1. Introduction
In 2017, nearly 123 million individuals were suspected of having alcohol-related cirrhosis worldwide [
1]. Mortality is twice as high in patients with alcohol-related cirrhosis as in those with cirrhosis from other causes [
2]. In 2019, heavy alcohol consumption was associated with 25% and 42% of cirrhosis mortality and 19% and 35% of liver cancer mortality, worldwide and in Europe, respectively [
3]. In 2019, the global age-standardized death rate for alcohol-related cirrhosis and liver cancer was 4.5 and 1.1 deaths per 100,000 population, respectively [
3].
Making the diagnosis of cirrhosis in subjects with heavy alcohol consumption is important from a prognostic and motivational point of view. In fact, in a population-based Danish hospital registry study of patients with predominantly alcoholic cirrhosis, survival 10 years after cirrhosis diagnosis was reported to be only 22%. [
4]. Survival also depends on the stage of the disease in which cirrhosis is diagnosed, with median values of 12 and 2 years respectively for subjects with compensated and decompensated cirrhosis [
5]. Furthermore, the diagnosis of cirrhosis may motivate patients to stop alcohol intake in order to improve the prognosis and, in more advanced cases, to be able to access liver transplantation.
The clinical diagnosis of liver cirrhosis in heavy alcohol users is based on the presence or history of complications of this disease, liver imaging, and blood tests of liver function and platelet counts [6.] Several easy-to-perform and low-cost non-invasive bioassays have also been developed to rule out advanced fibrosis or the presence of cirrhosis in alcoholics even when compensated, including Fibrosis-4 index (FIB-4) and AST to Platelet Ratio Index (APRI) [
7,
8,
9,
10,
11,
12].
The first aim of the present study was to improve the diagnostic accuracy of non-invasive tests for alcoholic cirrhosis. To do this we also considered the patient's detailed history of alcohol consumption, genetic predisposition to alcohol-induced liver injury and the presence of metabolic syndrome features. The amount of alcohol consumed by the patient over time is in fact associated with the development of cirrhosis. [
13] Genetic variants have also been described which, given the same alcohol consumption, could be associated with a different susceptibility to the development of alcoholic cirrhosis. Among them, PNPLA3, rs738409 C>G, p.I148M, and transmembrane 6 superfamily 2 (TM6SF2), rs58542926 (C/T) E167K variants have been associated with the development and progression of alcoholic liver disease [
14,
15]. In contrast, another gene variant, a splice variant named rs72613567 of the hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13) gene, was found to be associated with a protective effect against disease progression [
14]. Recent data suggest a clinical utility of polygenic risk scores for the diagnosis of advanced fibrosis or alcoholic cirrhosis, also taking into account the patients' metabolic variables [
16,
17]. In fact, the metabolic syndrome or its components interact with heavy alcohol consumption for the risk of liver disease [
18]. In addition, the genetic variants PNPLA3 rs738409, TM6SF2 rs58542926 and HSD17B13 rs72613567 are involved in lipid droplet metabolism in hepatocytes and have been associated with both alcoholic and nonalcoholic fatty liver disease [
16,
19,
20].
In addition to improving the non-invasive diagnostic accuracy for alcoholic cirrhosis, it would be important, both from the point of view of prevention and future cost calculation strategies, to also have a predictive score of the development of alcoholic cirrhosis over time. Thus, the second objective of the present study was to develop a prognostic score of subsequent development of alcoholic cirrhosis. We previously demonstrated that age at start of at-risk alcohol use and the PNPLA3 rs738409 variant were associated with future risk of developing cirrhosis [
21]. In the present study we developed a new model considering all three aforementioned genetic variants, adjusting for known variables at the time of onset of at-risk alcohol consumption, such as BMI and gender [
18,
22].
We found that our diagnostic models of alcoholic cirrhosis all performed better than FIB-4 and APRI. Furthermore, as far as the diagnosis of compensated cirrhosis is concerned, our model containing information on alcohol consumption and the one in which the PNPLA3 rs738409 and HSD17B13 rs72613567 variants are also considered achieve progressively greater accuracies.
Finally, the HSD17B13 rs72613567 variant, in addition to the PNPLA3 rs738409 variant, also allows to achieve a good accuracy of the prognostic score for the future development of alcoholic cirrhosis based on the age of onset of at-risk alcohol consumption.
2. Materials and Methods
2.1. Population and study design
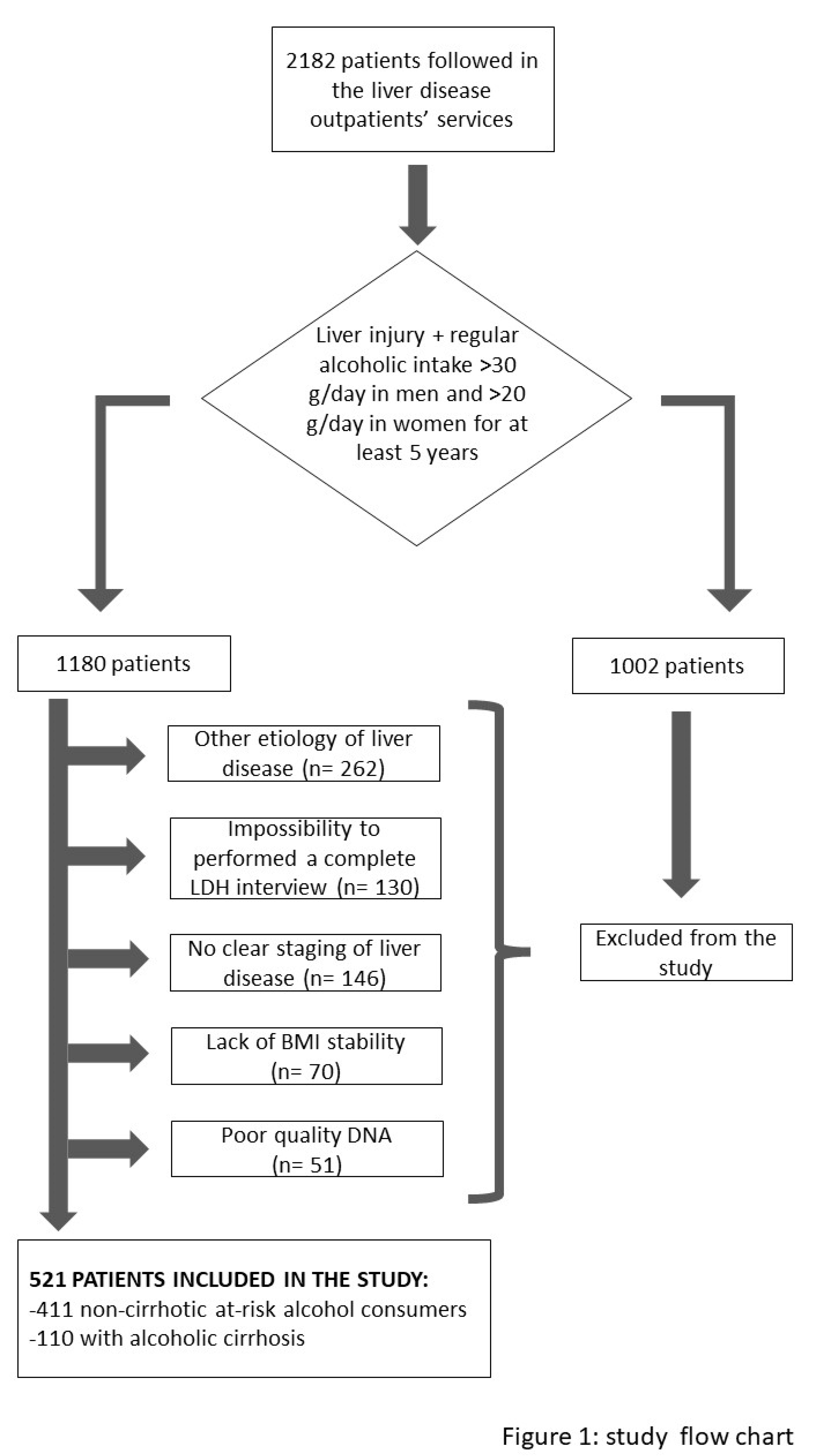
A total of 2182 consecutive Caucasian patients followed in the liver disease outpatients’ services of the Translational and Precision Medicine, Policlinico Umberto I, Rome, Italy between 2010 and 2021 were retrospectively analyzed. On the first visit, all patients underwent a detailed clinical examination and interview on the clinical history and lifestyle habits. At-risk alcohol consumption, defined as ≥3 and ≥2 alcohol units for men and women respectively for at least 5 years, was present in 1180 patients. One unit of alcohol was defined as 12 g of ethanol. In patients with at-risk alcohol use, a more thorough alcohol history was obtained by interview using the lifetime drinking history (LDH). In particular, the age reported at the beginning of the risky alcohol consumption, the duration in years of the risky alcohol consumption and the average daily intake of alcohol expressed as the number of alcoholic units per day were obtained. The eventual presence of cirrhosis and the time of first diagnosis of cirrhosis were also reported as previouly described [
23]. Body mass index (BMI) calculation based on dry weight was recorded. Patients were asked whether their body weight was stable (changes <5 kg) compared to that at age 25, at the first visit for noncirrhotic patients, and before the diagnosis of cirrhosis in cirrhotic patients. Subjects with a history of unstable BMI, a present or previous concurrent diagnosis of hepatitis B and/or C, autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, Wilson's disease, hemochromatosis were excluded from the study. We also excluded from the analyzes subjects with incomplete LDH data, incomplete clinical/biochemical data to obtain a diagnosis of cirrhosis and/or a definite age at diagnosis of cirrhosis, or with poor DNA quality. A total of 521 patients were included in the study, as describe in the flowchart (Figure 1).
Analyzing the entire study population in a cross-sectional way, we wanted to develop predictive indices for the diagnosis of cirrhosis that also took into account the patients' alcoholic history and their genetic predisposition to alcohol-induced liver damage. We then compared the accuracy of our predictive indices with that of other widely used indices such as the APRI and the FIB-4. Since the utility of a diagnostic model for cirrhosis is limited when cirrhosis is advanced and therefore clearly diagnosable clinically, we repeated the cross-sectional analysis limiting it, as far as cirrhotic patients are concerned, to those with compensated cirrhosis.
Finally, we aimed to develop a predictive risk model of future development of cirrhosis in heavy drinkers. To do this, we considered the variables inferable at the time of the onset of risky alcohol consumption, including genetic predisposition. Informed consent was obtained from all subjects involved in the study. The study was conducted in accordance with the Declaration of Helsinki and approval was obtained from the local ethical committee (Ref. number 1913/18.11.2010).
2.2. Polymorphisms Screening
Ethylene-diamine-tetra acetic acid (EDTA) blood, obtained by venepuncture, was collected to recover DNA.
DNA was obtained by Peripheral Blood Mononuclear Cells (PBMC), according to the manufacturer’s instructions (QIAmp® DNA Blood Kit , QIAGEN S.p.A, Milan, Italy). Purified DNA was quantified by O.D. 260 and directly used for polymorphisms screening.
The rs738409 (I148M, PNPLA3) and rs58542926 (E167K, TM6SF2) single-nucleotide polymorphisms (SNPs) were detected using a specific TaqMan® Predesigned SNP Genotyping Assays. Each assay includes two allele-specific TaqMan® MGB probes containing distinct fluorescent dyes and a PCR primer pair to detect specific the target. 1–20 ng purified genomic DNA was used per well (final concentration: 0.2 ng/μL).
The identification of the rs72613567 variant of the HSD17B13 gene was carried out using a Custom Taqman assay designed “in-house” and produced by Thermo Fisher Scientific Italy.
Genotyping data were confirmed through automatic sequencing. In particular, in order to generate an amplicon suitable for sequencing, Primer BLAST tool software (
https://www.ncbi.nlm.nih.gov/tools/primer-blast/) was used to design primers surrounding the sequence of interest and generating a 677 bp amplicon.
Basic Local Alignment Search Tool analyzed acquired sequences at NCBI website whereas alignments were performed with ClustalW2 at the EMBL-EBI website using default parameters.
For all variants, genotyping was performed in triplicate with 100% concordance between replicates.
2.3. Statistical analyses
The data are reported as medians with interquartile ranges (IQR) for continuous variables and as frequencies and percentages for categorical variables. Baseline characteristics were compared by Kruskal Wallis test or Chi Square, as appropriate. In cross-sectional analyzes for cirrhosis diagnosis, multivariable logistic regression model was estimated to establish the influence of covariates: sex, BMI; platelet count (103/µl), serum total bilirubin (mg/dL), ALT (U/L) and creatinine (mg/dL) and presence of diabetes and/or dyslipidemia and/or arterial hypertension on the outcome (Model 0). Model selection was performed by stepwise procedure based on the Akaike Information Criterion. To establish the influence on outcome of the history of alcohol, it was defined the Model 1 including the following covariates in Model 0: daily alcohol consumption (unit) and duration of at-risk alcohol consumption (years). The Model 2 included the Model 1 covariates and genetic components. The diagnostic accuracy of multivariable logistic regression models was assessed by the area under the curve (AUC) plotting receiver operating characteristic (ROC) curve that was designed to differentiate between the patients with and without cirrhosis. The ROC curves obtained with the different models were compared with Delong's test. The multivariable logistic regression models were validated by means cross validation methods using 10 fold cross validation.
In time-dependent longitudinal analyzes for the risk of future cirrhosis development, time of progression to cirrhosis was defined as the time to onset of at-risk alcohol consumption to diagnosis of cirrhosis. Patients who do not have a cirrhosis diagnosis were censored at the date they were last known to be alive. The cirrhosis probabilities were estimated in each group using the non-parametric Kaplan-Meier method and displayed graphically.
The groups difference in cumulative incidence across genotypes were assessed by Log-rank test. Multivariable Cox proportional hazard model was used to evaluate the effect of age at start at-risk alcohol consumption, PNPLA3 and HSD17B13 SNPs on risk of developing cirrhosis after adjusting for sex and BMI. The proportional hazards assumption was verified using graphical methods; scaled Schoenfeld residuals and graphical checks proposed by Klein and Moeschberger were performed.
A score to predict alcoholic cirrhosis incidence at 24 years from the onset of at-risk alcohol consumption was defined as the linear combination of the predictors in the Cox regression model where the weights were proportional to the corresponding rounded coefficients. The performance of prognostic score was assessed using time dependent receiver operating characteristic (ROC) curves.
Optimal cut-off value was chosen to maximize the sum of sensitivity and specificity (Youden index).
Internal validation was obtained by means of 10,000 bootstrap replicates. All analyses were performed using software R (version 4.2.2. R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Cross-sectional study in the entire study population
To define a predictive index for the diagnosis of cirrhosis we first analyzed the entire study population in a cross-sectional manner. As shown in
Table 1, we compared 411 non-cirrhotic patients with heavy alcohol consumption and 110 patients with alcoholic cirrhosis. As expected, cirrhotic compared with non-cirrhotic patients had significantly higher INR, serum bilirubin, creatinine and AST values. Cirrhotic patients, compared with non-cirrhotic patients, were significantly older, had a higher BMI, were more frequently affected by diabetes and arterial hypertension but less frequently by dyslipidemia. Regarding the history of alcohol consumption, cirrhotic patients, compared with non-cirrhotics, had lower daily alcohol consumption but the duration of at-risk alcohol consumption was longer. There was no difference between the two groups for age at onset of at-risk alcohol consumption. Regarding genetics, the group of cirrhotic patients, compared to that of non-cirrhotics, had a significantly higher frequency of the PNPLA3 rs738409 variant, particularly in homozygosity. The protective rs72613567 HSD17B13 variant (heterozygosity) was significantly less frequent in the cirrhotic patient group (
Table 1). No difference was observed for the rs58542926 TM6SF2 variant.
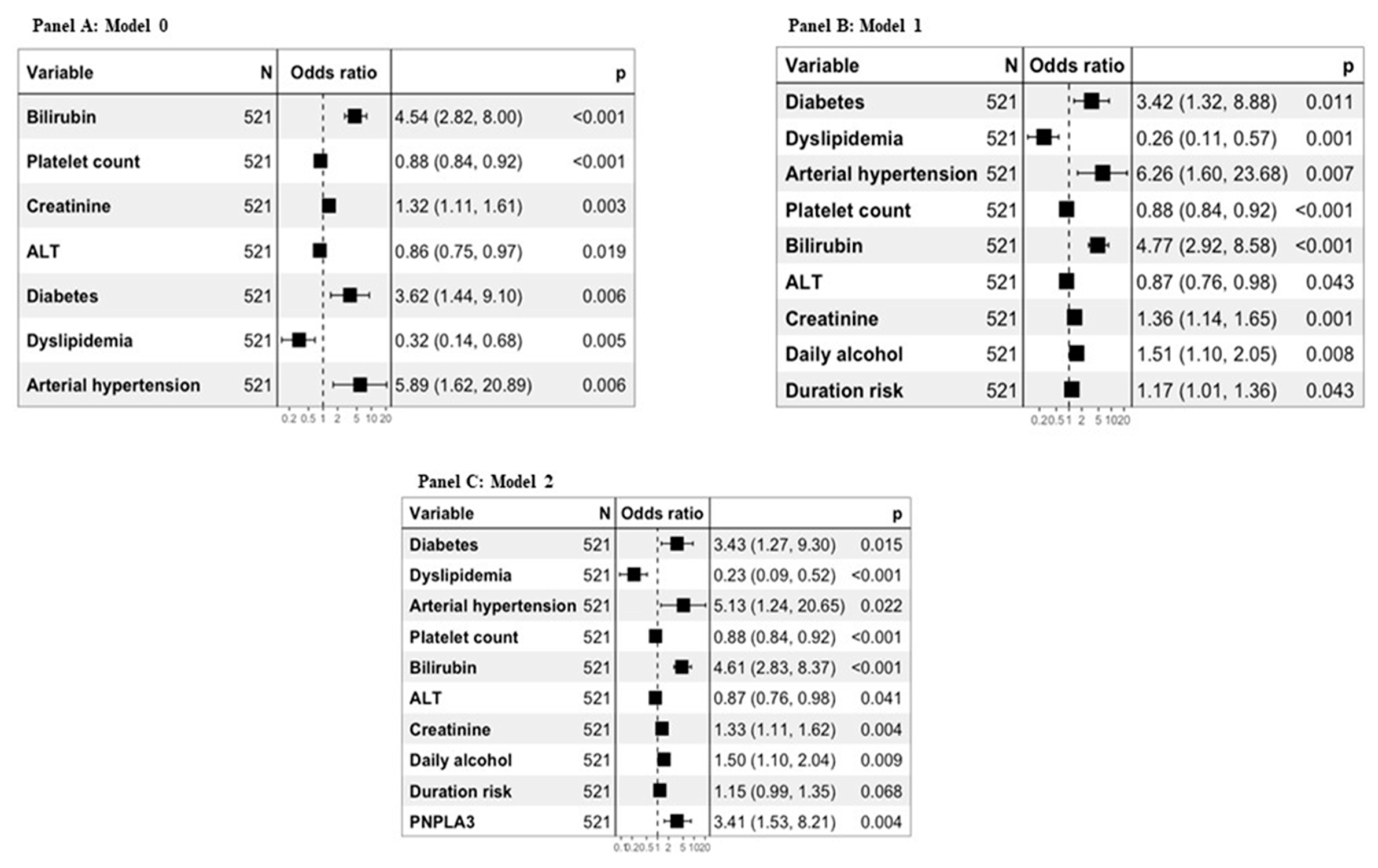
Diagnostic models for the presence of cirrhosis in the whole study population were defined. Model 0 was defined using only biochemical and clinical variables significantly associated with the diagnosis of cirrhosis. Model 1 was defined considering both biochemical and clinical variables as well as alcohol history variables significantly associated with the diagnosis of cirrhosis. Finally, Model 2 was defined considering both the biochemical and clinical variables, those relating to the history of alcohol and the genetic ones significantly associated with the diagnosis of cirrhosis (
Figure 2, Panels A, B, C).
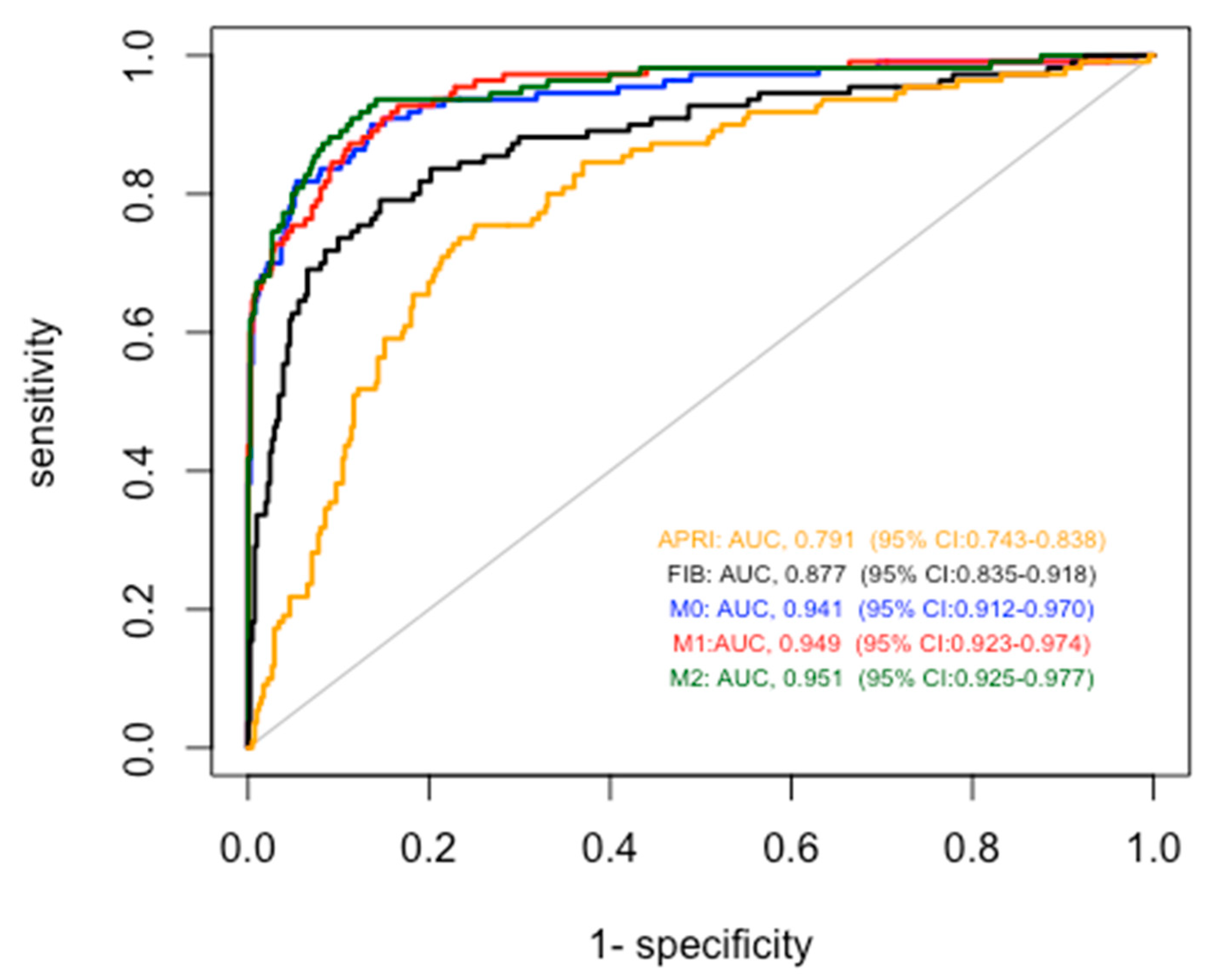
To test the accuracy for cirrhosis diagnosis of our models, we compared them with that of FIB4 and APRI in the entire study population (
Figure 3). As described in
Table 2, all the proposed models obtained significantly better results than FIB4 and APRI. In particular, the AUROCs were 0.791 for APRI, 0.877 for FIB-4, 0.941 for Model 0, 0.949 for Model 1 and 0.951 for Model 2.
When applied to the cross validation method considering 10 folds the AUROCs were 0.941 (95% CI: 0.913-0.968) for Model 0, 0.946 (95% CI: 0.921- 0.971) for Model 1 and 0.949 (95%CI: 0.923- 0.974) for Model 2.
3.2. Cross-sectional study limited to cirrhotic patients with compensated cirrhosis
Since the clinical utility of a diagnostic model for cirrhosis is greatest when the cirrhosis is not advanced, we repeated the analysis considering only cirrhotic patients with compensated cirrhosis. For this analysis, we therefore compared the 411 non-cirrhotic patients with heavy alcohol consumption and 38 patients with alcoholic cirrhosis without any previous or present cirrhosis complication. In the univariate analysis (
Table 1), there were the same differences between non-cirrhotic and cirrhotic patients as we had found considering the entire study population except that there were no significant differences in BMI, daily alcohol consumption and AST serum activity.
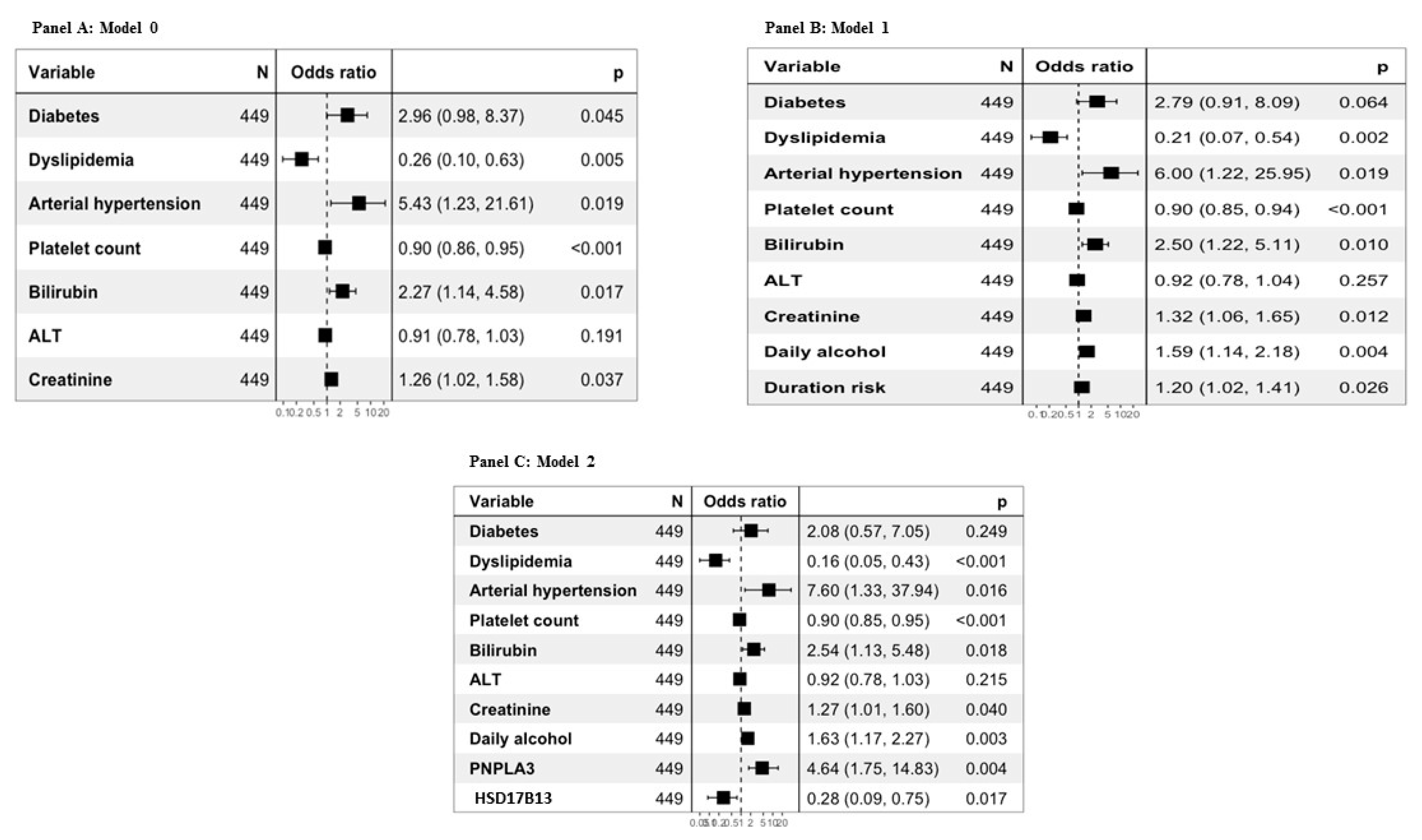
Diagnostic models for the presence of compensated cirrhosis were built (
Figure 4, panels A, B, C).
Model 0 for compensated cirrhosis was constructed using only biochemical and clinical variables significantly associated with the diagnosis of cirrhosis. Model 1 considered both biochemical and clinical variables, as well as alcohol history variables significantly associated with the diagnosis of cirrhosis and, finally, Model 2 included both biochemical and clinical variables, those related to alcohol history and genetic variables significantly associated with the diagnosis of cirrhosis.
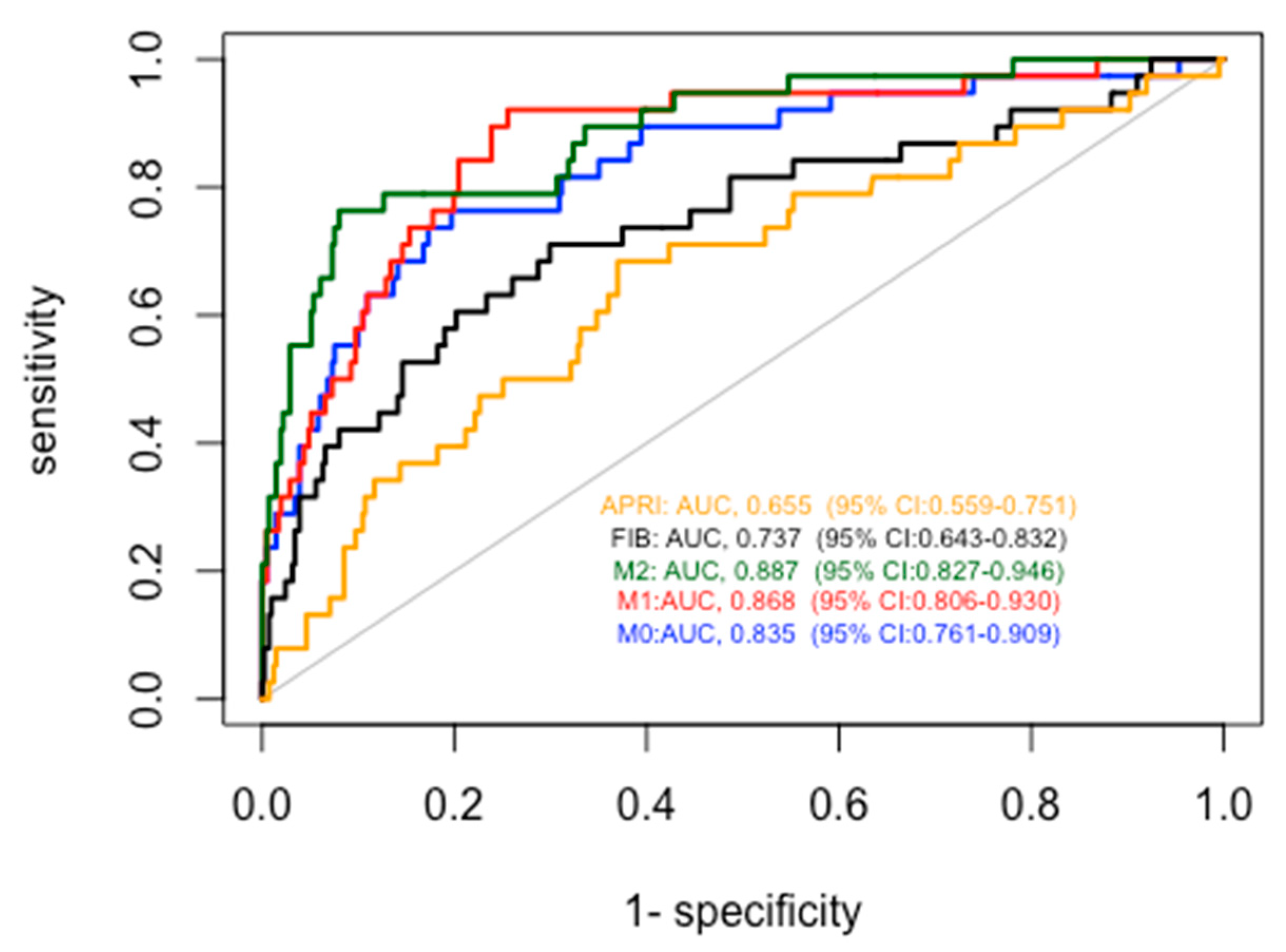
We then compared the diagnostic accuracy for cirrhosis of our models with that of FIB4 and APRI (
Figure 5). As described in
Table 3, all the proposed models obtained significantly better results than FIB4 and APRI. In particular, the AUROCs were 0.655 for APRI, 0.737 for FIB-4, 0.835 for Model 0, 0.868 for Model 1 and 0.887 for Model 2.
When applied to the cross validation method, considering 10 folds, the AUROCs were 0.837 (95% CI: 0.766-0.901) for Model 0, 0.866 (95% CI: 0.802- 0.929) for Model 1 and 0.890 (95%CI: 0.831- 0.950) for Model 2.
3.3. Longitudinal study
Since the rs738409 PNPLA3 and rs72613567 HSD17B13, but not the rs58542926 TM6SF2, were significantly associated with the development of liver cirrhosis over time (supplementary figure 1), we delineated a predictive model for cirrhosis development over time. This model considered the variables that can be inferred at the time of the onset of alcohol consumption.
Multivariable Cox regression model, adjusted for sex and BMI, shown the development of cirrhosis was significantly associated with age at start at-risk alcohol consumption (HR=1.10, 95%CI: 1.07-1.12, p<0.001), PNPLA3 (HR=2.36, 95%CI: 1.44-3.87, p <0.001) and HSD17B13 variants (HR=0.62, 95%CI: 0.40-0.96, p=0.03). The AUROC for the predictive model of cirrhosis development at 24 years was 0.836 (95% CI: 0.755-0.918). The prognostic score was defined as: (0.09*age at onset of at-risk alcohol consumption) + (0.86*number of PNPLA3 variant alleles) -0.47* number of HSD17B13 variant alleles). The threshold of 3.34 performed in predicting the risk of alcoholic cirrhosis development, with a sensitivity 0.74 and a specificity of 0.75. The score was internally validated by bootstrap sampling procedure, giving an AUC of 0.777 (95% CI: 0.702-0.845).
4. Discussion
In our study we retrospectively analyzed a cohort of heavy drinkers characterized in detail from the clinical hepatic point of view, with regard to the metabolic syndrome, the history of alcoholic habits and the genetic predisposition to alcoholic liver damage. We carefully subgrouped patients into non-cirrhotic and cirrhotic patients, excluding from the study those with unclear stage of liver disease. We then analyzed the data in a cross-sectional fashion to derive diagnostic models for cirrhosis and for compensated cirrhosis. Knowing the onset time of at-risk alcohol consumption and that of the eventual diagnosis of cirrhosis, we analyzed the data in longitudinal mode to develop a predictive model of the development of alcoholic cirrhosis over time.
The first result of our study, obtained with the cross-sectional analyses, is that we have developed diagnostic models for alcoholic cirrhosis that are more accurate than FIB-4 and APRI, two widely used indirect markers of liver fibrosis. Our basic model (model 0) is based on a few commonly used laboratory tests such as platelet count, serum bilirubin, creatinine and ALT associated with the presence or absence of diabetes, arterial hypertension and dyslipidemia. By adding to the basic model daily alcohol consumption and the duration of at-risk alcohol consumption (model 1) and also the presence or absence of predisposing genetic variants (model 2), we progressively increased the diagnostic accuracy. This progressive improvement in diagnostic accuracy was especially achieved for the diagnosis of compensated cirrhosis, where clinical diagnosis is more difficult. Indeed, while the AUC of FIB-4 was 0.737, this was 0.868 and 0.887 for model 1 and model 2, respectively. In non-specialised units where transient elastography is not available, blood tests recommended for diagnosing compensated alcoholic cirrhosis include, in addition to the platelet count and serum AST and ALT used to calculate FIB-4 and APRI, INR and bilirubin, albumin and serum creatinine [
10]. Our models save the costs of measuring INR and serum AST and albumin. Accurate alcohol history and the addition of genetic testing for PNPLA3 rs738409 and HSD17B13 rs72613567 variants allow our models to increase diagnostic accuracy for compensated alcoholic cirrhosis, with low additional costs. This is also true for model 2, because the cost of genetic testing is progressively decreasing.
The second result of our study, obtained with the longitudinal analysis, is that we have outlined a predictive model of the development of alcoholic cirrhosis in the 24 years following the onset of at-risk alcohol consumption. The model, adjusted for sex and BMI, is based on the patient's age at the onset of at-risk alcohol use and on PNPLA3 rs738409 and HSD17B13 rs72613567 variants. The score with the cut-off of 3.34 demonstrates a good sensitivity and specificity to predict the development of cirrhosis in patient with heavy alcohol consumption. We previously developed a prognostic model of future development of alcoholic cirrhosis in males that took into account BMI and the PNPLA3 rs738409 and cluster of differentiation 14 (CD14) rs2569190 variants [
23]. In the present study, although we did not include BMI in the predictive score we managed to obtain good predictive accuracy for both sexes by replacing the CD14 variant with the HSD17B13 variant rs72613567.
5. Conclusions
Our new predictive score is important both from a prevention point of view and for future costing strategies. In fact, knowing the prevalence of the PNPLA3 rs738409 and HSD17B13 rs72613567 variants and the average age of onset of at-risk alcohol consumption in a certain population, it will be possible to deduce projections over time of the burden represented by cirrhotic patients for the healthcare system. Indeed, the cost of alcohol-related cirrhosis exceeds all other etiologies of cirrhosis [
24]. Furthermore, the prognostic model can be used to motivate drinkers to stop drinking. Indeed, complete abstinence is the only effective therapeutic option for both compensated and decompensated cirrhosis and, when the disease progresses despite abstinence, liver transplantation is the only option but with very high costs [
24,
25].
The study has limitations due to its retrospective and monocentric nature. However, our diagnostic models, especially the one for compensated cirrhosis, even if inferred with a low number of cirrhotic patients, are useful in clinical practice. Our predictive score of future cirrhosis development is also useful in clinical practice, above all to stratify patients into subgroups in order to increase the targeted surveillance of the disease in subjects with genetic predisposition, in a precision medicine perspective.
Supplementary Materials
Supplementary figure 1, association between rs738409 PNPLA3 and rs72613567 HSD17B13 presence and liver cirrhosis development over time.
Author Contributions
Conceptualization, M.M., F.F., S.G.C.; Investigation, Formal Analysis and Methodology, M.M., F.F., S.G.C. and A.S..; Writing—Original Draft Preparation and Editing, A.S. Data Curation, M.M., C.C. and R.M.M. Methodology, A.A., M.G., S.P. Data Curation – Review and Editing, F.A. Supervising, Editing and Data Curation. Project administration: S.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by “Cet – Comitato Etico Lazio Area 1 (protocol code 1913/18.11.2010, date of approval 18/11/2010).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020, 5, 245–266. [CrossRef]
- Hirode G, Saab S, Wong RJ. Trends in the Burden of Chronic Liver Disease Among Hospitalized US Adults. JAMA Netw Open. 2020, 3, e201997. [CrossRef]
- Huang DQ, Mathurin P, Cortez‐Pinto H, Loomba R. Global epidemiology of alcohol‐associated cirrhosis
and HCC: trends, projections and risk factors. Nat Rev Gastroenterol Hepatol. 2023, 20, 37–49. [CrossRef]
- Jepsen P, Vilstrup H, Andersen PK, Lash TL, Sørensen HT. Comorbidity and survival of Danish cirrhosis
patients: a nationwide population‐based cohort study. Hepatology, 2008, 48, 214–20. [CrossRef]
- D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol, 2006, 44, 217–31. [CrossRef]
- Sharma, P. Value of Liver Function Tests in Cirrhosis. J Clin Exp Hepatol, 2022, 12, 948–964. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Easl clinical practice guidelines: Management of alcohol-related liver disease. J Hepatol 2018, 69, 154–81. [CrossRef]
- Singal AK, Mathurin P. Diagnosis and treatment of alcohol-associated liver disease: A review. Jama 2021, 326, 165–76. [CrossRef]
- Huang J, Yu J, Wang J, Liu J, Xie W, Li R, Wang C. Novel potential biomarkers for severe alcoholic liver disease. Front Immunol, 2022, 13, 1051353. [CrossRef]
- Krag A, Roskams T, Pinzani M, Mueller S. Diagnostic challenges in patients with alcohol-related liver disease. Z Gastroenterol. 2022, 60, 45–57. [CrossRef]
- Addolorato G, Abenavoli L, Dallio M, Federico A, Germani G, Gitto S, Leandro G, Loguercio C, Marra F, Stasi E. Alcohol associated liver disease 2020: A clinical practice guideline by the Italian Association for the Study of the Liver (AISF). Dig Liver Dis. 2020, 52, 374–391. [CrossRef]
- Louvet A, Trabut JB, Moreno C, Moirand R, Aubin HJ, Ntandja Wandji LC, Nourredine M, Ningarhari M, Ganne-Carrié N, Pageaux GP, Bailly F, Boursier J, Daeppen JB, Luquiens A, Nguyen-Khac E, Anty R, Orban T, Donnadieu-Rigole H, Mallat A, Bureau C, Pariente EA, Paupard T, Benyamina A, Perney P, Mathurin P, Rolland B; for the Groupe collaboratif AFEF-SFA Maladie du foie liée à l'alcool. Management of alcohol-related liver disease: the French Association for the Study of the Liver and the French Alcohol Society clinical guidelines. Liver Int. 2022, 42, 1330–1343. [CrossRef]
- Becker U, Deis A, Sørensen TI, Grønbaek M, Borch-Johnsen K, Müller CF, Schnohr P, Jensen G. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996, 23, 1025–1029. [CrossRef]
- Schwantes-An TH, Darlay R, Mathurin P, Masson S, Liangpunsakul S, Mueller S, Aithal GP, Eyer F, Gleeson D, Thompson A, Muellhaupt B, Stickel F, Soyka M, Goldman D, Liang T, Lumeng L, Pirmohamed M, Nalpas B, Jacquet JM, Moirand R, Nahon P, Naveau S, Perney P, Botwin G, Haber PS, Seitz HK, Day CP, Foroud TM, Daly AK, Cordell HJ, Whitfield JB, Morgan TR, Seth D; GenomALC Consortium. Genome-wide Association Study and Meta-analysis on Alcohol-Associated Liver Cirrhosis Identifies Genetic Risk Factors. Hepatology. 2021, 73, 1920–1931. [CrossRef]
- Balcar L, Scheiner B, Urheu M, Weinberger P, Paternostro R, Simbrunner B, Semmler G, Willheim C, Pinter M, Ferenci P, Trauner M, Reiberger T, Stättermayer AF, Mandorfer M. The impact of transmembrane 6 superfamily 2 (TM6SF2) rs58542926 on liver-related events in patients with advanced chronic liver disease. Dig Liver Dis. 2023, 28, S1590. [CrossRef]
- Whitfield JB, Schwantes-An TH, Darlay R, Aithal GP, Atkinson SR, Bataller R, Botwin G, Chalasani NP, Cordell HJ, Daly AK, Day CP, Eyer F, Foroud T, Gleeson D, Goldman D, Haber PS, Jacquet JM, Liang T, Liangpunsakul S, Masson S, Mathurin P, Moirand R, McQuillin A, Moreno C, Morgan MY, Mueller S, Müllhaupt B, Nagy LE, Nahon P, Nalpas B, Naveau S, Perney P, Pirmohamed M, Seitz HK, Soyka M, Stickel F, Thompson A, Thursz MR, Trépo E, Morgan TR, Seth D; GenomALC Consortium. Corrigendum to: 'A genetic risk score and diabetes predict development of alcohol-related cirrhosis in drinkers' J Hepatol. 2022, 76, 1244–1245. [CrossRef]
- Johansen S, Thiele M, Juel HB, Hansen T, Krag A. External validation of a genetic risk score that predicts development of alcohol-related cirrhosis. J Hepatol. 2022, 77, 1720–1721. [CrossRef]
- Åberg F, Färkkilä M, Männistö V. Interaction Between Alcohol Use and Metabolic Risk Factors for Liver Disease: A Critical Review of Epidemiological Studies. Alcohol Clin Exp Res. 2020, 44, 384–403. [CrossRef]
- Luukkonen PK, Färkkilä M, Jula A, Salomaa V, Männistö S, Lundqvist A, Perola M, Åberg F. Abdominal obesity and alcohol use modify the impact of genetic risk for incident advanced liver disease in the general population. Liver Int. 2023, 26, Epub ahead of print. [CrossRef]
- Ajmera V, Loomba R. Advances in the genetics of nonalcoholic fatty liver disease. Curr Opin Gastroenterol. 2023, 39, 150–155. [CrossRef]
- Burza MA, Molinaro A, Attilia ML, Rotondo C, Attilia F, Ceccanti M, Ferri F, Maldarelli F, Maffongelli A, De Santis A, Attili AF, Romeo S, Ginanni Corradini S. PNPLA3 I148M (rs738409) genetic variant and age at onset of at-risk alcohol consumption are independent risk factors for alcoholic cirrhosis. Liver Int. 2014, 34, 514–520. [CrossRef]
- Llamosas-Falcón L, Probst C, Buckley C, Jiang H, Lasserre AM, Puka K, Tran A, Rehm J. Sex-specific association between alcohol consumption and liver cirrhosis: An updated systematic review and meta-analysis. Front Gastroenterol (Lausanne). 2022, 1, 1005729. [CrossRef]
- Mancina RM, Ferri F, Farcomeni A, Molinaro A, Maffongelli A, Mischitelli M, Poli E, Parlati L, Burza MA, De Santis A, Attilia F, Rotondo C, Rando MM, Attilia ML, Ceccanti M, Ginanni Corradini S. A two gene-based risk score predicts alcoholic cirrhosis development in males with at-risk alcohol consumption. Appl Clin Genet. 2019, 12, 1–10. [CrossRef]
- Ntandja Wandji LC, Ningarhari M, Lassailly G, Dharancy S, Boleslawski E, Mathurin P, Louvet A. Liver Transplantation in Alcohol-related Liver Disease and Alcohol-related Hepatitis. J Clin Exp Hepatol. 2023, 13, 127–138. [CrossRef]
- Louvet A, Bourcier V, Archambeaud I, d'Alteroche L, Chaffaut C, Oberti F, Moreno C, Roulot D, Dao T, Moirand R, Duclos-Vallée JC, Goria O, Nguyen-Khac E, Pol S, Carbonell N, Gournay J, Elkrief L, Fouchard-Hubert I, Chevret S, Ganne-Carrié N; CIRRAL group. Low alcohol consumption influences outcomes in individuals with alcohol-related compensated cirrhosis in a French multicenter cohort. J Hepatol. 2023, 78, 501–512. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).