Submitted:
03 July 2023
Posted:
04 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Test time and place
2.2. Experimental design
2.3. Feed management
2.4. Sample collection and testing
2.4.1. Horse milk sample collection
2.4.2. Blood sample collection
2.4.3. Faecal sample collection
2.5. Sample testing
2.5.1. Measurement of lactation volume
2.5.2. Determination of milk composition
2.5.3. Determination of plasma biochemical indexes
2.5.4. Determination of diversity in the fecal microflora
2.6. Data analyses
3. Results
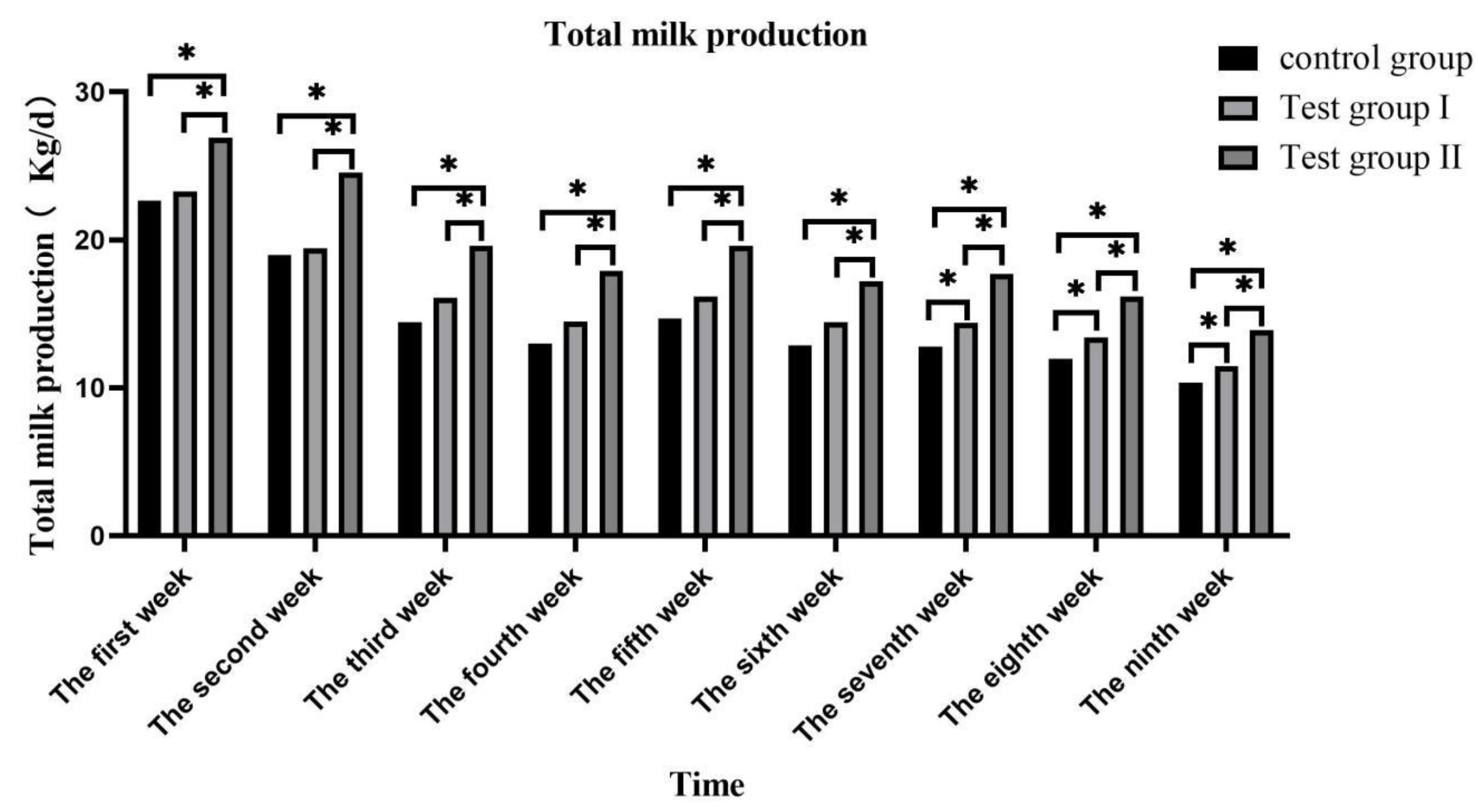
3.1. Effect of concentrate supplementation and fatty acids on lactation volume of grazing Yili horses
3.2. Effects of concentrate supplementation and fatty acids on milk composition of grazing Yili horses
3.3. Effects of concentrate supplementation and fatty acid on fatty acid constitute of grazing Yili horses
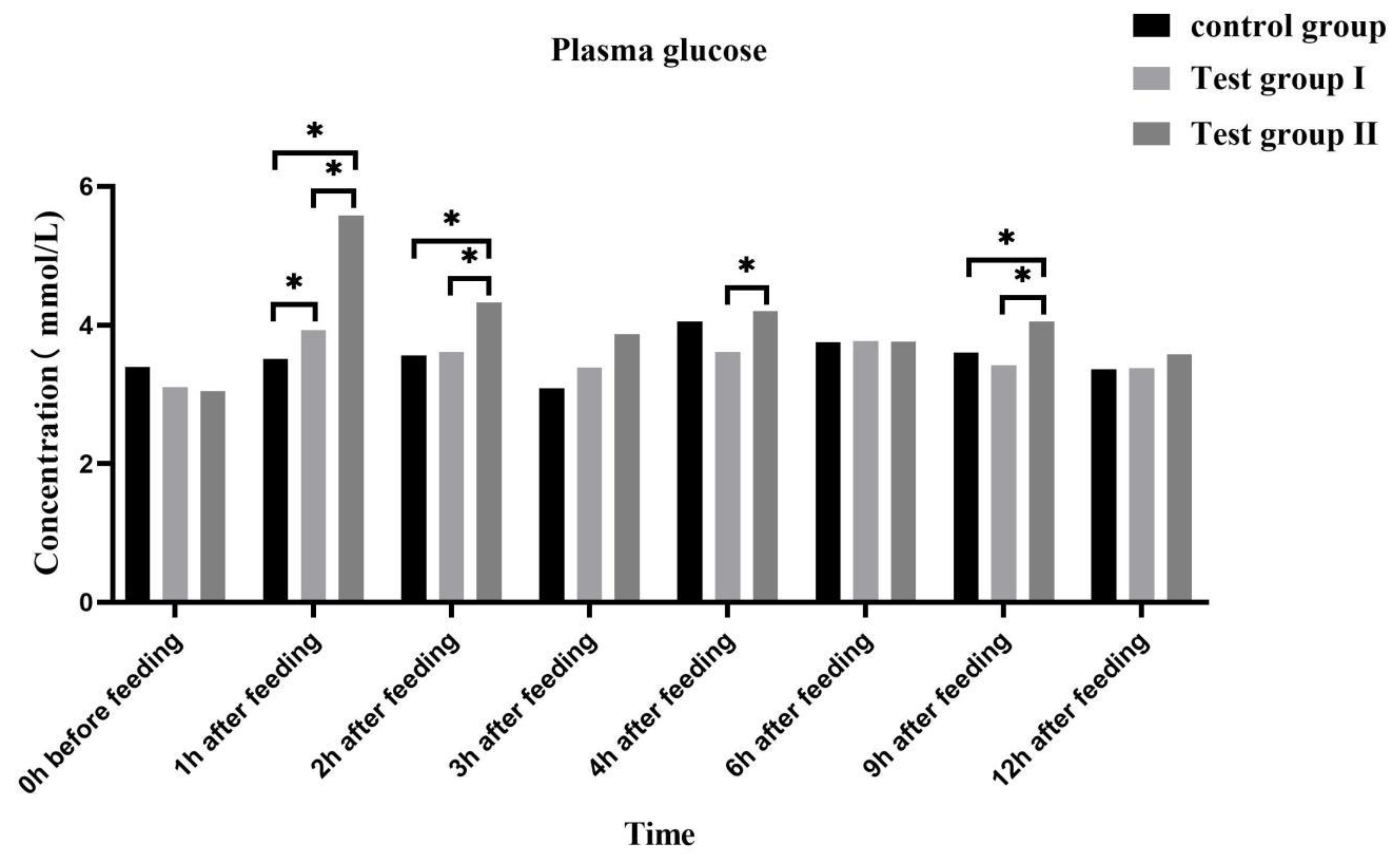
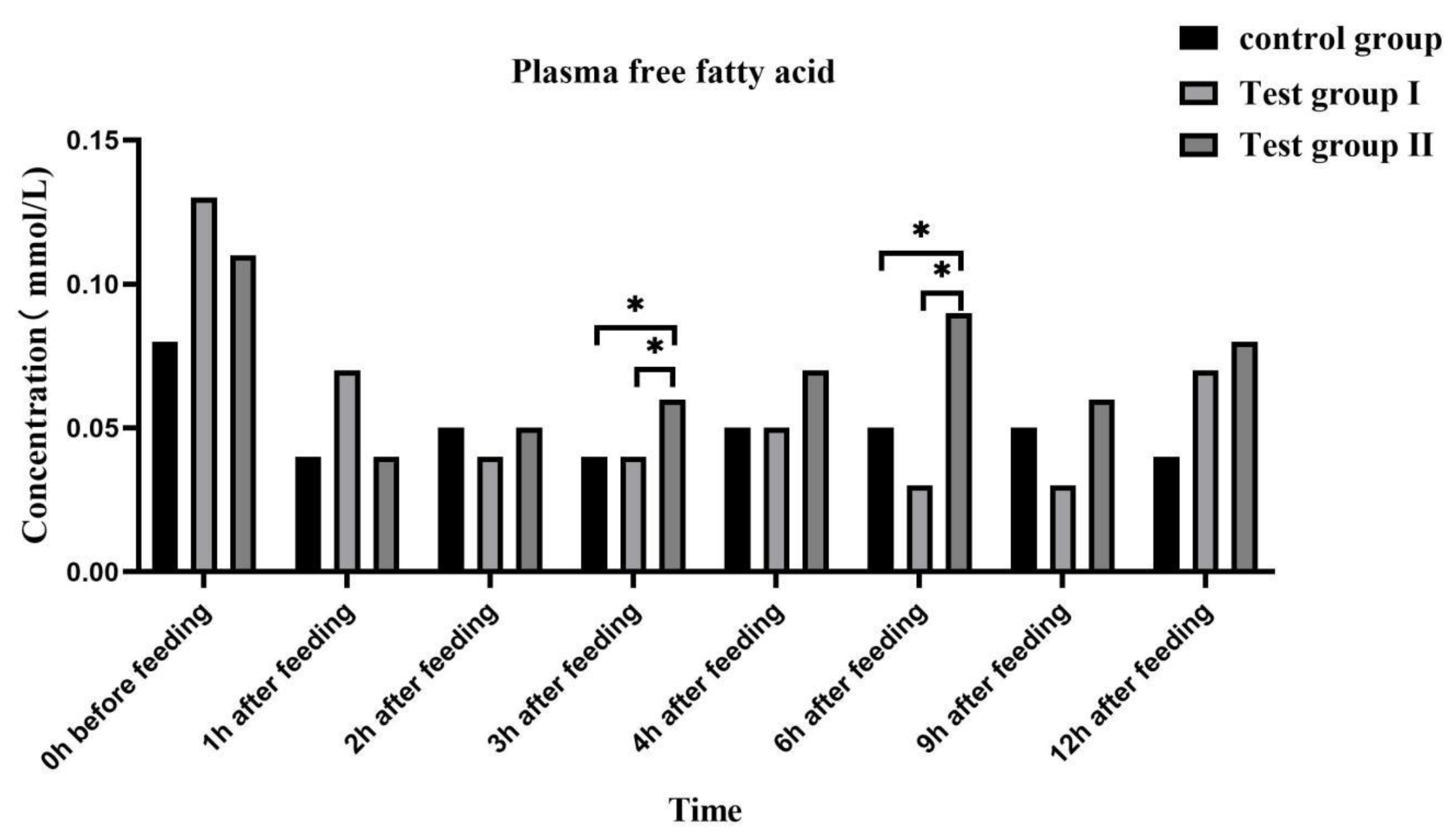
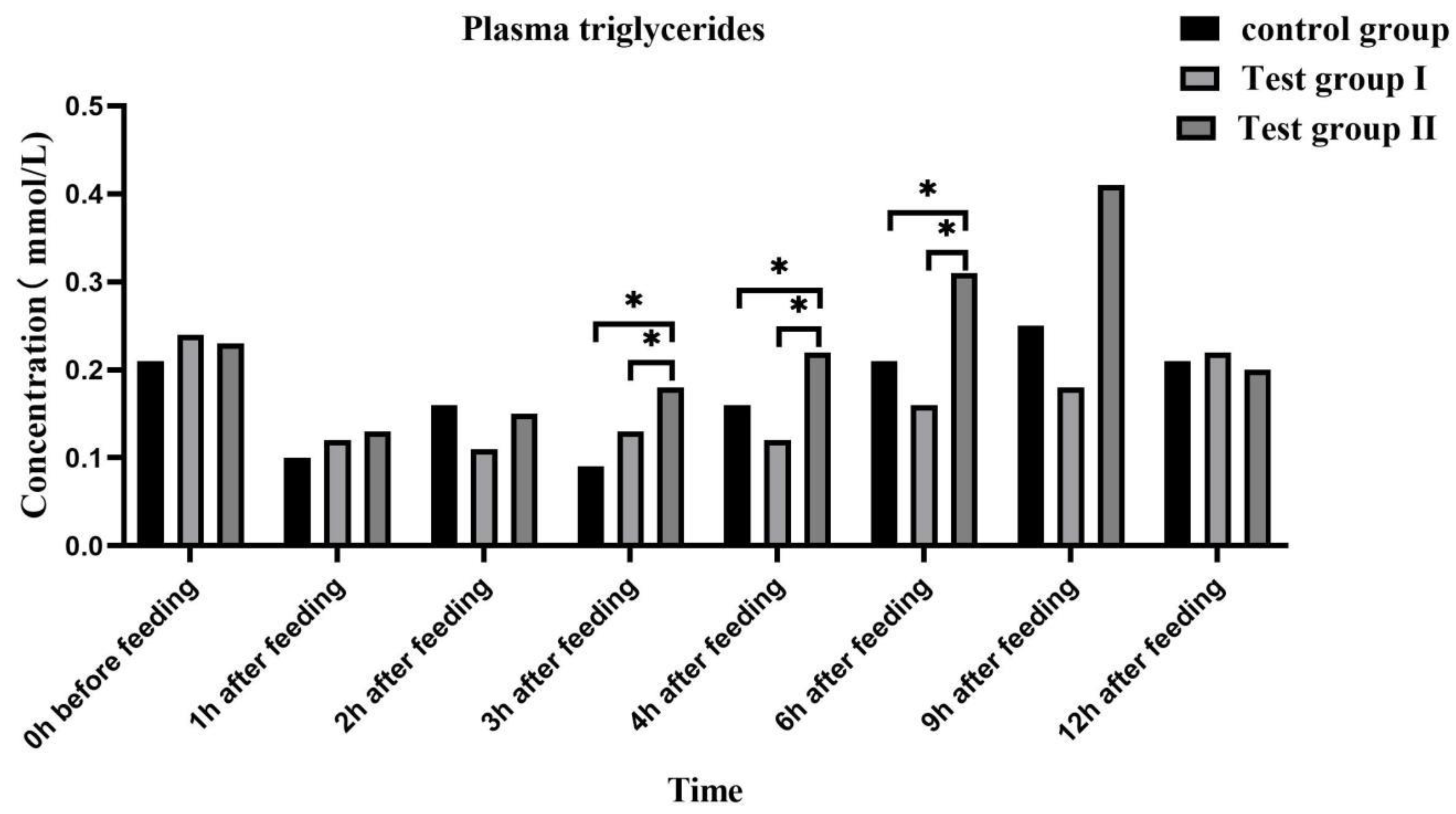
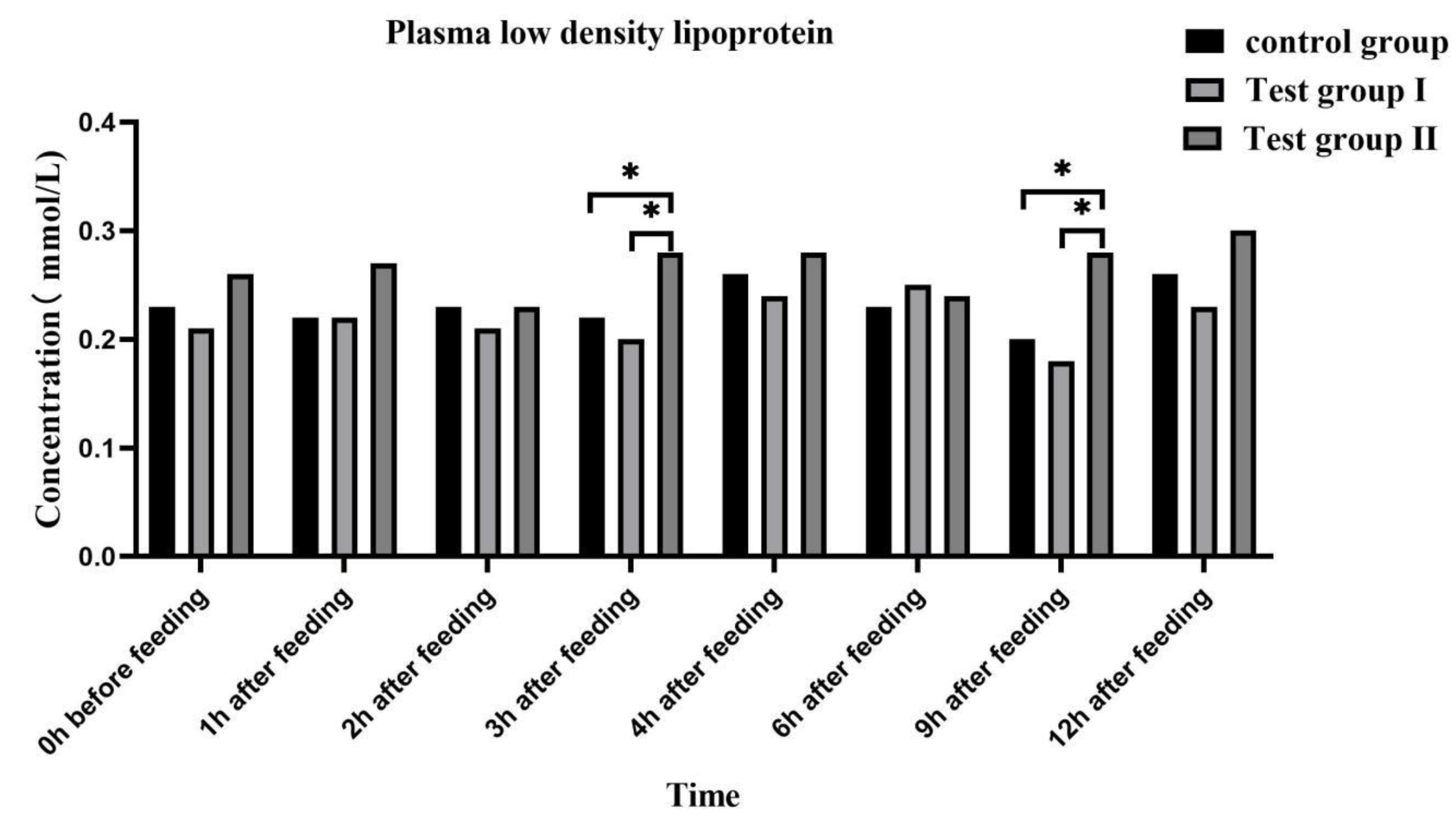
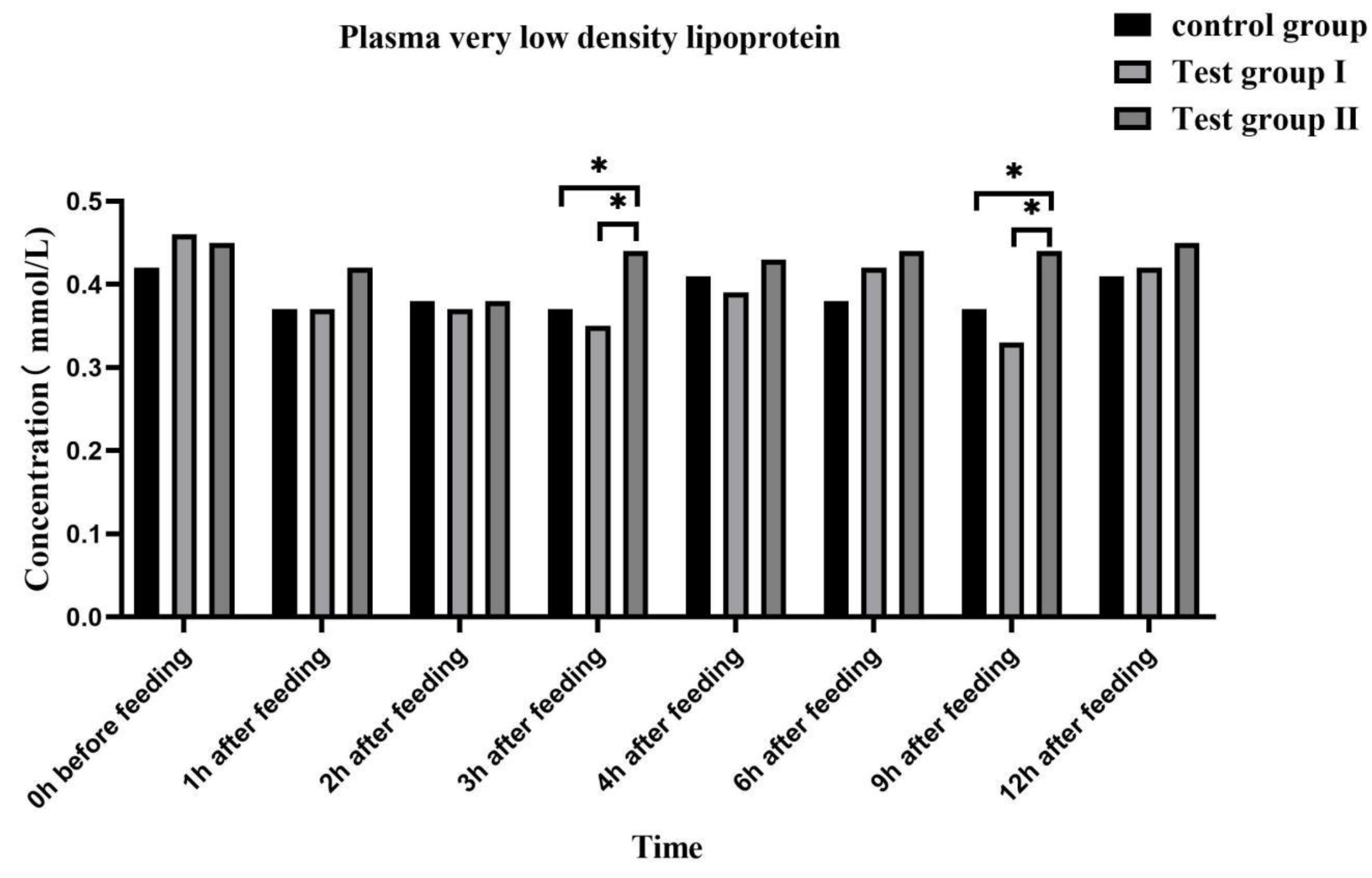
3.4. Effect of concentrate supplementation and fatty acids on plasma biochemical indexes of grazing Yili horses
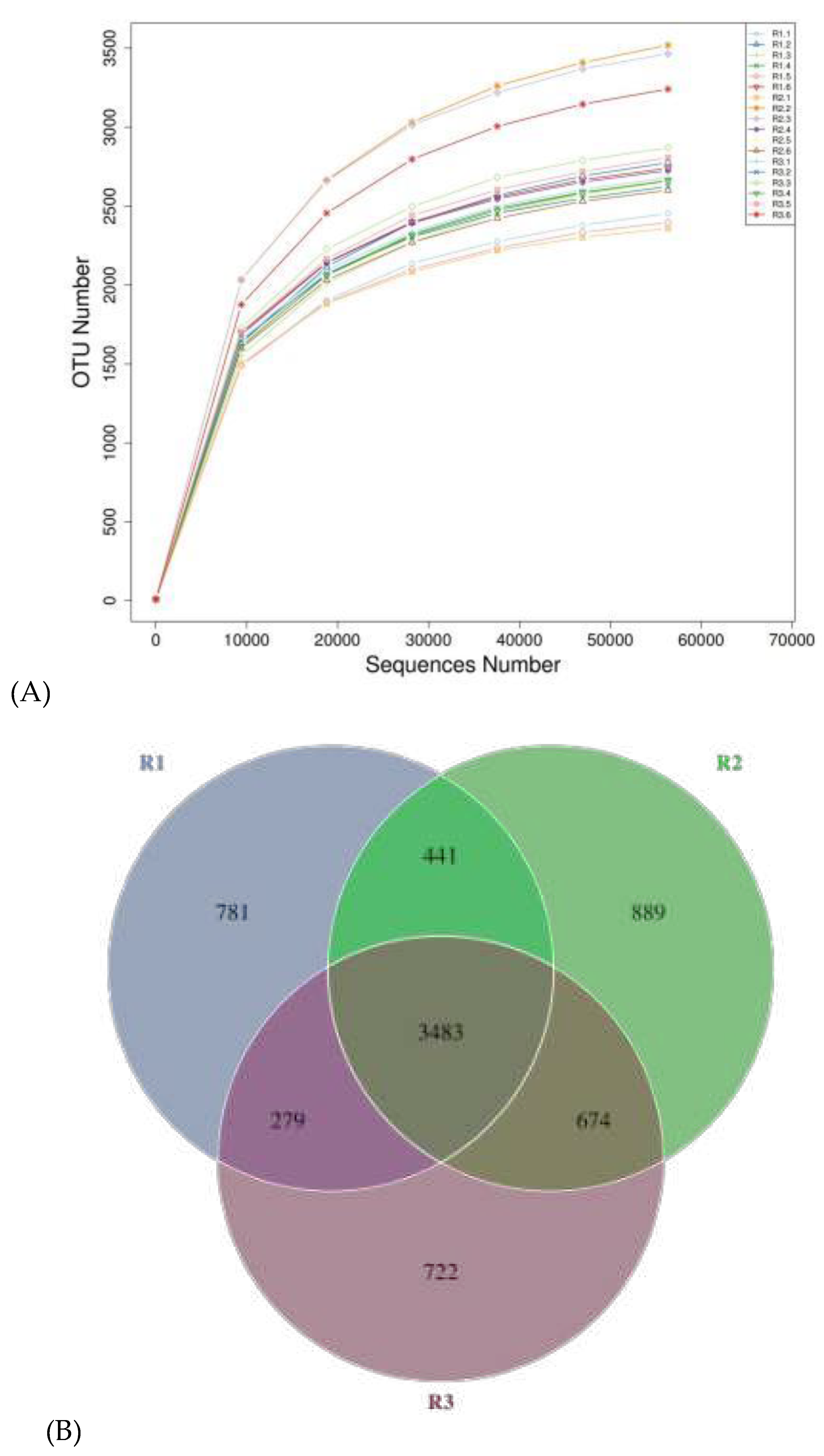
3.5. Effect of concentrate supplementation and fatty acids on the faecal flora diversity of grazing Yili horses
3.6. Effect of concentrate supplementation and fatty acids on the Alpha diversity analysis of fecal phylum flora in grazing Yili horses
3.7. Effect of concentrate supplementation and fatty acids on the abundance of fecal phylum flora in grazing Yili horses
3.8. Effect of concentrate supplementation and fatty acids on the abundance of bacteria in fecal families of grazing Yili horses
3.9. Effect of concentrate supplementation and fatty acids on the abundance of bacteria in genus level of grazing Yili horses
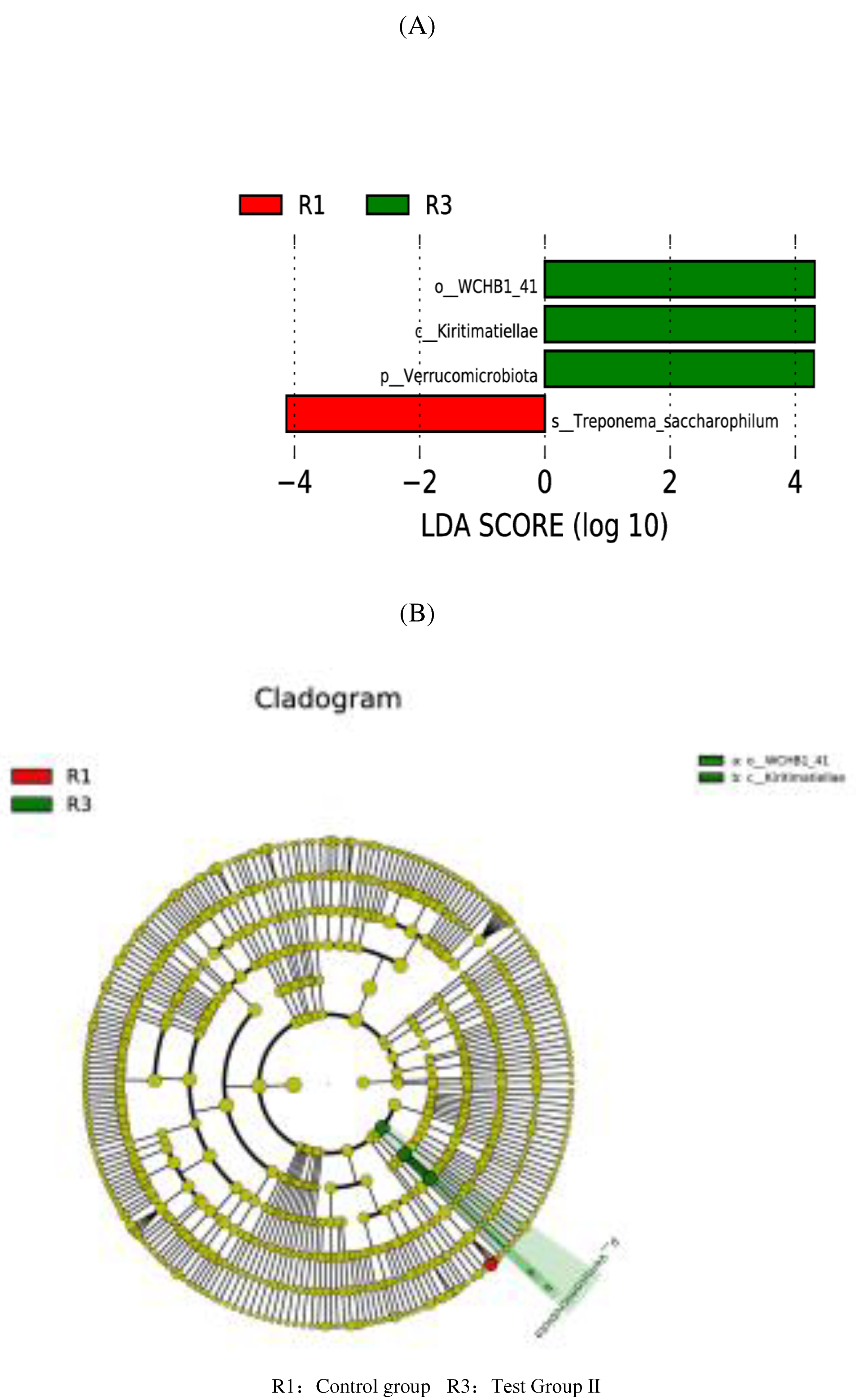
3.10. LEfSe analysis and Tax4Fun function prediction of feces on dietary Supplementary concentrate and fatty acids of grazing Yili horses
3.11. PICRUSt Functional prediction
4. Discussion
5. Conclusions
Author Contributions
Conflict of Interest
Acknowledgments
Ethics Approval and Consent to Participate
References
- Korosue K, Murase H, Sato F, et al. Successful induction of lactation in a barren Thoroughbred mare: growth of a foal raised on induced lactation and the corresponding maternal hormone profiles[J]. Journal of Veterinary Medical Science 2012, 74, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Li N, Xie Q, Chen Q, et al. Cow, Goat, and Mare Milk Diets Differentially Modulated the Immune System and Gut Microbiota of Mice Colonized by Healthy Infant Feces[J]. Journal of Agricultural and Food Chemistry 2020, 68, 15345–15357. [CrossRef] [PubMed]
- Yu X, Fang C, Liu L, et al. Transcriptome study underling difference of milk yield during peak lactation of Kazakh horse[J]. Journal of Equine Veterinary Science 2021, 102, 103424. [Google Scholar] [CrossRef] [PubMed]
- Coenen M, Kienzle E, Vervuert I, et al. Recent German Developments in the Formulation of Energy and Nutrient Requirements in Horses and the Resulting Feeding Recommendations[J]. Journal of Equine Veterinary Science 2011, 31, 219–229. [Google Scholar] [CrossRef]
- Mangwiro Y T, Cuffe J S, Vickers M H, et al. Maternal exercise alters rat fetoplacental stress response: Minimal effects of maternal growth restriction and high-fat feeding[J]. Placenta 2021, 104, 57–70. [Google Scholar] [CrossRef]
- Spers R C, Spers A, Fernandes W R, et al. Effect of dietary supplementation with coco-nut babaçu oil on performance of lactating mares[J]. Brazilian Journal of Veterinary Research & Animal Science 2006, 43, 120–128. [Google Scholar]
- Doreau M, Boulot S, Bauchart D, et al. Voluntary intake, milk production and plasma metabolites in nursing mares fed two different diets.[J]. Journal of Nutrition 1992, 122, 992–999. [Google Scholar] [CrossRef]
- Bernard L, Rouel J, Leroux C, et al. Mammary lipid metabolism and milk fatty acid secretion in Alpine goats fed vegetable lipids[J]. Journal of Dairy Science 2005, 88, 1478–1489. [Google Scholar] [CrossRef]
- Davison K E, Potter G D, Greene L W, et al. Lactation and reproductive performance of mares fed added dietary fat during late gestation and early lactation[J]. Journal of Equine Veterinary Science 1991, 11, 111–115. [Google Scholar] [CrossRef]
- Ericsson Aaron C, Johnson Philip J, Lopes Marco A, et al. A Microbiological Map of the Healthy Equine Gastrointestinal Tract[J]. PloS one 2016, 11, e0166523. [Google Scholar]
- Vermorel M, Martin-rosset W, Vernet J. Energy utilization of twelve forages or mixed diets for maintenance by sport horses[J]. Livestock Production Science 1997, 47, 157–167. [Google Scholar] [CrossRef]
- Frederic D, Jean-francois L, M R W, C N F. Allometric scaling of the elevation of maternal energy intake during lactation[J]. Frontiers in zoology 2016, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Stein H H, Nutrient requirementsof swine[J]. Feedstuffs 2018, 90 (Suppl. 1), 15–21.
- Miraglia N, Burger D, Kapron M, Flanagan J, Langlois B, Martin-Rosset W. eLocal animal ressources and products in sustainable development: role and potential of equids[C]. International Symposium on Livestock Farming Systems 2006, S8, 532. [Google Scholar]
- Hristov A N, Degaetano A T, Rotz C A, Hollinger DY. Climate change effects on livestock in the Northeast US and strategies for adaptation[J]. Springer Netherlands 2018, 146, 33–45. [Google Scholar]
- Laws J, Juniper D T, Lean I J, Amusquivar E, Herrera E, Dodds P F, Clarke L. Supplementing sow diets with palm oil during late gestation and lactation: effects on milk production, sow hormonal profiles and growth and development of her offspring[J]. Animal 2018, 12, 2578–2586. [Google Scholar] [CrossRef]
- Doreau M, Martin-Rosset W. Animals that Produce Dairy Foods | Horse[J]. Encyclopedia of Dairy Sciences (Second Edition) 2011, 40, 358–364. [Google Scholar]
- Davison K E, Potter G D, Greene L W, Evans J W, Mcmullan W C. Lactation and reproductive performance of mares fed added dietary fat during late gestation and early lactation[J]. Journal of Equine Veterinary Science 1991, 11, 111–115. [Google Scholar] [CrossRef]
- Harper M T, Oh J, Melgar A, Nedelkov K, Risnen S. Production effects of feeding extruded soybean meal to early-lactation dairy cows-ScienceDirect[J]. Journal of Dairy Science 2019, 102, 8999–9016. [Google Scholar] [CrossRef]
- Kadegowda A K G, Piperova L S, Delmonte P, Erdman R A. Abomasal Infusion of Butterfat Increases Milk Fat in Lactating Dairy Cows[J]. Journal of Dairy Science 2008, 91, 2370–2379. [Google Scholar] [CrossRef]
- Henry Dabadie, Evelyne Peuchant, Mireille Bernard, Pascale LeRuyet, François Mendy. Moderate intake of myristic acid in sn-2 position has beneficial lipidic effects and enhances DHA of cholesteryl esters in an interventional study[J]. The Journal of Nutritional Biochemistry 2005, 16, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Glasser F, Ferlay A, Chilliard Y. Oilseed Lipid Supplements and Fatty Acid Composition of Cow Milk: A Meta-Analysis[J]. Journal of Dairy Science 2008, 91, 4687–4703. [Google Scholar] [CrossRef] [PubMed]
- Malacarne Massimo, Francesca Martuzzi, Andrea Summer, Mariani Primo. Protein and fat composition of mare's milk: some nutritional remarks with reference to human and cow's milk[J]. International Dairy Journal 2002, 12, 869–877. [Google Scholar] [CrossRef]
- Zeyner A, Bessert J, Gropp J M. Effect of feeding exercised horses on high-starch or high-fat diets for 390 days[J]. Equine Veterinary Journal 2010, 34, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Krasnow S M, Steiner R A. Physiological Mechanisms Integrating Metabolism and Reproduction-ScienceDirect[J]. Knobil and Neill's Physiology of Reproduction(Third Edition) 2006, 2, 2553–2625. [Google Scholar]
- Han X F, Feng F J, Yu J P, Tang S X, Wang M. Effects of conjugated linoleic acid supplementation on growth, carcass characteristics and fatty acid profiles of muscle and fat in growing-finishing pigs[J]. Journal of Animal & Feed Sciences 2011, 20, 171–185. [Google Scholar]
- John Zhong Li, Yao Lei, Yue Wang, Yin xin Zhang, Jing Ye, Xia yu Xia, Xian ming Pan, Peng Li. Control of cholesterol biosynthesis, uptake and storage in hepatocytes by Cideb[J]. BBA-Molecular and Cell Biology of Lipids 2010, 1801, 577–586. [Google Scholar] [CrossRef]
- Huttenhower C, Gevers D, Knight R, Abubucker S, Jonathan H Badger. Structure, function and diversity of the healthy human microbiome[J]. Nature 2012, 486, 53–82. [Google Scholar]
- Thaiss C A, Zmora N, Levy M, Elinav E. The microbiome and innate immunity[J]. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Ruth E Ley, Micah Hamady, Catherine Lozupone, Peter J Turnbaugh, Rob Roy Ramey, J Stephen Bircher, Michael L Schlegel, Tammy A Tucker, Mark D Schrenzel, Rob Knight, Jeffrey I Gordon. Evolution of Mammals and Their Gut Microbes[J]. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Evans N J, Brown J M, Murray R D, Getty B, Birtles R J, Hart C A, Carter S D. Characterization of novel bovine gastrointestinal tract Treponema isolates and comparison with bovine digital dermatitis treponemes[J]. Appl Environ Microbiol 2011, 77, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Jiang F, Song P, Wang H, Zhang J, Liu D, Cai Z,; Gao H, Chi X, Zhang T. Comparative analysis of gut microbial composition and potential functions in captive forest and alpine musk deer[J]. Applied Microbiology and Biotechnology 2022, 106, 1325–1339. [Google Scholar] [CrossRef]
- Maslowski Kendle M, Vieira Angelica T, Ng Aylwin, Kranich Jan, Sierro Frederic, Yu Di, Schilter Heidi C, Rolph Michael S, Mackay Fabienne, Artis David, Xavier Ramnik J, Teixeira Mauro M, Mackay Charles R. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43[J]. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Caro-Quintero A, Ritalahti K M, Cusick K D, Löffler F E, Konstantinidis K T. The chimeric genome of Sphaerochaeta: nonspiral spirochetes that break with the prevalent dogma in spirochete biology[J]. mBio 2012, 3, 12–25. [Google Scholar]
- Su Shaofeng, Zhao Yiping, Liu Zongzheng, Liu Guiqin, Du Ming, Wu Jing, Bai Dongyi, Li Bei, Bou Gerelchimeg, Zhang Xinzhuang, Dugarjaviin Manglai. Characterization and comparison of the bacterial microbiota in different gastrointestinal tract compartments of Mongolian horses[J]. Microbiology Open 2020, 9, 1085–1101. [Google Scholar] [CrossRef] [PubMed]
- Gong L, Cao W, Chi H, Wang J, Zhang H, Liu J, Sun B. Whole cereal grains and potential health effects:involvement of the gut microbiota[J]. Food Research International 2018, 103, 84–102. [Google Scholar] [CrossRef]
- Yohei, Watanabe, Fumiko, Nagai, Masami, Morotomi. Characterization of Phascolarctobacterium succinatutens sp. nov, an Asaccharolytic, Succinate-Utilizing Bacterium Isolated from Human Feces[J]. Applied and Environmental Microbiology 2011, 78, 511–518. [Google Scholar]
- Zhao X H, Chen Z D, Zhou S, Song X Z, Ouyang K H, Pan K, Xu L J, Liu C J, Qu M R. Effects of daidzein on performance, serum metabolites, nutrient digestibility, and fecal bacterial community in bull calves[J]. Animal Feed Science and Technology 2017, 225, 87–96. [Google Scholar] [CrossRef]
- McGuckin Michael A, Lindén Sara K, Sutton Philip, Florin Timothy H. Mucin dynamics and enteric pathogens[J]. Nature reviews. Microbiology 2011, 9, 265–278. [Google Scholar] [CrossRef]
- McKinney Caroline A, Bedenice Daniela, Pacheco Ana P, Oliveira Bruno C M, Paradis MaryRose, Mazan Melissa, Widmer Giovanni. Assessment of clinical and microbiota responses to fecal microbial transplantation in adult horses with diarrhea[J]. PloS one 2021, 16, e0244381. [Google Scholar]
- Edwards J E, Shetty S A, van den Berg P, Burden F, van Doorn D A, Pellikaan W F, Dijkstra J, Smidt H. Multi-kingdom characterization of the core equine fecal microbiota based on multiple equine (sub) species[J]. Animal Microbiome 2020, 2, 231–268. [Google Scholar]
- McKinney Caroline A, Oliveira Bruno C M, Bedenice Daniela, Paradis Mary-Rose, Mazan Melissa, Sage Sophie, Sanchez Alfredo, Widmer Giovanni. The fecal microbiota of healthy donor horses and geriatric recipients undergoing fecal microbial transplantation for the treatment of diarrhea[J]. PloS one 2020, 15, e0230148. [Google Scholar]
- Byndloss Mariana X, Pernitzsch Sandy R, Bäumler Andreas J. Healthy hosts rule within: ecological forces shaping the gut microbiota[J]. Mucosal immunology 2018, 11, 1299–1305. [Google Scholar] [CrossRef]
- Lindenberg F, Krych L, Fielden J, Kot W, Frøkiær H, van Galen G, Nielsen D S, Hansen A K. Expression of immune regulatory genes correlate with the abundance of specific Clostridiales and Verrucomicrobia species in the equine ileum and cecum[J]. Scientific reports 2019, 9, 12674. [Google Scholar] [CrossRef] [PubMed]
- Quercia S, Freccero F, Castagnetti C, Soverini M, Turroni S, Biagi E, Rampelli S, Lanci A, Mariella J, Chinellato E, Brigidi P, Candela M. Early colonisation and temporal dynamics of the gut microbial ecosystem in Standardbred foals[J]. Equine Veterinary Journal 2019, 51, 231–237. [Google Scholar] [CrossRef] [PubMed]

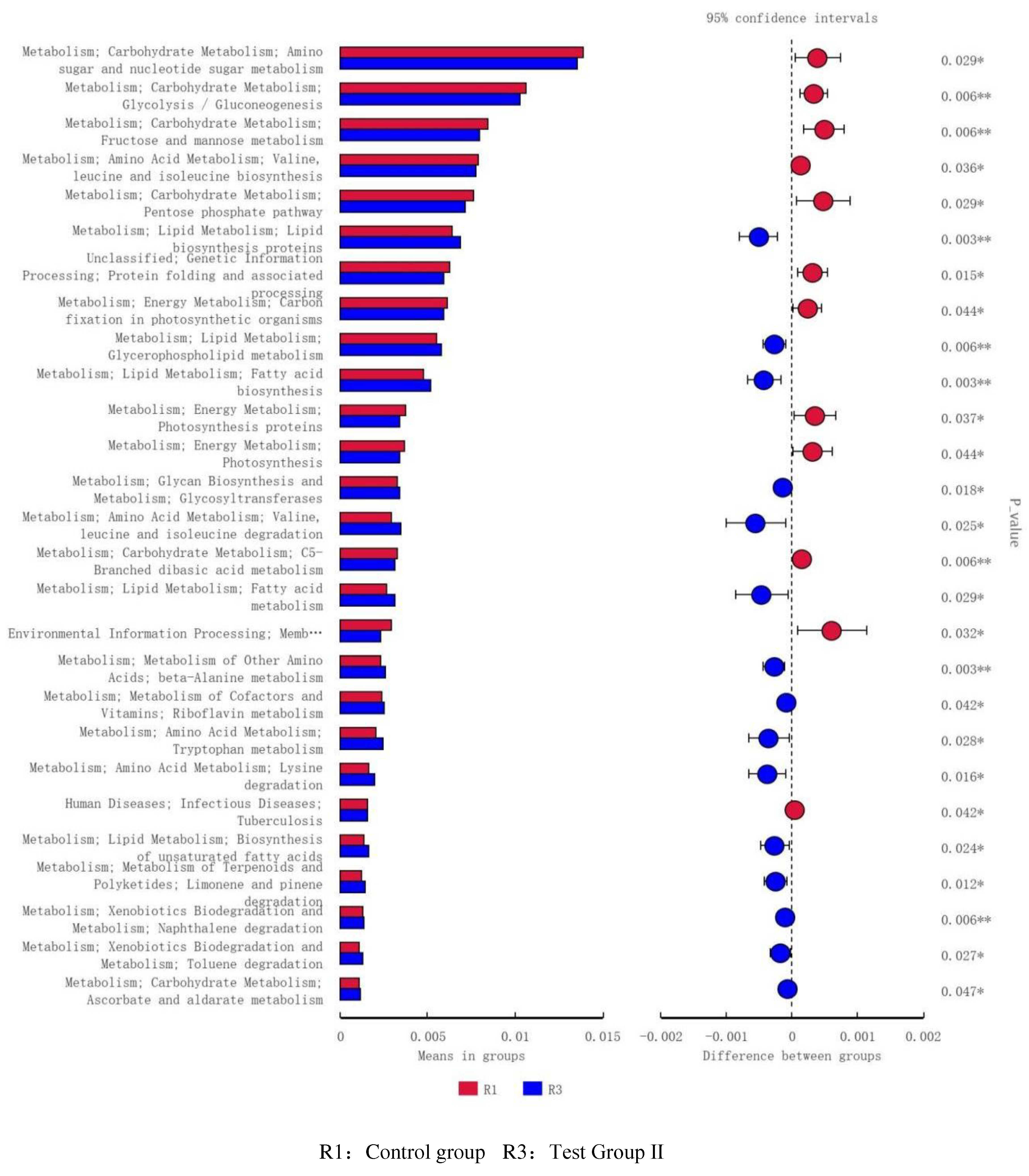
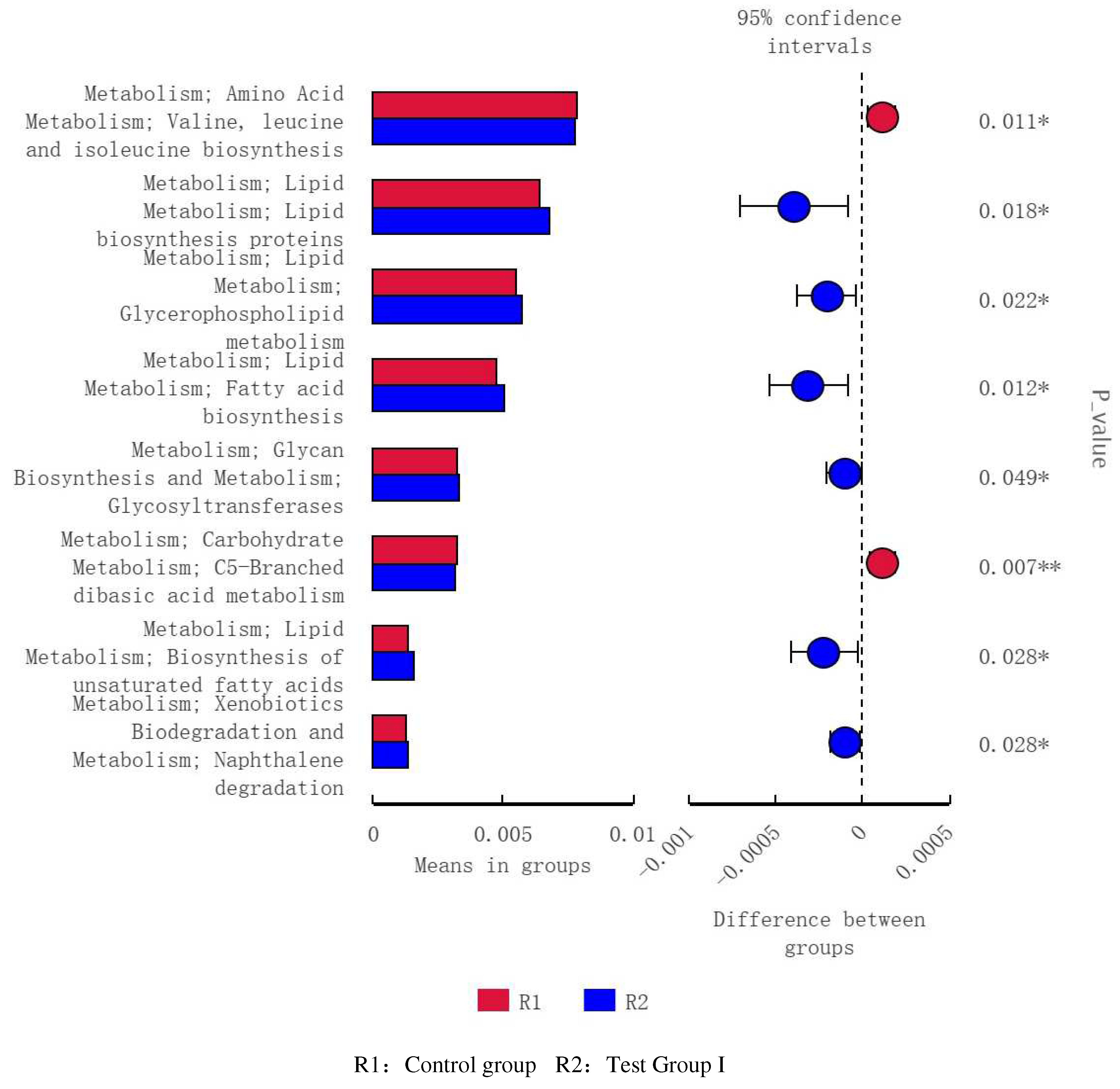
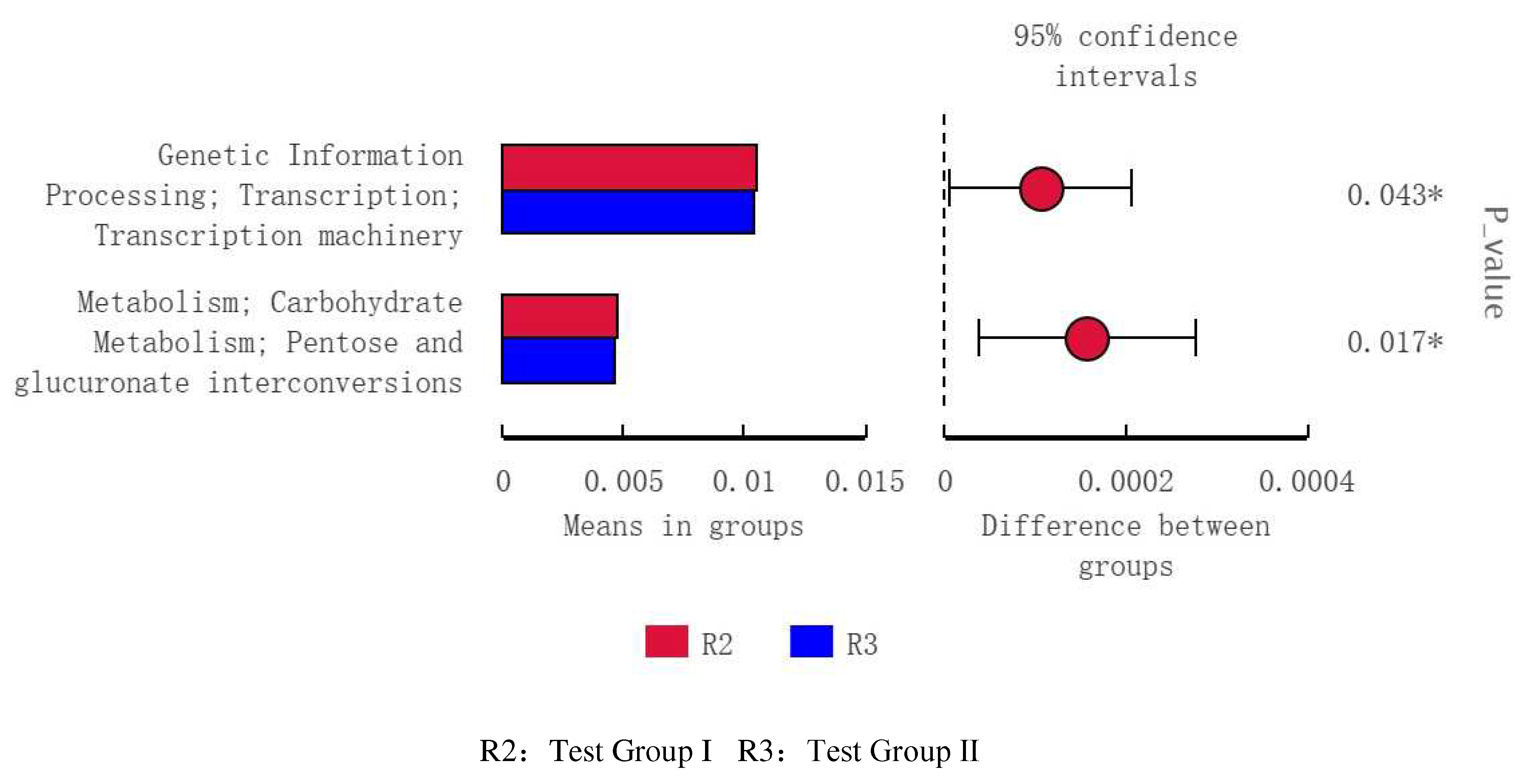
| Items | Content | Items | Content |
|---|---|---|---|
| Ingredients | Nutrient levels2) | ||
| Barley | 55.54 | DM | 89.25 |
| Corn | 36.00 | CP | 14.56 |
| Soybean meal | 6.00 | EE | 4.08 |
| CaHPO4 | 1.30 | OM | 97.18 |
| Premix1) | 1.16 | NDF | 13.36 |
| Total | 100.00 | ADF | 5.18 |
| Ash | 2.82 | ||
| Ca | 0.31 | ||
| P | 0.46 |
| Items | Concentrate | Forage | Coated fatty acids |
|---|---|---|---|
| Butyric acid C4:0 |
0.43 | 0.82 | 0.24 |
| Hexanoic acid C6:0 |
0.06 | 0.43 | 0.49 |
| Octoic acid C8:0 |
0.25 | 1.17 | 0.37 |
| Decanoic acid C10:0 |
0.00 | 0.00 | 5.25 |
| Ricinoleic acid C11:0 |
0.00 | 0.00 | 0.02 |
| Lauric acid C12:0 |
0.00 | 4.06 | 44.83 |
| Tetradecanoic acid C14:0 |
0.12 | 0.86 | 18.42 |
| Pentadecanoic acid C15:0 |
0.03 | 0.00 | 0.02 |
| Palmitic acid C16:0 |
13.66 | 13.61 | 12.20 |
| Palmitoleic acid C16:1 |
0.12 | 0.33 | 0.02 |
| Margaric acid C17:0 |
0.05 | 0.32 | 0.01 |
| Heptadecanoic acid monoenoic acid C17:1 |
0.03 | 0.30 | 0.00 |
| Stearic acid C18:0 |
1.54 | 1.97 | 3.97 |
| Elaidic acid C18:1n9t |
0.03 | 0.00 | 0.00 |
| Oleic acid C18:1n9c |
25.17 | 3.02 | 3.91 |
| Linoleic acid C18:2n6c |
45.40 | 13.60 | 2.20 |
| γ- Linolenic acid C18:3n6 |
0.45 | 4.07 | 0.05 |
| α- Linolenic acid C18:3n3 |
2.44 | 28.67 | 0.56 |
| Arachidic acid C20:0 |
0.39 | 2.66 | 0.12 |
| Eicosaenoic acid C20:1 |
0.56 | 0.00 | 0.04 |
| Cis-11,14-eicosadienoic acid C20:2 |
0.06 | 0.00 | 0.00 |
| Behenic acid C22:0 |
0.20 | 1.11 | 0.00 |
| Total saturated fatty acids ∑SFA |
16.74 | 27 | 85.94 |
| Total unsaturated fat acids ∑UFA |
74.27 | 49.99 | 6.78 |
| Total monounsaturated fatty acids ∑MUFA |
25.92 | 3.65 | 3.97 |
| Total unsaturated fat acids ∑PUFA |
48.34 | 46.34 | 2.80 |
| Total saturated fatty acid/total unsaturated fat acid ∑SFA/∑UFA |
0.23 | 0.54 | 12.68 |
| Items | Control group | Test Group I | Test Group Ⅱ | SEM | P-value | ||
|---|---|---|---|---|---|---|---|
| Grous | Time | G*T | |||||
| Butter-fat content (%) | 1.47 | 1.45 | 1.49 | 0.07 | 0.944 | 0.104 | 0.897 |
| Milk fat production (g/d) | 3.59B | 3.85B | 4.77A | 0.19 | <0.001 | 0.088 | 0.793 |
| Milk protein percentage (%) | 1.63 | 1.61 | 1.58 | 0.04 | 0.643 | 0.918 | 0.982 |
| Milk protein yield (g/d) | 3.97B | 4.27B | 5.06A | 0.11 | <0.001 | 0.905 | 0.981 |
| Lactose percentage (%) | 6.68 | 6.76 | 6.76 | 0.04 | 0.307 | 0.563 | 0.555 |
| Lactose production (g/d) | 16.29C | 17.90B | 21.70A | 0.11 | <0.001 | 0.625 | 0.608 |
| Total solids (%) | 9.86 | 9.90 | 9.88 | 0.10 | 0.963 | 0.67 | 0.469 |
| Somatic number (Thousand/mL) | 21.25 | 13.33 | 18.25 | 4.67 | 0.489 | 0.331 | 0.418 |
| Solid no fat (%) | 8.54 | 8.58 | 8.57 | 0.06 | 0.858 | 0.654 | 0.681 |
| Urea nitrogen (mg/dL) | 26.64aA | 24.24bB | 24.58bAB | 0.61 | 0.02 | <0.001 | 0.967 |
| Items | Control group | Test Group I | Test Group Ⅱ | SEM | P-value |
|---|---|---|---|---|---|
| Butyric acid C4:0 |
0.34 | 0.42 | 0.37 | 0.02 | 0.390 |
| Hexanoic acid C6:0 |
0.22 | 0.20 | 0.21 | 0.01 | 0.895 |
| Octoic acid C8:0 |
1.22 | 1.20 | 1.29 | 0.05 | 0.742 |
| Decanoic acid C10:0 |
2.83 | 2.94 | 3.26 | 0.13 | 0.380 |
| Ricinoleic acid C11:0 |
0.35 | 0.37 | 0.38 | 0.02 | 0.805 |
| Lauric acid C12:0 |
3.93b | 4.32b | 8.78a | 0.55 | <0.001 |
| Tetradecanoic acid C14:0 |
5.13b | 5.64b | 7.50a | 0.27 | <0.001 |
| Myristoleic acid C14:1 |
0.77 | 0.77 | 0.91 | 0.03 | 0.091 |
| Pentadecanoic acid C15:0 |
0.32a | 0.29ab | 0.23b | 0.01 | 0.013 |
| Palmitic acid C16:0 |
19.14 | 20.53 | 19.16 | 0.32 | 0.124 |
| Palmitoleic acid C16:1 |
7.98 | 7.77 | 6.93 | 0.23 | 0.139 |
| Margaric acid C17:0 |
0.18 | 0.17 | 0.16 | 0.01 | 0.738 |
| Heptadecanoic acid monoenoic acid C17:1 |
0.61a | 0.59a | 0.46b | 0.02 | 0.005 |
| Stearic acid C18:0 |
0.67 | 0.73 | 0.78 | 0.03 | 0.265 |
| Elaidic acid C18:1n9t |
0.19 | 0.21 | 0.17 | 0.01 | 0.268 |
| Oleic acid C18:1n9c |
12.66 | 12.63 | 13.31 | 0.20 | 0.304 |
| Linoleic acid C18:2n6c |
7.90a | 7.73ab | 7.24b | 0.12 | 0.056 |
| γ- Linolenic acid C18:3n6 |
8.31a | 7.90a | 6.32b | 0.26 | <0.001 |
| α- Linolenic acid C18:3n3 |
20.55a | 18.74a | 15.32b | 0.62 | <0.001 |
| Cis-11,14-eicosadienoic acid C20:2 |
0.15b | 0.13b | 0.18a | 0.01 | 0.002 |
| Cis-8,11,14-eicosotrienic acid C20:3n6 |
0.16 | 0.15 | 0.17 | 0.01 | 0.704 |
| Cis-11,14,17-eicosotrienic acid C20:3n3 |
0.41 | 0.40 | 0.39 | 0.01 | 0.928 |
| Total saturated fatty acids ∑SFA |
34.35b | 36.81b | 42.12a | 0.94 | <0.001 |
| Total unsaturated fat acids ∑UFA |
59.70a | 57.03a | 51.38b | 1.04 | <0.001 |
| Total monounsaturated fatty acids ∑MUFA |
22.22 | 21.97 | 21.77 | 0.28 | 0.824 |
| Total unsaturated fat acids ∑PUFA |
37.48a | 35.06a | 29.61b | 0.93 | <0.001 |
| Total saturated fatty acid/total unsaturated fat acid ∑SFA/∑UFA |
0.58b | 0.65b | 0.82a | 0.03 | <0.001 |
| Items | Control group | Test Group I | Test Group Ⅱ | SEM | P-value |
|---|---|---|---|---|---|
| Observed species | 2163.67 | 2884.33 | 2814.00 | 76.73 | 0.348 |
| Shannon index | 9.30 | 9.59 | 9.53 | 0.06 | 0.108 |
| Simpson index | 0.99 | 1.00 | 1.00 | 0.0006 | 0.426 |
| Chao1 index | 2811.71 | 3084.11 | 3026.10 | 81.91 | 0.382 |
| ACE index | 2829.28 | 3107.67 | 3045.80 | 83.87 | 0.386 |
| Goods coverage(%) | 0.99 | 0.99 | 0.99 | 0.0003 | 0.883 |
| Items | Control group | Test Group I | Test Group Ⅱ | SEM | P-value |
|---|---|---|---|---|---|
| Bacteroidetes | 44.20 | 42.75 | 43.51 | 1.30 | 0.911 |
| Firmicutes | 32.59 | 33.60 | 31.52 | 1.02 | 0.736 |
| Spirochaetes | 6.41 | 3.39 | 2.98 | 0.87 | 0.221 |
| Verrucomicrobia | 3.92c | 6.81b | 7.92a | 0.63 | 0.017 |
| Unidentified_Bacterri | 2.27 | 2.61 | 3.01 | 0.21 | 0.372 |
| Proteobacteria | 1.20 | 1.66 | 2.17 | 0.25 | 0.309 |
| Euryarchaeota | 0.66 | 0.43 | 0.52 | 0.14 | 0.817 |
| Halobacterota | 0.72 | 0.43 | 0.52 | 0.14 | 0.248 |
| Fibrobacterota | 1.25 | 0.87 | 1.00 | 0.09 | 0.186 |
| Acidobacteriota | 0.18 | 0.32 | 0.23 | 0.08 | 0.804 |
| Others | 6.60 | 7.14 | 7.05 | 0.35 | 0.817 |
| Items | Control group | Test Group I | Test Group Ⅱ | SEM | P-value |
|---|---|---|---|---|---|
| Rikenellaceae | 11.95 | 12.84 | 13.77 | 0.98 | 0.773 |
| Lachnospiraceae | 11.18 | 10.39 | 9.96 | 0.61 | 0.733 |
| Spirochaetaceae | 6.26 | 3.25 | 2.82 | 0.86 | 0.213 |
| Prevotellaceae | 8.39 | 6.42 | 5.88 | 0.54 | 0.138 |
| p-251-o5 | 8.72 | 6.34 | 6.44 | 0.67 | 0.279 |
| F082 | 6.04 | 7.80 | 7.62 | 0.39 | 0.123 |
| Bacteroidales_RF16_group | 2.37 | 2.30 | 2.95 | 0.29 | 0.632 |
| Oscillospiraceae | 3.55 | 4.36 | 4.25 | 0.17 | 0.107 |
| Clostridiaceae | 0.96 | 0.48 | 0.52 | 0.22 | 0.644 |
| Ruminococcaceae | 2.42 | 2.43 | 2.46 | 0.13 | 0.992 |
| Others | 38.16 | 43.38 | 43.32 | 1.16 | 0.104 |
| Items | Control group | Test Group I | Test Group Ⅱ | SEM | P-value |
|---|---|---|---|---|---|
| Treponema | 6.09 | 3.13 | 2.74 | 0.85 | 0.219 |
| Rikenellaceae_RC9_gut_group | 9.33 | 9.60 | 10.33 | 0.69 | 0.849 |
| Clostridium_sensu_stricto_1 | 0.90 | 0.34 | 0.34 | 0.22 | 0.518 |
| Prevotellaceae_UCG-001 | 1.78 | 1.11 | 1.02 | 0.18 | 0.171 |
| UCG-004 | 0.89 | 0.63 | 0.65 | 0.15 | 0.750 |
| Prevotellaceae_UCG-004 | 1.24 | 1.61 | 1.49 | 0.11 | 0.405 |
| Ruminococcus | 2.00 | 1.87 | 1.80 | 0.12 | 0.810 |
| Prevotellaceae_UCG-003 | 1.85 | 1.21 | 1.21 | 0.13 | 0.065 |
| Lachnospiraceae_UCG-009 | 1.21 | 0.89 | 0.71 | 0.12 | 0.230 |
| Faecalibaculum | 0.53 | 0.44 | 0.43 | 0.17 | 0.227 |
| Others | 73.98b | 79.17a | 79.72a | 1.00 | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





