Submitted:
04 July 2023
Posted:
05 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Synthesis of MnO2-nanozymes
2.2. Synthesis of gold-nanozymes
2.3. Steady-state kinetics studies
3. Results and discussion
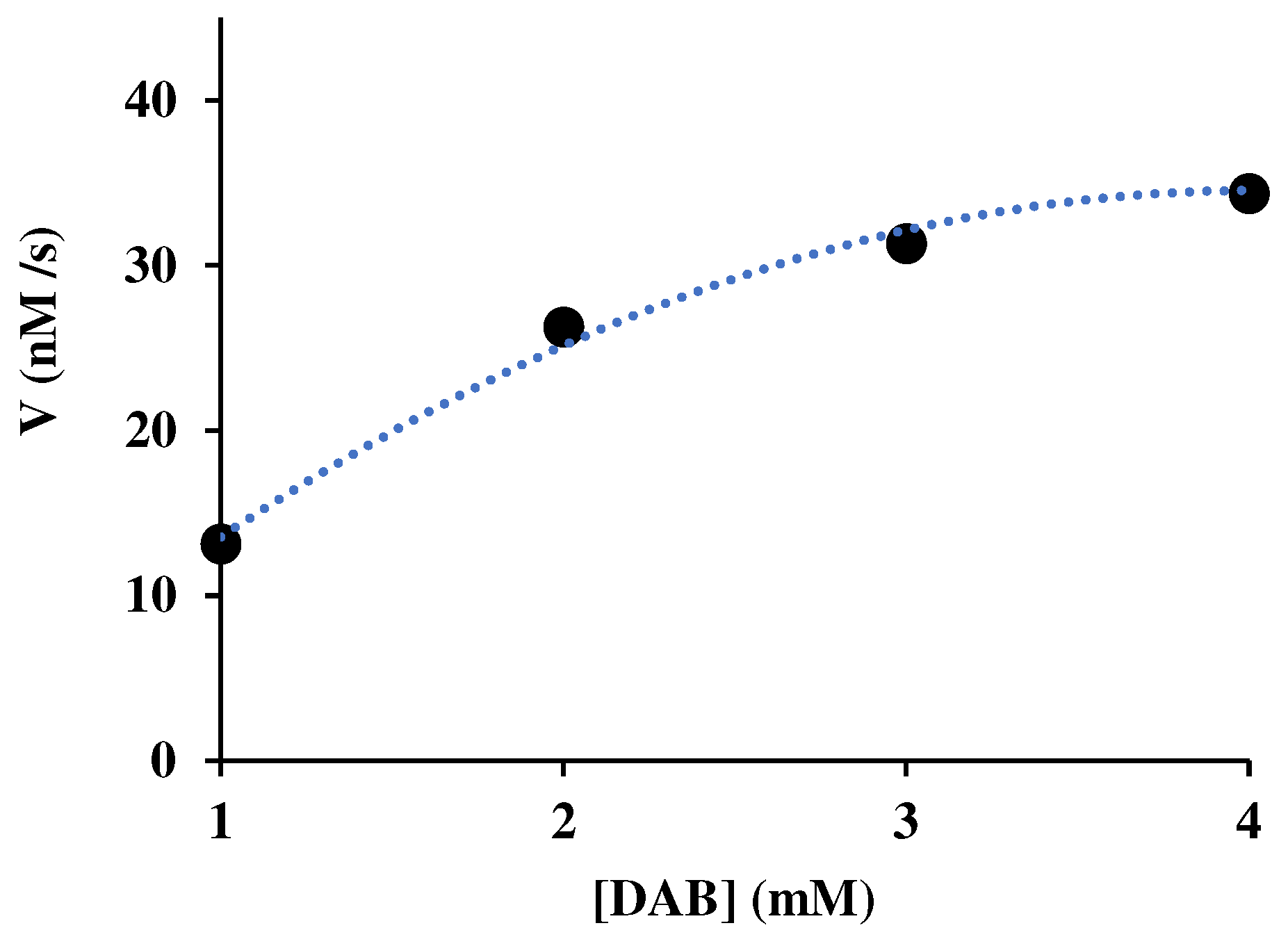
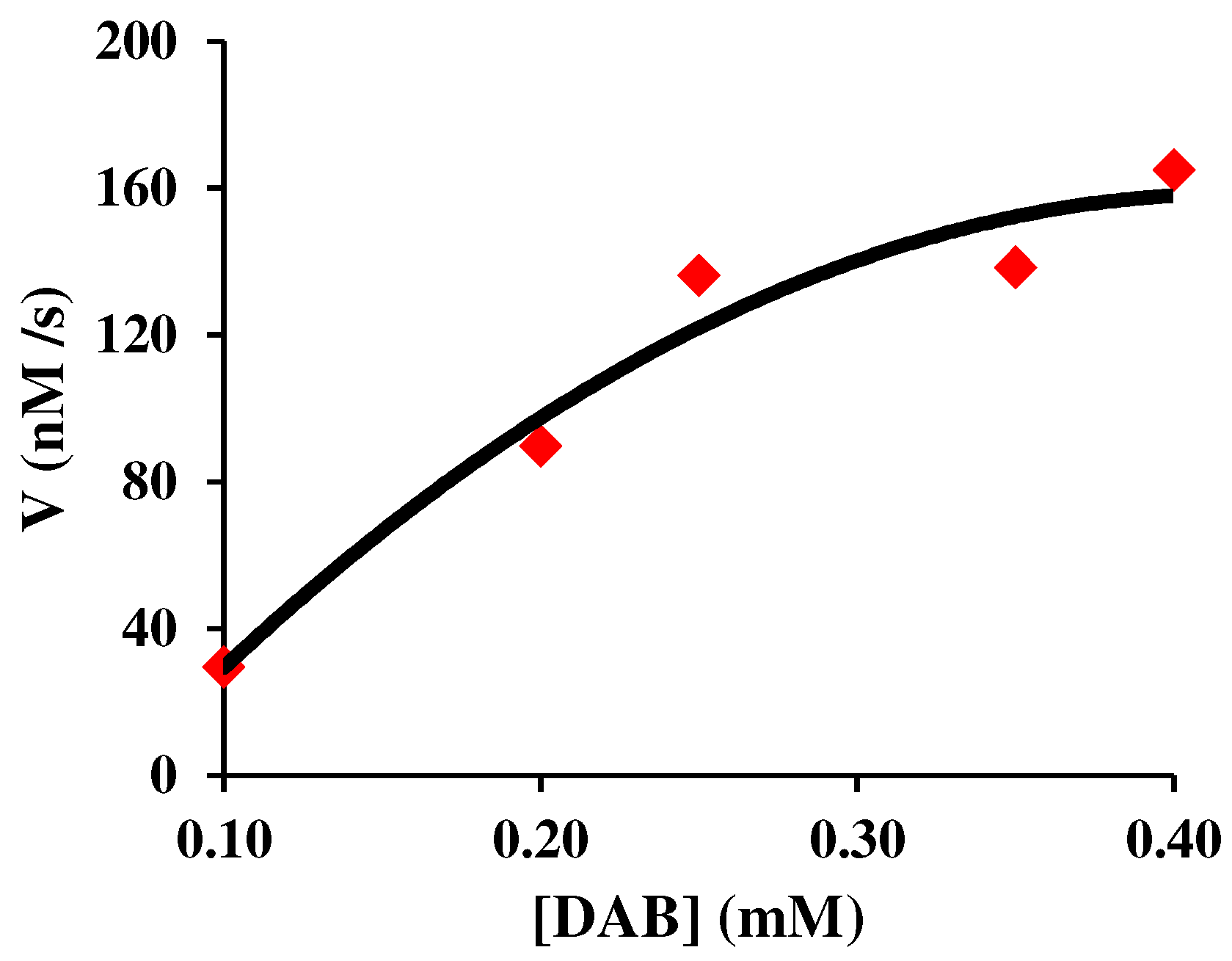
3.1. Steady-state saturation plots of the Michaelis-Menten model
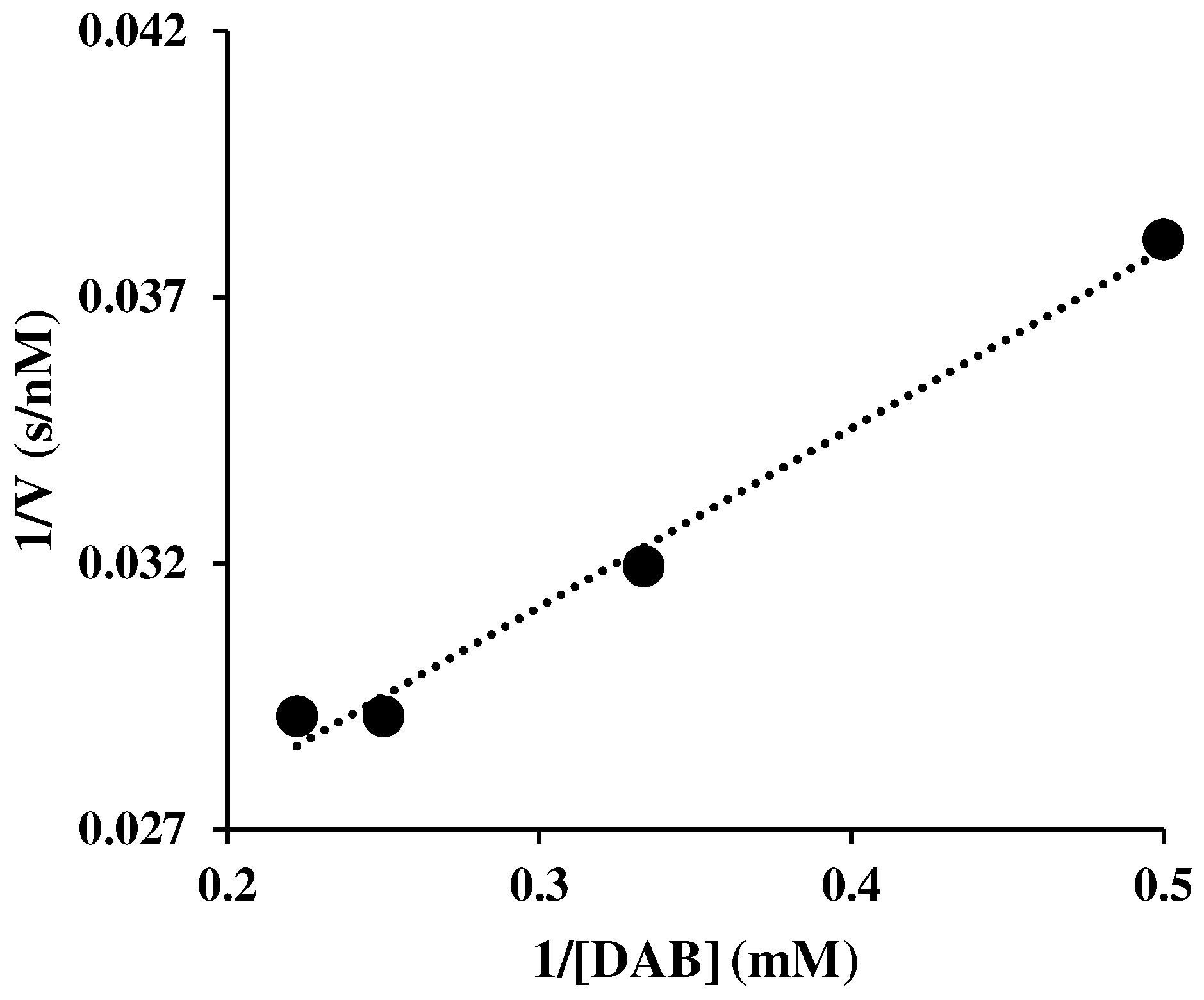
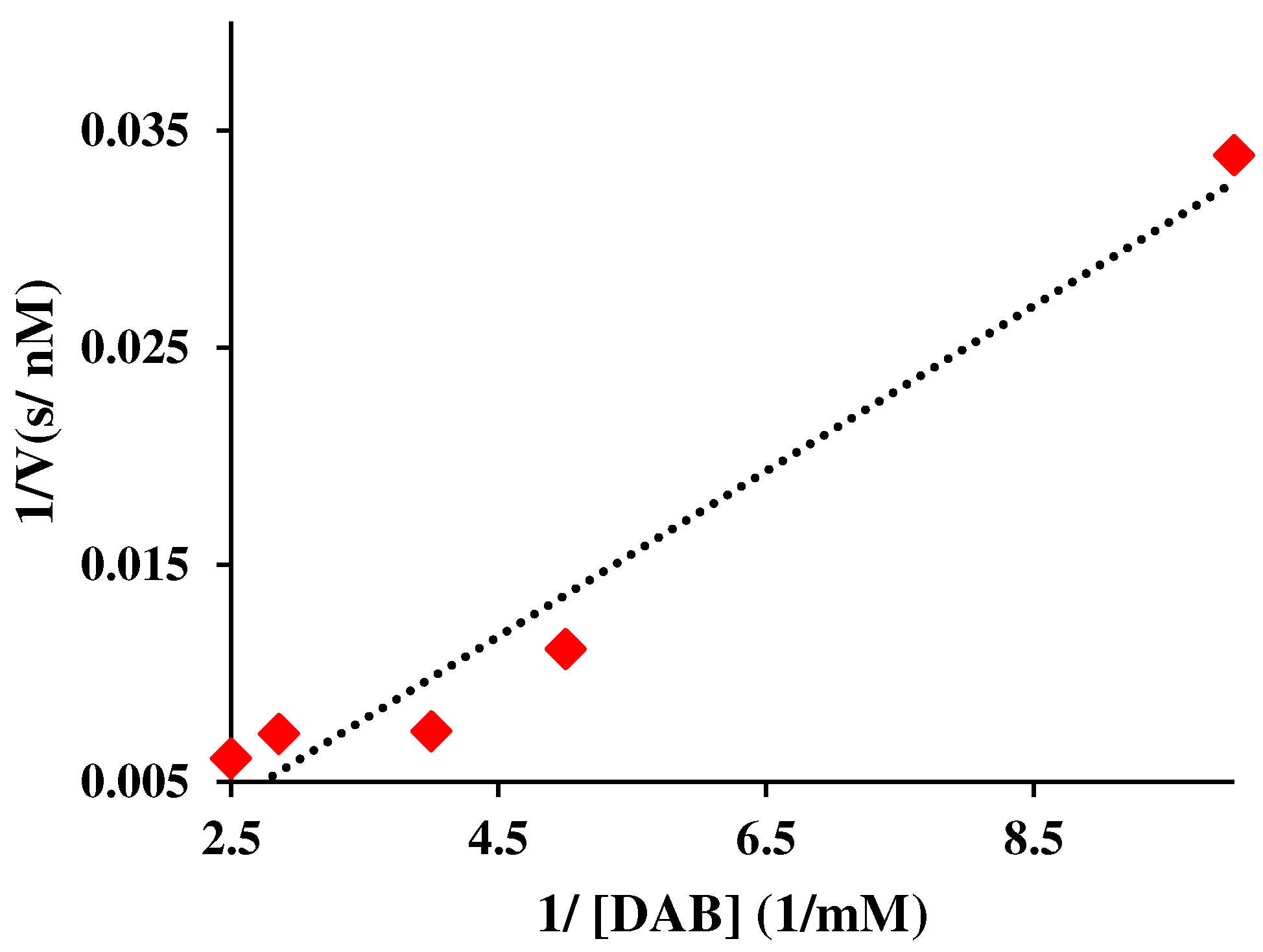
3.2. Quantification of kinetic parameters utilizing Lineweaver-Burk linear model
4. Conclusions
Acknowledgments
References
- Zhou, Y., Liu, B., Yang, R., & Liu, J. (2017). Filling in the gaps between nanozymes and enzymes: challenges and opportunities. Bioconjugate chemistry, 28(12), 2903-2909. [CrossRef]
- Hormozi Jangi, S. R., & Akhond, M. (2021). High throughput urease immobilization onto a new metal-organic framework called nanosized electroactive quasi-coral-340 (NEQC-340) for water treatment and safe blood cleaning. Process Biochemistry, 105, 79-90. [CrossRef]
- Hormozi Jangi, S. R., & Akhond, M. (2022). Introducing a covalent thiol-based protected immobilized acetylcholinesterase with enhanced enzymatic performances for biosynthesis of esters. Process Biochemistry, 120, 138-155. [CrossRef]
- Hormozi Jangi, S. R., Akhond, M., & Dehghani, Z. (2020). High throughput covalent immobilization process for improvement of shelf-life, operational cycles, relative activity in organic media and enzymatic kinetics of urease and its application for urea removal from water samples. Process Biochemistry, 90, 102-112. [CrossRef]
- Hormozi Jangi, S. R. (2023). Synthesis and characterization of magnesium-based metal-organic frameworks and investigating the effect of coordination solvent on their biocompatibility. Chemical Research and Nanomaterials, 1(4), 1-9.
- Hormozi Jangi, A. R., Hormozi Jangi, M. R., & Hormozi Jangi, S. R. (2020). Detection mechanism and classification of design principles of peroxidase mimic based colorimetric sensors: A brief overview. Chinese Journal of Chemical Engineering, 28(6), 1492-1503. [CrossRef]
- Hormozi Jangi, S. R. (2023). Low-temperature destructive hydrodechlorination of long-chain chlorinated paraffins to diesel and gasoline range hydrocarbons over a novel low-cost reusable ZSM-5@ Al-MCM nanocatalyst: a new approach toward reuse instead of common mineralization. Chemical Papers, 1-15. [CrossRef]
- Hormozi Jangi S. R.; Akhond M. (2020). High throughput green reduction of tris (p-nitrophenyl) amine at ambient temperature over homogenous AgNPs as H-transfer catalyst. Journal of Chemical Sciences, 132, 1-8.
- Zeng, X., Ruan, Y., Chen, Q., Yan, S., & Huang, W. (2023). Biocatalytic cascade in tumor microenvironment with a Fe2O3/Au hybrid nanozyme for synergistic treatment of triple negative breast cancer. Chemical Engineering Journal, 452, 138422.
- Hormozi Jangi, S. R., & Dehghani, Z. (2023). Kinetics and biochemical characterization of silver nanozymes and investigating impact of storage conditions on their activity and shelf-life. Chemical Research and Nanomaterials, 1(4), 25-33.
- Hormozi Jangi, S. R., & Dehghani, Z. (2023). Spectrophotometric quantification of hydrogen peroxide utilizing silver nanozyme. Chemical Research and Nanomaterials. https://crn.shiraz.iau.ir/article_701960.html?lang=en.
- Li, W., Chen, B., Zhang, H., Sun, Y., Wang, J., Zhang, J., & Fu, Y. (2015). BSA-stabilized Pt nanozyme for peroxidase mimetics and its application on colorimetric detection of mercury (II) ions. Biosensors and Bioelectronics, 66, 251-258. [CrossRef]
- Du, C., Gao, Y., Chen, H., Li, P., Zhu, S., Wang, J., ... & Chen, W. (2020). A Cu and Fe dual-atom nanozyme mimicking cytochrome c oxidase to boost the oxygen reduction reaction. Journal of Materials Chemistry A, 8(33), 16994-17001. [CrossRef]
- Hormozi Jangi, S. R., & Akhond, M. (2020). Synthesis and characterization of a novel metal-organic framework called nanosized electroactive quasi-coral-340 (NEQC-340) and its application for constructing a reusable nanozyme-based sensor for selective and sensitive glutathione quantification. Microchemical Journal, 158, 105328. [CrossRef]
- Hormozi Jangi, S. R., & Akhond, M. (2021). Ultrasensitive label-free enantioselective quantification of d-/l-leucine enantiomers with a novel detection mechanism using an ultra-small high-quantum yield N-doped CDs prepared by a novel highly fast solvent-free method. Sensors and Actuators B: Chemical, 339, 129901.
- Ahmadi-Leilakouhi, B., Hormozi Jangi, S. R., & Khorshidi, A. (2023). Introducing a novel photo-induced nanozymatic method for high throughput reusable biodegradation of organic dyes. Chemical Papers, 77(2), 1033-1046. [CrossRef]
- Akhond, M., Hormozi Jangi, S. R., Barzegar, S., & Absalan, G. (2020). Introducing a nanozyme-based sensor for selective and sensitive detection of mercury (II) using its inhibiting effect on production of an indamine polymer through a stable n-electron irreversible system. Chemical Papers, 74, 1321-1330. [CrossRef]
- Hormozi Jangi, S. R., Akhond, M., & Absalan, G. (2020). A novel selective and sensitive multinanozyme colorimetric method for glutathione detection by using an indamine polymer. Analytica Chimica Acta, 1127, 1-8. [CrossRef] [PubMed]
- Hormozi Jangi, S. R., Akhond, M., & Absalan, G. (2020). A field-applicable colorimetric assay for notorious explosive triacetone triperoxide through nanozyme-catalyzed irreversible oxidation of 3, 3′-diaminobenzidine. Microchimica Acta, 187, 431. [CrossRef]
- Hormozi Jangi, S. R. (2023). Effect of daylight and air oxygen on nanozymatic activity of unmodified silver nanoparticles: Shelf-stability. Qeios. [CrossRef]
- Hormozi Jangi, S. R., Davoudli, H. K., Delshad, Y., Hormozi Jangi, M. R., & Hormozi Jangi, A. R. H. (2020). A novel and reusable multinanozyme system for sensitive and selective quantification of hydrogen peroxide and highly efficient degradation of organic dye. Surfaces and Interfaces, 21, 100771.
- Chen, J., Hu, S., Cai, Y., Liu, X., Wu, Y., Dai, Y., & Wang, Z. (2022). Co–N/C-900 metal–organic framework-derived nanozyme as a H 2 O 2-free oxidase mimic for the colorimetric sensing of l-cysteine. Analyst, 147(5), 915-922.
- Sharma, B., Dangi, A. K., & Shukla, P. (2018). Contemporary enzyme based technologies for bioremediation: a review. Journal of environmental management, 210, 10-22. [CrossRef]
- Li, Z., Hu, J., Zhan, Y., Shao, Z., Gao, M., Yao, Q., ... & Wang, L. (2023). Coupling Bifunctional Nanozyme-Mediated Catalytic Signal Amplification and Label-Free SERS with Immunoassays for Ultrasensitive Detection of Pathogens in Milk Samples. Analytical Chemistry, 95(15), 6417-6424. [CrossRef]
- Li, T., Wang, Y., Liu, W., Fei, H., Guo, C., & Wei, H. (2023). Nanoconfinement-Guided Construction of Nanozymes for Determining H2O2 Produced by Sonication. Angewandte Chemie International Edition, 62(12), e202212438.
- Jangi, S. R. H. (2023). Determining kinetics parameters of bovine serum albumin-protected gold nanozymes toward different substrates. Qeios. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




