1. Introduction
The emergence of a new coronavirus in 2019, originating in the Chinese city of Wuhan and affecting the global population, was classified as a pandemic by the WHO on March 11, 2020. Such virus was named SARS-CoV-2 as it clinically presents as acute respiratory syndrome, causing cough, fever, and fatigue in infected individuals, and is transmitted through the respiratory route [
1]. The Covid-19 pandemic quickly became a global concern, affecting human and animal health, as well as the economies of countries, particularly emerging ones [
2,
3,
4,
5,
6].
SARS-CoV-2 is a single-stranded RNA virus belonging to the family Coronaviridae, and it is the seventh virus in the family capable of infecting humans [
2,
3]. Regarding its origin, it is believed that the virus has a zoonotic origin, given its similarity to other bat SARS-CoV and MERS-CoV viruses, and has subsequently been transmitted to humans through a spillover event followed by adaptation, or via possible intermediate mammalian hosts such as pangolins, cats, ferrets, or minks [
1,
5,
7].
The entry pathway of the virus into human cells occurs through the interaction between the virus's receptor-binding domain (RBD) and the angiotensin-converting enzyme 2 (ACE2) receptor, which has orthologous genes in other mammals [
1,
8,
9]. Since the S1 subunit and the RBD are the major sources of genetic variability in the virus, their genetic plasticity may allow SARS-CoV-2 to initially bind and subsequently adapt to the ACE2 receptor of various animal species, increasing the likelihood of the virus crossing the species barrier [
10,
11].
In addition to humans, several cases of animal infection with SARS-CoV-2 have been reported in various countries since the beginning of the pandemic in 2020, affecting domestic, wild, zoo, and production animals [
12,
13,
14,
15,
16,
17,
18]. At least 32 animal species in 32 countries have been infected by the virus [
11,
19]. The first confirmed cases occurred in domestic animals that had contact with humans infected with SARS-CoV-2, sparking debates about the virus's transmissibility from animals [
4]. According to studies by Shi et al., 2020, dogs were asymptomatic for respiratory symptoms of the disease, indicating inefficient viral replication in their bodies and thus showing low susceptibility to infection. On the other hand, cats affected by the disease showed respiratory symptoms and the ability to transmit the virus through their airways, suggesting high replication, transmission, and seroconversion [
3,
12]. As for production animals, studies indicate that cattle, chickens, and ducks have little to no susceptibility to the SARS-CoV-2 virus [
4,
8,
20,
21,
22].
In the last three decades, the emergence of zoonotic diseases has become an increasing risk, with outbreaks of Ebola, avian influenza, and the SARS and MERS coronavirus syndromes. The prevention strategies for these diseases are difficult to implement, as they involve various factors such as viral genetic evolution, demographic changes, interactions between wild, domestic, and human animals, as well as environmental and climatic conditions of the ecosystem [
23]. According to the editorial "Emerging Zoonoses: A One Health Challenge", the One Health approach is necessary to prevent the emergence of new zoonotic diseases. This approach promotes the interdependence of human, animal, and environmental health, involving multidisciplinary strategies such as the implementation of programs, monitoring policies, and research that can communicate with each other at the academic and public health levels [
24].
With the scarcity of research and articles on the transmission and effects of SARS-CoV-2 infection in animals, it is essential that new studies be conducted to understand their susceptibility to contamination. Some species may act as natural reservoirs of the virus, promoting its continuous replication and mutation. Consequently, this can interfere with the transmissibility, pathogenicity, and adaptation of the virus, as well as potentially compromising the efficacy of vaccines already administered against the novel coronavirus in humans [
6].
Therefore, this present study gathered information regarding this issue, having obtained approval from the Ministry of Agriculture, Livestock, and Supply for the importation of the direct ELISA kit for SARS-CoV-2 from the kit manufacturer. The general objective of this project is to conduct a serological survey in different species of animals, whether they are domestic or wild, in order to detect the presence of antibodies for SARS-CoV-2 and thus demonstrate the infection of animals by the novel coronavirus.
2. Materials and Methods
It is of great importance to report that the analyses conducted in this study were approved by the Ethics Committee for Animal Use (CEUA-IB) under protocol number 181/22. It is worth noting that this project is in accordance with the Ethical Principles in Animal Experimentation adopted by the Brazilian Society of Science in Laboratory Animals (SBCAL/COBEA), the National Council for the Control of Animal Experimentation (CONCEA), and the Brazilian Guideline for the Care and Use of Animals for Scientific and Educational Purposes (DBCA).
2.1. Sampling
For the laboratory analysis, a total of 950 animal serum samples were used. Among these samples, 886 are related to domestic animals, including bovine sera (n=367), canine sera (n=38) , and equine sera (n=481). The remaining 64 samples pertain to the portion of wild animals, with sera from tapirs (n=27) and from bats (n=37).
The bovine samples were taken from the sample bank of the Laboratory of Bovid Viruses at the Biological Institute of São Paulo, where the sera were stored under refrigeration and were previously used for routine examinations of these animals. These samples were randomly chosen and encompass sera from animals collected between 2020 and 2022, without distinction of sex and age. Regarding the canine and equine samples, they were collected from the kennel and cavalry of the Military Police of São Paulo by qualified veterinarians, between 2022 and 2023. Finally, the tapir samples were collected in August 2022 in the state of Mato Grosso do Sul, and the bat samples were collected in São Paulo in July 2022. All samples were then refrigerated and transported to the Laboratory of Bovine Viral Diseases at the Biological Institute of São Paulo for further laboratory analysis.
As the analyses were being conducted, additional sample collections were requested from animals belonging to the Military Police in order to monitor the presence of anti-SARS-CoV-2 antibodies. Therefore, the final number of analyses exceeded the number of animals from which sera were collected.
2.2. ELISA Test
The laboratorial analysis was conducted using the "ID Screen® SARS-CoV-2 Double Antigen Multi-Species" ELISA kit from IDVet®. This type of test is used to detect the presence of antibodies in serum or whole blood samples, where target antigens are adsorbed onto a plate with wells and samples are subsequently added for antibody screening [
25]. The specific kit used in this study has demonstrated a specificity of 99.1% and high sensitivity, as reported in previous studies [
21].
The antigen present on the plate is the purified recombinant N protein from the nucleocapsid of the SARS-CoV-2 virus. A volume of 25µL of animal serum was pipetted into each well, except for the first four wells, which were filled with 2 negative controls and 2 positive controls, respectively. The antigen-sample incubation time is 45 minutes ±5 minutes at 37ºC ± 2ºC. After 5 washes and drying of the plate, the conjugate solution was added, which contains anti-nucleoprotein and the peroxidase enzyme HRP, with a plate incubation time of 30 minutes ±3 minutes at 21°C ± 5°C. After this period, the plate was subjected to another 5 washes and dried again before adding the substrate solution, with an incubation time of 20 minutes ±2 minutes at 21°C ±5°C and in the absence of light. This step is responsible for the appearance of a blue color in samples positive for the presence of anti-SARS-CoV-2 antibodies. Then, the stop solution was added, which changed the color of the samples from blue to yellow due to the difference in pH. Samples that exhibit a yellow color indicate the presence of anti-SARS-CoV-2 antibodies. Finally, the spectrophotometric optical density was read at 450nm using a spectrophotometer to estimate the concentration of anti-SARS-CoV-2 antibodies in the sample.
2.1.1. Test Validation
Regarding the validation of the kit controls, for the positive control, the average value of its spectrophotometric reading should be greater than 0.35, and the ratio of the average value of the spectrophotometric reading of the positive control to the negative control should be greater than 3.
For the samples, the S/P% (sample to positive control ratio) of each one should be calculated using the formula:
where "ODsample" is the optical density of the sample, "ODnc" is the optical density of the negative control, and "ODpc" is the optical density of the positive control.
Samples with an S/P% value greater than or equal to 60 were considered reactive, while samples with a value less than or equal to 50 were considered non-reactive. Inconclusive samples were those that resulted in an S/P% value between 50 and 60, requiring confirmatory analysis through tests that are not performed in Brazil (viral neutralization and indirect immunofluorescence).
At the end of the laboratory analysis, the results were transferred to an Excel spreadsheet for evaluation in different domestic and wild animal species for statistical analysis.
Animals that tested positive in the ELISA had their serological analysis repeated in triplicate to confirm the initially observed result.
3. Results
3.1. Total Seroprevalence
After the laboratory analysis, 13 reactive animals were found. Among them, 1 canine, 7 equines, 4 bovines, and 1 bat. The serological samples from tapirs all resulted in non-reactive. Thus, out of the 950 tested samples, the overall seroprevalence rate was 1.37% (13/950). Regarding each species, the seroprevalence rates were 2.63%, 1.46%, 1.09%, 0%, and 2.70% for canines, equines, bovines, tapirs, and bats, respectively. The results can be found in
Table 1.
As the results were obtained, new samples were collected from the seropositive animals to monitor the antibody levels over time in these animals.
3.2. Bovine Seroprevalence
In order to study the relationship between respiratory symptoms and SARS-CoV-2 infection, samples from bovines that presented respiratory symptoms at the time of collection were analyzed and compared to the seroprevalence found in samples from healthy animals at the time of collection. Two seropositive animals for anti-SARS-CoV-2 antibodies were found in the first type of sample, and two seropositive animals were found in the second type. The results can be found in
Table 2.
For the animals with respiratory symptoms, the average O.D. and S/P% values were 2.06 (261.1%) for the first positive sample and 0.72 (122.2%) for the second positive sample, respectively. Regarding the animals that did not present respiratory symptoms, the average O.D. and S/P% values of the first positive sample were 0.45 (61.23%), and for the second positive sample, the average O.D. was 1.01 (175.15%).
3.3. Equine Seroprevalence
Among the samples from equines, 481 samples were tested, resulting in 7 (1.45%) seropositive animals. Out of these 7 animals, only 6 could be monitored for the persistence of anti-SARS-CoV-2 antibodies over time.
3.3.1. Equine Monitoring
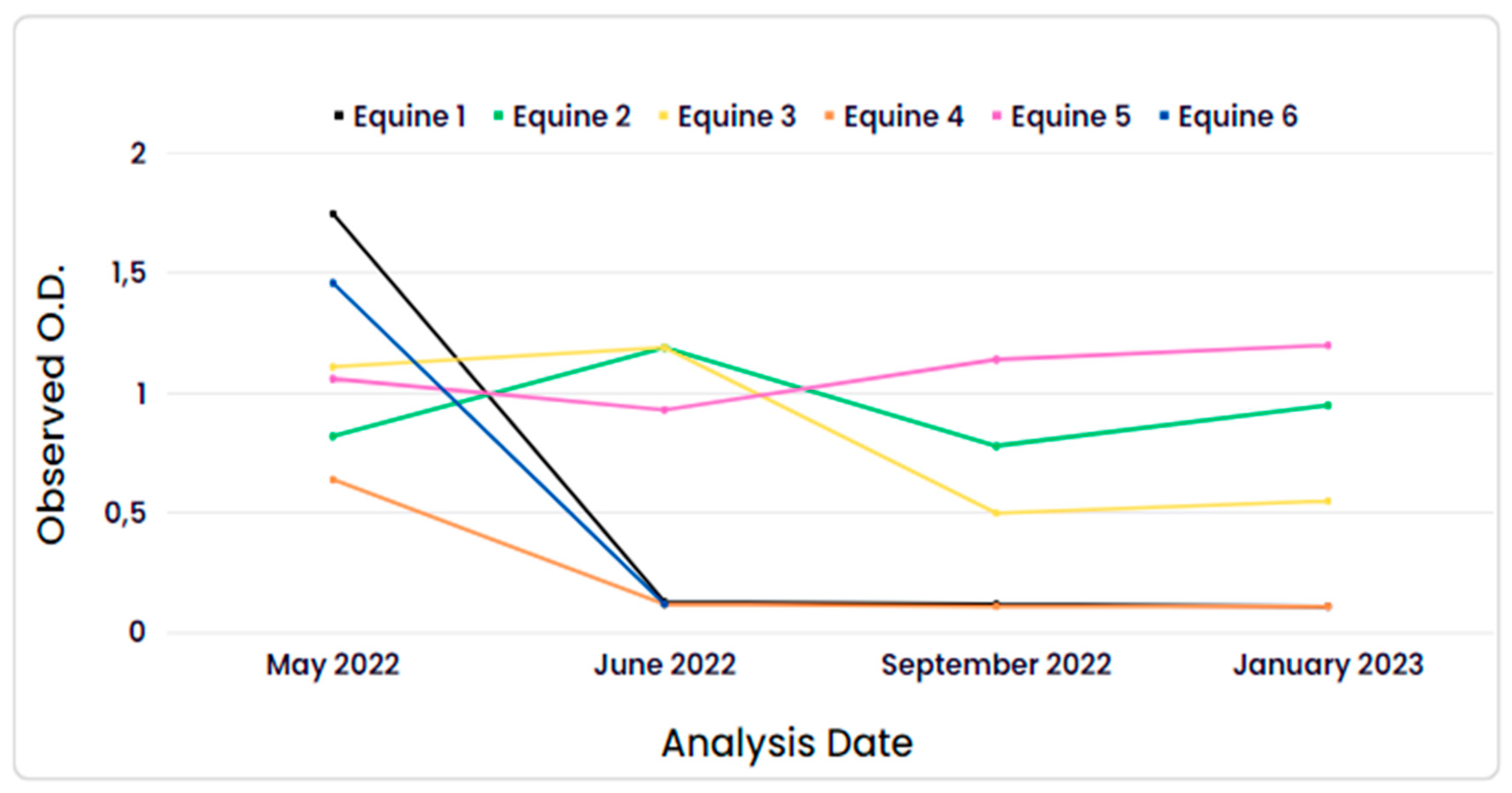
Regarding the monitored horses, the first collection was conducted on May 2022, the second in June 2022, the third in September 2022, and the last in January 2023. The data can be observed in
Figure 1. The mean observed O.D. (optical density) of the equine samples and their respective S/P% (sample-to-positive percentage) in the first analysis were 1.75 (151.1%), 0.82 (60.67%), 1.11 (88.69%), 0.64 (68.76%), 1.06 (126.22%), and 1.46 (178.29%) for equines 1, 2, 3, 4, 5, and 6, respectively. In the fourth analysis, the mean observed O.D. was 0.11 (-1.22%), 0.95 (101.8%), 0.55 (52.76%), 0.11 (-1.20%), and 1.20 (132.51%) for equines 1, 2, 3, 4, and 5, respectively. Equine 6 was not monitored in January 2023 due to it was transferred to another unit of the Military Police.
3.4. Canine Seroprevalence
Out of the 38 analyzed canine samples, only one tested positive, and its
analysis was repeated in triplicate for result confirmation.
3.4.1. Canine Monitoring
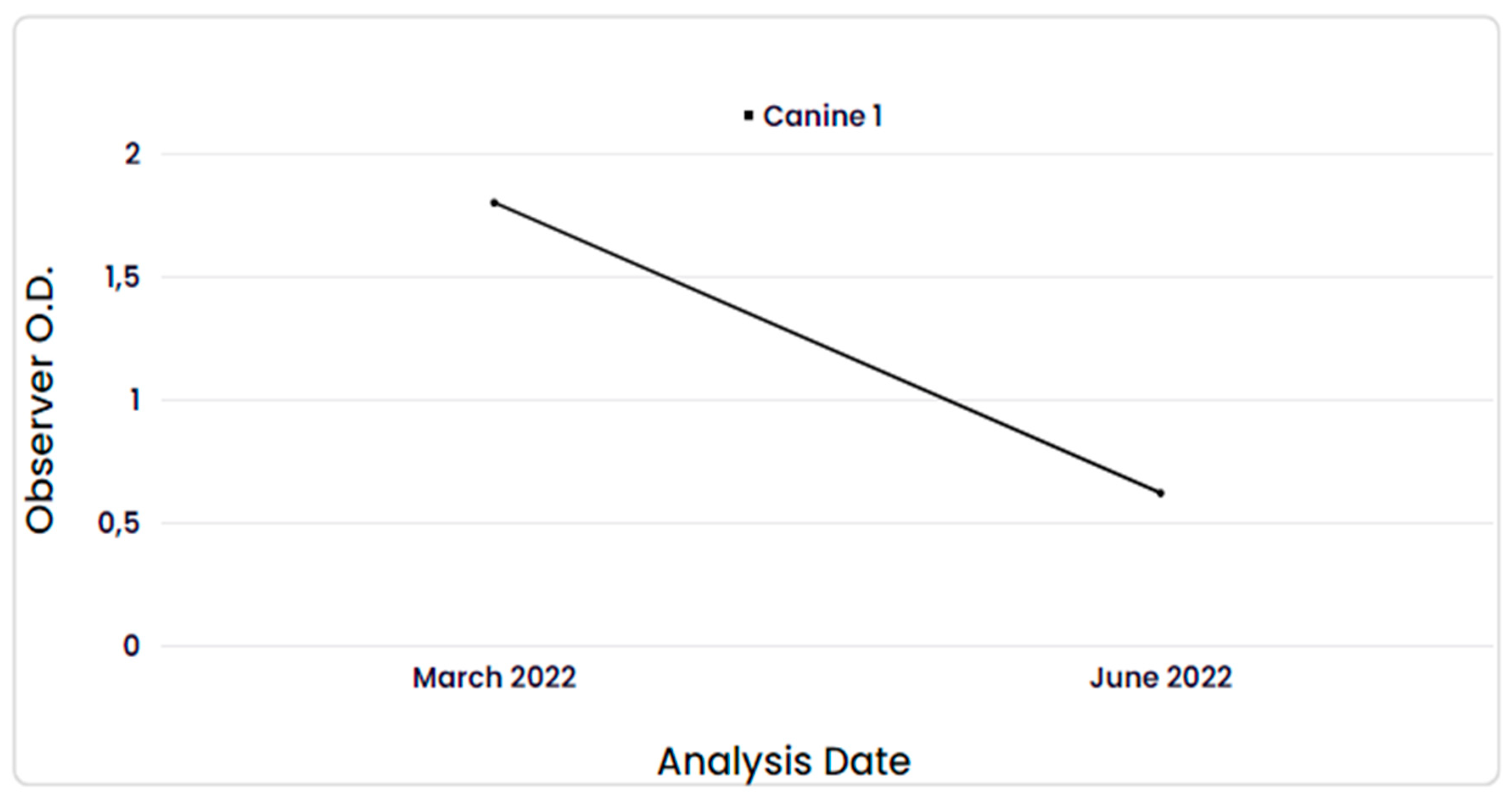
The dog that tested positive for anti-SARS-CoV-2 antibodies had only one additional sample collected, one month after the first one, and the result was negative, as shown in
Figure 2. The mean observed O.D. values after repetition in triplicate, along with their respective S/P% values, were 1.80 (228.80%) in the first collection and 0.62 (52.6%) in the second collection.
3.5. Tapir (Tapirus terrestris) and Bat Seroprevalence
Among the 27 tapir samples analyzed, no reactive animals for COVID-19 were found. The mean observed O.D. values ranged from 0.12 to 0.26, with a maximum S/P% ratio of 16.81.
Regarding the bats, one animal was identified with antibodies against SARS-CoV-2, which exhibited a mean O.D. value and its respective S/P% ratio of 0.615 (64.1%).
4. Discussion
4.1. Total Seroprevalence
The present study in domestic and wild animals covered the period of the pandemic from 2020 to 2023, represented by the oscillation of SARS-CoV-2 waves in humans in the state of São Paulo and the different variants detected over time.
This project identified the ability of SARS-CoV-2 to infect different animal species, whether domestic or wild, with or without clinical symptoms compatible with respiratory diseases.
The overall seroprevalence of anti-SARS-CoV-2 antibodies in domestic animals was significantly higher than that observed in wild animals. According to previous studies, the main mode of transmission of SARS-CoV-2 to animals is close contact with humans [
26,
27,
28,
29]. Therefore, as of June 2023, Brazil has reported more than 37 million cases of COVID-19, according to WHO data. With the high prevalence of cases among humans, the human-to-animal transmission of the virus is likely to be proportionally increased [
26,
27,
28,
29].
It is worth noting that the horses and dogs tested, as they are animals belonging to the Military Police of São Paulo, are responsible for patrolling rounds and have intense contact with other humans and police officers themselves, which may explain the higher prevalence of antibodies among them compared to other domestic animals such as cattle, for example.
4.2. Bovine Seroprevalence
The absence of severe symptoms related to COVID-19 in cattle can be explained by the immunological cross-protection that possibly occurs between SARS-CoV-2 and bovine coronavirus, due to the high homology observed between the spike protein epitopes of these two viruses, given that the latter already affects cattle [
30]. In the present study, we did not obtain information on whether the animals received a vaccine for bovine coronavirus or if there would be cross-reactivity in the SARS-CoV-2 ELISA test.
The seroprevalence found in cattle in this study was relatively higher than other serological findings in the literature. According to Wernike et al., the seroprevalence found in cattle analyzed from different farms was 1.1% (11/1000) [
31].
4.3. Equine Seroprevalence
Although few cases of SARS-CoV-2 infection in horses have been reported in previous research, studies indicate that the equine ACE2 receptor (eqACE2) shows affinity for the virus's RBD [
32,
33].
Previous studies have not detected SARS-CoV-2 in horses through RT-PCR, but they have shown the presence of anti-SARS-CoV-2 antibodies in these animals, indicating a possible absence of virus transmission by horses but probable infection [
34].
During the longitudinal analyses of the research, it was observed that 3 horses (equine 1, equine 4, equine 6) showed a decline in antibodies as early as the second collection, with an optical density (O.D.) below 0.12, becoming then no reactives. However, one animal (equine 3) tested positive in the first two analyses, but in the last two, its O.D. was at the cutoff point of the test (~0.5), resulting in an inconclusive result according to the test validation criteria. This may indicate that the antibody levels were already decreasing. Another 2 animals (equine 2 and equine 5) showed a seroconversion interval during the total monitoring period, with anti-SARS-CoV-2 antibodies detected up to 8 months after the first detection of these antibodies through ELISA analysis, with an O.D. greater than 0.95. There have been reports of a seroconversion interval of anti-SARS-CoV-2 antibodies in horses lasting at least 21 days [
33]. However, in this project, the obtained result is consistent with findings from longitudinal studies of neutralizing antibodies against SARS-CoV-2 in humans, which can last up to 10 months [
35].
4.4. Canine Seroprevalence
Similarly to what happens with cattle, the high genetic similarity between SARS-CoV-2 and canine respiratory coronavirus (CRCoV) may provide some immunological protection to dogs, preventing them from developing severe forms of COVID-19 [
30].
The humoral response in COVID-19 seropositive dogs was considered short-lived, as it showed a low antibody O.D. (0.62) in the month following the first collection (1.80). This finding contrasts with the results observed by Decaro et al., 2022, who found antibodies in the serum of dogs for at least 10 months after contact with the SARS-CoV-2 virus. In that study, the animals were monitored shortly after exposure and infection with the virus, confirmed by RT-PCR [
36].
The result found in this project may be due to the seropositive animal being exposed to the virus at some point long before the start of the collections or the possible fact that dogs receive the vaccine against canine coronavirus, which can provide cross-protection and, consequently, a more efficient response to SARS-CoV-2 infection. It is worth noting that the animal did not show any respiratory or enteric symptoms during the study period. Further studies should be conducted to evaluate these issues.
4.5. Tapir and Bat Seroprevalence
The results obtained from serological analysis of samples from wild animals indicate a low prevalence of anti-SARS-CoV-2 antibodies in these animals. Since it is believed that SARS-CoV-2 infection occurs after contact with infected humans, the lower prevalence of virus antibodies observed in wild animals can be explained by the fact that these animals have little or no contact with humans.
Anti-SARS-CoV-2 antibodies have been found in other species of wild animals, according to previous articles, but it is believed that these animals may have become infected while foraging in areas close to human contact [
37].
This is also one of the hypotheses used to explain the seropositive bat result, as the region where the sample was collected was not far from anthropic activity, suggesting possible direct or indirect contact with humans. However, tracking of wild animals is very challenging. The second hypothesis used is the possibility of cross-reaction between the antigen used in the ELISA test (nucleocapsid protein) and other naturally occurring SARS or MERS coronaviruses in bats, as they exhibit high genetic similarity.
5. Conclusions
This assay demonstrated that dogs, horses, cattle, and bats are susceptible to the SARS-CoV-2 virus, as evidenced by the identification of animals with anti-SARS-CoV-2 antibodies. A higher prevalence of the disease was observed in domestic animals compared to wild animals, likely due to the closer contact of domestic animals with humans, who are the primary targets and transmitters of the virus.
Furthermore, the findings regarding the seroconversion interval observed in horses can be compared to that observed in humans.
However, this project has certain limitations, such as the need for confirmatory tests for reactive animals, such as virus neutralization and indirect immunofluorescence. Additionally, there is a possibility of cross-reaction between the antigen used in the ELISA test and genetically similar types of animal coronaviruses. It is also necessary to evaluate cross-protection in vaccinated animals against canine and bovine coronaviruses.
This research has contributed to sharing new information about animal infection with SARS-CoV-2, aiming to better understand the susceptibility of animals to the virus and to prevent them from acting as natural reservoirs of the virus. It also seeks to prevent the emergence of new zoonotic diseases, following the principles of the One Health Approach.
Author Contributions
Conceptualization, L. H. Okuda; methodology, J. M. Dias, A. H. C. N. Romaldini; software, A. H. C. N. Romaldini; validation, A. H. C. N. Romaldini and M. O. Costa; formal analysis, A. H. C. N. Romaldini and L. H. Okuda.; investigation J. M. Dias, resources, A. O. de Souza, M. L. Faria, D. H. Golcman, M. B. Selim, P. Medici, G. S. Haga L. H. Okuda; data curation, J. M. Dias; writing—original draft preparation, L. H. Okuda and J. M. Dias; writing—review and editing, J. M. Dias, A. O. de Souza, D. H. Golcman, P. Medici, G. S. Haga and L. H. Okuda; visualization, A. H. C. N. Romaldini; supervision, L. H. Okuda; project administration, L. H. Okuda.; funding acquisition, L. H. Okuda. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Foundation of the Agriculture Research, grant number 409.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of the Biological Institute of São Paulo (protocol code 181/22, 05/10/2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Research was conducted in partnership with the Kennel and 9th of July Cavalry Regiment of the State of Sao Paulo, Institute for Ecological Research and Brazilian National Council of Scientific and Technological Development (CNPq; Brazil).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wernike, K.; Aebischer, A.; Michelitsch, A.; Hoffmann, D.; Freuling, C.; Balkema-Buschmann, A.; Graaf, A.; Müller, T.; Osterrieder, N.; Rissmann, M.; et al. Multi-species ELISA for the Detection of Antibodies against SARS-CoV-2 in Animals. Transbound Emerg Dis 2021, 68, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Dhama, K.; Sharun, K.; Iqbal Yatoo, Mohd. ; Malik, Y.S.; Singh, R.; Michalak, I.; Sah, R.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. COVID-19: Animals, Veterinary and Zoonotic Links. Veterinary Quarterly 2020, 40, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.Y.; Carrai, M.; Choi, Y.R.; Brackman, C.J.; Tam, K.W.S.; Law, P.Y.T.; Woodhouse, F.; Gray, J.; Kim, J.H.; Park, J.; et al. Low Prevalence of SARS-CoV-2 Antibodies in Canine and Feline Serum Samples Collected during the COVID-19 Pandemic in Hong Kong and Korea. Viruses 2023, 15, 582. [Google Scholar] [CrossRef] [PubMed]
- McNamara, T.; Richt, J.A.; Glickman, L. A Critical Needs Assessment for Research in Companion Animals and Livestock Following the Pandemic of COVID-19 in Humans. Vector-Borne and Zoonotic Diseases 2020, 20, 393–405. [Google Scholar] [CrossRef] [PubMed]
- F. Gao, G.; Wang, L.; Chinese Center for Disease Control and Prevention, Beijing, China; CAS Key Laboratory of Pathogen Microbiology and Immunology, Institute of Microbiology, Center for Influenza Research and Early-warning, CAS-TWAS Center of Excellence for Emerging Infectious Diseases, Chinese Academy of Sciences, Beijing, China COVID-19 Expands Its Territories from Humans to Animals. China CDC Weekly 2021, 3, 855–858. [Google Scholar] [CrossRef]
- Salajegheh Tazerji, S.; Magalhães Duarte, P.; Rahimi, P.; Shahabinejad, F.; Dhakal, S.; Singh Malik, Y.; Shehata, A.A.; Lama, J.; Klein, J.; Safdar, M.; et al. Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) to Animals: An Updated Review. J Transl Med 2020, 18, 358. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Han, J.; Lichtfouse, E. Backward Transmission of COVID-19 from Humans to Animals May Propagate Reinfections and Induce Vaccine Failure. Environ Chem Lett 2021, 19, 763–768. [Google Scholar] [CrossRef]
- Mahdy, M.A.A.; Younis, W.; Ewaida, Z. An Overview of SARS-CoV-2 and Animal Infection. Front. Vet. Sci. 2020, 7, 596391. [Google Scholar] [CrossRef]
- Sreenivasan, C.C.; Thomas, M.; Wang, D.; Li, F. Susceptibility of Livestock and Companion Animals to COVID-19. Journal of Medical Virology 2021, 93, 1351–1360. [Google Scholar] [CrossRef]
- Leroy, E.M.; Ar Gouilh, M.; Brugère-Picoux, J. The Risk of SARS-CoV-2 Transmission to Pets and Other Wild and Domestic Animals Strongly Mandates a One-Health Strategy to Control the COVID-19 Pandemic. One Health 2020, 10, 100133. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat Rev Mol Cell Biol 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Sit, T.H.C.; Brackman, C.J.; Ip, S.M.; Tam, K.W.S.; Law, P.Y.T.; To, E.M.W.; Yu, V.Y.T.; Sims, L.D.; Tsang, D.N.C.; Chu, D.K.W.; et al. Infection of Dogs with SARS-CoV-2. Nature 2020, 586, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Barrs, V.R.; Peiris, M.; Tam, K.W.S.; Law, P.Y.T.; Brackman, C.J.; To, E.M.W.; Yu, V.Y.T.; Chu, D.K.W.; Perera, R.A.P.M.; Sit, T.H.C. SARS-CoV-2 in Quarantined Domestic Cats from COVID-19 Households or Close Contacts, Hong Kong, China. Emerg. Infect. Dis. 2020, 26, 3071–3074. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.; Smith, D.; Ghai, R.R.; Wallace, R.M.; Torchetti, M.K.; Loiacono, C.; Murrell, L.S.; Carpenter, A.; Moroff, S.; Rooney, J.A.; et al. First Reported Cases of SARS-CoV-2 Infection in Companion Animals — New York, March–April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 710–713. [Google Scholar] [CrossRef]
- Kok, K.-H.; Wong, S.-C.; Chan, W.-M.; Wen, L.; Chu, A.W.-H.; Ip, J.D.; Lee, L.-K.; Wong, I.T.-F.; Lo, H.W.-H.; Cheng, V.C.-C.; et al. Co-Circulation of Two SARS-CoV-2 Variant Strains within Imported Pet Hamsters in Hong Kong. Emerging Microbes & Infections 2022, 11, 689–698. [Google Scholar] [CrossRef]
- Hale, V.L.; Dennis, P.M.; McBride, D.S.; Nolting, J.M.; Madden, C.; Huey, D.; Ehrlich, M.; Grieser, J.; Winston, J.; Lombardi, D.; et al. SARS-CoV-2 Infection in Free-Ranging White-Tailed Deer. Nature 2022, 602, 481–486. [Google Scholar] [CrossRef]
- Wernike, K.; Böttcher, J.; Amelung, S.; Albrecht, K.; Gärtner, T.; Donat, K.; Beer, M. Serological Screening Suggests Single SARS-CoV-2 Spillover Events to Cattle; Microbiology. 2022. [Google Scholar]
- McAloose, D.; Laverack, M.; Wang, L.; Killian, M.L.; Caserta, L.C.; Yuan, F.; Mitchell, P.K.; Queen, K.; Mauldin, M.R.; Cronk, B.D.; et al. From People to Panthera : Natural SARS-CoV-2 Infection in Tigers and Lions at the Bronx Zoo. mBio 2020, 11, e02220–20. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Ito, M.; Iwatsuki-Horimoto, K.; Yasuhara, A.; Okuda, M.; Hamabata, T.; Murakami, J.; Duong, C.; Yamamoto, T.; Kuroda, Y.; et al. Seroprevalence of SARS-CoV-2 Antibodies in Dogs and Cats during the Early and Mid-Pandemic Periods in Japan. One Health 2023, 17, 100588. [Google Scholar] [CrossRef]
- El Masry, I.; Al Makhladi, S.; Al Abdwany, M.; Al Subhi, A.; Eltahir, H.; Cheng, S.; Peiris, M.; Gardner, E.; Von Dobschuetz, S.; Soumare, B.; et al. Serological Evidence of SARS-CoV-2 Infection in Dromedary Camels and Domestic Bovids in Oman. Emerging Microbes & Infections 2023, 12, 2220577. [Google Scholar] [CrossRef]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of Ferrets, Cats, Dogs, and Other Domesticated Animals to SARS–Coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef]
- Ulrich, L.; Wernike, K.; Hoffmann, D.; Mettenleiter, T.C.; Beer, M. Experimental Infection of Cattle with SARS-CoV-2. Emerg. Infect. Dis. 2020, 26, 2979–2981. [Google Scholar] [CrossRef]
- Costagliola, A.; Liguori, G.; d’Angelo, D.; Costa, C.; Ciani, F.; Giordano, A. Do Animals Play a Role in the Transmission of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2)? A Commentary. Animals 2020, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Emerging Zoonoses: A One Health Challenge. EClinicalMedicine 2020, 19, 100300. [CrossRef] [PubMed]
- Alhajj, M.; Zubair, M.; Farhana, A. Enzyme Linked Immunosorbent Assay. StatPearls 2023.
- Colitti, B.; Bertolotti, L.; Mannelli, A.; Ferrara, G.; Vercelli, A.; Grassi, A.; Trentin, C.; Paltrinieri, S.; Nogarol, C.; Decaro, N.; et al. Cross-Sectional Serosurvey of Companion Animals Housed with SARS-CoV-2–Infected Owners, Italy. Emerg. Infect. Dis. 2021, 27, 1919–1922. [Google Scholar] [CrossRef]
- Tan, C.C.S.; Lam, S.D.; Richard, D.; Owen, C.J.; Berchtold, D.; Orengo, C.; Nair, M.S.; Kuchipudi, S.V.; Kapur, V.; Van Dorp, L.; et al. Transmission of SARS-CoV-2 from Humans to Animals and Potential Host Adaptation. Nat Commun 2022, 13, 2988. [Google Scholar] [CrossRef]
- Happi, A.N.; Ayinla, A.O.; Ogunsanya, O.A.; Sijuwola, A.E.; Saibu, F.M.; Akano, K.; George, U.E.; Sopeju, A.E.; Rabinowitz, P.M.; Ojo, K.K.; et al. Detection of SARS-CoV-2 in Terrestrial Animals in Southern Nigeria: Potential Cases of Reverse Zoonosis. Viruses 2023, 15, 1187. [Google Scholar] [CrossRef]
- Cupertino, M.D.C.; Freitas, A.N.D.; Meira, G.S.B.; Silva, P.A.M.D.; Pires, S.D.S.; Cosendey, T.D.A.; Fernandes, T.M.; Mayers, N.A.J.; Siqueira-Batista, R. COVID 19 and One Health: Potential Role of Human and Animals in SARS-COV-2 Life Cycle. Science in One Health 2023, 100017. [Google Scholar] [CrossRef]
- Tilocca, B.; Soggiu, A.; Musella, V.; Britti, D.; Sanguinetti, M.; Urbani, A.; Roncada, P. Molecular Basis of COVID-19 Relationships in Different Species: A One Health Perspective. Microbes and Infection 2020, 22, 218–220. [Google Scholar] [CrossRef]
- Wernike, K.; Böttcher, J.; Amelung, S.; Albrecht, K.; Gärtner, T.; Donat, K.; Beer, M. Antibodies against SARS-CoV-2 Suggestive of Single Events of Spillover to Cattle, Germany. Emerg. Infect. Dis. 2022, 28, 1916–1918. [Google Scholar] [CrossRef]
- Xu, Z.; Kang, X.; Han, P.; Du, P.; Li, L.; Zheng, A.; Deng, C.; Qi, J.; Zhao, X.; Wang, Q.; et al. Binding and Structural Basis of Equine ACE2 to RBDs from SARS-CoV, SARS-CoV-2 and Related Coronaviruses. Nat Commun 2022, 13, 3547. [Google Scholar] [CrossRef] [PubMed]
- Pusterla, N.; Chaillon, A.; Ignacio, C.; Smith, D.M.; Barnum, S.; Lawton, K.O.Y.; Smith, G.; Pickering, B. SARS-CoV-2 Seroconversion in an Adult Horse with Direct Contact to a COVID-19 Individual. Viruses 2022, 14, 1047. [Google Scholar] [CrossRef] [PubMed]
- Lawton, K.O.Y.; Arthur, R.M.; Moeller, B.C.; Barnum, S.; Pusterla, N. Investigation of the Role of Healthy and Sick Equids in the COVID-19 Pandemic through Serological and Molecular Testing. Animals 2022, 12, 614. [Google Scholar] [CrossRef]
- Sonnleitner, S.T.; Prelog, M.; Jansen, B.; Rodgarkia-Dara, C.; Gietl, S.; Schönegger, C.M.; Koblmüller, S.; Sturmbauer, C.; Posch, W.; Almanzar, G.; et al. Maintenance of Neutralizing Antibodies over Ten Months in Convalescent SARS-CoV-2 Afflicted Patients. Transbounding Emerging Dis 2022, 69, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Grassi, A.; Lorusso, E.; Patterson, E.I.; Lorusso, A.; Desario, C.; Anderson, E.R.; Vasinioti, V.; Wastika, C.E.; Hughes, G.L.; et al. Long-term Persistence of Neutralizing SARS-CoV-2 Antibodies in Pets. Transbounding Emerging Dis 2022, 69, 3073–3076. [Google Scholar] [CrossRef] [PubMed]
- Stoffella-Dutra, A.G.; De Campos, B.H.; Bastos E Silva, P.H.; Dias, K.L.; Da Silva Domingos, I.J.; Hemetrio, N.S.; Xavier, J.; Iani, F.; Fonseca, V.; Giovanetti, M.; et al. SARS-CoV-2 Spillback to Wild Coatis in Sylvatic–Urban Hotspot, Brazil. Emerg. Infect. Dis. 2023, 29, 664–667. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).