Submitted:
04 July 2023
Posted:
06 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
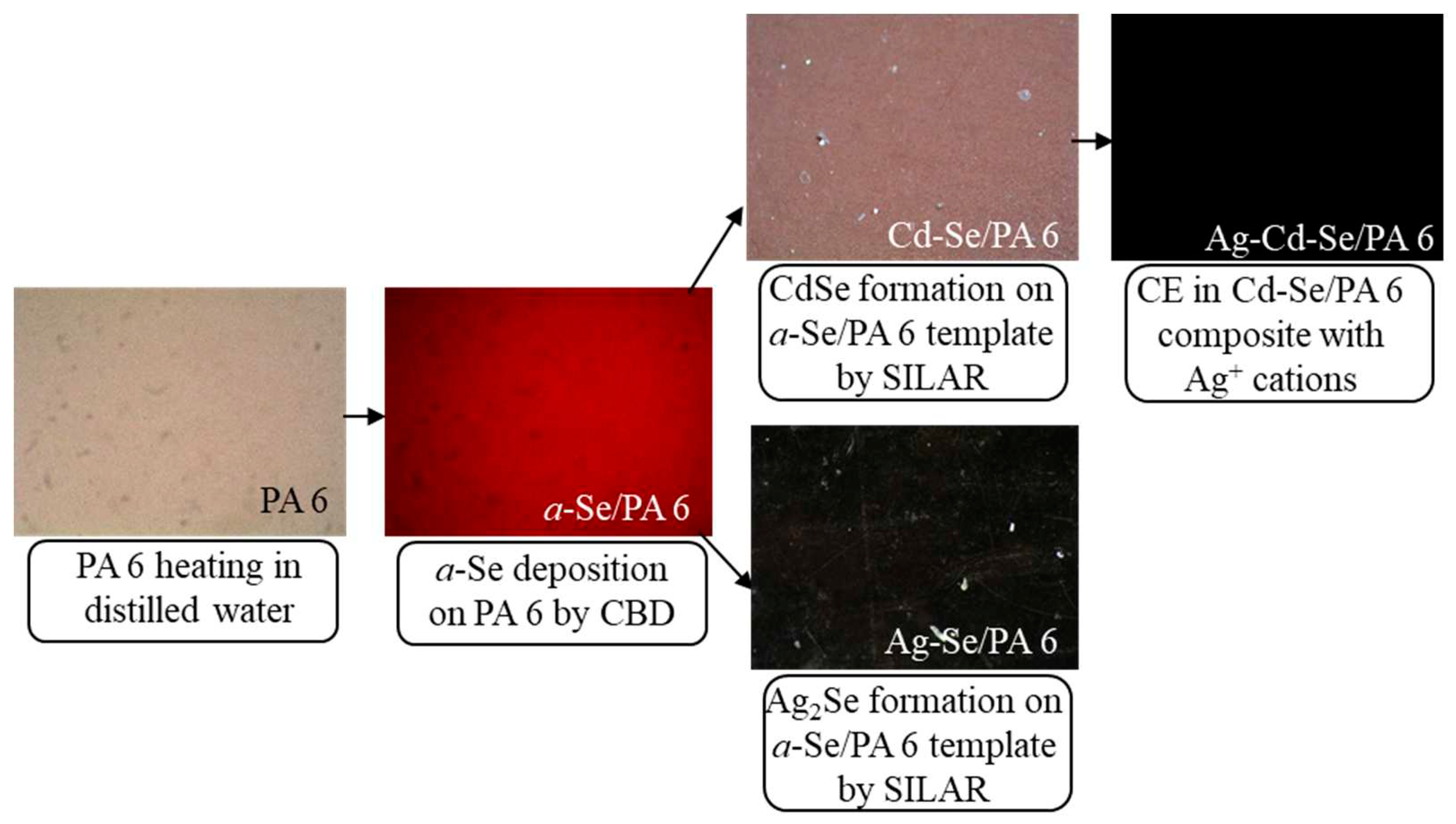
2. Materials and Methods
3. Results and Discussions
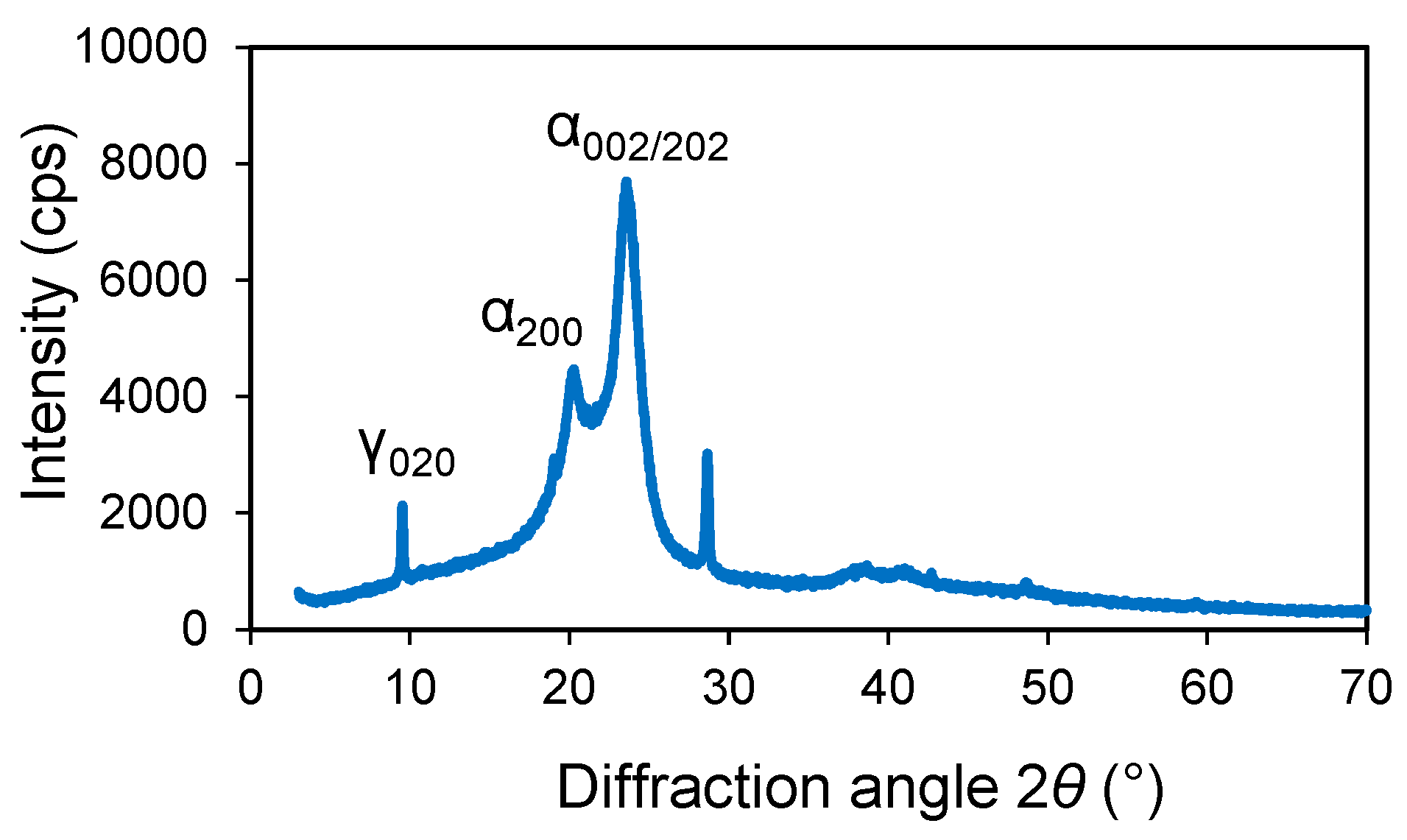
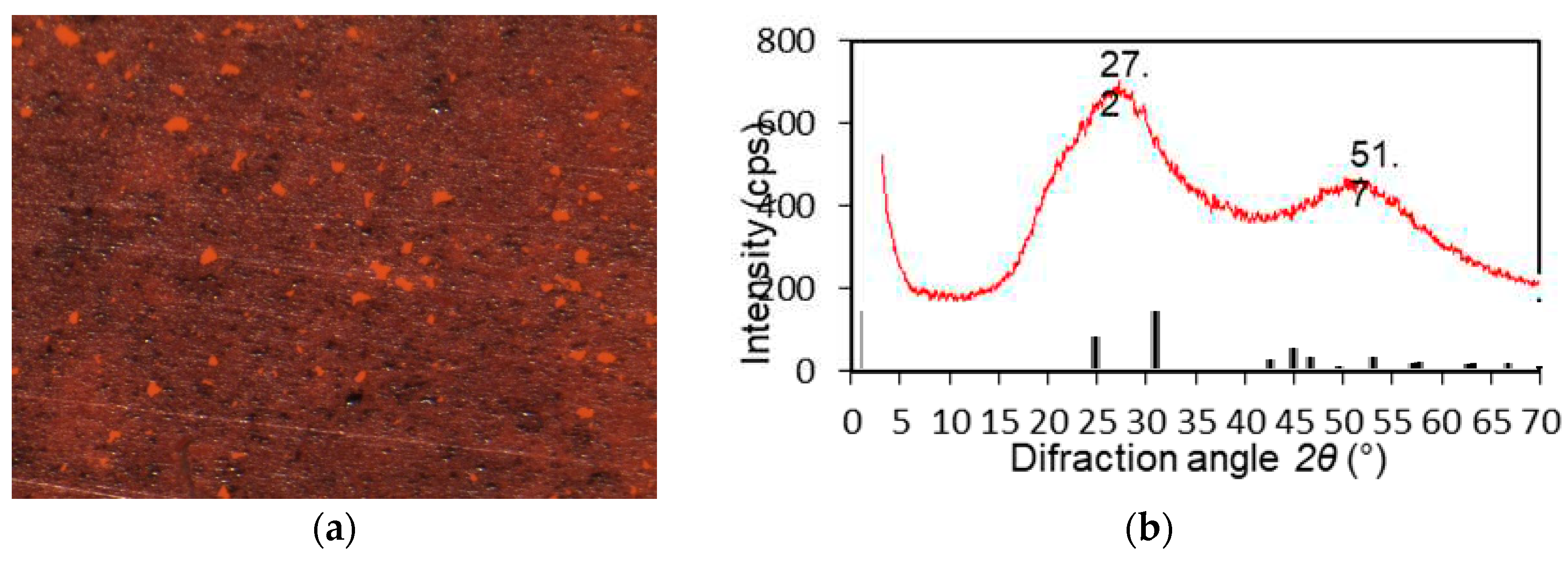
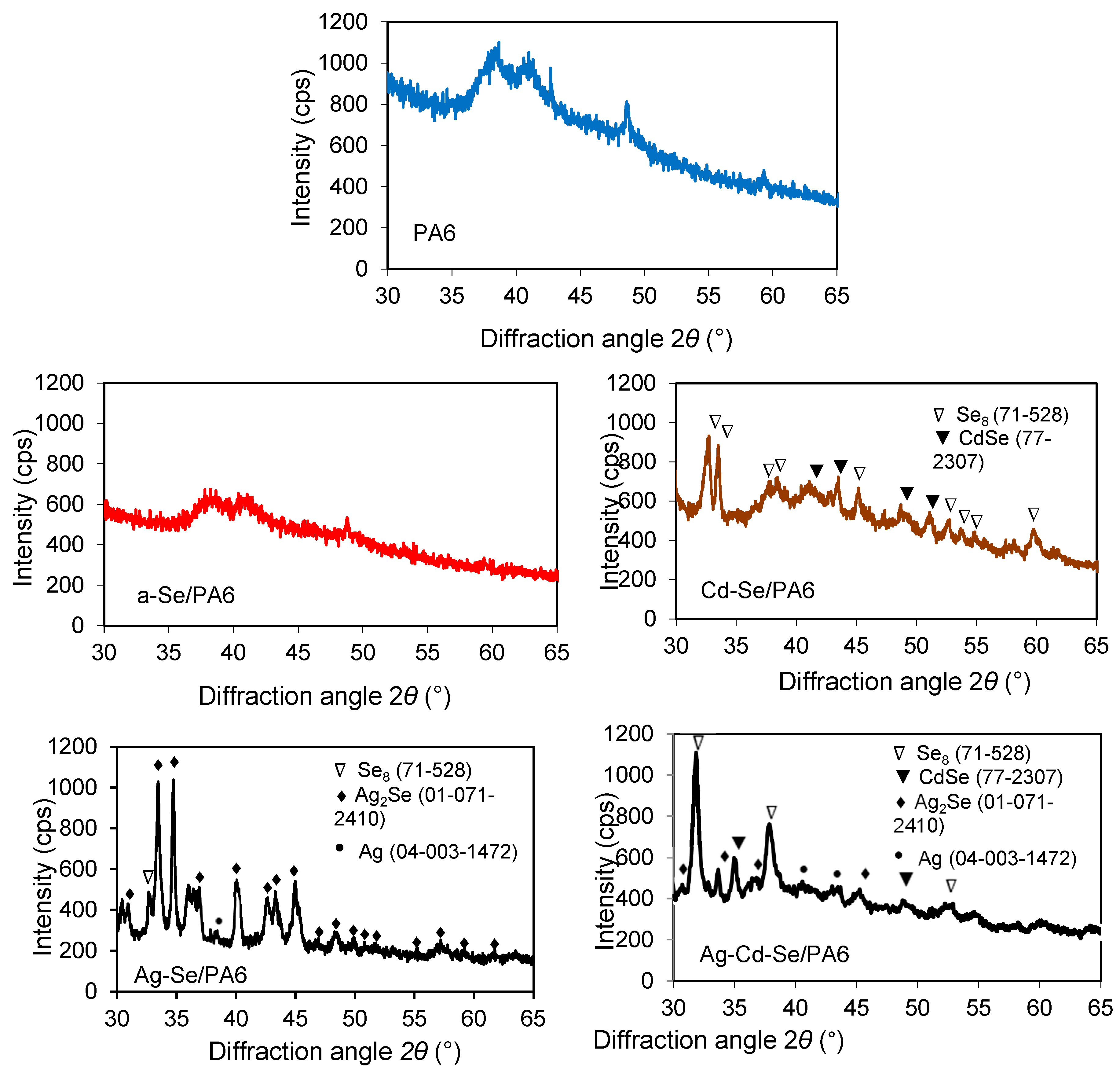
3.1. XRD Analysis
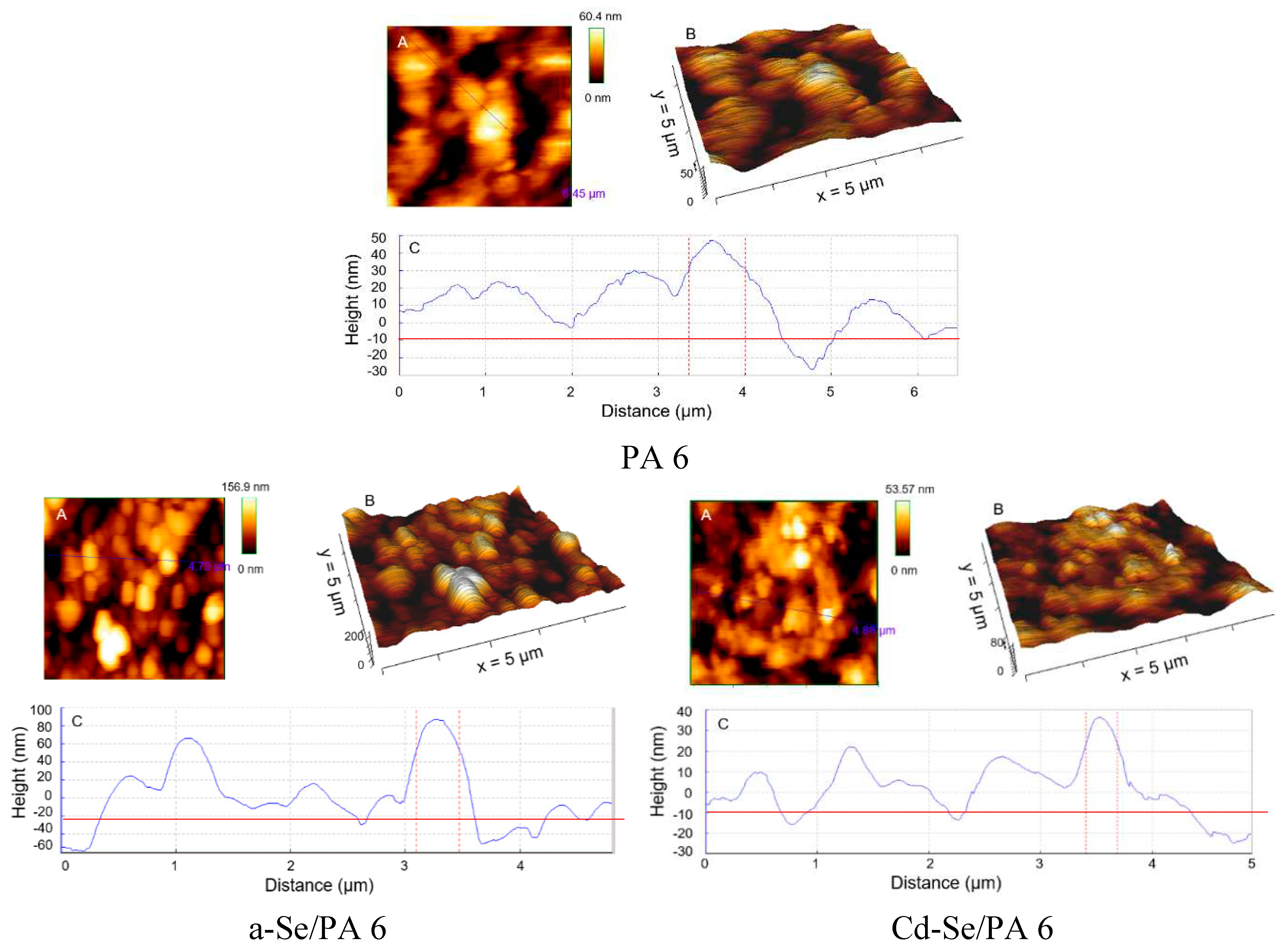
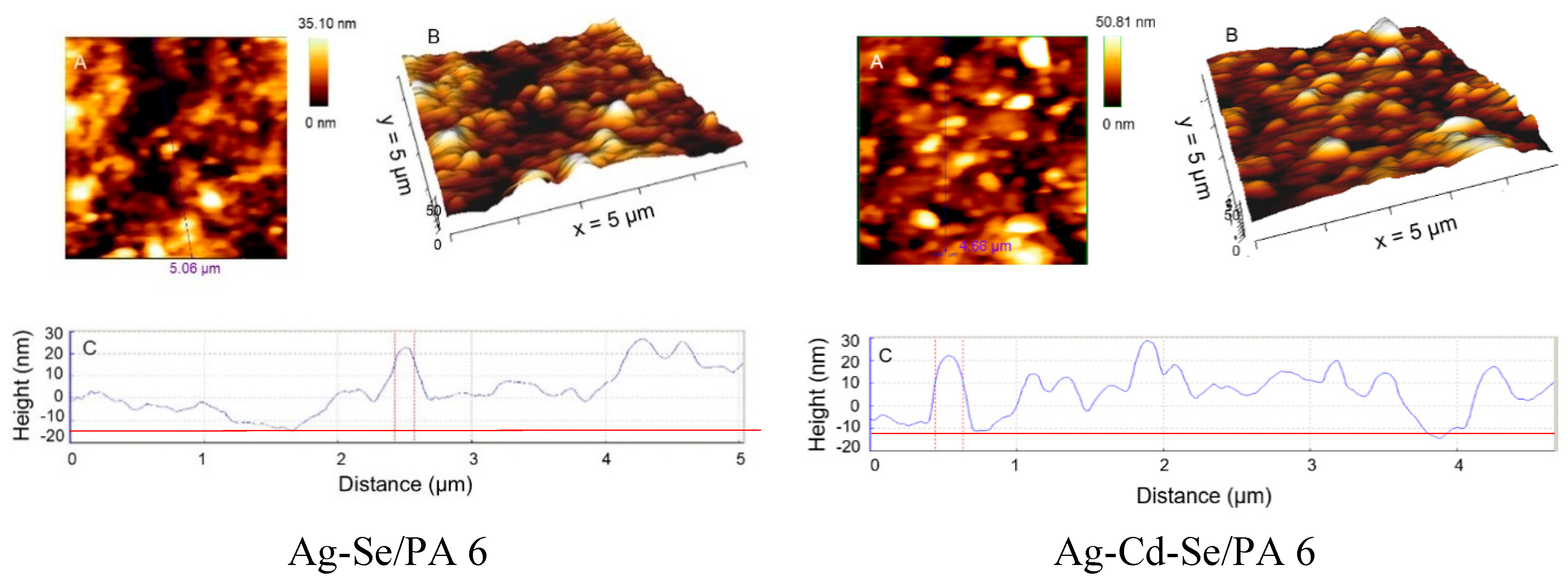
3.2. AFM Analysis
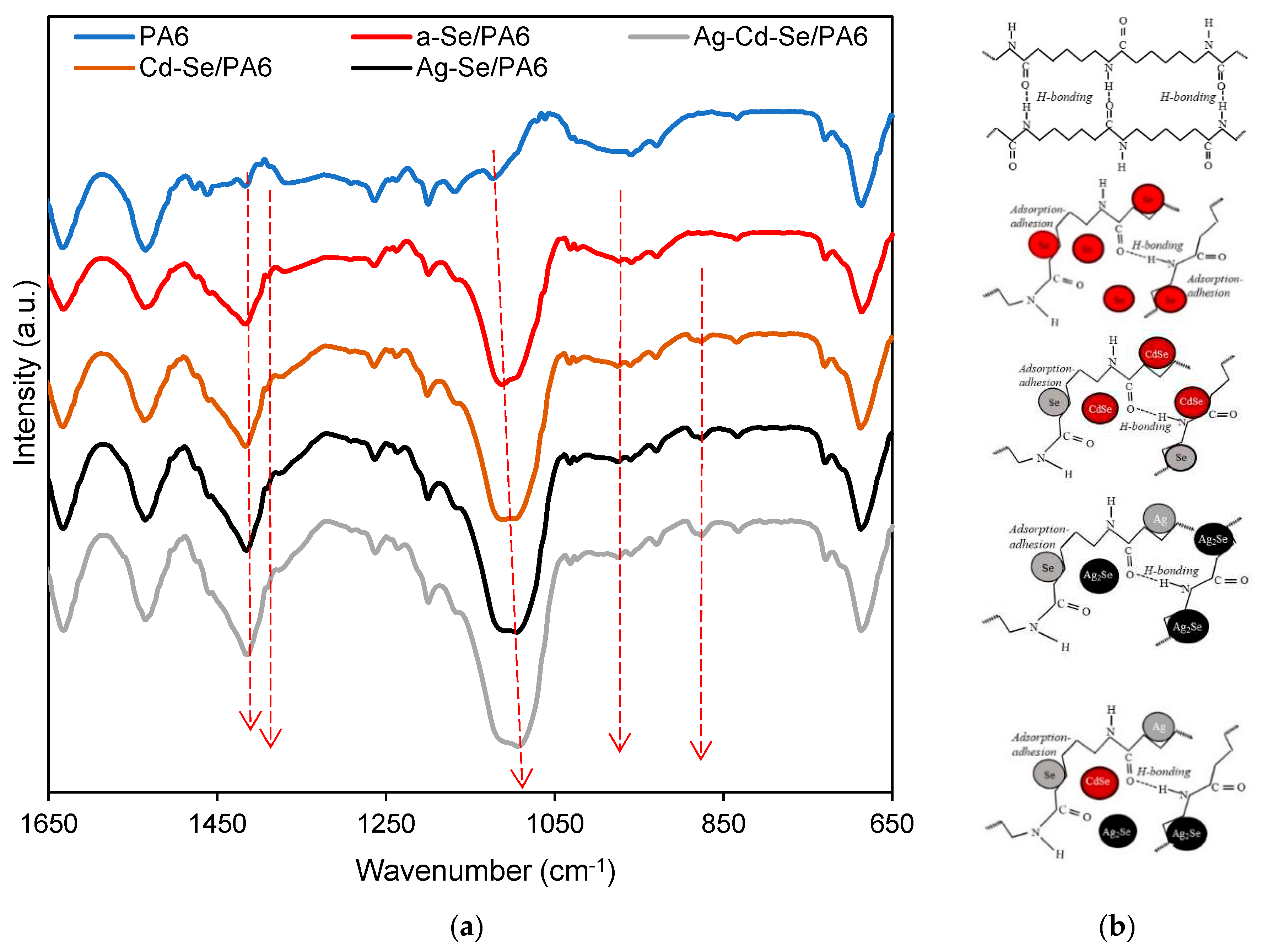
3.3. ATR-FTIR Analysis
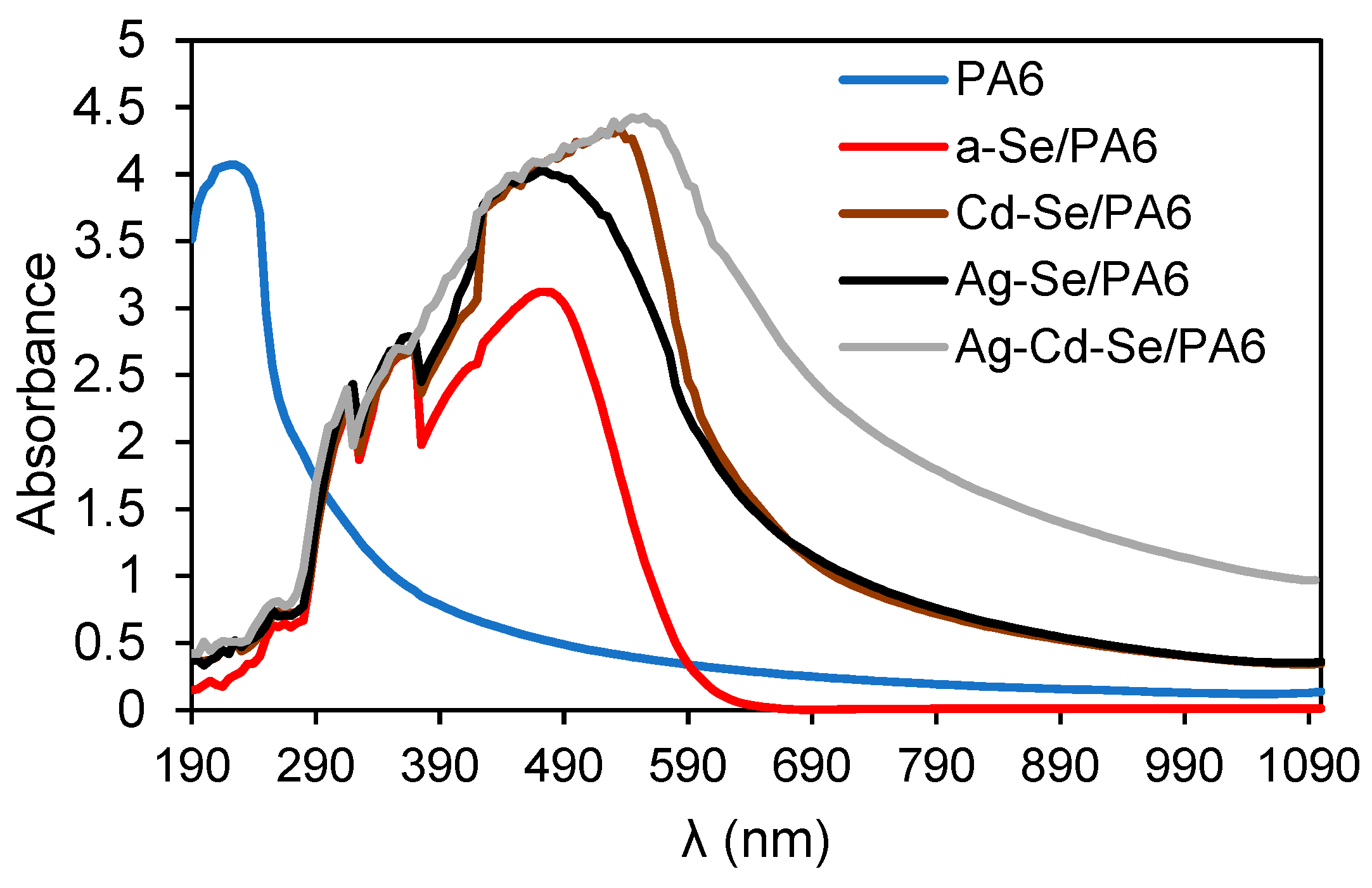
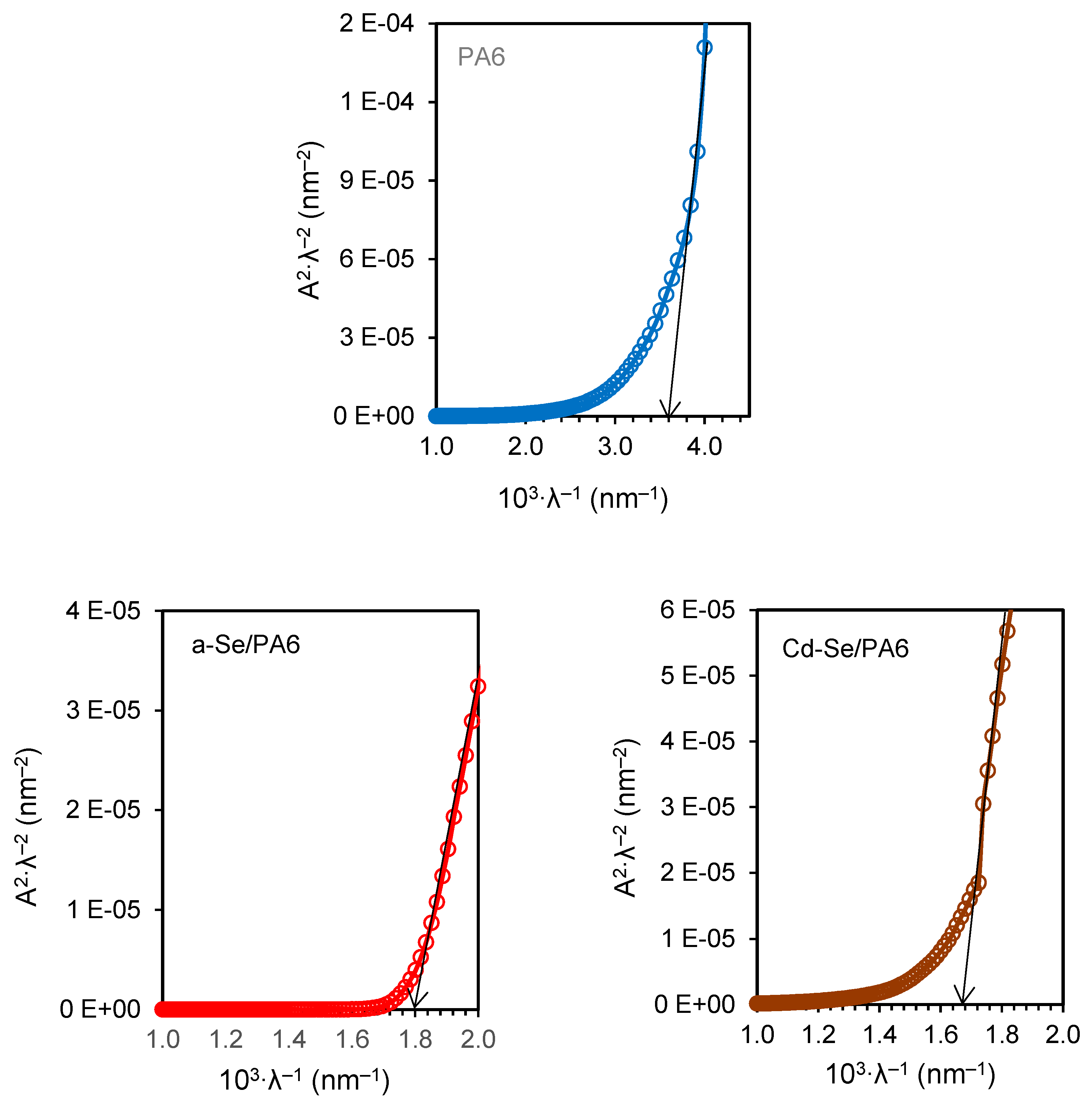
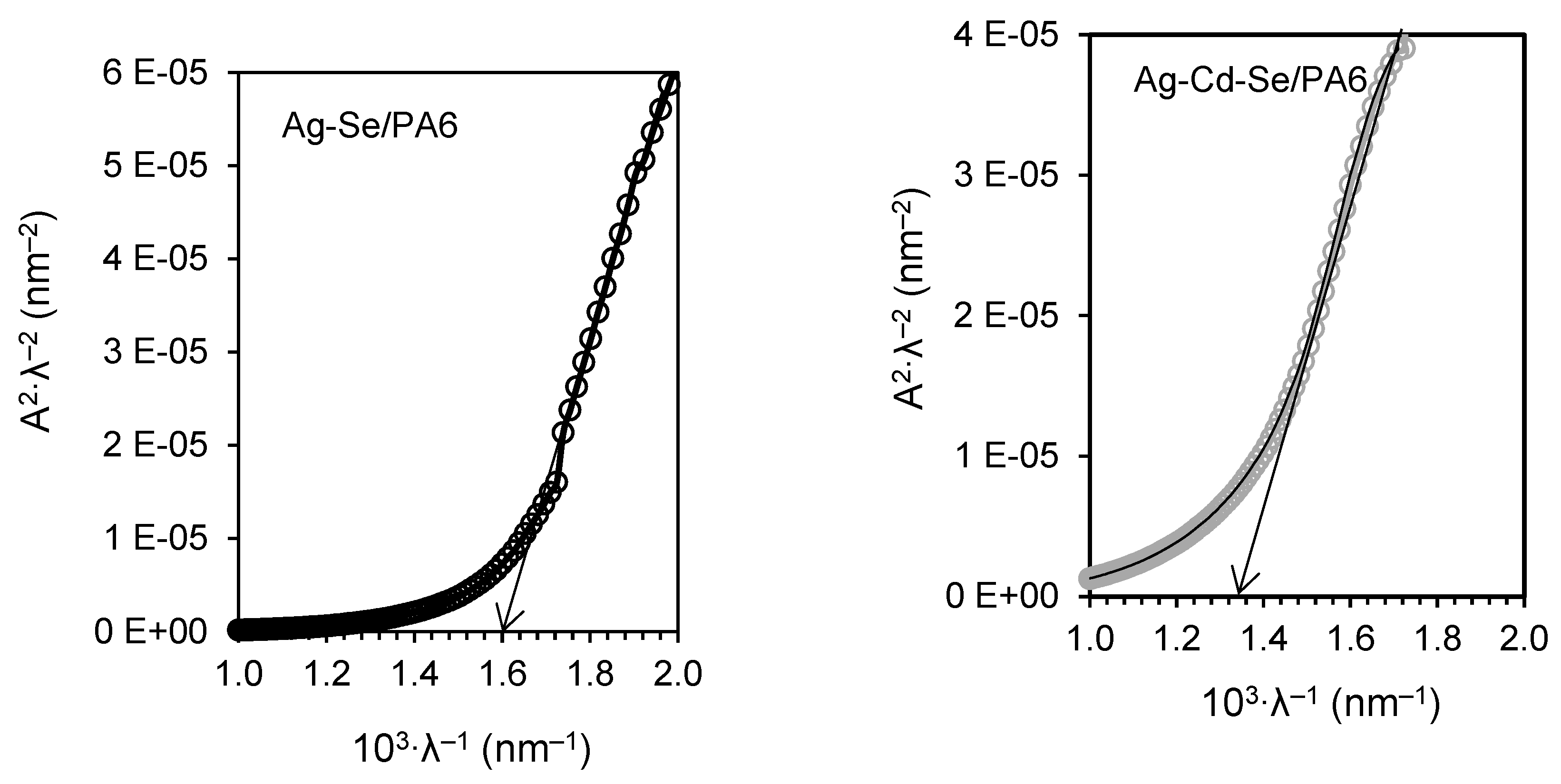
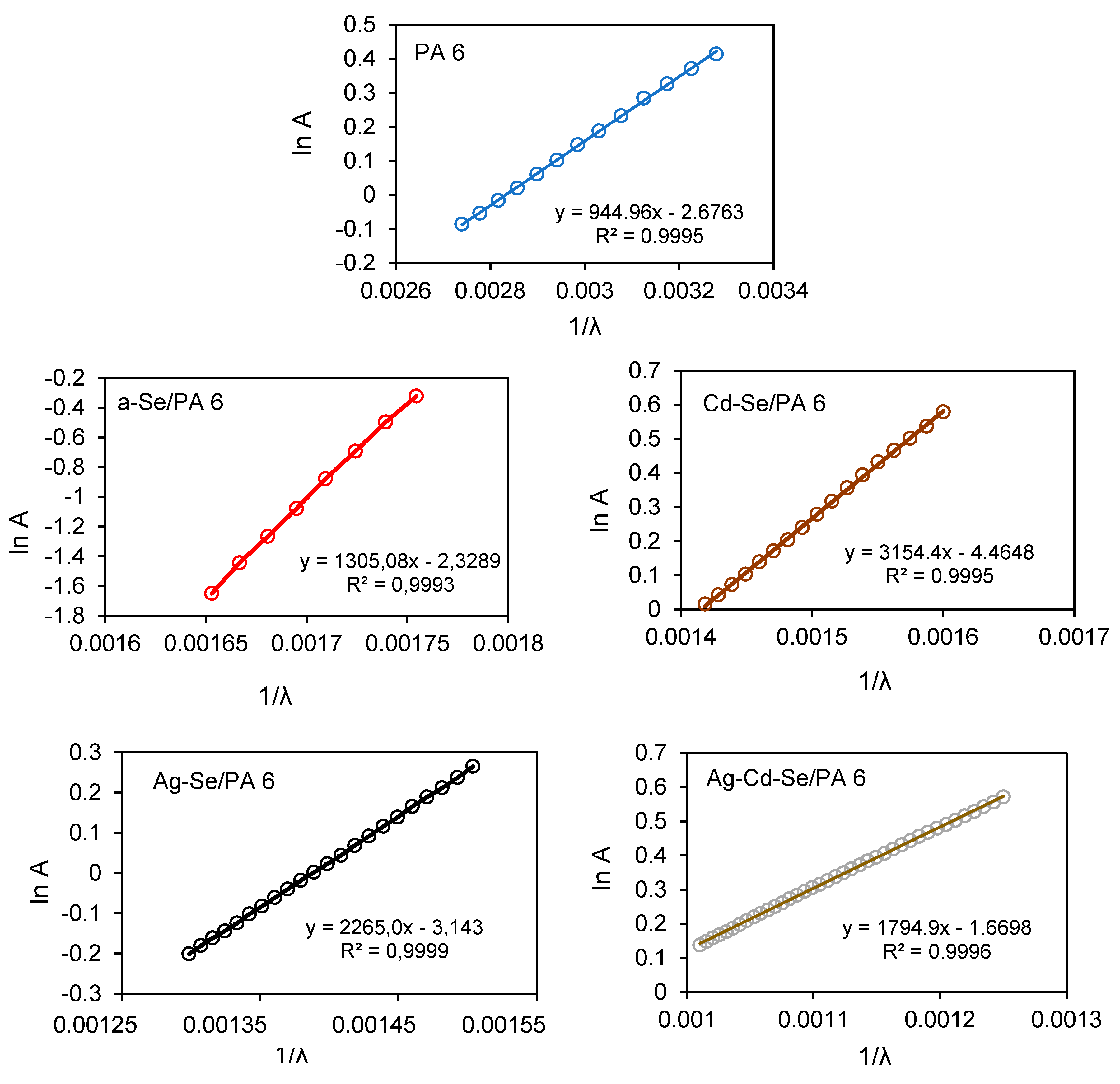
3.4. UV-Vis Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Park, Y.; Advincula, R.C. Hybrid semiconductor nanoparticles: π-conjugated ligands and nanostructured films. Chem. Mater. 2011, 23, 4273–4294. [Google Scholar] [CrossRef]
- Liu, R. Hybrid organic/inorganic nanocomposites for photovoltaic cells. Materials 2014, 7, 2747–2771. [Google Scholar] [CrossRef] [PubMed]
- Soler-Illia, G.J.; Azzaroni, O. Multifunctional hybrids by combining ordered mesoporous materials and macromolecular building blocks. Chem. Soc. Rev. 2011, 40, 1107–1150. [Google Scholar] [CrossRef]
- Willner, I.; Willner, B. Functional nanoparticle architectures for sensoric, optoelectronic, and bioelectronic applications. Pure Appl. Chem. 2002, 74, 1773–1783. [Google Scholar] [CrossRef]
- Di Luccio, T.; Piscopiello, E.; Laera, A.M.; Antisari, M.V. Structural studies of thin films of semiconducting nanoparticles in polymer matrices. Mater. Sci. Eng. C 2007, 27, 1372–1376. [Google Scholar] [CrossRef]
- Gates, B.; Mayers, B.; Cattle, B.; Xia, Y. Synthesis and characterization of uniform nanowires of trigonal selenium. Adv. Funct. Mater. 2002, 12(3), 219–227. [Google Scholar] [CrossRef]
- Jiang, F.; Cai, W.; Tan, G. Facile Synthesis and optical properties of small selenium nanocrystals and nanorods, Nanoscale Res. Lett. 2017, 12, 401. [Google Scholar] [CrossRef]
- Guha, S.; Munekata, H.; LeGoues, F.K.; Chang, L.L. Growth mode and dislocation distribution in the ZnSe/GaAs (100) system. Appl. Phys. Lett. 1992, 60, 3220. [Google Scholar] [CrossRef]
- Bhatnagar, A.K.; Venugopal Reddy, K.; Srivastava, V. Optical energy gap of amorphous selenium: effect of annealing. J. Phys. D: Appl. Phys. 1985, 18, 149–l53. Available online: http://iopscience.iop.org/0022-3727/18/9/001.
- Thayer, J.S. A Review of: Selenium. In Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry, Zingaro, R.A., Cooper, W.C. Eds.; Van Nostrand Reinhold, New York, 1974, xvii, 5(2) 159–160. [CrossRef]
- Afzaal, M.; O’Brien, P. Recent developments in II–VI and III–VI semiconductors and their applications in solar cells. J. Mater. Chem. 2006, 16, 1597–1602. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, W.; Lu, Y.; Cai, K.; Li, Y.; Wang, Z.; Wu, M.; Huang, C.; He, J. High power factor Ag/Ag2Se composite films for flexible thermoelectric generators. ACS Appl. Mater. Interface 2021, 13(12), 14327–14333. [Google Scholar] [CrossRef]
- Wu, M.; Cai, K.; Li, X.; Li, Y.; Liu, Y.; Lu, Y.; Wang, Z.; Zhao, W.; Wei, P. Ultraflexible and high-thermoelectric-performance sulfur-doped Ag2Se film on nylon for power generators. ACS Appl. Mater. Interfaces 2022, 14(3), 4307–4315. [Google Scholar] [CrossRef]
- Roy, S.; Tuinenga, C.; Fungura, F.; Dagtepe, P.; Chikan, V.; Jasinski, J. Progress toward producing n-type CdSe quantum dots: tin and indium doped CdSe quantum dots. J. Phys. Chem. C, 2009, 113(30), 130008–13015. [CrossRef]
- Liu, J.; Zhao, Q.; Liu, J.L.; Wu, Y.S.; Cheng, Y.; Ji, M.W.; Qian, M.H.; Hao, W.C.; Zhang, L.J.; Wei, X.J.; Wang, S.G.; Zhang, J.T.; Du, Y.; Dou, S.X.; Zhu, H.S. Heterovalent-doping-enabled efficient dopant luminescence and controllable electronic impurity via a new strategy of preparing II−VI nanocrystals. Adv. Mater. 2015, 27, 2753–2761. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.D.; Dong, A.G.; Buhro, W.E. Solution–liquid–solid synthesis, properties, and applications of one-dimensional colloidal semiconductor nanorods and nanowires. Chem. Rev., 2016, 116(18), 10888–10933. [CrossRef]
- Coughlan, C.; Ibanez, M.; Dobrozhan, O.; Singh, A.; Cabot, A.; Ryan, K.M. Compound copper chalcogenide nanocrystals. Chem. Rev. 2017, 117, 5865–6109. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, J. Nanointerface chemistry: lattice-mismatch-directed synthesis and application of hybrid nanocrystals. Chem. Rev. 2020, 120, 2123–2170. [Google Scholar] [CrossRef]
- Agranovich, V.M.; Gartstein, Y.N.; Litinskaya, M. Hybrid resonant organic–inorganic nanostructures for optoelectronic applications. Chem. Rev. 2011, 111, 5179–5214. [Google Scholar] [CrossRef]
- Mehta, C.; Abbas, J.M.; Saini, G.S.S.; Tripathi, S.K. Effect of deposition parameters on the optical and electrical properties of nanocrystalline CdSe. Chalcogenide Letters 2007, 4(11), 133–138. [Google Scholar]
- Pejova, B.; Najdoski, M.; Grozdanov, I.; Dey, S.K. Chemical bath deposition of nanocrystalline (111) textured Ag2Se thin films. Mater. Lett. 2000, 43, 269–273. [Google Scholar] [CrossRef]
- Gates, B.; Yin, Y.; Xia, Y. A Solution-phase approach to the synthesis of uniform nanowires of crystalline selenium with lateral dimensions in the range of 10-30 nm. J. Am. Chem. Soc. 2000, 122, 12582–12583. [Google Scholar] [CrossRef]
- Murty, A.S.R. Thiourea in analysis. I. Estimation of selenium and tellurium. Indian J. Chem. 1965, 3(7), 298–299. [Google Scholar]
- Li, Z.; Huang, D.; McDonald, L.M. Heterogeneous selenite reduction by zero valent iron steel wool. Water Sci. Technol. 2017, 75(4), 908–915. [Google Scholar] [CrossRef]
- Liang, L.; Yang, W.; Guan, X.; Li, J.; Xu, Z.; Wu, J.; Huang, Y.; Zhang, X. Kinetics and mechanisms of pH-dependent selenite removal by zero valent iron. Water Res. 2013, 47, 5846–5855. [Google Scholar] [CrossRef]
- Liang, L.P.; Jiang, X.; Yang, W.J.; Huang, Y.Y.; Guan, X.H.; Li, L.N. Kinetics of selenite reduction by zero-valent iron. Desalin. Water Treat. 2015, 53, 2540–2548. [Google Scholar] [CrossRef]
- Jain, R.; Jordan, N.; Schild, D.; van Hullebusch, E.D.; Weiss, S.; Franzen, C.; Farges, F.; Hübner, R.; Lens, P.N.L. Adsorption of zinc by biogenic elemental selenium nanoparticles. Chem. Eng. J. 2015, 260, 855–863. [Google Scholar] [CrossRef]
- Misra, G.J.; Tandon, J.P. Gravimetric determination of selenium using copper chloride as reducing agent. Indian J. Chem. 1967, 5(11), 560–562. [Google Scholar]
- Kalaparthi, R.; Korapu, S.; Gandam, H.; Kurimella, V.R. Synthesis of selenium nanoparticles using sodium selenite [Se(IV)] as a precursor and titanium(III) chloride as a reducing agent. Int. J. Eng. Res. 2020, 9(4), 359–363. [Google Scholar] [CrossRef]
- Geoffroy, N.; Demopoulos, G.P. Stannous chloride—an effective reducing agent for the removal of selenium(IV) from acidic solution. J. Chem. Technol. Biotechnol. 2012, 87, 983–989. [Google Scholar] [CrossRef]
- Geoffroy, N.; Demopoulos, G.P. Reductive precipitation of elemental selenium from selenious acidic solutions using sodium dithionite. Ind. Eng. Chem. Res. 2009, 48, 10240–10246. [Google Scholar] [CrossRef]
- Vieira, A.P.; Stein, E.M.; Andreguetti, D.X.; Cebrián-Torrejón, G.; Doménech-Carbó, A.; Colepicolod, P.; Ferreira, A.M.D.C. “Sweet Chemistry”: a green way for obtaining selenium nanoparticles active against cancer cells. J. Braz. Chem. Soc. 2017, 28(10), 2021–2027. [Google Scholar] [CrossRef]
- Vahdati, M.; Moghadam, T.T. Synthesis and characterization of selenium nanoparticles lysozyme nanohybrid system with synergistic antibacterial properties. Sci. Rep. 2020, 10(1), 510. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.E. Recovering selenium and tellurium from copper refinery slimes. JOM 1989, 41(7), 33–37. [Google Scholar] [CrossRef]
- Kunita, M.H.; Girotto, E.M.; Radovanovic, E.; Gonçalves, M.C.; Ferreira, O.P.; Muniz, E.C.; Rubira, A.F. Deposition of copper sulfide on modified low-density polyethylene surface: morphology and electrical characterization. Appl. Surf. Sci. 2002, 202(3–4), 223–231. [CrossRef]
- Essaidia, H.; Gantassi, A.; Touihria, S.; Ouerfelli, J. Tuning the structural, optical and electrical properties of AgInSe2 thin films prepared by sequentially deposited silver and indium nano-films under vacuum. Optik 2019, 182, 866–875. [Google Scholar] [CrossRef]
- Nguyen, H.Q. Synthesis and optical properties of CdSe nanocrystals and CdSe/ZnS core/shell nanostructures in non-coordinating solvents. Adv. Nat. Sci: Nanosci. Nanotechnol. 2010, 1, 25004. [Google Scholar] [CrossRef]
- Souri, D.; Tahan, Z.E. A new method for the determination of optical band gap and the nature of optical transitions in semiconductors. Appl. Phys. B: Lasers Opt. 2015, 119, 273–279. [Google Scholar] [CrossRef]
- Souri, D.; Shomalian, K. Band gap determination by absorption spectrum fitting method (ASF) and structural properties of different compositions of (60−x) V2O5–40TeO2–xSb2O3 glasses. J. Non Cryst. Solids 2009, 355, 1597–1601. [Google Scholar] [CrossRef]
- Solaymani, S.; Talu, S.; Nezafat, N.B.; Dejam, L.; Shafiekhani, A.; Ghaderi, A.; Zelati, A. Optical properties and surface dynamics analyses of homojunction and hetrojunction Q/ITO/ZnO/NZO and Q/ITO/ZnO/NiO thin films. Results Phys. 2021, 29, 104679. [Google Scholar] [CrossRef]
- Xu, S.; Ye, L. Synthesis and properties of monomer cast nylon-6-b-polyether amine copolymers with different structures, R. Soc. Chem. Adv. 2015, 5, 32460–32468. [Google Scholar] [CrossRef]
- 6—Selenium, Tellurium and Polonium, In Chemistry of the Elements, 2nd ed.; Greenwood, N.N.; Earnshaw, A. Eds.; Elsevier Ltd., Butterworth-Heinemann, 1997; Volume 16, pp. 747–788. [CrossRef]
- Mahieu, S.; Ghekiere, P.; Depla, D.; de Gryse, R. Biaxial alignment in sputter deposited thin films. Thin Solid Films 2006, 515(4), 1229–1249. [Google Scholar] [CrossRef]
- Park, S.H.; Choi, J.Y.; Lee, Y.H.; Park, J.T.; Song, H. Formation of metal selenide and metal-selenium nanoparticles using distinct reactivity between selenium and noble metals. Chem Asian J. 2015, 10(7), 1452–6. [Google Scholar] [CrossRef]
- Yanhui, Z.; Xu, M.; Liu, Y.; Bai, Y.; Deng, Y.; Liu, J.; Chen, L. Green synthesis of Se/Ru alloy nanoparticles using gallic acid and evaluation of their anti-invasive effects in HeLa cells. Colloids Surf. B 2016, 44, 118–124. [Google Scholar] [CrossRef]
- Olin, Å.; Noläng, B.; Osadchii, E.G.; Öhman, L.-O.; Rosén, E. Chemical Thermodynamics of Selenium, 1st ed.; Elsevier Science, 2005. 894 p. Available online: https://www.oecd-nea.org/dbtdb/pubs/vol7-selenium.pdf.
- Ozin, G.A.; Arsenault, A.C.; Cademartiri, L. Nanochemistry: A Chemical Approach to Nanomaterials, 2nd ed.RSC Publishing: Cambridge, UK, 2009; pp. 335–361. [Google Scholar]
- Mayers, B.T.; Liu, K.; Sunderland, D.; Xia, Y. Sonochemical synthesis of trigonal selenium nanowires. Chem. Mater. 2003, 15, 3852–3858. [Google Scholar] [CrossRef]
- Jiang, X.; Mayers, B.; Herricks, T.; Xia, Y. Direct synthesis of Se@CdSe nanocables and CdSe nanotubes by reacting cadmium salts with Se nanowires. Adv. Mater. 2003, 15(20), 1740–1743. [Google Scholar] [CrossRef]
- Gates, B.; Mayers, B.; Wu, Y.; Sun, Y.; Cattle, B.; Yang, P.; Xia, Y. Synthesis and characterization of crystalline Ag2Se nanowires through a template-engaged reaction at room temperature. Adv. Funct. Mater. 2002, 12, 679–686. [Google Scholar] [CrossRef]
- Ayele, D.W. A facile one-pot synthesis and characterization of Ag2Se nanoparticles at low temperature. Egypt. J. Basic Appl. Sci. 2016, 3, 149–154. [Google Scholar] [CrossRef]
- Kienle, L.; Duppel, V.; Mogwitz, B.; Janek, J.; Kreutzbruck, M.V.; Leineweber, A.; Simon, A. Synthesis–real structure–property: the showcase of silver-rich Ag2Se. Cryst. Growth Des. 2011, 11, 2412–2421. [Google Scholar] [CrossRef]
- Feng, S.; Xu, R. New materials in hydrothermal synthesis. Acc. Chem. Res. 2001, 34(3), 239–247. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E. Phase transformations in metals and alloys, 2nd. ed.; Chapman and Hall, 1992.
- Maskaeva, L.N.; Smirnova, Z.I.; Markov, V.F. Assessment of the ability of metal chalcogenides to enter into ion-exchange reactions in aqueous solutions. Russ. Chem. Bull. 2014, 63(7), 1515–1522. [Google Scholar] [CrossRef]
- Luo, Y.R. Comprehensive handbook of chemical bond energies, 1st ed.; CRC press, Boca Raton, 2007.
- Beberwyck, B.J.; Surendranath, Y.; Alivisatos, A.P. Cation exchange: a versatile tool for nanomaterials synthesis. J. Phys. Chem. C 2013, 117(39), 19759–19770. [Google Scholar] [CrossRef]
- Yokota, I. On the deviation from the Einstein relation observed for diffusion of Ag+ ions in α-Ag2S and others. J. Phys. Soc. Jpn. 1966, 21(3), 420–423. [Google Scholar] [CrossRef]
- Stehlik, S.; Shimakawa, K.; Wagner, T.; Frumar, M. Diffusion of Ag ions under random potential barriers in silver-containing chalcogenide glasses. J. Phys. D: Appl. Phys. 2012, 45(20), 205304. [CrossRef]
- Wu, Q.; Liu, X.; Berglund, L.A. FT-IR spectroscopic study of hydrogen bonding in PA6/clay nanocomposites. Polymer 2002, 43(8), 2445–2449. [Google Scholar] [CrossRef]
- Krylova, V.; Dukštienė, N. The structure of PA-Se-S-Cd composite materials probed with FTIR spectroscopy. Appl. Surf. Sci. 2019, 470, 462–471. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, X.; Yang, D.; Wangyang, P.; Gao, X.; Tian, H. An economical method for amorphous selenium thick films preparation: e-beam evaporation. Mater. Lett. 2016, 183, 94–96. [Google Scholar] [CrossRef]
- Tüzüner, S.; Demir, M.M. Dispersion of organophilic Ag nanoparticles in PS-PMMA blends. Mater. Chem. Phys. 2015, 162, 692–699. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-calculated IR spectrum amide I, II, and III band contributions of N-methylacetamide fine components. ACS Omega 2020, 5(15), 8572–8578. [Google Scholar] [CrossRef] [PubMed]
- Kotkata, M.F.; Masoud, A.E.; Mohamed, M.B.; Mahmoud, E.A. Structural characterization of chemically synthesized CdSe nanoparticles. Phys. E: Low-Dimens. 2009, 41, 640–645.
- Sun, Z.B.; Dong, X.Z.; Chen, W.Q.; Shoji, S.; Duan, X.M.; Kawata, S. Two and three dimensional micro/nanostructure pattering of CdS-polymer nanocomposites with a laser interference technique and in situ synthesis. Nanotechnology 2008, 19, 035611–035619. [Google Scholar] [CrossRef] [PubMed]
- Helios, K.; Pietraszko, A.; Zierkiewicz, W. Wojtowicz, H.; Michalska, D. The crystal structure, infrared, Raman and density functional studies of bis(2-aminophenyl) diselenide. Polyhedron 2011, 30, 2466–2472. [Google Scholar] [CrossRef]
- Sadtler, B.; Demchenko, D.O.; Zheng, H.; Hughes, S.M; . Merkle, M.G.; Dahmen, U.; Wang, L.-W.; Alivisatos, A.P. Selective Facet Reactivity during Cation Exchange in Cadmium Sulfide Nanorods. J. Am. Chem. Soc. 2009, 131, 5285–5293. [Google Scholar] [CrossRef]
- Li, Y.C.; Zhong, H.Z.; Li, R.; Zhou, Y.; Yang, C.H.; Li, Y.F. High-yield fabrication and electrochemical characterization of tetrapodal CdSe, CdTe, and CdSexTe1–x nanocrystals. Adv. Funct. Mater. 2006, 16, 1705–1716. [Google Scholar] [CrossRef]
- Ingole, A.R.; Thakare, S.R.; Khati, N.T.; Wankhade, A.V.; Burghate, D.K. Green synthesis of selenium nanoparticles under ambient condition. Chalcogenide Lett. 2010, 7(7), 485–489. [Google Scholar]
- Surana, K.; Salisu, I.T.; Mehra, R.M.; Bhattacharya, B. A simple synthesis route of low temperature CdSe-CdS core-shell quantum dots and its application in solar cell. Opt. Mater. 2018, 82, 135–140. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Abd El-Hameed, A.M. Characterization of CdSe-nanocrystals used in semiconductors for aerospace applications: Production and optical properties, NRIAG. J. Astron. Geophys. 2014, 3(1), 82–87. [Google Scholar] [CrossRef]
- Pejova, B.; Najdoski, M.; Grozdanov, I.; Dey, S.K. Chemical bath deposition of nanocrystalline (111) textured Ag2Se thin films. Mater. Lett. 2000, 43, 269–273. [Google Scholar] [CrossRef]
- Ramezanloo, B.; Molaei, M.; Karimipour, M. Red emissive Ag2Se quantum dots (QDs) with room-temperature synthesis of both orthorhombic and superionic cubic phases via stirring approach. J. Lumin. 2018, 204, 419–423. [Google Scholar] [CrossRef]
- Singh, S.; Bharti, A.; Meena, V.K. Green synthesis of multi-shaped silver nanoparticles: optical, morphological and antibacterial properties. J. Mater. Sci.: Mater. Electron. 2015, 26, 3638–3648. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Zhang, Y.; Li, P. Ion-exchange synthesis and improved photovoltaic performance of CdS/Ag2S heterostructures for inorganic-organic hybrid solar cells. Solid State Sci. 2016, 61, 195–200. [Google Scholar] [CrossRef]
- Di, T.; Cheng, B.; Ho, W.; Yu, J.; Tang, H. Hierarchically CdS–Ag2S nanocomposites for efficient photocatalytic H2 production. Appl. Surf. Sci. 2019, 470, 196–204. [Google Scholar] [CrossRef]
- Bubenov, S.S.; Dorofeev, S.G.; Kotin, P.A.; Znamenkov, K.O.; Kuznetsova, T.A. Oleic capped CdSe nanocrystals silver doped in the course of synthesis. Mendeleev Commun. 2014, 24(4), 250–252. [Google Scholar] [CrossRef]
- Asadpour-Zeynali, K.; Mollarasouli, F. A novel and facile synthesis of TGA-capped CdSe@Ag2Se core-shell quantum dots as a new substrate for high sensitive and selective methyldopa sensor. Sens. Actuator B Chem. 2016, 237, 387–399. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Khare, N. Effect of intrinsic strain on the optical band gap of single phase nanostructured Cu2ZnSnS4. Mater. Sci. Semicond. Process, 2017, 63, 220–226. [Google Scholar] [CrossRef]
- Purushotham, E.; Krishna, N.G. Effect of particle size and lattice strain on Debye-Waller factors of Fe3C nanoparticles. Bull. Mater. Sci. 2014, 37, 773–778. [Google Scholar] [CrossRef]
- Bhatnagar, A.K.; Venugopal Reddy, K.; Srivastava, V. Optical energy gap of amorphous selenium: effect of annealing. J. Phys. D: Appl. Phys. 1985, 18, 149–153. [Google Scholar] [CrossRef]
- Cody, G.D.; Tiedje, T.; Abeles, B.; Moustakas, T.D.; Brooks, B.; Goldstein, Y. Disorder and the optical-absorption edge of hydrogenated amorphous silicon. J. Phys. Colloques 1981, 42, C4–301. [Google Scholar] [CrossRef]
- Kaiser, O.J. Sandberg, N. Zarrabi et al. A universal Urbach rule for disordered organic semiconductors. Nat. Commun. 2021, 12, 3988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Mahadevan, S.; Yuan, J.; Ho, J.K.W.; Gao, Y.X.; Liu, W.; Zhong, H.; Yan, H.; Zou, Y.P.; Tsang, S.W.; So, S.K. Unraveling Urbach tail effects in high performance organic. photovoltaics: dynamic vs static disorder, ACS Energy Lett. 2022, 7, 1971–1979. [Google Scholar]
- Ambrosone, G.; Basa, D.K.; Coscia, U.; Fathallah, M. Study on the microstructural and overall disorder in hydrogenated amorphous silicon carbon films. J. Appl. Phys. 2008, 104, 123520. [Google Scholar] [CrossRef]
- Rani, S.; Sanghi, S.; Agarwal, A.; Seth, V.P. Study of optical band gap and FTIR spectroscopy of Li2O.Bi2O3.P2O5 glasses. Spectrochim. Acta A 2009, 74(3), 673–677. [Google Scholar] [CrossRef]
- Ananth Kumar, R.T.; Chithra Lekha, P.; Sanjeeviraja, C.; Pathinettam Padiyan, D. Evolution of structural disorder using Raman spectra and Urbach energy in GeSe0.5S1.5 thin films. J. Non-Cryst. 2014, 405, 21–26. [Google Scholar] [CrossRef]
- Kabir, H.; Rahman, M.M.; Uddin, K.M.; Bhuiyand, A.H. Structural, morphological, compositional and optical studies of plasma polymerized 2-furaldehyde amorphous thin films. Appl. Surf. Sci. 2017, 423, 983–994. [Google Scholar] [CrossRef]
| Sample | Phase | 2θ, ° | D, nm | δ×1015, line∙m–2 | ε×10–3, line–2∙m–4 |
|---|---|---|---|---|---|
| PA6 | PA6 | 23.5 | 16 | 3.70 | 2.11 |
| a-Se/PA6 | PA6 | 23.7 | 17 | 3.33 | 2.00 |
| a-Se | – | – | – | – | |
| Cd-Se/PA6 | PA6 | 23.5 | 17 | 3.38 | 2.01 |
| Se8 | 33.5 | 33 | 0.89 | 1.04 | |
| CdSe | 42.7 | 40 | 0.63 | 0.87 | |
| Ag-Se/PA6 | PA6 | 23.6 | 17 | 3.56 | 2.06 |
| Se8 | 31.8 | 31 | 0.91 | 1.07 | |
| Ag2Se | 33.8 | 34 | 0.85 | 0.98 | |
| Ag | 43.4 | 34 | 0.89 | 1.02 | |
| Ag-Cd-Se/PA6 | PA6 | 23.7 | 17 | 3.58 | 2.08 |
| Se8 | 31.8 | 30 | 1.10 | 1.15 | |
| CdSe | 48.7 | 37 | 0.74 | 0.94 | |
| Ag2Se | 33.6 | 35 | 0.84 | 1.00 | |
| Ag | 43.5 | 34 | 0.88 | 1.03 |
| Phase or ion | G, kJ/mol | BDE, kJ/mol | KSP, (mol/dm3)n | S, mol/dm3 |
|---|---|---|---|---|
| a-Se | 6.00± 2.2 | ‒ | ‒ | 2.2 10‒8 |
| cr-Se | 0.00 | 332.6 (Se‒Se) | ‒ | 1.5 10‒9 |
| Ag+ | 77.11 | ‒ | ‒ | ‒ |
| Cd2+ | ‒77.16 | ‒ | ‒ | ‒ |
| CdSe | ‒141.60 | 127.6 ± 25.1 | 1.0·10‒33 | 3.16·10‒17 |
| Ag2Se | ‒46.90 | 210.0 ± 14.6 | 3.1·10‒65 | 3.14·10‒22 |
| Band location, cm‒1 | Assignment | ||||
|---|---|---|---|---|---|
| PA6 | a-Se/PA6 | Cd-Se/PA6 | Ag-Se/PA6 | Ag-Cd-Se/PA6 | |
| 687 | 687 | 687 | 687 | 687 | C–C bending |
| 730 | 730 | 729 | 730 | 729 | CH2 waging |
| 834 | 835 | 833 | 833 | 833 | CO–NH in plane |
| – | – | 877 | 877 | 876 | Cd-Se or/and Ag-Se |
| 929 | 929 | 930 | 930 | 930 | CO–NH in plane |
| 960 | 959 | 961 | 960 | 960 | CO–NH in plane (α crystalline) |
| – | 974 | 976 | 975 | 975 | CO–NH in plane (γ crystalline) |
| 1023 | 1023 | 1025 | 1024 | 1024 | trans C–C backbone conformation |
| 1031 | 1031 | 1031 | 1032 | 1031 | CO–NH in plane (α crystalline) |
| 1124 | 1112 | 1112 | 1108 | 1094 | C–C stretching (sym) |
| 1169 | 1165 | 1166 | 1166 | 1165 | N–C–O groups/CH2 twisting |
| 1200 | 1200 | 1200 | 1200 | 1201 | C–CH bending (sym)CH2 twisting |
| 1238 | 1238 | 1236 | 1237 | 1235 | |
| 1264 | 1264 | 1261 | 1263 | 1263 | C–N stretching (amide III) |
| 1365 | 1371 | 1376 | 1377 | 1377 | CH2 twisting |
| – | 1390 | 1392 | 1392 | 1391 | C–C stretching (sym) |
| 1417 | 1417 | 1417 | 1416 | 1415 | CH2 scissoring next to >C=O group |
| 1463 | 1459 | 1460 | 1459 | 1459 | CH2 scissoring |
| 1476 | 1475 | 1476 | 1475 | 1475 | CH2 scissoring next to N–H group |
| 1535 | 1534 | 1536 | 1536 | 1535 | C–N stretching and N–H bending of hydrogen-bonded N–H groups (amide II) |
| 1633 | 1633 | 1633 | 1633 | 1632 | C=O stretching (amide I) |
| Composite | λg, nm | Eg, eV | EU, eV | σ | Ee-p, eV | χ |
|---|---|---|---|---|---|---|
| PA 6 | 278 | 4.46± 0.02 | 1.31 | 0.020 | 33.33 | 11.99 |
| a-Se/PA 6 | 556 | 2.23± 0.02 | 0.95 | 0.027 | 24.69 | 5.99 |
| Cd-Se/PA 6 | 605 | 2.05± 0.02 | 0.39 | 0.066 | 10.10 | 5.51 |
| Ag-Se/PA 6 | 625 | 1.98± 0.02 | 0.55 | 0.047 | 14.18 | 5.32 |
| Ag-Cd-Se/PA 6 | 756 | 1.64± 0.02 | 0.69 | 0.037 | 18.02 | 4.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





