2. Intrinsic versus extrinsic joint afferents
Typically, conclusions about the causes of pain in osteoarthritis (OA) revolve around abnormalities visible in imaging: eroded cartilage and modic changes in bone marrow for example, and reactive changes such as inflammation in synovial soft tissues and periosteum. For lack of better options, however, we have come grudgingly to accept the tenuous correlation between the images and the pain. CT and MRI images vividly reveal the pathology, but they only dimly account for the level of pain experienced by the patient. We are also forced to live with the less-than-optimal pain relief delivered by interventions that focus on cartilage repair or replacement, and by anti-inflammatory drugs. I posit that the explanation of this is straightforward. Simply put,
pain is not due to bones, cartilage, tendons or periosteum. Pain is due to nerves. And not just tissue innervation, but the electrical impulses that carry pain signals along nerves from peripheral tissues into the central nervous system (CNS). Analysis must be focused on the pain system itself. The pathology seen on imaging does not bear a direct relation to the processes responsible for impulse generation in nerve endings (electrogenesis) and the propagation of the resulting impulses to the CNS. If we were able to see in images of joints the molecules that cause neuronal hyperexcitability, primarily voltage-gated Na
+ channels [
1,
2], it would probably be trivial to sort joint pathology images into cases with and without pain. But today’s CT and MRI technologies are blind to the molecules that cause pain by generating pain-signaling nerve impulses. They don’t show the source of these impulses, or differences in neural activity among individual patients. The technology required to see these things still lies in the future.
There is, however, a proxy for seeing pain-provoking neural activity that is available today: micro-neurographic recording. But unfortunately, this accessible technology has not yet gained much clinical traction [
3,
4]. Even diagnostic nerve blocks are less routinely done than they should be. Rather, pain in OA is simply assumed to be driven by sensitized nociceptors in soft tissues of the joint and periosteum, and from the grinding of bone-on-bone during weight-bearing and movement following loss of the normal cartilaginous padding. But there is a problem here. There is no doubt that soft tissues of the joint and periosteum have nociceptive innervation that can drive pain. And correspondingly, there is no doubt that introducing a local anesthetic into the synovial space will transiently relieve the resulting pain. However, the (damaged) cartilage is
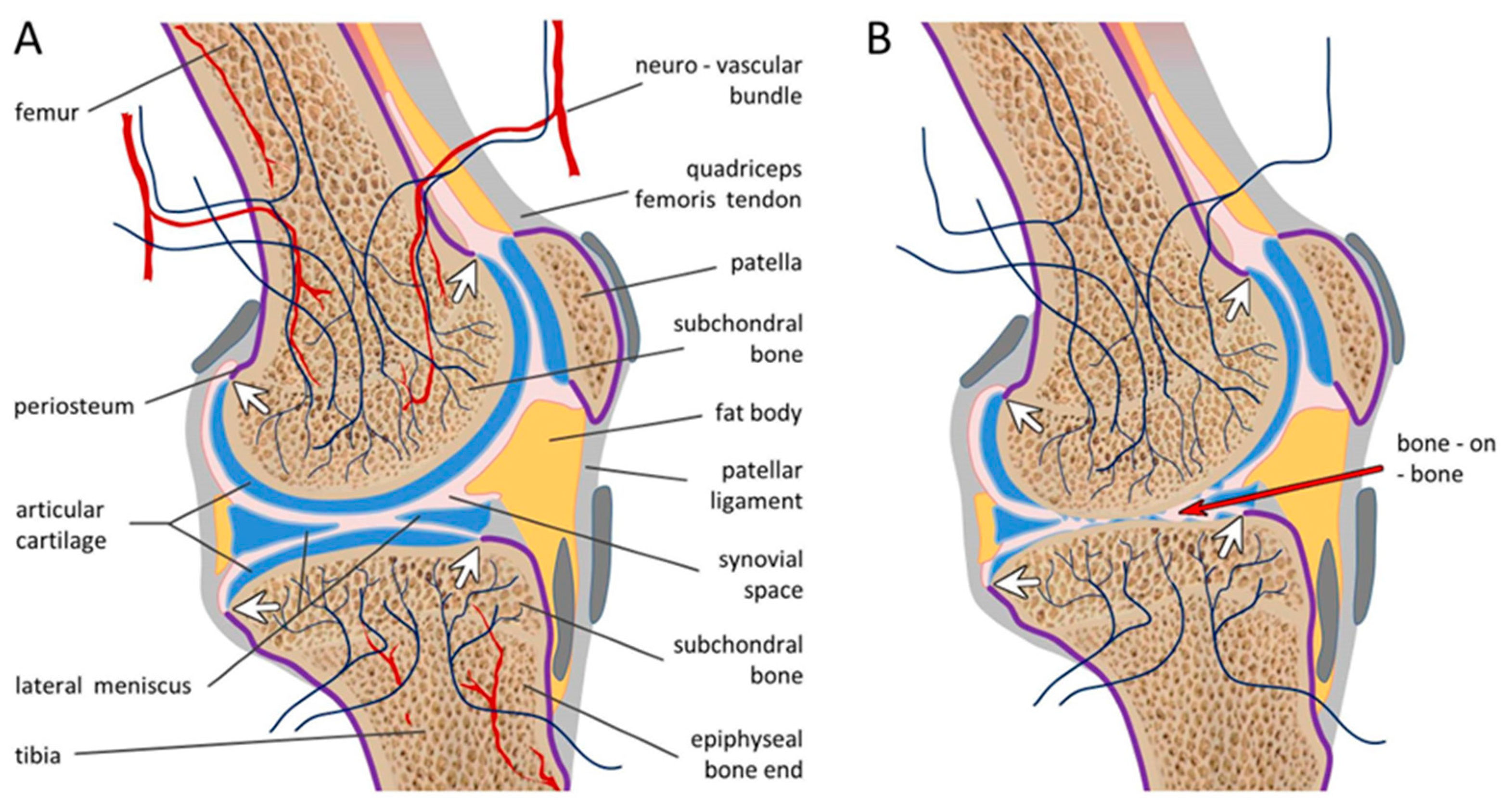
not itself innervated and the periosteum, that is innervated, does not extend over the surface of cartilage where its residue in OA might detect forces applied by bone grinding on bone. Nor is periosteum present under the cartilage (i.e., between the cartilage and the underlying subchondral bone) where it might otherwise be exposed in OA and serve as a pain sensor following cartilage erosion (
Figure 1A). Why, then, is bone-on-bone painful at all? What nerve fibers are present that could deliver a bone-on-bone pain signal to the CNS? And why does intra-synovial block (transiently) relieve OA pain?
The answer to these questions is that subchondral bone itself is innervated, not by the
extrinsic nociceptive nerve fibers that serve soft tissues and periosteum of the joint, but by
intrinsic nociceptive nerve fibers present within the bone marrow, especially at the epiphyseal bone end ([
5,
6]
Figure 1A). Like virtually all sensory nerve fibers, the cell bodies of these intrinsic bone nociceptors reside in a para-spinal dorsal root ganglion (DRG). From the DRG intrinsic bone afferents travel in peripheral nerves to their site of termination within the bone. For the spine, they enter dorsal ramus branches which serve both
extrinsic (periosteal) and
intrinsic elements of the vertebrae (basivertebral nerve) and disks. For the limbs, axons of DRG neurons enter the ventral ramus whose main branches to the leg, the sciatic and femoral nerves, carry sensory signals from all innervated hindlimb tissues into the spinal cord and brain. These large nerve trunks progressively split into large collateral branches including the tibial and common peroneal nerves, which in turn split into still smaller nerves, such as the dozen named genicular nerves that serve the knee. These genicular nerves, in turn, split into still finer nerve fascicles (which in general are not individually named) and which finally split into still finer terminal branches that end in the various component tissues of the knee. These delicate terminal branches provide the innervation of both
extrinsic and
intrinsic targets, external synovial tissues and the periosteum as well as structures within the bone marrow including the subchondral bone [
7,
8]. The
intrinsic sensory nerve fiber bundles, unnamed, enter the bone marrow as an element of neurovascular bundles which contain sensory afferents, sympathetic “nutritive” efferents and blood vessels. They gain access to the bone interior by passing through numerous small foramina located along the bone shaft, but mostly near the epiphyseal bone-end ([
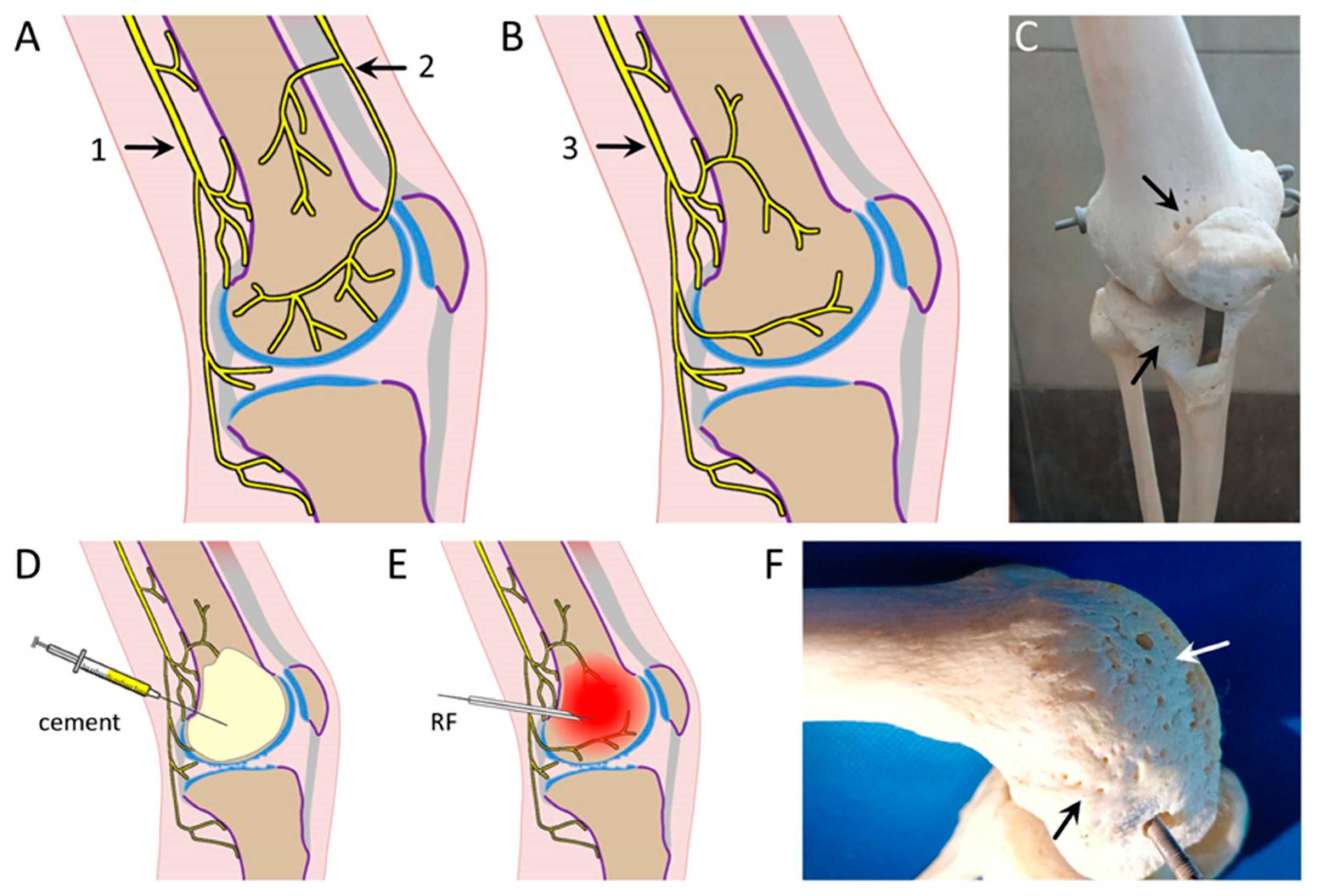
9]
Figure 2). The finer the nerve bundle, the more likely its exact path and contents will differ from individual to individual.
The afferent fibers that enter the marrow chamber along neurovascular bundles are mostly small diameter unmyelinated and lightly myelinated fibers that express markers such as substance P and CGRP suggesting that most, at least, are nociceptors [
5,
6,
7]. They distribute along the length of the bone but are enriched at the epiphyseal bone ends. The endings of these sensory axons are "electrogenic", meaning that they are specialized to generate action potentials capable of propagating centrally along the axon, past the DRG and into the CNS. These
intrinsic nociceptive nerve fiber endings are presumably responsible for pain associated with intrinsic tumors such as multiple myeloma, inflammation and, along with extrinsic periosteal innervation, fractures. A large fraction of these
intrinsic nociceptive axons, however, are directed to the epiphyseal bone end where they innervate the subchondral bone
from the inside. The anatomical layout alone leaves little doubt that bone-on-bone pain during weight bearing and movement in patients with OA is due to activation of the
intrinsic nociceptive subchondral nerve endings. The only other innervated structures in the vicinity are the laterally placed menisci (
Figure 1B).
Sensory endings
intrinsic to the bone ends might be sensitized by the mild inflammation often present in OA, but inflammation is probably not necessary [
10]. The mechanical forces associated with standing and walking in the absence of cartilaginous padding, or of bending finger joints, is enough to activate healthy, non-sensitized nociceptors. The reason that intra-synovial local anesthetic block provides transient pain relief is that the local anesthetics readily access the subchondral bone nociceptor endings from inside the synovial space, rendered accessible by fragmentation of the cartilage (
Figure 1B). Correspondingly, longer-term pain relief can be achieved in OA by substituting lidocaine with a neurotoxin such as capsaicin which causes axonal dying back [
11]. In time, however, these axons are expected to regenerate and re-innervate the subchondral bone, causing a return of pain.
Identification of the nociceptive fiber endings responsible for OA pain as intrinsic rather than extrinsic may bear on the greater success of hip arthroplasty compared to knee arthroplasty. In the hip, both articular surfaces are removed and replaced with metal and plastic; the proximal femoral head by a metallic ball and the ischial socket (acetabularium) by a plastic appliance. Innervated subchondral bone is distanced from both articular surfaces of the joint. In knee arthroplasty, in contrast, the subchondral bone of femur and tibia are trimmed, but not completely removed, and appliances are fastened to the still-innervated subchondral bone using pins and screws. In successful cases mechanical forces generated during weight-bearing and walking are distributed across the bone interface with minimal movement and minor activation of residual intrinsic bone afferents. In less successful cases, perhaps individuals with softer bone or with an unfortunate distribution of intrinsic nerve bundles, forces generated by use of the joint are likely transmitted to nerve endings in the subchondral bone, causing pain.
3. Why it matters that OA pain is subserved by intrinsic bone afferents
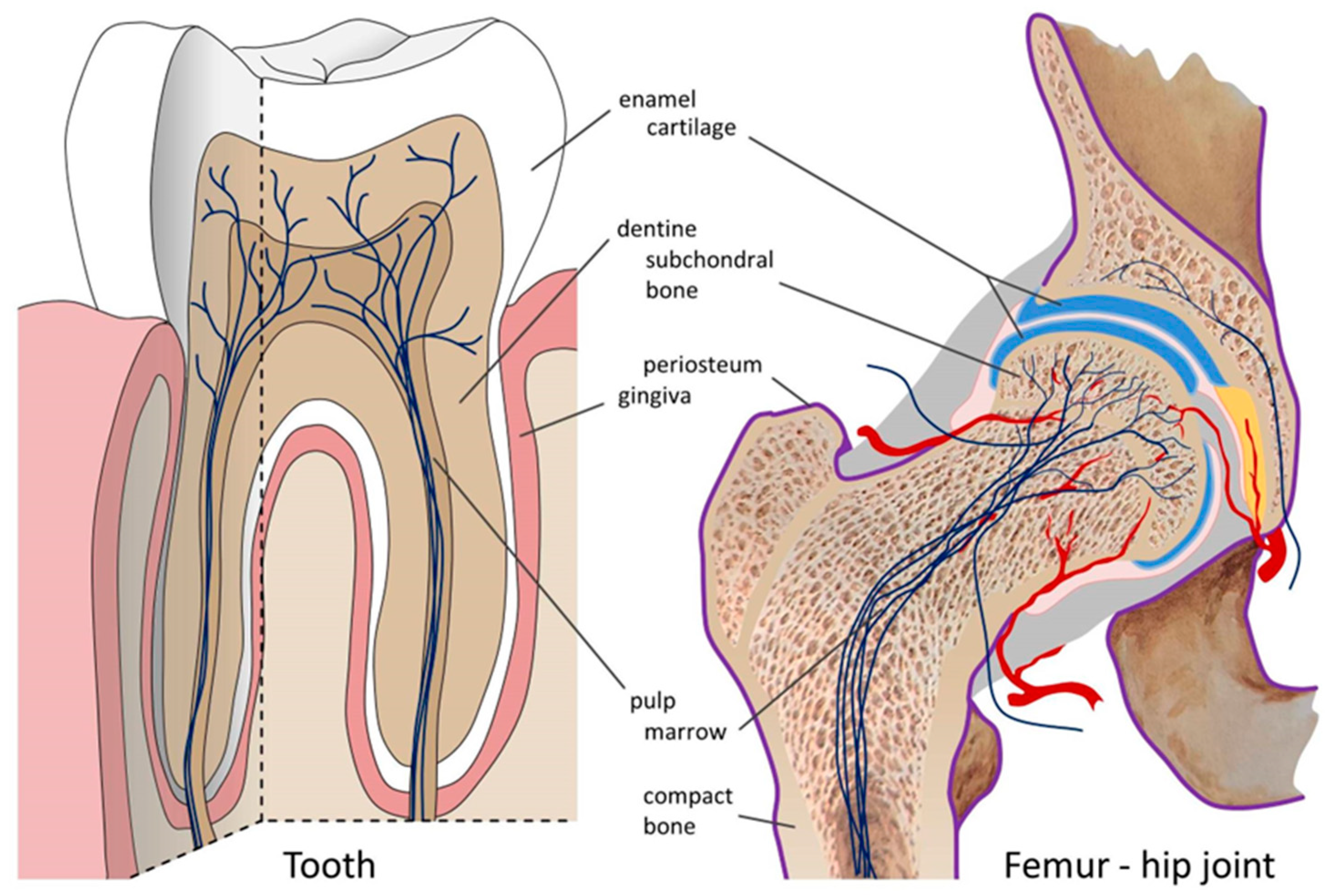
Consider an analogy taken from a distant medical discipline, but an accurate avatar of OA in many ways. I refer to the structure and innervation of teeth. Cartilage at the articular surfaces of joints is analogous to the enamel at the occlusive surfaces of teeth. They interface tooth-on-tooth, and neither is innervated. Below comes the subchondral bone, analogous to dentine. Both structures are innervated by thin nociceptive afferent axons that end within fine boney tubules only tens of microns from the interface with cartilage (bone) and enamel (teeth). And underneath is the bone marrow, filled with blood vessels and
intrinsic nerve fibers, analogous to the tooth pulp. Finally, synovial soft tissue and periosteum is analogous to gingiva. Both are served by
extrinsic nociceptive nerve fibers and become sources of pain in gingivitis and (rheumatoid) arthritis, respectively (
Figure 3). Joints, of course, differ from teeth in many ways. But the analogy is helpful, especially as the causes of tooth pain are better understood than of bone pain and the treatment of dental pain is more reliable and successful than that of joint pain.
What happens when the overlying non-innervated layer, tooth enamel or articular (hyaline) cartilage, is removed? Exposing dentine in this way renders teeth exceedingly sensitive to even very weak stimulation. Light touch, air-puff or thermal stimuli now evoke pain. This is due to nociceptor endings within the dentinal tubules, and perhaps also sensory endings of "algoneurons" that end in the dentine. Algoneurons are sensory neurons with myelinated axons that respond to weak, non-noxious stimuli (low threshold mechanoreceptor endings), but that signal pain to a conscious brain [
12,
13]. Pursuing the analogy, erosion of articular cartilage in OA exposes nerve fibers in the subchondral bone to biomechanical forces during weight bearing and joint movement. This, I propose, is the primary cause of pain in OA. Algoneurons have been identified in teeth, and are likely present also in bones and/or joints. But even if not, the mechanical forces impinging on knee joints, with bone grinding on bone, is surely sufficient to drive normal, healthy nociceptor endings. Prior sensitization by inflammatory mediators is not required. Dentists routinely remove enamel from healthy teeth in the process of anchoring bridgework, and the intact, non-inflamed dentine exposed in this way is highly sensitive. I venture that the same is true of subchondral bone following erosion of overlying cartilage. To be sure, when inflammation is present it can augment pain, but this factor is more important in gingivitis (teeth) and rheumatoid arthritis (joints).
Pursuing the tooth-joint analogy, the obvious way to obtain prolonged relief from pain in arthritic joints is to permanently ablate the
intrinsic innervation of the epiphyseal bone end and prevent reinnervation, a sort of root-canal treatment of the joint. A practical approach to doing this, proposed 20 years ago [
14], is to inject a bone cement such as polymethyl methacrylate (PMMA) transversely into the epiphyseal bone end(s), or perhaps via the synovial capsule (
Figure 2D). PMMA is injected into bone as a liquid at room temperature, but it sets in an exothermic reaction generating sufficient heat to cauterize nerve fibers and blood vessels (near 95°C). And as it cools it establishes a permanent bone-hard barrier to subchondral bone reinnervation. In the presence of osteoporosis, common in OA patients, there should be no need for the specialized needles used routinely for bone marrow aspiration [
15]. Injection of a local anesthetic prior to the PMMA, sural nerve block, or regional anesthesia should permit the procedure itself to be mostly painless. This is "marrow-canal treatment", accomplished using PMMA in place of amalgam. PMMA and other bone cements are biocompatible and cause minimal necrosis of adjacent compact bone. More important still, lesioning
intrinsic bone innervation is very unlikely to induce formation of a painful neuroma. These things are known, for example, from hip joint replacement where insertion of a titanium prosthesis often anchored by bone cement into the femoral bone canal has been standard practice in orthopedics for decades. Neither bone necrosis nor neuroma pain are common adverse events. In dental root-canal treatment as well, painful neuromas are a very rare complication. The reasons for this are uncertain [
2,
16]. For OA of the hip treating the femoral epiphysis might be sufficient, but for OA of the knee or digits both apposed bone ends would presumably need to be treated.
One potential concern associated with cauterizing intrinsic epiphyseal innervation using bone cement is that if it failed the presence of the cement might complicate subsequent execution of routine knee arthroplasty. With this in mind, initial trials should probably be carried out in patients who are not candidates for open knee surgery. Indeed, this concern might be bypassed entirely using one of numerous other possible means of denervating the epiphyseal bone end. For example, one might inject a neurolyic agent into the bone end or cauterize using a RF electrode ([
17,
18];
Figure 2E). Subchondral bone denervation might even be carried out non-invasively using radiological tomography or focused ultrasound (FUS, [
19,
20]. In early trials, before performing neurolysis, it might be wise to begin with diagnostic intra-epiphyseal block using injected bupivacaine or another relatively long-acting local anesthetic. Immediately following injection, the patient would be encouraged to use the joint in a way that normally evokes his/her typical pain and report on changes during the course of the block until the anesthetic effect fades. Quoting an authority in the field, "I can confirm that intraosseous injection of bupivacaine provides at least 2 hours of pain relief when injected in the hip epiphysis" (Prof. Phillippe Hernigou, Hôpital Henri Mondor, Creteil, France, with permission).
I am not aware of the intervention proposed, although published in a leading pain journal two decades ago, ever having been tried in OA patients, even in a diagnostic mode to test the concept that
intrinsic innervation is indeed the key driver of OA pain [
14,
21]. Unfortunately, animal models of arthropathy are so remote from clinical OA as to be unlikely to provide a useful prediction of efficacy. Indeed, reliable translation from rodents to man in the pain arena has tended to be iffy across the board [
22,
23]. However, beyond the fundamental logic of blocking intrinsic bone innervation (
Figure 1B), and the ability to predict efficacy in the individual patient by diagnostic intra-epiphyseal anesthesia, the promise of long-lasting pain relief can be evaluated on the basis of a related procedure: intraosseous injection of bone cement in the treatment of bone pain caused by malignancies. The primary aims of this procedure are structural, reducing the likelihood of fractures and reducing the volume of the tumor. However, substantial pain relief is frequently reported as a positive side-effect, including pain relief at rest and during movement. This is very likely due to incidental cautery of intrinsic bone innervation and not just increased bone stability [
24,
25].
4. Relation to genicular nerve section in the treatment of OA
A relatively recent randomized controlled trial (RCT) published by Choi et al. reported moderate success in relieving pain in OA by RF cauterization of three of the named genicular nerves: the superior lateral (SL), the superior medial (SM) and the inferior medial (IM) nerves [
26]. This minimally invasive procedure was rapidly adopted in the belief that it is a simple, painless and comparatively successful approach to the relief of OA of the knee [
27,
28]. The reliability of this belief, however, has been challenged recently with the suggestion that despite this (single) RCT, the numerous optimistic follow-up reports may largely reflect wishful thinking by patient and physician, i.e., placebo [
29]. Research is ongoing to test this and to determine whether lesioning a larger fraction of the genicular nerves might yield better and more reliable results. In light of this uncertainty, and the hypothesis laid out here, it is worth considering the mechanism whereby pain relief might be achieved by destroying 3, or perhaps more, genicular nerves.
The 3 genicular nerves targeted by Choi et al. undoubtedly provide innervation, including nociceptive innervation, to the soft tissues of the knee, including the periosteum. Partial destruction of this
extrinsic nociceptive innervation is presumed in the medical community to be the basis for the pain relief reported. However, as noted above, the
intrinsic innervation of the bone end is also provided by sciatic and femoral nerve branches that serve the knee, i.e., by genicular nerves and their fine collateral branches. Is it lesioning of the
extrinsic or the
intrinsic innervation of the knee that provides pain relief (if any) following RF cauterization of the SM, SL and IM genicular nerves? The answer to this question depends on whether: 1) These 3 named genicular nerves contain axons that serve
extrinsic tissues exclusively, particularly periosteum, 2) whether they serve
intrinsic subchondral bone exclusively (unlikely a priori), or 3) whether most of the nerve serves periosteum and other
extrinsic targets, but that small unnamed fascicles of one or all of the 3 peel off and enter the bone marrow along penetrating neurovascular bundles to serve
intrinsic targets inside, particularly subchondral bone (
Figure 2A,B arrows 1,2 and 3 respectively). The answer matters. If Cohen et al.'s [
29] critique that lesioning (these) 3 genicular nerves is not enough, their proposal to attack a larger number is indeed likely to result in better pain relief as this will surely increase the extent of subchondral bone denervation. However, the simultaneous massive denervation of the knee’s
extrinsic innervation might risk joint instability, painful genicular nerve-end neuromas, or even a Charcot joint.
I have been unable to establish whether the 3 specific genicular nerves lesioned using Choi et al.'s procedure do in fact carry
intrinsic bone afferent fibers at the location at which the RF lesions are made. However, as these lesions are made at some distance from the epiphyseal bone end where most neurovascular bundles enter the bone marrow, this is very likely. If so, the efficacy of the genicular nerve RF procedure as currently executed is probably due to severing these
intrinsic nerve fascicles at a point proximal to where they split off of the main genicular nerve. The degree of pain relief in the individual patient, however, would be expected to vary considerably with the specific branching pattern of the fine pre-terminal penetrant fascicles in the patient, and how their distribution matches with the exact sites of cartilage erosion. In any event, with ablation of only 3 nerves, subchondral bone denervation would surely be partial, and many healthy nerve fibers that do not contribute to the patient's pain would be cut unnecessarily. Ablating many more than 3, as noted, might risk severe adverse effects. The anatomical question concerning genicular nerve branching patterns should be straightforward to resolve by careful dissection in the cadaver lab. But as a first step, a strong hint is obtained by simply observing the location of the osseous foramina through which neurovascular bundles penetrate into the femur and tibia to access the subchondral bone. As shown in
Figure 2C,F the large bulk of these foramina are located at the epiphyseal bone end, directly adjacent to the innervated subchondral bone.
A more direct and likely safer approach to selectively lesioning the
intrinsic nociceptor innervation, sparing the
extrinsic, would involve making the RF lesions further distally along the relevant genicular nerve branches, closer to the point of exit of penetrating fascicles as they travel towards the osseous foramina and the epiphyseal bone end beyond. Moving the point of lesioning distally in this way would spare most of the
extrinsic innervation of the joint. Indeed, using a surgical exposure adjacent to the bone end itself (arrows,
Figure 2C,F) and proceeding with special care, it might be possible to selectively sever penetrating nerve bundles as they enter the bone while sparing both
extrinsic innervation and the accompanying nutritive blood vessels. This approach could be added to the list of selective
intrinsic denervation methods note above.
5. OA symptoms beyond arthritic pain
Pain relief by root canal treatment solves the dental patient’s acute pain problem, but a crown still needs to be fitted to the tooth stump to restore the tooth’s masticatory function. The marrow canal treatment proposed here is expected to relieve OA pain, but not the secondary symptoms of joint stiffness, loss of flexibility and limited range of motion. Indeed, in the absence of pain, increased use of the joint might even accelerate these degenerative processes, although there is reason to believe that if anything, it is best to encourage physical activity in OA patients [
21]. In practice, as OA patients tend to elderly, marrow canal treatment of hip, knee and/or digital joints might provide an effective lifelong solution. In individuals in whom pain relief did lead to accelerated functional disability a salvage option, if necessary, is available… moving on to conventional knee replacement surgery.
In dental practice, pain arising from
intrinsic innervation of the dentine, toothache, is very different from pain arising from
extrinsic innervation of inflamed gingiva. By analogy, pain due to the
intrinsic innervation of subchondral bone needs to be distinguished from pain felt in the external soft tissue of the joint, articular synovium and periosteum. These symptoms, due primarily to
extrinsic joint innervation, are more typical of inflammatory (rheumatoid) arthritis than OA (degenerative arthritis). Here too, consideration of the underlying neural substrate may point to improved therapeutic options. Dentists are angels of pain relief. The patient who enters a dental clinic not with gingivitis, but with a mind-gouging toothache, is almost certain to leave within an hour with the problem solved, the pain gone, forever, and with little likelihood of return. All the dentist needs to do is to denervate the articular dentine and fill the resulting cavity with an inert biocompatible material, amalgam. OA pain might be treated similarly and (hopefully) with equal success if more thought were given to nerves and nociceptive innervation of the relevant tissues. This is the proposal on the table [
14]. For good reasons a mature discipline like orthopedics might not always be fully open to new, untested ideas that come from elsewhere, pain science and dentistry in this case. But sometimes cross-fertilization yields tasty new fruit.