3.2. Redescription of currently recognized species
The following five species are redescribed here based on morphological data for newly available specimens, and previously reported voucher specimens which considerably increase the samples sizes:
M. femoralis (124 specimens),
M. ambreensis (46 specimens),
M. ambony (14 specimens),
M. mocquardi (148 specimens) and
M. zolitschka (10 specimens) (
Figure 2). We provide these redescriptions to facilitate comparison with the descriptions provided for the new and resurrected species. The specimens presented here with asterisk are the referee species for the building of the tree and also deposited in GENBANK (
Table S1).
3.2.1. Mantidatylus femoralis (Boulenger 1882)
Rana femoralis [
8] (p. 462) (Syntypes: BMNH 1947.2.22.65–68, according to [
27] (p. 26); BMNH 1947.2.22.65 designated lectotype by [
10] (p. 85);
Rana flavicrus [
28] (p. 245);
Mantidactylus flavicrus [
9] (p. 450);
Mantidactylus femoralis: [
29] (p. 393);
Mantidactylus
Lectotype: BMNH 1947.22.65 collected from the eastern slope of the Betsileo Region, Madagascar;
Paratype: BMNH 1947.22.66–68. same location as the lectotype;
Paralectotypes: BMNH 1947.22.66–68. same location as the lectotype.
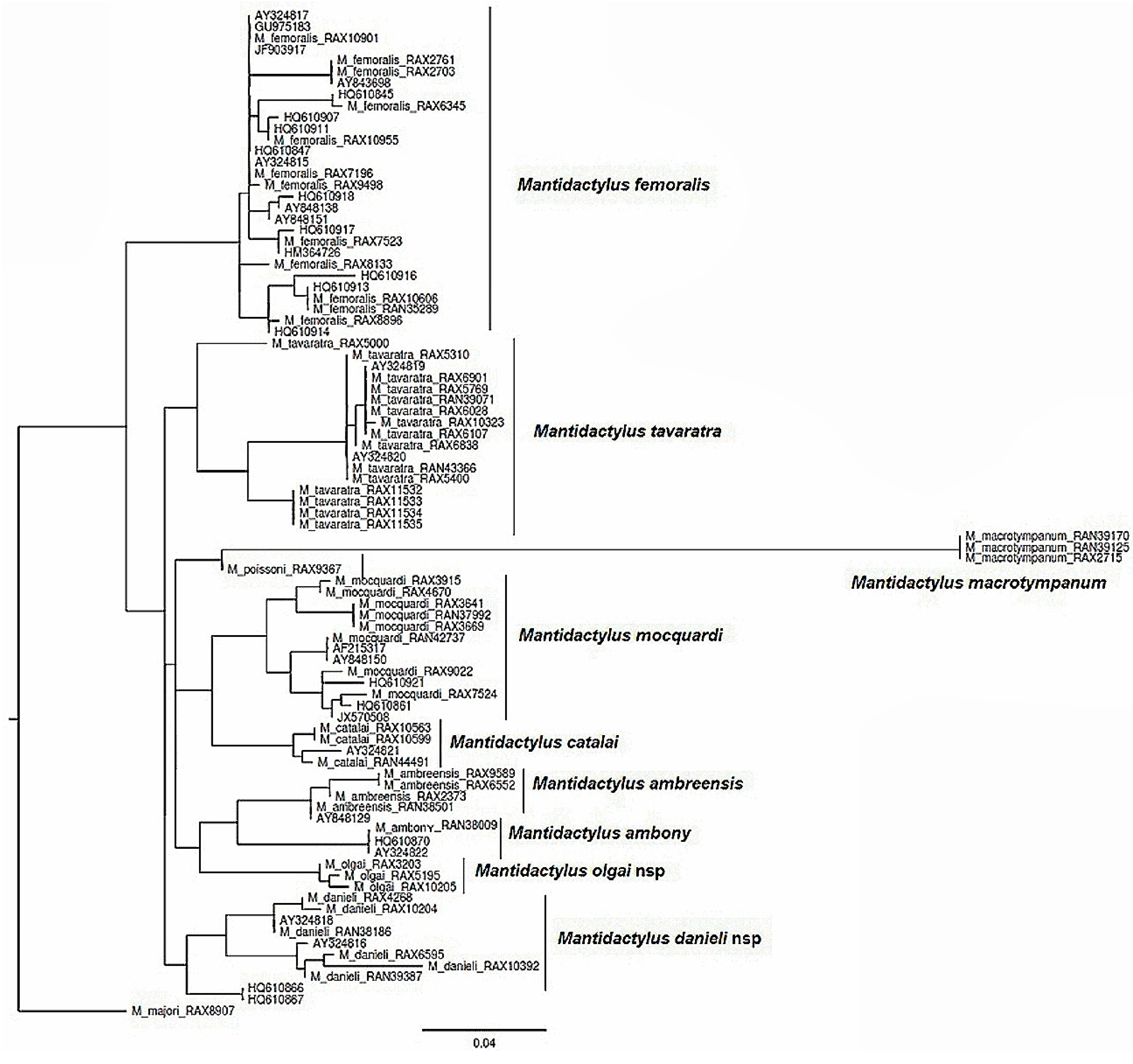
Figure 1.
Tree of Ochthomantis subgenus 16 S reduced taxa. All validate species have genetic divergence> 3% (Mantidactylus) flavicrus [
30] (p. 25); Mantidactylus catalai [
31] (p. 203); Mantidactylus poissoni [
32] (p. 178); Mantidactylus femoralis: [
33] (p. 235); Mantidactylus (Hylobatrachus) femoralis: [
34] (p. 312); Mantidactylus (Ochthomantis) femoralis: [
7] (p. 400); [
3] (p. 3).
Figure 1.
Tree of Ochthomantis subgenus 16 S reduced taxa. All validate species have genetic divergence> 3% (Mantidactylus) flavicrus [
30] (p. 25); Mantidactylus catalai [
31] (p. 203); Mantidactylus poissoni [
32] (p. 178); Mantidactylus femoralis: [
33] (p. 235); Mantidactylus (Hylobatrachus) femoralis: [
34] (p. 312); Mantidactylus (Ochthomantis) femoralis: [
7] (p. 400); [
3] (p. 3).
Specimens Examined: BMNH 1947.22.65–68: eastern slope of the Betsileo Region. AMNH A23781 and A50366: Moramanga, District Moramanga, Alaotra Mangoro Region, Madagascar. AMNH A157112 adult male: Ampanasana Ankolony [14°26,2’S 49°46,5’E] Marojejy National Park, Andapa District, Sava Region, Madagascar, Oct. 1998, A. Raselimanana. AMNH A167521* (RAX 6345) subadult: Analapakila Trois Lacs, District Bealanana [14°26.233’S 48°36.696’E, 1400 m], Mar. 12, 2003, N. Rabibisoa and S. Mahaviasy. AMNH A167580* (RAX 2703) juvenile: Antsahatelo [13°51.588’S 48°51.979’E, 800 m], Apr. 6, 2001, S. Mahaviasy, N. Rabibisoa, C. J. Raxworthy, A Razafimanantsoa, and A. Razafimanantsoa. AMNH A167581* (RAX 2761) adult male: Ramena river, Tsaratanana Reserve, Ambanja District [13°55.071’S 48°53.179’E; 750 m], Apr. 8, 2001, same collectors as previous. AMNH A174623* (RAX 7196) felale adult: Ankafina Tsarafidy, Ambohimasoa District, Haute Matsiatra Region, Madagascar [21°12.598’S 47°12.874’E, 1420 m], Feb. 16, 2004, N. Rabibisoa, M. Randriambahiniarime, F. Ranjanaharisoa, and C. J. Raxworthy. AMNH A174627* (RAX 7523) adult male: Betampona Reserve, Toamasina District, Atsinanana Region, Madagascar [17°55.866’S 49°12.190’E, 350 m]; Feb. 2, 2004, N. Rabibisoa, M. Randriambahiniarime, F. Ranjanaharisoa, and C. J. Raxworthy. AMNH A174646* (RAX 8133) adult female: Manasamena, Lakato, Moramanga District, Alaotra–Mangoro Region, Madagascar [19°02.637’S 48°20.910’, 950 m], Mar. 27, 2004, N. Rabibisoa and N. Rakotondrazafy. AMNH A174651* (RAX 8896) juvenile: Kianjavato–Vatovavy, Ranomafana District, Vatovavy–Fitovinany Region, Madagascar [21°22.791’S 47°52.052’E, 150 m], Feb. 18, 2006, N. Rabibisoa and C.J. Raxworthy. AMNH A174654* (RAX 9498) juvenile: Ambohibehivavy–Vasiana, Betafo District, Vakinankaratra Region, Madagascar [19°41.387’S 46°06.953’E, 850 m], March 28, 2006, N. Rabibisoa, N. Rakotondrazafy, and J. Rafanomezantsoa. AMNH A181735* (RAX 10606): Beampingaratsy Pass Anosy Mts, District Tolagnaro, Anosy Region, Madagascar [24°28.244’S 46°53.521’E, 520m], Feb. 13, 2009, C.J. Raxworthy. AMNH A187128* (RAX 10901): Ambatomenaloha/Itremo, Ambatofinandrahana District, Amorin’I Mania Region, Madagascar [20°37.130’S 46°33.347’E, 1650 m], Dec. 19, 2009, C.J. Raxworthy. UADBA 4517–18, 4520 (RAN 52471, 52470, 52109): Eminiminy, Andohahela National Park, Tolagnaro District, Anosy Region, Madagascar [24°37.55’S 46°45.92’E, 500 m], Oct. 21, 1995, A. Raselimanana and J. B. Ramanamanjato. UADBA 20478–79: Andriankely, Anjozorobe–Angavo National Parc, Anjozorobe District, Analamanga Region, Madagascar [18°25.225’S 47°56,245’E, 1250 m], Feb. 2, 2003, M. Anjeriniana. UADBA 26118, 26268–70 (NR 1866, 1819, 1858, 1859): Ampanatovana Lakato, Moramanga District, Alaotra–Mangoro Region, Madagascar [19°02.637’S 48°20.912’E, 1025 m], Nov. 29, 2003 and Dec. 6, 2003, N. Rabibisoa, N.A. Rakotondrazafy. UADBA 26249, 26262, 26389 (RAX7198, 7197, 7212): Ankafina Tsarafidy, Ambohimasoa District, Amoron’I Mania Region, Madagascar [21°12.598’S 47°12.874’E, 1150 m], Feb. 16, 2004, N. Rabibisoa, M. Randriambahiniarime, F. Ranjanaharisoa, and C.J. Raxworthy. UADBA 26250–51, 26263–65, 26402 (RAX 7961, 7553, 7945, 7555, 8002, 7618): Betampona Strict Natural Reserve, Toamasina II District, Atsinanana Region, Madagascar [17°55.866’S 49°12.190’E, 250–450 m], Feb. 28, 2004–Mar. 17, 2004, N. Rabibisoa, M. Randriambahiniarime, F. Ranjanaharisoa, and C. J. Raxworthy. UADBA 26252 (RAN 44674) adult male: Sahavatoy River, Andringitra National Park, Ambalavao District, Ihorombe Region, Madagascar [22°13.667’S 47°0.217’E, 810 m], Nov. 24, 1993, N. Rabibisoa, A. Razafimanantsoa, and C. J. Raxworthy. UADBA 26253–54, 26374, 26378 (RAN 45537, 45684, 45538, 44833): Rangovalo Ridge,
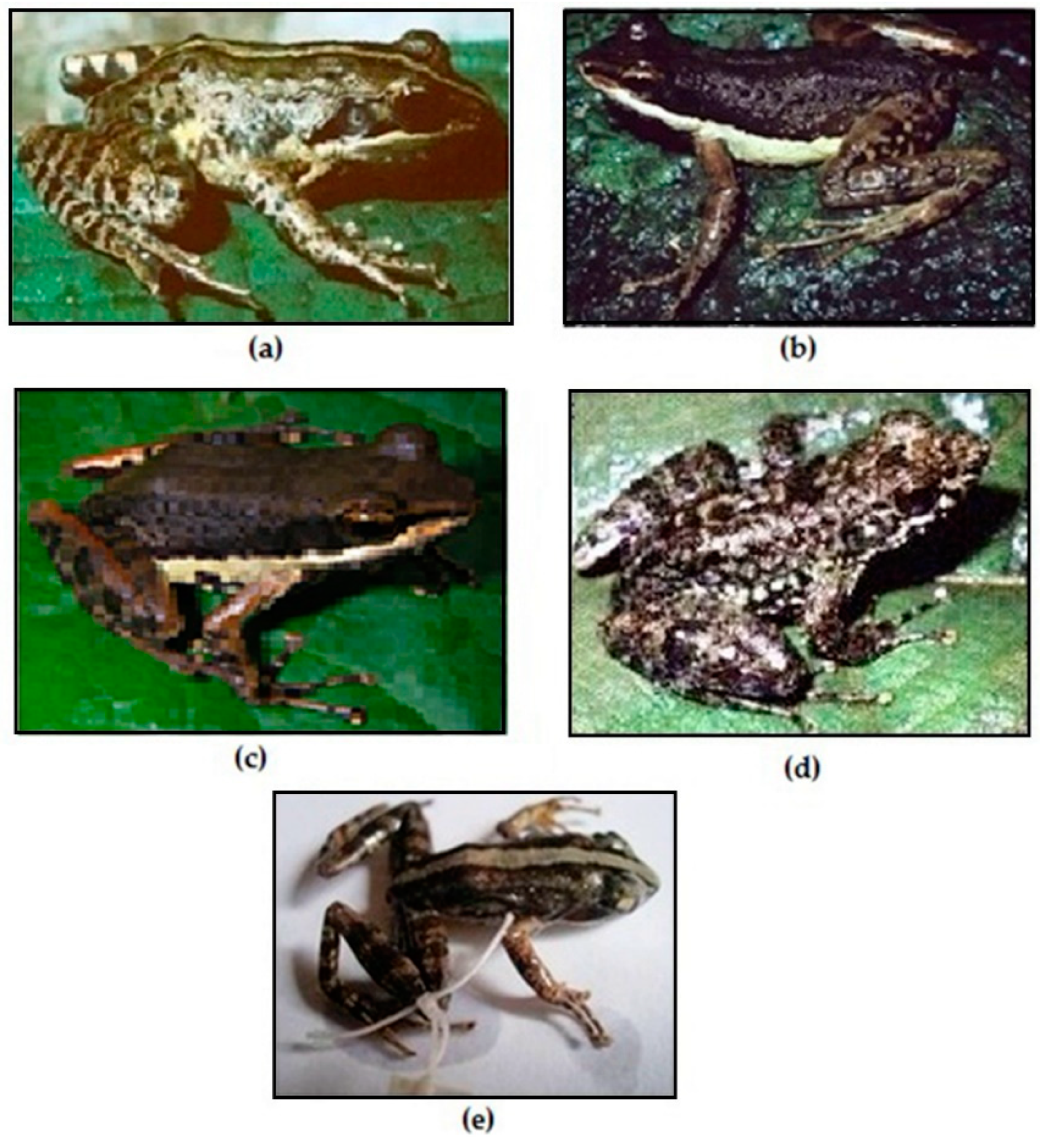
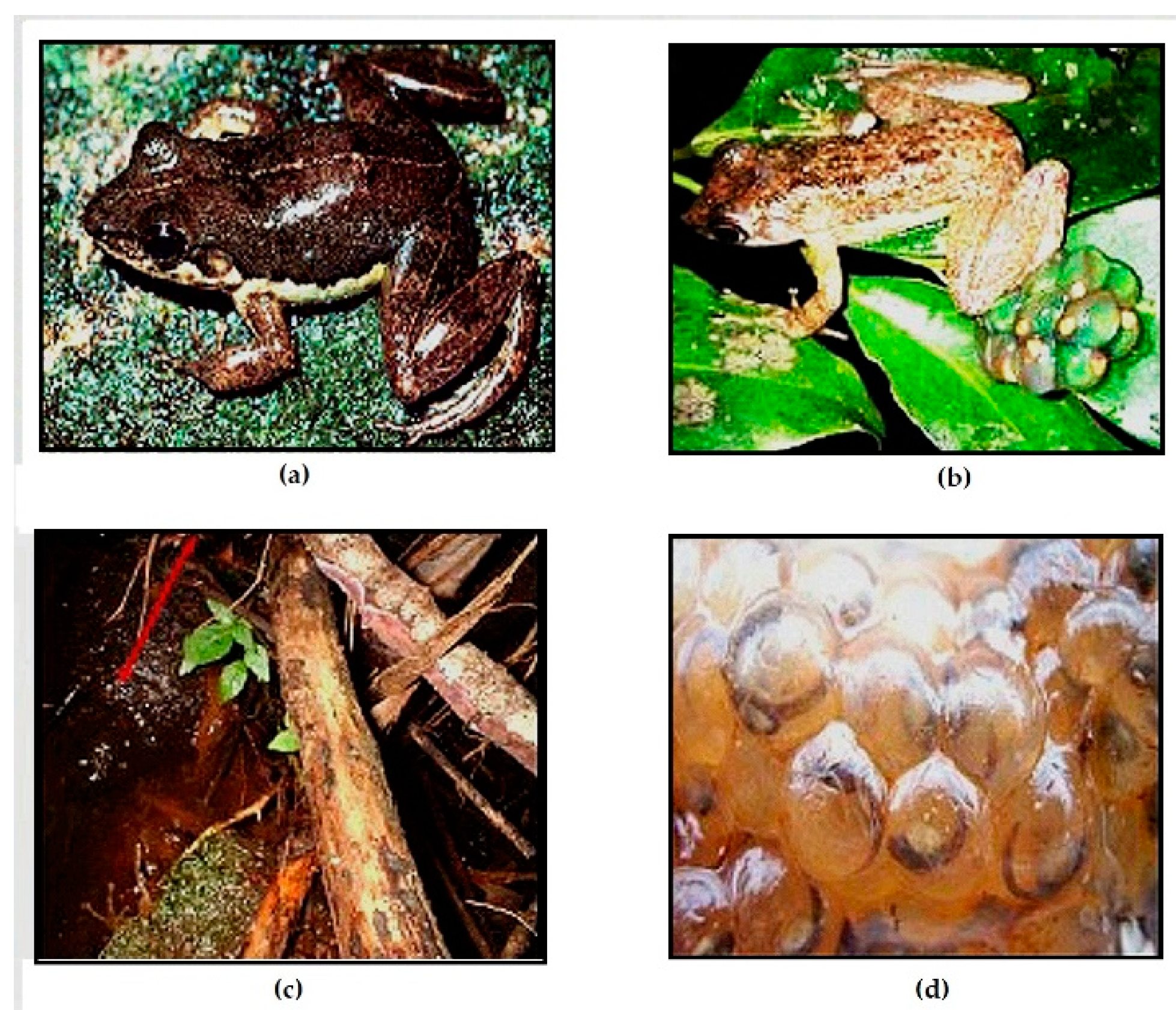
Figure 2.
Photos of the validate species of the subgenus Ochthomantis: a. Mantidactylus femoralis, Zahamena (CJR); b. Mantidactylus ambreensis, Montagne d’Ambre (CJR), c: Mantidactylus ambony, Montagne d’Ambre (CJR); d. Mantidactylus mocquardi, Betampona (NR), e. Mantidactylus zolitschka, An’ala (NR).
Figure 2.
Photos of the validate species of the subgenus Ochthomantis: a. Mantidactylus femoralis, Zahamena (CJR); b. Mantidactylus ambreensis, Montagne d’Ambre (CJR), c: Mantidactylus ambony, Montagne d’Ambre (CJR); d. Mantidactylus mocquardi, Betampona (NR), e. Mantidactylus zolitschka, An’ala (NR).
Zahamena National Park, Ambatondrazaka District, Alaotra–Mangoro Region, Madagascar [17°40,5’S 48°45.5’E, 1150 m], Mar. 4−8, 1994, J.B. Ramanamanjato, A. Raselimanana, C.J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa. UADBA 26255, 26380 (RAN47059, RAN47047): Andranomangoboka Ambohijanahary, Morafenobe District, Melaky Region, Madagascar [18°14.787’S 45°21.419’E, 730–950 m], Jan. 16, 1995, J. B. Ramanamanjato, A. Raselimanana, C. J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa. UADBA 26257 (RAN47663): Ambohimanana Tolongoina, Ikongo District, Vatovavy–Fitovavy Region, Madagascar [21°28.557’S 47°33.759’E, 600 m], Feb. 9, 1995, J. B. Ramanamanjato, A. Raselimanana, C. J. Raxworthy, A. Razafimanantsoa, and A Razafimanantsoa. UADBA 26258 (RAN47953): Sahamalio, Isalo National Park, Ranohira District, Ihorombe Region, Madagascar [22°26.315’S 45°15.648’E, 700 m], Feb. 18, 1995, C. J. Raxworthy, A. Raselimanana, J. B. Ramanamanjato, A Razafimanantsoa, and A Razafimanantsoa. UADBA 26259, 26284, 26260–61 (RAN47995, 47996, 48219, 48221): Canyon Singe, Isalo National Park, Ranohira District, Ihorombe Region, Madagascar [22°29.138’S 45°23.086’E, 600 m], Feb. 20, 1995, same collectors as previous. UADBA 26266, 26385 (RAX 8157, 8158): Manasamena Lakato, Moramanga District, Alaotra Mangoro Region, Madagascar [19°02.637’S 48°20.910’E, 950 m], Mar. 27, 2004, N. Rabibisoa and N. Rakotondrazafy. UADBA 26267 (RAN 45891): Namarafana, Zahamena National Park, Ambatondrazaka District, Alaotra Mangoro Region, Madagascar [17°44’S 48°58.5’E, 420 m], Mar. 16, 1994, J. B. Ramanamanjato, A. Raselimanana, C. J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa. UADBA 26281, 26376 (RAN 47280, 47248): Doany Ambohijanahary, Morafenobe District, Melaky Region, Madagascar [18°17.297’S 45°33.289’E, 1220 m], Jan. 24, 1995, same collectors as previous. UADBA 26282 (RAN 47580) adult female: Ambohitantely Special Reserve, Ankazobe District, Analamanga Region, Madagascar [18°11.158’S 47°16.757’E, 1580 m], Feb. 2, 1995, same collectors as previous. UADBA 26363 (JB 127) adult female: Andranomay, Anjozorobe District, Analamanga Region, Madagascar [18°28.8’S 47°57’E, 1300 m], Dec. 12, 1996, A. Raselimanana. UADBA 26377 (RAN 47631) same condition as UADBA 26257 except date of collection Feb. 8, 1995. UADBA 26375, 26379 (RAN 45395, 45364): Volontsagana River, Zahamena National Park, Ambatondrazaka District, Alaotra–Mangoro Ragion [17°42’S 48°46’E, 850 m], Feb. 28, 1994, J. B. Ramanamanjato, A. Raselimanana, C. J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa. UADBA RAX10955*: Mandraka, Manjakandriana District, Analamanga Region, Madagascar [18°57.733′S 47°55.050′E, 1140 m], Mar. 18, 2006, N. Rabibisoa, and J. Rafanomezantsoa. UMMZ 212835 (RAN 39921) adult male: Manantenina river, Marojezy National Park, Andapa District, Sava Region, Madagascar [14°26’S 49°46’E, 700 m], Nov. 25, 1992, R. A. Nussbaum, A. Razafimanantsoa, A Razafimanantsoa, and C J Raxworthy. UMMZ 197651* (RAN 35289): Manantantely Forest, District Tolagnaro, Anosy Region [24°39’S 46°55.083’E, 125 m], Nov. 6, 1990, J.B. Ramananjato, A Raselimanana, RA Naussbaum, and C J Raxworthy.
Diagnosis: A medium to large–sized
Ochthomantis (adult male SVL 31–43.4 mm; adult female 43.0–62.4 mm). tibio–tarsal articulation reaching beyond nostrils (or rarely between eye and nostril), 1.5–2 free phalanges on the internal edge of toe 4, the width of the digit terminal disc ≥ 1.70 disc base, a white stripe along the superior lip and a prominent yellow patch in the inguinal region.
Mantidactylus femoralis can be distinguished from all other subgenus
Ochthomantis species by the following combination of characters:
M. ambreensis by a yellow line or patch in the inguinal region;
M. poissoni by the absence under the eye of a large white spot or multiple partly fused white spots on the upper lip;
M. mocquardi, M. catalai,
M.
olgai, and
M. tavaratra by the presence of 1.5–2 free phalanges on the internal edge of toe 4, a yellow patch in the inguinal region, and the tibio–tarsal articulation reaching beyond the eye;
M. zolitschka by its large size (SVL ≥ 33 mm) and yellow patch in the inguinal region;
M.
danieli by the yellow patch in the inguinal region, and the width of the digit terminal disc ≥ 1.70 disc base; and
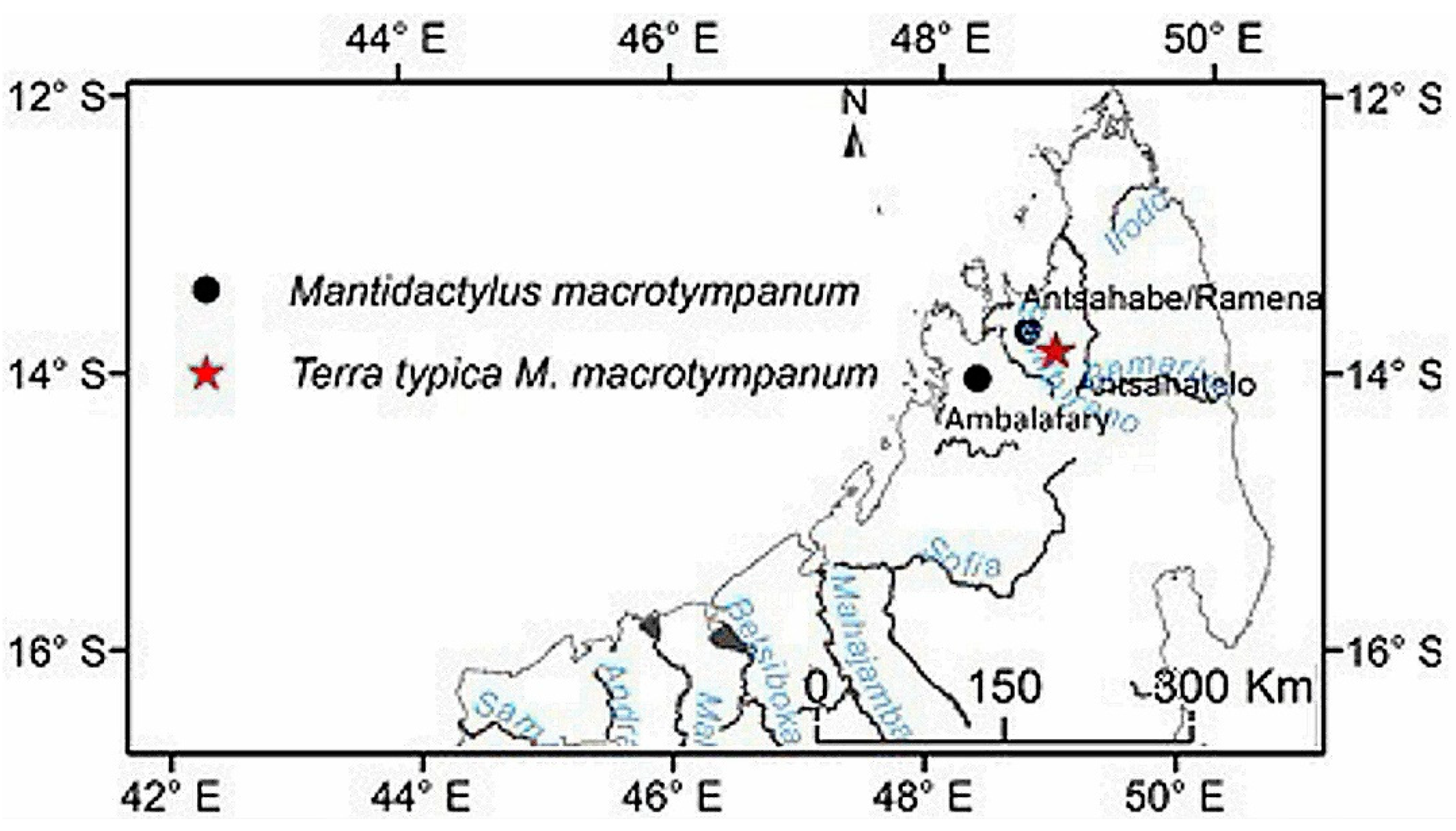
M. macrotympanum by the smaller adult male SVL (< 60 mm). The
Table 1 and
Table 2 summurizes the character diagnostics of this new species.
Description of
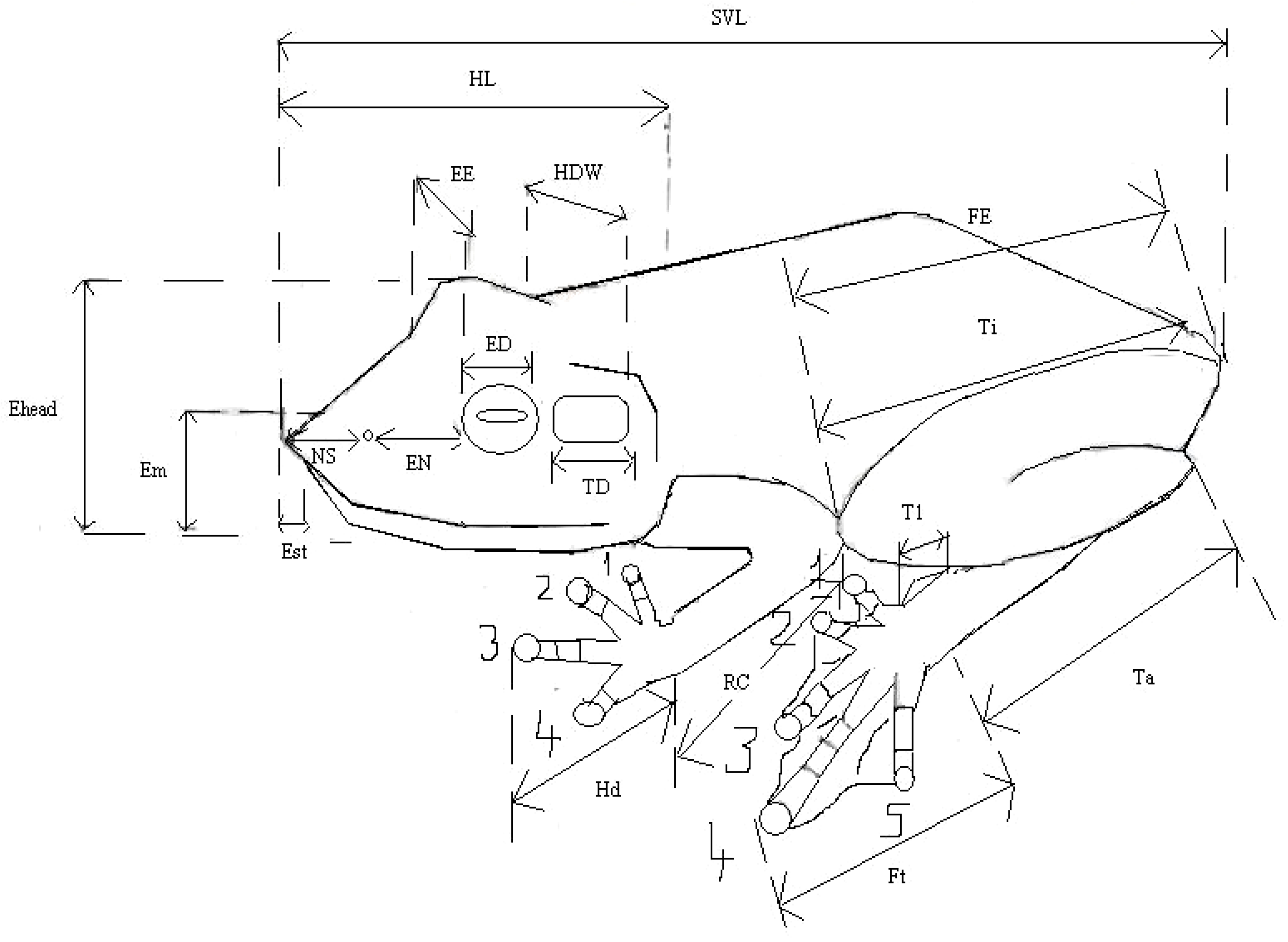
UADBA 19662 (NR 1724): Adult male (SVL = 37.45 mm) in an excellent state of preservation. Measurements are presented in
Table S2. In dorsal view and lateral view, snout pointed, and triangular in ventral appearance. Snout tip with a 1.75 mm ventral extension beyond mouth. Head 1.44 times longer than wide. Head length 0.48 times SVL. Canthus rostralis weakly evident. Tympanum diameter 0.88 times eye. Tympanum ovoid and distinct from the supratympanic ridge, which continues to above the insertion point of the forelimb. Internarial distance 0.28 times head width. Tongue ovoid anteriorly and bifid posteriorly. Nostrils rounded, and with lateral opening. Eye to nostril distance 1.45 times nostril to snout distance. Forearm length 0.50 times SVL. Hand length (including discs) 0.30 times SVL. Fingers not webbed. Inner and outer tubercule metacarpals very developed in granule-like. Fingers not webbed. Relative fingers lenght 1 < 2 < 4 < 3. Digits with large terminal discs (the widest part twice the width of the base). tibio–tarsal articulation reaches beyond snout tip. Lateral metatarsal separated. Hind limb 1.90 times SVL. Thigh length 0.95 times tibia length. Foot including tarsus 0.82 times SVL. Inner metatarsal tubercule shield-shaped (lenght 1.5 mm) at the base of the toe 1. Outer metatarsal tubercule is a small granule. Toes with extensive webbing, with webbing formula: 1 (1), 2i (1), 2e (0.5), 3i (1), 3e (0.5), 4i (2), 4e (1.5), 5 (0). and sum of free phalanges is 7.50 (WS): Relative toe lenght 1 < 2 < 3 < 5 < 4. The body flank region with small granules, and dorsal body almost without granules. Femoral glands on the ventral surface of thighs are oblong and slightly developed with the medio–proximal area having a pore surrounded by many granules, giving a crater-like form. Internally, the femoral glands are Type 3 [
2,
35]. In preservative, the dorsal head, body and limbs are dark brown with a thick pale brown vertebral line running from the snout tip to anus. Upperlip with a white band on its lower edge. Lowerlip with white spot. Dark brown eye mask posterior to the eye. Ventral face pale brown, spotted with darker brown flecks, with becomes lighter and less dense in the belly region. A pair of dark brown parallel stripes on the throat. The inguinal region has a prominent pale yellow oblique line. Dorsal transverse bands of darker brown on the hind and forelimbs.
Variation: Morphological diagnostic variation details are summarized in TS2. Sexual dimorphism is evident: males have smaller SVL (33–43 mm vs 43–62 mm), shorter tibia, larger toe 3, and the head is relatively smaller. The ratio of td/ed larger in males than females (0.69–1.00 vs 0.52–0.76). The femoral glands are more swollen in males, and are smaller and more circular in females. Black spots are also observed on the anal area of females. The free phalanges on the toes vary: toe 1 (0.25–1), internal edge of the toe 2 (1–1,50), external edge of the toe 2 (0–1) internal edge of the toe 3 (1–1,75), external edge of the toe 3 (0–1), internal edge of the toe 4 (1,50–2), external edge of the toe 4 (1–2), and in toe 5 (0–0,75).
Coloration in life: The iris has a golden ring on the outer area. The dorsal body may have a pale yellow vertebral line, or this may be completely absent. There is a distinct oblique and large yellow patch present in the inguinal region, with the yellow colour sometimes extending onto the ventral side. The ventral surface often is pale brown on anterior parts, and with yellowish-brown color on the belly. The throat may lack a pair of dark brown parallel stripes. The ventral surface of the thigh is brown color, and its posterior part with yellow spots. A dark brown transverse bar is present between the eyes. Hind limbs with alternating brown and black transverse bands.
Habits: A semi-aquatic rainforest species living close to rivers and small streams. Rarely, it can also be seen outside rainforest, along streams close to relict forests (e.g., Tsarafidy). This species is found on rocks, leaves, branches and on the ground along river banks between 9.00 am to 23.30 pm. However, it is most active and obvious at night, and appears to be nocturnal. During the day, it can be found hidden in holes and rock crevices along rivers. At night, the females are generally found sitting on leaves of shrubs along streams, or more rarely on the rocks in the middle of rivers. Males can be found in all areas along rivers. except on branches and plant stems. The vertical distribution of individuals on shrubs is different between the sexes: males up to 1 m above the ground, whereas females up to 2 m. Individuals are mainly observed near streams between 1–10 m width, with a maximum water depth of 10 to 150 cm, and with slow flowing water. Eggs are laid in masses outside of water on a leaf or a branch overhanging or close to the stream or river. The tadpoles live in the calm water far from torrents. The period of reproduction is during the cold winter season (e.g., June and July in Mantadia). The calls of males are low, and hardly audible. In the Moramanga region metamorphosis appears to occur in September to October. At Andranomanamponga in August 2002, tadpoles of different stages of development were observed in calm clear water, protected by rocks, in pools of 1 to 3 m2 surface area and 20–100 cm water depth. When disturbed, the tadpoles quickly swim obliquely to hide in mud, under dead leaves, or retreat into rock crevices.
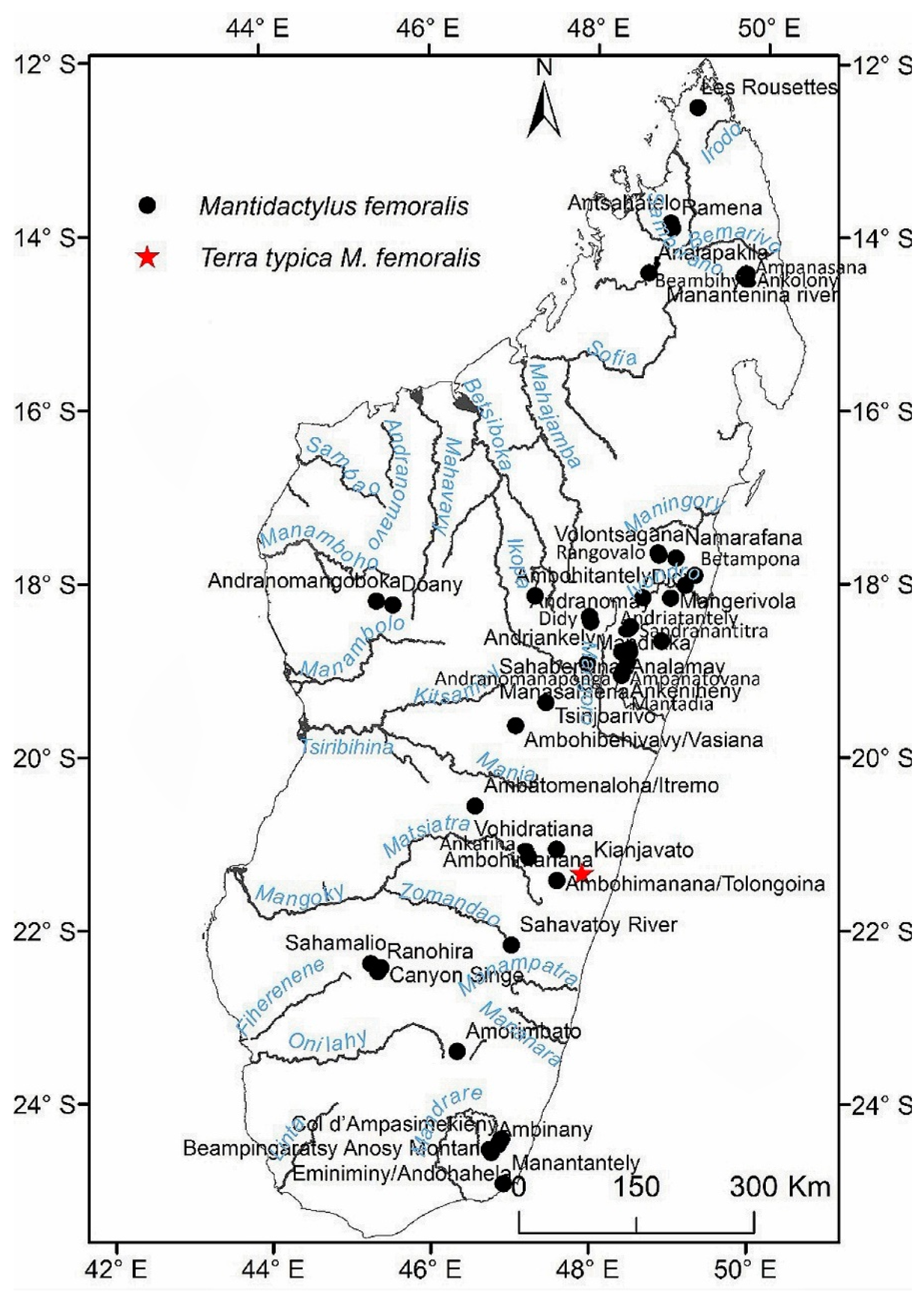
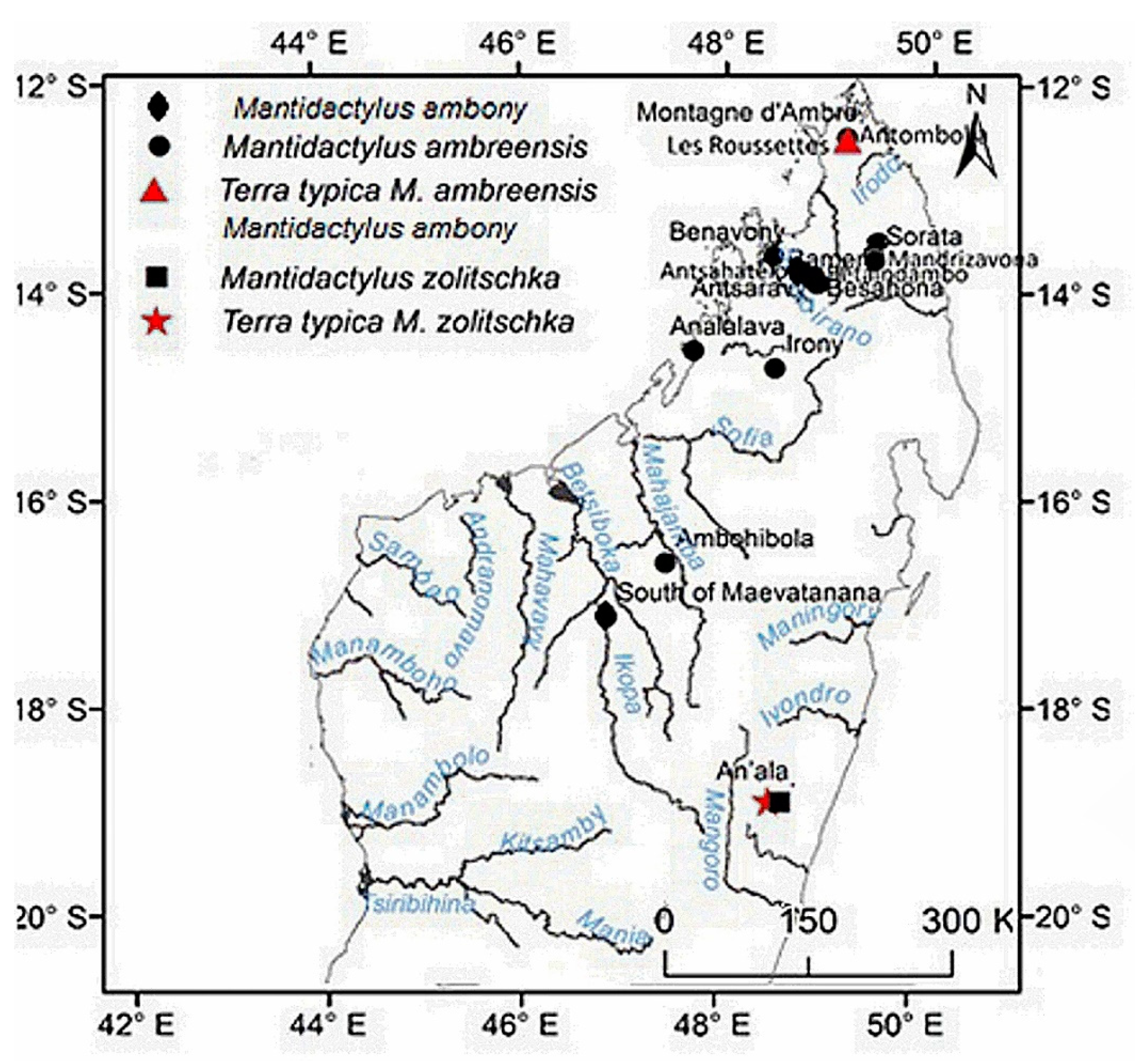
Distribution: Eastern and northern rain forest, including the High Plateau of Madagascar, with an elevation range between 230 to 1600 m. The species occurs as far north as the Sambirano Region (14°S) and as far south as the Anosy Mountains (25°S) (
Figure 3,
Table S13).
Comments: Our morphological description agrees with the
M. femoralis shown by [
36] (
Figure 2, p. 247) from Antoetra, and our molecular analyses groups all our
M. femoralis samples (
Table S1) with the
M. femoralis samples reported by [
10]: AY324815 (FGMV 2001.155) AY324817 (FGMV 2002.56); and [
11]: HQ610845 (ZSN 1630/2007), HQ610847 (ZSM 1643/2007), HQ610913–14 (FGZC 271, ZCMV 370) and HQ 610916–18 (ZCMV 464, 937, 5874).
M. flavicrus remains a junior synonym of
M. femoralis. The type locality for this taxa is Madagascar.
3.2.2. Mantidactylus ambreensis [37]
Mantidactylus ambreensis [
37] (p. 127). Validate as bonna species by [
38] (p. 67); Mantidactylus (Ochthomantis) ambreensis: [
7] (p. 400); [
3] (p. 3).
M. ambreensis being synonimized with
M. femoralis by [
27], and later recognized again as a good species by [
10,
38].
Holotype: MNHN 1893.241: Montagne d’Ambre, Madagascar.
Specimens examined: MNHN 1893.241 adult female: Montagne d’Ambre, Madagascar. AMNH A50521: Analalava District, Sofia Region, Madagascar, Jan. 21, 1971. AMNH A167482* (RAX 2373) adult male: Mandrizavona, Tsaratanana Reserve, Ambanja District, Diana Region, Madagascar [13°48.043’ S 48°44.78’ E, 650 m], Jan. 12, 2001, C. J. Raxworthy, A. Razafimanantsoa and A. Razafimanantsoa. AMNH A167485 (RAX 2468) adult male: Besahona, Tsaratanana Reserve, Ambanja District, Diana Region, Madagascar [13°54.372’S 48°52.425’E, 550–750 m], Jan. 25, 2001, same collectors as AMNH A167482*. AMNH A167486 (RAX 2720): Antsahatelo, Tsaratanana Reserve, Ambanja District [13°51.588’ S 48°51,979’ E, 700 m], Apr. 7, 2001, S. Mahaviasy, N. Rabibisoa, C. J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa. AMNH A167499–500 (RAX 3289, 3330, 3343): Antsaravy, RNI Tsaratanana, Ambanja District, Diana Region, Madagascar [13°55.560’S 48°54.353’E, 1150 m], Apr. 20, 2001, same collectors as AMNH A167486. AMNH A167501*–02*–04 (RAX 6552, 6557, 6584, 6558): Irony Relict Forest Camp, Bealanana District, Sofia Region, Madagascar [14°44.937’S 48°29.449’E, 930 m] collected Apr. 01, 2003, S. Mahaviasy, N. Rabibisoa, N. Rakotondrazafy, and C. J. Raxworthy. AMNH A 167565* (RAX 3203) juvenile: Antsaravy, RNI Tsaratanana, District Ambanja, Diana Region, Madagascar [13°55.560’S 48°54.353’E, 1150 m], Apr. 16, 200, N Rabibisoa, S. Mahaviasy A. Razafimanantsoa, and A. Razafimanantsoa. AMNH A174618* (RAX 9589) adult male: Ambohibola forest, Tsaratanana District, Betsiboka Region, Madagascar [16°38.358′S 47°26.165′E, 300 m], Apr. 10, 2006, N. Rabibisoa, and C. J. Raxworthy. UADBA 3714 (RAN 38503) adult male: Fitsahana Atomboka River, Montagne d’Ambre, Antsiranana District, Diana Region, Madagascar, [12°29.2’S 49°10,3’S, 1150 m], Dec. 25, 1991, by C. J. Raxworthy, A. Raselimanana, J. B. Ramanamanjato. UADBA 5647–50 (RAN 54050, 54105, 54130, 54054): Irony Relict Forest Camp, Bealanana District, Sofia Region, Madagascar [14°44.937’S 48°29.449’E, 930 m], February 21–24, 1996, C. J. Raxworthy, A. Razafimanantsoa, A. Razafimanantsoa. UADBA 7222–24: Benavony, Ambanja District, Diana Region, Madagascar, 200 m, Mar. 21, 1994, F. Glaw, N. Rabibisoa, and O. Ramilison. UADBA 8393, 8396, 8414 (RAX 2796, 2875, 3000): Ramena River Camp, Ambanja District, Diana Region, Madagascar [13°55.071’S 48°53.179’E, 730–750 m], Apr. 9–13, 2001, Mahaviasy, N. Rabibisoa, C. J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa. UADBA 8394, 8411, 8416 (RAX 2530, 2528, 2514): Besahona, Ambanja District, Diana Ragion [13°54.372’S 48°52.425’E, 550 m], Jan. 27, 2001, A. Razafimanantsoa, and Razafimanantsoa. UADBA 8395, 8398, 8412, 8415 (RAX 2661–58): Nirhy’s cascade Camp Analabe, Tsaratanana Reserve, Ambanja District, Diana Region, Madagascar [13°51.023’S 48°47.902’E, 760 m], Apr. 5, 2001, same collectors as UADBA 8393. UADBA 8397, 8413 (RAX 2557, 2401): Mandrizavona, Ambanja District, Diana Region, Madagascar [13°48.043’S 48°44.78’E, 450 m], Jan. 14, and Jan. 30, 2001, A. Razafimanantsoa, and A. Razafimanatsoa. UADBA 8399 (RAX 2554) adult female: Betaindambo, Ambanja District, Diana Region, Madagascar [13°51.932’S 48°49.189’E 550 m], Jan. 30, 2001, A. Razafimanantsoa, and A. Razafimanatsoa. UADBA 8401 (RAX 2702) adult female: Antsahatelo, Tsaratanana Reserve, Ambanja District, Diana Region, Madagascar [13°51.588’S 48°51.979’E, 800 m], Apr. 6, 2001, same collectors as UADBA 8393. UADBA 8408, 8410 (RAX 3195, 3236): Antsaravy Valley Camp, Tsaratanana Reserve, Ambanja District, Diana Region, Madagascar [13°55.560’S 48°54.353’E, 1150 m], Apr. 15, and Apr. 17, 2001, S. Mahaviasy, N. Rabibisoa, A. Razafimantsoa, and A. Razafimanantsoa. UADBA 9056–57 (NR 548, 547): Les Rousettes Camp, Montagne d’Ambre National Park, Antsiranana District, Diana Region, Madagascar [12°31’S 49°10’E, 1000 m], Mar. 3, 1996, N. Rabibisoa, D. Rakotomalala, and O. Ramilison. UADBA 26120, 26222–24, 26271–72 (RAX 6556, 6586, 6553, 6555, 6554, 6559): Irony Relict Forest Camp, District Antsohihy, Sofia Region, Madagascar [14°44.937’S 48°29.449’E, 930] and [14°45.140’S 48°29.690’E, 950 m], Apr. 1–2, 2003, S. Mahaviasy, N. Rabibisoa, N. Rakotondrazafy, and C. J. Raxworthy. UMMZ 212426* and 212435* (RAN 38501, 38009): Antomboka river, Montagne d’Ambre, Antsiranana District, Diana Region, Madagascar [12°32.3’S 49°10’S, 650–1150 m], Nov. 17 and Dec. 25, 1991, C.J. Raxworthy, and R.A. Nussbaum.
Diagnosis: A relatively small sized
Ochthomantis (adult male SVL 33,3–39 mm; adult female 38,2–42 mm) with a sharply defined lateral white strip along the side of the head and body, and which runs from the snout tip to groin area. The
Table 1 and
Table 2 summurize the character diagnostics of this current species.
Mantidactylus ambreensis can be diagnosed from M. ambony by its large size, and the rest of the other subgenus Ochthomantis by the presence of the sharply defined white lateral stripe, which is absent in all other species.
Description of
UADBA 8393 (RAX 2796): Adult male (SVL = 39.00 mm) in an excellent state of preservation. Measurements are presented in
Table S3. In dorsal view and lateral view, snout tip pointed. Snout tip with a 2.0 mm ventral extension beyond mouth. In dorsal view, head clearly longer than large (ratio hdw/hdl= 0.73). Head 1.38 times longer than wide. Head length 0.44 times SVL. Canthus rostralis indistinct. Loreal area concave. Tympanum diameter 1.04 times eye. Tympanum rounded, and touched with supratympanique along theirs borders except in posterior part. Supratympanic fold continues posteriorly to a point above three large granules above the insertion of the forearm. Dark tympanum with a small notch in its median superior area. Internarial distance 0.27 times head width. Tongue ovoid anteriorly and bifid in posterior part. Nostrils rounded, and with lateral opening. Eye to nostril distance 1.97 times nostril to snout distance. Forearm length 0.48 times SVL. Hand length (including discs) 0.17 times SVL. Inner metacarpal not obvious and outer metacarpal forms a flattened granule. Fingers not webbed. Relative finger length 1 < 2 < 4 < 3. Digits with large terminal discs (the widest part twice the width of the base). Tibio–tarsal articulation reaches between eye and nostril. Lateral metatarsal separated. Hind limb 1.77 times SVL. Thigh length 0.97 times tibia length. Foot including tarsus 0.74 times SVL. Inner metatarsal tubercule not obvious (length 1.80 mm) at the base of toe 1. Outer metatarsal tubercule absent. Toe with relative extensive webbing; with webbing formula: 1 (1) 2i (1) 2e (0.50), 3i (1.25) 3e (0.50) 4i (1.50) 4e (1.50) 5 (0.50), and sum of free phalanges is 7.75 (WS). Relative toe length 1 < 2 < 3 < 5 < 4. The bodies flank, venter, and sacral areas with very small granules. Femoral glands on the ventral surface of thighs are oblong and developed with the medio–proximal area having a pore surrounded by many granules, giving a crater-like form. Internally, the femoral gland is type 3 [
2,
35]. In preservative, the dorsal head, body and limbs are dark. Lateral body less dark than dorsal. Lips with white band colors continuing along the flank to the hind limb insertion. The iris dark surrounding by white ring. Ventral face whitish with dark marbling except in belly region. A pair of dark–brown parallel bands on the throat. Forelimb with some dark spots on the ventral face. Thigh with large dark spots except in the femoral gland region. Dorsal transverse bands, alternating dark and light, on the hind and forelimbs.
Variation: Morphometric variation diagnostic details are summarized in
Table S3. Sexual dimorphism is evident: males have smaller SVL (33,3–39 mm versus 38,2–42 mm), relatively longer tibia and feet, and shorter toe 3. The ratio of td/ed larger in males than females (0.80–1.16
vs 0.52–0.79). The femoral glands are more swollen in males, and are smaller and more circular in females. The free phalanges on the toes vary: toe 1 (0–1), internal edge of toe 2 (1), external edge of toe 2 (0–0.50), internal edge of toe 3 (1–1.50), external edge of toe 3 (0–1), internal edge of toe 4 (1–2), external edge of toe 4 (1–2), and in toe 5 (0–1). Small skin granules may be present on the dorsal body, and above the eyes. Granules are also found in some individuals in the sacral area.
Coloration in life: The iris has a gold ring on the outer area. The dorsal body is without vertebral line. A white or yellow band is running along the flank. The dorsal surfaces of the head, body, and limbs may be dark green, brown or grayish brown. The ventral surfaces are usually mottled brown, but some individuals show almost no mottling. Some individuals have a pair of short longitudinal dark bands on the throat that diverge, and fade on the thorax. Large round dark spots may also be present on the throat and the thorax. A dark crossbar may be present between the eyes.
Habits: A semi-aquatique forest species living next to flowing streams or rivers generally closed of the rocks. This species is observed between 9.00–23.00 hours, but it is rather nocturnal than diurnal. At night, females rest on leaves than on branches, and the day we observed them sometimes resting on the banks of the river and very rarely on the ground. For males, the day they rest on banks and at night, they rest on leaves and sometimes we can watch them on rocks. The vertical distribution of individuals on shrubs is different between both sexes: males between 10–200 cm, whereas females between 100–200 cm. Distinct to
M. femoralis, this taxon prefer rivers than small stream. At Montagne d’Ambre (Station les Roussettes), it is observed near the irrigation canals. Calling male was heard in March in the afternoon from the ground along forest brook [
7]. A clutch consisted of about 100–120 eggs are deposited on rock edge closed to calm and shallow stream in April 2006 at Vohibola Tsaratanana. An egg diameter is about 2–3 mm, and tadpoles hatched after two days in plastic bag.
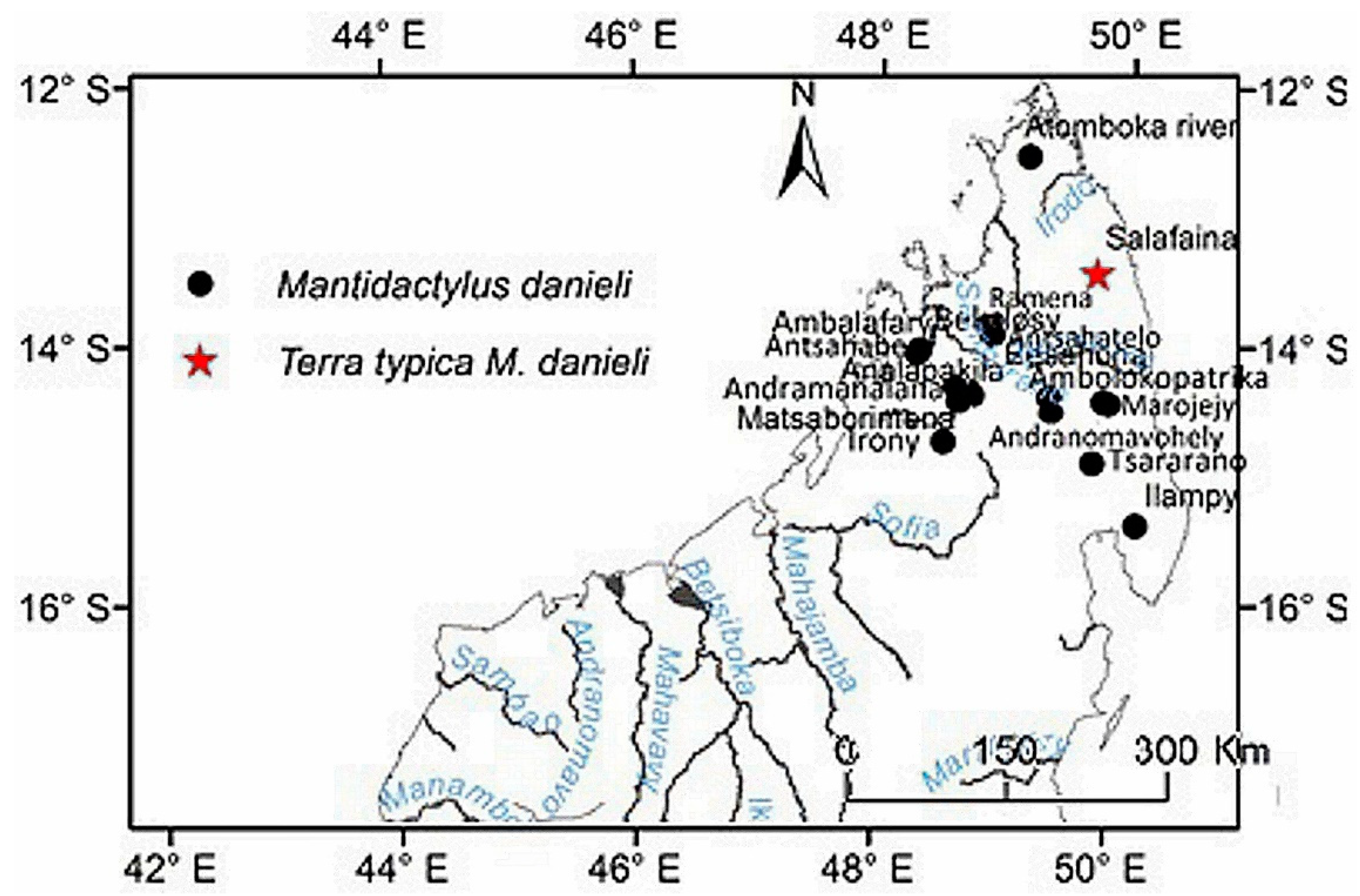
Distribution: species of low and mid-altitude forests of the North–western and northern of Madagascar, with an elevational range between 200 to 1150 m. This species is found as far north as Montagne d’Ambre, and across humid forests in the northern highlands such as Manongarivo, Tsaratanana, Andramanalana, Sorata; and extending to the northern limit of the High Plateau at Irony River, Ambohibola Forest (
Figure 4,
Table S13).
Comments: Our molecular analyses group all our
M. ambreensis samples with the
M. ambreensis samples reported by [
10]: AY324822 (ZSM 492/2000); [
11]: HQ610870 (FGMV 2002.1950); and by the holotype MNHN 1893.241 and their sequence tissus deposed to Genbank with the number MT982119, MT982173, and MT993842 and resgistered in zoobank with the Life Science Identifier (LSID): 54957B7E-BDB3-437F-93A7-3E86288477BF [
5].
3.2.3. Mantidactylus ambony [5]
Holotype
: ZSM 2078/2007 (FGZC 1039): adult female, Montagne dʼAmbre National Park, Antsiranana District [12.5280° S, 049.1720° E, 1050 m], Feb. 24, 2007, F. Glaw, P. Bora, H. Enting, J. Köhler, and A. Knoll [
5].
Specimens Examined: AMNH A167487 (RAX 2758): Antsahatelo, Tsaratanana Reserve, Ambanja District [13°51.588’ S 48°51,979’ E, 700 m], Apr. 7, 2001, S. Mahaviasy, N. Rabibisoa, C. J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa. AMNH A167490–95, 167497 (RAX 2816, 2872–74, 2876, 2874, 2932, 2970): Ramena, Ambanja District, Diana Region, Madagascar [13°55,071’S 48°53,179’E, 730–750 m], Apr. 11, 2001 except AMNH A167497 Apr.12, 2001, S. Mahaviasy, N. Rabibisoa, C. J. Raxworthy, A. Razafimanantsoa and A. Razafimanantsoa. AMNH A167498 (RAX 3289): Antsaravy, RNI Tsaratanana, Ambanja District, Diana Region, Madagascar [13°55.560’S 48°54.353’E, 1150 m], Apr. 20, 2001 Apr. 18, 2001, S. Mahaviasy, N. Rabibisoa, C. J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa. UADBA 7225–26: Les Roussetes Camp, Montagne d’Ambre National Park, Antsiranana District, Diana Region, Madagascar [12°31’S 49°10’E, 1000 m], Feb. 02, 1994, F. Glaw, N. Rabibisoa, and O. Ramilison. UADBA 8406 (RAX 2871): Ramena River Camp, Ambanja District, Diana Region, Madagascar [13°55.071’S 48°53.179’E, 730–750 m], Apr. 9–13, 2001, Mahaviasy, N. Rabibisoa, C. J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa. UADBA 9058 (NR 564): Les Rousettes Camp, Montagne d’Ambre National Park, Antsiranana District, Diana Region, Madagascar [12°31’S 49°10’E, 1000 m], Mar. 3, 1996, N. Rabibisoa, D. Rakotomalala, and O. Ramilison. UADBA 5726 (RAN 51382): 34 km South from Maevatanana, Maevatanana District, Betsiboka Region, Madagascar [17°09.092’S 46°51.365’E, 350 m], Jan. 24, 1996, A. Raselimanana, C. J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa.
Diagnosis: A small sized
Ochthomantis (adult male SVL 30.0–31,8 mm; adult female 34.0–37.9 mm) with a sharply defined lateral white to yellow strip along the side of the head and body, and which runs from the snout tip to groin area.
Mantidactylus ambony can be diagnosed from
M. ambreensis by its small size, and the rest of the other subgenus
Ochthomantis by the presence of the sharply defined white to yellow lateral stripe, which is absent in all other species. The
Table 1 and
Table 2 summurizes the character diagnostics of this ressurected species.
Description of
UADBA 8406 (RAX 2871): Adult male (SVL = 31.8 mm) in an excellent state of preservation. Measurements are presented in
Table S4. In dorsal view and lateral view, snout tip relatively pointed. Snout tip with a 1.7 mm ventral extension beyond mouth. In dorsal view, head clearly longer than large (ratio hdw/hdl= 0.74). Head 1.35 times longer than wide. Head length 0.47 times SVL. Canthus rostralis indistinct. Loreal area concave. Tympanum diameter 1.19 times eye. Tympanum rounded, and touched with indistinct supratympanique line along theirs borders, and continues posteriorly to a point above the insertion of the forearm. Large dark tympanum with a small notch in its median superior area. Internarial distance 0.30 times head width. Tongue ovoid anteriorly and bifid in posterior part. Nostrils rounded, and with lateral opening. Eye to nostril distance 1.40 times nostril to snout distance. Forearm length 0.47 times SVL. Hand length (including discs) 0.19 times SVL. Inner metacarpal not obvious and outer metacarpal forms an obvious granule. Fingers not webbed. Relative finger length 1 < 2 < 4 < 3. Digits with large terminal discs (the widest part twice the width of the base. Tibio–tarsal articulation reaches between eye and nostril.Lateral metatarsal separated. Hind limb 1.69 times SVL. Thigh length 0.90 times tibia length. Foot including tarsus 0.73 times SVL. Inner metatarsal tubercule not obvious (length 1.35 mm) at the base of toe 1. Outer metatarsal tubercule absent. Toe with less extensive webbing; with webbing formula: 1 (1) 2i (1) 2e (0.50), 3i (1.25) 3e (1) 4i (2) 4e (1.50) 5 (0.50), and sum of free phalanges is 8.75 (WS). Relative toe length 1 < 2 < 3 < 5 < 4. The bodies: flank, venter, and sacral areas with very small granules, and dorsal with obvious granules. Femoral glands on the ventral surface of thighs are oblong and developed with the medio–proximal area having a pore surrounded by many granules, giving a crater-like form. Internally, the femoral gland is type 3 [
2,
35]. In preservative, the dorsal head, body and limbs are dark. Lateral body less dark than dorsal. Lips with white band colors continuing along the flank to the hind limb insertion. The iris dark surrounding by white ring. Ventral face whitish with dark marbling except in belly region. A pair of dark–brown parallel bands on the throat. Forelimb with some dark spots on the ventral face. Thigh with large dark spots except in the femoral gland region. Dorsal transverse bands, alternating dark and light, on the hind and forelimbs.
Variation: Morphometric variation diagnostic details are summarized in
Table S4. Sexual dimorphism is evident: males have smaller SVL (30–31.8 mm versus 34–37.9 mm), relatively longer tibia and feet, and shorter toe 3. The ratio of td/ed larger in males than females (0.93–1.33
vs 0.59–0.79). The femoral glands are more swollen in males, and are smaller and more circular in females. The free phalanges on the toes vary: toe 1 (0,50–1), internal edge of toe 2 (1–1.25), external edge of toe 2 (0–0.75), internal edge of toe 3 (1.25–1.50), external edge of toe 3 (0.25–1), internal edge of toe 4 (1.50–2), external edge of toe 4 (1–2), and in toe 5 (0–1). Dorsal skin and above the eyes with obvious granules. Granules are also found in some individuals in the sacral area.
Coloration in life: The iris has a gold ring on the outer area. The dorsal body is without vertebral line. A white and yellow band is running along the flank. The dorsal surfaces of the head, body, and limbs may be dark green or brown. The ventral surfaces are usually mottled brown. All individuals have a pair of short longitudinal dark bands on the throat that diverge, and fade on the thorax. Large round dark spots absent on the throat and the thorax. A dark crossbar may be present between the eyes.
Habits: A semi-aquatique forest species living next to quiet river with rocks. It was observed between 11.00–20.00 hours. The male was terrestrial during the day and active in leaf litter. At night, females rest on leaves than on branches next to the calm water between 0-50 cm above the ground. Like to
M. ambreensis, this taxon prefers rivers than small stream. At Montagne d’Ambre (Station les Roussettes), it was observed near the irrigation canals. According [
5], it was rheophilous species and frequently terrestrial by day, sitting on the ground, on rocks, wood, lichen, or hiding under rocks. At night, often observed in height above the water and once up to 2 m height, and sitting on substrates like leaves, rocks, dead wood, and plant stems.
Distribution: Species of low and mid-altitude forests of the North-western and northern of Madagascar, with an elevational range between 300 to 1150 m a.s.l. From relict caduc forest in south of Maevatanana to humid forest at Montagne d’Ambre, extreme north of Madagascar (
Figure 4,
Table S13).
Comments: Our molecular analyses group all our
M. ambony samples with the
M. ambony samples reported by [
5]: holotype ZSM 2078/2007 (FGZC 1039) and paratypes, and their sequence tissus resgistered in zoobank with LSID: 2F3F14D1-6D0E49E5-9646-8167E64CFDF3.
3.2.4. Mantidactylus mocquardi [39]
Mantidactylus mocquardi [
39]: 359 (Holotype MNHN 1929.207, according to [
40]: 50, secondary homonym of
Rhacophurus mocquardi [
41];
Mantidactylus (Mantidactylus) mocquardi: [
30]: 37;
Mantidactylus (Hylobatrachus) mocquardi: [
34]: 312;
Mantidactylus (Ochthomantis) mocquardi: [
7]: 400, [
3]: 3.
Holotype: MNHN 1929.207 collected in Rogez, Moramanga District, Alaotra–Mangoro Region, Madagascar
Specimens examined: AMNH A157111 (APR 234) adult female: Ampanasana Ankolony, Marojejy National Park, Andapa District, Sava Region, Madagascar [14°26.2’S 49°46.5’E, 1300 m], Nov. 1998, A. Raselimanana and D. Rakotomalala. AMNH A157118–119 (APR 351, 354) adult male and female: Andapimbazaha, Marojejy National Park, Andapa District, Sava Region, Madagascar [14°26’S 49°46.7’E, 850 m], the same date and collector as AMNH A157111. AMNH A167583 (RAX3806) adult female and AMNH A167585, 167587* (RAX 3903, 3915) adult males: Bezavona, Vohémar District, Sava Region, Madagascar [13°31.962’S 49°51.954’E, 350 m], Feb. 2 and 8, 2002, S. Mahaviasy, N. Rabibisoa, and C. J. Raxworthy. AMNH A167588 (RAX 4687) adult male: Ankitsika, Vohemar District, Sava Region, Madagascar [13°52’20.6’’S 49°47’02.7’’E, 650 m], Mar. 22, 2002, N. Rabibisoa and S. Mahaviasy. AMNH A167589 (RAX 5298) adult female and AMNH A167597 (RAX 5297) adult male: Sorata, Vohémar District, Sava Region, Madagascar [13º41.986′S 49º26.687′E, 980 m], Apr. 22, 2002, S. Mahaviasy, N. Rabibisoa, C. J. Raxworthy. AMNH A174621* (RAX 3669) adult female: Ambolokopatrika, Andapa District, Sava Region, Madagascar [14°32’18.1’’S 49°26’14.6’’E, 850 m], Nov. 29, 2001, S. Mahaviasy, N. Rabibisoa, N. Rakotondrazafy, A. Razafimanantsoa and A. Razafimanantsoa. AMNH A174622* (RAX 4670): Ankitsika, Vohemar District, Sava Region, Madagascar [13°52’20,6’’S 49°47’02.7’’E, 650 m], Mar. 22, 2002, N. Rabibisoa, S. Mahaviasy, and N. Rakotondrazafy. AMNH A174628* (RAX 7524): Betampona Strict Reserve, Toamasina II District, Atsinanana Region, Madagascar [17°54.858’S 49°12.474’E 350 m], Feb 28, 2004, C. J. Raxworthy, N. Rabibisoa, M. Randriambahiniharime, and F. Ranjanaharisoa. AMNH A174652* (RAX 9022): Ambodiriana, Soanierana Ivongo District, Analanjirofo Region, Madagascar [16°40.469′S 49°42,167′E, 100 m], Mar. 3,2006, N. Rabibisoa. UADBA 7769 (MRJ 107) adult female: Ampanasantongotra, Marojejy National Park, Andapa District, Sava Region, Madagascar [14°26’S 49°46.5’E, 350 m], Oct. 10, 1994, N. Rabibisoa, J. B. Ramanamanjato, and O. Ramilison. UADBA 8118 (NR 285) adult female: Anjanaharibe–Sud Special Reserve, Andapa District, Sava Region, Madagascar [14°44.5’S 49°26.5’E, 1550 m], Nov. 11, 1994, N. Rabibisoa. UADBA 12312–13 (NR 1371–72) adult females: Sandranantitra, Toamasina District, Atsinanana Region, Madagascar [18°2.9’S 49°5.5’E, 450m], Jan. 10, 1999, J. Randrianirina and J. Razafimanantsoa. UADBA 19596 (RAX 3680) adult male: Ambolokopatrika River, Andapa District, Sava Region, Madagascar [14°32’18.1’’S 49°26’14.6’’E, 875 m], Nov. 30, 2001, S. Mahaviasy, N. Rabibisoa, C. J. Raxworthy, A. Razafimanantsoa and A. Razafimanantsoa. UADBA 19647* (RAX 3641) adult female: Ambolokopatrika, Andapa District, Sava Region, Madagascar [14°32’18.1’’S 49°26’14.6’’E, 850 m], Nov. 29, 2001, the same collectors as UADBA 19596. UADBA 26238 (RAX 8021) adult female, and UADBA 26287, 26290 (RAX 7539, 8036) adultes males: Betampona Strict Natural Reserve, Toamasina District, Atsinanana Region, Madagascar [17°54.858’S 49°12.474’E, 390–450 m], Mar. 8–18, 2004, C. J. Raxworthy, N. Rabibisoa, M. Randriambahiniharime, and F. Ranjanaharisoa. UADBA 26240, 26242 (RAN 45476, 45363) adult females, and UADBA 26298 (RAN 45370) adult male: Rangovalo, Zahamena National Park, Fenoarivo Atsinanana District, Analanjirofo Region, Madagascar [17°40.5’S 48°45.5’E, and 17°42’S 48°46’E, 850–1150 m], Feb. 28, 1994–Mar.3, 1994, F. Rabemananjara, J. B. Ramanamanjato, A. Raselimanana, A. Ravoninjatovo, C. J. Raxworthy, J. Razafimanantsoa, A. Razafimanantsoa, and A. Razafimanantsoa. UADBA 26283 (RAN 47954) adult female: Sahamalio, Isalo National Park, Ihorombe Region, Madagascar, [22°26,315’S 45°15,648’E, 700 m], Feb. 18, 1995, J. B. Ramanamanjato, A. Raselimanana, C. J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa. UMMZ 212824* (RAN 37992) juvenile: Manantenina River, closed to Marojejy National Park, Andapa District, Sava Region, Madagascar [14°26’S 49°46’E, 600 m], Nov. 16, 1992, R. A. Nussbaum, C. J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa. UMMZ 21235 (RAN 39291) adult male: Ambalafary, Ambanja District, Diana Region, Madagascar [14°04’S 48°17’E, 250 m], Fev. 24, 1992, C. J. Raxworthy, A. Raselimanana, J. B. Ramanamanjato, A. Razafimanantsoa, and A. Razafimanantsoa; UMMZ 212881* (RAN 42737) juvenile: Ankavanana River, Masoala National Park, Antalaha District, Sava Region, Madagascar [15°18,5’S/50°14’E, 70–100 m], Jan. 12, 1993, C. J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa.
Diagnosis: A medium to large sized
Ochthomantis (adult male 36–48 mm; adult female 44–65 mm). tibio–tarsal articulation reaching between eye and nostril, but sometimes beyond nostril. Body dark brown or black in color (black in preservative) with a few white spots scattered on the lateral body, upper lip of mouth paler brown with dark brown spots that are heaviest posteriorly, and snout tip pointed laterally and extending > 1.75 mm beyond lower jaw.
Mantidactylus mocquardi can be diagnosed from all other subgenus
Ochthomantis species by the blackish body coloration, the white spots along the lateral body, and the dark brown spotting on the pale upper lip. The
Table 1 and
Table 2 summurize the character diagnostics of this current species.
Description of UADBA 19596 (RAX 3680): Adult male (SVL = 39.90 mm) in excellent state of preservation. Measurements are presented in
Table S5. In dorsal view and lateral view snout tip very pointed. Snout tip with a 2.30 mm straight ventral extension beyond mouth. Head 1.52 times longer than wide. Head length 0.47 times SVL. Canthus rostralis obvious. Loreal indented. Tympanum diameter 0.80 times eye. Tympanum rounded with a small notch in its median superior area, and distinct from the supratympanic ridge, which umbrellas the tympanum and continues its final way slightly slanting to above the insertion point of the forelimb, and behind of a large clear granule. Anterior half part of the tympanum is light, and the posterior part is dark. Internarial distance 0.27 times head width. Tongue ovoid anteriorly and bifid posteriorly. Non–protruding nostril with relatively closed lateral opening. Eye to nostril distance 1.59 times nostril to snout distance. Forearm length 0.47 times SVL. Hand length (including discs) 0.29 times SVL. Fingers not webbed. Outer and inner metacarpals poorly developed. Finger relative length size 1 <2 <4 <3. Digits with large terminal disc (the widest part twice the width of the base of the disc). tibio–tarsal articulation reaches the nostril. Lateral metatarsal separated. Hind limb 1.71 times SVL. Thigh same length as tibia. Foot including tarsus 0.72 times SVL. Inner metatarsal tubercule in bell-like (length 1.6 mm) at the base of the toe 1. Outer metatarsal tubercule absent. Webbing formula 1 (0), 2i (1), 2e (0), 3i (1), 3e (0), 4i (1.5), 4e (1), 5 (0) and sum of free phalanges is 4 (WS). Relative Toe length 1 <2 <3 <5 <4. The importance and the repartition of the granule bodies vary in shape and color: side and edge of the dorsal face, and above the tympanum highly granulated; inguinal area, basal of the flanks, and posteriorly of the upper mouth with white evident granules; back with little striking, and belly finely granular. Femoral glands on on ventral surface of thighs are oblong and relatively developed with centro–distal area having a pore surrounded by many granules, giving a crater-like form. Internally, the femoral glands are type 3 [
2,
35]. In preservative, the dorsal face is black. Upper lip and flanks are dotted with clear spots. Throat and thorax with white and some silver reticulated dark brown pigments, and belly light yellow color. Thorax with parallel dark bands: in “
X-like
” in the left side and divided into two forms, spot and “
+/–like
” in his right side. The ventral side of the femur is partially mottled brown; which is weak in the femoral gland. The lower part of the hind limb completely pigmented. The ventral side of forelimb is clear yellowish. Evident dorsal transverse bands alternating Clear and dark on hindlimb, and unclear on forelimb, which has some pinkish reticles indifferently distributed in its dorsal face.
Variation: Morphometric variation diagnostic details are summarized in
Table S5. Sexual dimorphism is evident: males have smaller SVL (36.10–48.15 mm
vs 44.30–65.40 mm) with large eyes, higher head, broader terminal disc, shorter tibia and slithly developed nostril. Colors vary from blackish brown (e.g holotype, UADBA 12312, 26211, 26238, 26287, 26290, AMNH A157111, A157118, A167583, A167585) to full black one (e.g., UADBA 7769, 12313, 19647, 26240, 26242, 26298, AMNH A167588–89). The differences are also reflected in the amount of granules and pigments between these two groups of specimens: black specimens 1) granules are almost absent and body almost smooth except at the flanks, 2) stains rounded or other shapes are missing, 3) upper mouth and flank no silver and nor white spots, 4) the ventral face dark brown except in the belly that is clear; blackish brown specimens 1) granules very important in holotype 2) flanks with evident granules, 2) Some specimens with dorsal dark spots (UADBA 12313, 26238, 26240, 26290), 3) Flanks, upper mouth, throat, thorax with obvious spots, and 4) Thorax and throat dark browns with mottled white or silver spots, and belly no spot. Periphery of the tympanic region, smooth in male and finely granular in female. In male tympanum and supratympanic touch each other, except the holotype. In addition, free phalanges on toes varies: toe 1 (0–0.50) internal edge of toe 2, (0.50–1), internal edge of toe 3 (1–1.25), external edge of toe 3 (0–0.50), internal edge of toe 4 (1–2), and external edge of toe 4 (1–1.75).
Coloration in life: The iris has a golden ring on the outer area. The dorsal body is dark brown or completely black. Upper mouth dark brown or blackish. Whitish grey spots maybe present or not in inguinal region. Ventral face very heterogeneous: throat and thorax usually colored black with small white spots, and belly no pigmented; throat with two dark spots or without spots. Flank unicolor with evident or not white spots, and few granules. The ventral face of the thigh has at least some clear surface and no brown pigment. The dark cross band in rod-like are absent between the eyes, but may be present or not in dorsal with black mark in V or Y-shaped. Hind and forelimbs with alternating bands, black and brown.
Habits: A semi–aquatic rainforest species but can live in open and degraded forest especially in northern of Madagascar. This species living along riverbank and streams with the different stage of speeds between 9.00 a.m to 23.00 p.m. However, it appears rather diurnal than nocturnal. It prefers rocky than trees, and resting on the banks of the rivers. On tree, it prefers resting on leaves and branches.
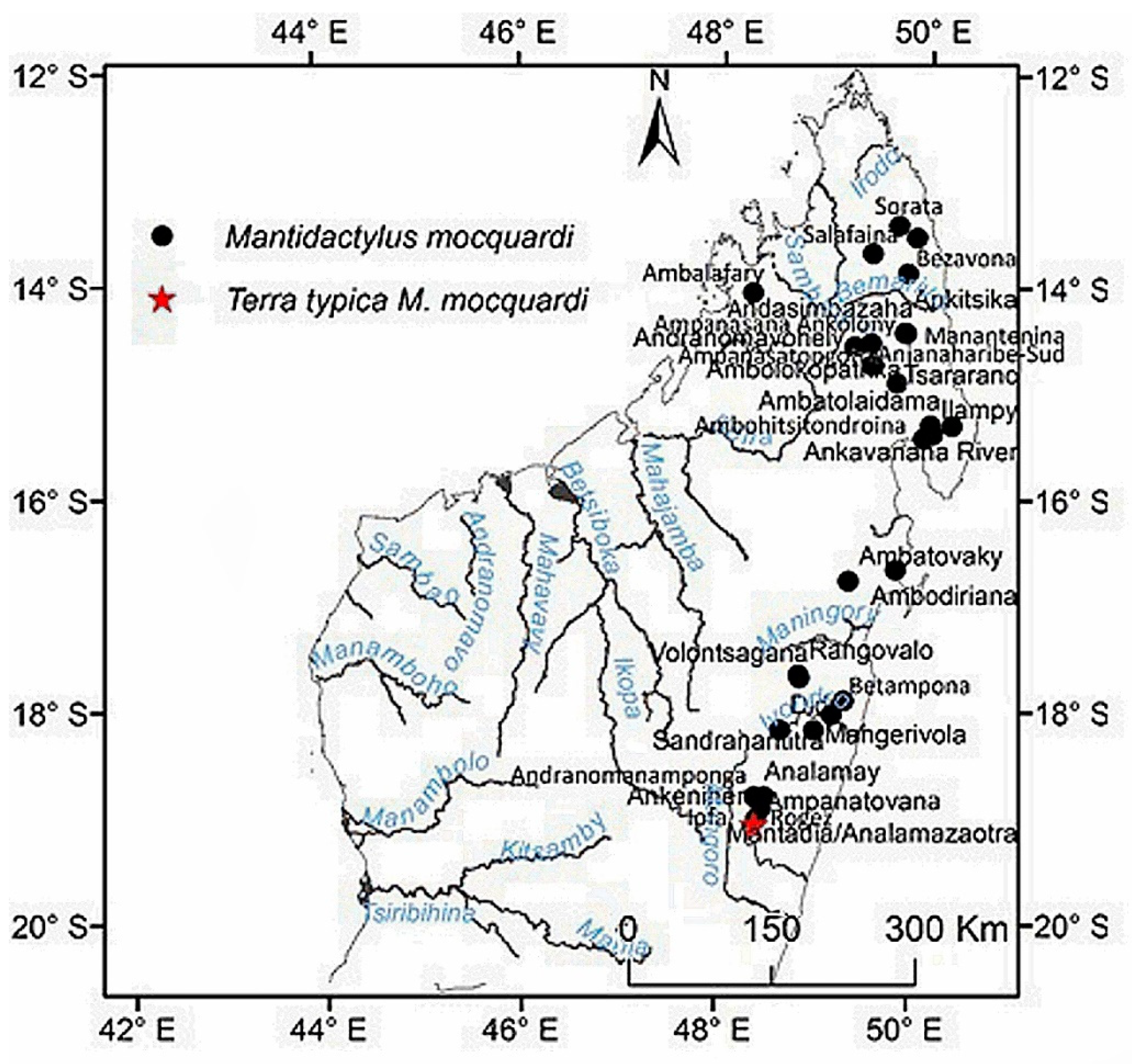
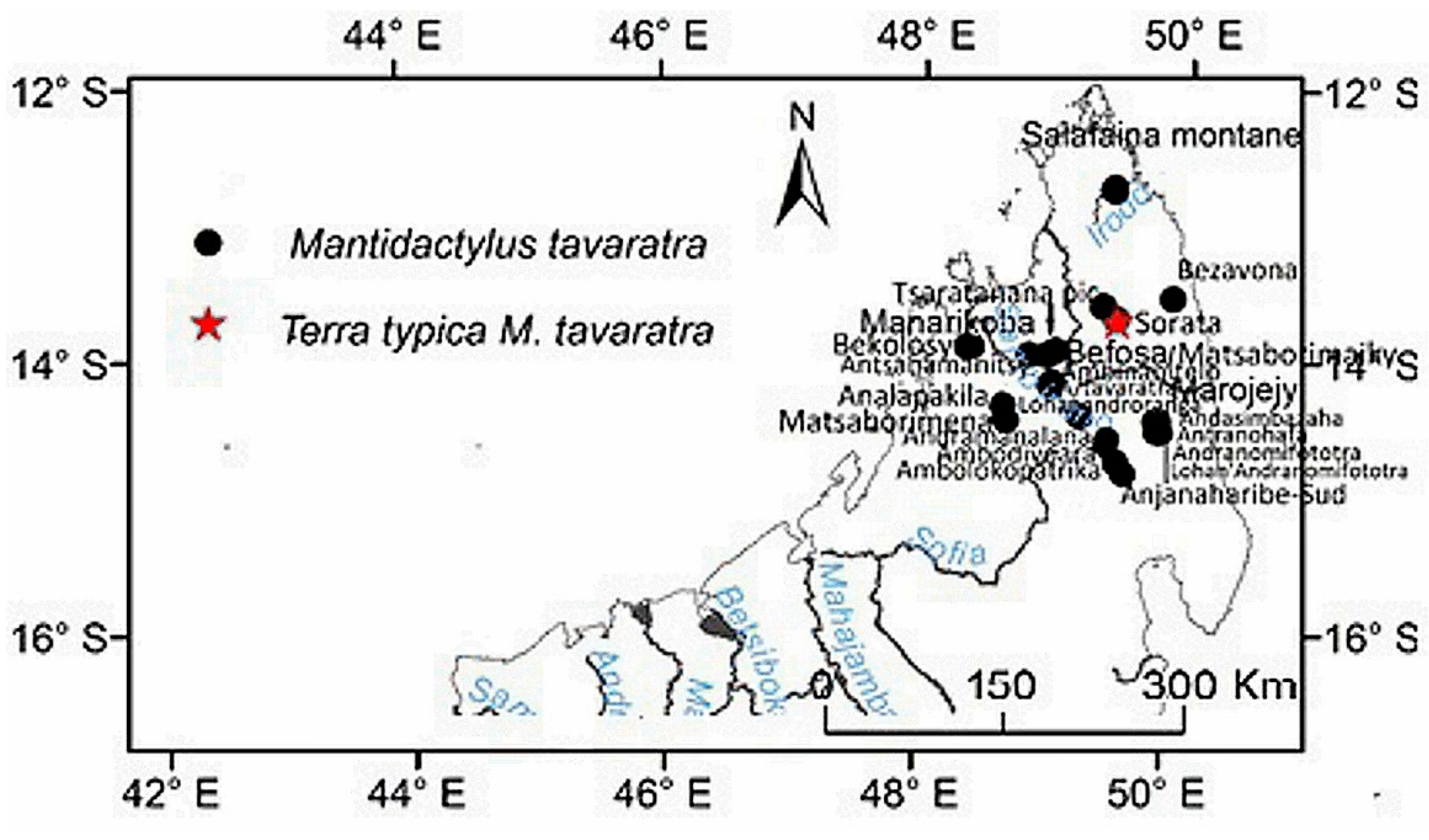
Distribution: Species of low and mid–altitudes in the Central–eastern, and northern forest of Madagascar, from 100 to 1550 m elevations (
Figure 5,
Table S13).
Comments: Our morphological description agrees with the
M. mocquardi specimen shown by [
36] (
Figure 1, p. 249) from Andasibe, and our molecular analyses group all our
M. mocquardi samples with the
M. mocquardi samples reported by [
10]: AF215317 (ZFMK 66668) from Ambato, Masoala; and [
11]: HQ610861 (ZSM 1846/2007) and HQ610921 (ZCMV 8818) from An’Ala and Mahasoa.
3.2.5. Mantidactylus zolitschka [10]
Holotype: ZFMK 60110: closed to An’Ala Forest [18°56′S 48°28′E, 840 m], Mar. 21, 1995, F. Glaw and D. Vallan.
Paratype: ZFMK 60112–60116, ZSM 939/2000, same data as holotype. ZSM 184/2003, same location as holotype, Mar. 2, 2003, G. Aprea, F. Glaw, M. Puente, L. Raharivololoniaina, R. D. Randrianiaina, and M. Thomas.
Specimens examined: UADBA 6965–66, same data as holotype.
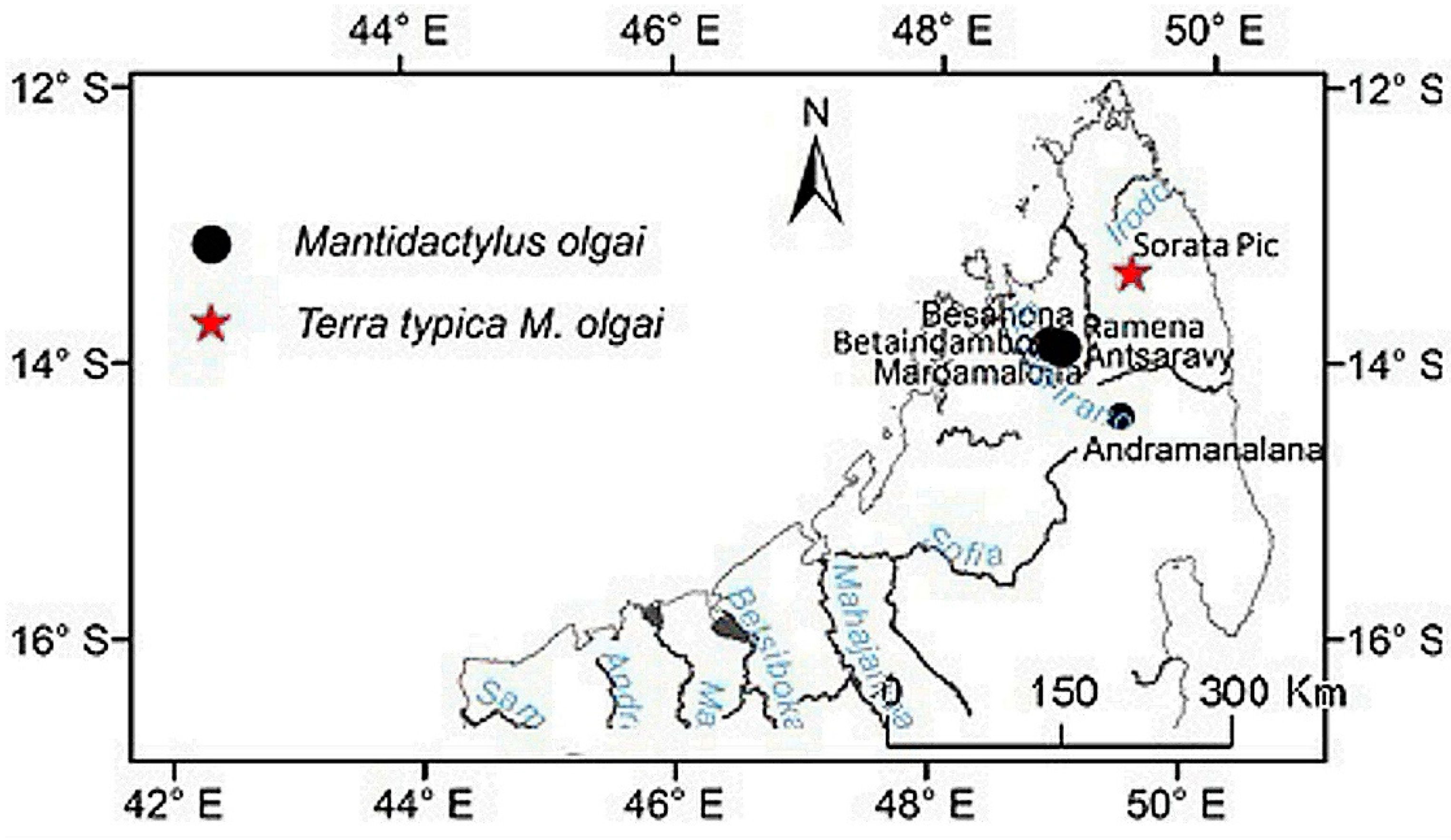
Diagnosis: A small sized
Ochthomantis (adult male SVL 29.6–30.6 mm, adult female SVL 37.6–37.7 mm). tibio–tarsal articulation reaching at least up to nostril. The width of the terminal disk 1.78 times disk base.
Mantidactylus zolitschka can be distinguished from all other subgenus
Ochthomantis species by its smaller size (adult male SVL < 31 mm, female < 38 mm), and foot with less well developed webbing (sum of free phalanges, WS > 9). The
Table 1 and
Table 2 summurizes the character diagnostics of this current species.
Desription of
UADBA 6966: Adult male (SVL= 27.65 mm) in good state. Measurements are presented in
Table S6. In dorsal view, body clearly slender. In lateral and dorsal view, snout tip pointed. Snout tip with a 1.20 mm ventral extension beyond mouth. Head 1.40 times longer than wide. Head length 0.47 times SVL. Canthus rostralis distinct and straight. Loreal weakly concave. Tympanum diameter 0.83 times eye diameter. Tympanum rounded and distinct to supratympanic fold, which continue starting straight with a rather distinct bend midway before towards the insertion point of the forelimb. Internarial distance 0.24 times head width. Tongue ovoid anteriorly and distinctly bifid posteriorly. Nostril small rounded and without protuberant lateral opening. Eye to nostril distance 1.44 times nostril to snout distance. Forearm length 0.52 times SVL. Hand length (including discs) 0.33 times SVL. Fingers not webbed. Inner and outer metacarpal tubercules present. Fingers not webbed. Relative finger lenght 1<2<4<3. Digits with slightly enlarged terminal discs (the widest part 1.78 times the width of the base). Legs slender, tibio–tarsal articulation reaches nostril. Lateral metatarsal separated. Hind limb 1.85 times SVL. Thigh same length as tibia. Foot including tarsus 0.78 times SVL. Inner metatarsal tubercule rather small (0.85 mm) at the base of toe 1. Metatarsal tubercule present. Webbing formula: 1(1), 2i (1.25), 2e (1), 3i (1.5), 3e (1), 4i (2), 4e (2), 5 (1), and sum of free phalanges is 9.75 (WS). Relative toe lenght 1<2<3<5<4. Skin rather smooth in its upper surface, and slightly granular on flanks. Ventral side smooth. The femoral glands on the ventral surface of thighs are obvious in contact in anal area, and which are sharply delimited by granules with irregular tubercle-like, and a mediane porus, giving a crater-like form. Internally, the femoral gland is type 3 [
2,
35]. In preservative, the dorsal is a grey-brownish with irregular dark, and light marblings. Upperlip and loreal area whitish. Tympanic region dark brown. The lower lip is indistinctly alternating with light, and dark spots. Sharp border between dark flanks and light ventral coloration, giving an overall impression of an irregularly flanks pattern. Ventral face varies: throat whitish, and becomming more yellowish on belly. Throat with two longitutidal brown marking from lip to thorax, and both merge at the pectoral girdle as “Y-like”. One light longitutdinal stripe runs from the inguinal area along dark brown flanks fading towards the forelimb insertion. Forelimbs, hands, hind limbs, and feet light brown with dark crossbands (about six crossbands on forelimb and hand includind third finger, four on femur, three on tibia, and five on tarsus and foot). Hindlimbs with irregular dark mottlings.
Variation: Morphometric variation diagnostics are summarized in
Table S6. Sexual dimorphism is evident: males have smaller SVL (26.5–30.6 mm
vs 33.6–37.7 mm), and large tympanums. The free phalanges on toes vary: toe 1 (0.5–1); internal edge of toe 2 (1–1.5); external edge of toe 2 (0.5–0.75); internal edge of toe 3 (1.75–2); external edge of toe 3 (0.75–1); external edge of toe 4 (1.75–2), and in toe 5 (0.5–0.75).
Coloration in life: The iris has a golden ring on the outer area. The body with a strong natural coloring. The dorsal body has a small light stripe. There is a clear band of bright yellow in the inguinal region.
Habits: A semi-aquatic rainforest species living close to stream, around An’Ala Forest. The female ZFMK 30116 contains 49 eggs with yellow and dark brown center markings, diameter 2 mm [
10].
Comments: We included genetic data for this species from [
11]: HQ610866 (ZSM 1768/2007) and HQ610867 (ZSM 1841/2007).
3.3. Ressurected species
Based on our molecular and morphological results, we find strong evidence to recognize two species of
Mantidactylus (Ochthomantis) that correspond to taxa that currently are considered as junior synonyms of
M. femoralis. After examining their type specimens and our new materials, we here recognize
Mantidactylus catalai [
31] (33 specimens) and
Mantidactylus poissoni [
32] (12 specimens) as valid species, and provide new descriptions for both species below (
Figure 6).
3.3.1. Mantidactylus catalai [31]
Mantidactylus catalai [
31] (p. 203) (Holotype MNHN 1935.153, according to the original publication and [
6] (p. 220);
Mantidactylus femoralis: [
27] (p. 26);
Mantidactylus (Hylobatrachus) femoralis: [
34] (p. 312);
Mantidactylus (Ochthomantis) femoralis: [
7] (p. 400), [
3] (p.3).
M. catalai is previously considered by [
27] as synonym of
M. femoralis, but [
10] had noted the considerable morphological differences between
M. catalai, and
M. femoralis of the southeast of Madagascar. However, lack of sufficient biological material they cannot pronounce.
Holotype: MNHN 1935.153: Isaka–Ivondro, Tolagnaro District, Anosy Region, Madagascar, 700 m, 1935, M. R. Catala. Specimen in good condition.
Specimens examined: AMNH 7881–82 A133689–90, AMNH 18019 A168364: Fianarantsoa–Ifanadiana Road, Southwest Ranomafana, Ifanadiana District, Vatovavy–Fitovinany Region, Madagascar, 900 m. AMNH A 181732*, A 181821* (RAX 10563, 10599): Beampingaratsy Pass, Anosy Montain, Tolagnaro District, Anosy Region, Madagascar, [24°28.244’S 46°53.521’E, 490 – 1140m], Feb. 12–13, 2009, S. Mahaviasy, N. Rakotondrazafy, and C. J. Raxworthy. UADBA 1419–21, 1423 (RAN 36377, 36434, 36446, 36505): Ampasimekieny Pass, Tolagnaro District, Anosy Region, Madagascar [24°32.0’S 46°51.0’E, 800–950 m], Dec. 24–28, 1990, J. B. Ramanamanjato, A. Raselimanana; C. J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa. UADBA 3706 (RAN 35091): Manatantely Forest, Tolagnaro District, Anosy Region, Madagascar [29°59.0’S 46°55.083’E, 125 m], October 30, 1990, same collectors as UADBA 1419. UADBA 4513–14; 4516, 4521, 4523 (RAN 52831, 52699, 52762, 52807, 52830): Eminiminy, Andohahela National Park, Tolagnaro District, Anosy Region, Madagascar [24°35.04’S 46°44.08’E, 1000–1100 m], Nov. 11–15, 1995, J. B. Ramanamanjato, and A. Raselimanana. UADBA 4522 (RAN 52472): Ambinany, Andohahela National Park, Tolagnaro District, Anosy Region, Madagascar [24°35.6’S/46°44.3’E, 820 m], Nov. 14, 1995, J. B. Ramanamanjato, and A. Raselimanana. UADBA 9772–74; 9782 (RAN 57002, 56723, 56937, 57006): Amorimbato Forest; Kalambatritra Special Reserve, Iakora District, South–Est Region, Madagascar [23°27.44’S 46°20.02’E, 1150–1300 m], Oct. 30–Nov. 8, 1996, J B Ramanamanjato, R. A. Nussbaum, and J. Spannring. UADBA 26403–05 (RAN 44835, 44672, 44701): Sahavatoy and Volontsagana Rivers, Andringitra National Park, Ihorombe Region, Madagascar [22°13.667’S 47°0.217’E, 810–1240 m], Nov. 24–30, 1993, N. Rabibisoa, A. Razafimanantsoa, and C. J. Raxworthy. UMMZ 191515–16 (RAN 32567, 32597): Sainte Luce, Tolagnaro District, Anosy Region, Madagascar [24°45’S 47°11’E, 20 m], Oct. 7 and 10, 1989, R. A. Nussbaum, and C. J. Raxworthy. UMMZ 197662–64 (RAN 35686, 35706–07): Nahampoana, Tolagnaro District, Anosy Region, Madagascar [24°58’S 46°58’E, 75–300 m], Nov. 23–24, 1990, R. A. Nussbaum, J. B. Ramanamanjato, and A. Raselimanana. UMMZ 197676 (RAN 36626): Manangotry, Tolagnaro District, Anosy Region, Madagascar [24°45’S 46°52’E, 850 m], Jan. 3, 1991, J. B. Ramanamanjato, A. Raselimanana, and C. J. Raxworthy. UMMZ 212890* (RAN 44491): Iatara river, Andringitra National Park, Ivohibe District, Atsimo Atsinanana Region, Madagascar [22°13.333’S 47°01.483’E, 720 m], Nov. 18, 1993, same collectors as UADBA 26403.
Diagnosis: A medium to large sized
Ochthomantis (adult male SVL 41–45 mm, female SVL 51–62 mm). tibio–tarsal articulation reaching between eyes and nostril (or very rarely at snout), toes fully webbed to discs, except on toe 4 where 1–1.5 phalanges are free, no stripe line along the superior lip and clear area in the inguinal region, snout tip very pointed in lateral view with a large extension beyond the mouth (1.75–3.45 mm), and the head wider and flattened but very sharp as “fish–like”.
Mantidactylus catalai can be distinguished from the other
Ochthomantis species by the following:
M. ambreensis by the absence of white stripes along the side of the body;
M. femoralis,
M. zolitschka, and
M. danieli by the number of free phalanges in the internal edge of toe 4 (1–1.5), and absence of the prominent pale yellow or white stripes in the inguinal region (horizontal or oblique);
M. mocquardi by the number of free phalanges in the internal edge of toe 4 (1–1.5), and flanks without whitish spots;
M. olgai by the absence of the obvious black granules on the flanks, and in preservative the absence of crossbars in V or Y–shaped on the back;
M. tavaratra by digits with large terminal discs (the widest part > 1.80 times the width of the disc base), the lack of a prominent pale inguinal streak, and the absence of a white strip on the superior lip;
M. poissoni by the absence of white spots below the eye, and tibio–tarsal articulation reaching between eyes and nostrils; and
M. macrotympanum by the smaller adult male SVL (< 60 mm). The
Table 3 and
Table 4 summurize the character diagnostics of this ressurected species.
Description of
UADBA 1419 (RAN 36377): Adult male (SVL = 42.60 mm) in good state of preservation. Measurements presented in
Table S7. In dorsal and lateral view, snout tip pointed. Snout tip with a 2.60 mm ventral extension beyond mouth. Head 1.40 times longer than wide. Head length 0.50 times SVL. Canthus rostralis well distinct. Loreal region with evident indentation. Tympanum diameter 1.03 times eye. Tympanum slightly round and touch from the supratympanic along their borders except the porterior part, which continues posterioly to a half of the tympanum, and then split obliquely to above a small granule, and reaching the insertion point of the forelimb. Tympanum with a small notch in its median superior area. Internarinal distance 0.27 times head width. Tongue ovoid anteriorly and bifid posteriorly. Nostrils with distinct cutaneous fold, and with lateral oblique opening. Eye to nostril distance 1.58 times nostril to snout distance. Forearm length 0.61 times SVL. Hand length (including discs) 0.42 times SVL. Fingers not webbed. Inner and outer metacarpal tubercules evident. Fingers not webbed. Relative fingers lenght 1 < 2 < 4 < 3. Digits with large terminal discs (the widest part 2.07 times the width of the base). tibio–tarsal articulation reaches between eye–nostril. Lateral metatarsal separated. Hind limb 1.84 times SVL. Thigh same length as tibia. Foot including tarsus 0.78 times SVL. Inner metatarsal tubercule obvious (2.15 mm), along of toe 1. Outer metatarsal tubercule in small granule–shapped. Webbing formula:
1 (0.75)
2i (1)
2e (0.25),
3i (1.25)
3e (0.25)
4i (1.5)
4e (1)
5 (0), and sum of free phalanges is 6 (WS). Relative toe lenght 1 < 2 < 3 < 5 < 4. Body granules vary: flank with obvious granules; dorsal with granules irregularly distributed, which is more concentrated above the superior part of tympanum, and sacral area, and belly finely granulated. Femoral glands on ventral surface of thighs are oblong and relatively developed, with medio–proximal portion having a pore surrounded by many granules, giving a crater-like form. Internally, the femoral gland type 3 [
2,
35]. In preservative, the body brown clear with white longitudinal vertebral band running from the snout tip to anus. Lips with two vertical light stripes on the loreal, and a clear band continue behind the eyes. Ventral face orange–brown and clear part. A pair of parallel dark–browned spots on the throat (in front of the scapular belt). Inguinal region with white spot or not. Forearm with clear ventral face bordered by brown–sided. Fore and hindlimbs with obvious alternating crossed band, dark and clear.
Variation: Morphometric variation diagnostic details are summarized in
Table S7. Sexual dimorphism is evident, males have smaller SVL (41.10–45.40 mm versus 51.85–61.50 mm), wider head, bigger tympanum, longer fore and hindlimbs, and metatarsal tubercule is not evident. The ratio of td/ed in males than females (0.89–1.08
vs 0.50–0.69). The femoral glands are more swollen in males, and are smaller and more circular in females. A majority of all specimens are brown darker except the holotype, and UADBAs (1419–21, 4513, 9773, 26403–04) which are brun lights. In dorsal view, granules in plate-like are observed except for UADBAs (4523, 26403, 26405). A vertebral line is absent except for UADBAs (1419, 4513, 4523). The throat with white spots in males except for UDBAs (1419, 1421). The free phalanges of toes vary: toe 1 (0–1), internal edge of toe 2 (1–1.25), external edge of the toe 2 (0–0.25), internal edge of toe 3 (1–1.25), external edge of toe 3 (0–0.50), internal edge of toe 4 (1–2), external edge of toe 4 (1–1.50) and in toe 5 (0–0.25).
Coloration in life: Dorsal body is dark brown. Superior lips with light dots. Round tympanum with central dark color surrounded by darkness background. Male without yellowish vertebral line. There is a small spots or not in inguinal region with or not small spots. A reddish brown ventral face with smalll spots, and darker punctuations.
Habits: Semi-aquatic rainforests species living in bamboo forests. This species is a diurnal and/or nocturnal observed between from 9.00 a.m to 0.15 a.m, which is adapted to a “burrowing life” through holes, and interstice of rocks but closed to the waters (stagnant water to river but very rarely in fast stream). It is mainly observed on rocks, and sometimes on a ground, and especially during the day. No indivuduals seen on leaves, branches, and one individual UADBA 4521 collected on roots.
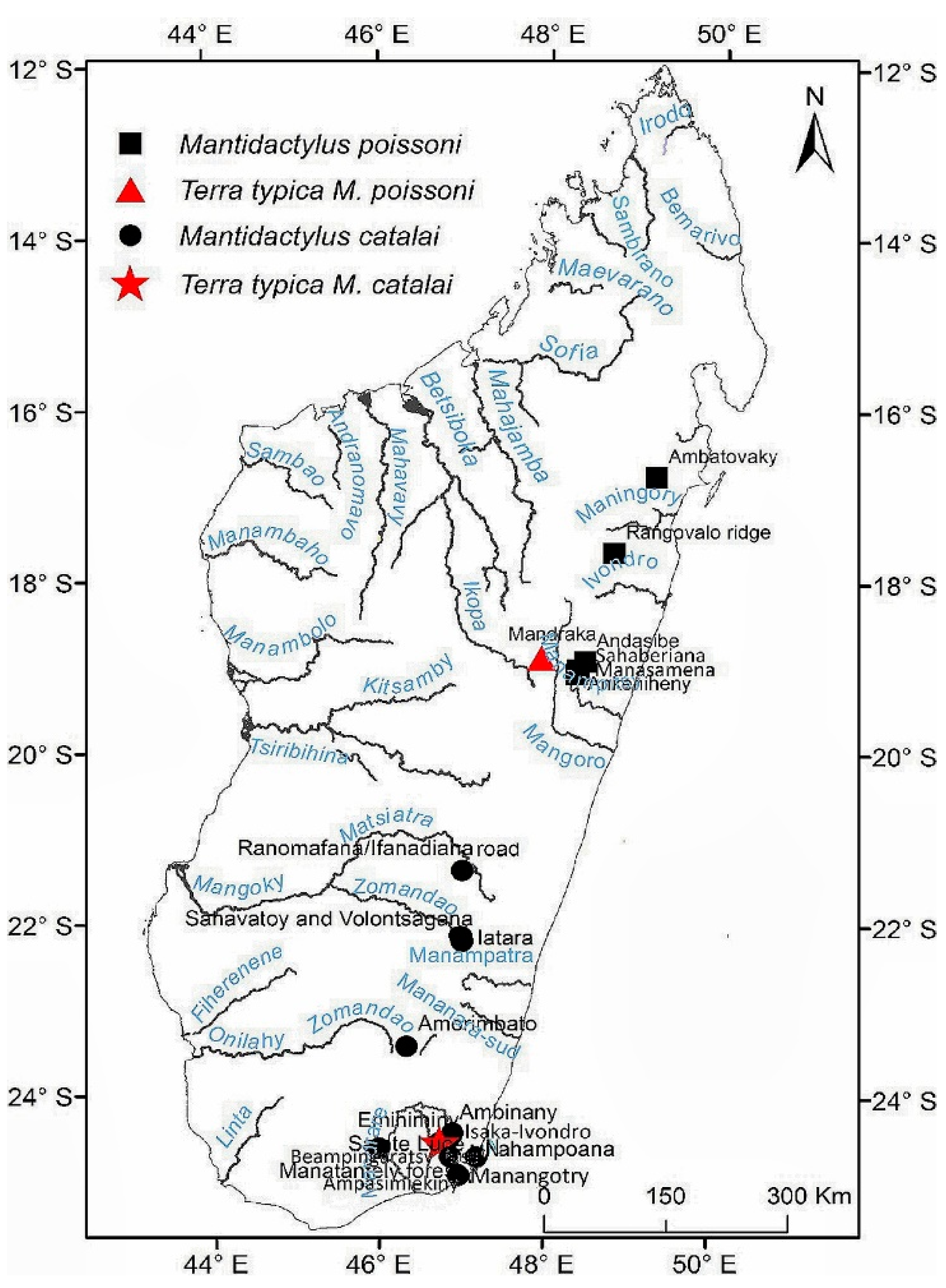
Distribution: known only from rainforest in southeast of Madagascar, at low and medium elevations (
Figure 7,
Table S13):
Comments: Our morphological description agrees with the
M. sp. aff.
mocquardi shown by [
36] (
Figure 3, p. 249) from Ambatolahy, near Ranomafana, and our molecular analyses group all our
M. catalai samples (
Table S1) with the Ranomafana
M. cf.
mocquardi sample reported by [
11]: AY324821 (FGMV 2002.173). This specimen has more recently been referred to as ‘Confirmed Candidate Species (CCS) sp. 47′ [
11].
3.3.2. Mantidactylus poissoni [32]
Mantidactylus poissoni [
32] (p. 178) (Holotype MNHN 1937.1)
Mantidactylus femoralis: [
27] (p. 26);
Mantidactylus (Hylobatrachus) femoralis: [
34] (p. 312);
Mantidactylus femoralis: [
42]: (p. 278);
Mantidactylus (Ochthomantis) femoralis: [
7] (p. 400), [
3] (p. 3).
Holotype: MNHN 1937.1: Mandraka forest, 70 km from Antananarivo, Manjakandriana District, Analamanga Region, Madagascar, 1937, collected by M. H. Poisson. [
27] (p. 45) reports that this type is in a poor state of preservation, requiring its replacement by a neotype from same closed locality.
Neotype: AMNH A174653* (RAX 9367): Mandraka, Manjakandriana District, Analamanga Region, Madagascar, [18°54.727′S 47°55.174′E, 1250 m], Mar. 18, 2006, N. Rabibisoa, J. Rafanomezantsoa, N. A. Rakotondrazafy, and P. Razafimahatratra. Same locality as the holotype
Specimens examined: MNHN 1937.1: Mandraka forest, 70 km from Antananarivo, Manjakandriana District, Analamanga Region, Madagascar,1937, M. H. Poisson. AMNH A50362 adult male: Madagascar, 1971, Guibé. AMNH A174649-50 (RAX 8198–99) adult females: Manasamena River, Lakato, Moramanga District, Alaotra Mangoro Region, Madagascar [19°02’38.2’’S 48°20’54.6’’E 950 m], Mar. 29, 2004, N. Rabibisoa, M. Randriambahiniarime, and F. Ranjanaharisoa. AMNH A174653* (RAX 9367): Mandraka, Manjakandriana District, Analamanga Region, Madagascar [18°54.727′S 47°55.174′E, 1250 m], Mar. 18, 2006, N. Rabibisoa, J. Rafanomezantsoa, N. A. Rakotondrazafy, and P. Razafimahatratra. UADBA 6876, 7125 adult females: Ankeniheny and Andasibe, Moramanga District, Alaotra Mangoro Region, Madagascar [19°05.850’S 48°19.910’E, 950 m, and 18°57’S 48°26’S, 900 m], Dec. 28, 1994 and Dec. 15, 1997, N. Rabibisoa, and S. Ramilison. UADBA 11899 (NR 1196) adult male: Sahaberiana, Mantadia National Park, Moramanga District, AlaotraMangoro Region, Madagascar [18°47,503’S 48°25,572’E, 895 m], Nov. 20, 1998, J. Rafanomezantsoa, and N. Rabibisoa. UADBA 19786 (LV77) subadult female: Ambatovaky Special Reserve, Soanierana Ivongo District, Analanjirofo Region, Madagascar [16°46.910’S 49°14.417’E, 600 m], Aug. 5, 1999, by N. Rabibisoa, and S. Ramilison. UADBA 26409 (RAN 45665) adult female: Rangovalo Ridge, Zahamena National Park, Fenoarivo Atsinanana District, Analanjirofo Region, Madagascar [17°40.5’S 48°45.5’E, 1150 m], Mar. 4, 1994, J. B. Ramanamanjato, A. Raselimanana, C. J. Raxworthy, and A. Razafimanantsoa. UADBA 26411–12 (RAX 8190, 8155) adult females: Manasamena River, Lakato, Moramanga District, Alaotra Mangoro Region, Madagascar [19°02.637’S 48°20.910’E 950 m], Mar. 29 and 27, 2004, N. Rabibisoa, M. Randriambahiniarime, and F. Ranjanaharisoa. UADBA 39000 (RAX 9368) adult male: same data as neotype.
Diagnosis: A medium to large–sized
Ochthomantis (adult male SVL 39.7–48.2 mm, adult female 53–66 mm). tibio–tarsal articulation reaching at least nostril. The width of the digit terminal disk ≥1.70. There is a large white spot under the eye for the females, and numerous white spots for the males, which do not fuse to form a pale continuous line on the superior lip.
Mantidactylus poissoni can be distinguished from all other subgenus
Ochthomantis species by the following combination of characters:
M. ambreensis by a lack of white continuous lateral line on the side the head and body;
M. femoralis by the absence of a prominent pale inguinal patch or line, and absence of a white stripe on the upper lip;
M. mocquardi by the lack of white spots along the lateral body, and the body coloration neither black nor very dark brown;
M. catalai by the presence of white spots below the eye, and tibio–tarsal articulation reaching at least nostril;
M.
olgai by the absence of black granules on the dorsal head and body flanks;
M. tavaratra and
M.
danieli by the presence of white spots below the eye;
M. zolitschka by its large adult size (SVL ≥ 39.7 mm); and
M. macrotympanum by its smaller adult male size (SVL < 49 mm). The
Table 3 and
Table 4 summarize the character diagnostics of this ressurected species.
Description
of UADBA 39000 (RAX 9368): Adult male (SVL = 48 mm) in an excellent state of preservation. Measurements are presented in
Table S8. In dorsal view and lateral view, snout tip relatively obtuse. Snout tip with a 1.35 mm ventral extension beyond mouth. Head 1.25 times longer than wide. Head length 0.45 times SVL. Canthus rostralis distinct. Loreal with groove. Tympanum diameter 0.98 times eye. Slightly round tympanum with a clear small notch in its medium superior part and in contact from supratympanic in the anterior part, and separate in the posterior, which continues to before the insertion point of the forelimb. Internarial distance 0.25 times head width. Tongue ovoid anteriorly and bifid posteriorly. Nostrils rounded distinctly in cutaneous fold, and with lateral opening. Eye to nostril distance 1.81 times nostril to snout distance. Forearm length 0.49 times SVL. Hand length (including discs) 0.28 times SVL. Fingers not webbed. Inner and outer metacarpals present. Fingers not weebed. Relative finger length 1 <2 <4 <3. Digits with large terminal discs (the widest part > 1.70 times the width of the base). tibio–tarsal articulation reaches between nostril and snout tip. Lateral metatarsal separated. Hind limb 1.76 times SVL. Thigh same length as tibia. Foot including tarsus 0.72 times SVL. Inner metatarsal tubercule shield–shapped (length 2 mm) at the base of the toe 1. Outer metatarsal tubercule in small granule. Webbing formula: 1 (0), 2i (1), 2e (0), 3i (1.25), 3e (0.50), 4i (1.75), 4e (1.75), 5 (0.25), and sum of free phalanges is 6.50 (WS). Relative toe lenghts 1 < 2 < 3 < 5 < 4. The body flank and dorsal completely with granules. Femoral glands on ventral surface of thighs are elongated, and a little swollen with the medio–distale area having a pore surrounded by many granules, giving a crater-like form. Internally, the femoral glands are type 3 [
2,
35]. In preservative, dorsal blackish brown. Upper lip with clear tranverse and interrupted band oriented to the eyes. Belly and ventral side of the femur with yellowish white spots. Obvious white pigments in border of the thorax, and form together with abdomen an 8-like. Thorax and throat almost pigmented by whites with a few scattered brown spots, and Thorax with two brownish parallel dark bands, which open laterally at the insertion of the forearm. The inguinal region has a whitish marked in L-shaped bed. Dorsal tranverse bands rather indistinct on the hind and forelimbs.
Variation: Morphometric variation diagnostic details are summarized in
Table S8. Sexual dimorphism is evident: males have a smaller SVL (39.7–46.8 mm), thicker snout, shorter hand, larger terminal disc, and loreale less elongated. The ration of td/ed in males than females (0.62-0.82
vs 0.51-0.68). tibio–tarsal articulation reaching between nostril and snout tip except UADBAs (19786, 26411, 26412), and AMNH A174650 beyond snout tip. The femoral glands are more swollen in males than females, and are smaller in females. The free phalanges on the toes vary: toe 1 (0–0.75), external edge of toe 2 (0–0.25), internal edge of toe 3 (1–1.50), external edge of the toe 3 (0–0.25), internal edge of the toe 4 (1.25–1.75), external edge of the toe 4 (1–1.50), and in toe 5 (0–0.25).
Coloration in life: The iris has a golden ring on the outer area with few black spots. The dorsal body has more small granules, and dark brown color. There is a more or less rounded yellow spots in the inguinal area in females, and stick-like in males. Ventral pigmented by white color with a yellowish border bands in 8–shaped. Hind and fore limbs with alternating dark and clear brown transverse bands.
Habits: A semi-aquatic rainforest species living close to small streams with rock. Stream depth not reaches 1 m. All specimens were observed during the day and night between 16.00 pm to 21.30 pm. In daytime observed between 5–10 m far from the bank on the ground, and at night 3–5 m far from the river, and roosting on leaves between 50–100 cm height. UADBA 7125 seen on rocks in the middle of the river, where water is very speed, maybe there is here by misfortune. It is rather than ground and tree–dwelling frogs than aquatic.
Distribution: Mid and high elevations of the Eastern slope forest of Madagascar (
Figure 6,
Table S13).
Comments: The only genetic data known for this species is from the neotype AMNH A174653 (RAX 9367) (
Table S1).
3.4. New species descriptions
Based on our molecular and morphological results, we find strong evidence to recognize four species of
Mantidactylus (Ochthomantis) that correspond to taxa that cannot be assigned to any exisiting described species (including taxa previously considered as junior synonyms). After examining our new materials, and developing diagnoses for each taxon, we here provide descriptions for each of these new species, such as
Mantidactylus danieli n.sp (54 specimens),
M. macrotympanum n.sp (5 specimens),
M. olgai n.sp (60 specimens), and
M. tavaratra n.sp. (150 specimens) (
Figure 8).
3.4.1. Mantidactylus danieli, new species
Holotype: AMNH A167590 (RAX 4268) adult female collected 22 Febuary 2002 at Salafaina Forest, District Vohemar, Sava Region, Madagascar, 400 m, 13°26.257′S 49°43.001′E by S. Mahaviasy and N. Rabibisoa.
Paratypes: AMNH A167523 (RAX 6595) juvenile collected 2 April 2003 at the relict Irony Forest, Antsohihy District, Sofia Region, Madagascar, 950 m, 14°45.140’S 48°29.690’E by S. Mahaviasy, N. Rabibisoa, N. Rakotondrazafy and C. J. Raxworthy; AMNH A167582 (RAX 2999) adult female collected 9 April 2001 at Ramena river, Ambanja District, Diana Region, Madagascar, 750 m, 13°55.071’S 48°53.179’E, by S. Mahaviasy, N. Rabibisoa, C. J. Raxworthy, A Razafimanantsoa, and A. Razafimanantsoa; AMNH A 167592 (RAX 4371) adult female, AMNH A 167591 (RAX 4339) and UADBA 19595 (RAX 4372) adult males collected 24–25 February 2002, the same locality as the holotype; AMNH A 167517 (RAX 6110) and UADBA 26359 (RAX 6118) adult males, collected 11 March 2003 at Matsaborimena Trois Lacs, 1550 m, 14°19.859’ S 48°35.240’ E, Bealanana District, Sofia Region by S. Mahaviasy, N. Rakotondrazafy, and N. Rabibisoa; AMNH A181773 (RAX 10204) collected 5 April 2008 at Andramanalana, Andapa district, Sava Region, Madagascar, 850m, 14°22.351′S 49° 21.747′ by S. Mahaviasy and N. Rakotondrazafy; AMNH A181731 (RAX 10392) collected 16 April 2008, Tsararano, Anjanaharibe-Sud/Masoala corridor, Analanjirofo Region, Madagascar, 490 m, 14°54.667’S 49°41.383’E, by S. Mahaviasy and N. Rakotondrazafy; UADBA 3716 (RAN 39507) adult male, collected 9 March 1992 at Bekolosy Manongarivo, Ambanja District, Diana Region, Madagascar, 1200 m, 14°02.5’S 48°18’E by J-B. Ramanamanjato, A. Raselimanana, and C. J. Raxworthy; UADBA 7770 (MRJ 108) juvenile, collected 12 October 1992 at Ampanasatongotra, Marojezy National Park, Andapa District, Sava Region, Madagascar, 600 m, 14°26.2’S 49°46.5’E by N. Rabibisoa, J. B. Ramanamanjato, and O. Ramilison; UADBA 8382 (RAX 2737) adult female, collected 9 April 2001: at Ramena river Analabe, Tsaratanana Reserve, Ambanja District, 750 m, 13°55.071’S 48°53.179’E by S. Mahaviasy, N. Rabibisoa, C. J. Raxworthy, A Razafimanantsoa, and A. Razafimanantsoa; UADBA 19593 (RAX 3454) adult female, collected 4 December 2006 at Ambolokopatrika, Anjanaharibe-Sud/Marojejy Corridor, Andapa District, Madagascar, 880 m, 14°32.302’S 49°26.243’E, the same collectors as holotype; UADBA 19594 (RAX 3785), adult male collected 15 December 2001 at Andranomavohely, Andapa District, Sava region, Madagascar, 800 m, 14°34.165’S 49°16.568’E, by S. Mahaviasy, N. Rabibisoa, N. Rakotondrazafy, A. Razafimanantsoa, and A. Razafimanantsoa; UADBA 26361 (RAX 6482) adult female, collected 21 March 2003, Analapakila, Trois Lacs, 1450 m, 14°26.233’ S 48°36.696’ E, District Bealanana, Sofia Region by S. Mahaviasy, N. Rakotondrazafy and N. Rabibisoa; UADBA 26366–68, 26373 (RD 918, 839-840, 881), collected November 2000 at Ambolokopatrika, 880 m, 14°32.302’S 49°26.243’E, Andapa, Sava Region by D. Rakotomalala; UADBA 26371 (RAX 3783) adult male, the same condition as the holotype; UMMZ 212827 (RAN 38186) collected 22 November 1991 at Antomboka River, Montagne d’Ambre, Antsiranana, Diana Region, 1150 m, 12°32.3’S 49°10’S by C J Raxworthy, J B Ramanamanjato, and A Raselimanana; UMMZ 212835 (RAN 39291) adult male: collected 24 Feb. 1992 at Ambalafary, Manongarivo Special Reserve, Ambilobe District, Diana Region, Madagascar, 250 m, 14°04’S 48°17’E, by C. J. Raxworthy, A. Raselimanana, J. B. Ramanamanjato, A. Razafimanantsoa, and A. Razafimanantsoa; UMMZ 212836 (RAN 39387) collected 2 March 1992, Antsahabe River, Manongarivo Special Reserve, Antsiranana, Diana region, 1200m, 14°02.5’S 48°18’E by C. J. Raxworthy, J. B. Ramanamanjato, and A. Raselimanana
Diagnosis: A small to medium sized
Mantidactylus (Ochthomantis) species (adult SVL male 33–42 mm, female 42–59 mm), with a dark brown dorsal body color often with black spotted granules; a weakly developed pale yellow inguinal streak that may be partly broken up or narrow; the absence of a continuous pale stripe along the lower flank; the lack of an obvious white spot below the eye; moderately developed foot webbing with 1.5–2 free phalanges at the internal edge of toe 4, and WS> 7; tibio-tarsal articulation extends beyond nostril, and maximum width of terminal disc on fingers <1.70 disc width base.
Mantidactylus danieli can be distinguished from the following species:
M. mocquardi,
M. catalai,
M. olgai sp. nov., and
M. tavaratra sp. nov. by the more developed webbing: WS> 7, 1.5-2 free phalanges at the internal edge of toe 4, tibio–tarsal articulation reaching beyond the nostril, and the presence of a pale yellow inguinal streak that that may be partly broken up or narrow;
M. poissoni by the lack of white spots below the eye;
M. femoralis by the maximum width of terminal disc on fingers <1.70 base width, and by the inguinal pale yellow streak that may be broken up and narrow;
M. zolitschka by the adult SVL > 32 mm;
M. ambreensis by the absence of a continuous pale stripe along the lower flank; and
M. macrotympanum sp. nov. by the smaller adult SVL (< 60 mm) and the male tympanum/eye diamater < 0.94. The
Table 5 and
Table 6 summurize the character diagnostics of this new species.
Description of holotype: Adult female (SVL 50.6 mm) in excellent state of preservation. Measurements are presented in
Table S9. In dorsal view, head longer than wide. Head length 1.18 times width length. Head length 0,41 times SVL. In dorsal view, snout tip pointed; in lateral view blunt and with a 3 mm ventral extension beyond lower lip mouth. Canthus rostralis evident. Loreal concave. Tympanum and supratympanic ridge distinct, with the ridge wrapping around the tympanum anterior to the forearm insertion. Round tympanum and tympanum diameter 0.74 times eye diameter. Tongue ovoid anteriorly and bifid posteriorly. Internarial distance 0.21 head width. Nostril round with lateral opening. Eye-nostril distance 1.27 times nostril-snout distance. Forearm length 0.51 times SVL. Hand length (including discs) 0.26 times SVL. Fingers not webbed. Outer and inner metacarpal tubercules developed. Relative finger lenght 1 <2 <4 <3. Terminal disc relatively large (the widest part 1.45 width of the base of the disc). Tibio–tarsal articulation reaches between nostril and snout tip. Hindlimb 2.01 times SVL. Thigh length 0.98 times tibia length. Foot including tarsus 0.92 times SVL. Lateral metatarsal separated. Inner metatarsal tubercule as a skin-like with three-sided (1.35 mm) at the base of toe 1. Outer metatarsal tubercule present. Webbing formula: 1 (1), 2i (1), 2 (0.25), 3i (1.75), 3e (0.50), 4i (2), 4e (2), 5 (0.50), and sum of free phalanges is 9 (WS). Relative toe lenght 1<2<3<5<4. Skin granules on the dorsal surface of thigh, posterior area above eye and above tympanum, and around the femoral glands. The femoral glands on the ventral surface of thighs are rounded, and poorly developed with the central pore. Internally, the femoral gland is type 3 [
2], but without the small granules in proximal area characteristics of the male femoral gland [
35]. In preservative, coloration dark brown dorsally with small darker spots. Upper lip dark brown except the part below the tympanic region which is clear one. Throat pale brown with two dark parallel bands in an L-shape directed outward. Venter pale brown, and almost entirely covered with fine dark brown spots, which decrease in density posteriorly. Forelimbs are brown without darker bands. Hindlimb are brown with darker transverse dorsally, and the pale brown ventral face with dark–brown spots. Inguinal region with a pale broad oblique streak that is broken, and not well developed.
Variation: Morphometric variation diagnostic details are summarized in
Table S9. Sexual dimorphism is obvious in this species, with males being smaller sized and with a relatively larger tympanum. Males have a smaller SVL than females (33–42mm
vs 42–59 mm); males have larger tympanum diameter than females (0.66–1.12 times eye diameter in males
vs 0.49–0.76 times eye diameter in females); and have a shorter hindlimb, and larger terminal disc. Free phalanges on the toes vary: toe 1 (0.50–1), internal edge of toe 2 (0.50–1.50), external edge of toe 2 (0–0.75), internal edge of toe 3 (1–1.75), external edge of toe 3 (0–1), internal edge of toe 4 (1.75–2), external edge of toe 4 (1.50–2), and in toe 5 (0–0.50).
Coloration in life: The iris golden in the superior area, and fading to lower area of eye. The dorsal head, limbs, and body are dark brown, with black spots sometimes for females. The upperlip has a white band, that is most developed in the posterior area. Darker brown cross-bars in a V-shape are present dorsally between eyes. Ventral surfaces with pigmented dark brown spotting on a cream or yellowish background but almost uniformly dark reddish in anterior part: the throat may be reddish, but the belly is always paler. Two dark reddish parallel stripes may be present on the throat, with postrior part narrowing and fading on the thorax (absent in UADBA 8382, 26361, 26366, 26367, 26371, 26373). Ventral face of the thigh completely covered with dark reddish brown spots. Inguinal region with a pale yellow oblique streak that is typically broken, and not well developed (not broken in UADBA 19595, 26359, 26367). Transverse darker brown bands present on the dorsal hind limbs. For very dark specimens (e.g UADBA 26406) the alternating transverse bands on the hind limbs are difficult to see. One adult female (UADBA 3770) has a pale vertebral line running from the snout to anus.
Habits: This species lives in rainforest between 350 - 1580 m elevation, and is a semi-aquatic, occupying the banks of small streams and rivers. We observed it between 10.30 – 23.00 hours, although it is more active and obvious at night, and appears to be mostly nocturnal. Unlike other Ochthomantis species, males rest mostly on rivers banks. More rarely, we observed males on leaves of trees and bushes, and very rarely on rocks. Females are also more tree-dwelling than rupicolous. The vertical distribution varies for both sexes: males observed between 20-100 cm, whereas females between 50-300 cm above the ground. This species appears to prefer rivers to small fast flowing streams, and females were observed mostly in areas of slow moving water.
Etymology: The specific name danieli honors Daniel Rakontondravony for his substantial contributions to the knowledge of the Malagasy fauna.
Distribution: Low and mid altitude northern and Sambirano region of Madagascar (180–1200 m), from subhumid to humid forests (
Figure 9,
Table S13).
Comments: Our morphological description agrees with the
M. sp. aff.
femoralis shown by [
36] (pp. 178-179) from Tsaratanana. Our molecular analyses group all our
M. danieli samples (
Table S1) with the Montagne d’Ambre AY324818 (FGMV 2002.929) and Manongarivo AY324816 (FGMV 2002.825).
Mantidactylus cf.
femoralis samples reported by [
10]. FGMV 2002.929 has more recently been referred to as ‘CCS sp. 42′, and FGMV 2002.825 as a ‘possible additional Unconfirmed Candidate Species (UCS) from Manongarivo’ [
11], (
Figure 1 and
Figure 2, UCS sp 63 Tsaratanana, p. 24).
3.4.2. Mantidactylus macrotympanum new species
Holotype: AMNH A 167589* (RAX 2715): adult male at Antsahatelo, Western Slope Tsaratanana Strict Reserve, Ambanja District, Diana Region, Madagascar, 800m, 13°51.588′S 48°51.979′E, Apr. 6, 2001, N. Harilanto, S. Mahaviasy, N. Rabibisoa, C. J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa.
Paratypes: AMNH 167598 (RAX 2603): adult male at Antsahabe, Ramena River, Ambanja District, Diana Region, Madagascar, 180 m, 13°43.272’S 48°39.304’E, Apr. 4, 2001, N. Harilanto, S. Mahaviasy, N. Rabibisoa, C. J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa. UMMZ 201416*–418 (RAN 39170–71, 39470), UMMZ 213447* (RAN 39125): adult males, Ambalafary, Manongarivo Special Reserve, Ambilobe District, Diana Region, Madagascar, 250 m, 14°04’S 48°17’E, Feb. 20, 1992 and Mar. 3, 1992, A. Raselimanana, J. B. Ramanamanjato, C. J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa.
Diagnosis: A large sized
Mantidactylus (Ochthomantis) species (adult male SVL 57–62 mm, female unknown but presumably, like other
Ochthomantis species, larger than males > 62 mm), with a dark brown dorsal head and body color, and skin granules form a weak dorsolateral ridge on the body; no pale inguinal streak; males with a large diameter tympanum ≥ 7.6 mm; tibio–tarsal articulation extends beyond the snout tip; and well developed webbing with sum of the free phalanges (WS) 3.75.
Mantidactylus macrotympanum can be distinguished from all subgenus
Ochthomantis by the following characters: big tympanum diameter ≥ 7.6 mm, large adult male SVL ≥ 57 mm, and the presence of skin granules forming a weak dorsolateral ridge on the body. The
Table 5 and
Table 6 summarize the character diagnostics of this new species.
Description of holotype
: Adult male (SVL 61 mm) in an excellent state of preservation. Measurements are presented in
Table S10. In dorsal view, head longer than wid. Head length 1.29 times head width. Head length 0.44 times SVL 0.44. In dorsal view, snout tip pointed, and in lateral view almost acuminate and curved down with a 2.20 mm ventral extension beyond lower lip mouth. Obvious canthus rostralis and concave loreal which make lip well evident. Tympanum and supratympanic ridge distinct, which the ridge continues posteriorly its direction almost vertical to the one evident granule above the insertion point of the forearm. Round tympanum and tympanum diameter 0.94 times eye diameter. Tongue ovoid anteriorly and bifid posteriorly. Internarial distance 0.23 times head width. Non–protruding nostril has a lateral slanting opening. Eye-nostril distance 1.86 times nostril-snout tip distance. Forearm length 0.50 times SVL. Hand length (including discs) 0.32 times SVL. Fingers not webbed. Outer and inner metacarpal tubercules are flattened and widened. Relative fingers lengths 1 < 2 <4 <3. Terminal discs large (the widest part 2.20 the width of the base of the disc). Tibio–tarsal articulation largely beyond the snout tip. Hind limb 1.89 times SVL. Thigh and tibia the same length. Foot including tarsus 0.76 times SVL. Lateral metatarsal separated. Outer metatarsal tubercule absent. Inner metatarsal tubercule not evident at the base of the toe 1. Webbing formula 1 (0), 2i (0.5), 2e (0), 3i (1), 3e (0), 4i (1.25), 4e (1), 5 (0), and sum of the free phalanges is 3.75 (WS). Relative toe length 1 < 2 < 3 < 5 < 4. Flanks with few prominent granules in its superior part, and white spot-like posteriorly. Some epidermis granules focused in sacral area, and small granules above the eyes. Femoral glands on ventral surface of thighs are not swollen and rounded both sides of the thighs. Internally, the femoral gland is type 4 [
2,
35]. In preservative
, the dorsal face is blackish marbled with some clear spots. The ventral face and superior lip are homogenous with brownish color. Throat darker with two parallel bands. Fore and hind limbs with alternating transversal bands clear and dark. The inguinal region, has some scattered darker spots.
Variation
: This species is only known from six male specimens. Morphometric variation diagnostic details are summarized in
Table S10. The SVL varies between 57.90 to 62.10 mm. The ventral face is heterogeneous: individuals with clear belly have black spots, and those can be either present or not with dark belly specimens. The flanks are clear brownish background with white plate in central area like holotype, but sometimes white spots observed for specimens which have black flanks background. Three internal phalanges vary between 0.50–1 for toe 2i and 1–1.50 for toe 4i.
Coloration in life: The iris with constant black color surrounding by white color in his superior, and lower parts. The body is dark brownish. Superior lip with black crossbars anteriorly. Above the eyes with white line. The belly is darker with black spot. Thorax with two parallel bands. The brown clear flanks have a white plate–like. A white oblique line can be present in inguinal area. Two alternate bands clear and dark are rather distinct in fore and hind limbs.
Habits: This is a rainforest species living along calm stream and fast rivers with spotted rocks. This can be inhabited outside the forest like plantation. The individuals observed between 12.00–20.00 hours but they are nocturnal than diurnal. The night, they roost and rest on branches between 10–200 cm height, and the days they rest on the rocks. All the known specimens observed are males, so they have a spatio-temporal behavior from the other subgenus, this is scansorial the night, and rock–dwelling the day.
Etymology: The specific name macrotympanum is given to its very big tympanum feature versus the others Ochthomantis subgenus, and all Malagasy amphibians documented till now.
Distribution
: Known only from relict forest close to Ambalafary village, in the Manongarivo Special Reserve at 250m; and from the Ramena River (Tsaratanana Reserve) between 180–800 m (
Figure 10,
Table S13).
Comments: The only genetic data known for this species is from AMNH A167589 (RAX 2715), UMMZ 201416 (RAN 39170), and UMMZ 213447 (RAN 39125) (
Table S1).
3.4.3. Mantidactylus olgai, new species
Holotype: AMNH A167596* (RAX 5195), subadult female collected 19 April 2002 at Sorata pic Forest, Vohemar District, Sava Region, Madagascar, 1700 m, 13°41.147’S 49°26.511’E, S. Mahaviasy, N. Rakotondrazafy, N. Rabibisoa, and C.J. Raxworthy.
Paratypes: AMNH A167555 (RAX 2475) adult male, and UADBA 8383, 8388 (RAX 2473, 2472) adult females collected 25 January 2001 at Besahona, Analabe forest, Ambanja District, Diana Region, Madagascar, 700 m, 13°54.372’S 48°44.785’E, A. Razafimanantsoa, and A. Razafimanantsoa; AMNH A167556 (RAX 2552) adult female collected 29 January 2001 at Ramena, Analabe forest, Ambanja District, Diana Region, Madagascar, 700 m, 13°42,707’S 48°34,156’E, same collectors as previous. AMNH A167557 (RAX 2555) adult male collected 29 January 2001 at Betaindambo, Analabe forest, Ambanja District, Diana Region, Madagascar, 600 m, 13°51.932’S 48°49.189’E, same collectors as previous; AMNH A167558 (RAX 2620) adult female collected 4 April 2001 at Ramena, Analabe forest, Ambanja District, Diana Region, Madagascar, 700 m, 13°42.707’S 48°34.156’E, N. Harilanto, S. Mahaviasy, N. Rabibisoa, C.J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa; AMNH A167560 (RAX 2655) adult male, and AMNH A167561–62 (RAX 2662–63) adult females collected 5 April 2001 at Maroamalona, Analabe forest Ambanja District, Diana Region, Madagascar, 600 m, 13°51.023’S 48°47,902’E, same collectors as previous. AMNH A167565* (RAX 3203) juvenile, and AMNH A167567–68 (RAX 3230, 3232) adult males, collected 15–16 April 2001 at Antsaravy Analabe forest, Ambanja District, Diana Region, Madagascar, 1150 m, 13°55.560’S 48°54.353’E, N. Harilanto, S. Mahaviasy, N. Rabibisoa, A. Razafimanantsoa, and A. Razafimanantsoa; AMNH A181726* (RAX 10205) collected 5 April 2008 at Andramanalana, Andapa District, Sava Region, Madagascar, 850m, 14°24′24”S 49°20’13”E, S. Mahaviasy, and N. Rakotondrazafy; UADBA 8231, 8387, 8389–90 (RAX 3231, 3228, 3196–97) adult females, collected 15–16 April 2001 at Antsaravy Analabe forest, Ambanja District, Diana Region, Madagascar, 1150 m, 13°55.560’S 48°54.353’E, N. Harilanto, S. Mahaviasy, N. Rabibisoa, C.J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa; UADBA 8404 (RAX 3312) adult male, collected 19 April 2001 at Antsaravy, Analabe forest, Ambanja District, Diana Region, Madagascar,1150 m, 13°55.560’S 48°54.353’E, N. Harilanto, S. Mahaviasy, N. Rabibisoa, A. Razafimanantsoa, and A. Razafimanantsoa.
Diagnosis: A small to medium sized
Mantidactylus (Ochthomantis) species (adult SVL male SVL 34–40 mm, female SVL 45–52 mm), with a black granules on a grey dorsal body and lateral body; no pale yellow streak in the inguinal region; no broad pale line or white spots on the body flank; the upper lip with a pale line; the presence of the crossbars in V or Y–like on the back; the snout tip pointed in lateral view; well developed foot webbing with 1 free phalange on the internal edge of toe 4, and with WS< 7; tibio–tarsal articulation reaching between eye and nostril; and maximum width of terminal disc on fingers > 1.8 disc width base.
Mantidactylus olgai can be distinguished from all others subgenus
Ochthomantis species by the presence of obvious black skin granules both on the dorsal and lateral bodies, and from the following characters:
M. femoralis by 1 free phalange on the internal edge of toe 4, no yellow patch in the inguinal region, and the tibio–tarsal articulation reaching between eye and nostril;
M. ambreensis by a lack of a broad pale line on the body flank;
M. mocquardi by the grey body coloration, and no white spots on the body flanks;
M. zolitschka by larger adult male SVL > 33 mm and female > 44 mm;
M. poissoni by the lack of white spots below the eye;
M. catalai by the presence of crossbars in V or Y–like on the back;
M. danieli by the more developed webbing: WS< 7, 1 free phalange at the internal edge of toe 4, no pale yellow streak in the inguinal region, and the tibio–tarsal articulation reaching between eye and nostril;
M. macrotympanum by smaller adult male SVL < 60 mm, and the male tympanum-eye diamater < 0.94; and
M. tavaratra by larger terminal disk, maximum width of terminal discs on finger > 1.8 disc width base. The
Table 5 and
Table 6 summurize the character diagnostics of this new species.
Description of holotype: subadult female (SVL = 40.70 mm) in an excellent state of preservation. Measurement are presented in
Table S11. In dorsal view, head rather longer than wide. Head length 1.23 times head width 1.23. Head length 0.46 times SVL. In dorsal and lateral view, snout pointed and with a ventral extension 2.20 mm evident ventrally beyond lower lip mouth, and giving it shell–like form. Canthus rostralis not evident. Loreal furrowed. Tympanum distinct from the well developped suratympanic ridge which continues to before the insertion point of the forearm. Tympanum diameter 0.56 eye diameter 0.56. Tongue ovoid anteriorly and bifid posteriorly. Internarial distance 0.21 times head width. Nostrils round in cutaneous fold with antero–lateral opening. Eye-nostril distance 1.74 times nostril-snout distance. Forearm length 0.55 times SVL. Hand length (including discs) 0.33 times SVL. Fingers not webbed. Outer and inner tubercule metacarpals poorly developed. Relative fingers lenght 1 < 2 < 4 <3. Terminal disc large (the widest part twice width of the base of the disc). Tibio–tarsal articulation reaches between eye and nostril. Hindlimb 1,98 times SVL. Thigh length times 1.07 the tibia length. Foot including tarsus 0.77 times SVL. Lateral metatarsal separated. Inner metatarsal tubercule in half moon–like (1.3 mm lenght) at the base of the toe 1. Outer metatarsal tubercule in small granule. Webbing formula: 1 (0), 2i (1), 2e (0), 3i (1), 3e (0), 4i (1.25), 4e (1), 5 (0) and sum of free phalanges is 4.25. Relative toe lenght 1 < 2 < 3 < 5 < 4. Skin granules on flank. The femoral on ventral surface of thighs are rounded, and poorly developed with the central pore. Internally, the femoral gland is type 3 [
2], but without the small granules in proximal area characteristics of the male femoral gland [
35]. In preservative, coloration grayish dorsally with
Y-like mark. Upper lip with white pigment. Throat with two dark parallel bands. Belly, ventral surface of thigh and forelimb are clears. Hind and fore limbs are grey with transversal bands dorsally alternating clear and dark. Inguinal region without pale inguinal streak. Flank with some black granules.
Variation: Morphometric variation diagnostic details are summarized in
Table S11. Sexual dimorphism is obvious in this species, with males being smaller sized, and larger tympanum. Sexual dimorphism is evident: males have a smaller SVL than females (34.00–39.55 mm
vs 45.75–51.80 mm); males have a larger tympanum diameter than females (0.80–1.30 times eyer diameter in males
vs 0.40–0.69 times eye diameter in females); and larger hindlimb; 90 % of specimens have a large large femur between 0.91–1.18 times tibia length and the rest < 0.91. Free phalanges vary: toe 1 (0–0.50), internal edge of toe 3 (1–1.25), external edge of toe 3 (0–0.50), internal edge of toe 4 (1–1.50).
Coloration in life: The iris has a golden ring on outer area. Body coloration grey except AMNH A167560, and UADBA (8391, 8477) brown one. The upperlip is white dirty except the holotype white color. No dark parallel bands on throat except UADBA 8407. All of the specimens have V or Y–shapped dark spots dorsally except UADBA (8384, 8387, 8389) with X–shapped. Ventral surface is clear except juvenile which is dirty, and some specimens have a throat with white pigments including holotype and AMNH (A167557, A167564, A167568–69, A167571, A167573–74). Inguinal region without a pale yellow streak. Transverse darker brown bands present on the dorsal hind limbs. Some specimens have a thick pale brown vertebral line.
Habits: Low and mid–altitude rainforest species closed in edge of stream or river with rocky bottom. We observed it between 10.00–22.30 hours, and mainly nocturnal except juvenile in various habitats (rock, ground, riverbank, and edge of water). Generally, females rest on rocks at night and some specimens overhanging on leaves between 20–50 cm above the ground. Males are more tree–dwelling than rupicola, and resting on leaves of shrubs, between10–30 cm height. Specimens observed at all of type of water, but they have a particular preference for streams or fast rivers.
Etymology: The specific name olgai honors Professor Olga Ramilijaona, the thesis Director to N.R., in recognition of her substantial mentorship.
Distribution: Species inhabits along Ramena river (Tsaratanana Strict Natural Reserve, and Analabe), Andramanalana, and Sorata in the North of Madagascar. The elevation ranges between 600–1700 m (
Figure 11,
Table S13).
Comments: The only genetic data known for this species is presented in
Table S1. Although
M. olgai has been recorded at Andramanalana, no genetic or morphogical samples have yet been confirmed from Marojejy, which is a well-sampled site about 40 km to the west.
3.4.4. Mantidactylus tavaratra, new species
Holotype: AMNH A 167505* (RAX 5310) adult male collected 22 April 2002 at Sorata, Vohemar District, Sava Region, Madagascar, 980m, 13°41.986’S 49°26.687’E, by S. Mahaviasy, N. Rabibisoa, and C. J. Raxworthy.
Paratypes: AMNH A 167506* (RAX 5400) juvenile, AMNH A167507 (RAX 5403) adult female, and UADBA 26306 (RAX 5599) adult male collected 3 February 2003 at Matsaborimaiky Lake, Tsarananana Strict Natural Reserve, Bealanana, Sofia Region, Madagascar, 1950 m, 14°09.175’S 48°57.431’E, by S. Mahaviasy, N. Rabibisoa, N. Rakotondrazafy, and C. J. Raxworthy. AMNH A167510* (RAX 5769) juvenile collected 23 February 2003 at Befosa River, Tsaratanana Strict Natural Reserve, Bealanana District, Sofia Region, Madagascar, 1680 m, 14°10.455’S 48°56.708’E, by S. Mahaviasy, N. Rabibisoa, N. Rakotondrazafy, and C. J. Raxworthy. AMNH A167515*–16* (RAX 6028, 6107) adult males collected 11 March 2003 at Matsaborimena, Trois Lacs, Bealanana District, Sofia Region, Madagascar,1600 m, 14°19.859’S 48°35.240’E, by S. Mahaviasy, N. Rabibisoa, and N. Rakotondrazafy. AMNH 167524*–25* (RAX 6838, 6901) adult males, and AMNH A167529 (RAX 7120), UADBA 26326, 26335, 26394 (RAX 7009, 6903, 6952) adult females collected 13–17 April 2003 at Lohanandroranga River ridge, and Ambatotavaratra River, Corridor Tsaratanana Anjanaharibe Sud, Bealanana District, Sofia Region, Madagascar, 1430–1650 m, 14°24.076’S 49°10.253’E, the same collectors as AMNH A167510. AMNH A167593* (RAX 5000) adult male collected 13 April, 2002 at Sorata, Vohemar District, Sava Region, Madagascar,1330 m, 13°41.147’S 49°26.511′E, by S. Mahaviasy, N. Rabibisoa, and C. J. Raxworthy. AMNH A181730* (RAX 10323) juvenile 10 April 2008 at Andramanalana, Andapa, Sava Region, Madagascar, 1300 m, 14°24.400’S 49°20.21’E, by S. Mahaviasy, and N. Rakotondrazafy. AMNH A187088*–89* (RAX 11534–35), and UADBA RAX 11532*–33* juveniles collected 6 May 2010 at Marojejy, Andapa District, Sava Region, Madagascar, 1550 m, 14°32.302’S 49°26.243’E, by S. Mahaviasy, N. Rakotondrazafy, and C.J. Raxworthy. UADBA 19628, 19634 (RAX 4993, 4999): adult females, and UADBA 19630 (RAX 4995) adult male collected 13 April 2002 at Sorata, Vohemar District, Sava Region, Madagascar, 1300 m, 13°41.147’S 49°26.511′E, by S. Mahaviasy, N. Rabibisoa, and C. J. Raxworthy. UADBA 19625, 19631, 26307–08 (RAX 4951, 4996, 5771, 5778) adult males, and UADBA 26328, 26331 (RAX 5402, 5753) adult females collected 22–23 February 2003 at Befosa River, Tsaratanana Strict Reserve, Bealanana District, Sofia Region, Madagascar, 1600 m, 14°10.455’S 48°56.708’E, by S. Mahaviasy, N. Rabibisoa, and N. Rakotondrazafy. UADBA 19650 (RAX 3980) adult female collected 11 Februray, 2002 at Bezavona, Vohémar District, Sava Region, Madagascar, 350 m, 13°31.962’S 49°51.954’E, by S. Mahaviasy, N. Rabibisoa, and C. J. Raxworthy. UMMZ 212440* (RAN 39071) juvenile collected 16 February 1992 at Bekolosy, Manongarivo Special Reserve, Ambanja District, Diana Region, Madagascar, 1100 m, 14°02.5’S 48°18’E, by A Raselimanana, J. B. Ramanamanjato, and C. J. Raxworthy. UMMZ 212889* (RAN 43366) adult female collected 1 Apr. 1993 at Befosa River, Tsaratanana Natural Reserve, Bealanana District, Sofia Region, Madagascar, 1630 m, 14°10.455’S 48°56.708’E, by J. B. Ramanamanjato, A. Raselimanana, C. J. Raxworthy, A. Razafimanantsoa, and A. Razafimanantsoa.
Diagnosis: A medium to large sized
Mantidactylus (Ochthomantis) species (adult SVL male 35–52 mm, female 42–63 mm), with a brown dorsal body color without black granules, and laterally flanks typically with a straight border from the dark to pale venter coloration; a thin pale white or yellow streak in the inguinal region; the upper lip with a pale stripe; webbing with 1–1.5 free phalanges at the external edge of toe 4 and no free phalange at the external edge of toe 2; tibio–tarsal articulation reaches between eye and nostril; and maximum width of terminal disks on fingers ≤ 1.8 disc width base.
Mantidactylus tavaratra can be distinguished from all other
Ochthomantis species by the following characters:
M. ambreensis by the lack of white continuous lateral line on the side of the head and body;
M. poissoni by the lack of white spots below the eye;
M. catalai by digits with smaller terminal discs (the widest part ≤ 1.8 disc base), and the inguinal region marked by a thin pale white or yellow oblique line, and a white stripe on the upper lip;
M. femoralis, and
M. danieli by the presence of 1–1.5 free phalanges at the external edge of toe 4, and tibio–tarsal articulation reaches as further the nostril;
M. mocquardi by the presence of a thin pale continuous line on the upper lip, the absence of white spots on the flank, and absence of a grey body coloration;
M. olgai by a flank typically with a straight border from the dark to pale venter coloration, and a maximum width of terminal disk ≤ 1.80 disc width base;
M. zolitschka by larger adult SVL > 35 mm ; and
M. macrotympanum by smaller adult male SVL < 60 mm, and the male tympanum-eye diamater < 0.94. The
Table 5 and
Table 6 summurize the character diagnostics of this new species
Description of holotype: Adult male (SVL = 37 mm) in an excellent state of preservation. Measurements are presented in
Table S12. In dorsal view, head larger than wide. Head length 1.34 times head width 1.34. Head length 0.44 times SVL. In dorsal view, snout tip slightly pointed and almost straight in lateral view, and with a 1.55 mm ventral extension beyond lower lip mouth, which makes snout tip in obtuse appearance. Canthus rostralis obvious, and loreal concave make jaw well evidente. Tympanum, and supratympanic ridge distinct, which continues almost vertical to a three evident white granules before reaching the forearm insertion. Round tympanum and tympanum diameter 0.62 times eye diameter. Tongue ovoid anteriorly and bifid posteriorly. Internarial distance 0.30 times head width. Round nostril with a lateral opening non–protruding. Eye-nostril distance equal nostril-snout distance. Forearm length 0.46 times SVL. Hand length (including discs) 0.30 times SVL. Fingers not webbed. Outer and inner and metacarpal tubercules are flattened and widened. Relative finger lengtht 1 < 2 <4 <3. Fingers not webbed. Terminal disc relatively large (the widest part 1.40 width of the base of the disc). Tibio–tarsal articulation reaches between eye and nostril. Ventral surface of the forearm with 3 granules on its internal ridge at the base of the metacarpal. Hindlimb 1.90 times SVL. Thigh length almost equal of tibia length. Foot including tarsus 0.77 times SVL. Lateral metatarsal separated. Outer metatarsal tubercule indistinct. Inner metatarsal tubercule flattened at the base of the toe 1. Webbing formula is: 1 (0.25), 2i (1), 2e (0), 3i (1.25), 3e (0.25), 4i (1.5), 4e (1.5), 5 (0), and sum of the free phalanges is 5.75 (WS). Relative toe lenght 1 < 2 < 3 < 5 < 4. Dorsal with small granules on their edge, and in sacral region. The femoral glands on the ventral surface of thighs are oblong and relatively developed with the centro–distal portion having a pore surrounded by many granules, giving a crater-like form. Internallys, femoral gland is type 3 [
2,
35]. In preservative, coloration brown dorsally with some clear or depigmented portions, especially posteriorly. Upperlip with finely dark brown pigment posteriorly and completely stained anteriorly. An indistinct clear band runs behind the eyes and crumbles in the anterior half of the back. Throat with two dark short parallel stripes. Ventral surface homogeneous and clear. Ventral face of the throat, and thorax with silver–white, and some reticulated dark brown pigments, belly whitish with some brown fine round spots, femur and forearm a clear part. Inguinal region with fine streak. The lower portion of hinlimb with a few scattered brown spots. Hindlimb with evident alternate bands, thick dark bands and fine clear bands. Forelimb with indistinct alternate bands.
Variation: Morphometric variation diagnostic details are summarized in
Table S12. Sexual dimorphism is obvious in this species, with males being smaller sized, and with a larger tympanum. Males have smaller SVL than females (35.65–52.40 mm
vs 42.70–63.25 mm), males have a larger tympanum diameter than females (0.82–1.12 times eye diameter in males
vs 0.43–0.73 times eye diameter in females). Body coloration vary from grayish brown to reddish brown UADBA 26331 (RAX 5753) or light brown UADBA 19628, 26308 (RAX 4993, 5778). In males, half of specimens (including holotype) have a snout tip ≤ 2mm ventral extension beyond lower lip mouth, and the other half > 2mm. Throat with obvious dark brown parallel bands except with some specimens with clear ventral face or indistinct or absent: e.g UADBA (26238, 26371, 26394). Throat and thorax with white or white silver coloration, but there is clear with some specimens UADBA (26238, 26308, 26331, and 26394). Dorsal dark crossbar
V or
Y–shaped absent except in the holotype, and the Paratypes UADBA (19625, 19628, 19630–31, 26308, 26394). Flanks with obvious black and white granules observed on Paratype UADBA 26391, but do not reach roughness of
M. olgai species. Toe 3 slightly short compared to toe 5 in males, whereas it is shorter in females except UADBA 26326 (RAX 7009). Free phalanges vary: toe 1 (0–0.75), external edge of toe 2 (0–0.75), internal edge of toe 3 (0.25–1.50), external edge of toe 3 (0–0.50), internal edge of toe 4 (1–2), and external edge of toe 4 (1–1.50).
Coloration in life: The iris has a golden ring on the outer area. The body is grayish brown, light or dark brown, or sometimes reddish brown. The upper lip has an evident white clear band or not. Darker brown cross-bars in a rod are present dorsally between eyes, and dorsal body with dark brown cross-bars in a V or Y–shape. The throat and thorax are silvery white or whire color, and the belly is clear with a smaller white spots. The throat is always with distinct dark brown parallel stripes. Ventral face of thighs with some dark reticulations. Inguinal area with or without a little white or yellowish line, but if present it is narrower shape. White brownish bands can be present in the superior part of the forelimbs, and alternating bands, white and brown, in the lower part of the forelimbs. The alternating transverse bands, light and dark browns, present on the dorsal hind limbs. The black granules are present or not on the white flank, or concentrated in the sacral area of the dorsal, and on the sides of the forearm, but they are not very obvious like M. olgai.
Habits: This is a rainforest and savannah species living along the riverbank. It inhabits almost of all kinds of aquatic habitats: stream, river, ponds, and shallow marshes except waterfalls. We observed it between 9.30–23.00 hours, but mainly nocturnal except juveniles. Juveniles observed during the day. At night, they rest both on rocks, and on leaves, but rarely on branches, and accidentally on aerial roots and Pandanus sp.. The females prefer roosting on rocks rather than on leaves, whereas the males are indifferente of those. The vertical distribution varies between the both sexes: males between 5–100 cm, and females between 5–150 cm above the ground.
Etymology: The specific name tavaratra refers to the Malagasy word for ‘‘the north’’. This name used as a nonlatinized specific epithet, and is given in reference to the known distribution of this species in northern of Madagascar
Distribution: Northern endemic species of Madagascar, elevation varies from 80 to 2450 m elevation (
Figure 12,
Table S13).
Comments: Our morphological description agrees with the
M. sp. aff.
mocquardi shown by [
36] (
Figure 2, p. 249) from Marojejy. Our molecular analyses group all our
M. tavaratra samples (
Table S1) with the Manongarivo AY324819 (FGMV 2002.824) and Tsaratanana AY324820 (ZSM 643/2001)
M. cf.
mocquardi samples reported by [
10]. FGMV 2002.824 from Manongarivo has more recently been referred to as ‘UCS sp. 63 Tsaratanana’, and this taxon also includes another specimen (FGMV 2001.114) from Tsaratanana [
11].
3.5. Identification key
In addition of identification key, we are giving the morphological diagnostic features for all
Ochthomantis species (see
Table 1) to facilitate their specific character.
1. Lateral head and body with a broad pale line running continuously from the snout tip to the groin area. M. ambreensis (SVL, adult male between 33–39 mm and adult female 38–42 mm), and M. ambony (SVL, adult male between 30–32 mm and adult female 34–38 mm).
– Lateral head and body without a broad pale line running continuously from the snout tip to the groin area. 2.
2. Adult male SVL <31 mm, female SVL <38 mm, reduced foot webbing with ≥2 free phalanges on the internal edge of toe 4 and WS > 9, male tympanum diameter ≤ 7 mm. M. zolitschka.
– Adult male SVL 31–55 mm, female SVL 38–67 mm, more extensive foot webbing with ≤2 free phalanges on the internal edge of toe 4 and WS < 9, male tympanum diameter < 7 mm. 3
– Adult male SVL > 56 mm (female unknown, but likely ≥ 67 mm), more extensive foot webbing with 1.25 free phalanges on the internal edge of toe 4 and WS < 4, male tympanum diameter ≥ 7 mm. M. macrotympanum.
3. Tibio–tarsal articulation reaches between nostril and snout tip, or beyond. 4
– Tibio–tarsal articulation reaches between eye and nostril. 6.
4. Upper lip with a large pale spot under the eye (female) or multiple white spots (male), groin area lacks a short pale line or spots. M. poissoni.
– Upper lip lacks a large pale spot under the eye (female) or multiple white spots (male), groin area with a short pale line or spots. 5.
5. Large finger pads with disc width > 1.7 x disc base, upper lip with a thin pale continuous line, groin area with a short bold pale line that may be continuous or broken (in life, yellow). M. femoralis.
– Smaller finger pads with disc width < 1.7 x disc base, upper lip without a thin pale continuous line, groin area with a short weak pale line that is often broken or even absent. M. danieli.
6. Upper lip with a thin pale continuous line. 7
– Upper lip without a thin pale continuous line, but may have small pale spots. 8
7. Snout tip sharply angular in lateral view, flanks with an irregular border from the dark to pale venter coloration, dorsal body grey in life (and often in preservation) with black granules, larger finger pads, with disc width > 1.8 x disc base. M. olgai.
– Snout tip not sharply angular in lateral view, flanks typically with a straight border from the dark to pale venter coloration, dorsal body brown without black granules, smaller finger pads, with disc width ≤ 1.8 x disc base. M. tavaratra.
8. Snout tip angular in lateral view, body dark brown (or black in preservation) with distinct white spots on flanks, groin area with a few or lacking pale spots, foot webbing with 1.5 free phalanges on the internal edge of toe 4 and WS 4. M. mocquardi.
– Snout tip not angular shape in lateral view, body medium brown (never black in preservation) and lacks white spots on flanks, groin area with a prominent short pale line or spots, foot webbing with 1–1.5 free phalanges on the internal edge of toe 4 and WS 6. M. catalai.