1. Introduction
In many countries, universal neonatal hearing screening is recommended or required by law. Since the birth of the Commission for the Early Detection of Hearing Loss (CODEPEH) in 1995, various initiatives have been carried out with the aim of promoting and prioritizing the application of neonatal hearing screening programs throughout Spain. In 2003, a consensus was reached with the Ministry of Health and the Autonomous Communities to implement it, which has evolved to the present day [
1]. The Valencian Community has a neonatal screening program since 2001 [
2].
Newborn hearing screening protocols vary by country and health system, but generally follow a similar process. Usually, the program consists in screening all newborns for hearing loss before they leave the hospital or maternity center, although there are also out-of-hospital protocols. Many protocols use two stage screening. The first stage is a quick and simple screening test, such as the automated auditory brainstem response (ABR) or otoacoustic emission (OAE) test. If the baby does not pass the first stage, he will be referred for rescreening with these or other techniques a few days or weeks later (second stage). The rationale for this is that if a newborn fails a hearing screening test, it does not necessarily mean he has a hearing loss [
3,
4].
Confirmatory testing in the form of a retest before one month of life is established in most neonatal hearing screening programs in order to reduce the number of infants referred for otorhinolaryngology (ENT) assessment (in order not to exceed 4%) [
1] and to increase the specificity and sensitivity of the first step [
3].
To fail in the first step tests is highly variable and depends on several factors. There are many reasons why an infant may fail the initial screening test, such as amniotic fluid in the middle ear, bad neonatal condition during the test, environmental noise, the age at discharge, the staff that performs the test, and the used technique among others [
2].
Recommended follow-up after a failed hearing screening test is important as it can help to detect hearing loss early and ensure that the child receives appropriate treatment and intervention services [
5]. Children who do not receive timely intervention for hearing loss may experience delays in speech and language development, social and emotional development, and academic performance. [
2]. Detecting hearing loss early increases the likelihood of successful intervention and optimal outcomes for the child. Depending on the severity and type of hearing loss, treatment options may include hearing aids, cochlear implants and/or speech therapy [
6].
Second-level hospitals face peculiarities that hinder the implementation of the hearing screening protocol, which are not uncommon in other settings but may be aggravated by time and staffing constraints. In third-level hospitals, teams of several professionals involved in the screening can be formed with sufficient time allocated, but in second-level hospitals this is not so frequent causing a worse performance, delays in the recommended protocol and in the final result. This can be particularly serious in the rescreening process.
Although the heterogeneity of the screening programs currently in place prevents generalizations on the basis of one study, it is true that many of the problems encountered in a second-level hospital setting are common to different hospitals and protocols. The aim of this study is to analyze the hearing rescreening process under the point of view of the quality criteria and objectives of the programs generally accepted by specific committees such as CODEPEH and JCIH (Joint Committee of Infant Hearing) [
1,
7].
The aim of this study is to find out and evaluate:
The volume of rescreening and the origin of the neonates;
The performance of rescreening in neonates born in the center’s maternity ward;
The performance of screening and rescreening in neonates born outside the center’s maternity ward;
To determine the rescreening times in both groups;
To know the final ENT diagnosis.
2. Materials and Methods
Inclusion criteria: all newborns born in this or other hospitals who have undergone one or more neonatal hearing rescreening tests in outpatient clinic of a second level Hospital, from 1 January 2015 to 31 December 2022.
Exclusion criteria: newborns who have not undergone any test in outpatient clinic.
2.1. Protocol
The screening protocol discerns between neonates with risk factors and those without risk factors. The risk factor definitions provided by CODEPEH were used, based on the recommendations of the Joint Committee on Infant Hearing (JCIH) [
1,
7].
In the case of healthy newborns with no risk factors, it is carried out in three steps. The first one consists in performing a bilateral OAE test (1st test), specifically transient evoked otoacoustic emissions (TEOAEs), at around 48 hours of life, always within the possibilities of the service. This is carried out every day of the week, depending on the availability of the service and the workload of all the maternity ward nurses. Once parental consent has been obtained, the TEOAE test is carried out in the same room as the newborn lies, making sure that the environmental noise is as low as possible. In order to be sure that the newborn was calm and therefore to avoid sedation in all cases, the test was performed after the newborn had been fed. In the case of altered results, an outpatient appointment is made before one month of life to repeat the test (2nd test). If it is normal, the baby is discharged and if the results are altered, an automatic brain response (ABR) is performed (3rd test). If the results are also altered, a referral is made to otorhinolaryngology (ENT) to carry out the diagnostic process and appropriate treatment, if needed [
2].
In the case of neonates with risk factors, the first step is always the performance of ABR (1st test). In the case of altered results, an outpatient appointment is made before one month of life to repeat the test (2nd test). If these also show altered results, a referral is made to ENT for diagnosis and treatment, if needed [
2].
Both techniques are performed bilaterally; if one of the ears is altered, it is considered as an altered test and, therefore, in the next test, both ears will be retested.
2.2. Techniques or Equipment
2.2.1. Transient evoked otoacoustic emissions (TEOAE).
From 2015 to 2018 TEOAE were collected using ECOCHECK OAE Screener® and from January 2019 they were collected using OtoNova Screener®, both are based on the ILO 88 system (Otodynamics Ltd.), together with the ILO ECP® neonatal probe (Otodynamics Ltd). The technique consists of a 1ms stimulus whose intensity is 84±3 decibels (dB) at a frequency of 80 cycles/s. An acceptable result requires a signal greater than 6 dB. above the ambient noise. The results are showed as a pass or fail. Only a result that is a bilateral pass will be accepted as a normal result. [
2,
8]
2.2.2. Automatic Auditory Brainstem Response (ABR)
In the case of ABRs an AccuScreen® MADSEN evoked potentials device (GN Otometrics, Denmark) was used for this purpose till 2018. Since 2019, the OtoNova Screener® device has been used. Three electrodes will be placed (forehead, cheek and neck) and a series of stimulus is performed in each ear at an intensity of 35dB. Threshold is the lowest level at which the wave V can be measured. An impaired test result is considered when this threshold is higher than 35dB. The results are shown as a pass or fail [
9].
2.2.3. Data analysis
Data analysis was performed using an Excel 2010® spreadsheet (Microsoft Co.) and the SPSS 20.0® software platform (IBM Co.).
2.3. Study variables
TEOAE test result in first and second test (normal/altered);
ABR test result (normal/altered);
Hospital of birth (internal/external);
Test in otorhinolaryngology (ENT) (normal/altered);
Intervals in days between the different phases of the screening.
3. Results
The distribution of cases is shown in
Table 1 with total counts.
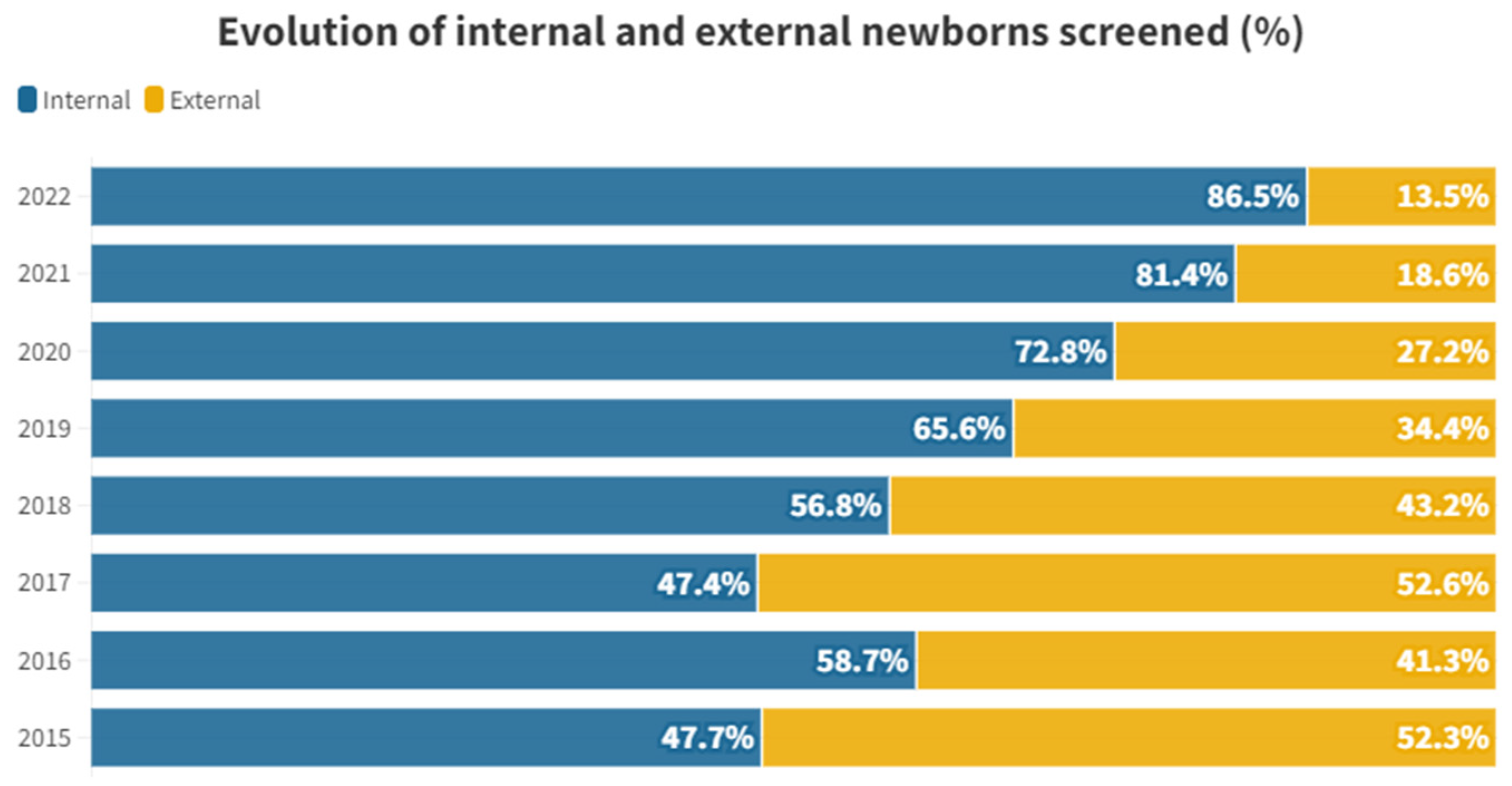
The proportional distribution of each year between internal and external individuals is shown in
Figure 1.
Table 2 shows the mean time in days after birth for both groups and different tests.
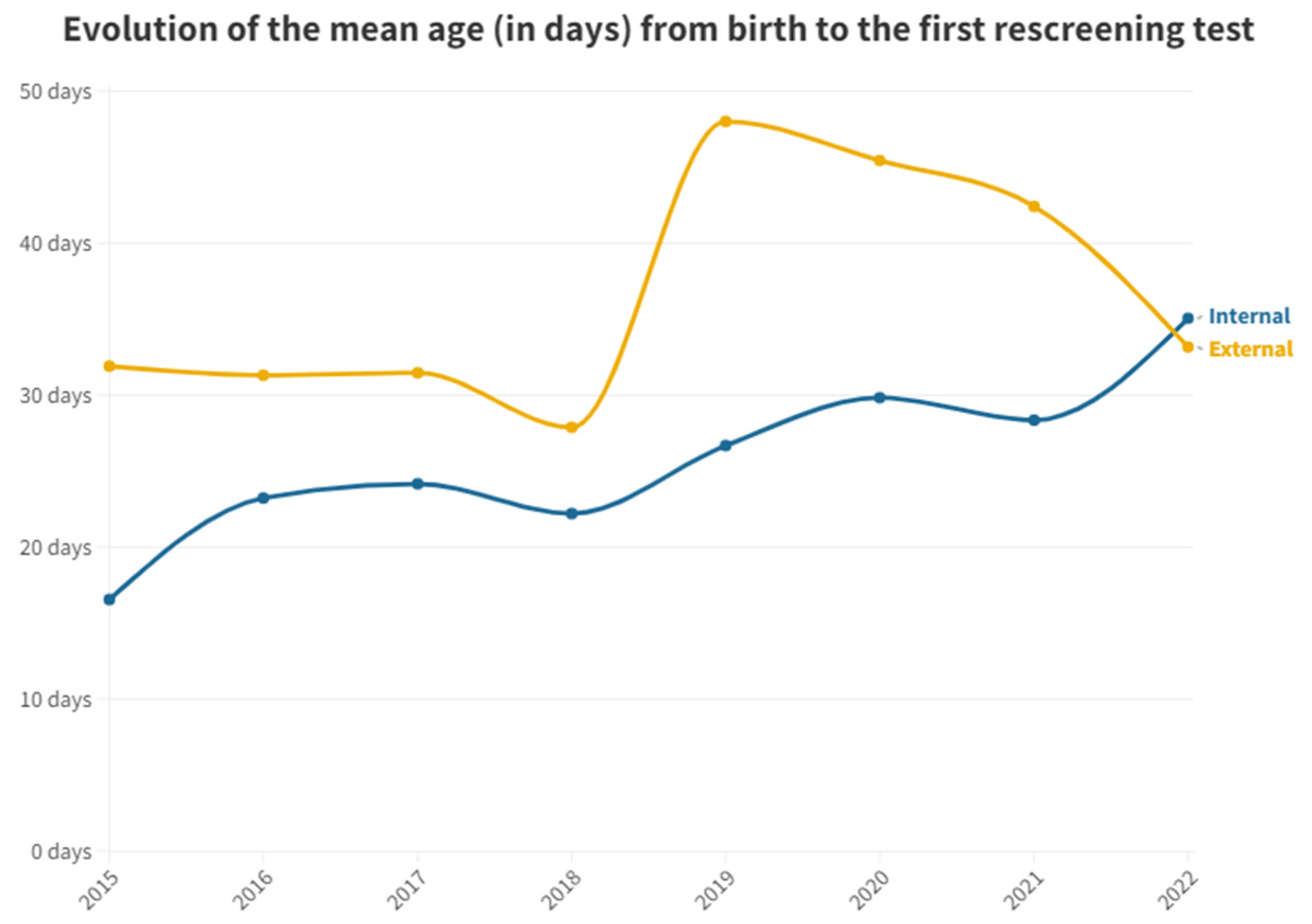
Figure 2 shows the mean time in days after birth for both groups and first outpatient rescreening test.
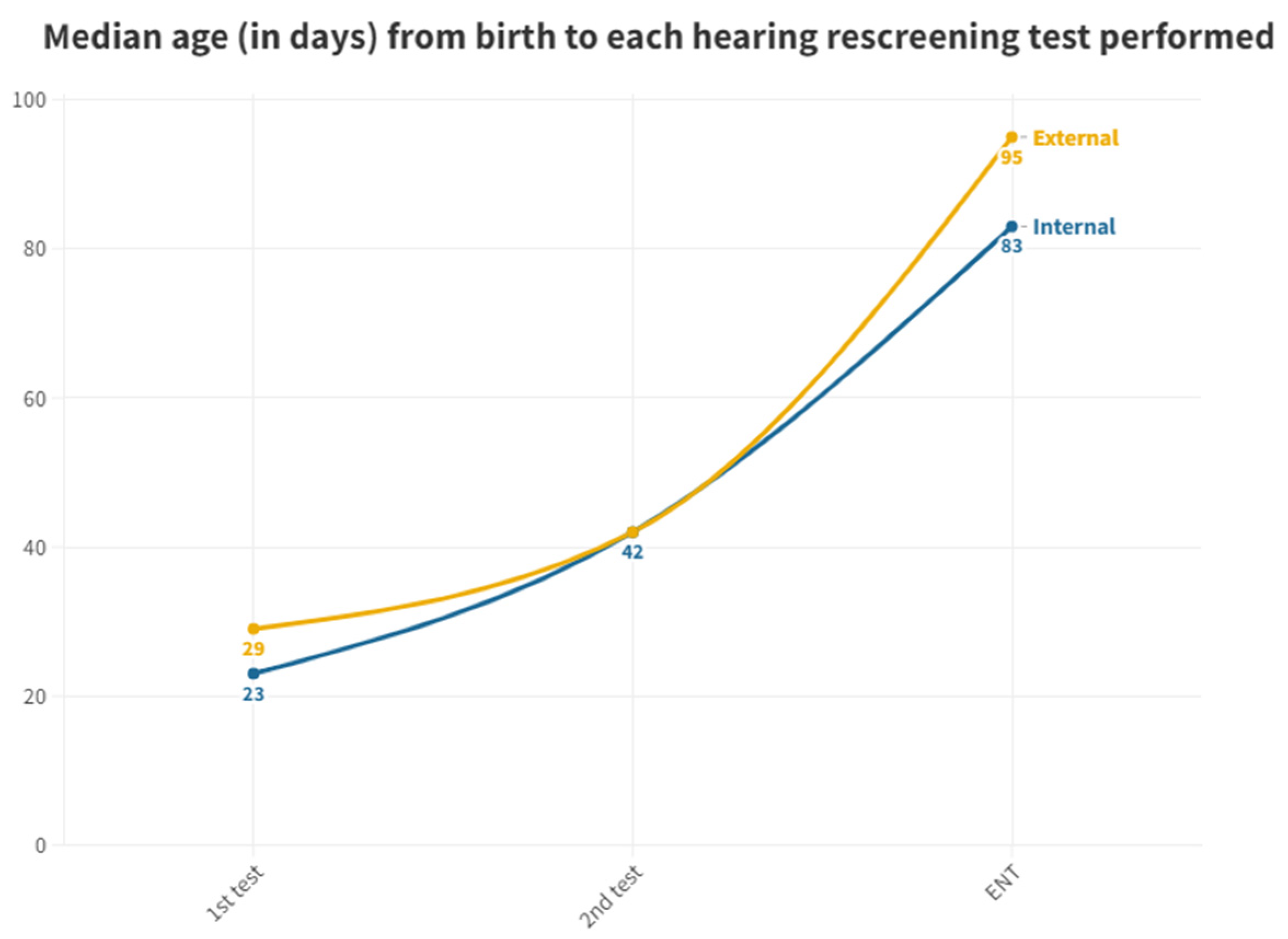
Due to the presence of outliers in screened neonates who take too long to be tested due to very specific circumstances, the
Figure 3 has been performed with the median, as it is a more representative value.
Time elapsed between tests is shown in
Table 3.
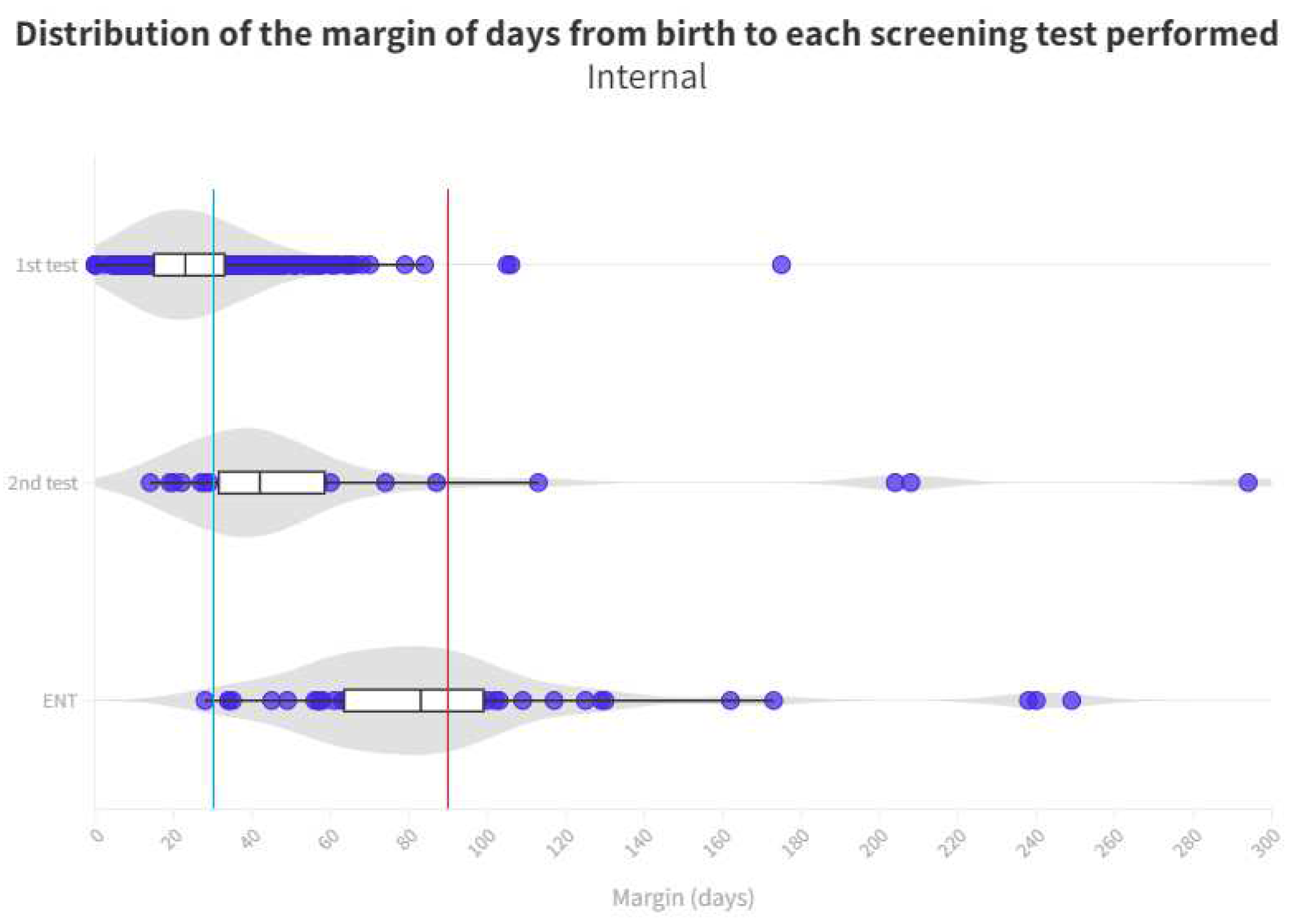
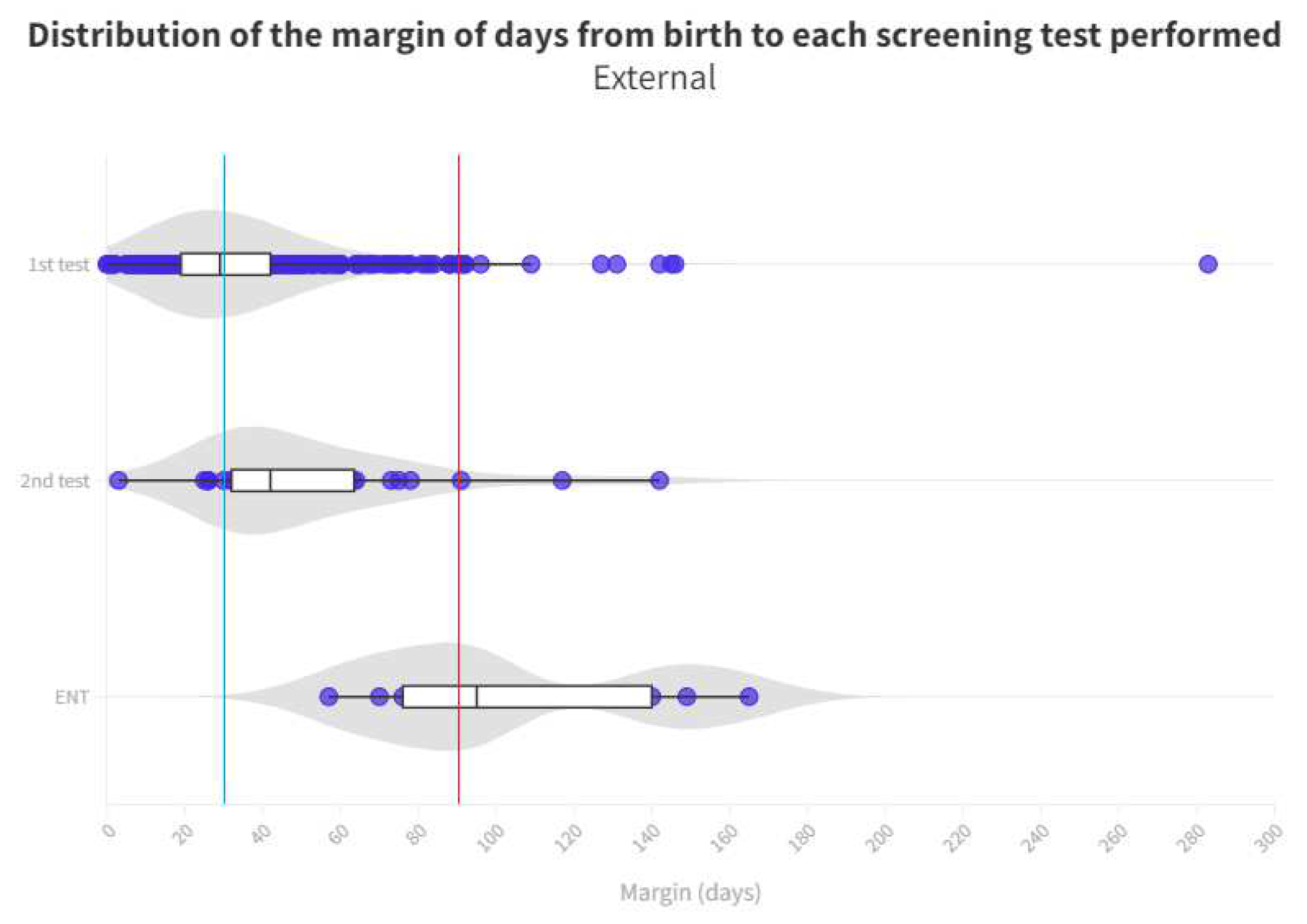
Time distribution for each test is shown in
Figure 4 and
Figure 5. Limit for screening process (1 month) represents the blue line whereas the red line is the limit for ENT diagnosis (3 months).
Cases referred to ENT and results are shown in
Table 4.
Hearing loss cases after ENT evaluation are shown in
Table 5.
Analysis of lost to follow up in both groups by phase is shown in
Table 6.
4. Discussion
Neonatal hearing rescreening is an essential component of the overall screening process that helps to improve the results and ensure accurate identification of hearing loss in infants offering opportunities for early intervention and support, but its development has challenges that we will discuss below.
4.1. Hospital of birth of screened newborns
The hearing loss screening includes both newborns born at the hospital and those born at other hospitals. Between 2015 and 2022, a total of 8563 individuals were born in this hospital. Of these inpatients (“internal”), 690 (8.06%) were referred to outpatient clinic for further hearing impairment screening, either because they failed their first test in the maternity ward before discharge (n=679) or because they were referred directly to outpatient clinic (n=11) because the first test was not performed. There were 440 individuals born in other hospitals (“outpatients/external”), arriving directly to our hospital's outpatient clinic from primary care (
Table 1 and
Figure 1). A total of 1130 individuals were included in the study.
If we study the proportional distribution of each year between internal and external individuals, we can see that there is a certain stability between 2015 and 2018. From 2019 onwards, tests on internal individuals begin to be proportionally higher until reaching the 86.44% in 2022. This has not implied an increase in the internal individuals tested but has been due to a decrease in the number of external newborns that have taken part in the rescreening. For external neonates, a peak is observed in 2017, from which year it begins to decline sharply to only 12 tests performed in 2022 (
Table 1 and
Figure 1).
This is probably due to the publication of decree 218/2018 of November the 30th by the Regional Department of Universal and Public Health [
10]. This decree regulates the screening programs in the territory of the Valencian Community, emphasizing that the first hearing loss screening test must be carried out in the hospital of birth, just before discharge. This caused private hospitals to start performing this test instead of referring it to the outpatient clinic of the public hospitals to which the newborn belongs, greatly reducing the burden in the rescreening process of public hospitals, since while in the years 2015-2018 of the study external screenings stood at an average of 47.35% per year, between 2019 and 2022 it remains at only 23.42% with a clear dynamic of continuing its reduction (
Figure 1). This reflects the importance of having legislation that supports universal screening across the community.
4.2. Timing of rescreening
During the rescreening process, it is very important to comply with the time horizons of each test to ensure a good performance of the program.
In terms of the mean number of days between birth and the first test performed in the inpatient and outpatient groups, there is a large difference between the two groups. While the inpatients by protocol are tested very soon after birth (2.57 days on average), the outpatients take 33.91 days on average to perform their first test at the outpatient clinic (
Table 2). Inpatients who have failed and need to be rescreened (n=679) or did not get tested at maternity ward (n=11), take 25.41 days to get their test done at the outpatient clinic. Thus, inpatients can perform two hearing screening tests before outpatients are able to get their first one.
The median has been used as a test to reduce the effect of outliers from those patients who, due to specific circumstances, have had an abnormal delay in the completion of their tests.
According to the protocol, the first screening test should be performed before discharge from the maternity ward. While 98.41% of neonates born at the hospital have a first test in the maternity ward, all those born in other centers referred to outpatient clinic from primary care did not have this first test performed before discharge. This causes a significant delay, while neonates born in the hospital have a median age of 2 days when the first otoemissions are performed in the neonatal ward, those born outside the hospital do not get their first test until a median of 29 days later and directly in the outpatient department. The first outpatient test of those born in our hospital (from the group of those who got an altered result at maternity or those who had not been tested) is performed at a median of 23 days after birth. So those born outside the hospital take 6 days more to get their first result (
Figure 3).
In the second test performed in outpatient clinics, the medians are equal at 42 days for both groups. This is due to the fact that, because of the delay in the outpatient group, pediatricians try to schedule them as soon as possible to perform a screening test to confirm or rule out the altered result. On the other hand, for the inpatient group, the following visits take longer, and this is the reason for this equality shown (
Figure 3). It is important to highlight the evolution of the time elapsed before the first outpatient test is done in neonates born in this hospital. It has been on an upward trend since data were collected in this study. This indicates an increased pressure on the medical services and a reduced capacity to cope with the workload and show the need of more staff dedicated to hearing screening.
Therefore, it is important for healthcare facilities to prioritize hearing screening for neonates and allocate the necessary resources to ensure timely and effective screening. This may include hiring additional staff, providing ongoing training and education for existing staff, and implementing quality improvement initiatives to streamline the screening process. Early detection and intervention of hearing loss in neonates can have a significant impact on their overall development and quality of life, and as such, should be a priority for all healthcare providers.
As for neonates referred to ENT, there is again a significant difference between those born in the hospital, taking a median of 83 days and those born outside taking a median of 95 days. In the analysis for the ENT consultation, where the definitive diagnosis is given to patients who have failed their tests, there is a margin of 12 days in favor of those born in the hospital, so that they can begin their treatment and adaptation earlier than the other group. We cannot explain this difference because both groups follow the same ENT referral process. It is noteworthy that the third quartile in the outpatient group is 140 days, well above the 90-day limit for ENT process [
11]. At the end, this means that, although more than 50% are on time, there are many infants who do not receive their final result in a timely manner, with the third quartile being 99 days. In the case of those born outside the hospital, the median is already 95 days, above the limit we indicated (
Figure 5).
Adequate and timely rescreening is an essential component of newborn hearing screening to identify hearing loss in newborns. However, there are issues that can become barriers that delay diagnosis and intervention.
Health facilities may not have the staff or resources to perform diagnostic evaluations within the required time limit, leading to delays in diagnosis and intervention. In addition, diagnostic hearing assessments can be complex, requiring specialized equipment and expertise. If the equipment is not correctly calibrated, or if the test is not properly performed, inaccurate results can occur making it necessary to repeat it later.
False positives sometimes occur, indicating a hearing loss in the newborn when in fact it is not. This can be due to a variety of factors such as environmental noise or the presence of debris in the ear canal. It can also be caused by a variety of factors such as an unstable sleep pattern or overly active movement on the part of the newborn that makes it impossible to test accurately. These false positives cause insecurity and anxiety on families, as well as the need for retesting. Too many newborns with false positive results increase the staff pressure and delay. In this situation, the age at which the first test is carried out is essential because the first few days of life are more likely to produce a fail because middle ear effusions affect greatly the TEOAEs.
Some other reasons for this delay are discussed in another studies. Deng [
12] found that delayed diagnosis of hearing loss was associated with maternal education, maternal race/ethnicity, and admission into a neonatal intensive care unit (NICU). Nikolopoulos [
13] found that delayed diagnosis and management, auditory neuropathy, late-onset deafness, and socioeconomic factors were major weaknesses of neonatal hearing screening programs and Chapman [
14] found that the presence of co-occurring birth defects prolonged the time of the initial infant hearing screening, which contributed to further delays in the subsequent diagnosis of hearing loss.
4.3. Quality of care
In a screening where follow-up is so important, quality of care should be considered a relevant indicator to observe how easy it is for families to remain involved in the process. To measure this, we have used the difference between the median number of days between one test and the next to avoid the effect of residual values in exceptional cases. The shorter these periods, the higher the quality of care.
As discussed earlier, according to the results obtained in first test, neonates born in this hospital are tested before those born outside, taking the day of birth as a reference. Those born in other hospitals take up 6 days longer to receive this first result (
Table 3).
From the first to the second test, inpatients take a median of 19 days, considerably increasing the time taken, while outpatients take 13 days, perhaps because they begin the screening later and in them it was more necessary to shorten the time limits and specialists try to fix a new appointment as soon as possible when a significant delay in the first test is noticed. Once outpatients enter the screening process, they end up being scheduled earlier than those born in hospital.
The ENT tests are carried out 41 days after the last test in the case of internal and 53 days in the case of external, either from the second test or if they are sent directly from the first (
Table 3). There is a median delay in the final ENT diagnosis of 12 days for neonates born in other hospitals compared to those born in our maternity ward (
Figure 3). So, the interns again take less than the median number of days of externals to reach this step in order to receive a definitive diagnosis and to start with the adaptation in case of an altered result. This difference in days can be crucial for the development of the newborn and the beginning of treatment. As aforementioned, we have no explanation for this.
It is noteworthy that the years of the COVID pandemic have not significantly altered the course of the screening protocol.
4.4. Results of screening and ENT
From the analysis of ENT final test results among the neonates born in the hospital between the years studied, 58 have needed an appointment in ENT. These 58 patients represent an 8.41% of newborns who entered the rescreening process (n=690) and a 0.68% over every birth in the period studied (n=8563). 5 (8.62%) were lost to follow up (LFU). Among the 53 who got a result, 64.15% (n=34) obtained a normal result, while 35.85% (n=19) were altered, a good result for screening processes. At the end, in the group of those born in our hospital, 97.21% of individuals had normal results and 2.79% failed ones for the whole period. This represents a hearing loss incidence of 2.2‰ over the total births in our hospital for this period, a figure that is in accordance with various studies (
Table 4 and
Table 5).
Only 9 of those born outside the hospital had to go to the ENT center, a 2% of the total rescreened (n=440). There were no missed appointments. As for the tests, 7 of them (77.78%) obtained a normal result and only 2 (22.22%) finally obtained a diagnosis of hearing loss, a result that is not to be underestimated. At the end, in the case of those not born in our hospital, 99.54% of individuals had normal results and 0.46% had abnormal results. Both the trend of normal and abnormal results seems to be maintained over time for both patient groups. However, in the case of outpatients it becomes less relevant from 2018 onwards, as the number of individuals who are screened for the first time in this hospital decreases (
Table 4 and 5).
Regarding the outpatient ENT consultation, where the final diagnosis is given to the patients, the difference between the percentage of referred inpatients and outpatients is remarkable. While 8.41% (n=58) of the rescreened patients born in this hospital reach this stage, only 2% (n=9) of the outpatients do so. The explanation for this can be that outpatients are tested with more days of life and this can improve the response to the first test because a better condition of middle ear leaving less individuals to rescreen and less to refer to ENT (
Table 4 and
Table 5).
Taking these data into account, there is not an excess of neonates reaching the confirmatory test in ENT, as the global rate should be less than 4% and in our case it is 0.68% [
11].
4.5. Losses (Lost to follow up)
Loss to follow-up in hearing screening refers to a situation where a neonate does not complete the recommended follow-up after an initial hearing screening test. Diverse studies [
13] found that the percentage of newborns who fail the initial testing and then are lost to follow-up are major weaknesses of neonatal hearing screening programs.
Rescreening of infants who fail the first test represents a risk of loss to the process, with reported figures of 5-25%. Above 20%, the validity of the program is compromised [
15] [
16].
A known reason for loss to follow-up derives from birth in hospitals that do not screen at birth and therefore these infants must be recruited from primary care for screening which may result in them not entering the screening process or, at best, the time limits set for adequate screening may be easily exceeded (
Figure 4 and
Figure 5)
According to our results, total losses amounted to 13 (1.2%) of the 1130 patients studied (
Table 6). This figure is within the range recommended by CODEPEH, which limits the maximum losses of the process to 5% [
15].
With regard to the losses in each of the tests, the inverse trend in the two groups is noteworthy. At some stage, 1.45% of the inpatients have abandoned the rescreening process. The trend shows that as the test’s phases advance, the percentage of LFU cases also increase. Those born in other hospitals do not attend the first tests to be performed in a similar ratio and have double percentage of LFU on second test but after that, they do not generally miss their future appointments. Only 0.68% of the outpatients tested abandon the screening process (
Table 5).
Of the 58 inborn neonates referred to ENT, there were 5 losses, 8.62% of the patients referred, a percentage considerably higher than the losses in the first test (0.58%) and in the second (1.54%). In contrast, of the 9 patients born outside our hospital who were rescreened and referred to ENT, there were 0 losses in this step.
Some studies suggest that there are several other causes for losing the follow-up in neonatal hearing screening. For example, Luz [
17] found that forgetting the date of the retest and ignorance about the importance of retesting were significant factors for non-compliance with newborn hearing screening retests. Davis [
18] found that poor information systems were a major problem in current UK practice.
Overall, the studies suggest that improvements in information systems, tracking systems, and public awareness are crucial for successful program implementation and reducing lost to follow-up in neonatal hearing screening. Some families may not understand the importance of having these tests or having them on time. They may also be unaware that their child is at risk of hearing loss and the consequences of delaying diagnosis or not coming for testing and missing follow-up. It is therefore crucial for healthcare providers to educate families about the importance of follow-up appointments and monitoring for any potential signs of illness in their newborn, even if the initial screening was normal.
In addition to awareness, there is also a problem of access to testing. If the newborn fails the initial test, it is necessary to continue testing over the following weeks to determine the extent and type of hearing loss, if any. However, some families may find it difficult to access these follow-up tests.
In the case of the analyzed health area, one of the barriers is transport to the hospital, especially if families live in country or remote areas or do not have access to reliable transport. Ours is a health area with a large and widely dispersed population and this is a known factor [
19].
On the other hand, language barriers may prevent families who do not speak the main language of the health facility from understanding the screening process and the instructions received. In the case of the studied health department, 17.17% of the population is immigrant, a 5.53% higher than the national average (11.64%) [
20]. The work-family balance of the caregiver is also relevant, as it could make it difficult to attend check-up appointments.
Several measures are available to address these barriers and ensure that check-ups are carried out on time. Families need to be well informed about the importance of repeat testing if necessary, and be available to answer any questions they may have.
Being able to offer translated material or an interpreting service to families facilitates communication with the center’s staff and ensures that information about the tests is transmitted correctly.
In relation to work-family reconciliation, flexible schedules for testing should be offered, as well as providing transport systems or other resources for families to get to appointments.
It is important to collaborate with community organizations and outreach programs to identify and address these and similar issues that impede proper neonatal screening.
If an infant is lost to follow-up hearing screening, health professionals should make an effort to contact the family and encourage them to make a follow-up appointment. In some cases, outreach programs or community resources can help to connect families with the necessary services [
21].
5. Limitations
This study did not consider the hearing risk factors of the neonates analyzed since, regardless of the protocol followed, the aim of the study was to evaluate the volume and performance of neonatal hearing screening performed in a second level hospital. In addition, the difference between the protocols is that in those with risk factors, the first test is performed as a ABR test, but if the results are altered in the first test, they are also rescreened in outpatient clinics.
Both the results obtained for TEOAE with the Echocheck Screener® and the OtoNova Screener® and the results obtained for ABR with the AccuScreen Madsen® and the OtoNova Screener® are for passing or fail the test and not real values for the amplitude of the response. However, even if this response is significantly lower, it may be of sufficient intensity for neonates to pass the test. For the TEOAE test to be considered impaired, there must be a hearing loss greater than 30 dB HL, and in the case of ABR greater than 35 dB.
The study presented here reflects what was done in a specific hospital and, given the heterogeneity of screening protocols and programs in different hospitals and autonomous communities, it does not represent the current state of affairs at a general level, but many of the problems exposed do affect different protocols, hence its usefulness.
As the techniques used in hearing screening explore a specific frequency range (from 0 to 6 kHz) at an intensity of 30-35 dB, more studies are needed to explore other frequency ranges or intensities that could be affected, but which are not detected with these devices and therefore are not referred for rescreening.
Passing or failing the TEOAE test reflects the hearing status of the neonate; however, although the signal emitted is directly related to the inner ear and cochlea, the middle and outer ear also play a role, as the signal travels through them and therefore a disturbance in these would also be reflected in the OAE test. There are differences in the number of false-positive screening results depending on the test performed, being lower in ABRs than in TEOAE, which is the technique mostly used in this study.
The children referred from other hospitals may not be all eligible since we do not know their overall number or the number of tests performed or those lost to follow-up on first test.
6. Conclusions
Hearing screening in a second-level hospital is difficult because of the staffing and time constraints inherent to medium-sized hospitals. This results in longer than recommended turnaround times and interferes with the timely detection of hearing loss. Strong support from the administration is needed for the adequate provision of any screening program because of its potential cost-saving capacity.
Screening times vary from patient to patient due to several factors, but one of the most important is the newborn’s birth hospital. Late referral of outpatients from primary care to screen makes it mandatory to perform the first test and rescreening in their birth hospital or, alternatively, the creation of a shortened circuit for outpatients from primary care or direct referral from the birth hospital to the reference hospital.
The delay of rescreen process is based in the fact that the appointments are usually made around one month after birth (the time limit for first step hearing screening), so that unforeseen events and repetitions lead easily to exceed the recommended screening times. If an appointment were made within 15 days of life for rescreening, it would have time to do additional tests before one-month limit, if necessary.
Referral of children with impaired screening to out-of-town ENT referral centers leads to unacceptable loss to follow-up in these high-risk children.
A legislative support for all these rescreening issues is absolutely necessary.
Author Contributions
Conceptualization, J.M.S.C., M.G.D.; methodology and formal analysis, A.L.M., V.A.C., J.M.S.C., A.M.S.; writing—original draft preparation and editing, A.L.M., V.A.C., J.M.S.C.; reviewing and editing, A.M.S., J.M.S.S., J.M.S.C., M.G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
This study could not have been conducted without the collaboration of the Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana (FISABIO) through the EPRIEX2022 program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Trinidad-Ramos, G.; de Aguilar, V.A.; Jaudenes-Casaubón, C.; Núñez-Batalla, F.; Sequí-Canet, J.M. Early hearing detection and intervention: 2010 CODEPEH recommendation. Acta Otorrinolaringol Esp 2010, 61(1), 69-77. https://doi.org/10.1016/j.otorri.2009.09.008. [CrossRef]
- Sequi Canet, J.M.; Sala Langa, M.J.; Collar Del Castillo, J.I. Análisis crítico de una década de cribado neonatal de hipoacusia en un hospital comarcal [Results from ten years newborn hearing screening in a secondary hospital]. An Pediatr 2016, 85(4), 189-196. https://doi.org/10.1016/j.anpedi.2015.11.00. [CrossRef]
- Bussé, A.M.L.; Mackey, A.R.; Hoeve, H.L.J.; Goedegebure, A.; Carr, G.; Uhlén, I.M.; Simonsz, H.J. Assessment of hearing screening programmes across 47 countries or regions I: provision of newborn hearing screening. International journal of audiology 2021, 60(11), 821-830. https://doi.org/10.1080/14992027.2021.1886350. [CrossRef]
- Kanji, A.; Khoza-Shangase, K.; Moroe, N. Newborn hearing screening protocols and their outcomes: A systematic review. International journal of pediatric otorhinolaryngology 2018, 115, 104-109. https://doi.org/10.1016/j.ijporl.2018.09.026. [CrossRef]
- Organización Panamericana de la Salud. Informe mundial sobre la audición 2021. https://doi.org/10.37774/9789275324677. [CrossRef]
- Núñez-Batalla, F.; Jaudenes-Casaubón, C.; Sequí-Canet, J.M.; Vivanco-Allende, A.; Zubicaray-Ugarteche, J. Actualización de los programas de detección precoz de la sordera infantil: recomendaciones CODEPEH 2018 (Nivel 1 Detección). Revista Española de Discapacidad 2019, 7 (I), 201-220.
- Trinidad, G.; Jaudenes-Casaubón, C. Sordera Infantil. Del diagnóstico precoz a la inclusión educativa. Guía práctica para el abordaje interdisciplinar (2ª Ed.). Madrid, Confederación Española de Familias de Personas Sordas-FIAPAS (2012). http://www.fiapas.es/sordera-infantil-del-diagnostico-precoz-la-inclusion-educativa.
- Kemp D.T.; Ryan S.; Bray P. A guide to the effective use of otoacoustic emissions. Ear Hear 1990, 11(2), 93-105. https://doi.org/10.1097/00003446-199004000-00004. [CrossRef]
- Shang, Y.; Hao, W.; Gao, Z.; Xu, C.; Ru, Y.; Ni, D. An effective compromise between cost and referral rate: A sequential hearing screening protocol using TEOAEs and AABRs for healthy newborns. Int J Pediatr Otorhinolaryngol 2016, 91, 141–5. https://doi.org/10.1016/j.ijporl.2016.10.025. [CrossRef]
- Generalitat Valenciana. Decreto 218/2018, de 30 de Noviembre, del Consell, por el que se regulan los programas de cribados neonatales en la Comunitat Valenciana, detección precoz de la hipoacusia neonatal y cribado neonatal de enfermedades congénitas. DOGV: Valencia, España, 2018. https://dogv.gva.es/es/eli/es-vc/d/2018/11/30/218/.
- Benito-Orejas, J.I.; Poncela-Blanco, M.; García-Vicario, F.; Benito-González, F.; Martín-Sigüenza, G.; San Román-Carbajo, J. ¿Es fácil encargarse de coordinar un programa de hipoacusia infantil? Revista ORL 2016, 7(2), 77–90. https://doi.org/10.14201/orl.14237. [CrossRef]
- Deng, X.; Ema, S.; Mason, C.; Nash, A.; Carbone, E.; Gaffney, M. Receipt and Timeliness of Newborn Hearing Screening and Diagnostic Services Among Babies Born in 2017 in 9 States. J Public Health Manag Pract 2022, 28(1), E100-E108. https://doi.org/10.1097/PHH.0000000000001232. [CrossRef]
- Nikolopoulos, T.P. Neonatal hearing screening: what we have achieved and what needs to be improved. Int J Pediatr Otorhinolaryngol 2015, 79(5), 635-7. https://doi.org/10.1016/j.ijporl.2015.02.010. [CrossRef]
- Chapman, D.A.; Stampfel, C.C.; Bodurtha, J.N.; Dodson, K.M.; Pandya, A.; Lynch, K.B.; Kirby, R.S. Impact of co-occurring birth defects on the timing of newborn hearing screening and diagnosis. Am J Audiol 2011, 20(2), 132-9. https://doi.org/10.1044/1059-0889(2011/10-0049). [CrossRef]
- Núñez-Batalla, F.; Jáudenes-Casaubón, C.; Sequí-Canet, J.M.; Vivanco-Allende A.; Zubicaray-Ugarteche J.; Olleta Lascarro I. Programas de cribado de la hipoacusia congénita en 2020: recomendaciones CODEPEH. Acta Otorrinolaringol Esp 2020, 72(5), 312-323. doi: 10.1016/j.otorri.2020.06.009. [CrossRef]
- Sequi-Canet JM.; Brines-Solanes J. Keypoints to Successful Newborn Hearing Screening. Thirty Years of Experience and Innovations. Healthcare (Basel). 2021, Oct 25;9(11),1436. https://doi.org/10.3390/healthcare9111436. [CrossRef]
- Luz, I.; Ribas, A.; Kozlowski, L.; Willig, M.; Berberian, A.P. Newborn Hearing Screening in a Public Maternity Ward in Curitiba, Brazil: Determining Factors for Not Retesting. Int Arch Otorhinolaryngol 2016, 20(4), 300-304. https://doi.org/10.1055/s-0035-1567866. [CrossRef]
- Davis, A.; Bamford, J.; Wilson, I.; Ramkalawan, T.; Forshaw, M.; Wright, S. A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment. Health Technol Assess 1997, 1(10), 1-176.
- Generalitat Valenciana. Decreto 205/2018, de 16 de Noviembre, del Consell, por el que aprueba el mapa sanitario de la Comunitat Valenciana. DOGV: Valencia, España, 2018. https://dogv.gva.es/portal/es/eli/es-vc/d/2018/11/16/205.
- INEbase [Internet]. Madrid: Instituto Nacional de Estadística; 2022. Población extranjera por Nacionalidad, comunidades, Sexo y Año [Comunidad Valenciana, Ambos sexos, 2022]; https://www.ine.es/jaxi/Datos.htm?path=/t20/e245/p08/l0/&file=02005.px.
- Findlen, UM.; Davenport CA.; Cadieux J.; Gehred A.; Frush Holt R.; Vaughn LM.; Houston D.; Hunter LL. Barriers to and Facilitators of Early Hearing Detection and Intervention in the United States: A Systematic Review. Ear Hear. 2023, May-Jun, 01,44(3),448-459. https://doi.org/10.1097/AUD.0000000000001312. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).