Introduction
One of the most important challenges in the 21st century is to provide reliable, renewable and environmentally friendly sources of energy. Moreover, the current geopolitical situation forces invention of an alternative solution to traditional energy sources even more urgently. One of the answers is improvement of the renewable biofuels production. Currently, bioethanol is still mostly produced as a first-generation biofuel from edible plants. As a result, it reduces food sources and thus is questionable. Although lignocellulosic biomass is widely obtained from tree plantations or agricultural waste, only ~3% of bioethanol is produced from this source as second-generation bioethanol [
1,
2,
3,
4]. The main limitation of efficient, industrial production of the second-generation bioethanol is its complex composition and the structure of plant cell wall. Mostly composed of cellulose, the cell wall is highly heterogeneous as it also contains hemicelluloses, pectin and lignin. The outcome structure is determined by the function of secondary cell walls [
5,
6,
7]. During technological processing, the lignocellulosic biomass requires separation of its components and breaking down complex polysaccharides into fermentable sugars [
8,
9]. In particular, lignin presents a great challenge as it affects the activity of cellulases [
9,
10] and also because phenolic compounds are inhibitors of alcohol fermentation in brewers’ yeast (
Saccharomyces cerevisiae) [
11]. Pretreatments to reduce recalcitrance increase the cost of production and negatively impact the environment with toxic wastes [
12].
Plants are capable of efficient saccharification
in vivo during induction of certain types of the Programed Cell Death (PCD) [
13] e.g., during lysigenous aerenchyma formation in response to root hypoxia [
14]. Discovered of proteins witch regulated lysigenous aerenchyma formation opened a new pathway to study this form of plants adaptation to stress as well as study autolysis of plant cell wall. We also postulated that ability of plants to degradation of its cell wall can be an interesting target to improve the second-generation bioethanol production. According to our knowledge, plant enzymes involved in the natural saccharification of the cell wall have not been explored in the context of their use as a potential source for industrial enzymes. Similarly, the role of genes encoding molecular PCD regulators have not been studied in the cell wall lignification and cellulose fiber polymerization related processes.
Here we present the biotechnological potential of the conditional PCD regulators [
15,
16,
17] i.e., LESION SIMULATING DISEASE 1 (LSD1), ENHANCED DISEASE SUSCEPTIBILITY 1 (EDS1), and PHYTOALEXIN DEFFICIENT 4 (PAD4). The very regulators were earlier recognized as involved in the lysigenous aerenchyma formation in response to root hypoxia stress in Arabidopsis thaliana [
14]. Conditional PCD regulation by LSD1, EDS1 and PAD4 was demonstrated of runaway PCD during growth of
lsd1 mutant in ambient laboratory conditions and in field condition [
15,
16], which affected in its seed yield and productivity. Based on the results in stable transgenic aspen lines with deregulated PtEDS1, PtLSD1, PtPAD4, we obtained four independent transgenic lines with lower lignin content and a higher cellulose polymerization degree in four-year-old aspen wood. Higher efficiency of the industrial bioethanol production from transgenic wood with the lowest lignin content was found.
Results
In Vitro Cellulolytic and Xylanolytic Activities of Enzymes Isolated from eds1 Hypocotyls
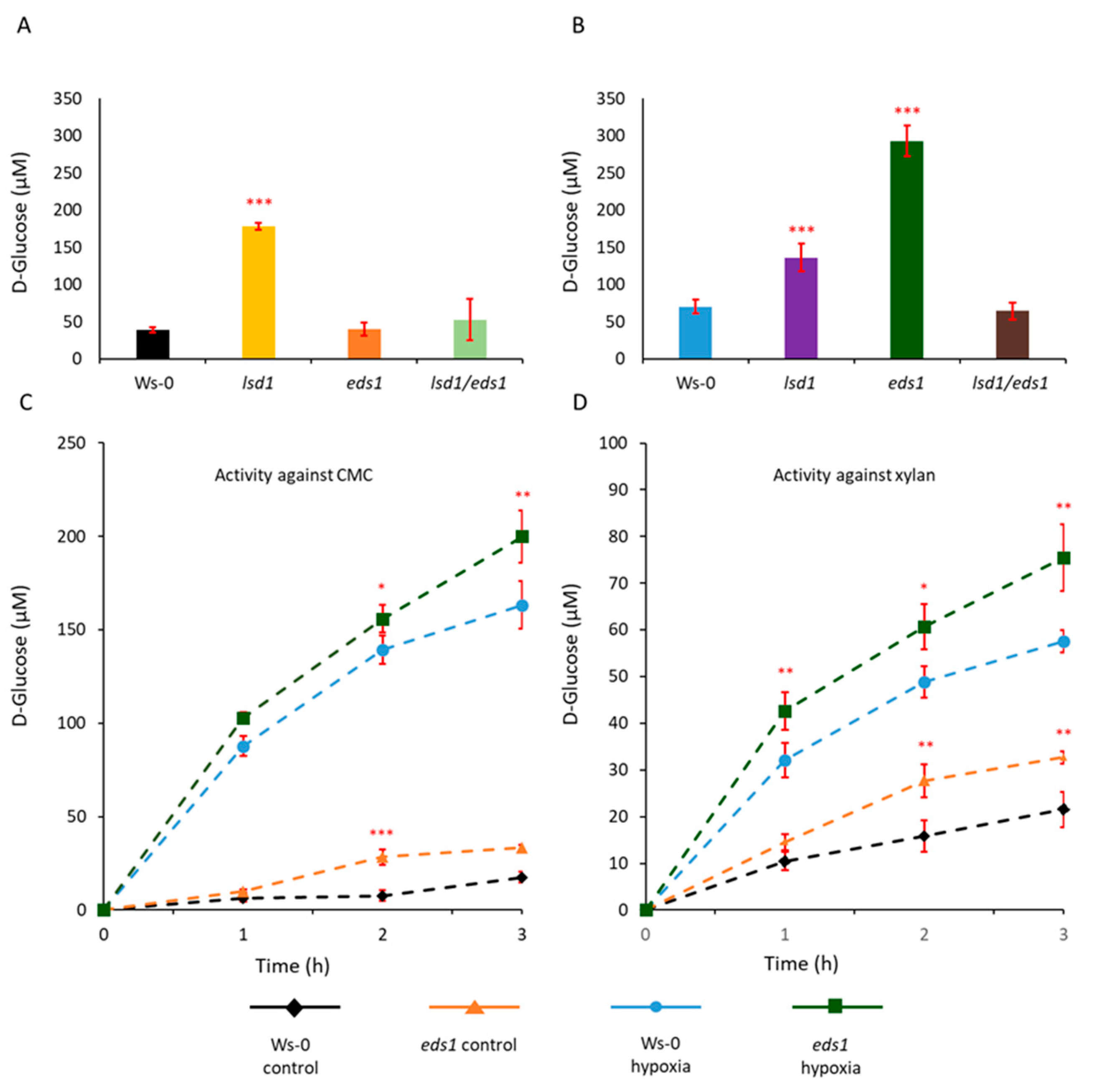
Two different substrates i.e., carboxymethyl cellulose (CMC) and beechwood xylan were added to proteins extracted from Ws-0 and
eds1 hypocotyls. CMC is a substrate for β-D-glucanase hydrolyzing β-1-4 glycosidic linkages between glucopyranose units (in amorphous cellulose), while xylan is a substrate for xylanase acting on β-1-4 glycosidic linkages between xylopyranose units in xylan backbone. The
Arabidopsis thaliana enzymes induced in roots and hypocotyls during hypoxia stress have the potential to break down both cellulose and xylan, however, proteins extracted from
eds1 mutant exhibited higher activity than those extracted from Ws-0 (
Figure 1C,D). In contrast, enzymatic extracts from
lsd1 and
lsd1/
eds1 double mutant had lower activity against both CMC and xylan than
eds1 mutant and wild-type plants (
Figure S2A–D). The high reducing sugar content after stress and stable enzymatic activity of extracts of
eds1 mutant indicated that cellulase, xylanases and also cell wall loosening (CWL)proteins were intact and unaffected probably by endogenous proteolysis. Similarly, higher activity was observed in the protein extracts from
eds1 in Col-0 background examined in zymography activity assay (
Figure S1C; proteins were separated with Native-PAGE in presence of 1% CMC as substrate). The ecotype Col-0 showed lower activity than Ws-0 (
Figure S1C) and, in the same way, it was observed in the
eds1 mutant. Generally, proteins with molecular weight higher than 150 kDa had enzymatic activity.
Cell Walls of Aspen with Deregulated PCD Growing in the Field Contain Less Lignin and Longer Cellulose Fibers
It was demonstrated earlier that transgenic aspen with an individually lowered expression of
PtLSD1, PtEDS1, or
PtPAD4, differentially modified wood density, wood swelling and cell wall thickness [
20,
21] in comparison to the wild type in the field conditions. Here four transgenic lines with reduced expression of
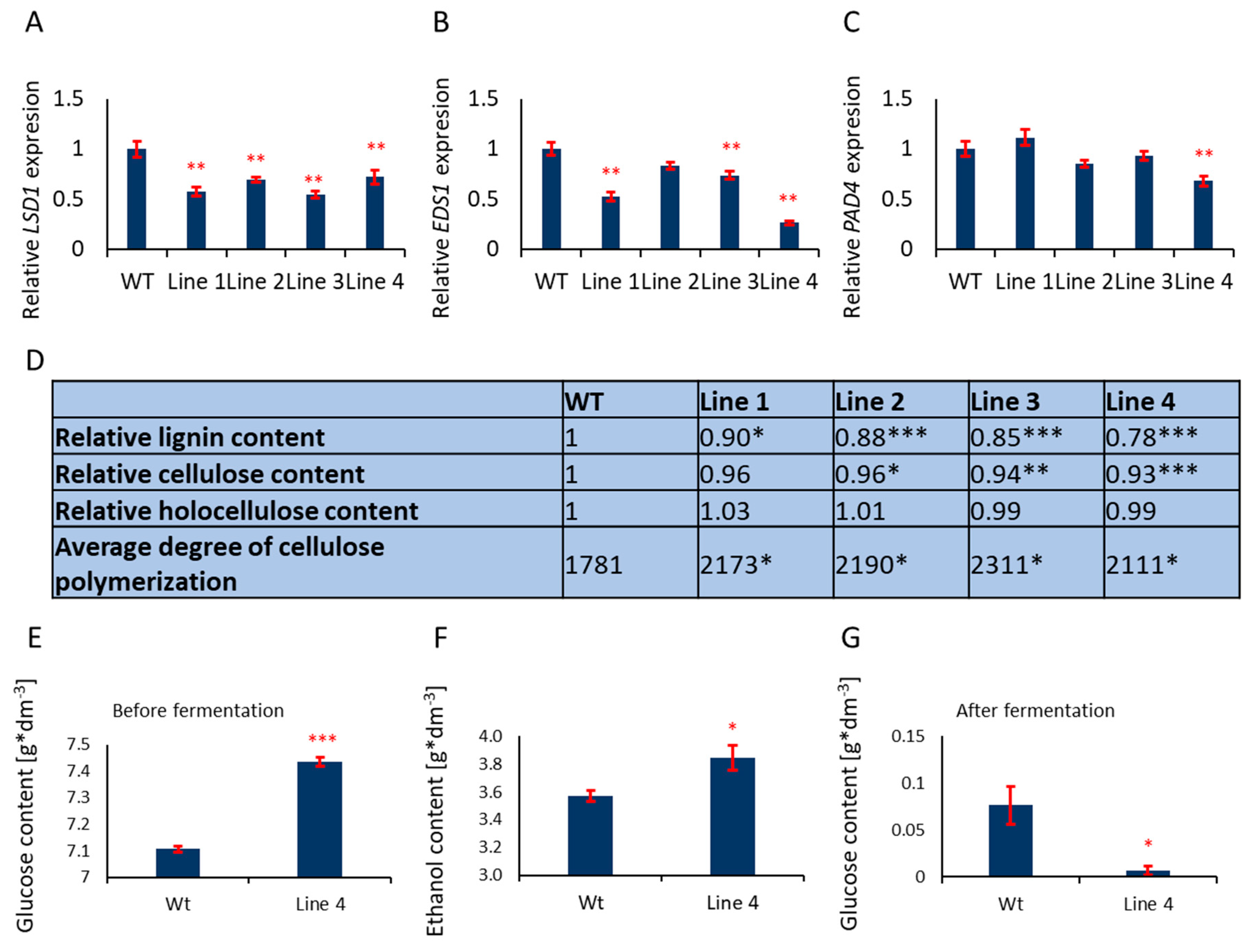
PtLSD1, PtEDS1, and PtPAD4 (lines: Line 1, Line 2, Line 3 and Line 4) (
Figure 3A–C) were tested. All three genes, i.e.,
PtLSD1, PtEDS1, and PtPAD4 were deregulated regardless of the double transgene combination (even if the third gene was not modified in double transgenic line), probably because these genes are conditionally co-regulated, while LSD1 and PAD4 interact with EDS1 protein [
15,
16,
17,
20,
21] (
Figure 3A–C). Transgenic trees exhibited significantly lowered lignin content in the wood and higher cellulose fiber polymerization degree (
Figure 3D). This phenotype (feature) correlated with the expression level of
LSD1,
EDS1, and
PAD4 (
Figure S5). The relative reduction of lignin (Wt = 100%) was in the range of 10% in Line 1 to 22% in Line 4 (
Figure 3D). Cellulose content was also significantly lower (up to 7% in Line 4), however, its polymerization degree was significantly higher up to 23% (
Figure 3D).
Strong Reduction of Lignin and Higher Cellulose Polymerization Degree in the Wood of Line 4 Allows High Fermentation Efficiency
Lignin is an inhibitor of alcoholic fermentation [
22]. Therefore, we confirm that Line 4 was the most suitable for bioethanol production. Higher glucose yield after alkaline pre-treatment and enzymatic hydrolysis of aspen wood (wild type plants and Line 4) (
Figure 3E) as well as higher ethanol yield after fermentation (
Figure 3F) were obtained. The high effectiveness of the alcohol fermentation process was confirmed, as almost whole glucose obtained from enzymatic hydrolysis was converted to ethanol for Line 4 wood but not for the wild type (
Figure 3G).
PtLSD1, PtEDS1, and PtPAD4 Strongly Influence Cambium and Xylem Transcriptome in Line 4
Having analyzed transcriptome of cambium and differentiating xylem tissue in Line 4 we found that it differed from the wild type tree (
Figure S6) with 2648 differentially expressed genes (DEG) (
Table S7) of which 2299 were known (recognized by
Panther.org,
Table S8) and 342 were unknown (
Table S9). For all the known genes gene ontology analysis was performed considering their molecular function (
Table S10 and Figure S7) or biological process (
Table S11 and Figure S8). Many differentially expressed genes encoded putative enzymes (
Figure S8). The overrepresentation of genes assigned to the regulation of the phenylpropanoid metabolic process, which is related to lignin biosynthesis [
23], was found (
Table S11). Some of the 63 genes (ca. 3.2%) were involved in lignin and cellulose synthesis/metabolism, cell wall biogenesis and cell wall organization (
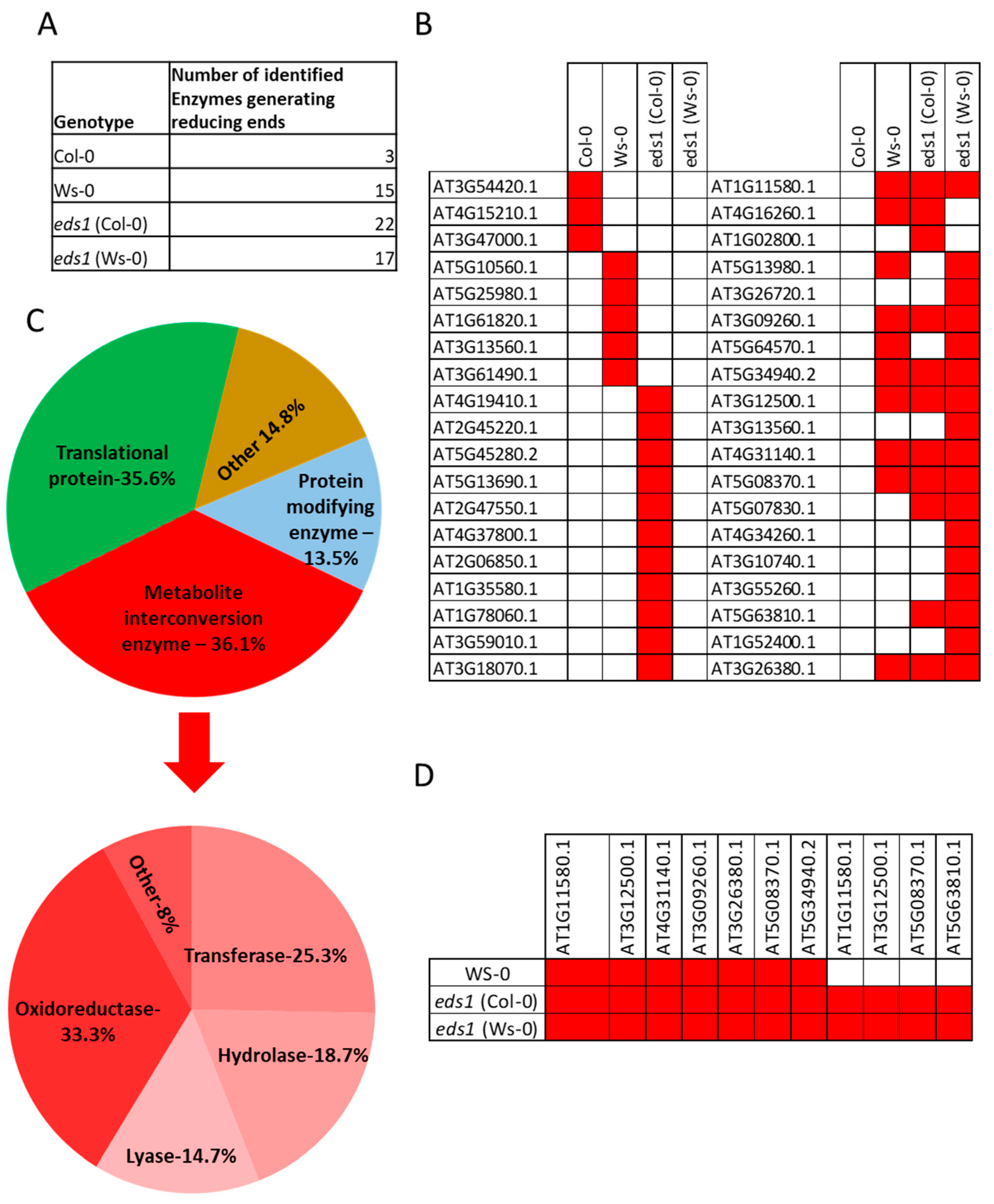
Table S12). Moreover, we compared genes encoding enzymes identified in the Maldi-TOF experiment in
Arabidopsis thaliana (
Table S2) with their homologs among genes deregulated in transgenic Line 4. For 19 Line 4 DEGs its
Arabidopsis homologs were identified in the Maldi-TOF experiment (
Table S13). This list included two cell wall-loosening proteins and enzymes generating reducing sugars; ALPHA-L-FUCOSIDASE 2 and BETA-D-XYLOSIDASE 7, which could be interesting targets for the biofuel industry. In order to find some gene clusters, which can be particularly important in the context of aspen wood quality, we compared Line 4 DEGs with known CAZY family protein data base (
Table S14) [
24] to genes involved in the cell wall integrity (malectin and malectin-like domain-containing proteins (
Table S15) [
25]. In Line 4 DEGs 252 we found genes encoding proteins from CAZY families and 23 genes encoding malectin and malectin-like domain-containing proteins. 14 genes from Line 4 DEGs were found in both groups (
Table S16 and Figure S9). These results strongly indicate that conditional PCD regulators LSD1, EDS1 and PAD4 are developmental regulators of the secondary cell wall structure in the woody plants.
Transgenic Lines Exhibited Similar Phenotype and Development Level as Wild Type Trees
Insignificant differences in tree height, stem fresh weight and stem diameter during annual growth were measured. In contrast, CO2 assimilation was changed in transgenic lines, even if differences were not found in chlorophyll content (
Figure S10).
Discussion
Cell wall biotechnological ameliorations with decreased lignin content in the wood is a goal of many studies [
3,
26]. However, the knowledge about cell wall structure and composition regulators, plant cellulolytic and CWL enzymes naturally decomposing cell walls is limited [
27,
28]. Wood tissue and cell wall development require the occurrence of PCD [
29]. Therefore, conditional regulators of PCD and lysigenous aerenchyma formation i.e. LSD1, EDS1, and PAD4 [
15,
16,
17,
30,
31] were studied in both
Arabidopsis thalian and aspen for their biotechnological potential in biofuels production.
Based on the results of this study, we report that formation of lisogenous aerenchyma in response to root hypoxia stress is regulated by LSD1 and EDS1 and involves at least 37 CWL enzymes in the cell wall saccharification and decomposition process. We also report that conditional PCD regulators (LSD1, EDS1 and PAD4) are developmental regulators in woody plants of the secondary cell wall, since lines with the reduced expression of these genes, growing in four subsequent seasons in the field conditions, in stable transgenic aspen significantly reduced lignin content and significantly increased cellulose fiber polymerization degree. Most importantly these results are strongly supported by significant similarities (homology) between proteins identified in MALDI-TOF experiment on Arabidopsis thaliana and DEGs identified in cambium of transgenic aspen Line 4 (
Tables S2, S8 and S13).
Usually, the transgenic plants with modified wood composition exhibited impaired growth, especially in the field. Downregulation of cinnamate 4-hydroxylase (an enzyme involved in an early stage of phenylpropanoid synthesis pathway) by RNAi resulted in ~30% less lignin content in hybrid aspen, but also reduced tree growth and wood mechanical properties [
32]. Another widely used target for genetic manipulation of lignin content is 4-coumarate: coenzyme A ligase (4CL). Suppression of this enzyme leads to a 40-45% reduction in lignin content in aspen [
33,
34,
35], however, this phenotype was confirmed in the greenhouse conditions [
36]. During experiments carried out in the field, the decrease in lignin content was accompanied by stunted growth [
37,
38,
39]. One of the most promising field experiments carried out on poplar with RNAi of 4CL and CCoAOMT resulted in 28% less lignin content and unchanged growth parameters, however, the experiment was performed on 1-year-old plants (one vegetation season) [
40]. To sum up, the reduction of lignin is considered often as a defense trait against biotic stress. It is not usually studied in the context of plant growth [
41,
42,
43] or reduced-lignin phenotype when growing in the greenhouse. It is also significantly weakened in fluctuating field conditions [
15,
16,
44]. Here, we demonstrate that the deregulation of genes encoding these proteins rather than the lignin biosynthesis pathway itself appears much more promising in biotechnological improvements of trees growing for several subsequent seasons in the field. Most importantly, these regulators control the phenylpropanoid pathway and the genes involved in CWL and lignin biosynthesis (e.g. CAD) in
Arabidopsis thaliana and aspen (GO:0045551,
Tables S5, S12 and S14). Moreover, it is shown that deregulation of the conditional PCD regulators results also in the deregulation of many genes from CAZY family in the cambium of transgenic aspen Line 4 in the field conditions [
45,
46,
47].
Lowering the lignin level up to 22% in transgenic aspen lines reduced their growth only slightly in comparison to the wild type (
Figure S12). Additionally, a higher degree of cellulose polymerization (up to 23%) was found in all transgenic lines. The higher cellulose polymerization facilitates the formation of hydrogen bonds among neighboring cellulose fiber and thus plays a pivotal role in increasing the mechanical performance of wood, easier separation of lignin and hemicellulose from cellulose fibers, simultaneously suppressing the decomposition of cellulose during lignin removal [
48]. These features were desirable to produce bioethanol from the wood of trees growing in the field for four subsequent seasons (optimal period for harvesting of fast-growing aspen plantation). Indeed, it was confirmed during experiments on a semi-industrial scale. Considering the above, the deregulation of well-known conditional PCD regulators (
LSD1, EDS1 and
PAD4) [
17,
30,
31,
49] gives an unexpected effect in a completely new context i.e., important for the biofuel industry.
Apart from improving wood as a raw technological material, the improvement of the enzymatic hydrolysis and fermentation process could be considered through the identification of unknow so far plant CWL enzymes. In industry, the most abundant enzymes are those obtained from the fungus Aspergillus niger. Many of the identified Arabidopsis thaliana proteins taking part in the lysigenous aerenchyma formation and aspen genes involved in the cell wall modifications have many unknown functions, thus they give us a wide range of opportunities to search for new biotechnological innovations for bioethanol, paper, production process or improvement of wood quality.
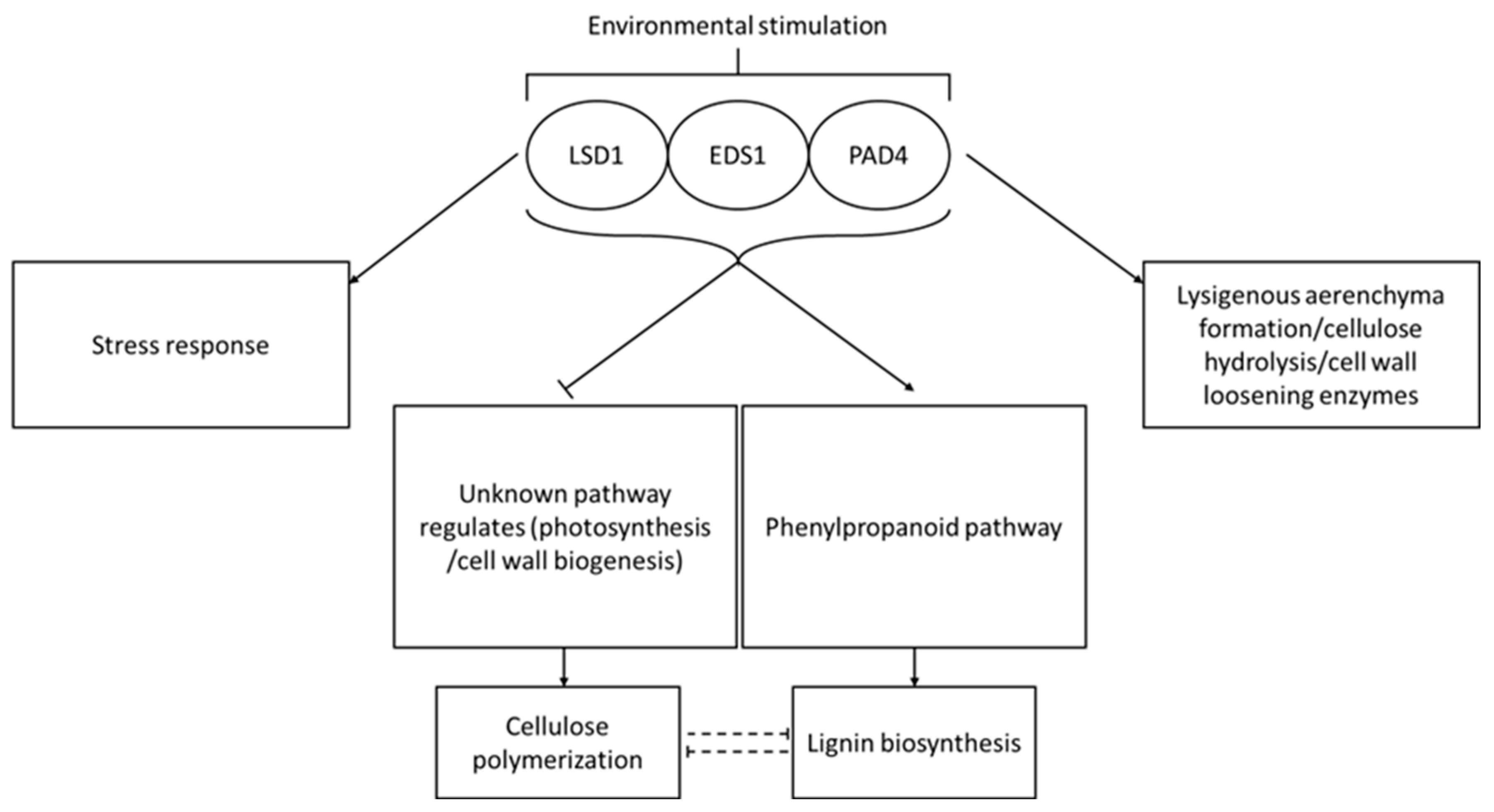
To sum up, we demonstrate for the first time that the deregulation of genes encoding conditional PCD regulators i.e., LSD1, EDS1 and PAD4 can be a breakthrough biotechnology for the biofuel industry. Although it needs to be further investigated, some of our discoveries can be applied directly to improve bioethanol production. Our hypothetical model of the role of LSD1, EDS1 and PAD4 in conditional regulation of cell death, stress responses and desired utility feature of wood (level of lignin and cellulose polymerization degree) is presented in
Figure 4.
Materials and Methods
Arabidopsis thaliana Plant Material
Arabidopsis thaliana seeds used in this study were available in our lab. Experiments were performed using lsd1, eds1, lsd1/eds1 mutants in two ecotypes, Col-0 and Ws-0.
Aspen Growth Condition
Transgenic aspen lines were propagated under
in vitro conditions. Plants were grown in phytotron during a long photoperiod (16 h/8 h day/night) with a light intensity 120 μmol photons m
−2 s
−1, temperature 25 °C/20 °C day/night and relative humidity 55%. Every 2 weeks, plants were transferred to a fresh Murashige and Skoog (MS) medium[
52]. Well-rooted plants were transferred to the greenhouse, kept 3 days in open jars to acclimatize and then transferred to pots with soil. Transgenic and control aspen plants were grown in the greenhouse under natural light supported with low-pressure sodium lamps (Philips, Amsterdam, Netherlands) emitting light intensity (300 μmol photons m
−2 s
−1). After 9 months the transgenic and control lines were transplanted to a field and grown in the Wolica experimental field (52°8′30″N, 21°4′12″E) in 2017. Experiments and measurements were performed on 4-years-old transgenic and wild-type aspen plants. Trees were randomized in the experimental field to minimalize the effect of placement on observed phenotype. The scheme of the field experiment is shown in the figure (
Figure S4). We performed analysis on all four independent transgenic lines (with minimum six trees
per Line) obtained from independent transformation events because we wanted to be sure that phenotype that we observe is a consequence of targeted genetic manipulation and not a side effect of transgene integration into aspen genome.
Determining to Reduce Sugar Content in Arabidopsis thaliana Hypocotyls
Reducing sugar content were determined using Amplex Red Glucose Assay kit (ThermoFisher scientific, USA) accordingly to the manufacturers protocol.
Cellulase and Xylanase Activity Assays
Proteins were extracted using the standard method and the PBS buffor. Protein content in samples was measured using the Bradford method. For other analysis we used 20 ug of total protein. Protein extraction efficiency did not differ much between the samples, so we also were able to use a similar volume of each sample for different analysis. Cellulase and xylanase activities were measured against CMC (PROVIDER) and beechwood xylan (PROVIDER) as substrates, both at 1% (W:W or W:V, PLEasE SPECIFY) concentration. We measured reducing sugar content in each sample before adding substrate and normalized results so that they could reflect how much of reducing sugars were produced from substrate during assay and not during the stress itself.
Native-PAGE Separation and Zymographic Activity Assay
We separated 20 ug of total proteins from each sample on 8% polyacrylamide gel. As we wanted to visualize the enzymatic activity, we did not use any reducing or denaturizing agents such as SDS, β-mercaptoethanol or DTT. Gels were supplemented with 1% CMC as substrate. After separation (2h, 60-70 V) in 4 °C each gel was submerged in PBS and incubated in room temperature for 18 hours. The gels were stained with Congo Red (Merck, Germany) which binds to glycosidic bonds in cellulose. 15 mins later the gels were detained using 2 M NaCl until clear zones were observed on the gel which indicate activity of cellulases.
Maldi-TOF Analysis of Isolated Proteins
Bands exhibiting cellulase activity were cut off from each sample and send to analysis on Maldi-TOF to The Environmental Mass Spectrometry Laboratory in the Department of Biophysics of the IBB PAN (Warsaw, Poland). Samples were treated with trypsin before separating and analyzing them on a mass spectrometer. The results were analyzed using the Mascot software and trimmed so there would be no more than 1% of false positives in each sample. The equipment was sponsored partially by the Centre for Preclinical Research and Technology (CePT), in frames of the European Regional Development Fund and Innovative Economy, The National Cohesion Strategy of Poland.
RNA Isolation and qPCR Analysis
RNA isolation, cDNA synthesis and qPCR analysis were performed as described before [
18,
54]. UBQ (LP-GCTTGAAGATGGGAGGACTCT, RP-AAATCTGCATTCCTCCACGG), PP2A (LP-ACTCTCTGCACTGTTGAGGAA, RP-ACCAATCAACCAGGTCAGTCT) and ACT (LP-TTACATGTTCACCACCACTGC RP-CTGCTCATAGTCAAGGGCAAC) were used as reference gens. For checking LSD1, EDS1 and PAD4 the following primers were used: LSD1 (LP-GACTCAAACTGTCGTTGTGGA, RP-CTGTAGTGACACCAACAACAAC, EDS1 (LP-CGACACCTCAAGAATGAAGA, RP-GTGCATTTATACCGTCTTGG), PAD4 (LP-CATCACCTTTGGCTCTCCAT, RP-AAATTTGCCACCCCATCTTT).
RNAseq Analysis
Fastq files were trimmed using TrimGalore 0.6.6 (
https://github.com/FelixKrueger/TrimGalore) with the following settings: --paired -j 4 --fastqc --quality 30 --length 50 --clip_R1 10 --clipR2 10 --three_prime_clip_R1 10 --three_prime_clip_R2 10. The quality of trimmed reads was assessed using FastQC version (
https://github.com/s-andrews/FastQC). Reads were mapped to the Populus trichocarpa v3.0 genome [
53] with annotations in version 4.1 downloaded from JGI (
https://data.jgi.doe.gov/) using STAR aligner 2.7.6a [
54] with the following settings: --outFilterScoreMinOverLread 0.3 --outFilterMatchNminOverLread 0.3 --outFilterMultimapNmax 100 --outReadsUnmapped Fastx --alignIntronMax 11,000 --quantMode GeneCounts. After mapping reads were counted using HTseq with the settings: -f bam -r pos -s no. Differentially expressed genes were identified with DEseq2 [
55].
Biometric Parameters and Gas Exchanging Measurement
Trees were weighed and measured using an industrial scale and a commercially available measure. CO
2 measurement was performed using LI-6400XT Portable Photosynthesis System (Li-COR Inc., Lincoln, NE, USA) just as described before [
54].
Determination of Photosynthetic Pigments Content
The photosynthetic pigments measurements were determined as precisely described in the previous chapters[
57]. Pigments extracted from the frozen tissue (grinded in liquid nitrogen before and stored in −80°C) were separated on a SynergiTM 4 μm Max-RP 80Å 250 × 4.6 mm column (Phenomenex, Torrance, CA, USA) at 30°C using Shimadzu HPLC System (Shimadzu, Kyoto, Japan). The results were expressed as peak area per μg of fresh weight.
Cellulose, Hemicellulose and Lignin Content
Dry wood material was milled using Retsch mill (SM 200, Retsch, Germany ) and sieved (AS 200, Retsch, Germany). Wood powder with particle size from 0.43 mm to 1.02 mm was used for chemical analyses. Material was extracted in Soxhlet apparatus with mixture of chloroform-ethanol 93:7w/w during 10 h before analyses. Cellulose content was determined by the Kürschner–Hoffer method, holocellulose content was analyzed according to Wise [
58]. Mineral substances content was determined by [
59] on dust fraction (under 0.43 mm) and lignin content was determined according to TAPPI UM 250 (1985) and TAPPI T222 om-02 (2006).
The Alkaline Pre-Treatment and Enzymatic Hydrolysis of Aspen Wood
For the alkaline pre-treatment a 2% aqueous NaOH solution was used in the proportions of 100 cm
3 of sodium hydroxide per 5 g of dry aspen wood powder from the fraction of 0.43 mm to 1.02 mm. The alkaline pre-treatment was performed in an autoclave under the following conditions: 121 °C, p = 0.1 MPa, 30 min. Afterwards the activated material was subjected to the enzymatic hydrolysis according to the procedure described by us previously[
60] with some changes specified below. To the hydrolysis Cellic CTec2 enzyme (Novozymes, Denmark) was used which an activity was 148 FPU/cm
3 determined by NREL method[
61]. The enzymatic hydrolysis process was carried out in the Erlenmeyer flasks and the total volume of the mixture was 100 cm
3. In the process 50 cm
3 of 0.1M citrate buffer solution at pH=4,8 was used and due to the use of yeast in the next step, no sodium azide was added during the hydrolysis. In the hydrolysis process 3.325 cm
3 of 25% (v/v) Cellic CTec2 enzyme solution on each sample was used in the ratio of 1 g of concentrated enzyme to 1 g of absolutely dry biomass. The hydrolysis was proceeded in an incubator (IKA KS 3000i control, IKA, Poland) with shaking of 150 rpm at 50 °C. The total time of enzymatic hydrolysis was 72 h. After the very process, glucose and xylose concentrations in the supernatant were analysed by the HPLC method.
The Ethanol Fermentation Process
Saccharomyces cerevisiae KKP 50 yeast strain on YPD agar plate obtained from the Institute of Agricultural and Food Biotechnology culture collection (Warsaw, Poland) was used in the fermentation study. Yeast inoculum was prepared by transferring single colonies from YPD agar plate using a sterile loop into 25 cm3 of autoclaved (15 min at 121 °C) liquid YPD medium (Millipore, Germany) in 100 cm3 Erlenmeyer flask and incubating flask overnight at 30°C with shaking of 240 rpm (LAB Companion IST-4075R, USA). Fermentation broths were prepared as follows: 20 cm3 of each enzymatic hydrolysate in 100 cm3 Erlenmeyer flasks were supplemented with 0.2 g of yeast extract (Biocorp, French) and autoclaved for 15 min at 121 °C. Subsequently 1 cm3 of inoculum cultured overnight was added into each flask. Batch cultures were carried out in triplicate at 30 °C with shaking of 240 rpm for 47.5 h. During cultivation 0.5 cm3 samples were taken from fermentation broths to 1.5 cm3 Eppendorf tubes, cooled in ice water and then centrifuged for 10 min (MPW-56 Laboratory Centrifuge, Poland) to remove the yeast biomass. The obtained supernatants were frozen until the HPLC analysis.
The HPLC Analysis
The concentration of glucose and xylose in the enzymatic hydrolysates (before fermentation) or glucose, xylose and ethanol in the fermentation broths (after fermentation) were determined using the high-performance liquid chromatography (HPLC, Varian 635 CL System, USA) with a refractive index (RI) detector (Smartline 2300, Knauer, Germany) and thermostated at 60°C Rezex ROA Organic acid H+ (8%) (300 × 7.8 mm) column with Security Guard Cartridge Carbo-H (4 × 3.0 mm) (Phenomenex, USA) using 0.001 N H2SO4 as the mobile phase at a flow rate of 0.4 cm3 min-1. All the HPLC analyses were done in triplicate. The concentrations were expressed in g per dm3 of hydrolysates or fermentation broths.
The Analysis of Cellulose Polymerization Degree
The degree of cellulose polymerization was determined using the SEC (Size Exclusion Chromatography) technique. Cellulose was isolated from aspen wood with the Kürschner-Hoffer method, dissolved in 8% lithium chloride/N,N-dimethylacetamide system and analyzed as described in detail in previous publications [
8,
15].All of the SEC analyses were done in triplicate or six times.
Statistic
For the aspen wood properties, biometric measurement and Arabidopsis thaliana the statistical analysis was performed using the Tukey honest significant difference (HSD) test. The number of biological and technical repetitions can be found in the description of the Figures. The qPCR data statistical analysis were performed using LinReg.
Supplementary Materials
Figure S1. Technical details of experiments. Figure S2. Glucanase and xylanase activities in roots and hypocotyls measured after hypoxia stress. Figure S3. Protein classes analysis by Panther.org. Figure S4. Scheme of aspen cultivation and field experiment. Figure S5. Correlation of wood lignin content and deregulation. Figure S6. RNAseq analysis of RNA isolated from cambium and differentiating xylem of wild type and transgenic. Figure S7. Gene ontology analysis of deregulated genes in Line 4 in term of its molecular function. Figure S8. Gene ontology analysis of deregulated genes in Line 4 in term of biological processes. Figure S9. Results of Line 4 DEGs and its comparison to genes encoding proteins from CAZY families and genes encoding malectin and malectin-like domain-containing proteins. Figure S10. Biometric parameters, CO2 assimilation, and chlorophyll content in transgenic lines. Figure S11. Vector pH7GWIWG2(I) used for transformation of P. tremula x tremuloides. Figure. S12. cDNA sequences of LSD1, EDS1 and PAD4. Additional file 2. Table S1 List of all genes encoding proteins identified in the Maldi-TOF analysis of 150 kDa protein fraction from Arabidopsis hypocotyls and roots samples of Col-0, Ws-0 and eds 1 mutants collected 7 days after start of root hypoxia stress. Table S2 List of all genes encoding proteins identified in Table S1 using Maldi-TOF analysis of 150 kD protein fraction after removing those present in two or more genotypes. Table S3 List of all genes encoding cell wall loosening proteins and enzymes generating reducing sugars identified in each ecotype (Col-0, Ws-0) and in eds1 mutants. Table S4 List of all genes encoding CWL proteins and enzymes generating reducing sugars. Table S5 Overrepresentation test of genes encoding proteins identified in Maldi-TOF analysis of 150 kDa fraction of proteins from hypocotyls and roots samples collected 7 days after the start of root hypoxia stress. Table S6 List of genes encoding proteins detected in Maldi-TOF analisis of 150 kDa fraction (Table S2) and not identified by PANTHER.ORG. Table S7 List of all differentially expressed genes (DEGs) compared to wild-type in cambium and differentiating xylem tissues isolated from 4-years-old transgenic aspen Line 4 growing in the field conditions. Table S8 List of differentially expressed genes (DEGs) detected in cambium and differentiating xylem tissues isolated from 4-years-old transgenic aspen Line 4 growing in the field conditions with PANTHER family/subfamily annotation. Table S9 List of differentially expressed genes (DEGs) detected in cambium and differentiating xylem tissues isolated from 4-years-old transgenic aspen Line 4 growing in the field conditions not identified in PANTHER.ORG. Table S10 Enrichment of molecular function analysis of differentially expressed genes (DEGs) detected in cambium and differentiating xylem tissues isolated from 4-years-old transgenic aspen Line 4 growing in the field condition using PANTHER.ORG software. Table S11 Enrichment of biological process analysis of differentially expressed genes (DEGs) detected in cambium and differentiating xylem tissues isolated from 4-years-old transgenic aspen Line 4 growing in the field condition using PNTHER.ORG software. Table S12 The list of differentially expressed genes (DEGs) detected in cambium and differentiating xylem tissues isolated from 4-years-old transgenic aspen Line 4 growing in the field condition involved in cellulose and lignin biosynthesis, cell wall construction and organization and other processes that may have contributed to better utility values. Table S13 List of differentially regulated genes in Line 4 (Table S8) and their corresponding orthologs or homologs identified in the Maldi-TOF analysis of 150 kDa protein fraction from Arabidopsis hypocotyls and roots (Table S2). Table S14 Genes encoding CAZY family proteins in poplar. Table S15 Poplar genes encoding proteins with malectin and malectin-like domains and their predicted characteristics. Table S16 Comparison of DEGs from cambium and differentiating xylem tissues in transgenic aspen Line 4 with genes coding proteins from CAZY families and proteins with malectin and malectin-like domains in poplar.
Author Contributions
SK gave idea for all the experiments, formulated the hypothesis and conceived the general research plan; SK, MSH, JZ and WS supervised the research and experiments; MJB was involved in the aspen field experiments, bioinformatic and other data analysis and writing of the whole manuscript; JM carried out the roots hypoxia experiments, determination of reduction sugar content, cellulases activity assays and samples preparation for Maldi-TOF; MM, AA, MD and JZ were involved in the wood chemistry and in the cell wall analysis; DW was involved in the hypoxia experiment and biometric measurement of aspen in the field; JDB was involved in the plasmids preparation for aspen transformation, biometric aspen analysis and preparation of aspen cambium and xylem samples for RNAseq and in vitro aspen propagation; PG was involved in the bioinformatic of RNAseq analysis and participated in writing of the manuscript; PB was involved in the LSD1, EDS1, and PAD4 mRNA level analysis in all aspen lines; AR was responsible for the foliar chlorophyll content determination and in vitro aspen propagation; EM was involved in supervision of transgenic aspen lines generation in UPSC and the manuscript writing; KDS performed the alkaline pretreatment and enzymatic hydrolysis of aspen and ethanol fermentation and its efficiency analysis; MJB, JM, MSH, EM, JZ, WS and SK interpreted results and wrote the very manuscript with participation of AA and MD. All authors reviewed and/or revised the paper.
Funding
This work was supported partially privately by Stanisław Karpiński and also in part by the “Biostrateg 2” project (BIOSTRATEG2/298241/10/NCBR/2016) granted to Stanisław Karpiński by the National Center for Research and Development and by the “OPUS 15” project (UMO-2018/29/B/NZ3/01198) granted to Stanisław Karpiński by the National Science Centre.
Acknowledgments
We would like to thank Alex Białas for his technical help in dendrometry measurements. Also, we would like to express our gratitude to Aleksandra Lechańska for proofreading.
Conflicts of Interest
The authors declare that they have no competing interests.
Availability of data and materials
All data generated or analyzed during this study are included in the very published article and its
Supplementary files.
Declarations
Ethics approval and consent to participate. Not applicable.
Consent for publication
Not applicable.
Abbreviations
| LSD1 |
LESION SIMULATING DISEASE 1 |
| PAD4 |
PHYTOALEXIN DEFICIENT 4 |
| EDS1 |
ENHANCED DISEASE SUSCEPTIBILITY |
| PCD |
Programed Cell Death |
| CMC |
Carboxymethyl cellulose |
| DEG |
Differential expressed genes |
| 4CL |
4-coumarate:coenzyme A ligase |
| CWL |
cell wall loosening |
| DEG |
differentially expressed genes |
| MS |
Murashige and Skoog medium |
References
- Hoengenaert, L.; Wouters, M.; Kim, H.; De Meester, B.; Morreel, K.; Vandersyppe, S.; et al. Overexpression of the scopoletin biosynthetic pathway enhances lignocellulosic biomass processing. Science Advances. 2022, 8, eabo5738. [Google Scholar] [CrossRef] [PubMed]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Frontiers in Chemistry 2019, 7. Available online: https://www.frontiersin.org/articles/10.3389/fchem.2019.00874 (accessed on 6 July 2022). [CrossRef]
- Shukla, A.; Kumar, D.; Girdhar, M.; Kumar, A.; Goyal, A.; Malik, T.; et al. Strategies of pretreatment of feedstocks for optimized bioethanol production: Distinct and integrated approaches. Biotechnology for Biofuels and Bioproducts. 2023, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Szyszlak-Bargłowicz, J.; Hawrot-Paw, M.; Koniuszy, A.; Zając, G.; Jaklewicz, J. Production of second generation bioethanolfrom straw during simultaneous microbial saccharification and fermentation. Archives of Environmental Protection 2020, 46, 47–52. Available online: https://journals.pan.pl/dlibra/publication/132525/edition/115791 (accessed on 26 June 2023).
- Turumtay, H. Cell Wall Engineering by Heterologous Expression of Cell Wall-Degrading Enzymes for Better Conversion of Lignocellulosic Biomass into Biofuels. Bioenerg Res. 2015, 8, 1574–1588. [Google Scholar] [CrossRef]
- Khan, I.; Akhtar, M.W. BioenergyProduction From Plant Biomass: Bioethanol From Concept To Reality. Nat Prec. 2011;1–1.
- Alonso, D.M.; Hakim, S.H.; Zhou, S.; Won, W.; Hosseinaei, O.; Tao, J.; et al. Increasing the revenue from lignocellulosic biomass: Maximizing feedstock utilization. Science Advances. 2017, 3, e1603301. [Google Scholar] [CrossRef] [PubMed]
- Sidana, A.; Yadav, S.K. Recent developments in lignocellulosic biomass pretreatment with a focus on eco-friendly, non-conventional methods. Journal of Cleaner Production. 2022, 335, 130286. [Google Scholar] [CrossRef]
- Antczak, A.; Szadkowski, J.; Szadkowska, D.; Zawadzki, J. Assessment of the effectiveness of liquid hot water and steam explosion pretreatments of fast-growing poplar (Populus trichocarpa) wood. Wood Sci Technol. 2022, 56, 87–109. [Google Scholar] [CrossRef]
- Lu, X.; Zheng, X.; Li, X.; Zhao, J. Adsorption and mechanism of cellulase enzymes onto lignin isolated from corn stover pretreated with liquid hot water. Biotechnology for Biofuels. 2016, 9, 118. [Google Scholar] [CrossRef]
- Fletcher, E.; Baetz, K. Multi-Faceted Systems Biology Approaches Present a Cellular Landscape of Phenolic Compound Inhibition in Saccharomyces cerevisiae. Frontiers in Bioengineering and Biotechnology 2020, 8. Available online: https://www.frontiersin.org/articles/10.3389/fbioe.2020.539902 (accessed on 6 July 2022). [CrossRef]
- Li, Y.; Fu, Q.; Rojas, R.; Yan, M.; Lawoko, M.; Berglund, L. Lignin-Retaining Transparent Wood. ChemSusChem. 2017, 10, 3445–3451. [Google Scholar] [CrossRef] [PubMed]
- Escamez, S.; Tuominen, H. Contribution of cellular autolysis to tissular functions during plant development. Current Opinion in Plant Biology. 2017, 35, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Mühlenbock, P.; Plaszczyca, M.; Plaszczyca, M.; Mellerowicz, E.; Karpinski, S. Lysigenous Aerenchyma Formation in Arabidopsis Is Controlled by LESION SIMULATING DISEASE1. Plant Cell. 2007, 19, 3819–3830. [Google Scholar] [CrossRef] [PubMed]
- Wituszyńska, W.; Ślesak, I.; Vanderauwera, S.; Szechyńska-Hebda, M.; Kornaś, A.; Kelen, K.V.D.; et al. LESION SIMULATING DISEASE1, ENHANCED DISEASE SUSCEPTIBILITY1, and PHYTOALEXIN DEFICIENT4 Conditionally Regulate Cellular Signaling Homeostasis, Photosynthesis, Water Use Efficiency, and Seed Yield in Arabidopsis. Plant Physiol. 2013, 161, 1795–1805. [Google Scholar] [CrossRef]
- Bernacki, M.J.; Czarnocka, W.; Rusaczonek, A.; Witoń, D.; Kęska, S.; Czyż, J.; et al. LSD1, EDS1 and PAD4-dependent conditional correlation among salicylic acid, hydrogen peroxide, water use efficiency, and seed yield in Arabidopsis thaliana. Physiologia Plantarum 2018. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/ppl.12863 (accessed on 11 December 2018).
- Czarnocka, W.; Van Der Kelen, K.; Willems, P.; Szechyńska-Hebda, M.; Shahnejat-Bushehri, S.; Balazadeh, S.; et al. The dual role of LESION SIMULATING DISEASE 1 as a condition-dependent scaffold protein and transcription regulator. Plant Cell Environ. 2017, 40, 2644–2662. [Google Scholar] [CrossRef]
- Mateo, A.; Mühlenbock, P.; Rustérucci, C.; Chang, C.C.-C.; Miszalski, Z.; Karpinska, B.; et al. LESION SIMULATING DISEASE 1 is required for acclimation to conditions that promote excess excitation energy. Plant Physiol. 2004, 136, 2818–2830. [Google Scholar] [CrossRef]
- Feys, B.J.; Moisan, L.J.; Newman, M.-A.; Parker, J.E. Direct interaction between the Arabidopsis disease resistance signaling proteins, EDS1 and PAD4. EMBO J. 2001, 20, 5400–5411. [Google Scholar] [CrossRef]
- Ślesak, I.; Szechyńska-Hebda, M.; Fedak, H.; Sidoruk, N.; Dąbrowska-Bronk, J.; Witoń, D.; et al. PHYTOALEXIN DEFICIENT 4 affects reactive oxygen species metabolism, cell wall and wood properties in hybrid aspen (Populus tremula L. × tremuloides). Plant Cell Environ. 2015, 38, 1275–1284. [Google Scholar] [CrossRef]
- Szechyńska-Hebda, M.; Czarnocka, W.; Hebda, M.; Karpiński, S. PAD4, LSD1 and EDS1 regulate drought tolerance, plant biomass production, and cell wall properties. Plant Cell Rep. 2016, 35, 527–539. [Google Scholar] [CrossRef]
- Pinto, A.S.S.; Brondi, M.G.; de Freitas, J.V.; Furlan, F.F.; Ribeiro, M.P.A.; Giordano, R.C.; et al. Mitigating the negative impact of soluble and insoluble lignin in biorefineries. Renewable Energy. 2021, 173, 1017–1026. [Google Scholar] [CrossRef]
- Douglas, C.J. Phenylpropanoid metabolism and lignin biosynthesis: From weeds to trees. Trends in Plant Science. 1996, 1, 171–178. [Google Scholar] [CrossRef]
- Kumar, V.; Hainaut, M.; Delhomme, N.; Mannapperuma, C.; Immerzeel, P.; Street, N.R.; et al. Poplar carbohydrate-active enzymes: Whole-genome annotation and functional analyses based on RNA expression data. Plant J. 2019, 99, 589–609. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Donev, E.N.; Barbut, F.R.; Kushwah, S.; Mannapperuma, C.; Urbancsok, J.; et al. Genome-Wide Identification of Populus Malectin/Malectin-Like Domain-Containing Proteins and Expression Analyses Reveal Novel Candidates for Signaling and Regulation of Wood Development. Frontiers in Plant Science 2020, 11. Available online: https://www.frontiersin.org/articles/10.3389/fpls.2020.588846 (accessed on 9 December 2022). [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. International Journal of Molecular Sciences. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Li, C.; Yin, B.; Liu, X.; Guo, X.; et al. PtomtAPX is an autonomous lignification peroxidase during the earliest stage of secondary wall formation in Populus tomentosa Carr. Nat Plants. 2022, 8, 828–839. [Google Scholar] [CrossRef]
- Taylor-Teeples, M.; Lin, L.; de Lucas, M.; Turco, G.; Toal, T.W.; Gaudinier, A.; et al. An Arabidopsis gene regulatory network for secondary cell wall synthesis. Nature. 2015, 517, 571–575. [Google Scholar] [CrossRef]
- Schuetz, M.; Smith, R.; Ellis, B. Xylem tissue specification, patterning, and differentiation mechanisms. Journal of Experimental Botany. 2013, 64, 11–31. [Google Scholar] [CrossRef]
- Karpiński, S.; Szechyńska-Hebda, M.; Wituszyńska, W.; Burdiak, P. Light acclimation, retrograde signalling, cell death and immune defences in plants. Plant, Cell & Environment. 2013, 36, 736–744. [Google Scholar]
- Mühlenbock, P.; Szechynska-Hebda, M.; Plaszczyca, M.; Baudo, M.; Mateo, A.; Mullineaux, P.M.; et al. Chloroplast signaling and LESION SIMULATING DISEASE1 regulate crosstalk between light acclimation and immunity in Arabidopsis. Plant Cell. 2008, 20, 2339–2356. [Google Scholar] [CrossRef]
- Bjurhager, I.; Olsson, A.-M.; Zhang, B.; Gerber, L.; Kumar, M.; Berglund, L.A.; et al. Ultrastructure and Mechanical Properties of Populus Wood with Reduced Lignin Content Caused by Transgenic Down-Regulation of Cinnamate 4-Hydroxylase. Biomacromolecules. 2010, 11, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-J.; Harding, S.A.; Lung, J.; Popko, J.L.; Ralph, J.; Stokke, D.D.; et al. Repression of lignin biosynthesis promotes cellulose accumulation and growth in transgenic trees. Nat Biotechnol. 1999, 17, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Zhao, H.; Wang, H.; Xing, Z.; Du, K.; Song, Y.; et al. Obtaining the transgenic poplars with low lignin content through down-regulation of4CL. Chin Sci Bull. 2004, 49, 905–909. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Y.; Cheng, X.; Sun, J.; Marita, J.M.; Ralph, J.; et al. Combinatorial modification of multiple lignin traits in trees through multigene cotransformation. Proc Natl Acad Sci U S A. 2003, 100, 4939–4944. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.K.; Min, D.; Savithri, D.; Lu, F.; Jameel, H.; Chiang, V.; et al. Field-Grown Transgenic Hybrid Poplar with Modified Lignin Biosynthesis to Improve Enzymatic Saccharification Efficiency. ACS Sustainable Chem Eng. 2017, 5, 2407–2414. [Google Scholar]
- Stout, A.T.; Davis, A.A.; Domec, J.-C.; Yang, C.; Shi, R.; King, J.S. Growth under field conditions affects lignin content and productivity in transgenic Populus trichocarpa with altered lignin biosynthesis. Biomass and Bioenergy. 2014, 68, 228–239. [Google Scholar] [CrossRef]
- Leplé, J.-C.; Dauwe, R.; Morreel, K.; Storme, V.; Lapierre, C.; Pollet, B.; et al. Downregulation of Cinnamoyl-Coenzyme A Reductase in Poplar: Multiple-Level Phenotyping Reveals Effects on Cell Wall Polymer Metabolism and Structure. The Plant Cell. 2007, 19, 3669–3691. [Google Scholar] [CrossRef]
- Van Acker, R.; Leplé, J.-C.; Aerts, D.; Storme, V.; Goeminne, G.; Ivens, B.; et al. Improved saccharification and ethanol yield from field-grown transgenic poplar deficient in cinnamoyl-CoA reductase. Proc Natl Acad Sci U S A. 2014, 111, 845–850. [Google Scholar] [CrossRef]
- Tian, X.; Xie, J.; Zhao, Y.; Lu, H.; Liu, S.; Qu, L.; et al. Sense-, antisense- and RNAi-4CL1 regulate soluble phenolic acids, cell wall components and growth in transgenic Populus tomentosa Carr. Plant Physiology and Biochemistry. 2013, 65, 111–119. [Google Scholar] [CrossRef]
- Min, D.; Li, Q.; Jameel, H.; Chiang, V.; Chang, H. The cellulase-mediated saccharification on wood derived from transgenic low-lignin lines of black cottonwood (Populus trichocarpa). Appl Biochem Biotechnol. 2012, 168, 947–955. [Google Scholar] [CrossRef]
- Min, D.; Yang, C.; Chiang, V.; Jameel, H.; Chang, H. The influence of lignin–carbohydrate complexes on the cellulase-mediated saccharification II: Transgenic hybrid poplars (Populus nigra L. and Populus maximowiczii A.). Fuel. 2014, 116, 56–62. [Google Scholar] [CrossRef]
- Coleman, H.D.; Park, J.-Y.; Nair, R.; Chapple, C.; Mansfield, S.D. RNAi-mediated suppression of p-coumaroyl-CoA 3’-hydroxylase in hybrid poplar impacts lignin deposition and soluble secondary metabolism. Proc Natl Acad Sci U S A. 2008, 105, 4501–4506. [Google Scholar] [CrossRef] [PubMed]
- Wituszyńska, W.; Gałązka, K.; Rusaczonek, A.; Vanderauwera, S.; Van Breusegem, F.; Karpiński, S. Multivariable environmental conditions promote photosynthetic adaptation potential in Arabidopsis thaliana. J Plant Physiol. 2013, 170, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Shu, X.; Ali, M.B.; Howard, S.; Li, N.; Winterhagen, P.; et al. A functional EDS1 ortholog is differentially regulated in powdery mildew resistant and susceptible grapevines and complements an Arabidopsis eds1 mutant. Planta. 2010, 231, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Bai, P.; Yang, Q.; Liu, F.; Wang, X.; Huang, L.; et al. Wheat zinc finger protein TaLSD1, a negative regulator of programmed cell death, is involved in wheat resistance against stripe rust fungus. Plant Physiology and Biochemistry. 2013, 71, 164–172. [Google Scholar] [CrossRef]
- Guan, Q.; Ma, H.; Wang, Z.; Wang, Z.; Bu, Q.; Liu, S. A rice LSD1-like-type ZFP gene OsLOL5 enhances saline-alkaline tolerance in transgenic Arabidopsis thaliana, yeast and rice. BMC Genomics. 2016, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Li, B.; Liu, Y.; Zhu, J.; Li, G.; Hou, G.; et al. Critical Role of Degree of Polymerization of Cellulose in Super-Strong Nanocellulose Films. Matter. 2020, 2, 1000–1014. [Google Scholar] [CrossRef]
- Dietrich, R.A.; Richberg, M.H.; Schmidt, R.; Dean, C.; Dangl, J.L. A Novel Zinc Finger Protein Is Encoded by the Arabidopsis LSD1 Gene and Functions as a Negative Regulator of Plant Cell Death. Cell. 1997, 88, 685–694. [Google Scholar] [CrossRef]
- Karimi, M.; Inzé, D.; Depicker, A. GATEWAYTM vectors for Agrobacterium-mediated plant transformation. Trends in Plant Science. 2002, 7, 193–195. [Google Scholar] [CrossRef]
- Nilsson, O.; Aldén, T.; Sitbon, F.; Little, C.H.A.; Chalupa, V.; Sandberg, G.; et al. Spatial pattern of cauliflower mosaic virus 35S promoter-luciferase expression in transgenic hybrid aspen trees monitored by enzymatic assay and non-destructive imaging. Transgenic Research. 1992, 1, 209–220. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiologia Plantarum. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Tuskan, G.A.; DiFazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; et al. The Genome of Black Cottonwood, Populus trichocarpa (Torr. & Gray). Science. 2006, 313, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics. 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014, 15, 550. [Google Scholar] [CrossRef]
- Bernacki, M.J.; Czarnocka, W.; Witoń, D.; Rusaczonek, A.; Szechyńska-Hebda, M.; Ślesak, I.; et al. ENHANCED DISEASE SUSCEPTIBILITY 1 (EDS1) affects development, photosynthesis, and hormonal homeostasis in hybrid aspen (Populus tremula L. × P. tremuloides). Journal of Plant Physiology. 2018, 226, 91–102. [Google Scholar] [CrossRef]
- Rusaczonek, A.; Czarnocka, W.; Kacprzak, S.; Witoń, D.; Ślesak, I.; Szechyńska-Hebda, M.; et al. Role of phytochromes A and B in the regulation of cell death and acclimatory responses to UV stress in Arabidopsis thaliana. J Exp Bot. 2015, erv375. [Google Scholar]
- Wise, L.E.; Murphy, M.; Addieco, A.A.d’. Chlorite holocellulose, its fractionation and bearing on summative wood analysis and studies on the hemicelluloses. 1946. [Google Scholar]
- Sluiter, A. Determination of Extractives in Biomass: Laboratory Analytical Procedure (LAP); Issue Date 7/17/2005. Technical Report. 2008;12.
- Antczak, A.; Marchwicka, M.; Szadkowski, J.; Drożdżek, M.; Gawron, J.; Radomski, A.; et al. Sugars Yield Obtained after Acid and Enzymatic Hydrolysis of Fast-growing Poplar Wood Species. BioResources. 2018, 13, 8629–8645. [Google Scholar] [CrossRef]
- Adney, B.; Baker, J. Measurement of Cellulase Activities: Laboratory Analytical Procedure (LAP); Issue Date: 08/12/1996. Technical Report. 2008;11.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).