Submitted:
04 July 2023
Posted:
05 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Catalyst
2.3. Characterization
2.4. Catalytic Experiments
2.4.1. Calculations
3. Results and Discussion
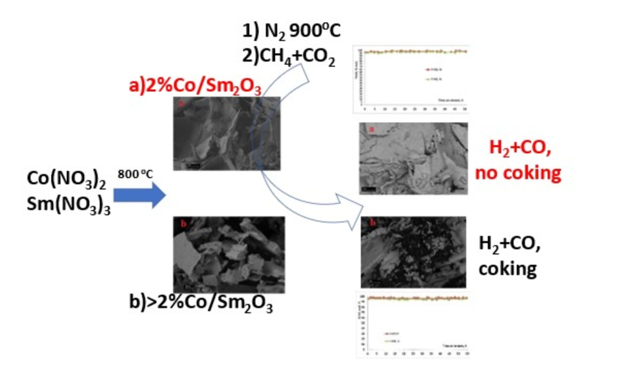
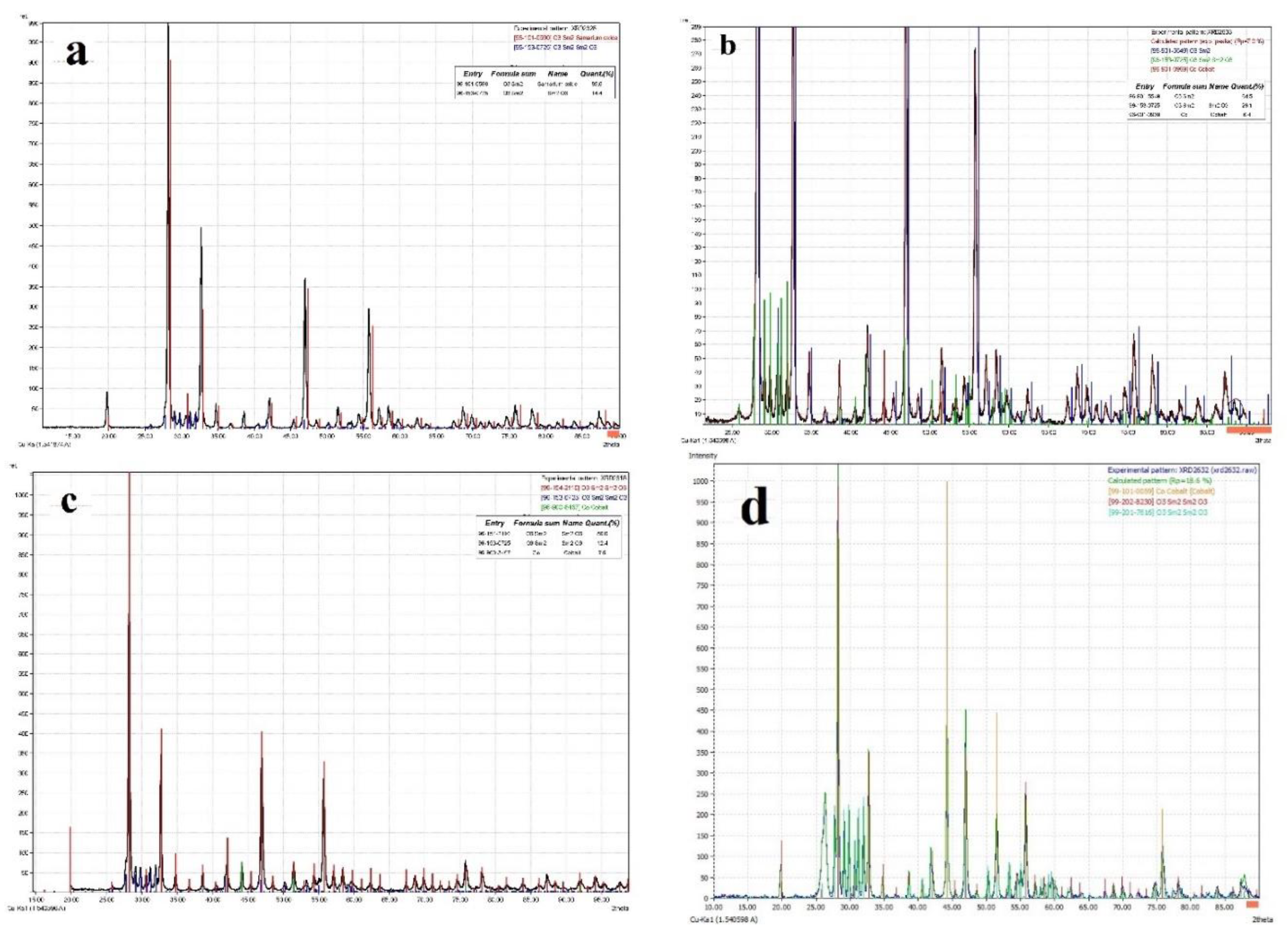
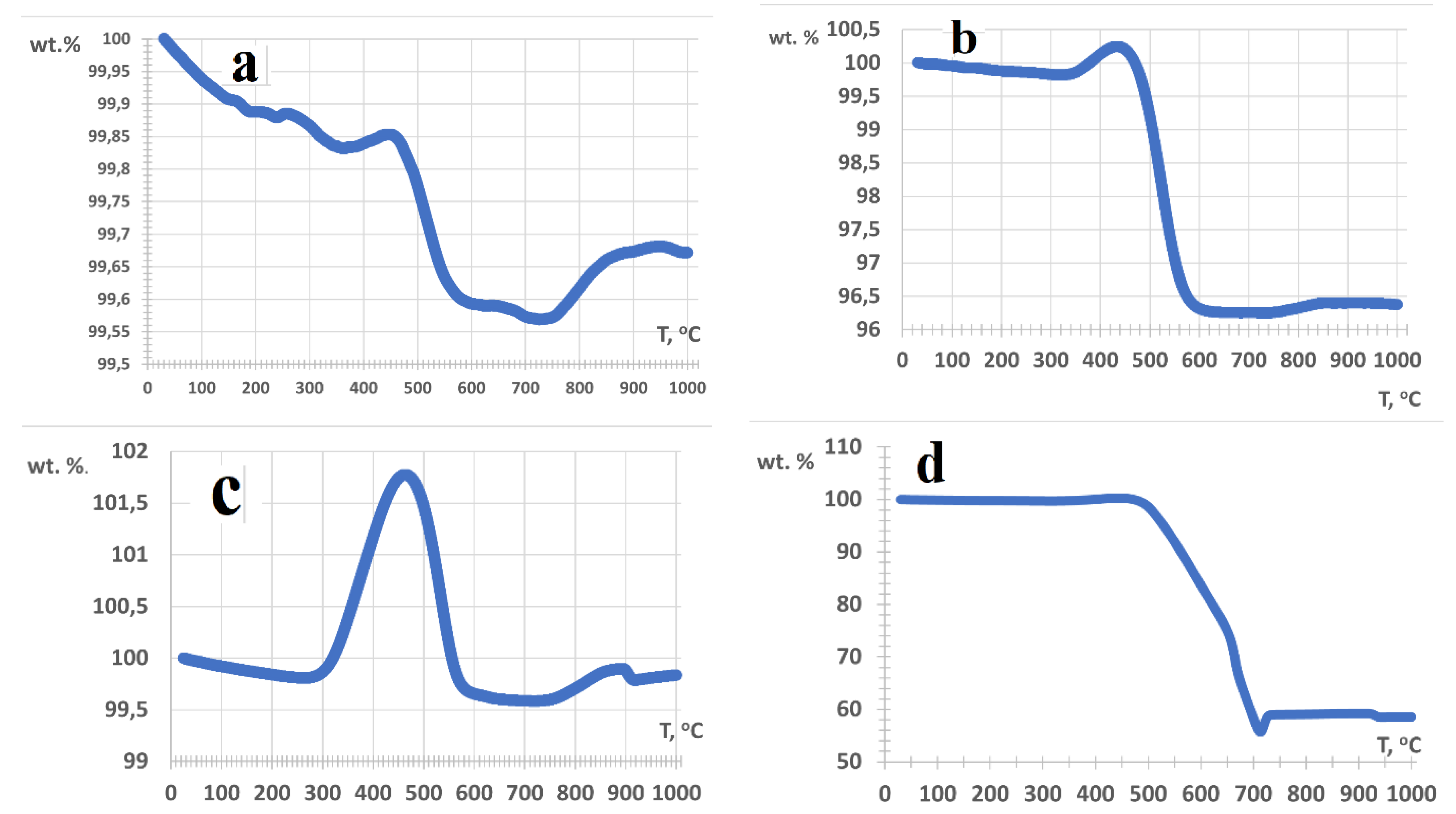
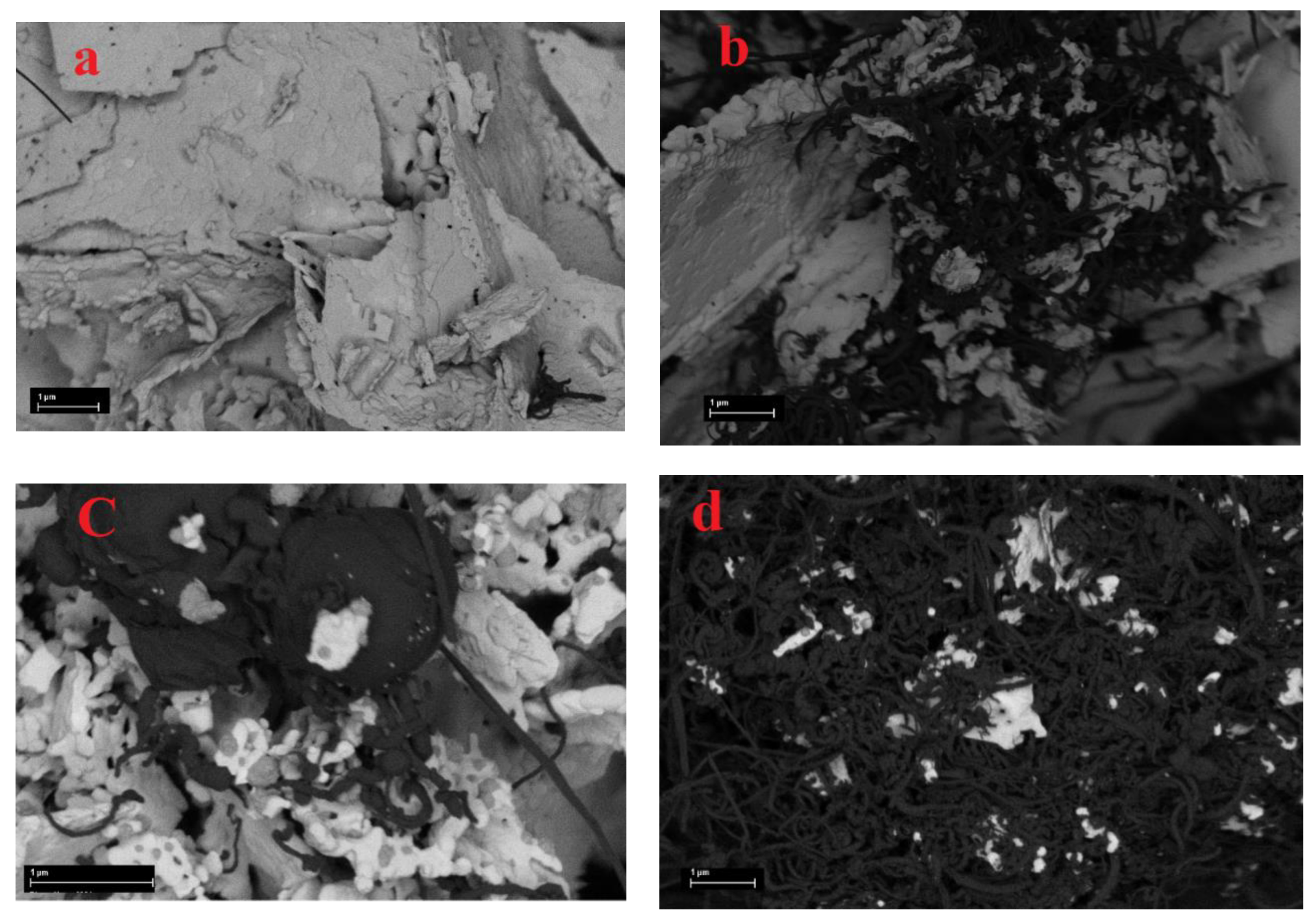
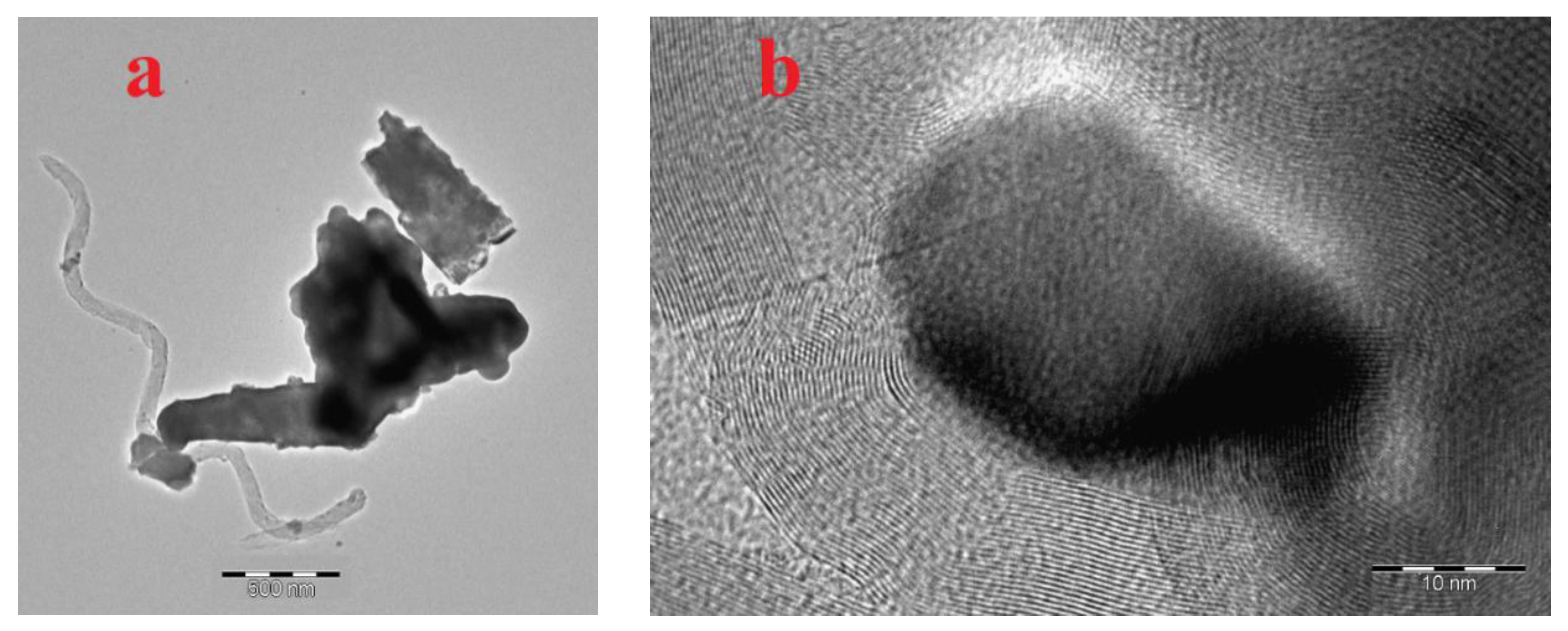
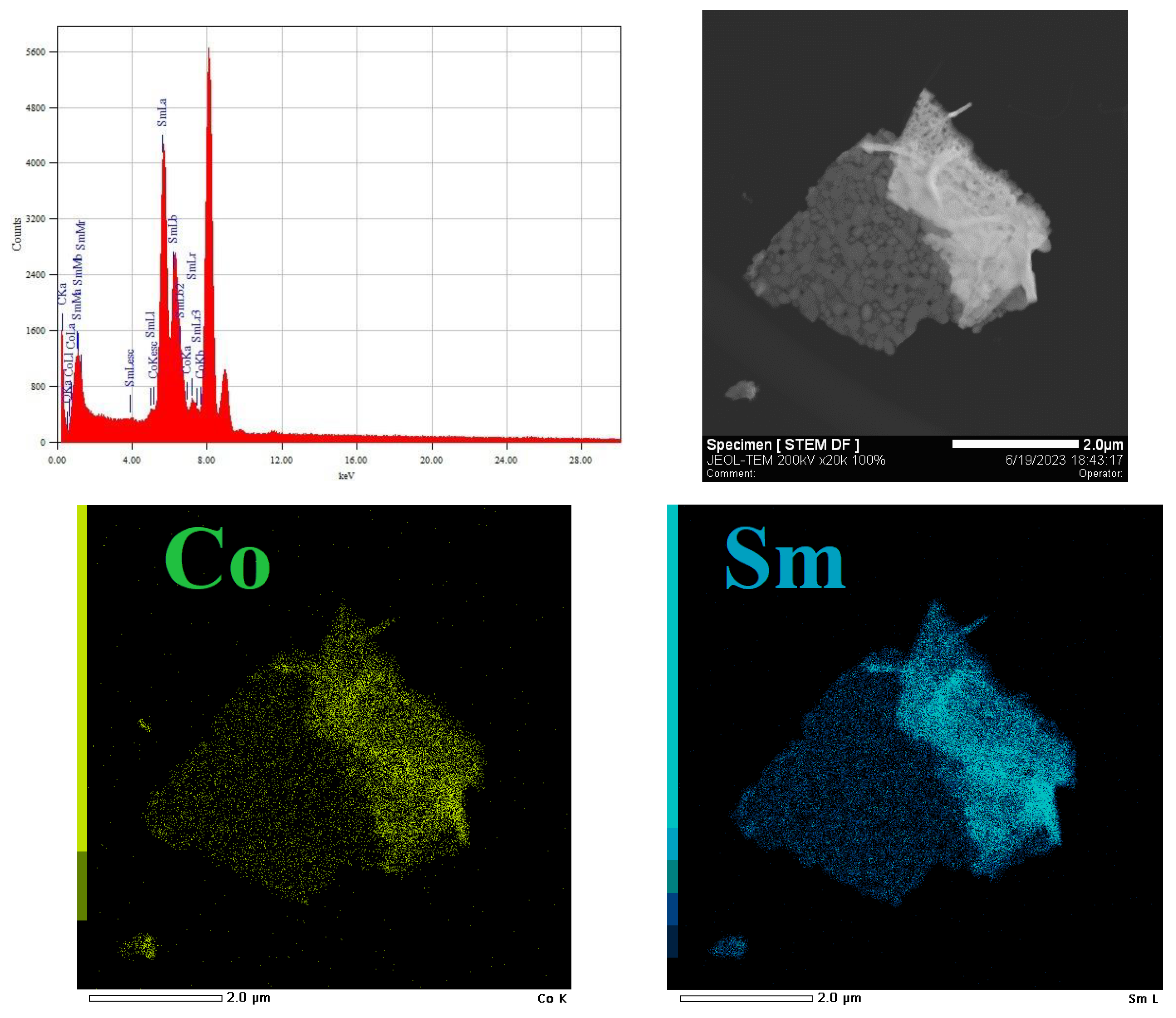
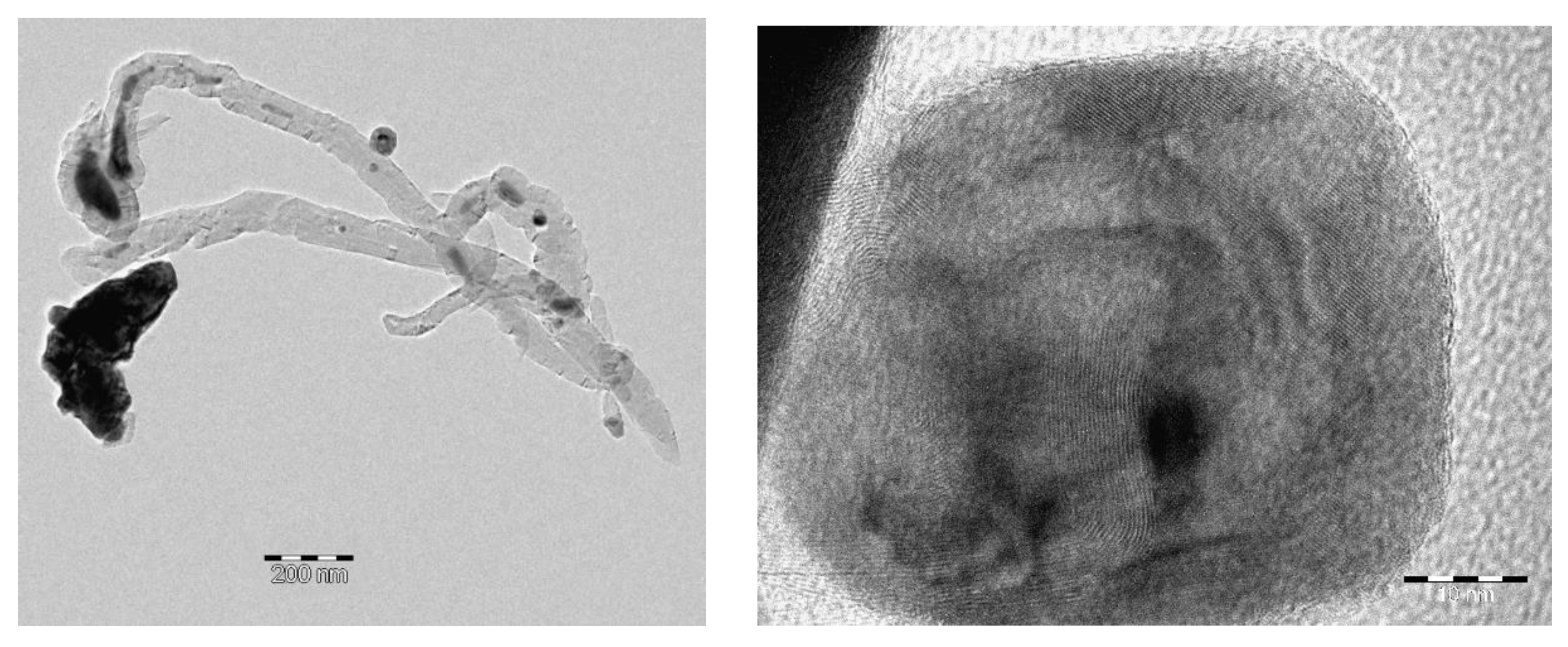
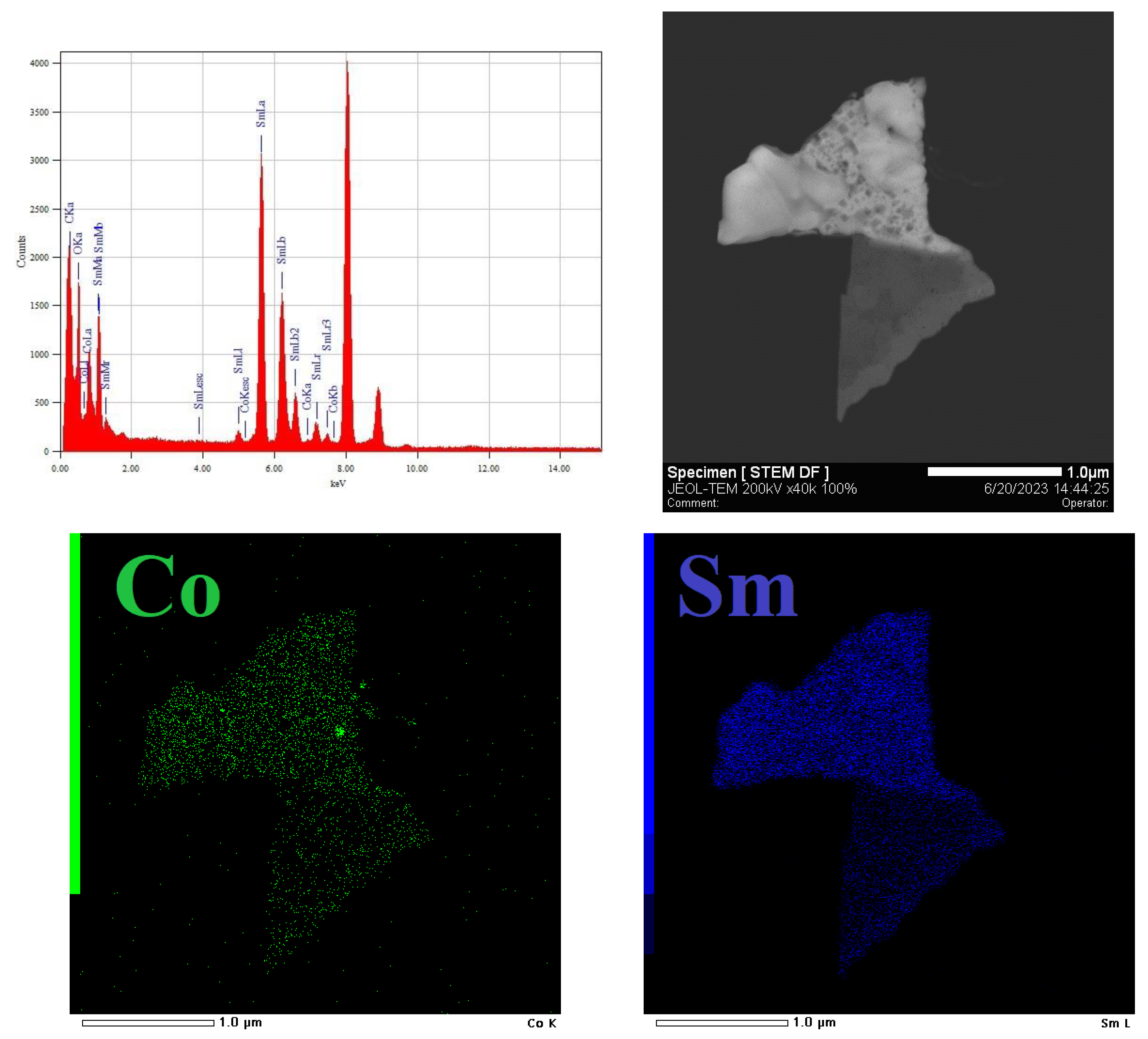
3.1. Characterization of freshly prepared materials
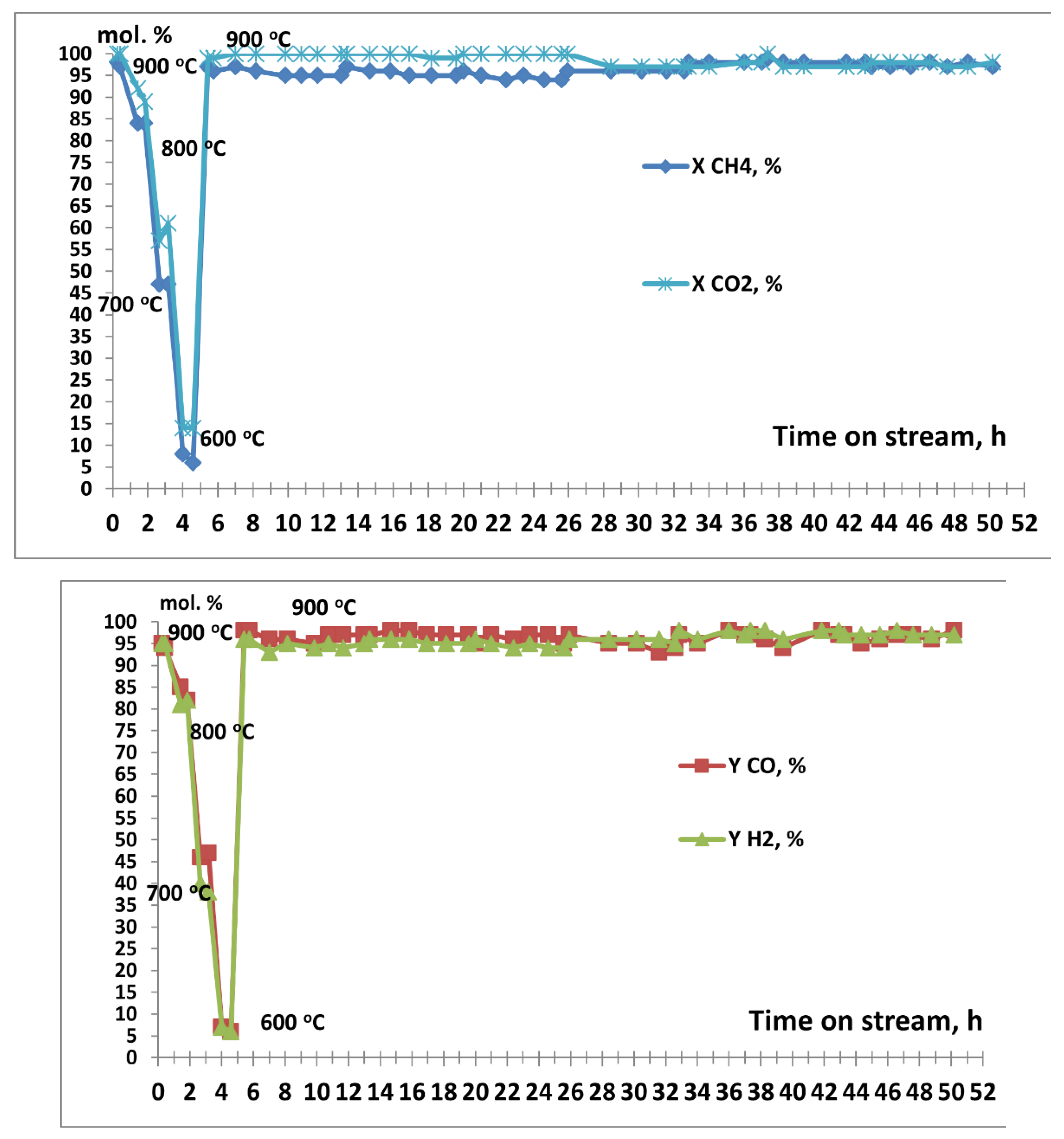
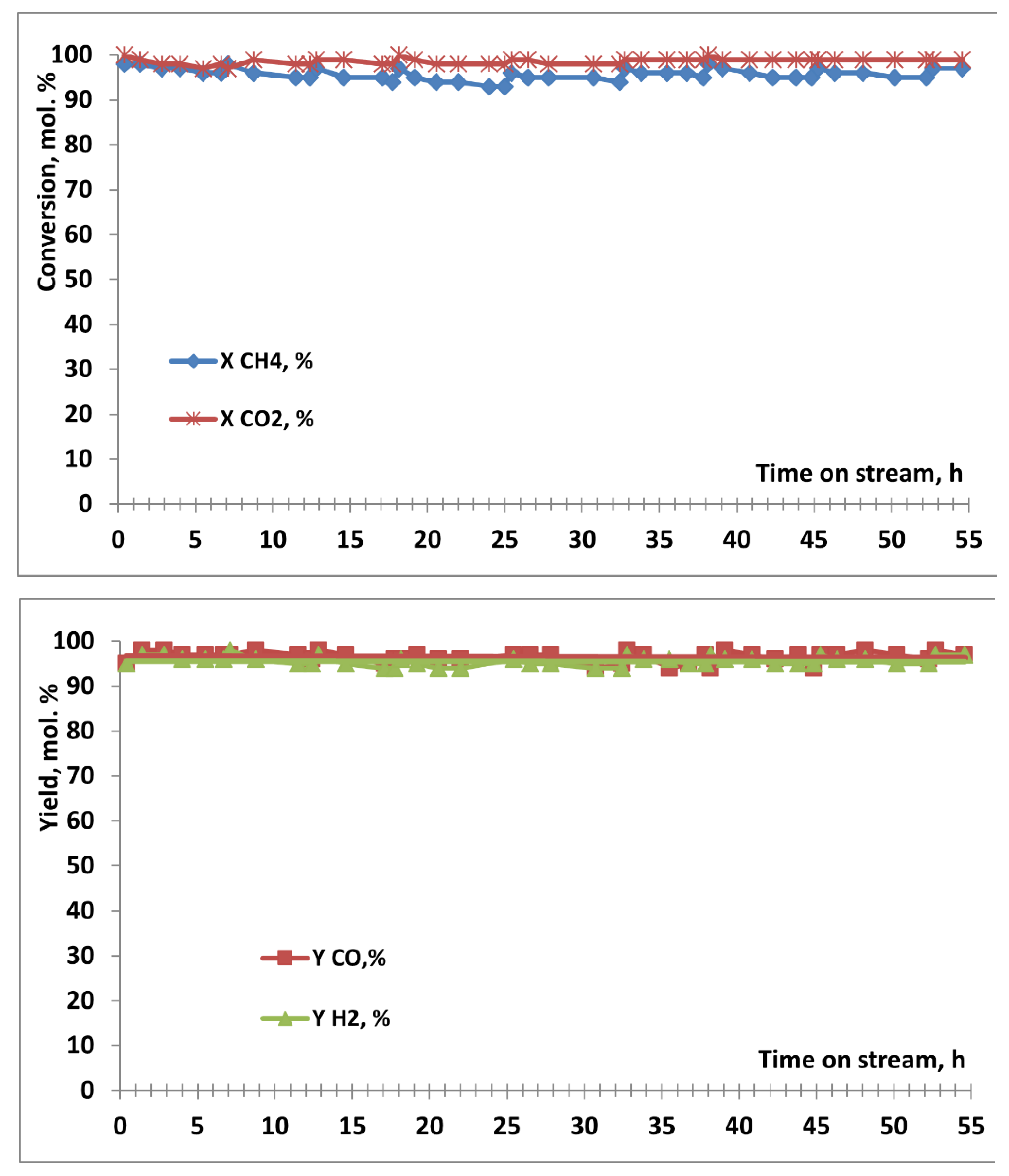
3.2. Results of Catalytic Experiments
4. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jang, W.-J.; Shim, J.-O.; Kim, H.-M.; Yoo, S.-Y.; Roh, H.-S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Su, B.; Wang, Y.; Xu, Z.; Han, W.; Jin, H.; Wang, H. Novel ways for hydrogen production based on methane steam and dry reforming integrated with carbon capture. Energy Conversion and Management 2022, 270, 116199. [Google Scholar] [CrossRef]
- Singh, R.; Dhir, A.; Mohapatra, S.K.; Mahla, S.K. Dry reforming of methane using various catalysts in the process: review. Biomass Conv. Bioref. 2020, 10, 567–587. [Google Scholar] [CrossRef]
- Holmen, A. Direct Conversion of Methane to Fuels and Chemicals. Catal. Today 2009, 142, 2–8. [Google Scholar] [CrossRef]
- Moiseev, I.I.; Loktev, A.S.; Shlyakhtin, O.A.; Mazo, G.N.; Dedov, A.G. New approaches to the design of nickel, cobalt, and nickel–cobalt catalysts for partial oxidation and dry reforming of methane to synthesis gas. Petrol. Chem. 2019, 59 Suppl. 1, S1–S27. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic Hydrogen Production from Methane: A Review on Recent Progress and Prospect. Catalysts 2020, 10, 858–876. [Google Scholar] [CrossRef]
- Elbadawi, A.H.; Ge, L.; Li, Z.; Liu, S.; Wang, S.; Zhu, Z. Catalytic partial oxidation of methane to syngas: review of perovskite catalysts and membrane reactors. Catal. Rev. 2021, 63, 1–67. [Google Scholar] [CrossRef]
- Al-Sayari, S.A. Recent Developments in the Partial Oxidation of Methane to Syngas. Open Catalysis J. 2013, 6, 17–28. [Google Scholar] [CrossRef]
- Alhassan, M.; Jalil, A.A.; Nabgan, W.; Hamid, M.Y.S.; Bahari, M.B.; Ikram, M. Bibliometric studies and impediments to valorization of dry reforming of methane for hydrogen pro-duction. Fuel 2022, 328, 125240. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Pal, A. Overview of hydrogen production from biogas reforming: Technological advancement. Intern. J. Hydrogen Energy 2022, 47, 34831–34855. [Google Scholar] [CrossRef]
- Bhattar, S.; Abedin, Md.A.; Kanitkar, S.; Spivey, J.J. A review on dry reforming of methane over perovskite derived catalysts. Catal. Today 2021, 365, 2–23. [Google Scholar] [CrossRef]
- The, L.P.; Setiabudi, H.D.; Timmiati, S.N.; Aziz, M.A.A.; Annuar, N.H.R.; Ruslan, N.N. Recent progress in ceria-based catalysts for the dry reforming of methane: A review. Chem. Engineering Sci. 2021, 242, 116606. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulations. Appl. Catal. B: Environmental 2021, 296, 120210. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Chen, M.; Liang, D.; Yang, Z.; Cheng, W.; Tang, Z.; Wang, J.; Zhang, H. Recent advances during CH4 dry reforming for syngas production: A mini review. Intern. J. of Hydrogen Energy, 2021, 46, 5852–5874. [Google Scholar] [CrossRef]
- le Saché, E.; Reina, T.R. Analysis of dry reforming as direct route for gas phase CO2 conversion. The past, the present and future of catalytic DRM technologies. Progr. in Energy and Combustion Sci. 2022, 89, 100970. [Google Scholar] [CrossRef]
- Hambali, H.U.; Jalil, A.A.; Abdulrasheed, A.A.; Siang, T.J.; Gambo, Y.; Umar, A.A. Zeolite and clay based catalysts for CO2 reforming of methane to syngas: A review. Intern. J. of Hydrogen Energy 2022, 47, 30759–30787. [Google Scholar] [CrossRef]
- Baharudin, L.; Rahmat, N.; Othman, N.H.; Shah, N.; Syed-Hassan, S.S.A. Formation, control, and elimination of carbon on Ni-based catalyst during CO2 and CH4 conversion via dry reforming process: A review. J. of CO2 Utilization 2022, 61, 102050. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Dybkjær, I. Industrial scale experience on steam reforming of CO2-rich gas. Appl. Catal. A: General 2015, 495, 141–151. [Google Scholar] [CrossRef]
- Teuner, S.C.; Neumann, P.; Von Linde, F. The Calcor standard and Calcor economy processes. OIL GAS European Magazine 2001, № 3, 44–46. https://www.caloric.com/wp-content/uploads/2016/11/Caloric-Downloads-Calcor.pdf.
- Usman, M.; Wan Daud, W.M.A.; Abbas, H.F. Dry reforming of methane: Influence of process parameters. A review. Renewable and Sustainable Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef]
- Schulz, L.A.; Kahle, L.C.S.; Delgado, K.H.; Schunk, S.A.; Jentys, A.; Deutschmann, O.; Lercher, J.A. On the coke deposition in dry reforming of methane at elevated pressures. Appl. Catal. A: General 2015, 504, 599–607. [Google Scholar] [CrossRef]
- Jang, W.-J.; Jeong, D.-W.; Shim, J.-O.; Kim, H.-M.; Roh, H.-S.; Son, I.H.; Lee, S.J. Combined steam and carbon dioxide reforming of methane and side reactions: Thermodynamic equilibrium analysis and experimental application. Appl. Energy 2016, 173, 80–91. [Google Scholar] [CrossRef]
- Yusuf, M.; Farooqi, A.S.; Keong, L.K.; Hellgardt, K.; Abdullah, B. Contemporary trends in composite Ni-based catalysts for CO2 reforming of methane. Chem. Engineering Science 2021, 229, 116072. [Google Scholar] [CrossRef]
- Jafarbegloo, M.; Tarlani, A.; Wahid Mesbah, A.; Sahebdelfar, S. Thermodynamic analysis of carbon dioxide reforming of methane and its practical relevance. Intern. J. of Hydrogen Energy 2015, 40, 2445–2451. [Google Scholar] [CrossRef]
- Khoshtinat Nikoo, M.; Amin, N.A.S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Setiabudi, H.D.; Teh, L.P.; Annuar, N.H.R.; Jalil, A.A. A review of heterogeneous catalysts for syngas production via dry reforming. J. of the Taiwan Institute of Chem. Engineers 2019, 101, 139–158. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Mondal, K.C.; Mamman, A.S.; Joshi, U.A. Carbon-free dry reforming of methane to syngas over NdCoO3 perovskite-type mixed metal oxide catalyst. Catal. Lett. 2005, 100, 271–276. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef]
- Gavrikov, A.V.; Loktev, A.S.; Ilyukhin, A.B.; Mukhin, I.E.; Bykov, M.A.; Maslakov, K.I.; Vorobei, A.M.; Parenago, O.O.; Sadovnikov, A.A.; Dedov, A.G. Supercritical Fluid Assisted Modification combined with the Resynthesis of SmCoO3 as Effective Tool to Enhance Long-term Performance of SmCoO3-derived Catalysts for Dry Reforming of Methane to Syngas. Dalton Trans. 2022, 51, 18446–18461. [Google Scholar] [CrossRef] [PubMed]
- Gavrikov, A.V.; Ilyukhin, A.B.; Belova, E.V.; Yapryntsev, A.D.; Dobrokhotova, Z.V.; Khrushcheva, A.V.; Efimov, N.N. Rapid preparation of SmCoO3 perovskite via uncommon though efficient precursors: Composition matters! Ceram. Int. 2020, 46, 13014–13024. [Google Scholar] [CrossRef]
- Osazuwa, O.U.; Cheng, C.K. Catalytic conversion of methane and carbon dioxide (greenhouse gases. into syngas over samarium-cobalt-trioxides perovskite catalyst. J. of Cleaner Production 2017, 148, 202–211. [Google Scholar] [CrossRef]
- Salaev, M.A.; Liotta, L.F.; Vodyankina, O.V. Lanthanoid-containing Ni-based catalysts for dry reforming of methane: A review. Int. J. of Hydrogen Energy 2022, 47, 4489–4535. [Google Scholar] [CrossRef]
- de Lira Lima, D.C.; Lemos, I.P.; Gomes, Rodrigues, R.S.; L.M.T.S.; Fréty, R.T.; Resini, C.; Junior, R.B.S.; Brandão, S.T. Study of LaNi1-xCoxO3 Perovskites-Type Oxides Either Pure or Mixed with SiO2 as Catalytic Precursors Applied in CH4 Dry-Reforming. Catal Lett. 2023, 153, 2137–2148. Catal Lett. [CrossRef]
- Dedov, A.G.; Loktev, A.S.; Ivanov, V.K.; Bykov, M.A.; Mukhin, I.E.; Lidzhiev, M.M.; Rogaleva, E.V.; Moiseev, I.I. Selective oxidation of methane to synthesis gas: Cobalt- and nickel-based catalysts. Doklady Phys. Chemistry 2015, 461, 73–79. [Google Scholar] [CrossRef]
- Loktev, A.S.; Mukhin, I.E.; Bykov, M.A.; Sadovnikov, A.A.; Osipov, A.K.; Dedov, A.G. Novel high-performance catalysts for partial oxidation and dry reforming of methane to synthesis gas. Petrol. Chemistry 2022, 62, 526–543. [Google Scholar] [CrossRef]
- Loktev, A.S.; Arkhipova, V.A.; Bykov, M.A.; Sadovnikov, A.A.; Dedov, A.G. Cobalt–Samarium Oxide Composite as a Novel High-Performance Catalyst for Partial Oxidation and Dry Reforming of Methane into Synthesis Gas. Petrol. Chemistry 2023. Open access publication. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. of Appl. Crystallography 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Olusola, J.O.; Sudip, M. Temperature programme reduction (TPR) studies of cobalt phases in γ-alumina supported cobalt catalysts. J. of Petrol. Technol. and Alternative Fuels 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Ma, F.; Chen, Y.; Lou, H. Characterization of perovskite-type oxide catalysts RECoO3 by TPR. React. Kinet. Catal. Lett. 1986, 31, 47–53. [Google Scholar]
- Dedov, A.G.; Loktev, A.S.; Mukhin, I.E.; Baranchikov, A.E.; Ivanov, V.K.; Bykov, M.A.; Solodova, E.V.; Moiseev, I.I. Effect of the Support Nature on Stability of Nickel and Nickel–Cobalt Catalysts for Partial Oxidation and Dry Reforming of Methane to Synthesis Gas. Petrol. Chemistry 2019, 59, 385–393. [Google Scholar] [CrossRef]


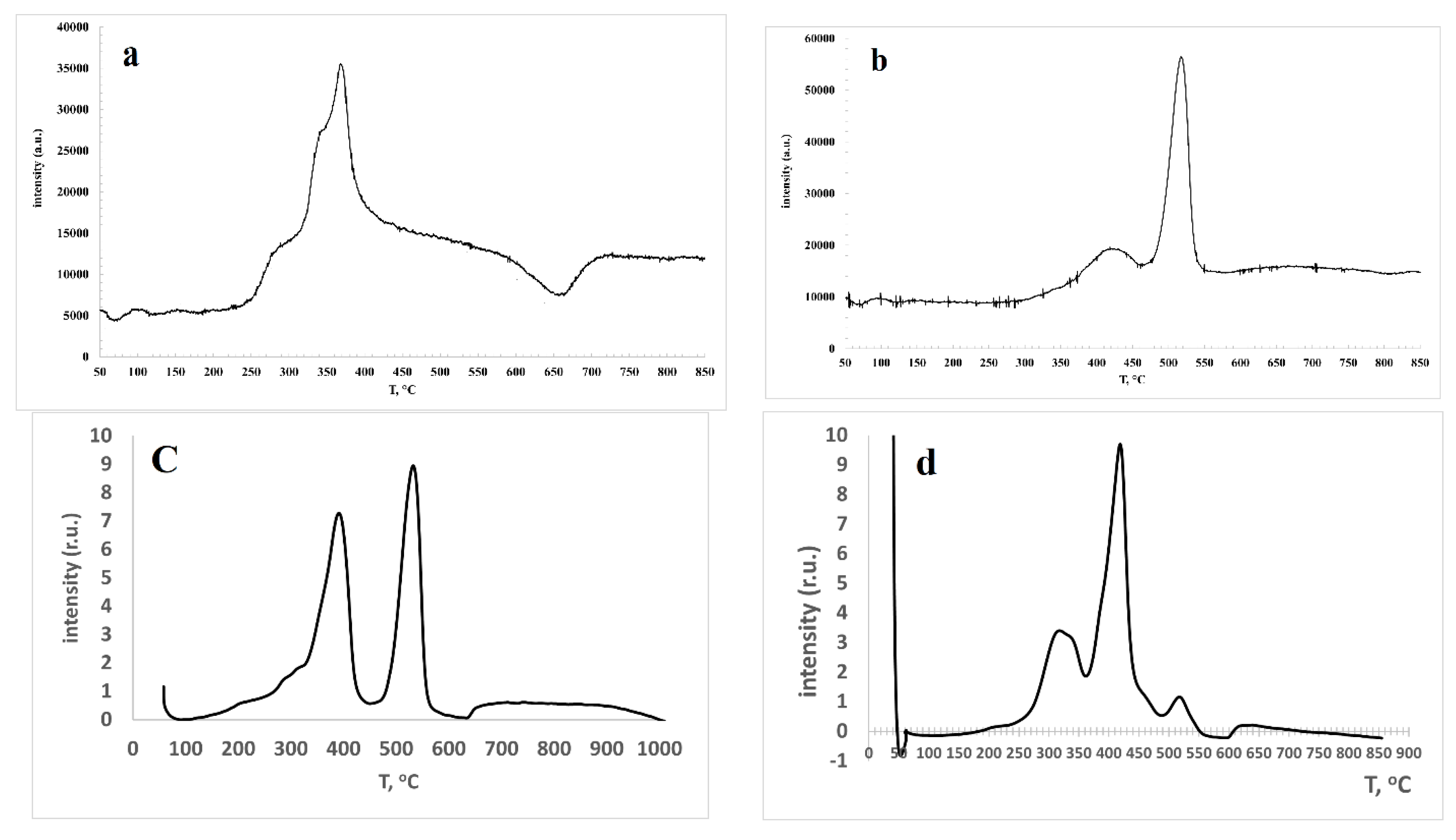
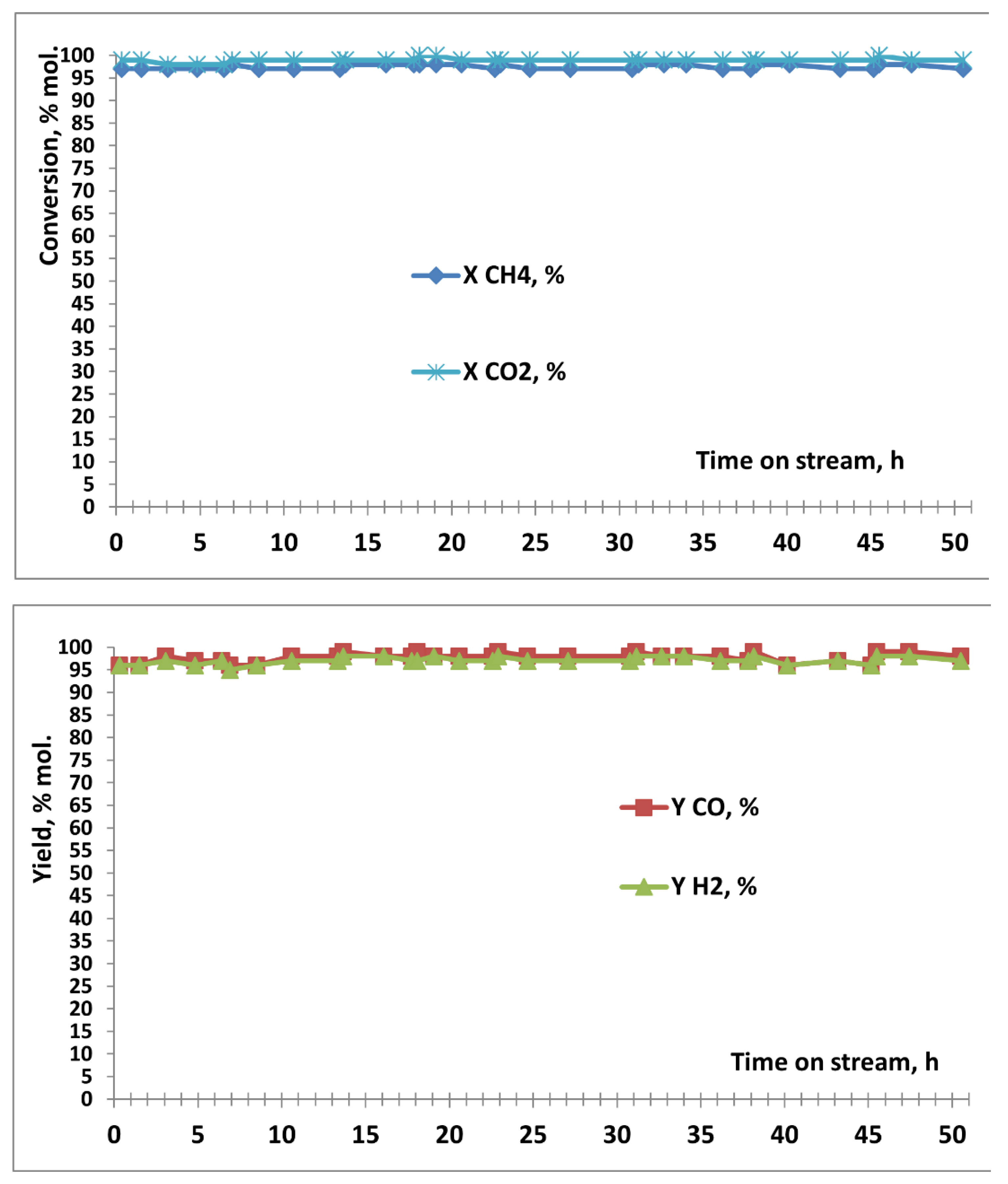
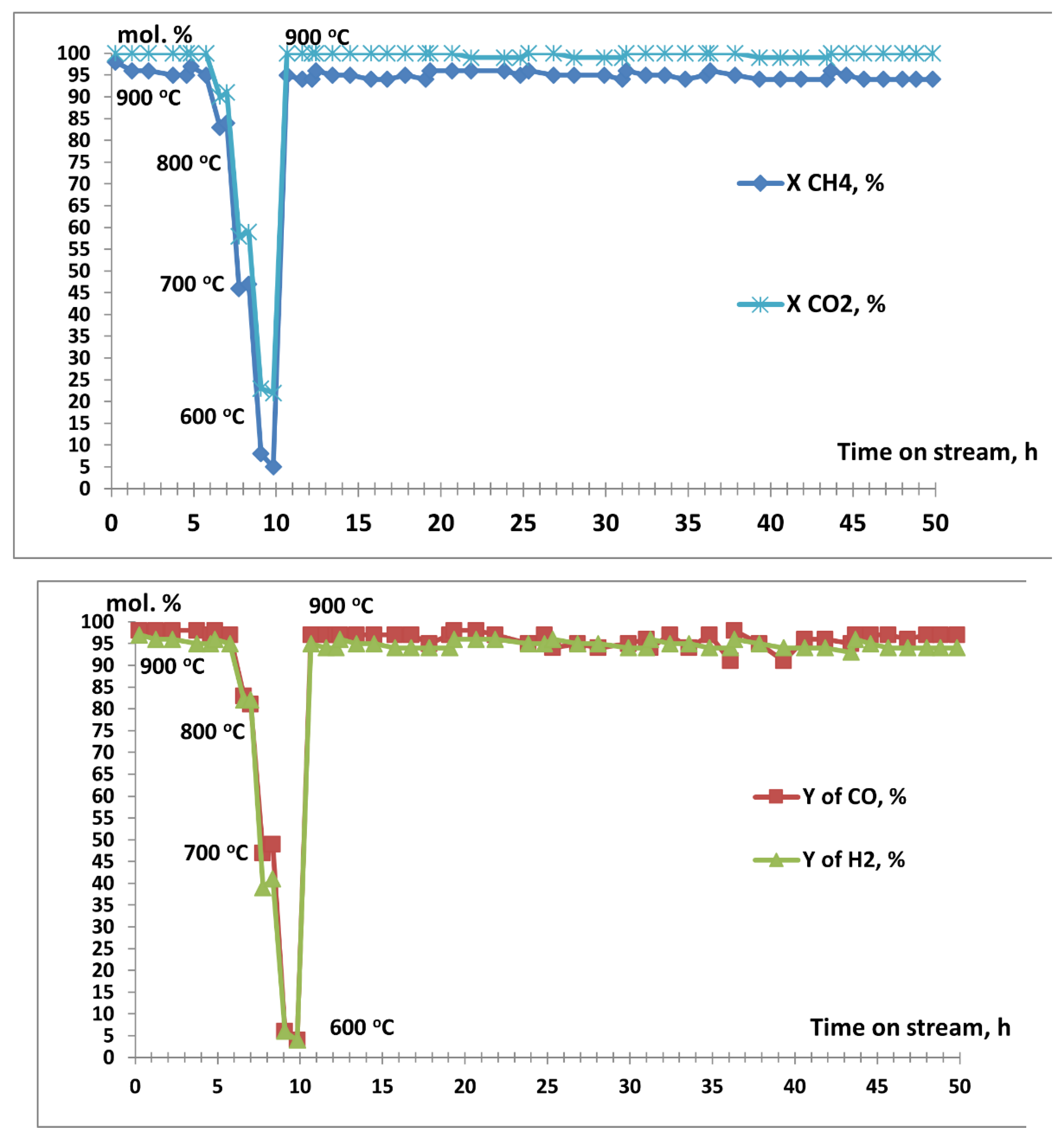
| Code of catalysts | Mass of Сo(NO3)2•6H2O, g | Mass of Sm(NO3)3•6H2O, g | SBET, m2/g |
|---|---|---|---|
| 2%Co/Sm2O3 | 0.49 | 12.49 | 1.79 |
| 5%Co/Sm2O3 | 1.23 | 12.11 | 2.82 |
| 10%Co/Sm2O3 | 2.47 | 11.47 | 3.45 |
| 23%Co/Sm2O3 | 2.91 | 4.47 | 3.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





