1. Introduction
Many challenges remain to understand the basis, and lack of success, in the treatment of polycystic ovary syndrome (PCOS), a clinical heterogeneous condition characterized by ovarian dysfunction, hyperandrogenism and polycystic ovaries [
1,
2]. This is so in part because there are still knowledge gaps regarding the pathophysiology of the syndrome, but also because patients with PCOS use to seek medical attention only if they experience severe clinical manifestations, or for reproductive reasons and, consequently, the treatment is frequently tailored to improve specific manifestations or to comply particular requirements of individual needs. Thus, the existence of metabolic conditions commonly associated with the syndrome, such as diabetes, dyslipidemia, and cardiovascular and hepatic diseases [
2,
3] are usually not investigated. Scientific evidence suggests that androgen excess play a critical role in the pathophysiology of metabolic disturbances [
3,
4], but it is unclear if such disturbances are related with obesity instead, since PCOS usually coexist with this phenotype. Identifying the contribution of obesity and hyperandrogenemia to the risk of metabolic clinical conditions, and recognizing the importance of the identification of early metabolic alterations is a priority, since these would allow to take prompt actions to delineate the appropriate therapeutic approach to modify the natural history of the syndrome.
The metabolic PCOS-related morbidities have been extensively assessed in population-based studies and in a number of meta-analyses [
5,
6,
7,
8,
9,
10]. Although studies coincide in that patients with PCOS are at risk of metabolic disorders, almost neither of those studies identify the isolated contribution of androgen excess or obesity. Few studies have made some approach by eliminating the effect of obesity comparing lean-PCOS with lean-controls [
5], or adjusting by nutritional status [
6,
10], but results remain inconsistent.
From the clinical perspective, that information is relevant for choosing the appropriate medical treatment. However, to properly assign the deleterious effect either to obesity or to hyperandrogenemia, it is essential to exclude the influence of other factors also implicated in the development of metabolic conditions, such as age, sedentary life styles, dyslipidemia, smoking, and the type of dietary lipids intake [
3,
11,
12,
13]. Nevertheless, this is not an easy task, because most of those factors are interrelated.
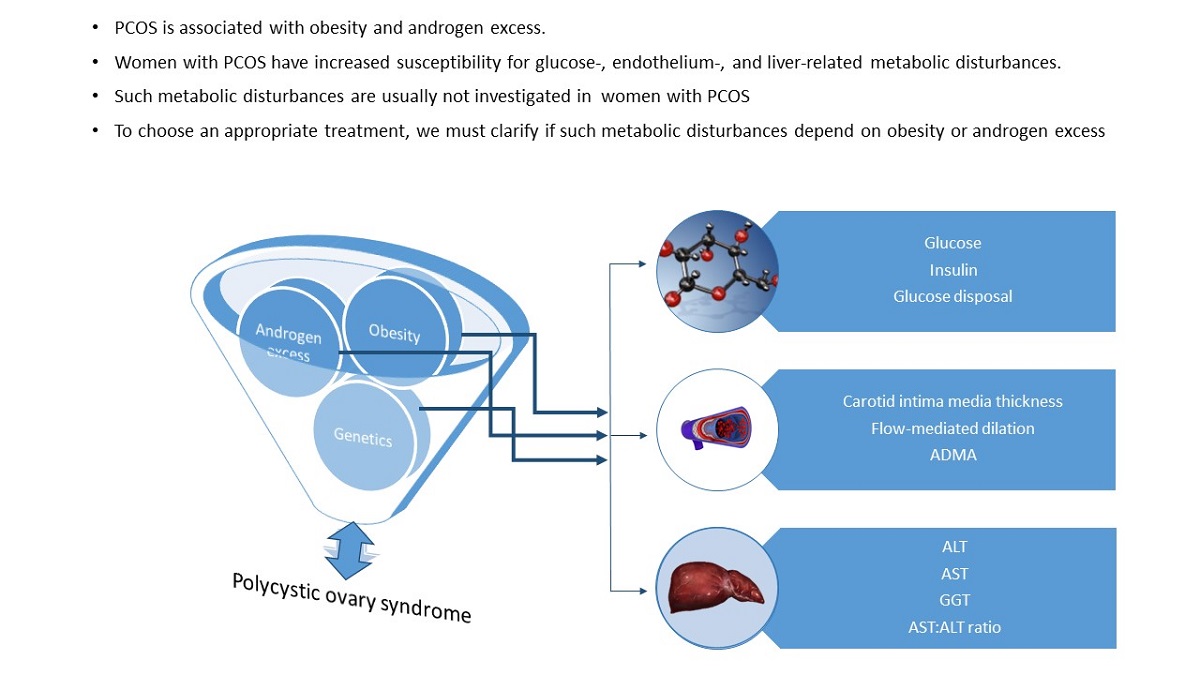
Under the hypothesis that androgen excess is the main contributor of metabolic alterations in PCOS patients, in the present study we aimed to separate the contribution of androgen excess and obesity on the susceptibility to develop metabolic disorders in women with PCOS. The contribution of hyperandrogenemia and obesity to the risk of developing glucose-, lipids-, endothelium-, and liver-related metabolic disturbances was analyzed considering the influence of important confounders. The frequency of metabolic disorders was compared among women with or without hyperandrogenemia, obese or lean.
2. Materials and Methods
2.1. Design
A cross-sectional study in PCOS patients and unrelated controls was conducted in hospitals from the Instituto Mexicano del Seguro Social (IMSS) in Mexico City. Participants were enrolled between September 2018 and January 2020. PCOS patients were recruited in the Department of Reproductive Medicine from a Gynecologic hospital, the research procedures in the Unit of Research in Medical Nutrition, and the ultrasound measurements in the Department of Health Research and Education of a Cardiologic hospital. Non-PCOS women were recruited in the waiting rooms of participating hospitals. The protocol was authorized by the National Committee of Scientific Research of the IMSS (R-2018-785-101). Written informed consent was obtained from all participants. The study follows the STROB reporting guideline.
We enrolled women with or without PCOS according with the Rotterdam criteria [
1], with obesity (BMI >30 kg/m2) or lean (BMI <25 kg/m2). For sampling, we recruited women diagnosed with PCOS, with or without obesity and, in parallel, an intentional search for women with obesity or lean but without the syndrome was made. Selected women were 18-38 years old, not taking medication, hormones or supplements, non-smokers, without known diabetes, hypertension, or cardiovascular disease. We excluded non-PCOS women who were kin of a PCOS patient. Field workers were trained and standardized for all the study procedures. A first appointment was arranged to explain the protocol procedures, measuring blood pressure and anthropometry, and signing the informed consent form. A second appointment was scheduled within a week at 7:00 AM to measure ultrasound variables, apply a 24-h recall questionnaire, and conduct a hyperinsulinemic-euglycemic clamp. All women underwent a pregnancy test before starting the clamp. Fasting blood samples were obtained, centrifuged at 3000 rpm, and serum aliquots reserved at -20 °C until biochemical determinations.
2.2. Measurements
To determine the common carotid intima-media thickness (CIMT), women were placed in supine position with the neck extended and rotated 45 degrees. The transductor was positioned at 45-50 degrees inclination (Samsung Medison, Sonoace R3 model 7.0-13 MHz linear transductor. Seoul, Korea). The measurement point was set in the proximal carotid at 3-4 cm from the bulb with the ultrasound beam directed perpendicularly to the carotid to identify the intima media. Carotid intima media thickness (CIMT) was measured from the intima blood interface to the adventitia media interface [
14]. Right and left sides were measured, the highest value was used for analysis. To determine the brachial artery flow-mediated dilation (FMD), women were placed on their left side with the right arm extended. Blood pressure was measured with a manual sphygmomanometer (Check A Teck by Hergom, B2_D model), the ultrasound transductor positioned on the right arm, the brachial artery identified, and the diameter registered. Immediately after, the sphygmomanometer cuff was placed 2 cm above the antecubital fold and insufflated during 5 minutes 50 mmHg above the systolic blood pressure taken at the beginning. After 60 seconds of cuff deflating, the diameter of the artery was measured again. FMD was calculated with the formula: D1-D0/D0x100 (where D0 and D1 are the first and second measured diameter) [
15].
Multiple-pass 24-h recall questionnaires were obtained in one occasion [
16]. Nutrient quantification was performed with the Food Processor software (v11.7, 2000, ESHA Research Inc., Salem, OR). Energy and nutrients intake were expressed as the percentage of recommendations based on Recommended Dietary Intake [
17]. Women were asked if they were involved in any structured exercise routine, the expected response was yes or no. If the response was yes, the time in minutes was registered.
Clamps were conducted as proposed by DeFronzo [
18] at 8:00 morning after 10h fasting. An antecubital venous catheter was placed to administer glucose and insulin infusions. A second retrograde catheter was placed in the opposite hand for sample collection while the hand was kept in a heating device. Blood samples were drawn at 5-min intervals for glucose determinations (0.5 ml). Insulin infusion started at 80 mU/m2 body surface area (BSA) during 10 min, followed by a constant infusion of 40 mU/m2 BSA. A solution of 20% glucose was administered at a variable rate to maintain plasma glucose at 90 mg/dl. The mean glucose infusion rate (M, mg/kg/min) was assessed during the last 30 min when the steady-state was reached (glucose concentration at 90 ± 3 mg/dL).
Serum glucose, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transferase (GGT), triglycerides, total cholesterol, HDL and VLDL (Spinreact. Sant Esteve De Bas, Spain) were determined by enzymatic analysis (YSI 2300 Stat Plus Glucose Analyzer, YSI Inc., Yellow Springs OH, USA). LDL was estimated with the Friedewald method [
19]. Insulin and the steroid hormone binding globulin (SHBG) were measured by chemiluminesence (Immulite, Siemens; UK), arginine and asymmetric dimethyl arginine (ADMA) by high-performance liquid chromatography (Waters, ACQUITY UPLC system; Milford MA, USA), and total testosterone (TT) by UPLC-MS/MS (Waters Xevo TQD Acquity UPLC H; Milford MA, USA). Free testosterone (FT) was calculated with the Vermeulen method [
20]. Variation coefficients of biochemical assays were between 5-10%.
2.3. Diagnosis of Metabolic Disorders
The cutoff points used for diagnosis were: M <5.7 mg/kg/min for insulin resistance [
21]; fasting glucose >100 mg/dL for prediabetes [
22]; ADMA >0.88 μmol/L [
23], CIMT >0.5 mm [
14] or FMD <10% [
15] for endothelial dysfunction; ALT >30 U/L [
24] or AST:ALT ratio <1.0 for hepatic damage [
25]; non-HDL >144 mg/dL for dyslipidemia [
13], and blood pressure >120/80 for hypertension [
26]. FT >5.6 pg/mL was used to identify hyperandrogenemia [
27] and women were classified with obesity if BMI ≥30 kg/m2, or lean if BMI ≤25 kg/m2 [
28].
2.4. Statistical Analysis
To calculate sample size we used the information reported by Pradisi et al (29), who assessed lipid profile, insulin, and endothelial function in PCOS and non-PCOS women with obesity. A correlation coefficient between leg blood flow and free testosterone -0.52, 80% beta, 0.05 alpha, as well as a mean lipoproteins difference of 8 mg/dL between PCOS and non-PCOS overweight women were used. The calculated sample size was 51 PCOS and 51 non-PCOS women. Forty women were added to conduct a stratified analysis according with obesity and hyperandrogenemia statuses. The estimated sample size was 142 women.
The Minitab statistical package (v19, State College, Pennsylvania) was used for analysis. A p-value ≤0.05 was considered for statistical significance. Quantitative variables were described with means, standard errors (SEM) or 95% confidence intervals (95%CI), and qualitative variables with proportions. Equality of variances was analyzed with the Levene test. Differences between two independent groups were analyzed with Student t-test, and multiple comparisons with one-way ANOVA and Bonferroni post-hoc test. Pearson correlation analyses were carried to identify univariate associations between biomarkers. Associations among qualitative variables were evaluated with χ2 analyses.
The influence of obesity and hyperandrogenemia on metabolic disturbances was analyzed with multivariate linear regression models introducing biomarkers as dependent variables and BMI and FT as predictor. All models were adjusted by confounders including age, non-HDL concentration, and the dietary intake of saturated fat, and ω-3 and ω-6 fatty acids. Because dietary information was not available for four women, these models were carried with and without these variables, but coefficients and statistical significance did not change. Interactions between BMI and FT were assessed. The variance inflation factor was used to evaluate collinearity. In a different analytical approach, the association between FT and biomarkers was analyzed introducing obesity as a covariate.
To separate the influence of obesity (OB) and hyperandrogenemia (HA) and their interaction, on the studied metabolic disorders, women were categorized into four groups: Healthy (no-HA, lean), HA (HA, lean), OB (no-HA, OB) and HAOB (HA and OB). The risk of glucose-, lipids- , endothelial-, and hepatic-related disorders were assessed with logistic regression analyses, introducing the stratified groups as predictors. Models were adjusted by age, dyslipidemia, and dietary lipids (total lipids, saturated fat, cholesterol, ω-3 and 6 fatty acids).
3. Results
3.1. General Characteristics
We studied 140 women, 29 ± 5.4 years old, BMI 30.4 ± 6.4 kg/m2, daily energy intake 2387 ± 214 kcal, total lipids intake 113 ± 6% of recommendations, saturated fat 112 ± 7%, and omega-3 fatty acids 45.4 ± 4.4%. None of the women reported to be engaged in a structured exercise routine. As expected, significant interrelations were observed among the studied variables in simple correlation analyses (
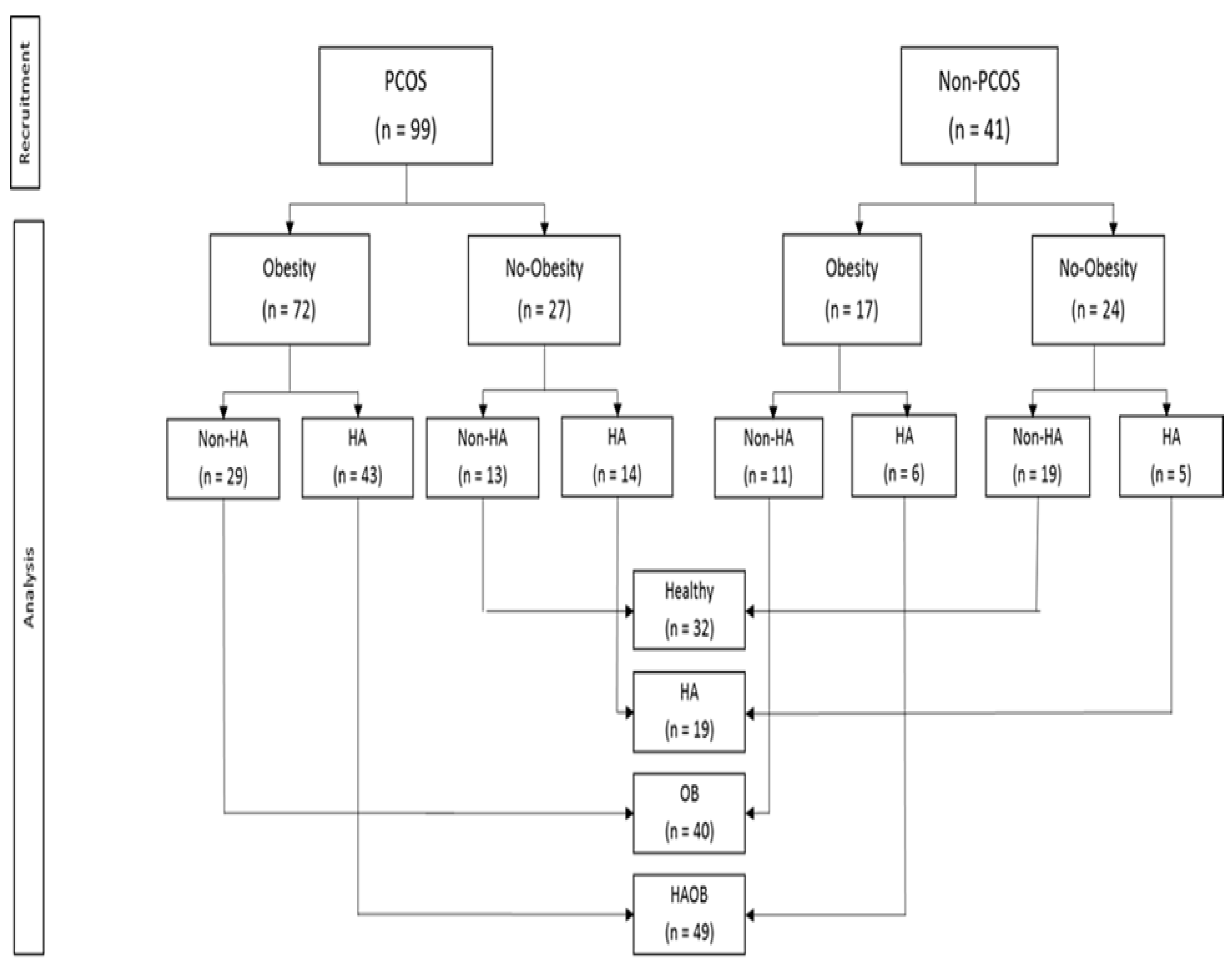
Table S1: Univariate correlations). Ninety-nine women met the diagnostic criteria for PCOS.
The average of most measurements in PCOS patients were different than controls and out of reference limits (
Table S2: Clinical and biochemical characteristics of woemn with or without PCOS). The frequency of insulin resistance (68% vs 22%), dyslipidemia (32% vs 12%), endothelial dysfunction (79% vs 61%), carotid intima media thickening (58% vs 39%), and liver disturbances (89% vs 63%) were significantly higher in PCOS than in non-PCOS groups. Mean (±SE) BMI (31.77 ± 0.59 vs 27.18 ± 1.0 kg/m2, p <0.001) and free testosterone (8.2 ± 0.60 vs 4.6 ± 0.63 pg/mL, p <0.001)) were also higher in PCOS than in non-PCOS. Obesity was observed in 72 (73%) PCOS and 17 (42%) non-PCOS, and hyperandrogenemia in 57 (58%) and 11 (27%) respectively (
Figure 1).
3.2. Associations between Free Testosterone and BMI with Biomarkers of Metabolic Disorders
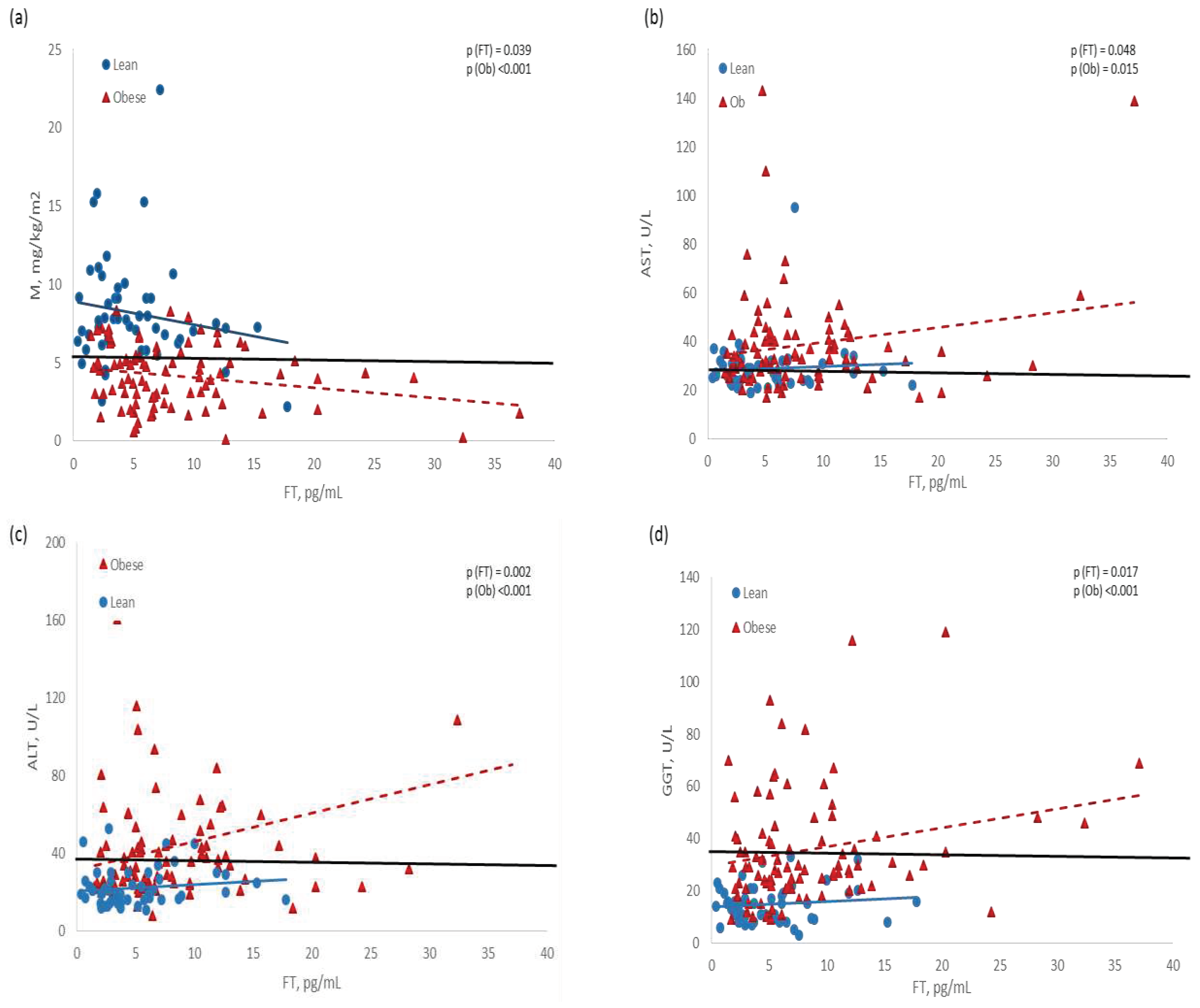
Adjusted linear models demonstrated that BMI was related with almost all the metabolic biomarkers, while FT was associated just with glucose- and liver-related biomarkers (
Table 1). Dietary intake of ω-3 fatty acids was associated with insulin sensitivity (M = 0.011 ± 0.005, p = 0.020), and non-HDL with liver enzymes (GGT = 13.92 ± 3.53, p <0.001), but these variables did not modify the associations of BMI and FT with biomarkers.
With another statistical approach, we observed that FT was inversely associated with M, and directly with liver enzymes, but women with obesity exhibited lower M values, and higher concentration of enzymes than lean women, at the same level of FT. The regression line of normal weight women was mostly within normal ranges (
Figure 2).
3.3. Contribution of Obesity and Hyperandrogenemia to the Susceptibility of Metabolic Disorders
The studied biomarkers were compared among 32 Healthy, 19 HA, 40 OB and 49 HAOB women. Most of these biomarkers were comparable between lean groups (Healthy vs HA), and between the groups with obesity (OB vs HAOB), but different between lean and obese (
Table 2). Hypertension (8%) and prediabetes (11%) were observed exclusively in the two groups with obesity. In general, the proportion of women with measurements out of reference limits was higher in the two groups with obesity than in lean groups (
Table 3).
Accordingly, the risk of metabolic disturbances was several orders of magnitude higher in OB and HAOB groups than in Healthy, and the risk of exhibiting M, HDL, CIMT and liver enzymes out of normal ranges in women with obesity was also greater than those with hyperandrogenemia but lean. The risk of showing elevated liver enzymes was associated with dyslipidemia, but the risks of the obesity groups remained unchanged. Finally, the susceptibility for metabolic disturbances was comparable between OB and HAOB groups (
Table 3).
4. Discussion
In the present study we demonstrated that obesity is the main contributor to the development of metabolic disorders in patients with PCOS. Yet, we also identified a mild but consistent effect of androgens on glucose- and hepatic-related biomarkers that was independent of obesity. We also confirmed the high frequency of metabolic disorders in women with PCOS, as reported by others [
2,
5,
8,
9,
10], and additionally, we provide data concerning the high frequency of metabolic alterations that have not yet been revealed as a pathological entity.
Although the harmful effect of both obesity and androgen excess in patients with PCOS has been already reported [
2,
3,
4], our stratified analysis allowed to discriminate the contribution of each to the risk of metabolic disturbances, which is paramount to choose the appropriate therapeutic approach. This analysis revealed that the influence of obesity is stronger than that of androgen excess, since the two groups with obesity showed comparable risks of metabolic disturbances regardless hyperandrogenemia, and higher values than the lean ones. Such results show the predominant participation of obesity in the development of metabolic diseases in PCOS patients. Nevertheless, since the group of women with hyperandrogenemia but lean showed consistently intermediate risks between Healthy and OB, the participation of androgen excess on the susceptibility of metabolic disturbances could not be ruled out. Indeed, the potential influence of androgens on metabolism was observed in the linear regression models, which demonstrated a dose-response association between androgens and glucose- and liver-related biomarkers, that is., the higher the concentration of FT, the lower the M and the higher the hepatic enzymes concentration, even within normal ranges. We interpret these results as an evidence of a mild but consistent influence of androgens on metabolism which, if persists, may progress toward a pathological condition.
Our study confirmed the high frequency of clinical conditions in PCOS patients, including dyslipidemia, thickened carotid and hepatic damage, reported in other studies [
2,
5,
8,
9,
10], but we also provide additional evidence regarding the high frequency of metabolic alterations such as decreased insulin sensitivity and increased biomarkers of endothelial and hepatic alterations, which have not reached the cutoff to diagnose a clinical condition, but which are precursors of chronic pathologies. This is clinically relevant because it underlies the need to intentionally search for these disturbances in order to prevent progression toward an irreversible condition such as diabetes, endothelial dysfunction and NAFLD.
We recognize some limitations, mainly the small sample of women in the HA group. Although this circumstance may lead to lack of power to detect differences among groups as observed in the stratified analysis, other statistical approaches used in our study, which utilized continuous data instead of stratification, permitted identifying the androgenic effect with a reasonable certainty. Another limitation is the identification of hepatic alterations using liver enzymes concentration. We are aware that these enzymes increase in a non-specific way in response to liver injuries from different etiologies (e.g. metabolic, viral, toxic, and alcoholic), and therefore, their elevation is not a specific indication of metabolic hepatic damage. In our study, none of participants recognized alcohol consumption, but viral infection or toxic damage were not investigated. Nevertheless, we think that by using an ALT cutoff point proposed to identify healthy population, derived from a hug sample of individuals without risk factors for liver disease, and validated for its ability to predict liver damage [
24], increases the probability that our result are correctly interpreted. Besides, the association of liver enzymes with obesity and hyperandrogenemia was consistent throughout the different analysis, suggesting the plausibility of our interpretation. Finally, we recognize that we studied a selected particular population since the participants were recruited from a hospital that attends fertility cases, and they were intentionally selected if meeting the selection criteria until completing the proposed sample size. Therefore, our results may be generalized only to women from similar characteristics.
Yet, our study have important strengths worth to highlight. The stratified analysis allowed to disentangle the role of obesity and hyperandrogenemia, providing robust evidence re the predominant role of obesity over that of androgen excess. This is relevant from the clinical perspective because it offers the basis to select the most appropriate therapeutic approach. In addition, we used cutting edge technology to measure important variables, obtaining therefore accurate data of insulin sensitivity (clamp), testosterone (mass-spectrometry), and endothelial dysfunction (two biochemical -ADMA and arginine, and two ultrasound -CIMT and FMD), which improved the opportunity to detecting any possible effect. Moreover, we excluded women with overweight to increase the probability of identifying differences in the metabolic profiles of women with obesity or lean. Furthermore, we considered the influence of important confounders like dyslipidemia and dietary lipids, that are usually associated with the risk of metabolic disorders, but which are not usually considered in similar studies. We think that all these characteristics made our results robust and reliable.
The recommendations of the American College of Obstetricians and Gynecologists for the management of patients with PCOS include the evaluation of BMI, blood pressure, and laboratory documentation of hyperandrogenemia and metabolic abnormalities such as a two-hour glucose tolerance test, as well as fasting lipids and lipoprotein concentrations [
30]. Our results support these recommendations and, additionally, provides sufficient information to encourage an intentional search for liver abnormalities. We are certain that giving specific recommendations for the management of PCOS is beyond the scope of our study, yet it is important to point out the importance to treat obesity. On this regard, an issue that deserves attention is the effect of a low intake of omega-3 polyunsaturated fatty acids on insulin sensitivity observed in our study. Despite the dietary information obtained with just one 24-h recall questionnaire is a limitation to interpret the influence of nutrients intake, our results are consistent with others found in studies conducted in Mexico by our team [
12] and by others [
31], suggesting that the extremely low intake of these fatty acids in Mexican population is a reliable circumstance. The topic is important because evidence from experimental studies demonstrate that the long-chain polyunsaturated fatty acids omega-3 are involved in insulin sensitivity and in the protection of endothelium and liver [
32,
33]. On this regard, we previously reported that erythrocytes eicosapentanoic acid, and the supplementation with docosahexaenoic and eicosapentaenoic acid, were associated with an improved androgenic profile of pubertal girls with obesity [
34]. We should have this in mind when planning the treatment of PCOS patients. On the other hand, the lack of association between metabolic alterations and the saturated fat intake is probably explained by the close to recommendations intake of these nutrients.
5. Conclusions
In conclusion, our results have potential clinical implications in the choice of treatment for PCOS patients, as well as in the prevention of metabolic disturbances and the progression to irreversible conditions. Our study also highlight the need for a major effort aimed at improving the detection of metabolic disturbances in clinical settings. It is expected that the focus on these situations will help clinicians, and health authorities, to make better decisions in the management of PCOS patients to limit the evolution to irreversible metabolic conditions.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Table S1: Univariate correlations; Table S2: Clinical and biochemical characteristics.
Author Contributions
Conceptualization, MLA and VSVR; methodology, MLA and VSVR; validation, EAG and JMH; formal analysis, MLA; resources, MLA, VSVR and EAG; data curation, JMH, SFC, RZCL and JMDS; writing—original draft preparation, MLA, VSVR and ASBV; writing—review and editing, MLA, VSVR and RZCL; visualization and supervision JVB, DCM and ASBV; project administration, MLA.; funding acquisition, MLA. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council of Science and Technology (CONACYT) Mexico (grant #: FOSSIS 2017-1-290399).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the National Committee of Scientific Research of the Instituto Mexicano del Seguro Social in August, 2018 (R-2018-785-101).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results”.
References
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;19:41-7. [CrossRef]
- American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 194: Polycystic Ovary Syndrome. Obstet Gynecol 2018;131:e157-e171. [CrossRef]
- Moghetti P, Tosi F, Bonin C, Di Sarra D, Fiers T, Kaufman JM, et al. Divergences in insulin resistance between the different phenotypes of the polycystic ovary syndrome. J Clin Endocrinol Metab 2013;98:E628-37. [CrossRef]
- Kakoly NS, Earnest A, Teede HJ, Moran LJ, Joham AE. The Impact of Obesity on the Incidence of Type 2 Diabetes among women with polycystic ovary syndrome. Diabetes Care 2019;42:560-7. [CrossRef]
- Zhu S, Zhang B, Jiang X, Li Z, Zhao S, Cui L, et al. Metabolic disturbances in non-obese women with polycystic ovary syndrome: a systematic review and meta-analysis. Fertil Steril 2019;111:168-77. [CrossRef]
- Ollila MM, West S, Keinänen-Kiukaanniemi S, Jokelainen J, Auvinen J, Puukka K, et al. Overweight and obese but not normal weight women with PCOS are at increased risk of Type 2 diabetes mellitus-a prospective, population-based cohort study. Hum Reprod 2017;32:423-31. [CrossRef]
- Zhou Y, Wang X, Jiang Y, Ma H, Chen L, Lai C, et al. Association between polycystic ovary syndrome and the risk of stroke and all-cause mortality: insights from a meta-analysis. Gynecol Endocrinol 2017;33:904-10. [CrossRef]
- Wekker V, van Dammen L, Koning A, Heida KY, Painter RC, Limpens J, et al. Long-term cardiometabolic disease risk in women with PCOS: a systematic review and meta-analysis. Hum Reprod Update 2020;26:942-960. [CrossRef]
- Rocha ALL, Faria LC, Guimarães TCM, Moreira GV, Cândido AL, Couto CA, et al. Non-alcoholic fatty liver disease in women with polycystic ovary syndrome: systematic review and meta-analysis. J Endocrinol Invest 2017;40:1279-88. [CrossRef]
- Sarkar M, Terrault N, Chan W, Cedars MI, Huddleston HG, Duwaerts CC, et al. Polycystic ovary syndrome (PCOS) is associated with NASH severity and advanced fibrosis. Liver Int 2020;40:355-9. [CrossRef]
- Nettleton JA, Villalpando S, Cassani RS, Elmadfa I. Health significance of fat quality in the diet. Ann Nutr Metab 2013;63:96-102. [CrossRef]
- López-Alarcón M, Perichart-Perera O, Flores-Huerta S, Inda-Icaza P, Rodríguez-Cruz M, Armenta-Álvarez A, et al. Excessive refined carbohydrates and scarce micronutrients intakes increase inflammatory mediators and insulin resistance in prepubertal and pubertal obese children independently of obesity. Mediators Inflamm 2014;2014:849031. [CrossRef]
- Brunner FJ, Waldeyer C, Ojeda F, Salomaa V, Kee F, Sans S, et al. Application of non-HDL cholesterol for population-based cardiovascular risk stratification: results from the Multinational Cardiovascular Risk Consortium. Lancet 2019;394:2173-83. [CrossRef]
- Touboul PJ, Grobbee DE, den Ruijter H. Assessment of subclinical atherosclerosis by carotid intima media thickness: technical issues. Eur J Prev Cardiol 2012;19:18-24. [CrossRef]
- Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 2009;120:502-9. [CrossRef]
- Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: an observational validation study. J Am Diet Asoc 2004;104:595-603. [CrossRef]
- FAO and IOM, Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). 1st Ed, National Institutes of Health, Washington, DC, USA, National Academy Press, 2005.
- DeFronzo RA, Tobin JD, Andrews R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214-23. [CrossRef]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499-502. [CrossRef]
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666-72. [CrossRef]
- Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocrine Rev 1985;6:45-86. [CrossRef]
- American Diabetes Association. Diagnosing Diabetes and Learning about Prediabetes. (Accessed on 28 May 2021); Available online: http://www.diabetes.org/diabetes-basics/diagnosis/.
- Németh B, Ajtay Z, Hejjel L, Ferenci T, Ábrám Z, Murányi E, et al. The issue of plasma asymmetric dimethylarginine reference range - A systematic review and meta-analysis. PLoS One 2017;12:e0177493. [CrossRef]
- Valenti L, Pelusi S, Bianco C, Ceriotti F, Berzuini A, Prat LI, et al. Definition of healthy ranges for alanine aminotransferase levels: A 2021 Update. Hepatol Commun 2021;5:1824-32. [CrossRef]
- Botros M, Sikaris KA. The De Ritis ratio: The test of time. Clin Biochem Rev 2013;34:117-130.
- World Health Organization. Diagnosis and management for patients with hypertension. World Health Organization 2017. ISBN 978 92 9061 797 6 (Accessed on 17 Feb 2022). https://apps.who.int › iris › rest › bitstreams › retrievepdf.
- Braunstein GD, Reitz RE, Buch A, Schnell D, Caulfield MP. Testosterone reference ranges in normal cycling healthy premenopausal women. J Sex Med 2011;8:2924-34. [CrossRef]
- World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation, 894. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1-253. (Accessed 17 March 2023). Available online https://apps.who.int/iris/handle/10665/42330.
- Paradisi G, Steinberg HO, Hempfling A, Cronin J, Hook G, Shepard MK, Baron AD. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation 2001;103:1410-5. [CrossRef]
- American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 194: Polycystic Ovary Syndrome. Obstet Gynecol 2018;131:e157-e171. [CrossRef]
- Ramírez-Silva I, Villalpando S, Moreno-Saracho JE, Bernal-Medina D. Fatty acids intake in the Mexican population. Results of the National Nutrition Survey 2006. Nutr Metab 2011;8:33-43. [CrossRef]
- Vafeiadou K, Weech M, Sharma V, Yaqoob P, Todd S, Williams CM, et al. A review of the evidence for the effects of total dietary fat, saturated, monounsaturated and n-6 polyunsaturated fatty acids on vascular function, endothelial progenitor cells and microparticles. Br J Nutr 2012;107:303-24. [CrossRef]
- Yang J, Fernández-Galilea M, Martínez-Fernández L, González-Muniesa P, Pérez-Chávez A, Martínez JA, et al. Oxidative stress and non-alcoholic fatty liver disease: Effects of omega-3 fatty acid supplementation. Nutrients 2019;11:872. [CrossRef]
- López-Alarcón MG, Vital-Reyes VS, Hernández-Hernández FI, Maldonado-Hernández J. The role of LCPUFA-ω3 on the obesity-associated hyperandrogenemia of pubertal girls: secondary analysis of a randomized clinical trial. J Pediatr Endocrinol Metab 2020;33:347-54. [CrossRef]
- Ceriotti F, Henny J, Queraltó J, Ziyu S, Ozarda Y, Chen B, et al. Common reference intervals for aspartate aminotransferase (AST), alanine aminotransferase (ALT) and g-glutamyl transferase (GGT) in serum: results from an IFCC multicenter study. Clin Chem Lab Med 2010;48:1593–1601.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).