miraghajani, M.; Dehsoukhteh, S.S. Tumor Cells Growth and Survival Time with the Ketogenic Diet in Animal Models: A Systematic Review. Int J Prev Med 2017, 8, 35. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).