1. Introduction
Coronaviruses are a classification of viral agents that have the ability to induce sickness both in human beings and animals. Some of these viruses can be transmitted from animals to humans. COVID-19, which emerged in 2019, is one of these viruses, known as SARS-CoV-2 and declared a pandemic by the World Health Organization (WHO) in 2020 [
1]. Fever, cough, pneumonia, and dyspnea, are key symptoms, and comorbidities such as cardiovascular diseases, chronic neurological illnesses, and type 2 diabetes mellitus increase the severity of illness [
2]. Although many people who are infected with the virus may not have any symptoms, making it difficult to control the spread of the disease [
3] or have experienced mild symptoms, however, up to 20% of hospitalized patients die and 18%–33% require mechanical ventilation [
4] and older patients with comorbidities have the highest risk of hospitalization or death [
4,
5]. Older age and higher Charlson comorbidity index scores are strongly associated with death and in immunosuppressed patients, 20.9% died or required ICU admission [
6,
7].
The current global pandemic has led to an urgent need for effective diagnostic and therapeutic interventions. Despite extensive efforts to control the spread of the SARS-CoV-2 virus, many regions of the world are still struggling to contain the infection as of 2023. Consequently, numerous research studies have been conducted to explore potential treatments for COVID-19. One promising approach that has received considerable attention is the use of interferons for the treatment of SARS-CoV-2 infection. While several studies have already been published on this topic, this paper provides an updated review of recent developments in the field. To accomplish this, a thorough screening of research papers and meta-analyses published between 2020 and 2023 was conducted, with only 15 papers being selected as potential sources of information. The selection criteria for these papers included factors such as sample size, study duration, geographic location, and scholarly acceptance. Notable contributions to the literature were identified, such as a meta-analysis by Lei Yang and colleagues in 2021 [
8] and a study by Jhuti et al. in the year 2022 [
9].
The objective of this study is to review the current understanding of the potential of interferons (IFNs) as a therapeutic drug for SARS-CoV-2. The review aims to cover the mechanisms of action of IFNs, the available data on the use of IFNs in COVID-19, and the potential, limitations, and challenges of IFN therapy. Additionally, it highlights the ongoing clinical trials investigating the use of IFNs in COVID-19 and their results. Although many ongoing studies on the treatment of SARS-CoV-2 are found during this paper writing and the optimal dosing and duration of IFN therapy still remain to be determined [
10]. However, further randomized controlled clinical trials are required to measure the safety and efficacy of IFN therapy in SARS-CoV-2 treatment. Because data available today is based on small-size studies and therefore not sufficient to conclude the right doses. However, the interferon theory offers a promising option for the management of SARS-CoV-2, but further research is needed to fully understand the potential benefits and limitations of IFN therapy [
10].
2. Interferon and its therapeutical roles
Interferons are a vital component of the innate immune system's response to viral infections. These cytokines act as signalling molecules by binding to specific receptors on the surface of cells, initiating the transcription of a wide range of interferon-stimulated genes [
11]. The proteins encoded by these genes exhibit a variety of functions, including the ability to impede viral replication, inhibit the growth of microorganisms and tumours, and modulate the immune response [
12,
13]. Over 25 distinct protein and IFN genes have been identified across various species, including humans. The classification of human interferons is based on receptor signalling and comprises three primary types: Type I, Type II, and Type III [
13]. IFN I and III are expressed in response to the detection of viral molecular patterns by endosomal and cytoplasmic receptors and are produced by almost every cell type. On the other hand, IFN type II is triggered by cytokines such as IL-12 and is primarily produced by natural killer (NK) and T cells [
14].
Type I (α/β) IFN binds to the IFNα/β receptors also known as IFNAR, which are composed of two important chains, IFNAR1 and IFNAR2 [
15]. The IFNAR receptor is a type I transmembrane protein that is involved in the signalling pathway of interferons (IFNs). Upon binding, the IFNAR receptor complex activates various downstream signalling pathways, including the JAK-STAT pathway, leading to the phosphorylation and nuclear translocation of STAT1 and STAT2 [
16,
17,
18]. These proteins then bind to the DNA sequences specific to that and are also known as ISREs (interferon-stimulated response elements), leading to the transcriptional activation of antiviral genes such as those encoding for the 2'-5' oligoadenylate synthase (OAS) and the RNase L enzymes.
Type II IFNs, also known as IFN-γ, bind to its receptor, known as IFN-γ receptor (IFNGR) and are made up of 2 chains, IFNGR 1 and 2. Upon binding, the IFNGR receptor complex activates similar downstream signalling pathways as Type I IFNs, but also activates additional pathways, including the MAPK and PI3K pathways, leading to the various transcription factors activation like NF-κB and AP-1, and the translations of genes encoding for antiviral proteins, such as MHC class I and II molecules [
19,
20].
Type III (CRF2-4 and CRF2-12) signal via IL10R2 and IFNLR1 and play a role in certain viral or fungal infections [
21]. Type III interferons, also known as interleukin-28 and interleukin-29, are more recently discovered and have been shown to have antiviral activity against several different viruses. In combating viral infections, the IFN systems efficiencies are evident in numerous inhibitors of IFN action or induction that are started by several viruses, which hinders their elimination and is responsible for the ongoing coexistence of vertebrates and viruses [
22].
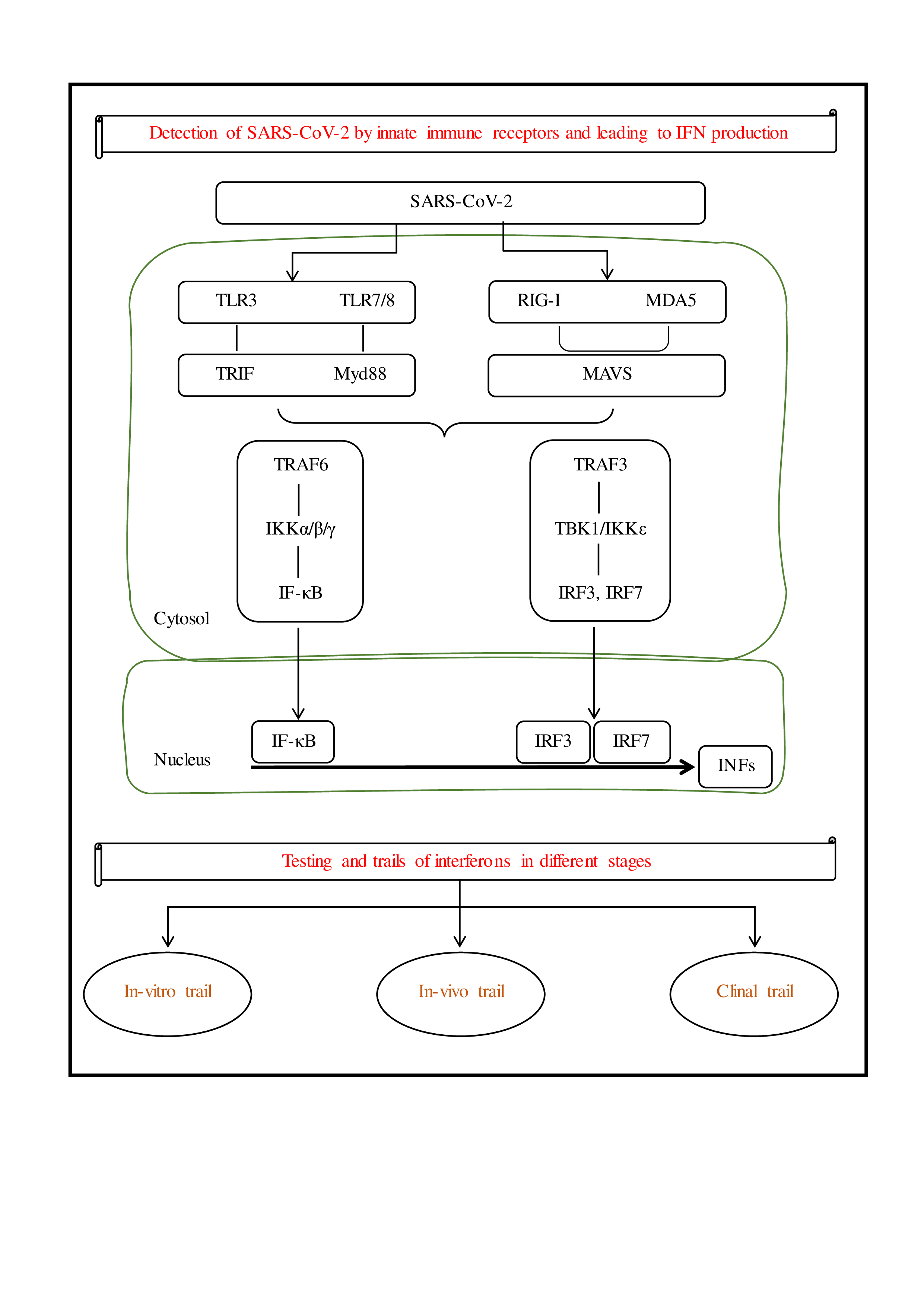
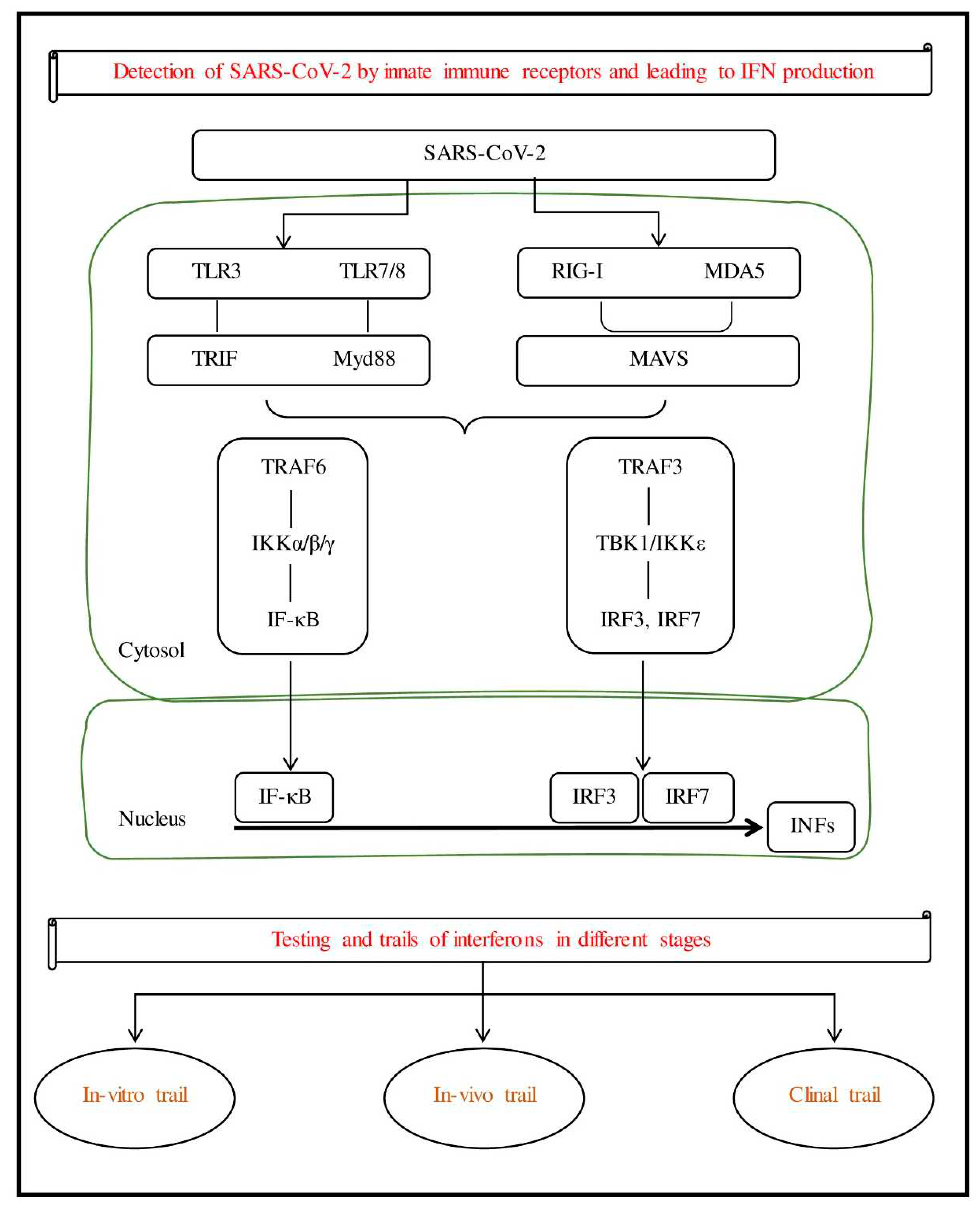
Figure 1.
Presentation of SARS-Cov-2 leads to IFN production and different stages of clinical trials.
Figure 1.
Presentation of SARS-Cov-2 leads to IFN production and different stages of clinical trials.
As the above discussion, IFNs act by binding to specific receptors on the surface of cells. This binding initiates a cascade of downstream signalling events that ultimately leads to the activation of interferon-stimulated genes (ISGs), which have antiviral properties [
23]. One of the key mechanisms by which IFNs exert their antiviral effects is through the induction of the ISG, interferon-induced transmembrane protein 3 (IFITM3). This protein acts as a barrier to viral entry by inhibiting the fusion of viral and host cell membranes. Additionally, IFNs can also inhibit viral replication by upregulating the expression of other ISGs, such as OAS1 and MxA, which have antiviral activity against the SARS-CoV-2 virus [
22]. In addition to their direct antiviral effects, IFNs have also been shown to have immunomodulatory properties that may be beneficial in the treatment of SARS-CoV-2. IFNs have been shown to inhibit viral replication by activating the signalling pathway of JAK-STAT, which leads to hundreds of antiviral genes expression [
24]. This results in the production of proteins such as 2',5'-oligoadenylate synthase (OAS), and RNase L, which directly target and degrade viral RNA. Additionally, IFNs can also stimulate the activation and proliferation of immune cells like T cells and NK (natural killer) cells, which play a crucial role in the clearance of viral infection [
24,
25]. Recombinant interferon can be administered through various routes, including subcutaneous injection, intramuscular injection, and intravenous infusion [
22]. It mimics the action of the natural interferons and activates the same immune response mechanisms to combat the viral infection [
23]. It is a well-established treatment for viral infections such as; Interferon alfa-2b and beta-1b reducing the risk of disease progression in patients with moderate COVID-19 and the need for mechanical ventilation and shortened hospital stays in patients with severe COVID-19 respectively [
26]. Some experts believe that interferon therapy may be most effective when administered early in the course of the disease before severe lung damage occurs [
9]. However, other studies have shown mixed results, and the optimal timing and dosage of interferon therapy for COVID-19 are still being investigated [
27]. Additionally, it has also been associated with several side effects, including flu-like symptoms, fatigue, and depression. Despite these side effects, interferon therapy remains an important tool in the fight against viral infections and continues to be used in the treatment of a variety of viral infections today.
3. Recent studies on interferon as therapy in SARS-CoV-2:
IFNs have been studied extensively in the context of SARA-CoV-2, with a growing body of data writing down their potential as therapeutic agents for the treatment of this disease. This is thought that inhibition of virus replication is mediated by the activation of ISGs which stand for IFN-stimulated genes that play a key role in the host’s defines against viral infections. Several studies have reported that IFNs can effectively suppress the replication of SARS-CoV-2 in both in-vitro and in-vivo conditions.
3.1. In-vitro trails
Preclinical and clinical studies have supplied evidence that IFN could be used as another therapy option for SARS-CoV-2 treatment. For example, a study in the year 2020 by Bosi et. al., found that treatment with IFN-alpha2a and IFN-beta1a significantly reduced the viral load in human lung epithelial cells infected with SARS-CoV-2 [
28]. A similar study in 2020 was reported by Dinnon et al., which found that IFN-beta treatment was able to reduce SARS-CoV-2 viral loads and showed improvement in the lung function of a mouse model [
29].
Another study reported by Pituch et. al., 2022, found that treatment with IFN-alpha2b significantly increased the number of natural killer cells in SARS-CoV-2-infected human peripheral blood mononuclear cells [
30]. A similar study published in the same year found that treatment with IFN-beta1a resulted in a significant increase in T cells number of SARS-CoV-2-infected human peripheral blood mononuclear cells.
These findings suggest that IFNs may be effective in reducing viral replication and potentially preventing the progression of SARS-CoV-2. In addition to their antiviral properties, IFNs have also been shown to modulate the host immune response to viral infections. This is thought to be mediated by the immune cells, like T cells and NK cells, which are critical for the clearance of viral infections. Several studies have reported that IFN treatment can enhance the host immune response against SARS-CoV-2, potentially improving the prognosis of COVID-19 patients.
3.2. In-vivo trails
Recent studies have investigated the potential of IFNs as a treatable drug for SARS-CoV-2 and the results of these studies suggest that IFNs may have a therapeutic benefit in COVID-19 treatment. The potential of IFNs in the tackle of SARS-CoV-2 has been demonstrated in multiple preclinical studies [
31]. For example, a study conducted in cell culture showed that treatment with IFN-α could inhibit the SARS-CoV-2 virus replication, resulting in a reduction of viral titers. Another study in mice showed that treatment with IFN-β was able to reduce the viral load and improve lung pathology in animals infected with the virus [
28]. Despite the promising preclinical data, the potential of IFNs as therapy remains to be fully evaluated in clinical trials. However, these studies state that IFNs may have therapeutic potential in the SARS-CoV-2 treatment, particularly at the initial stages of viral infection when replication is high. To fully understand the action mechanisms of IFNs in SARS-CoV-2 and to determine the optimal dosing and administration strategies for their use as therapeutics, further research studies are important [
32].
3.3. Clinical trails
In a recent systematic review and meta-analysis, researchers analyzed the available data on the use of IFNs in COVID-19 patients. For example, a Phase 2 clinical trial of the IFN-beta-1b drug showed markable recovery in mild to moderate patients of COVID-19 [
33,
34]. Another study included 20 randomized controlled trials (RCTs) involving a total of 2,059 participants. The results of the meta-analysis showed that the use of IFNs in patients infected with SARS-CoV-2 caused a significant reduction in the duration of viral shedding (p < 0.001), as well as a significant reduction in the risk of severe disease (p = 0.02). Additionally, the administration of IFNs was linked to a noteworthy decrease in the probability of hospitalization (p = 0.03) and a marked decline in the likelihood of mortality (p = 0.01) [
31].
In 2020, a study was published to assess the effectiveness of IFN-alpha2b in treating patients diagnosed with COVID-19. The research aimed to evaluate the efficacy of this treatment on a sample of 40 patients who presented mild to moderate symptoms of the disease and receive either IFN-alpha2b or a placebo. This study’s results exhibited that IFN-alpha2b as a drug offers a significant reduction in the viral shedding duration (p = 0.03) and a momentous decrease in the risk of progression to severe disease (p = 0.03). Additionally, the researchers discovered that the use of IFN-alpha2b correlated with a notable decrease in the concentrations of inflammatory indicators (p < 0.05) [
35].
In the above progress, another study published in the same year assessed the efficacy and safety of IFN-beta1a in the COVID-19 treatment. The study included 60 patients with COVID-19, who were randomized to receive either IFN-beta1a or placebo. This study’s results exhibited that the use of IFN-beta1a resulted in a significant reduction in the duration of viral shedding (p < 0.001) and a significant reduction in the hospitalization risk (p = 0.03). Additionally, this study also testified that IFN-beta1a use was linked to a significant decrease in the levels of inflammatory markers (p < 0.05) [
36].
In summary, the available data on the use of IFNs in COVID-19 suggests that they may be effective in reducing viral replication and modulating the host immune response. IFNs have been shown to decrease the duration of viral flaking, reduce the risk of severe sickness, reduce the risk of hospitalization, and reduce the risk of death. However, it is important to note that medical trials are presently ongoing to evaluate the efficacy and safety of IFN therapy for SARS-CoV-2.
Table 1.
Important ongoing clinical trials of IFN mentioned within the text and from outside, have been listed below.
Table 1.
Important ongoing clinical trials of IFN mentioned within the text and from outside, have been listed below.
| S. No. |
PMID |
Title of the study/trails |
Treatment groups |
Treatment administration stage |
Sample Size |
Initial outcomes |
Country and location |
| 1 |
32,758,689 |
“SARS-CoV-2 clearance in SARS-CoV-2 patients with novaferon treatment: A randomized, open-label, parallel-group trial” [37] |
(a) Novaferon
(b) Lopinavir/ ritonavir
(c) Novaferon + lopinavir/ritonavir |
Clinically classified, hospitalized SARS-CoV-2
patients from moderate-severe |
89 |
The lopinavir/ ritonavir plus novaferon combination showed higher viral clearance rates on day 9 compared to novaferon alone, with a difference of 13-18%, but the difference was not statistically significant (p-value=0.2839). |
Chinese’s Hunan Province |
| 2 |
32,862,111 |
“Interferon b-1b in randomized clinical trial” 8,28 |
(a) Treatment regimen involving interferon-b 1b with either hydroxychloroquine and lopinavir/ ritonavir or atazanavir/ritonavir
(b) Treatment regimen without interferon-b 1b |
Hospitalized patients with
severe infection of SARS-CoV-2 |
99 |
The study measured time to clinical improvement and found it significantly shorter in treatment a [(9(6-10)] (IFN group) than in treatment b [(11(9-15)] (control group) (p = 0.002). |
Tehran Province of
Iran |
| 3 |
33,264,556 |
“Repurposed antiviral drugs for SARS- CoV-2—Interim WHO Solidarity Trial Results” [38] |
(a) Remdesivir
(b) Hydroxychloroquine
(c) Lopinavir
(d) Interferon-b-1a |
Hospitalized patients
|
2050 |
The study found a higher in-hospital mortality rate for patients receiving interferon compared to the control group. The 16.8 odds ratio with 113.3 a variance. 1.16 was reported as the rate ratio of fatality (0.96–1.39). |
This study involved 405 hospitals located in 30 different countries including,
Austria, Albania, Colombia, Argentina, Brazil, Belgium, Egypt, France, Finland, India, Honduras, Indonesia, Iran, Italy, Ireland, Kuwait, Lithuania, Lebanon, Malaysia, Luxembourg, Norway, North Macedonia, Peru, Pakistan, Saudi Arabia, Philippines, Spain, South Africa, and Switzerland. |
| 4 |
33,189,161 |
“Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for
treatment of SARS-CoV-2
infection: a randomised, double-blind, placebo-controlled, phase 2 trial” [39] |
(a) Interferon beta-1a
(b) Placebo |
Mild-severe SARS-CoV-2 |
101 |
The clinical status of individuals was evaluated using the WHO Ordinal Measure for Medical Progress
on Day 15/16 and Day 28, yielding odds ratios of 2.32 [95% CI 1.07–5.04] (p = 0.033) and 3.15 [1.39–7.14] (p = 0.006), respectively. The likelihood of improvement was found to be significantly higher in the group treated with IFN compared to those receiving a placebo. |
The study involved collecting data from a total of 20 locations across England and Northern Ireland, including Hull, Birmingham, Cottingham, Leicester, Oxford, Bradford, Manchester, Nottingham, Salisbury, Southampton, Belfast, and Maidenhead. |
| 5 |
32,661,006 |
“A randomized
clinical trial of the efficacy and safety of interferon b-1a in treatment of severe SARS-CoV- 2” [40] |
(a) The treatment plan involves interferon-b-1a with either hydroxychloroquine or lopinavir-ritonavir or atazanavir-ritonavir.
(b) Treatment plan A excludes the use of interferon-b-1b. |
Severe condition of an infected patient with SARS-CoV-2 |
81 |
The study evaluated the clinical response time after initiating interventions in two groups, one treated with IFN and the other in a control group. The mean duration for the IFN group was 9.74 ± 5.8, whereas the control group had a mean duration of 8.39 ± 4.9. The hazard ratio was 1.10 with a 95% confidence interval of 0.64 to 1.87, indicating no significant difference between the two groups (p=0.72). |
Tehran Province of
Iran |
| 6 |
33,556,319 |
“Peginterferon lambda for the treatment of outpatients with SARS-CoV-2: a phase 2, placebo-controlled randomised trial” [41] |
(a) Peginterferon lambda-1a
(b) Placebo |
Individuals who tested positive within 7 days with SARS-CoV-2 and showed symptoms or taking a test (if asymptomatic) at an early stage. |
60 |
On day 7, the ratio of people with a (-ve) mid-turbinate swab was evaluated. Out of the total participants, 80% in the treatment group and 63% in the placebo group tested negative. However, there was no significant difference between the groups (p=0.15) and the unadjusted odds ratio for peginterferon lambda vs. placebo was 2.32 (0.74-7.81, p=0.15). |
Ontario,
Toronto, Canada |
4. Discussion on challenges, limitations and opportunities:
Interferon (IFN) therapy has been widely discussed as another promising treatment option for SARS-CoV-2 and IFNs have displayed antiviral activity against a broad range of viruses, including coronaviruses. However, the efficacy and safety of IFN therapy in the treatment of SARS-CoV-2 have been limited by several factors [
42,
43]. One limitation of IFN therapy is its relatively low efficacy in reducing viral load in patients with SARS-CoV-2. Studies have shown that IFN therapy can reduce viral load in some patients, but the effect is often not sustained and viral load tends to rebound after treatment is discontinued. This suggests that IFN therapy may be more effective as an adjunct to other treatments, rather than as a standalone therapy [
44]. Another limitation of IFN therapy is its potential to cause side effects, particularly in patients who are already critically ill. IFN therapy can cause flu-like symptoms, such as fever, fatigue, and muscle aches, which can exacerbate the symptoms of SARS-CoV-2. Additionally, IFN therapy can also cause more serious side effects, such as liver or kidney damage, which can be particularly dangerous in critically ill patients [
45]. A further limitation of IFN therapy is its relatively high cost, which may limit its accessibility to patients in low-income countries; for example, the cost of a single course of treatment with interferon beta-1a can range from
$4,000 to
$10,000. The cost of IFN therapy can be a significant burden for patients and their families, particularly in countries where healthcare resources are limited [
46]. IFN can be administered in different ways, including intravenously (IV) or subcutaneously (SC), which means through injection therefore it can be a barrier for patients. Although it is safe and decreases the burdens of medical staff and doctors but also associated with several reasons why the injectable route of administration may be a barrier for patients [
47]. For example, some patients may have a fear of needles or may experience pain or discomfort during the injection process. Others may have physical or medical conditions that make it difficult to receive injections, such as poor vein access or a bleeding disorder. This means that some patients may find it difficult or uncomfortable to receive injections, which could limit their ability or willingness to use interferons as a treatment option [
48,
49].
Overall, while IFN therapy has shown promise as a potential treatment for SARS-CoV-2, however, more research is needed to fully understand its efficacy and safety. Studies and clinical trials are ongoing and the final results are needed to be analyzed and evaluated before any conclusions can be made about the effectiveness of interferon as another optional therapy for the SARS-CoV-2 treatment [
50]. Additionally, IFN therapy is a promising treatment option for SARS-CoV-2, but it is associated with several challenges, including limited availability of data on efficacy and safety, the potential for serious side effects, and the high cost of IFN. Additional research is required for an improved understanding of the safety and efficacy of IFN therapy in the SARS-CoV-2 treatments and to classify the most appropriate IFN regimen for use in clinical practice.
5. Conclusion
In conclusion, Interferon (IFN) therapy as an antiviral drug medication has been used for decades to treat several viral infections and work by triggering an antiviral response in infected cells, thereby preventing the replication of the virus. Recent studies on interferon as therapy in COVID-19 have yielded mixed results. However, the majority of these studies have had small sample sizes and have not been adequately powered to detect significant differences in outcomes. Additionally, many of the studies have used different IFN regimens and have not been able to provide consistent results. Therefore, more research is required to improve our understanding of the safety and effectiveness of IFN therapy for treating SARS-CoV-2, as well as to determine the most suitable IFN treatment regimen for clinical use. Despite the challenges, the potential of Interferons as a therapeutic agent in COVID-19 patients cannot be overlooked as they have a proven track record in treating other viral infections. Furthermore, the use of IFN as a therapeutic medicine in the SARS-CoV-2 treatment may have additional benefits, such as reducing the duration of hospitalization and the risk of severe disease. However, it is important to note that IFN therapy is also associated with several challenges, including limited availability of data on efficacy and safety, the potential for serious side effects, and high cost. Therefore, careful monitoring of patients receiving IFN therapy is essential to minimize the risk of serious side effects. Additionally, the cost-effectiveness of IFN therapy in the treatment of SARS-CoV-2 must be carefully considered before its implementation in clinical practice.
Author Contributions
PY is working on the day of manuscript writing, formatting, revision and communicating to yet with all authors for writing and publications. Dr VC is supervising for conducting of this study and mentoring the first author and others. VR, AY, SA, AY and VMT contributed to writing of manuscript and finding of data and its analysis. Together, the authors provided a thorough review of interferon potential as a therapeutic approach for Sars-CoV-2.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Consent for Publication
Yes, all authors agreed to publish their manuscripts according to journal publication guidelines.
Ethical Approval and Consent to Participate
Not applicable.
Data Availability Statement
Not applicable, however, all the data presented in this manuscript has been cited and credited to the corresponding individuals and publications.
Acknowledgements
Not applicable.
Conflicts of Interest
The authors declared no conflict of interest.
References
- Bonilauri, P.; Rugna, G. Animal Coronaviruses and SARS-COV-2 In Animals, What Do We Actually Know? Life 2021, 11, 123. [Google Scholar] [CrossRef]
- Petrosillo, N.; Viceconte, G.; Ergonul, O.; Ippolito, G.; Petersen, E. COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. 2020, 26, 729–734. [Google Scholar] [CrossRef]
- Yadav, P. Interferon Production and Potentiality as a therapeutic drug for SARS-CoV-2. Pharmacoepidemiol. Drug Saf. 2023, 12, 288. [Google Scholar]
- Gili, T.; Benelli, G.; Buscarini, E.; Canetta, C.; La Piana, G.; Merli, G.; Scartabellati, A.; Viganò, G.; Sfogliarini, R.; Melilli, G.; et al. SARS-COV-2 comorbidity network and outcome in hospitalized patients in Crema, Italy. PLOS ONE 2021, 16, e0248498. [Google Scholar] [CrossRef]
- Iftimie, S.; López-Azcona, A.F.; Vicente-Miralles, M.; Descarrega-Reina, R.; Hernández-Aguilera, A.; Riu, F.; Simó, J.M.; Garrido, P.; Joven, J.; Camps, J.; et al. Risk factors associated with mortality in hospitalized patients with SARS-CoV-2 infection. A prospective, longitudinal, unicenter study in Reus, Spain. PLOS ONE 2020, 15, e0234452. [Google Scholar] [CrossRef]
- Lai, Q.; Spoletini, G.; Bianco, G.; Graceffa, D.; Agnes, S.; Rossi, M.; Lerut, J. SARS-CoV2 and immunosuppression: A double-edged sword. Transpl. Infect. Dis. 2020, 22, e13404. [Google Scholar] [CrossRef]
- Minotti, C.; Tirelli, F.; Barbieri, E.; Giaquinto, C.; Donà, D. How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection? A systematic review. J. Infect. 2020, 81, e61–e66. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Hui, P.; Yarovinsky, T.O.; Badeti, S.; Pham, K.; Liu, C. Potential role of IFN-α in COVID-19 patients and its underlying treatment options. Appl. Microbiol. Biotechnol. 2021, 105, 4005–4015. [Google Scholar] [CrossRef]
- Jhuti, D.; Rawat, A.; Guo, C.M.; Wilson, L.A.; Mills, E.J.; Forrest, J.I. Interferon Treatments for SARS-CoV-2: Challenges and Opportunities. Infect Dis. Ther. 2022, 11, 953–72. [Google Scholar] [CrossRef]
- Nakhlband, A.; Fakhari, A.; Azizi, H. Interferon-beta offers promising avenues to COVID-19 treatment: a systematic review and meta-analysis of clinical trial studies. Naunyn-Schmiedeberg's Arch. Pharmacol. 2021, 394, 829–838. [Google Scholar] [CrossRef]
- Rubinstein, M.; Katsoulidis, E.; Kaur, S.; Platanias, L.C.; Tamir, A.; Jordan, W.J.; Ritter, M.; Habib, N.; Lechler, R.I.; Foster, G.R.; et al. Multiple Interferon Subtypes: The Phenomenon and Its Relevance. J. Interf. Res. 1987, 7, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Khanna NR, Gerriets V. Interferon. Handbook of Hormones: Comparative Endocrinology for Basic and Clinical Research 2022:447–52. [CrossRef]
- Parmar, S.; Platanias, L.C. Interferons: mechanisms of action and clinical applications. Curr. Opin. Oncol. 2003, 15, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Taylor MW. Viruses and man: A history of interactions. Viruses and Man: A History of Interactions 2015:1–430. [CrossRef]
- Dorrington, M.G.; Bradfield, C.J.; Lack, J.B.; Lin, B.; Liang, J.J.; Starr, T.; Ernst, O.; Gross, J.L.; Sun, J.; Miller, A.H.; et al. Type I IFNs facilitate innate immune control of the opportunistic bacteria Burkholderia cenocepacia in the macrophage cytosol. PLOS Pathog. 2021, 17, e1009395. [Google Scholar] [CrossRef]
- Farrar, J.D.; Smith, J.D.; Murphy, T.L.; Murphy, K.M. Recruitment of Stat4 to the Human Interferon-α/β Receptor Requires Activated Stat2. J. Biol. Chem. 2000, 275, 2693–2697. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, D.S.; Horvath, C.M. A Road Map for Those Who Don't Know JAK-STAT. Science 2002, 296, 1653–1655. [Google Scholar] [CrossRef] [PubMed]
- Matikainen, S.; Sareneva, T.; Ronni, T.; Lehtonen, A.; Koskinen, P.J.; Julkunen, I. Interferon- Activates Multiple STAT Proteins and Upregulates Proliferation-Associated IL-2R, c-myc, and pim-1 Genes in Human T Cells. Blood 1999, 93, 1980–1991. [Google Scholar] [CrossRef]
- Platanias, L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005, 5, 375–386. [Google Scholar] [CrossRef]
- Boehm, U.; Klamp, T.; Groot, M.; Howard, J.C. Cellular responses to interferon-γ. Annu. Rev. Immunol. 1997, 15, 749–795. [Google Scholar] [CrossRef]
- Lin, F.-C.; Young, H.A. Interferons: Success in anti-viral immunotherapy. Cytokine Growth Factor Rev. 2014, 25, 369–376. [Google Scholar] [CrossRef]
- Friedman, R.M. Clinical uses of interferons. Br. J. Clin. Pharmacol. 2008, 65, 158–162. [Google Scholar] [CrossRef]
- George, P.M.; Badiger, R.; Alazawi, W.; Foster, G.R.; Mitchell, J.A. Pharmacology and therapeutic potential of interferons. Pharmacol. Ther. 2012, 135, 44–53. [Google Scholar] [CrossRef]
- Kotenko, S.V.; Gallagher, G.; Baurin, V.V.; Lewis-Antes, A.; Shen, M.; Shah, N.K.; Langer, J.A.; Sheikh, F.; Dickensheets, H.; Donnelly, R.P. IFN-λs mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 2003, 4, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Ozato, K.; Uno, K.; Iwakura, Y.; Buchbinder, E.I.; McDermott, D.F.; Owens, T.; Khorooshi, R.; Wlodarczyk, A.; Asgari, N.; Nunnally, M.E.; et al. Another Road to Interferon: Yasuichi Nagano's Journey. J. Interf. Cytokine Res. 2007, 27, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, H.; Davoudi-Monfared, E.; Nourian, A.; Khalili, H.; Hajizadeh, N.; Jalalabadi, N.Z.; Fazeli, M.R.; Ghazaeian, M.; Yekaninejad, M.S. Interferon β-1b in treatment of severe COVID-19: A randomized clinical trial. Int. Immunopharmacol. 2020, 88, 106903–106903. [Google Scholar] [CrossRef]
- Interferons | COVID-19 Treatment Guidelines n.d. Available online: https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/interferons/ (accessed on 17 March 2023).
- Bosi, E.; Bosi, C.; Querini, P.R.; Mancini, N.; Calori, G.; Ruggeri, A.; Canzonieri, C.; Callegaro, L.; Clementi, M.; De Cobelli, F.; et al. Interferon β-1a (IFNβ-1a) in COVID-19 patients (INTERCOP): study protocol for a randomized controlled trial. Trials 2020, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dinnon, K.H.; Leist, S.R.; Schäfer, A.; Edwards, C.E.; Martinez, D.R.; Montgomery, S.A.; West, A.; Yount, B.L., Jr.; Hou, Y.J.; Adams, L.E.; et al. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature 2020, 586, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Pituch-Noworolska, A.M. NK cells in SARS-CoV-2 infection. Central Eur. J. Immunol. 2022, 47, 95. [Google Scholar] [CrossRef]
- Emani, V.R.; Goswami, S.; Nandanoor, D.; Emani, S.R.; Reddy, N.K.; Reddy, R. Randomised controlled trials for COVID-19: evaluation of optimal randomisation methodologies—need for data validation of the completed trials and to improve ongoing and future randomised trial designs. Int. J. Antimicrob. Agents 2020, 57, 106222. [Google Scholar] [CrossRef]
- Bencze, D.; Fekete, T.; Pázmándi, K. Correlation between Type I Interferon Associated Factors and COVID-19 Severity. Int. J. Mol. Sci. 2022, 23, 10968. [Google Scholar] [CrossRef]
- Sosa, J.P.; Caceres, M.M.F.; Comptis, J.R.; Quiros, J.; Príncipe-Meneses, F.S.; Riva-Moscoso, A.; Belizaire, M.-P.; Malanyaon, F.Q.; Agadi, K.; Jaffery, S.S.; et al. Effects of Interferon Beta in COVID-19 adult patients: Systematic Review. Infect. Chemother. 2021, 53, 247–260. [Google Scholar] [CrossRef]
- Shalhoub, S. Interferon beta-1b for COVID-19. Lancet 2020, 395, 1670–1671. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, V.; Shannon, C.P.; Wei, X.S.; Xiang, X.; Wang, X. , Wang, Z.-H.; Tebbutt, S.J.; Kollmann, T.R.; Fish, E.N.; et al. Interferon-α2b Treatment for COVID-19. Front Immunol. 2020, 11, 1061. [Google Scholar] [CrossRef] [PubMed]
- Aricò, E.; Castiello, L.; Bracci, L.; Urbani, F.; Lombardo, F.; Bacigalupo, I.; Ancidoni, A.; Vanacore, N.; Falcione, A.; Reggiani, C.; et al. Antiviral and immunomodulatory interferon-beta in high-risk COVID-19 patients: a structured summary of a study protocol for a randomised controlled trial. Trials 2021, 22, 1–4. [Google Scholar] [CrossRef]
- Zheng, F.; Zhou, Y.; Zhou, Z.; Ye, F.; Huang, B.; Huang, Y.; Ma, J.; Zuo, Q.; Tan, X.; Xie, J.; et al. SARS-CoV-2 clearance in COVID-19 patients with Novaferon treatment: A randomized, open-label, parallel-group trial. Int. J. Infect. Dis. 2020, 99, 84–91. [Google Scholar] [CrossRef] [PubMed]
- H P, R P, AM H-R, MP P, V S, Q AK, et al. Repurposed Antiviral Drugs for COVID-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [CrossRef]
- Monk, P.D.; Marsden, R.J.; Tear, V.J.; Brookes, J.; Batten, T.N.; Mankowski, M.; Gabbay, F.J.; E Davies, D.; Holgate, S.T.; Ho, L.-P.; et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2020, 9, 196–206. [Google Scholar] [CrossRef]
- Davoudi-Monfared, E.; Rahmani, H.; Khalili, H.; Hajiabdolbaghi, M.; Salehi, M.; Abbasian, L.; Kazemzadeh, H.; Yekaninejad, M.S. A Randomized Clinical Trial of the Efficacy and Safety of Interferon β-1a in Treatment of Severe COVID-19. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Feld, J.J.; Kandel, C.; Biondi, M.J.; A Kozak, R.; Zahoor, M.A.; Lemieux, C.; Borgia, S.M.; Boggild, A.K.; Powis, J.; McCready, J.; et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir. Med. 2021, 9, 498–510. [Google Scholar] [CrossRef]
- Sallard, E.; Lescure, F.-X.; Yazdanpanah, Y.; Mentre, F.; Peiffer-Smadja, N. Type 1 interferons as a potential treatment against COVID-19. Antivir. Res. 2020, 178, 104791–104791. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Alothman, A.; Balkhy, H.H.; Al-Dawood, A.; Aljohani, S.; Al Harbi, S.; Kojan, S.; Aljeraisy, M.; Deeb, A.M.; Assiri, A.M.; et al. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials 2018, 19, 81. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Asiri, A.Y.; Assiri, A.M.; Abdullah, M.L.; Aljami, H.A.; Balkhy, H.H.; Al Jeraisy, M.; Mandourah, Y.; AlJohani, S.; Al Harbi, S.; et al. Heterogeneity of treatment effect of interferon-β1b and lopinavir–ritonavir in patients with Middle East respiratory syndrome by cytokine levels. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Darazam, I.A.; Shokouhi, S.; Pourhoseingholi, M.A.; Irvani, S.S.N.; Mokhtari, M.; Shabani, M.; Amirdosara, M.; Torabinavid, P.; Golmohammadi, M.; Hashemi, S.; et al. Role of interferon therapy in severe COVID-19: the COVIFERON randomized controlled trial. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Nguyen, H.A.; Cooke, G.S.; Day, J.N.; Flower, B.; Phuong, L.T.; Hung, T.M.; Dung, N.T.; Khoa, D.B.; Hung, L.M.; Kestelyn, E.; et al. The direct-medical costs associated with interferon-based treatment for Hepatitis C in Vietnam. Wellcome Open Res. 2020, 4, 129. [Google Scholar] [CrossRef]
- Bittner, B.; Richter, W.; Schmidt, J. Subcutaneous Administration of Biotherapeutics: An Overview of Current Challenges and Opportunities. BioDrugs 2018, 32, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Interferon Beta-1a Subcutaneous Injection: MedlinePlus Drug Information n.d. Available online: https://medlineplus.gov/druginfo/meds/a604005.html (accessed on 24 March 2023).
- Hauschild, A.; Gogas, H.; Tarhini, A.; Middleton, M.R.; Testori, A.; Dréno, B.; Kirkwood, J.M. Practical guidelines for the management of interferon-α-2b side effects in patients receiving adjuvant treatment for melanoma. Cancer 2008, 112, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; Shin, E.-C. Type I and III interferon responses in SARS-CoV-2 infection. Exp. Mol. Med. 2021, 53, 750–760. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).