1. Introduction
Vitamin E plays a critical role in animal nutrition by serving as a potent lipid-soluble antioxidant, as well as contributing to anti-inflammation, immune function, and gene expression regulation. As an antioxidant, it protects cell membranes and other lipid-containing structures from oxidative damage caused by free radicals (Doğru Pekiner, 2003). Thereby, vitamin E is the major chain-breaking antioxidant inhibiting lipid peroxidation, a physiological function that is not provided by other dietary or endogenous antioxidants (Burton et al., 1982). This makes it crucial in preserving cell integrity, particularly in tissues that are susceptible to oxidative stress, such as the liver, lungs, and muscles (Traber and Atkinson, 2007).
The significance of vitamin E in animal nutrition cannot be overestimated, as it has been recognized as an indispensable micronutrient for optimal health, growth, and development in livestock (Idamokoro et al., 2020). Throughout the past century, numerous studies and advancements have been made in understanding the crucial role vitamin E plays in livestock production. A deficiency of vitamin E can impair immune responses and increase the susceptibility of animals to infectious diseases. Furthermore, hypovitaminosis E has been linked to reduced reproductive performance in animals, including decreased fertility rates and increased embryonic mortality (Xiao et al., 2021).
For livestock, optimizing vitamin E status is particularly important for animal health and production. In dairy cattle, supplementation with vitamin E has been demonstrated to enhance milk yield and lower the occurrence of mastitis (Hogan et al., 1993; Cusack et al., 2009; Chandra et al., 2013). In poultry, it has been associated with better growth rates, egg production, and hatchability (Rengaraj and Hong, 2015; Surai et al., 2016). Likewise, in swine, vitamin E supplementation has been proven to enhance meat quality, reduce stress, and increase growth rates (Cheah et al., 1995; Corino et al., 1999; Lu et al., 2014; Wang et al., 2022).
To ensure that animals receive adequate amounts of vitamin E, it is common practice to add this micronutrient as synthetic retinyl acetate to animal feeds. However, determining the optimal level of vitamin E supplementation can be challenging, as the requirements for this nutrient can vary depending on the species, age, health status of the animal, as well as other factors (McDowell, 2006).
In this review publication, we aim to provide a comprehensive overview of the early milestones in vitamin E research, as well as its current understanding and future directions. By examining the historical advancements in vitamin E exploration, we hope to provide insight into the evolution of our knowledge and understanding of this essential micronutrient, and how it has shaped animal production and health over the past century. Additionally, we will highlight road of the vitamin E chemical synthesis as well as recent and future research on the role of vitamin E in animal nutrition. Through this review, we aspire to emphasize the continuing importance of vitamin E in animal nutrition and the need for ongoing research to fully understand its potential in supporting animal health and productivity in the future.
2. Early Discoveries and Understanding of Vitamin E
Last year marked the 100th anniversary of the discovery of vitamin E in 1922, which was made by Herbert McLean Evans, an embryologist and endocrinologist, and his co-worker Kathrine Julia Scott Bishop, a medical physician and trained anatomist, while working at Berkeley University in California/USA. The two scientists observed that female rats fed on a purified diet had good growth and development and stayed healthy, but could not reproduce, as the embryos died and were resorbed after some 10 days of gravidity. However, when the semi-synthetic diet was supplemented with fresh green leaves of lettuce or dried alfalfa meal, a sudden restoration of fertility in previously sterile rats could be observed (Evans and Bishop, 1922). At first, the researchers believed that Vitamin C, which had already been discovered at that time and was known not to be essential for growth, was necessary for pregnancy. However, they quickly realized that only the fat-soluble components of the leaves had caused the good result. After testing the hydro- and lipophilic extract of various wheat by-products of a nearby flour mill, the two scientists discovered a new fat-soluble dietary lipophilic compound which causes sterility in rats when lacking in the feed (Evans and Bishop, 1922).
The unknown dietary substance was initially called factor X, but it was soon renamed vitamin E by Barnett Sure (Sure, 1924) and Herbert Evans (Evans, 1925). Evans and Bishop later demonstrated that male rats with diets lacking the new fat-soluble vitamin E also experienced sterility (Evans, 1925), leading to the vitamin’s subsequent designation as the “anti-sterility vitamin”. In the same year, Evans and his co-worker George Burr prepared a potent concentrate of vitamin E by saponification of wheat germ oil, which proved to possess high biological potency (Evans and Burr, 1925). Wheat-germ oil-based concentrates were used in many further experiments on vitamin E and served as a source for the development of the first commercial vitamin E products in the 1930s.
In 1936, Evans and his co-workers isolated two compounds with vitamin E activity from wheat germ oil, for which they proposed the names α-tocopherol and ß-tocopherol (Evans et al., 1936). Soon afterward, a third active factor, γ-tocopherol, was found in cottonseed oil by Evans’ working group (Emerson et al., 1937), and in 1947, a fourth tocopherol, named δ-tocopherol, was isolated from soybean oil (Stern et al., 1947). In 1936, Evans and his co-workers suggested the nomenclature α-tocopherol, the childbirth-bearing alcohol, for the new compound based on the Greek terms “tokos” for childbirth, “phero” for to bear, and “-ol” indicating an alcohol. This designation was proposed by George Miller Calhoun, a classical philologist and professor of Greek at the University of California (Evans et al., 1936).
Therefore, the discovery of vitamin E by Evans and Bishop in 1922 resulted in the identification and isolation of several tocopherols. Their dedication and contributions to the study of vitamin E will continue to be celebrated and studied for years to come.
3. Vitamin E’s Chemical Structure and Biological Activity
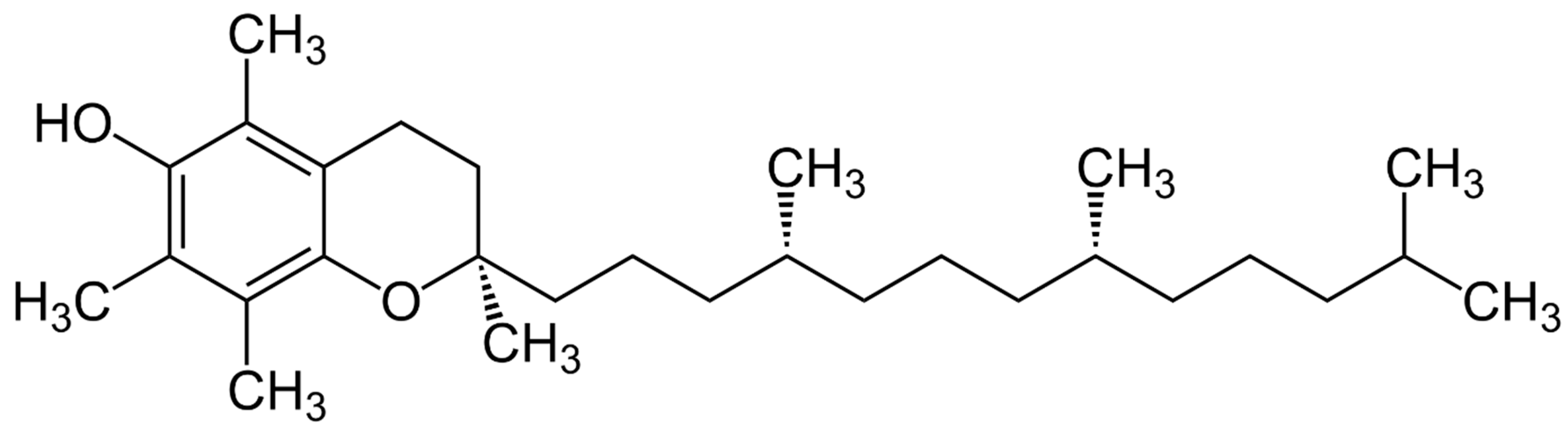
The chemical structure of vitamin E was elucidated by the German chemist Erhard Fernholz in 1938 while working in the USA. Fernholz proposed a structural formula which regarded α-tocopherol as a substituted 6-hydrocarbone with a long aliphatic sidechain attached to a pyran ring (
Figure 1). Prior to this, in 1937, Fernholz had studied the thermal decomposition of α-tocopherol and formed durohydro quinone and an aliphatic hydrocarbon. Shortly after Fernholz’s proposal, the Swiss chemist Paul Karrer achieved the chemical synthesis of α-tocopherol for the first time (Karrer, 1938; Karrer, 1939). Karrer condensed trimethyl hydroquinone with phytol bromide derived from natural phytol using zinc chloride as a catalyst. However, Karrer was not sure at that time regarding the chemical structure of the molecule he synthesized. He tended to assume a coumaran ring instead of the proposed chroman ring by Fernholz.
The first semi-synthetic tocopherol synthesized by Karrer consisted of two different stereoisomers and was initially called d,l-α-tocopherol or 2-ambo-α-tocopherol. Shortly after the first synthesis of α-tocopherol, Bergel and co-workers of Lister-Institute in London, UK, and Lee Irvin Smith and co-workers of the University of Minnesota, USA, accomplished the synthesis of α-tocopherol as well (Bergel et al., 1938; Smith et al., 1938).
The biological activity of the synthesized compound in the common rat resorption-gestation test was confirmed by Otto Isler (Isler, 1938), who accomplished an analog synthesis of vitamin E as Paul Karrer simultaneously.
Finally, the chroman ring as a constituent of α-tocopherol was confirmed with the help of UV-spectra and other comparative model tests by Walter John at Göttingen University in Germany (John, 1938; John, 1939). Furthermore, Walter John validated the chemical structure of α-tocopherol proposed by Fernholz and isolated ß-tocopherol simultaneously. John showed that ß-tocopherol differs from α-tocopherol only by one methyl group less at the chroman ring. He published more than 24 papers and book chapters on vitamin E-related topics in his short scientific career between 1937-1942.
In conclusion, the discovery of vitamin E’s chemical structure by Fernholz and synthesis of α-tocopherol by Karrer were significant milestones for this essential micronutrient. Walter John’s confirmation of the chroman ring in α-tocopherol and work on synthesizing vitamin E derivatives contributed to scientific understanding, though his work is largely unrecognized outside of German journals.
4. The Discovery of Vitamin E’s Unique Physiological Function as Chain-Breaking Antioxidant and the Antioxidant Network
In 1924, Henry Albright Mattill, a biochemist from the University of Iowa in the USA, conducted a study on the effects of milk consumption on reproduction. Along with his colleagues, he observed that rats became sterile when lard was added to their milk-based regimen. This led them to conclude that the fat content of a diet, in addition to vitamin E, affects reproduction. They proposed the hypothesis that the requirement for vitamin E increases with the amount of fat in the nutritional intake (Mattill et al., 1924).
Three years later, Mattill reported another finding: the destruction of vitamin E in the presence of fat, particularly unsaturated fats (Mattill, 1927). Building on this discovery, Mattill delved into further research on the autoxidation of fats. In collaboration with Marian Cummings, he put forward the idea that the oxidation of vitamin E could potentially safeguard other substances, such as vitamin A, from oxidation. They suggested that vitamin E possesses "anti-oxidant activity" and posited that its physiological role may lie in its ability to counteract oxidation (Cummings et al., 1931). It is worth noting that these early studies demonstrated the physiological consequences of the absence of antioxidant protection in lipids, namely, the sterility of rats.
A significant breakthrough in understanding the antioxidant role of vitamin E came from the research conducted by Aloys Tappel, a food biochemist at the University of California Davis/USA, during the 1950s. Alongside his colleagues, Tappel demonstrated that vitamin E effectively inhibits lipid peroxidation in living organisms. Through experiments conducted on isolated mitochondria and vitamin E-deficient animals, they observed elevated levels of lipid peroxidation in the liver, resulting in compromised mitochondrial stability (Tappel and Zalkin, 1959 and 1960).
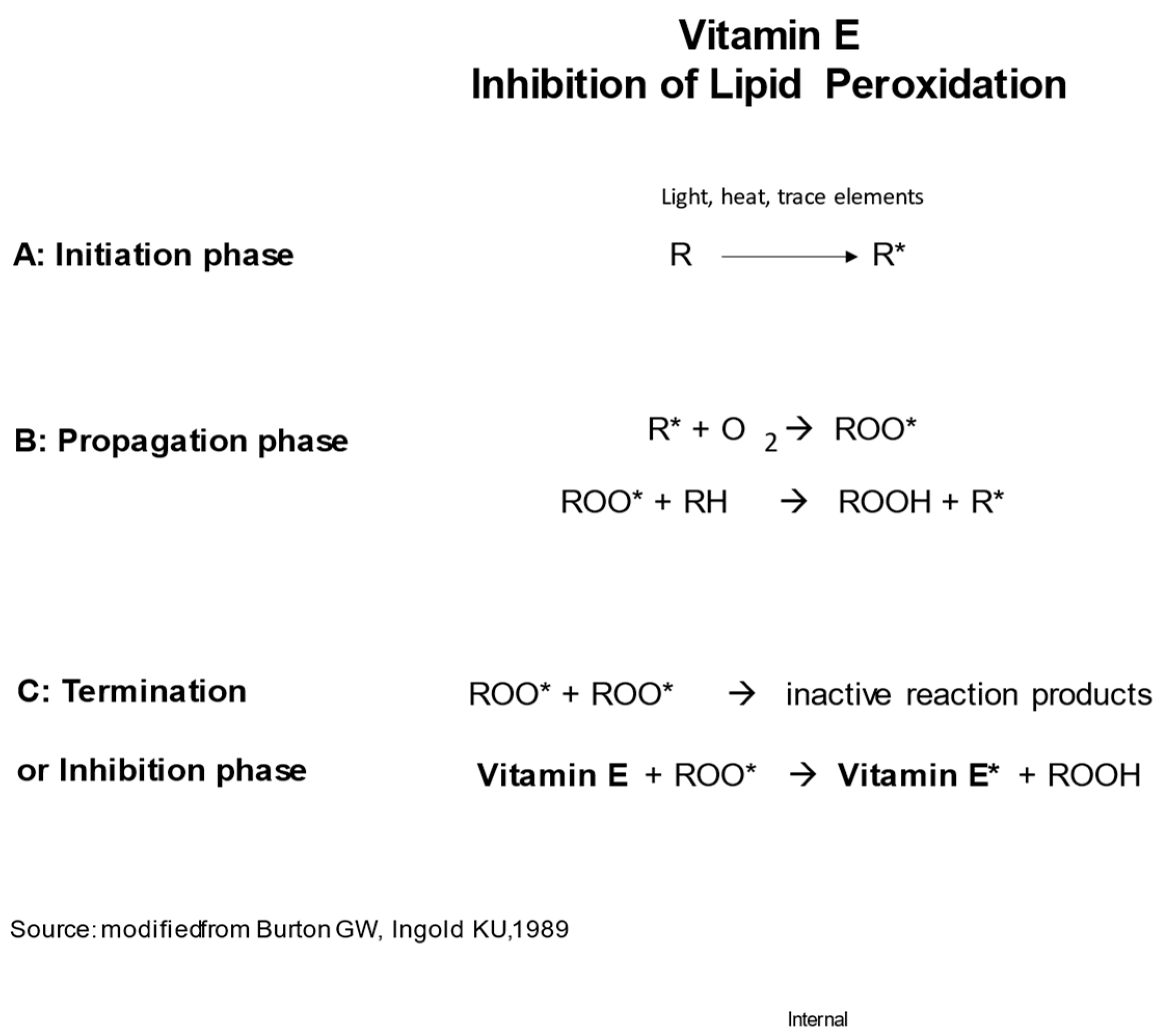
In the early 1980s, Graham Burton and Kathrin Ingold, researchers from the National Research Council of Canada, conducted chemical investigations into the antioxidant properties of vitamin E and other phenolic compounds. They elucidated the chemical structure of α-tocopherol, which proved to be optimal for scavenging peroxyl radicals due to its hydroxylated chromanol ring with significant methylation. Furthermore, they noted that α-tocopherol possesses ideal characteristics for in vivo localization alongside lipids, thanks to its phytyl side chain. Based on their findings, Burton and Ingold proposed that the primary, if not sole, function of α-tocopherol in living organisms is to act as an antioxidant. They even presented a reaction scheme for α-tocopherol in
Figure 2 (Burton and Ingold, 1981). Subsequently, through studies involving individuals deficient in vitamin E, Burton and his colleagues demonstrated that α-tocopherol serves as the predominant chain-breaking antioxidant in vivo (Burton et al., 1982).
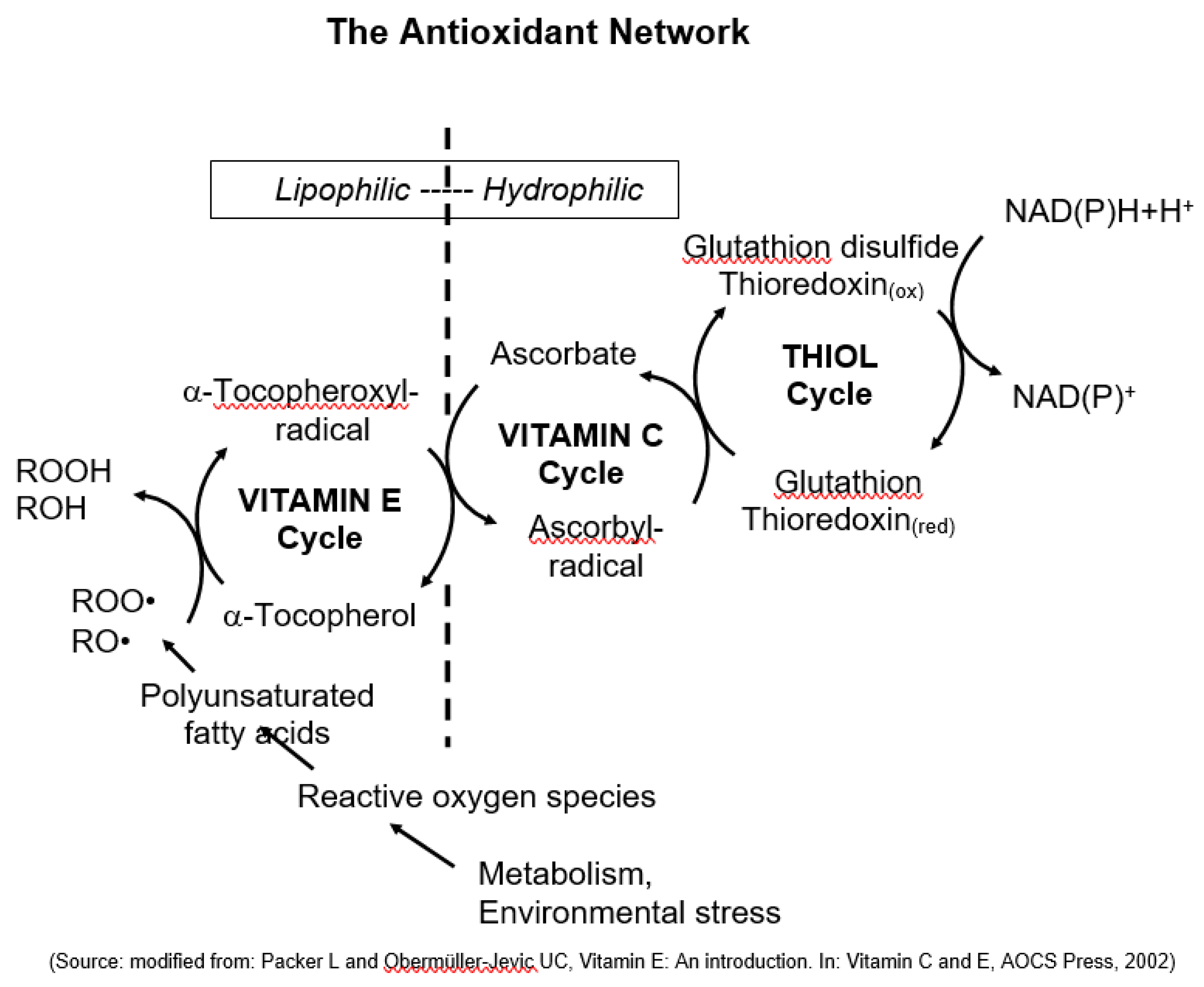
Finally, Lester Packer, a molecular and cell biologist from the University of California Berkeley/USA, made a significant observation regarding the combat against oxidative stress in cells. He recognized the importance of multiple antioxidants working together in what he referred to as “the antioxidant network” (
Figure 3). Packer's findings revealed that vitamin E and other antioxidants undergo oxidation but are subsequently recycled, forming a highly effective and precise defense system that adapts to oxidative stress (Packer et al., 1977; Packer and Landvik, 1989; Packer and Obermüller-Jevic, 2002).
5. The Evolution of Vitamin E Production: From Natural Sources to Synthetic Pathways and Standardized Potency Units
Otto Isler realized that acetylation was necessary to stabilize tocopherol as the vitamin E alcohol was not stable enough for practical use. He collaborated with Paul Karrer, in August 1938, to scale up this process (Isler, 1938; Fürst et al., 1993). The method suggested by Paul Karrer involved synthesizing α-tocopherol, which was then reacted with acetic acid anhydride to produce α-tocopheryl acetate, a vitamin E ester.
Isler conducted several tests and proved that α-tocopheryl acetate maintained its biological potency over time, whereas non-acetylated α-tocopherol lost its biological effect during storage (Isler, 1938). Subsequently, the world’s first synthetic vitamin E product (“Ephynal Acetate”, d,l-α-tocopheryl acetate) was launched for the treatment of all diseases associated with vitamin E hypovitaminoses in 1939 (Karrer, 1939). It was only available with a prescription from a physician.
From today’s perspective, it is remarkable that it took only two years from the clarification of the chemical structure to the development of a chemical synthesis pathway and the launch of the very first commercial vitamin E product for medical purposes. However, the production of “Ephynal Acetate” relied on natural phytol as a precursor, which was painfully extracted from hundreds of kilos of stinging nettles (Eggersdorfer et al., 2012). Therefore, preparations made from wheat-germ oil were initially used as a vitamin E source to combat hypovitaminosis and fertility problems in human and animal nutrition, as well as in human medicine and veterinary science.
The British pharmaceutical company Glaxo Laboratories Ltd. launched capsules of wheat-germ oil extract in 1933, which were claimed to be 15-30 times as potent as wheat-germ oil itself and assigned the brand name “Viteolin” in 1937 (Anonymous, 1940). Initially, the main application of wheat-germ oil-based vitamin E preparations in agriculture was to combat sterility in female animals. Two intramuscular injections of sterilized wheat-germ oil were recommended, and they were reported to cure 75% of the affected animals (Grandel, 1939). In cattle, wheat-germ oil was successfully used to combat brucellosis, also known as “Abortus Bang”, according to reports from Zurich University quoted by Karrer in 1939 (Karrer, 1939).
In the 1930s, the marketing of various commercial vitamin E preparations, which were derived from wheat-germ oil extract, required a standardized description of their vitamin E potency. To achieve this, three different biological dimensions were employed to describe and standardize the vitamin E potency of these products. All three were based on the fetal resorption-gestation test in rats, which determines the minimum dosage of a potential vitamin E source required for the restoration of fertility in sterile female rats. The Rat Unit according to Bomskov, the Fertility Dosage, and the Pacini-Linn Unit were used.
The Rat Unit according to Bomskov quantifies the single dosage of a preparation that ensures the normal gravidity of female rats fed on a vitamin E-free diet and prevents resorption of embryos. The Fertility Dosage was defined as the minimum daily allowance orally administered to sterile female rats during gravidity that results in at least one living offspring. The Pacini-Linn Unit was calculated by the equation 1000 divided by the minimum daily allowance expressed in mg required to prevent or remove sterility in female rats fed on a vitamin E deficient diet (Grandel, 1939).
These biological dimensions have become less relevant due to the reduced use of wheat-germ oil-based vitamin E products and the increasing adoption of synthetic vitamin E preparations in later years. In 1941, the Health Organization of the League of Nations established an international standard for vitamin E, similar to that for vitamin A, B, C and D, based on fetal resorption-gestation tests in rats. The international standard adopted was synthetic d,l-α-tocopheryl acetate derived from natural phythol, with a specific activity of 1 mg administered orally defined as 1 International Unit (IU) (Hume, 1941). This definition remained in place until 1956 when the supply of the semi-synthetic compound ran out, causing the International Standard for vitamin E to become obsolete (Brubacher et al., 1972).
Nevertheless, for labelling purposes, the concept of a unit of biological activity continues to be used, but it has been redefined as 1 mg of synthetic all-rac-α-tocopheryl acetate, which is the current USP reference standard, and renamed “USP Vitamin E-Unit”. This unit is numerically equal to the discontinued International Unit, with a weight/unit relationship of 1 mg all-rac-α-tocopheryl acetate = 1 USP-Unit (Kappus and Diplock, 1992;
Table 1).
In 1962, the Animal Nutrition Research Council (ANRC) in the United States selected a stabilized gelatin beadlet containing all-rac-α-tocopheryl acetate as the standard for animal nutrition (Anonymous, 1962).
In conclusion, after the chemical structure of vitamin E was clarified, the development of a synthesis pathway and launch of the first commercial medical product happened quickly. Wheat-germ oil-based vitamin E was used in human and animal nutrition, medicine, and veterinary science, and standardized using three biological dimensions. But, as synthetic vitamin E became more popular and cheaper, the unit of biological activity was redefined and standardized.
6. Early Experiments on Vitamin E and Its Effects on Animal Health
During the period from 1930-1950, several animal disorders were found to be caused by a deficiency in vitamin E. The first reports of nutritional muscular dystrophy in guinea pigs and rabbits, as well as encephalomalacia in chickens and ducklings, resulting from a vitamin E-deficient diet were made by Marianne Goettsch and Alwin Pappenheimer of Columbia University in New York in 1931 and 1934 (Goettsch and Pappenheimer, 1931; Pappenheimer and Goettsch, 1931; Goettsch and Pappenheimer, 1934). Dam and Glavind described the occurrence of exudative diathesis in chickens due to vitamin E deficiency a few years later in 1938 (Dam and Glavind, 1938). However, it has been challenging to produce a single vitamin E-deficiency symptom without the presence of other symptoms. During the 1950s and early 1960s, several significant observations were made that clarified the nutritional factors involved. In 1961, Machlin and Gordon compiled the interacting nutrients affecting the development of exudative diathesis, encephalomalacia, and muscular dystrophy in chickens (Machlin and Gordon, 1961).
In a series of fetal resorption-gestation studies spanning over two years from 1945 to 1947 and involving more than 700 rats, Harris and Ludwig compared the biopotency of natural RRR-α-tocopherol with that of synthetic all-rac-α-tocopherol or their respective acetate esters. They found that the natural and synthetic sources had a relative substitution rate of 1.36:1, which has been used in animal nutrition to this day (Harris and Ludwig, 1949).
In the 1950s and early 1960s, several other compounds with vitamin E activity were discovered in plants by various scientific groups in the USA and Europe using fetal resorption-gestation tests in rats. These compounds have a chemical structure similar to that of tocopherol but have an unsaturated isoprenoid side chain instead of a saturated phytyl side chain. They are called tocotrienols, as proposed by Green and colleagues in 1962 (Green et al., 1962). Tocotrienols exist as α-, β-, γ-, and δ-homologs, depending on the number and position of their methyl groups at the chroman ring.
While tocotrienols have a lower biopotency than tocopherols, they do not have any practical relevance in animal nutrition since their effectiveness is considerably diminished in fetal resorption-gestation tests or erythrocytes hemolysis tests in rats (
Table 2).
In the final analysis, the discovery of tocotrienols in the 1950s and 1960s provided further insight into the activity of vitamin E, but their lower biopotency limited their practical relevance in animal nutrition. However, the discovery of vitamin E deficiency as the cause of various animal disorders in the 1930s was a significant breakthrough that paved the way for further research on the nutritional factors involved.
Table 3 presents a comprehensive list of diseases caused by vitamin E deficiency.
7. Current Status and Future Research of Vitamin E in Animal Nutrition
Currently, vitamin E is the second most important vitamin in animal nutrition in terms of quantity. A recent market study indicates that global vitamin E consumption for animal nutrition was second only to choline chloride, with an estimated consumption of around 65,000 tons in 2020, equivalent to 130,000 tons of vitamin E 50% adsorbate (Vitamins, IHS Market Study, 2021). In terms of turnover, vitamin E was ranked second only to vitamin A, with an estimated market size of approximately €930 million in animal nutrition in 2020 (Vitamins, IHS Market Study, March 2021). It is evident that vitamin E is not only of great nutritional importance as an essential micronutrient but also commercially significant.
The latest research on vitamin E in animal nutrition has brought to light some intriguing and potentially revolutionary discoveries. A recent study featured in the Journal of Animal Science indicated that dietary vitamin E level may impede the growth of intestinal epithelial cells in weaned piglets, affecting intestinal structure and performance (Chen et al., 2019). In addition, the results of Choi et al.’s study in 2020 suggest that a deficiency in vitamin E can alter the gut microbiota of animals. These discoveries have significant implications for animal nutrition and highlight the potential for further research into the use of vitamin E to enhance the health and overall well-being of animals.
Another exciting area of research on vitamin E in animal nutrition involves its potential to improve the efficiency and well-being of ruminants. In a dose-response experiment using batch cultures, it was found that vitamin E supplementation had a positive impact on rumen fermentation, as evidenced by increased gas production and total VFA (Belanche et al., 2016). Furthermore, when α-tocopheryl acetate was supplied as the source of vitamin E, protozoal activity was higher compared to α-tocopherol. Interestingly, α-tocopheryl acetate also resulted in an increase in feed degradability by 8%. This effect may be attributed to the antioxidant properties of vitamin E, which led to higher levels of bacterial and protozoal activity in the rumen.
According to a study conducted by Wu et al. in 2023, high-dose vitamin E supplementation was found to have a positive impact on rumen fermentation and blood metabolism in dairy cows. This effect was achieved by modulating the relative abundance of rumen microorganisms, which helped to mitigate a range of adverse effects that are typically associated with subacute ruminal acidosis.
Moreover, the use of vitamin E as a dietary supplement may also reduce the need for antibiotics and other pharmaceuticals in animal production, which can help to address concerns around antimicrobial resistance and improve the overall sustainability of animal agriculture (Lee and Han, 2018; Hartmann et al., 2020; Hosain et al., 2021). These emerging findings suggest that vitamin E may have important implications for the future of animal nutrition and production.
In addition to its direct effects on animal health and productivity, vitamin E may also play a role in shaping the nutritional quality and safety of animal-derived foods. For example, research has shown that supplementing animal diets with vitamin E can increase the vitamin E content of meat, milk, and eggs, improving their nutritional value for consumers (Idamokoro et al., 2020). Furthermore, vitamin E has been proven to have a protective effect against lipid oxidation, which can cause off-flavors and reduce the shelf life of animal-derived foods (Zdanowska-Sąsiadek et al., 2016; Belles et al., 2019; Xu et al., 2021; Trombetti et al., 2022). This suggests that vitamin E may be an important tool for enhancing the quality and safety of animal-derived foods, particularly in the context of modern food systems where food safety and quality are major concerns. Overall, these recent findings suggest that vitamin E has a wide range of potential applications in animal nutrition, health, and food safety, and highlight the need for continued research in this area.
8. Conclusions
Looking back at the historical perspective of the discovery and understanding of vitamin E provides a foundation for further research and development of vitamin E in various fields, including animal health, food quality, and animal production efficiency. More research could focus on synthesizing vitamin E compounds with more stable and practical use than tocopheryl acetate, which could improve the production, biological value and availability of vitamin E for various applications.
In addition, further research could be conducted to explore the potential health benefits of vitamin E, such as its antioxidant properties, anti-inflammatory effects, and its role in immune function and gut health. This would help us understand the mechanisms of action of vitamin E and its potential application advantages for a variety of conditions.
Overall, the discovery of vitamin E and subsequent research has opened up new avenues for exploring its potential benefits and developing new applications. Further research is needed to fully understand the scope of its upsides and to develop more effective and practical uses for this important micronutrient.
References
- Anonymous. 1940. Vitamin E. Nature 3670: 345.
- Anonymous. 1962. Feedstuffs 3: 1-6.
- AWT (Arbeitsgemeinschaft für Wirkstoffe in der Tierernährung e.V.). 2002. Vitamin E in Vitamins in animal nutrition, pp. 15, AgriMedia, Germany. ISBN 3-86037-167-3.
- Belanche, A.; Kingston-Smith, A.H.; Newbold, C.J. An Integrated Multi-Omics Approach Reveals the Effects of Supplementing Grass or Grass Hay with Vitamin E on the Rumen Microbiome and Its Function. Front. Microbiol. 2016, 7, 905. [Google Scholar] [CrossRef] [PubMed]
- Bellés, M.; Campo, M.d.M.; Roncalés, P.; Beltrán, J.A. Supranutritional doses of vitamin E to improve lamb meat quality. Meat Sci. 2019, 149, 14–23. [Google Scholar] [CrossRef]
- Bergel F, Jacob A, Todd AR, Work TS. 1938. Vitamin E Synthesis of α-Tocopherol Nature, Vol. 142, 36.
- Blaxter, K.L. The significance of selenium and vitamin E in nutrition. Muscular dystrophy in farm animals: its cause and prevention. Proc. Nutr. Soc. 1962, 21, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Brubacher G, Wiss O. 1972. Tocopherols: Standardization of Activity. In The Vitamins (eds. Sebrell H., Harris R. S). p 248-251. Academic Press. [CrossRef]
- Burton GW, Ingold KU. 1981. Autoxidation of biological molecules. 1. The antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro 1. J Amer Chem Soc. 103: 6472-6477.
- Burton, G.; Joyce, A.; Ingold, K. First proof that vitamin E is major lipid-soluble, chain-breaking antioxidant in human blood plasma. Lancet 1982, 320, 327–327. [Google Scholar] [CrossRef] [PubMed]
- Chandra, G.; Aggarwal, A.; Singh, A.K.; Kumar, M.; Upadhyay, R.C. Effect of Vitamin E and Zinc Supplementation on Energy Metabolites, Lipid Peroxidation, and Milk Production in Peripartum Sahiwal Cows. Asian-Australasian J. Anim. Sci. 2013, 26, 1569–1576. [Google Scholar] [CrossRef]
- Cheah, K.; Cheah, A.; Krausgrill, D. Effect of dietary supplementation of vitamin E on pig meat quality. Meat Sci. 1995, 39, 255–264. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Li, J.; Li, Y.; Huang, P.; Ding, X.; Yin, J.; He, S.; Yang, H.; Yin, Y. Dietary vitamin E affects small intestinal histomorphology, digestive enzyme activity, and the expression of nutrient transporters by inhibiting proliferation of intestinal epithelial cells within jejunum in weaned piglets1. J. Anim. Sci. 2019, 97, 1212–1221. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.; Kim, S.; Lee, J.; Ha, J.; Oh, H.; Lee, Y.; Kim, Y.; Yoon, Y. Vitamin E (α-tocopherol) consumption influences gut microbiota composition. Int. J. Food Sci. Nutr. 2020, 71, 221–225. [Google Scholar] [CrossRef]
- Corino, C.; Oriani, G.; Pantaleo, L.; Pastorelli, G.; Salvatori, G. Influence of dietary vitamin E supplementation on "heavy" pig carcass characteristics, meat quality, and vitamin E status. J. Anim. Sci. 1999, 77, 1755–1761. [Google Scholar] [CrossRef]
- Cummings, M.J.; Mattill, H.A. The Auto-Oxidation of Fats with Reference to Their Destructive Effect on Vitamin E. J. Nutr. 1931, 3, 421–432. [Google Scholar] [CrossRef]
- Cusack, P.; McMeniman, N.; Rabiee, A.; Lean, I. Assessment of the effects of supplementation with vitamin E on health and production of feedlot cattle using meta-analysis. Prev. Veter- Med. 2009, 88, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Dam, H.; Glavind, J. Alimentary Exudative Diathesis. Nature 1938, 142, 1077–1078. [Google Scholar] [CrossRef]
- Peki̇ner, B.D. Vitamin E as an antioxidant. J Fac Pharm Ankara 2003, 32, 243–267. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Laudert, D.; Létinois, U.; McClymont, T.; Medlock, J.; Netscher, T.; Bonrath, W. One Hundred Years of Vitamins-A Success Story of the Natural Sciences. Angew. Chem. Int. Ed. 2012, 51, 12960–12990. [Google Scholar] [CrossRef]
- Emerson, O.H.; Emersox, G.A.; Mohammad, A.; Evans, H.M. The chemistry of vitamin E - tocopherols from various sources. J Biol Chem 1937, 122, 99–107. [Google Scholar] [CrossRef]
- Evans, H.M. The Pioneer History of Vitamin E. Vitam Horm. 1962, 20, 379–387. [Google Scholar] [CrossRef]
- Evans Herbert, M.; Emerson Oliver, H.; Emerson Gladys, A. The isolation from wheat germ oil of an alcohol, α-tocopherol, having the properties of vitamin E. Helv Chim Acta 1936, 113, 319–332. [Google Scholar]
- Evans, H.M.; Bishop, K.S. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 1922, 56, 650–651. [Google Scholar] [CrossRef]
- Evans HM, Burr GO. 1925. The anti-sterility fat soluble vitamin E. Proc Natl Acad Sci USA 11: 334-341.
- Evans, H.M.; Emerson, O.H.; Emerson, G.A. The isolation from wheat germ oil of an alcohol, α-tocopherol, having the properties of vitamin E. J. Biol. Chem. 1936, 113, 319–332. [Google Scholar] [CrossRef]
- Evans, H.M. Invariable Occurrence of Male Sterility with Dietaries Lacking Fat Soluble Vitamine E. Proc. Natl. Acad. Sci. 1925, 11, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Fernholz, E. The thermal decomposition of α-tocopherol. J. Am. Chem. Soc. 1937, 59, 1154–1155. [Google Scholar] [CrossRef]
- Fernholz, E. On the constitution of α-tocopherol. J Am Chem Soc. 1938, 60, 700–705. [Google Scholar] [CrossRef]
- Fryer, M.J. Vitamin E as a protective antioxidant in progressive renal failure. Nephrology 2000, 5, 1–7. [Google Scholar] [CrossRef]
- Fürst, A.; Brubacher, G.; Meier, W.; Rüttimann, A. DieHelvetica Chimica Acta und die Vitamine. Helv Chim Acta 1993, 577–635. [Google Scholar] [CrossRef]
- Gitler, C.; Sunde, M.L.; Baumann, C.A. Effect of Certain Necrosis-Preventing Factors on Hemolysis in Vitamin E-Deficient Rats and Chicks. J. Nutr. 1958, 65, 397–407. [Google Scholar] [CrossRef]
- Goettsch, M.; Pappenheimer, A.M. Nutritional muscular dystrophy in the guinea pig and rabbit. J. Exp. Med. 1931, 54, 145–165. [Google Scholar] [CrossRef]
- Pappenheimer, A.M.; Goettsch, M.; Alexieff, W.T.A.O.A. Nutritional myopathy in ducklings. J. Exp. Med. 1934, 59, 35–42. [Google Scholar] [CrossRef]
- Goettsch, M, Pappenheimer AW. Nutritional muscular dystrophy in the guinea pig and rabbit. J Exp Med. 1931, 54, 145–165. [Google Scholar] [CrossRef]
- Grandel, F. Das Vitamin E, seine Bedeutung bei Mensch, Tier und Pflanze. Angew. Chem. 1939, 52, 420–426. [Google Scholar] [CrossRef]
- Hackett, M. 2021. Vitamins. Chemical Economics Handbook, 38-39 Editor: IHS Markit.
- Harris, P.L.; Ludwig, M.I. Relative vitamin e potency of natural and of synthetic α-tocopherol. J. Biol. Chem. 1949, 179, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.S.; Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Vitamin E as promising adjunct treatment option in the combat of infectious diseases caused by bacterial including multi-drug resistant pathogens – Results from a comprehensive literature survey. Eur. J. Microbiol. Immunol. 2020, 10, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Hogan, J.; Weiss, W.; Smith, K. Role of Vitamin E and Selenium in Host Defense Against Mastitis. J. Dairy Sci. 1993, 76, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Hosain, Z.; Kabir, S.M.L.; Kamal, M. Antimicrobial uses for livestock production in developing countries. Veter- World 2021, 14, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Hume, E.M. STANDARDIZATION OF VITAMIN E. Nature 1941, 148, 472–473. [Google Scholar] [CrossRef]
- Idamokoro, E.M.; Falowo, A.B.; Oyeagu, C.E.; Afolayan, A.J. Multifunctional activity of vitamin E in animal and animal products: A review. Anim. Sci. J. 2020, 91, e13352. [Google Scholar] [CrossRef]
- Isler, O. Die Stabilisierung von d,l-α-Tocopherol. Helv Chim Acta. 1938, 21, 1756–1759. [Google Scholar]
- John, W. Über das Cumo-tokopherol, einen neuen Faktor der Vitamin E-Gruppe. . 1937, 250, 11–24. [Google Scholar] [CrossRef]
- John, W.; Dietzel, E.; Günther, P.; Emte, W. Zum Beweis der Chromanstruktur des α-Tokopherols. Sci. Nat. 1938, 26, 366–367. [Google Scholar] [CrossRef]
- 5 (26): 21-22.
- Kappus A, Diplock HT. 1992. Tolerance and safety of Vitamin E: A toxicological position report. Free Radic. Biol. Med. 13: 55-74.
- Karrer, P. Vitamin E und verwandte Verbindungen. Helvetica Chim. Acta 1939, 22, 334–350. [Google Scholar] [CrossRef]
- Karrer P, Fritzsehe H, Ringier BH, Salomon H. α-Tocopherol. Helv Chim Acta 1938, 21, 520–525. [Google Scholar] [CrossRef]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Harper, A.F.; Zhao, J.; Estienne, M.J.; Dalloul, R. Supplementing antioxidants to pigs fed diets high in oxidants: I. Effects on growth performance, liver function, and oxidative status1. J. Anim. Sci. 2014, 92, 5455–5463. [Google Scholar] [CrossRef] [PubMed]
- Machlin, L.J.; Gordon, R.S. Etiology of Exudative Diathesis, Encephalomalacia, and Muscular Degeneration in the Chicken. Poult. Sci. 1962, 41, 473–477. [Google Scholar] [CrossRef]
- Marsh, J.A.; Dietert, R.R.; Combs, G.F. Influence of Dietary Selenium and Vitamin E on the Humoral Immune Response of the Chick. Exp. Biol. Med. 1981, 166, 228–236. [Google Scholar] [CrossRef]
- Mattill HA, Carman JS, Clayton MM. . The nutritive properties of milk. III. The effectiveness of the X substance in preventing sterility in rats on milk rations high in fat. J Biol Chem 1924, 61, 729–740.
- Mattill, HA. The oxidative destruction of vitamins A and E and the protective action of certain vegetable oils. J Am Med Assoc. 1927, 89, 1505–1508. [Google Scholar] [CrossRef]
- Mayer H, Schudel P, Rüegg R, Isler O. . Die absolute Konfiguration des natürlichen α-Tocopherols. Helv Chim Acta. 1963, 46, 963–982. [CrossRef]
- McDowell, L.R. Vitamin nutrition of livestock animals: Overview from vitamin discovery to today. Can. J. Anim. Sci. 2006, 86, 171–179. [Google Scholar] [CrossRef]
- Menzies, P.; Langs, L.; Boermans, H.; Martin, J.; McNally, J. Myopathy and hepatic lipidosis in weaned lambs due to vitamin E deficiency. Can. Veter- J. = La Rev. Veter- Can. 2004, 45, 244–7. [Google Scholar]
- Muth, O.H. White muscle disease (myopathy) in lambs and calves. I. Occurrence and nature of the disease under Oregon conditions. J. Am. Veter- Med Assoc. 1955, 126. [Google Scholar]
- Nafstad, I.; Tollersrud, S. The Vitamin E-Deficiency Syndrome in Pigs. Acta Veter- Scand. 1970, 11, 452–480. [Google Scholar] [CrossRef] [PubMed]
- Obel, A.L. Studies on the morphology and etiology of so-called toxic liver dystrophy (hepatosis diaetetica) in swine. Acta Pathol. et Microbiol. Scand. Suppl. 1953, 94. [Google Scholar]
- Olcott HS, Mattill HA. . The unsaponifiable lipids of lettuce: II. Fractionation. J Biol Chem. 1931a, 93, 59–64.
- Olcott HS, Mattill HA. The unsaponifiable lipids of lettuce: III. Antioxidant. J Biol Chem. 1931b, 93, 65–70. [CrossRef]
- Olson, R.E. Vitamin E and Its Relation to Heart Disease. Circulation 1973, 48, 179–184. [Google Scholar] [CrossRef]
- Packer, L.; Smith, J.R. Extension of the lifespan of cultured normal human diploid cells by vitamin E: a reevaluation. Proc. Natl. Acad. Sci. 1977, 74, 1640–1641. [Google Scholar] [CrossRef]
- Packer, L.; Landvik, S. Vitamin E: Introduction to Biochemistry and Health Benefits. Ann. New York Acad. Sci. 1989, 570, 1–6. [Google Scholar] [CrossRef]
- Packer L, Obermüller-Jevic UC. 2002. Vitamin E: An introduction. In: Packer L, Traber MG, Kramer K et al, Hrsg. The antioxidant vitamins C and E. Champaign, IL: AOCS Press; 2002: 133-151.
- Pappenheimer, A.M.; Goettsch, M.; Pappenheimer, W.T.A.O.A. A cerebellar disorder in chicks, apparently of nutritional origin. J. Exp. Med. 1931, 53, 11–26. [Google Scholar] [CrossRef]
- Pappenheimer, AW. Certain nutritional disorders of laboratory animals due to vitamin E deficiency. J Mt Sinai Hosp 1940, 7, 65. [Google Scholar]
- Peplowski, M.A.; Mahan, D.C.; Murray, F.A.; Moxon, A.L.; Cantor, A.H.; Ekstrom, K.E. Effect of Dietary and Injectable Vitamin E and Selenium in Weanling Swine Antigenically Challenged with Sheep Red Blood Cells. J. Anim. Sci. 1981, 51, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Rengaraj, D.; Hong, Y.H. Effects of Dietary Vitamin E on Fertility Functions in Poultry Species. Int. J. Mol. Sci. 2015, 16, 9910–9921. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.L. Advances in our understanding of vitamin E. Fed. Proc. 1980, 39. [Google Scholar]
- Smith LI, Ungnade HE, Prichard WW. The Chemistry of Vitamin E. I. The Structure and Synthesis of α-Tocopherol. Science 1938, 88, 37–38. [Google Scholar] [CrossRef]
- Stern, M.H.; Robeson, C.D.; Weisler, L.; Baxter, J.G. δ-Tocopherol. I. Isolation from Soybean Oil and Properties1. J. Am. Chem. Soc. 1947, 69, 869–874. [Google Scholar] [CrossRef]
- Surai, P.F.; Fisinin, V.I.; Karadas, F. Antioxidant systems in chick embryo development. Part 1. Vitamin E, carotenoids and selenium. Anim. Nutr. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- Sure, B. Dietary requirements for reproduction. II. The existence of a specific vitamin for reproduction. J Biol Chem. 1924, 58, 693–703. [Google Scholar] [CrossRef]
- Tappel, A.; Zalkin, H. Inhibition of lipide peroxidation in mitochondria by vitamin E. Arch. Biochem. Biophys. 1959, 80, 333–336. [Google Scholar] [CrossRef]
- Tappel, A.L.; Zalkin, H. Inhibition of Lipid Peroxidation in Microsomes by Vitamin E. Nature 1960, 185, 35–35. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free. Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- Trombetti, F.; Minardi, P.; Mordenti, A.L.; Badiani, A.; Ventrella, V.; Albonetti, S. The Evaluation of the Effects of Dietary Vitamin E or Selenium on Lipid Oxidation in Rabbit Hamburgers: Comparing TBARS and Hexanal SPME-GC Analyses. Foods 2022, 11, 1911. [Google Scholar] [CrossRef] [PubMed]
- Vos, J.; Hulstaert, C.; Molenaar, I. Nutritional Myopathy in Ducklings: a Growth Rate-Dependent Symptom of ‘Tissue Peroxidosis’ due to a Net Nutritional Shortage of Vitamin E plus Selenium in Skeletal Muscle. Ann. Nutr. Metab. 1981, 25, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jang, Y.D.; Rentfrow, G.K.; Azain, M.J.; Lindemann, M.D. Effects of dietary vitamin E and fat supplementation in growing-finishing swine fed to a heavy slaughter weight of 150 kg: I. Growth performance, lean growth, organ size, carcass characteristics, primal cuts, and pork quality. J. Anim. Sci. 2022, 100. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G. The Discovery of the Antioxidant Function of Vitamin E: the contribution of Henry A. Mattill. J. Nutr. 2005, 135, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Guo, Y.; Zhang, J.; Deng, M.; Xian, Z.; Xiong, H.; Liu, D.; Sun, B. High-Dose Vitamin E Supplementation Can Alleviate the Negative Effect of Subacute Ruminal Acidosis in Dairy Cows. Animals 2023, 13, 486. [Google Scholar] [CrossRef]
- Xiao, J.; Khan, M.Z.; Ma, Y.; Alugongo, G.M.; Ma, J.; Chen, T.; Khan, A.; Cao, Z. The Antioxidant Properties of Selenium and Vitamin E; Their Role in Periparturient Dairy Cattle Health Regulation. Antioxidants 2021, 10, 1555. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Zhang, H.; Wu, S.; Yue, H.; Wan, X.; Yang, H.; Wang, Z.; Qi, G. Vitamin E Supplementation Enhances Lipid Oxidative Stability via Increasing Vitamin E Retention, Rather Than Gene Expression of MAPK-Nrf2 Signaling Pathway in Muscles of Broilers. Foods 2021, 10, 2555. [Google Scholar] [CrossRef]
- Zagoriy, V. 2017. Mass spectrometry in chemical biology – Evolving applications Edited by Norberto Preporine Lopes and Ricardo Roberto da Silva. Royal Society of Chemistry Chapter 1 “Introduction”, 1-16.
- Gozdowski, D.; Zdanowska-Sąsiadek. ; Michalczuk, M.; Damaziak, K.; Niemiec, J.; Poławska, E.; Gozdowski, D.; Różańska, E. Effect of vitamin E supplementation on growth performance and chicken meat quality. Eur. Poult. Sci. (eps) 2016, 80. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).