Submitted:
04 July 2023
Posted:
04 July 2023
You are already at the latest version
Abstract
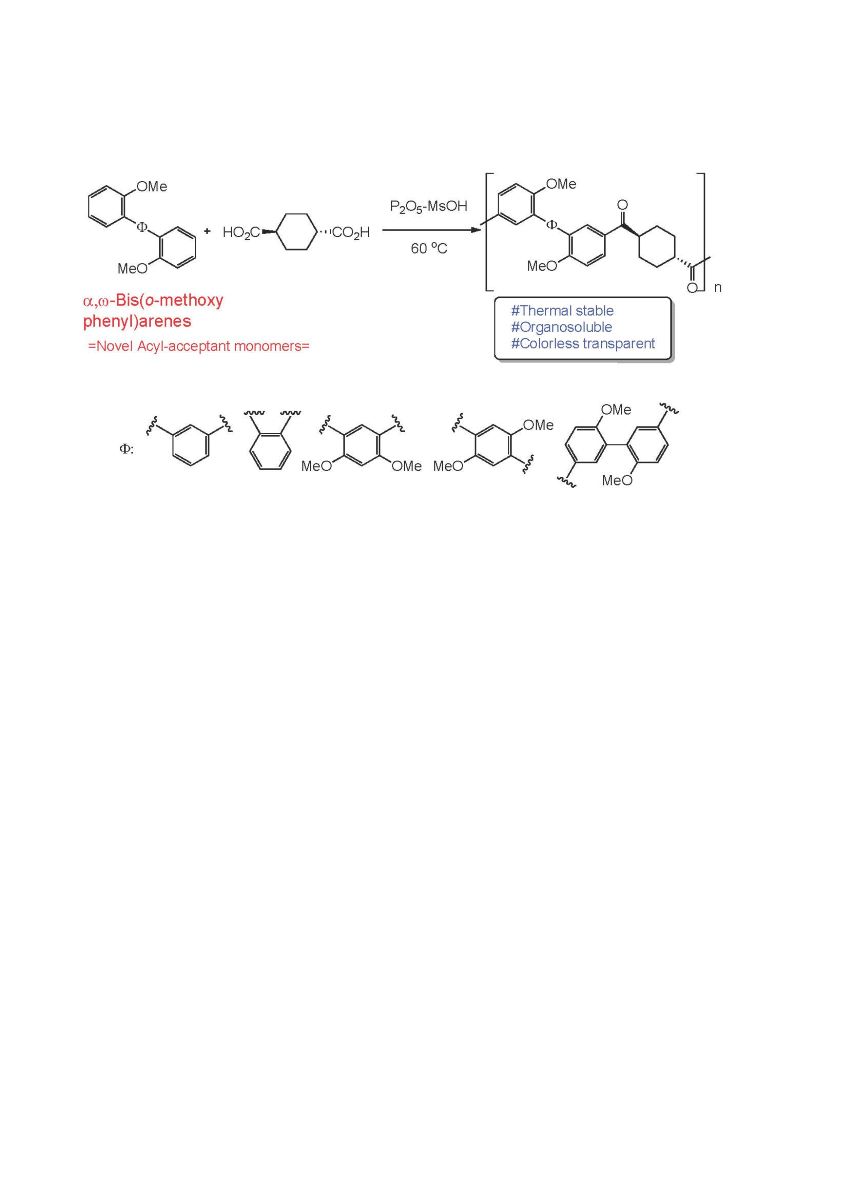
Keywords:
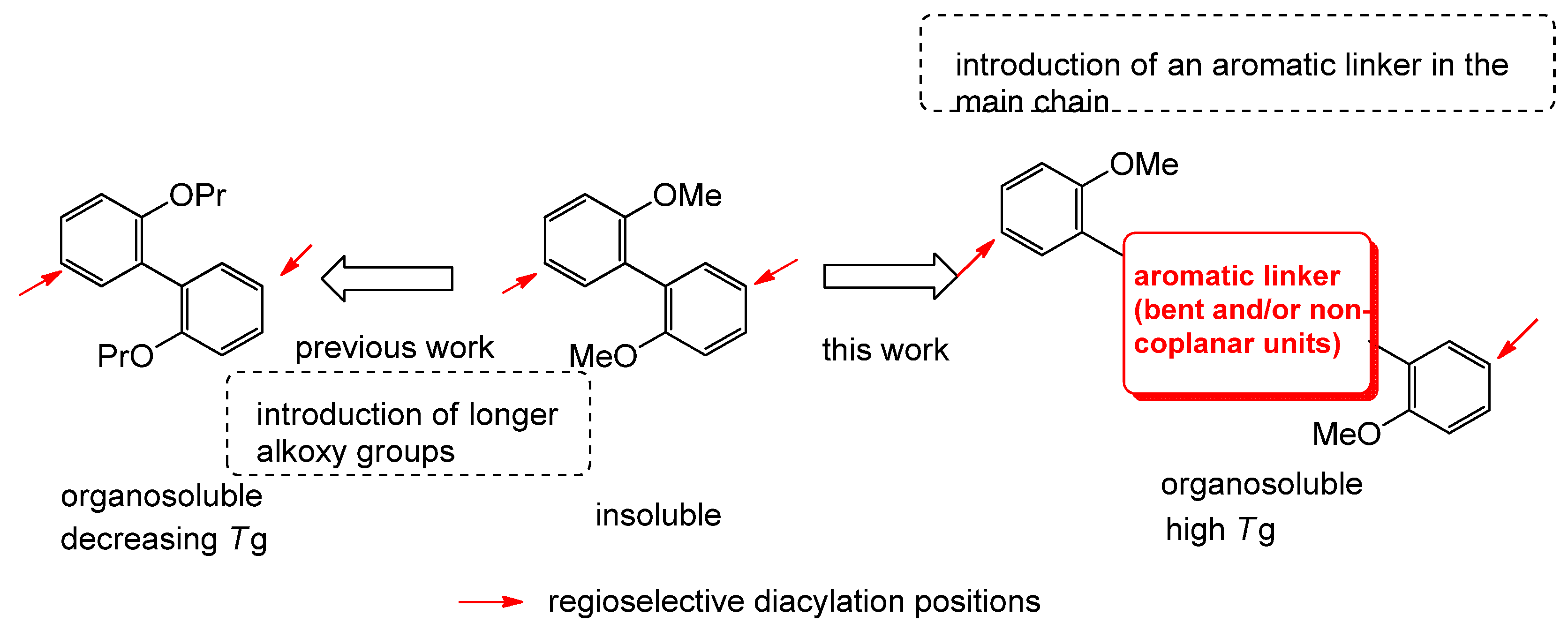
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Preparation of monomers 3a-e
2.4. Synthesis of aromatic polyketones 5a-e
3. Results and Discussion
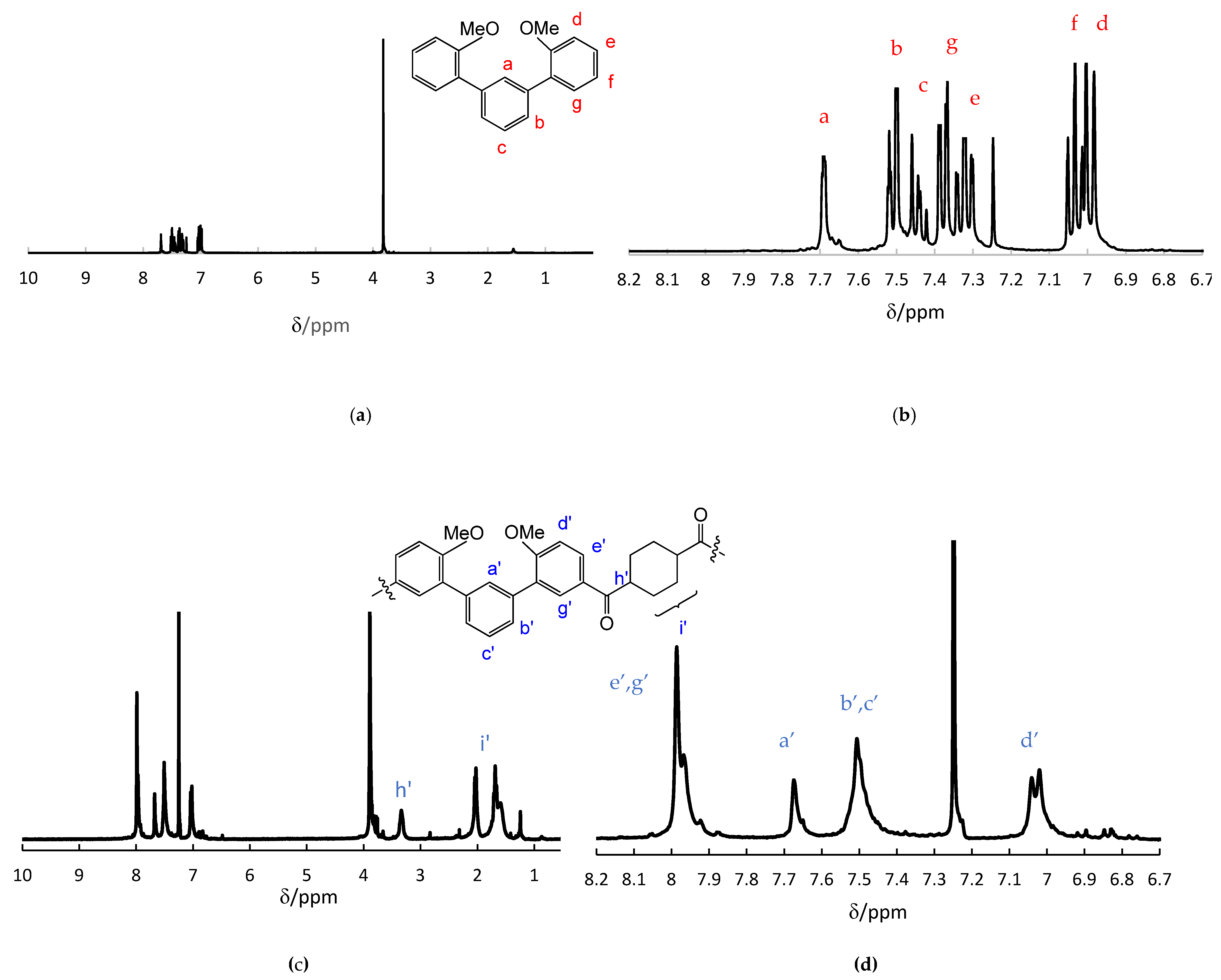
3.1. Preparation of monomers 3a-e
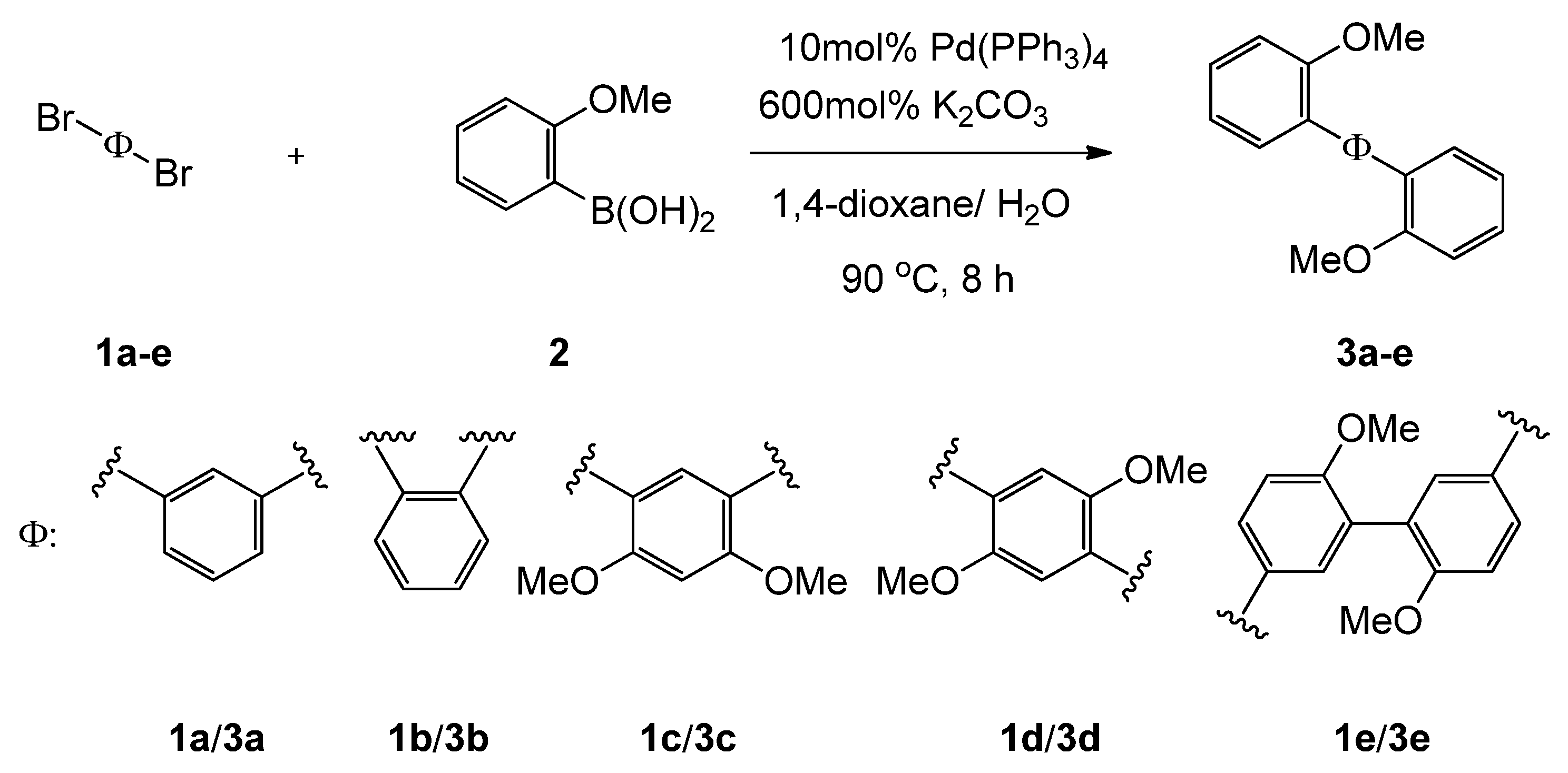
| 1 | 3 | Yield/% |
|---|---|---|
| 1a | 3a | 94 |
| 1b | 3b | 97 |
| 1c | 3c | 87 |
| 1d | 3d | 58 |
| 1e | 3e | 69 |
3.2. Synthesis of aromatic polyketones 5a-e
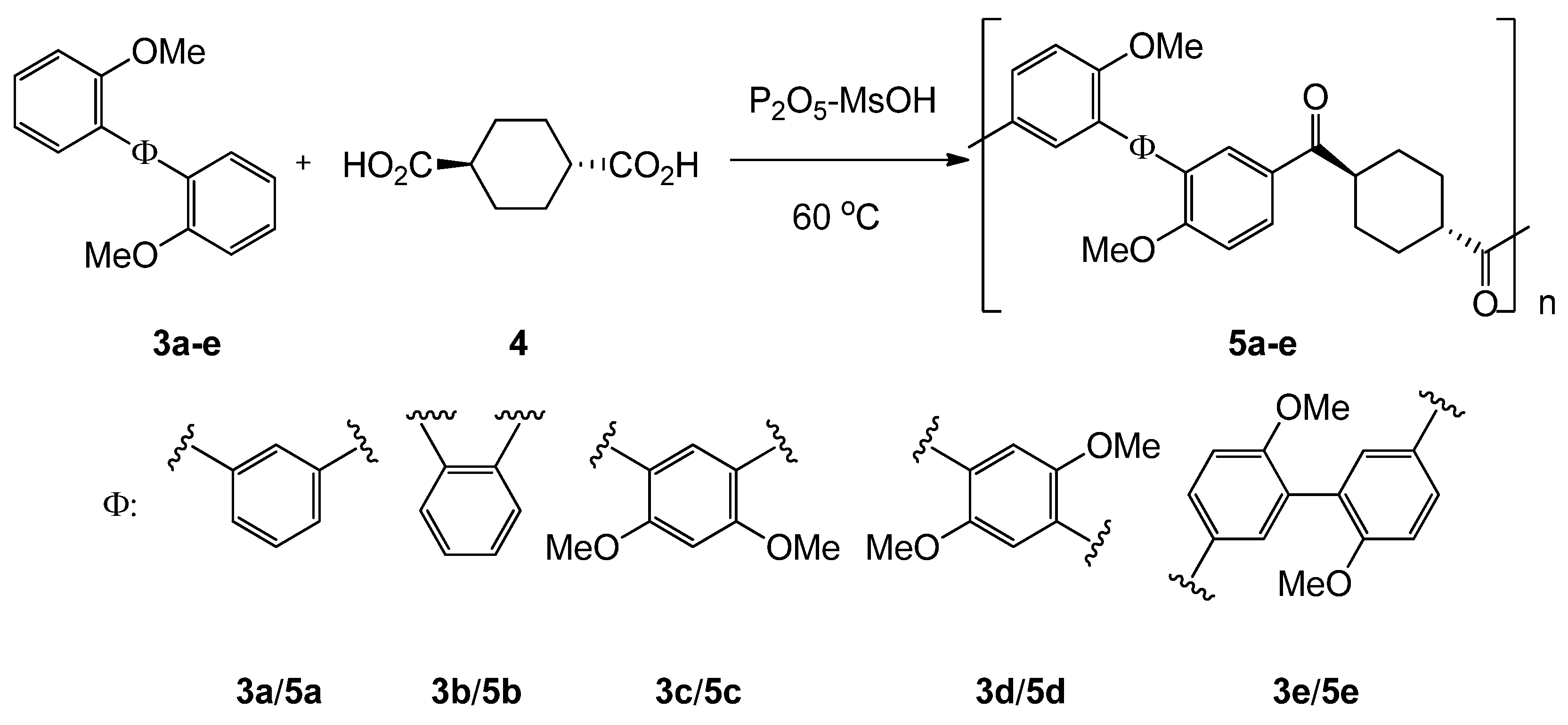
| 3 | 5 | Time/h | Yield/% | Mn2 | Mw2 | Mw /Mn 2 |
|---|---|---|---|---|---|---|
| 3a | 5a | 24 | 86 | 6900 | 28200 | 4.1 |
| 3b | 5b | 24 | 92 | 8200 | 14200 | 1.7 |
| 3c | 5c | 24 | 86 | 13200 | 35200 | 2.7 |
| 3d | 5d | 24 | 95 | 7100 | 25400 | 3.6 |
| 3e | 5e | 8 | 87 | 6000 | 9600 | 1.6 |
3.3. Thermal properties of aromatic polyketones 5a-e
| 5 | Solubility1 | Tg/oC2 | Td10/oC3 | ||||
| THF | CHCl3 | DMF | DMSO | NMP | |||
| 5a | ++ | ++ | ++ | +- | ++ | 201 | 456 |
| 5b | ++ | + | ++ | + | ++ | 188 | 447 |
| 5c | ++ | ++ | ++ | + | ++ | 219 | 411 |
| 5d | ++ | ++ | ++ | + | ++ | 205 | 428 |
| 5e | ++ | ++ | ++ | +- | ++ | 214 | 442 |
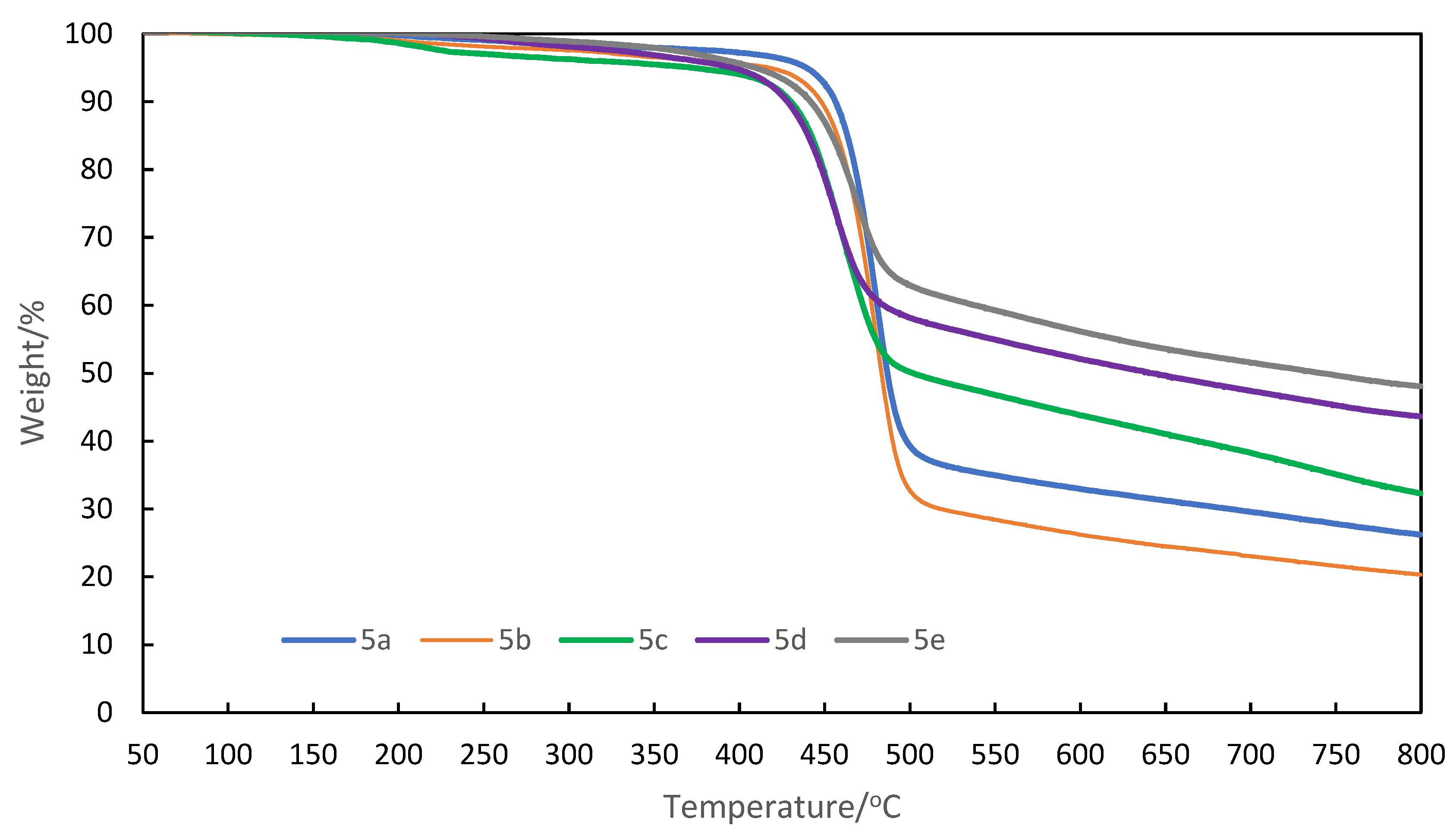
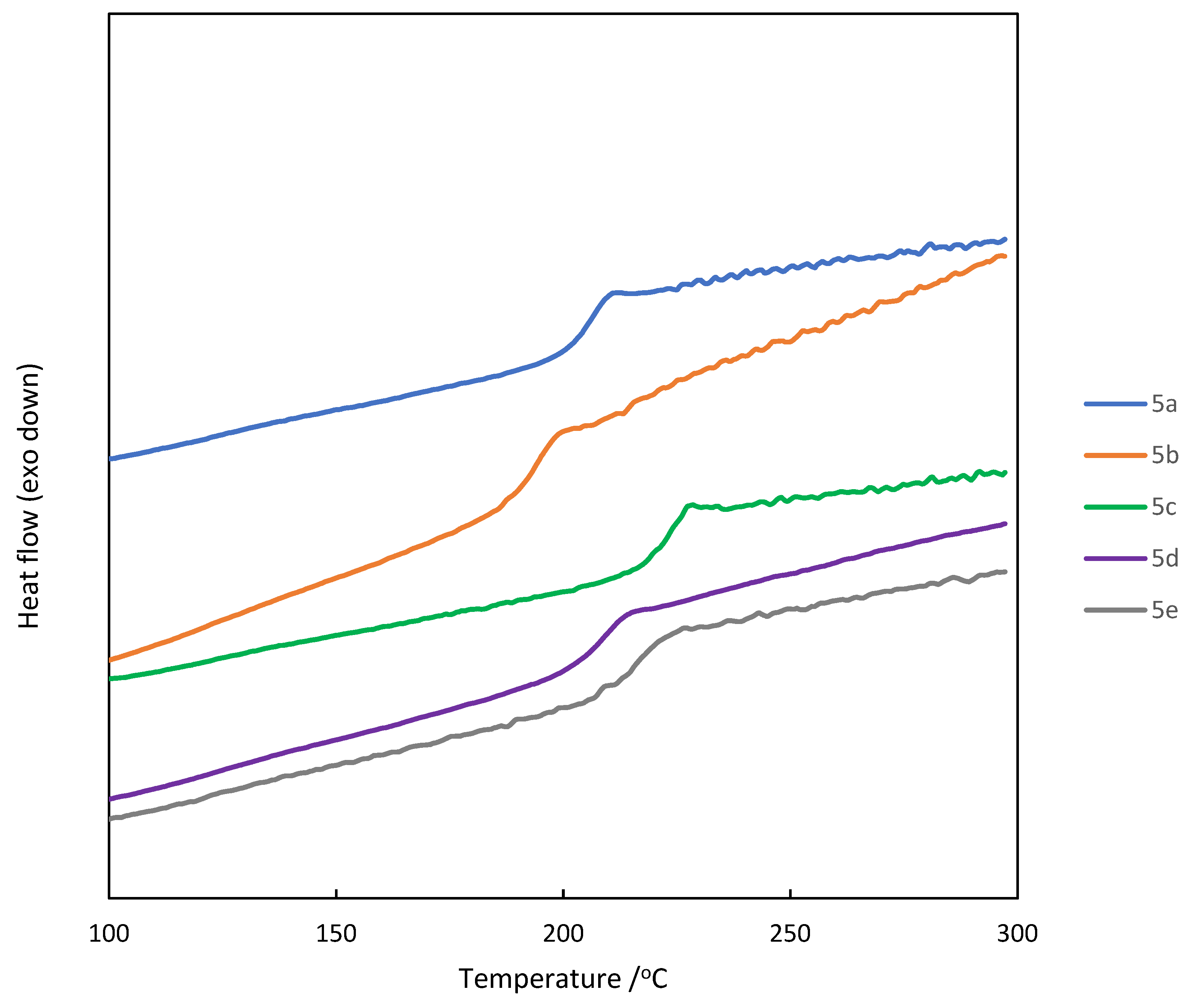
3.4. Optical properties of aromatic polyketones 5a-e
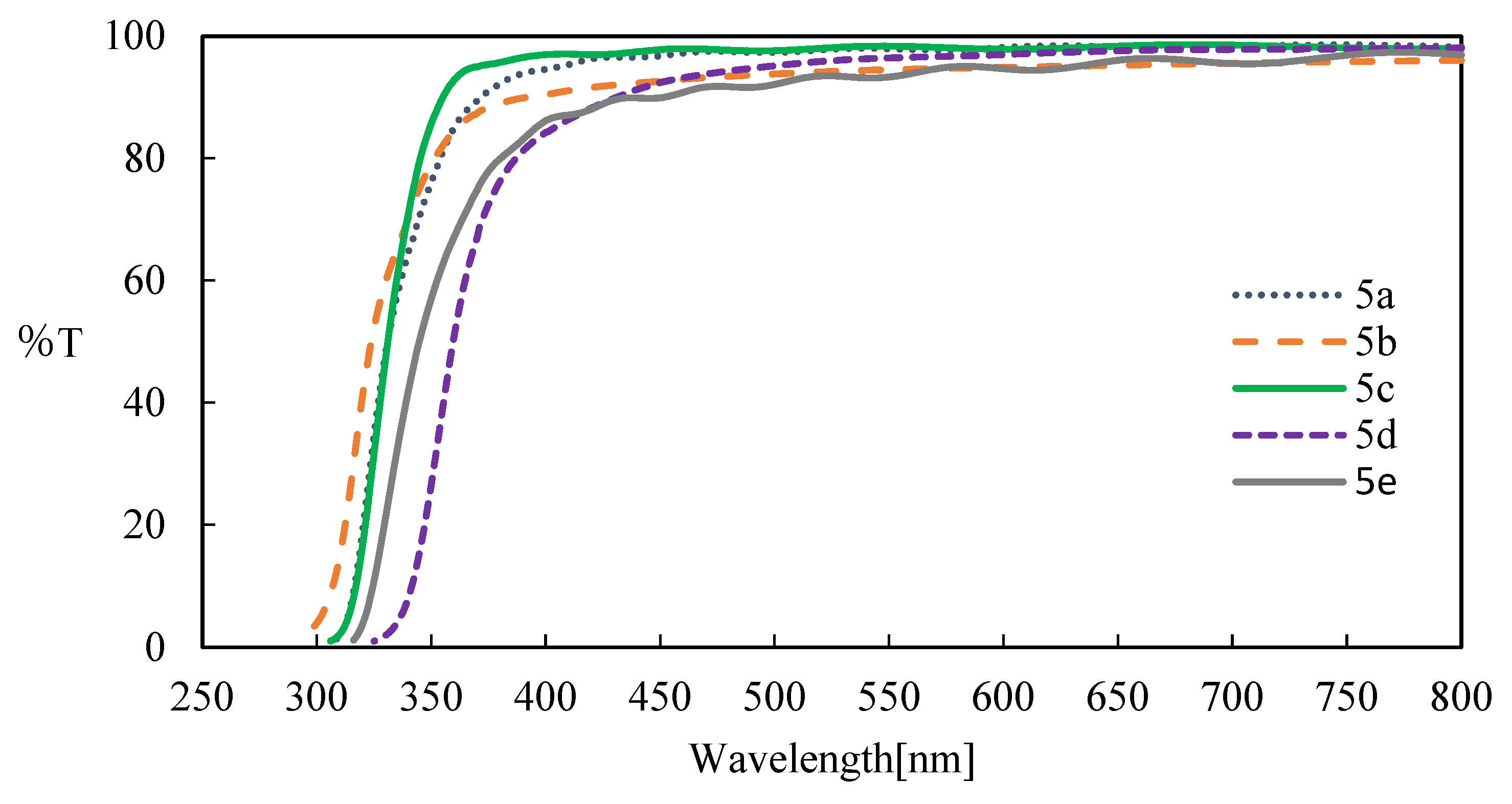
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Attwood, T.; Dawson, P.; Freeman, J.; Hoy, L.; Rose, J.; Staniland, P. Synthesis and properties of polyaryletherketones. Polymer 1981, 22, 1096–1103. [Google Scholar] [CrossRef]
- Maeyama, K. Synthesis of Wholly Aromatic Polyketones Based on Fine Molecular Designing. J. Synth. Org. Chem. Jpn. 2005, 63, 616–626. [Google Scholar] [CrossRef]
- Maeyama, K. Technology trends of aromatic poly(ether ketone)s. Kino Zairyo 2007, 27, 47–53. [Google Scholar]
- Yonezawa, N.; Mori, S.-I.; Miyata, S.; Ueha-Anyashiki, Y.; Wu, S.M.; Maeyama, K. Electrophilic Aromatic Acylation Synthesis of Wholly Aromatic Polyketones Composed of 2,2’-Dimethoxybiphenylene Units. Polym. J. 2003, 35, 998–1002. [Google Scholar] [CrossRef]
- Maeyama, K.; Ohe, T.; Nakamura, H.; Yonezawa, N. Successful Synthesis of Fully Aromatic Polyketones without ether linkages in the main chain. Nickel Complex-Mediated Aromatic Coupling Polymerization of Bis(chlorobenzoylated) o-Terphenyls. Polym. J. 2003, 35, 1009–1015. [Google Scholar] [CrossRef]
- Maeyama, K.; Sekimura, S.; Takano, M.; Yonezawa, N. Nickel-complex-mediated copolymerization of regioisomeric 5,5’-bis(chlorobenzoyl)-2,2’-dimethoxybiphenyls: synthesis of wholly aromatic polyketones. React. Funct. Polym. 2004, 58, 111–115. [Google Scholar] [CrossRef]
- Maeyama, K.; Tagata, Y.; Yonezawa, N. 2,2′-Bis(4-benzoylphenoxy)biphenyl: A Novel Efficient Acyl-acceptant Monomer Yielding Wholly Aromatic Polyketones via Friedel–Crafts Type Polymerization with Arenedicarbonyl Chloride. Polym. J. 2004, 36, 146–150. [Google Scholar] [CrossRef]
- Maeyama, K.; Hikiji, I.; Ogura, K.; Okamoto, A.; Ogino, K.; Saito, H.; Yonezawa, N. Synthesis of Optically Active Aromatic Poly(ether ketone)s via Nucleophilic Aromatic Substitution Polymerization. Polym. J. 2005, 37, 707–710. [Google Scholar] [CrossRef]
- Maeyama, K.; Ogura, K.; Okamoto, A.; Ogino, K.; Saito, H.; Yonezawa, N. Nickel Complex-Mediated Synthesis of Optically Active Wholly Aromatic Polyketones Bearing 2,2′-Dimethoxy-1,1′-binaphthyl-6,6′-ene Units. Polym. J. 2005, 37, 736–741. [Google Scholar] [CrossRef]
- Maeyama, K.; Maeda, S.; Ogino, K.; Saito, H.; Yonezawa, N. Synthesis of optically active aromatic poly(ether ketone)s containing 2,2′-bis (4-benzoylphenoxy)-1,1′-binaphthyl-6,6′-ene backbones. React. Funct. Polym. 2005, 65, 229–237. [Google Scholar] [CrossRef]
- Okamoto, A.; Mitsui, R.; Maeyama, K.; Saito, H.; Oike, H.; Murakami, Y.; Yonezawa, N. Electrophilic aromatic acylation polymerization synthesis of wholly aromatic polyketones composed of 2,2’-dimethoxy-1,1’-binaphthylene moiety. React. Funct. Polym. 2007, 67, 1243–1251. [Google Scholar] [CrossRef]
- Maeyama, K.; Tsukamoto, T.; Suzuki, M.; Higashibayashi, S.; Sakurai, H. Nanosized palladium-catalyzed Suzuki–Miyaura coupling polymerization: synthesis of soluble aromatic poly(ether ketone)s. Polym. J. 2012, 45, 401–405. [Google Scholar] [CrossRef]
- Maeyama, K.; Suzuki, M.; Tsukamoto, T.; Higashibayashi, S.; Sakurai, H. Synthesis of thermally stable, wholly aromatic polyketones with 2,2’-dimethoxy-1,1’-binaphthyl-6,6’-diyl units through nanosized-palladium-cluster-catalyzed Suzuki-Miyaura coupling polymerization. React. Funct. Polym. 2014, 79, 24–28. [Google Scholar] [CrossRef]
- Maeyama, K.; Kumagai, H. Synthesis of water-soluble wholly aromatic polyketones bearing 1,1′-binaphthyl units. React. Funct. Polym. 2015, 93, 18–21. [Google Scholar] [CrossRef]
- Maeyama, K.; Kamura, T.; Akiba, K.; Saito, H. Convenient synthesis of aromatic poly(ether ketone)s containing alicyclic units, Polym. J. 2008, 40, 861–866. [Google Scholar]
- Maeyama, K.; Katada, A.; Mizuguchi, N.; Matsutani, H. P2O5-MsOH-mediated facile synthesis of semi-aromatic polyketones bearing 1,4-cyclohexanediyl units, Chem. Lett., 2015, 44, 1783–1785. [Google Scholar]
- Eaton, P.E.; Carlson, G.R.; Lee, J.T. Phosphorus pentoxide-methanesulfonic acid. Convenient alternative to polyphosphoric acid. J. Org. Chem. 1973, 38, 4071–4073. [Google Scholar] [CrossRef]
- Du, C.J.F.; Hart, H.; Ng, K.K.D. A one-pot synthesis of m-terphenyls, via a two-aryne sequence. J. Org. Chem. 1986, 51, 3162–3165. [Google Scholar] [CrossRef]
- King, B.T.; Kroulík, J.; Robertson, C.R.; Rempala, P.; Hilton, C.L.; Korinek, J.D.; Gortari, L.M. Controlling the Scholl Reaction. J. Org. Chem. 2007, 72, 2279–2288. [Google Scholar] [CrossRef] [PubMed]
- Pring, B.G. Complex dibenzofurans XIV. Acid-catalyzed demethylation and dehydration of some tetramethoxyterphenyls. Acta Chemica Scandinavica, 1973, 27, 3873-3880.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





