1. Introduction
Hematopoiesis is closely related to bone. The bone marrow fills small and irregular marrow cavities produced by mesh-like networks of trabeculae called spongy bones, which are surrounded by compact bones. The hematopoietic stem cells (HSC) then attach to osteoblasts in the endosteum on the inside of the compact bone and on the surface of the trabeculae, which is called the endosteal niche, a preferential site of residence for the most potent HSC [
1]. Therefore, it is possible that a rich bone mass supports robust hematopoiesis, and studies have focused on the relationship between the number of white blood cells (WBC), red blood cells (RBC), and platelets (Plt), which reflect hematopoiesis, and bone mineral density (BMD). However, published work on the possible correlation between BMD and WBC, RBC, and particularly Plt levels have demonstrated conflicting results.
Several reports have shown a negative correlation between Plt count and BMD [
2,
3,
4]. Kristjansdottir HL, et al. conducted an analysis of 1,005 men (median age 75.3 years, range 69-81) and found that the relationship between lumbar spine BMD (L1-L4) and Plt count was r=-0.06, p=0.041, and that between total hip BMD and Plt count was r=-0.11, p=0.003 [
2]. Kim J, et al. examined 8,634 subjects, including men over 50 years of age and postmenopausal women [
4], and showed that in the highest Plt quartile, the odds ratio (95% confidence interval) of osteopenia vs. normal BMD was 1.39 (1.03-1.88), and that of osteoporosis vs. normal BMD was 1.60 (1.07-2.37). Furthermore, Li L, et al. analyzed 673 postmenopausal women and reported that the correlation between femur neck BMD and Plt count was r = -0.153, p = 0.001 [
3]. Several hypotheses have been proposed for the potential mechanism responsible for the negative correlation between Plt count and BMD, including the involvement of serotonin [
2], which is known to be negatively correlated with BMD [
5] and is found in abundance in Plt [
6].
On the other hand, there are published results that are not consistent with the findings above. Work by Valderrábano RJ, et al. [
7,
8]. The 2017 report included 2,571 men aged 65 years and older [
7], and the 2019 report included 5,888 men and women also aged 65 years and older [
8]. Meanwhile, other studies revealed a positive correlation between Plt count and BMD, with one by Schyrr F, et al. involving 143 patients prior to chemotherapy for breast cancer [
9] and another by Kim HL, et al. involving 338 postmenopausal women [
10].
Thus far, there has therefore been no definitive conclusion on the association between Plt count and BMD, nor has there been any analysis of the possible biological mechanism behind any reported association. In this paper, we demonstrate a negative correlation between BMD and Plt count in men, with women being strongly affected by age factors. We propose that the underlying biological mechanism may involve the receptor activator of nuclear factor-kappa B ligand (RANKL), which is secreted by megakaryocytes responsible for the production of platelets.
2. Results
2.1. Patient Characteristics
Table 1 presents the clinical characteristics of the 65 patients in our study (33 females and 32 males). There was no difference in age between men and women. All patients have malignant lymphomas, the majority of whom have the histologic type diffuse large B cell lymphoma (DLBCL). The proportion of patients with advanced disease (clinical stage III/IV) as compared to those with limited stage disease (clinical stage I/II) was higher in the female group than in the male group (p=0.031). For BMD, the female group was clearly lower than the male group for L2-L4, L1-L4, total hip, and femur neck (p<0.001). On the other hand, levels of WBC, neutrophils (Neu), lymphocytes (Lymph), RBC, hemoglobin (Hb), reticulocyte count (Ret), Plt, and tartrate-resistant acid phosphatase-5b (TRACP-5b), a marker of osteoclast number and bone resorption, showed no statistically significant difference between the sexes.
2.2. Correlation between BMD and Plt Count
As shown in
Table 2, all four BMDs decreased with age in the female group. TRACP-5b level was negatively correlated with BMD in L2-L4, L1-L4, and total hip. For CBC, positive correlations were observed between BMD and Lymph for L2-L4, L1-L4, and Total hip, but no correlation was observed between BMD and Plt. Results for the male group are presented in
Table 3. BMD did not decrease with age; on the contrary, BMD in L2-L4 and L1-L4 increased with age. TRACP-5b level was negatively correlated with BMD in L1-L4 and total hip. Negative correlations were found between BMD and WBC and Neu in total hip and femur neck with respect to CBC. Negative correlations were also found between BMD and Plt for total hip and femur neck (both r=-0.365, p=0.040). Since the negative correlation between BMD and Plt was confirmed only in male patients, the sample of female patients was not included in the subsequent analyses.
2.3. Correlation between BMD and Megakaryocyte-Produced RANKL
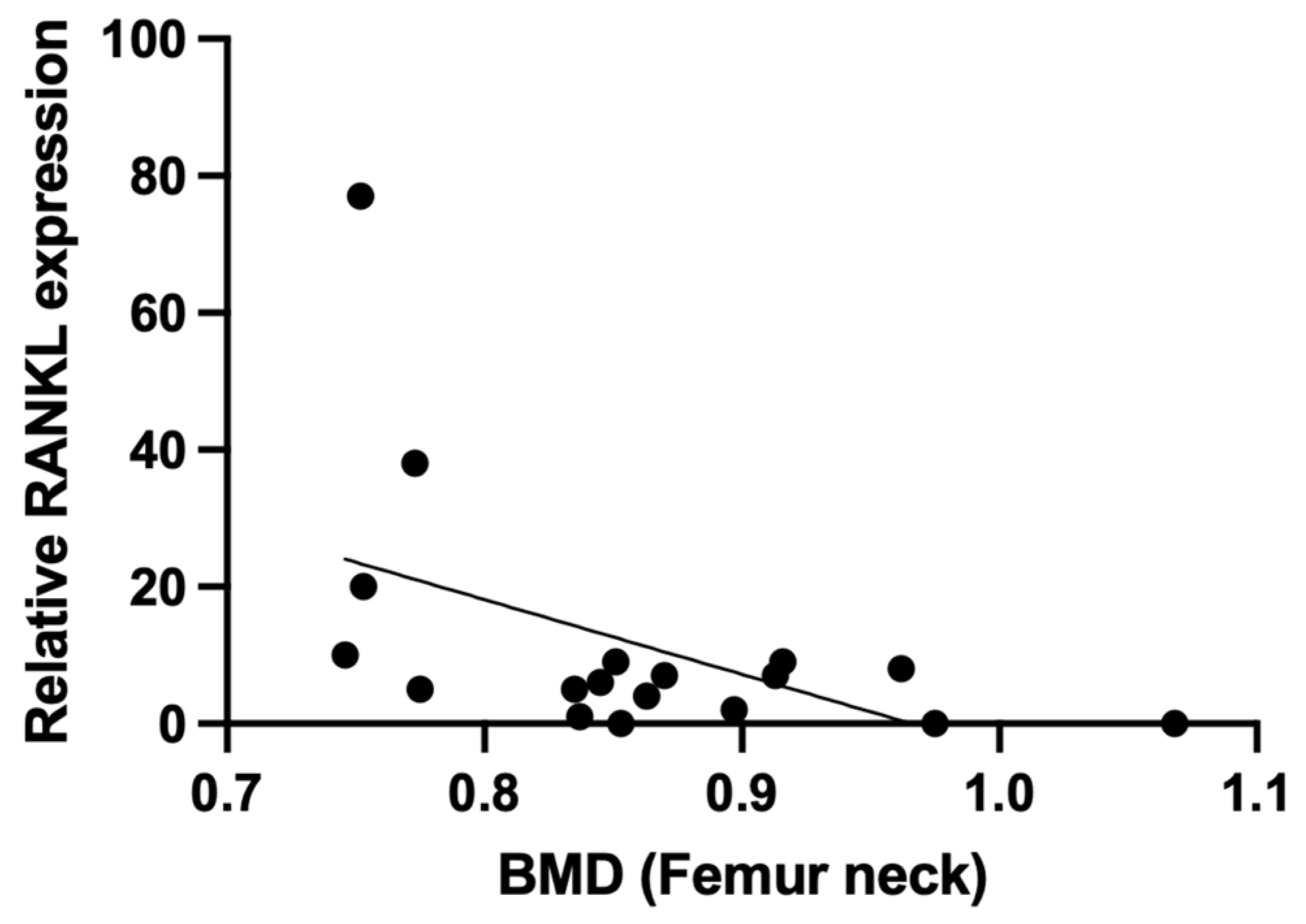
To evaluate for potential biological mechanism involved in the observed negative correlation between Plt count and BMD, we focused on BMD regulators secreted by megakaryocytes (MGK), which are responsible for Plt production. Bone marrow mononuclear cells (BM-MNC) from male patients were induced to differentiate into MGK, and the production of RANKL, osteoprotegerin (OPG), and macrophage colony stimulating factor (M-CSF) were examined as BMD regulators (
Table 4). MGK differentiation resulted in enhanced expression of CD41a, a marker of MGK (
Figure 1). Importantly, we observed a negative correlation between RANKL and BMD in the femur neck (r=-0.502, p=0.034) (
Table 4,
Figure 2).
3. Discussion
By excluding women and focusing only on men in our present study, we were able to find a negative correlation between Plt count and BMD. It is our assumption that such a correlation between Plt count and BMD is also present in women as well. However, since aging has an extremely potent effect on BMD in women (
Table 2), it may be difficult to delineate the relationship between BMD and other factors, independent of age. It is likely that a factor linking aging to lower BMD in women is lower level of estrogen [
11,
12], with age-related estrogen withdrawal in women leading to decreased BMD through a variety of mechanisms [
13].
In the female group in our study, there was a positive correlation between BMD and Lymph count (
Table 2), which may also be due to an estrogen effect. In a previous paper, it was reported that hormone replacement therapy with estrogen led to increased lymphocyte ratio in postmenopausal women [
14]. Therefore, it is possible that aging lowers estrogen levels, resulting in lymphocytopenia.
When considering the potential biological mechanism involved in the negative correlation between Plt count and BMD in the male group, we focused on MGK, given the dependence of Plt production on MGK. In addition, previous work [
15] demonstrated that increased MGK resulted in greater MGK-derived RANKL production, associated with higher level of osteoclasts in older mice than in younger mice. Since osteoclasts are responsible for BMD reduction, a factor linking increased Plt count with BMD reduction may be RANKL production by MGK. To evaluate this hypothesis, we examined the production of cytokines by MGK which can affect BMD, specifically RANKL, OPG, and M-CSF. RANKL and M-CSF induce osteoclast differentiation and decrease BMD, while OPG inhibits RANKL [
16]. Our work revealed a negative correlation between RANKL production and BMD (
Table 4) while level of TRACP-5b, an activation marker of osteoclasts induced to differentiate by RANKL and others, was negatively correlated with total hip BMD (
Table 3).
Besides Plt count, a negative correlation with BMD was observed for WBC in the male group, especially Neu count (
Table 3). This relationship between Neu count and BMD in a male cohort (n=2,571) was also reported by another group [
7]. Specifically, femur neck BMD was lower in men with higher Neu counts than in men with lower Neu counts (p=0.016), and the decrease in total hip and femur neck BMD over time was greater (p<0.001). This observed phenomenon may also be due to the involvement of RANKL, since a relationship between RANKL production in peripheral neutrophils and BMD loss has been reported in 59 male patients with chronic obstructive pulmonary disease (COPD) [
17].
In our study, the inverse association between Plt count and BMD was revealed by excluding women who are greatly affected by aging, associated with a decrease in estrogen. Searching for a potential biological mechanism, we focused on MGK, the source of platelet production, and found that MGK production of RANKL was inversely correlated with BMD. This meant that the production of platelets and RANKL by MGK was behind the inverse correlation between Plt count and BMD. However, there are limitations to our conclusion. Specifically, we did not formally examine the MGK population itself, but rather the MGK-like cells induced to differentiate from BM-MNCs. In the present study, we utilized BM-MNC isolated and cryopreserved from residual bone marrow samples as a retrospective analysis. These BM-MNC were then induced to differentiate into MGK, since BM-MNC isolated by the standard Ficoll purification method do not contain MGK. In the future, we plan to isolate MGK directly from fresh bone marrow samples as part of a prospective study to further confirm our present conclusions.
Furthermore, it may be difficult to fully analyze the role of bone marrow-derived cells in studies of human BMD, since BMD measurements and bone marrow aspiration tests are typically never conducted in the same time frame. One exception would be the pretreatment systemic search for disease involvement performed on patients diagnosed with malignant lymphoma. Bone marrow aspiration tests as part of the pretreatment staging studies are done to evaluate for potential bone marrow infiltration of lymphoma cells, and pretreatment BMD measurements are obtained as baseline levels, out of concern for exacerbation of osteoporosis caused by the corticosteroids that are included in standard anti-lymphoma therapies such as R-CHOP and others.
4. Materials and Methods
4.1. Patient Samples
Patients with malignant lymphoma who had bone density tests (dual-energy x-ray absorptiometry; DEXA) using PRODIGY (GE HealthCare) [
18], blood tests for complete blood counts (CBC) and bone metabolism markers (tartrate-resistant acid phosphatase-5b; TRACP-5b), and bone marrow tests performed at the Department of Hematology, University of Toyama Hospital between April 2019 and November 2021 were included. This study was conducted according to the Declaration of Helsinki and was approved by the ethics committees of Toyama University Hospital (reference number R2021127). Written informed consent was obtained from all patients prior to study participation.
4.2. Cell Culture
The method for inducing differentiation of bone marrow mononuclear cells (BM-MNC) into megakaryocytes (MGK)
was previously described [
19]. Lymphoprep
TM (Serumwerk Bernburg AG) was used to isolate BM-MNC from bone marrow puncture fluid. Isolated BM-MNC were cultured in MegaCult
TM-C Medium with Cytokines (STEMCELL technologies) for 14 days. It contains the following: Iscove's Modified Dulbecco's Medium, bovine serum albumin, recombinant human (rh) insulin, human transferrin (iron-saturated), 2-Mercaptoethanol, rh thrombopoietin, rh interleukin (IL)-6, rh IL-3, and supplements.
4.3. Flow Cytometry
For flow cytometric analyses, samples were collected using a FACSCanto II flow cytometer (BD Biosciences) and analyzed with FlowJo software (Treestar). Expression of CD41a was analyzed using an FITC mouse anti-human CD41a, clone HIP8, antibody (BD Biosciences).
4.4. Quantitative Real-Time RT-PCR
Following stimulation for MGK-differentiation, BM-MNC were lysed and total RNA was extracted by the use of RNeasy Plus Mini Kit (QIAGEN) according to the manufacturer’s instructions. Total RNA (1 μg) was reverse transcribed using QuantiTect Rev. Transcription Kit (QIAGEN). Quantification of mRNA was performed using the iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad) and QuantiTect SYBR Green PCR Kit (QIAGEN). Data obtained were analyzed with iQ™5 Optical System Software, Version 2.1 (Bio-Rad), being normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. The following PCR primers were designed: 5’-TGA GAC ACC TCT CCA GTT GCT G-3’ and 5’-GCA ATC AGG CTT GGT CAC CAC A-3’ for M-CSF; 5’-GGT CTC CTG CTA ACT CAG AAA GG-3’ and 5’-CAG CAA ACC TGA AGA ATG CCT CC-3’ for OPG; 5’-GCC TTT CAA GGA GCT GTG CAA AA-3’ and 5’-GAG CAA AAG GCT GAG CTT CAA GC-3’ for RANKL; 5’-GTC TCC TCT GAC TTC AAC AGC G-3’ and 5’-ACC ACC CTG TTG CTG TAG CCA A-3’ for GAPDH.
5. Conclusions
In this study, we investigated the association between CBC and BMD, and found that Plt count was a parameter demonstrating a negative correlation with BMD, especially in the total hip and femur neck regions of middle-aged and elderly men. We also demonstrated a negative correlation between RANKL production by MGK and BMD in the femur neck. These findings suggest that the negative correlation between Plt count and BMD may be mediated by RANKL production by MGK, which is responsible for Plt production. Our study provides new insights into RANKL production by MGK in osteoporosis and may have implications for the prevention and treatment of this disease.
Author Contributions
Investigation, methodology, S.K., A.W., Y.K., I.Y., D.K., K.K., R.H., T.F., Y.N., and T.M.; writing―review and editing, N.H.D.; funding acquisition, writing—original draft, T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Japan Science and Technology Agency (JST) Moonshot R&D Grant Number JPMJMS2021, Japan Agency for Medical Research and Development (AMED) Grant Number 21lm0203005j0005 (A145), 22ym0126807j0001 (A160), 21am0401024h0003, and Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research Grant Number 21K07237.
Institutional Review Board Statement
This study was approved by the ethics committees of Toyama University Hospital (reference number R2021127).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available on reasonable request. All data relevant to the study are included in the article.
Acknowledgments
We gratefully thank Toyomi Kozawa for her help in performing this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lévesque, J.P.; Helwani, F.M.; Winkler, I.G. The Endosteal Osteoblastic Niche and Its Role in Hematopoietic Stem Cell Homing and Mobilization. Leukemia 2010, 24, 1979–1992. [Google Scholar] [CrossRef]
- Kristjansdottir, H.L.; Mellström, D.; Johansson, P.; Karlsson, M.; Vandenput, L.; Lorentzon, M.; Herlitz, H.; Ohlsson, C.; Lerner, U.H.; Lewerin, C. High Platelet Count Is Associated with Low Bone Mineral Density: The MrOS Sweden Cohort. Osteoporosis International 2021, 32, 865–871. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.R.; Chen, J.; Ye, Y.J.; Xu, P.C.; Li, J.Y. Association of Bone Mineral Density with Peripheral Blood Cell Counts and Hemoglobin in Chinese Postmenopausal Women: A Retrospective Study. Medicine 2020, 99, e20906. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.S.; Lee, H.S.; Kwon, Y.J. The Relationship between Platelet Count and Bone Mineral Density: Results from Two Independent Population-Based Studies. Arch Osteoporos 2020, 15, 43. [Google Scholar] [CrossRef]
- Mödder, U.I.; Achenbach, S.J.; Amin, S.; Riggs, B.L.; Melton, L.J.; Khosla, S. Relation of Serum Serotonin Levels to Bone Density and Structural Parameters in Women. Journal of Bone and Mineral Research 2010, 25, 415–422. [Google Scholar] [CrossRef]
- Bader, M. Inhibition of Serotonin Synthesis: A Novel Therapeutic Paradigm. Pharmacol Ther 2020, 205, 107423. [Google Scholar] [CrossRef]
- Valderrábano, R.J.; Lui, L.Y.; Lee, J.; Cummings, S.R.; Orwoll, E.S.; Hoffman, A.R.; Wu, J.Y. Bone Density Loss Is Associated With Blood Cell Counts. Journal of Bone and Mineral Research 2017, 32, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Valderrábano, R.J.; Buzkova, P.; Chang, P.Y.; Zakai, N.A.; Fink, H.A.; Robbins, J.A.; Lee, J.S.; Wu, J.Y. Association of Bone Mineral Density with Hemoglobin and Change in Hemoglobin among Older Men and Women: The Cardiovascular Health Study. Bone 2019, 120, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Schyrr, F.; Wolfer, A.; Pasquier, J.; Nicoulaz, A.L.; Lamy, O.; Naveiras, O. Correlation Study between Osteoporosis and Hematopoiesis in the Context of Adjuvant Chemotherapy for Breast Cancer. Ann Hematol 2018, 97, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Cho, H.Y.; Park, I.Y.; Choi, J.M.; Kim, M.; Jang, H.J.; Hwang, S.M. The Positive Association between Peripheral Blood Cell Counts and Bone Mineral Density in Postmenopausal Women. Yonsei Med J 2011, 52, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A. Estrogen and Bone Health in Men and Women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Levin, V.A.; Jiang, X.; Kagan, R. Estrogen Therapy for Osteoporosis in the Modern Era. Osteoporosis International 2018, 29, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, X.; Lu, L.; Yu, X. The Relationship between Bone Marrow Adipose Tissue and Bone Metabolism in Postmenopausal Osteoporosis. Cytokine Growth Factor Rev 2020, 52, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Kamada, M.; Irahara, M.; Maegawa, M.; Yasui, T.; Takeji, T.; Yamada, M.; Tezuka, M.; Kasai, Y.; Aono, T. Effect of Hormone Replacement Therapy on Post-Menopausal Changes of Lymphocytes and T Cell Subsets. J Endocrinol Invest 2000, 23, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Kanagasabapathy D; Blosser RJ; Maupin KA; Hong JM; Alvarez M; Ghosh J; Mohamad SF; Aguilar-Perez A; Srour EF; Kacena MA; et al. Megakaryocytes Promote Osteoclastogenesis in Aging. Aging (Albany NY) 2020, 12, 15121–15133.
- Kandahari, A.M.; Yang, X.; Laroche, K.A.; Dighe, A.S.; Pan, D.; Cui, Q. A Review of UHMWPE Wear-Induced Osteolysis: The Role for Early Detection of the Immune Response. Bone Res 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.L.; Sun, Y.; Xu, W.; Lin, T.; Zeng, H. Expression of RANKL by Peripheral Neutrophils and Its Association with Bone Mineral Density in COPD. Respirology 2017, 22, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Hull, H.; He, Q.; Thornton, J.; Javed, F.; Allen, L.; Wang, J.; Pierson, R.N.; Gallagher, D. IDXA, Prodigy, and DPXL Dual-Energy X-Ray Absorptiometry Whole-Body Scans: A Cross-Calibration Study. Journal of Clinical Densitometry 2009, 12, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Faulhaber, M.; Wörmann, B.; Ganser, A.; Verbeek, W. In Vitro Response of Myelodysplastic Megakaryocytopoiesis to Megakaryocyte Growth and Development Factor (MGDF). Ann Hematol 2002, 81, 695–700. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).