Submitted:
05 July 2023
Posted:
06 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Case selection
2.2. Pathological data
2.3. Molecular analysis
2.4. Follow-up data
2.5. Statistical analysis
3. Results
3.1. Patient’s characteristics
3.2. Prevalence of BRAFV600E mutation and relationship with demographic, pathological and outcomes characteristics
3.3. Predictive factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xing, M. Molecular Pathogenesis and Mechanisms of Thyroid Cancer. Nat Rev Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef]
- Holmes, D. Thyroid Cancer: Incidence Trends in the USA. Nat Rev Endocrinol 2016, 12, 312. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Xing, M. Recent Incidences and Differential Trends of Thyroid Cancer in the USA. Endocr Relat Cancer 2016, 23, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Amphlett, B.; Lawson, Z.; Abdulrahman, G.O.; White, C.; Bailey, R.; Premawardhana, L.D.; Okosieme, O.E. Recent Trends in the Incidence, Geographical Distribution, and Survival from Thyroid Cancer in Wales, 1985-2010. Thyroid 2013, 23, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, A.A.; Teixeira, E.; Bella-Cueto, M.R.; Melo, M.; Oliveira, M.J.; Sobrinho-Simões, M.; Maciel, J.; Soares, P. Clinicopathological Features as Prognostic Predictors of Poor Outcome in Papillary Thyroid Carcinoma. Cancers (Basel) 2020, 12, 1–16. [Google Scholar] [CrossRef]
- Ulisse, S.; Baldini, E.; Lauro, A.; Pironi, D.; Tripodi, D.; Lori, E.; Ferent, I.C.; Amabile, M.I.; Catania, A.; Di Matteo, F.M.; et al. Papillary Thyroid Cancer Prognosis: An Evolving Field. Cancers (Basel) 2021, 13, 5567. [Google Scholar] [CrossRef]
- Bournaud, C.; Descotes, F.; Decaussin-Petrucci, M.; Berthiller, J.; de la Fouchardière, C.; Giraudet, A.L.; Bertholon-Gregoire, M.; Robinson, P.; Lifante, J.C.; Lopez, J.; et al. TERT Promoter Mutations Identify a High-Risk Group in Metastasis-Free Advanced Thyroid Carcinoma. Eur J Cancer 2019, 108, 41–49. [Google Scholar] [CrossRef]
- Liu, C.; Chen, T.; Liu, Z. Associations between BRAF and Prognostic Factors and Poor Outcomes in Papillary Thyroid Carcinoma: A Meta-Analysis. World J Surg Oncol 2016, 14, 241. [Google Scholar] [CrossRef]
- Garnett, M.J.; Marais, R. Guilty as Charged: B-RAF Is a Human Oncogene. Cancer Cell 2004, 6, 313–319. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF Gene in Human Cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Hilger, R.A.; Scheulen, M.E.; Strumberg, D. The Ras-Raf-MEK-ERK Pathway in the Treatment of Cancer. Onkologie 2002, 25, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Shong, Y.K.; Kim, T.Y.; Viola, D.; Elisei, R.; Bendlová, B.; Yip, L.; Mian, C.; et al. Association between BRAF V600E Mutation and Recurrence of Papillary Thyroid Cancer. J Clin Oncol 2015, 33, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, K.; Lin, X.; Zhao, L.; An, W.; Wang, C.; Liu, X. The Association between BRAF (V600E) Mutation and Pathological Features in PTC. Eur Arch Otorhinolaryngol 2014, 271, 3041–3052. [Google Scholar] [CrossRef] [PubMed]
- Attia, A.S.; Hussein, M.; Issa, P.P.; Elnahla, A.; Farhoud, A.; Magazine, B.M.; Youssef, M.R.; Aboueisha, M.; Shama, M.; Toraih, E.; et al. Association of BRAFV600E Mutation with the Aggressive Behavior of Papillary Thyroid Microcarcinoma: A Meta-Analysis of 33 Studies. Int J Mol Sci 2022, 23, 15626. [Google Scholar] [CrossRef]
- Kim, T.H.; Park, Y.J.; Lim, J.A.; Ahn, H.Y.; Lee, E.K.; Lee, Y.J.; Kim, K.W.; Hahn, S.K.; Youn, Y.K.; Kim, K.H.; et al. The Association of the BRAF(V600E) Mutation with Prognostic Factors and Poor Clinical Outcome in Papillary Thyroid Cancer: A Meta-Analysis. Cancer 2012, 118, 1764–1773. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. BRAF Mutation in Papillary Thyroid Cancer: Pathogenic Role, Molecular Bases, and Clinical Implications. Endocr Rev 2007, 28, 742–762. [Google Scholar] [CrossRef]
- Vuong, H.G.; Duong, U.N.P.; Altibi, A.M.A.; Ngo, H.T.T.; Pham, T.Q.; Tran, H.M.; Gandolfi, G.; Hassell, L. A Meta-Analysis of Prognostic Roles of Molecular Markers in Papillary Thyroid Carcinoma. Endocr Connect 2017, 6, R8–R17. [Google Scholar] [CrossRef] [PubMed]
- Ulisse, S.; Baldini, E.; Sorrenti, S.; Barollo, S.; Prinzi, N.; Catania, A.; Nesca, A.; Gnessi, L.; Pelizzo, M.R.; Mian, C.; et al. In Papillary Thyroid Carcinoma BRAFV600E Is Associated with Increased Expression of the Urokinase Plasminogen Activator and Its Cognate Receptor, but Not with Disease-Free Interval. Clin Endocrinol (Oxf) 2012, 77, 780–786. [Google Scholar] [CrossRef]
- Gan, X.; Shen, F.; Deng, X.; Feng, J.; Lu, J.; Cai, W.; Peng, L.; Zheng, W.; Wang, W.; Huang, P.; et al. Prognostic Implications of the BRAF-V600E Mutation in Papillary Thyroid Carcinoma Based on a New Cut-off Age Stratification. Oncol Lett 2020, 19, 631–640. [Google Scholar] [CrossRef]
- Lloyd, R. V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs; Lloyd, R. V. , Osamura, R.Y., Klöppel, G., Rosai, J., Eds.; International Agency for Research on Cancer, 2017. ISBN 978-92-832-4493-6.
- American Joint Committee on, Cancer.; Amin, M.B. American Joint Committee on Cancer.; Amin, M.B. AJCC Cancer Staging Manual. 2017.
- Nechifor-Boila, A.; Loghin, A.; Descotes, F.; Decaussin-Petrucci, M.; Borda, A. Evaluation of a DNA Extraction and Purification Protocol Using Archived Formalin-Fixed Paraffin-Embedded Tissues for BRAF Mutations Analysis in Papillary Thyroid Microcarcinomas. Appl Immunohistochem Mol Morphol 2019, 27, 70–76. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Costa, V.; Esposito, R.; Pallante, P.; Ciccodicola, A.; Fusco, A. The “next-Generation” Knowledge of Papillary Thyroid Carcinoma. Cell Cycle 2015, 14, 2018–2021. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Kucukodaci, Z.; Akar, E.; Haholu, A.; Baloglu, H. A Valuable Adjunct to FNA Diagnosis of Papillary Thyroid Carcinoma: In-House PCR Assay for BRAF T1799A (V600E). Diagn Cytopathol 2011, 39, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, X.; Xiong, J.; Li, C.; Liao, Y.; Zhu, Y.; Mao, J. Risk and Prognostic Factors for BRAFV600E Mutations in Papillary Thyroid Carcinoma. Biomed Res Int 2022, 2022. [Google Scholar] [CrossRef]
- Daliri, M.; Abbaszadegan, M.R.; Mehrabi Bahar, M.; Arabi, A.; Yadollahi, M.; Ghafari, A.; Taghehchian, N.; Zakavi, S.R. The Role of BRAF V600E Mutation as a Potential Marker for Prognostic Stratification of Papillary Thyroid Carcinoma: A Long-Term Follow-up Study. Endocr Res 2014, 39, 189–193. [Google Scholar] [CrossRef]
- Al-Masri, M.; Al-Shobaki, T.; Al-Najjar, H.; Iskanderian, R.; Younis, E.; Abdallah, N.; Tbakhi, A.; Haddad, H.; Al-Masri, M.; Obeid, Z.; et al. BRAF V600E Mutation in Papillary Thyroid Carcinoma: It’s Relation to Clinical Features and Oncologic Outcomes in a Single Cancer Centre Experience. Endocr Connect 2021, 10, 1531–1537. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.Z.; Guan, Y.X.; Chen, Q.J.; Zhu, Q.Y. Meta-Analyses of Association Between BRAF(V600E) Mutation and Clinicopathological Features of Papillary Thyroid Carcinoma. Cell Physiol Biochem 2016, 38, 763–776. [Google Scholar] [CrossRef]
- Xing, M.; Westra, W.H.; Tufano, R.P.; Cohen, Y.; Rosenbaum, E.; Rhoden, K.J.; Carson, K.A.; Vasko, V.; Larin, A.; Tallini, G.; et al. BRAF Mutation Predicts a Poorer Clinical Prognosis for Papillary Thyroid Cancer. J Clin Endocrinol Metab 2005, 90, 6373–6379. [Google Scholar] [CrossRef]
- Damiani, L.; Lupo, S.; Rossi, R.; Bruni, S.; Bartolomei, M.; Panareo, S.; Franceschetti, P.; Carcoforo, P.; Lanza, G.; Pelucchi, S.; et al. Evaluation of the Role of BRAF V600E Somatic Mutation on Papillary Thyroid Cancer Disease Persistence: A Prospective Study. Eur Thyroid J 2018, 7, 251–257. [Google Scholar] [CrossRef]
- Li, X.; Kwon, H. The Impact of BRAF Mutation on the Recurrence of Papillary Thyroid Carcinoma: A Meta-Analysis. Cancers (Basel) 2020, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Scheffel, R.S.; Dora, J.M.; Maia, A.L. BRAF Mutations in Thyroid Cancer. Curr Opin Oncol 2022, 34, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Elisei, R.; Viola, D.; Torregrossa, L.; Giannini, R.; Romei, C.; Ugolini, C.; Molinaro, E.; Agate, L.; Biagini, A.; Lupi, C.; et al. The BRAF(V600E) Mutation Is an Independent, Poor Prognostic Factor for the Outcome of Patients with Low-Risk Intrathyroid Papillary Thyroid Carcinoma: Single-Institution Results from a Large Cohort Study. J Clin Endocrinol Metab 2012, 97, 4390–4398. [Google Scholar] [CrossRef] [PubMed]
- Romei, C.; Elisei, R. A Narrative Review of Genetic Alterations in Primary Thyroid Epithelial Cancer. Int J Mol Sci 2021, 22, 1–16. [Google Scholar] [CrossRef]
- Guenter, R.; Patel, Z.; Chen, H. Notch Signaling in Thyroid Cancer. Adv Exp Med Biol 2021, 1287, 155–168. [Google Scholar] [CrossRef]
- Boufraqech, M.; Patel, D.; Nilubol, N.; Powers, A.; King, T.; Shell, J.; Lack, J.; Zhang, L.; Gara, S.K.; Gunda, V.; et al. Lysyl Oxidase Is a Key Player in BRAF/MAPK Pathway-Driven Thyroid Cancer Aggressiveness. Thyroid 2019, 29, 79–92. [Google Scholar] [CrossRef]
- Saqcena, M.; Leandro-Garcia, L.J.; Maag, J.L.V.; Tchekmedyian, V.; Krishnamoorthy, G.P.; Tamarapu, P.P.; Tiedje, V.; Reuter, V.; Knauf, J.A.; de Stanchina, E.; et al. SWI/SNF Complex Mutations Promote Thyroid Tumor Progression and Insensitivity to Redifferentiation Therapies. Cancer Discov 2021, 11, 1158–1175. [Google Scholar] [CrossRef]
| Factors | Total n= 127 |
BRAFV600E wild-type n=60 |
BRAFV600E mutated n=67 |
pa |
|---|---|---|---|---|
| Age at surgery (mean±SD, years) | 48.6 ± 1.28 | 46.18 ± 1.17 | 50.76 ± 1.72 | 0.075* |
| Age (n, %) | ||||
| < 55 years | 79 (62.2) | 43 (71.7) | 36 (53.7) | 0.037 |
| ≥ 55 years | 48 (37.8) | 17 (28.3) | 31 (46.3) | 0.037 |
| Gender, female (n, %) | 110 (86.6) | 56 (93.3) | 54 (80.6) | 0.035 |
| Tumor size (mean±SD, mm) | 22.88 ±1.5 | 23.35 ± 1.42 | 21.90 ± 1.33 | 0.458* |
| Tumor size (n, %) | ||||
| 11-20 mm | 67 (52.8) | 29 (48.3) | 38 (56.7) | 0.442 |
| 21-40 mm | 51 (40.2) | 27 (45.0) | 24 (35.8) | 0.225 |
| >40 mm | 9 (7.1) | 4 (6.7) | 5 (7.5) | 0.864 |
| Multifocality (n, %) | 51 (40.2) | 20 (33.3) | 31 (46.3) | 0.138 |
| Histological variant (n, %) | ||||
| Conventional | 88 (69.3) | 32 (53.3) | 56 (83.6) | 0.0005 |
| Tall cell variant | 9 (7.1) | 3 (5) | 6 (9) | 0.596 |
| Warthin-like | 7 (5.5) | 3 (5) | 4 (6) | 0.886 |
| Oncocytic | 3 (2.4) | 3 (5) | 0 | 0.205 |
| Solid | 2 (1.6) | 2 (3.3) | 0 | 0.434 |
| Follicular variant, infiltrative | 13 (10.2) | 12 (20) | 1 (1.5) | 0.001 |
| Follicular variant, encapsulated, invasive | 5 (3.9) | 5 (8.3) | 0 | 0.051 |
| Extrathyroidal extension (n, %) | 24 (18.9) | 5 (8.3) | 19 (28.4) | 0.004 |
| Primary tumor, pT (n, %) | ||||
| 1b | 51 (40.2) | 25 (41.7) | 26 (38.8) | 0.879 |
| 2 | 44 (34.6) | 25 (41.7) | 19 (28.4) | 0.165 |
| 3a | 8 (6.3) | 5 (8.3) | 3 (4.5) | 0.607 |
| 3b | 24 (18.9) | 5 (8.3) | 19 (28.4) | 0.007 |
| Lymph node involvement | 26/39 | 2/8 (25) | 24/31 (77.4) | 0.001 |
| Vascular invasion (n, %) | 4 (3.3) | 2 (3.1) | 2 (3) | 0.911 |
| Positive surgical resection margin | 18 (14.2) | 4 (6.7) | 14 (20.9) | 0.022 |
| Stage grouping | ||||
| I | 101 (79.5) | 54 (90) | 47 (70.1) | 0.010 |
| II | 22 (17.3) | 6 (10) | 16 (23.9) | 0.067 |
| III | 4 (3.1) | 0 | 4 (6) | 0.155 |
| Type of surgery | ||||
| Lobectomy | 0 | 0 | 0 | - |
| Total thyroidectomy | 88 (69.3) | 52 (86.7) | 36 (53.7) | 0.0001 |
| Total thyroidectomy with lymph node dissection | 39 (30.7) | 8 (13.3) | 31 (46.2) | 0.0001 |
| Follow-up data (n, median, months) | 57 (CI:9-130) | 58 (CI:17-114) | 57 (CI: 9-130) | - |
| I131 therapy (n, %) | 133 (100) | 66 (100) | 67 (100) | - |
| Disease free (n, %) | 107 (84.3) | 56 (93.4) | 51 (76.1) | 0.008 |
| Persistent disease (n, %) | 14 (11) | 2 (3.3) | 12 (17.9) | 0.009 |
| Recurrence (n, %) | 6 (4.7) | 2 (3.3) | 4 (6) | 0.484 |
| Distant metastasis (n, %) | 5 (3.9) | 0 | 5 (7.5) | 0.031 |
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Factors | BRAFV600E positive (67)/N | OR | [95% CI] | p | OR | [95% CI] | p |
| Age ≥55 years | 31/48 | 2.18 | [1.04 - 4.56] | 0.039 | 2.20 | [0.90- 5.37] | 0.081 |
| Sex, male | 13/17 | 3.37 | [1.03 -10.98] | 0.043 | 2.81 | [0.67 - 11.72] | 0.155 |
| PTC, conventional | 56/88 | 4.44 | [1.95- 10.13] | 0.008 | 6.33 | [2.18 - 18.40] | <0.001 |
| PTC, “tall cell” | 4/9 | 1.88 | [0.44 -7.82] | 0.392 | |||
| Tumor size >40mm | 5/9 | 1.13 | [0.28- 4.41] | 0.861 | |||
| Extrathyroidal extension | 19/24 | 4.35 | [1.51 -12.54] | 0.006 | 5.83 | [1.60- 21.27] | 0.007 |
| Multifocality | 31/51 | 1.72 | [0.83 - 3.53] | 0.139 | |||
| Lymph node metastasis | 24/26 | 7.81 | [2.52 - 24.20] | <0.001 | 4.77 | [1.44 - 15.79] | 0.010 |
| Positive resection margin | 14/18 | 3.69 | [1.14- 11.95] | 0.028 | 2.25 | [0.56 - 9.00] | 0.249 |
| BRAFV600E wild type | BRAFV600E mutated | Log Rank | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factors |
*12 months (%) (95%CI) |
*24 months (%) (95%CI) |
*48 months (%) (95%CI) |
*60 months (%) (95%IC) |
*12 months (%) (95%CI) |
*24 months (%) (95%CI) |
*48 months (%) (95%CI) |
*60 months (%) (95%CI) |
||
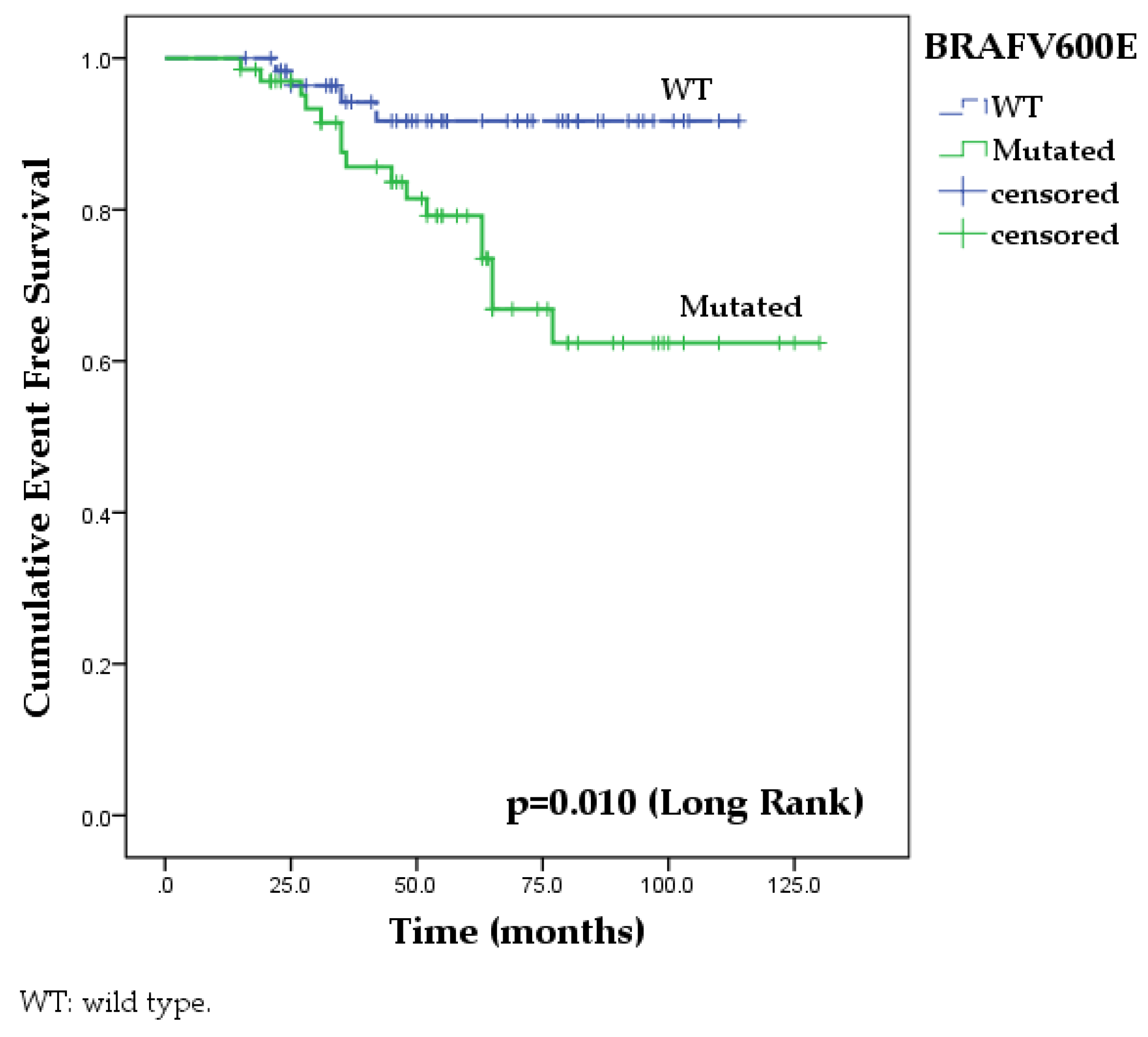
| Total | 98.3 (96.6-100) | 96.4 (93.9- 98.4) | 94.2 (90.9-97.5) | 91.7 (87.7-95.7) | 98.5 (97-100) | 95.1 (92.3-97.9) | 81.4 (76-86.8) | 62.4 (54.2-70.6) | 6.581 | 0.010 |
| Age (n,%) | ||||||||||
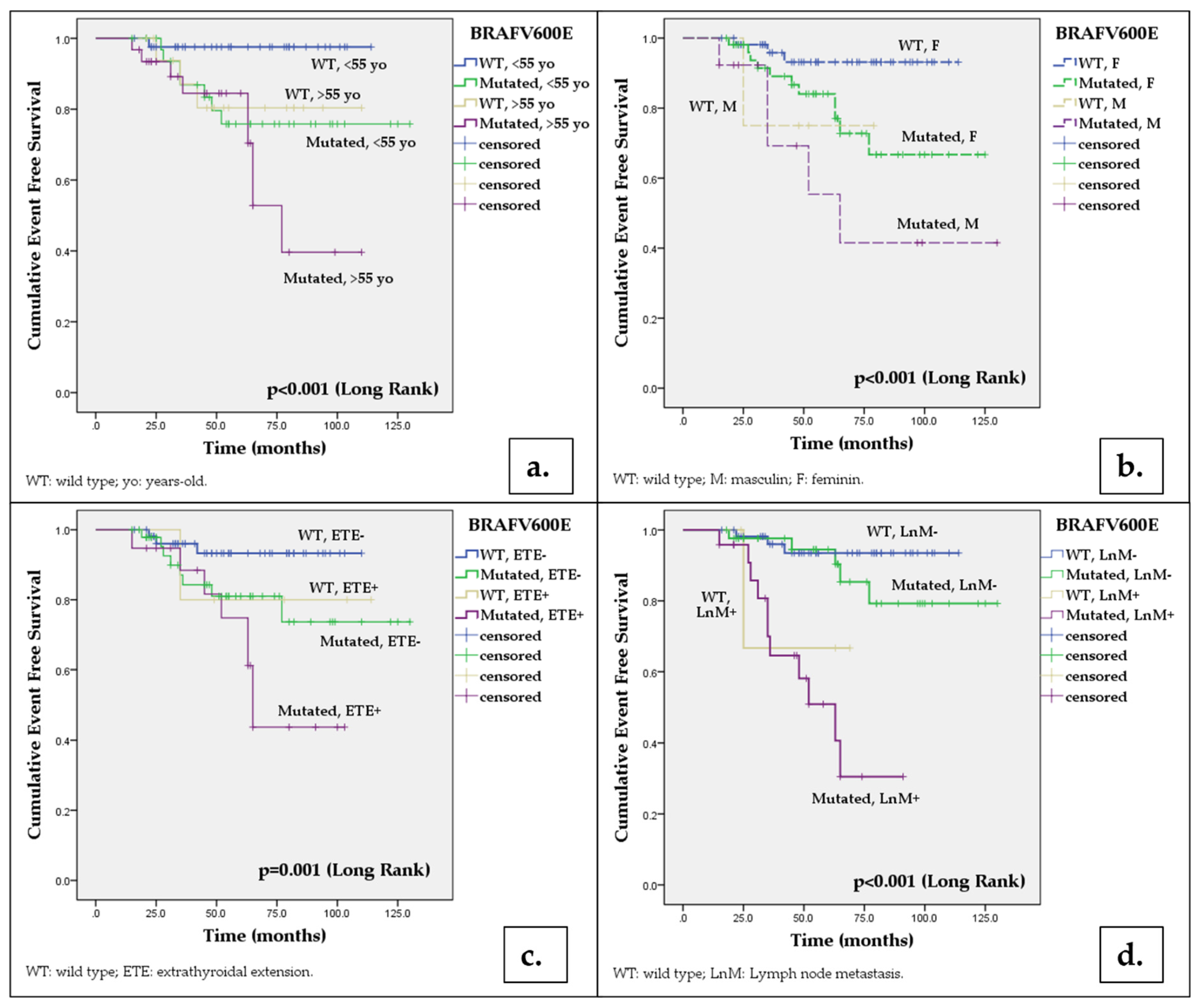
| < 55 years | 97.6 (95.2-100) | 97.6 (95.2-100) | 97.6 (95.2-100) | 97.6 (95.2-100) | 96.8 (96.4-100) | 93.5 (89.1-97.8) | 79.6 (72.1-87.1) | 75.8 (67.8-83.8) | 5.314a | 0.021 |
| ≥ 55 years | 93.8 (93.1-100) | 93.8 (93.1-100) | 80.4 (70.2-90.6) | 80.4 (70.2-90.6) | 96.8 (93.6-100) | 93.4 (88.9-97.9) | 84.5 (77.2-91.8) | 52.8 (39.3-66.3) | 12.641a | <0.001 |
| Gender (n, %) | ||||||||||
| Female | 98.1 (96.3-100) | 98.1 (96.3-100) | 93.1 (89.2-97) | 93.1 (89.2-97) | 98.1 (96.2-100) | 95.9 (93-98.8) | 84 (78.4-89.6) | 72.8 (65-80.6) | 5.274b | 0.022 |
| Male | 75 (53.3-96.7) | 75 (53.3-96.7) | 75 (53.3-96.7) | 75 (53.3-96.7) | 92.3 (84.9-100) | 92.3 (84,9-100) | 69.2 (47.7-78.1) | 55.4 (38.1-72.7) | 13.427b | <0.001 |
| Histology | ||||||||||
| PTC conventional | 96.7 (93.4-100) | 94.3 (90-98.6) | 92.1 (87.2-97) | 89.3 (83.7-94.9) | 98.1 (96.2-100) | 93.6 (90-97.2) | 78.6 (72.2-85) | 68.5 (59.8-77.2) | 7.973 | 0.005 |
| PTC tall cell variant | 98.4 (96.8-100) | 85.4 (81.5-89.3) | 66.7 (39.5-93.9) | 66.7 (39.5-93.9) | 83.3 (68.1-98.5) | 83.3 (68.1-96.5) | 62.5 (41.2-83.8) | 41.7 (19.5-63.9) | 5.997 | 0.014 |
| Tumor size | ||||||||||
| ≤ 40 mm | 98.2(96.4-100) | 96.2 (93.5-98,9) | 91.2 (86.9-95.5) | 91.2(86.9-95.5) | 98.4(96.8-100) | 96.7 (94.4-99) | 81.7 (76.1-87.3) | 68.3 (60.6-76) | 4.505c | 0.034 |
| > 40 mm | - | - | - | - | - | - | 80 (62.1-97.9) | 26.7 (4.1-49.3) | 8.221c | 0.004 |
| Extrathyroidal extension | ||||||||||
| Absent | 98.1 (96.3-100) | 96 (93.2-98.8) | 93.3 (89.5-97.1) | 93.3 (89.5-97.1) | 97.8 (95,6-100) | 95.2 (91.9-98.5) | 81 (74.5-87.5) | 73.7 (64.5-82.9) | 3.608d | 0.057 |
| Present | 99 (98-100) | 80 (62.1-97.9) | 80 (62.1-97.9) | 80 (62.1-97.9) | 94.7 (89.6-100) | 88,4 (80.6-96.2) | 74.8 (63.8-85.8) | 43.7 (29.9-57.5) | 11.243d | 0.001 |
| Multifocality | ||||||||||
| Absent | 97.2 (94.5-100) | 97.2 (94.5-100) | 97.2 (94.5-100) | 97.2 (94.5-100) | 97.2 (94.5-100) | 97.2 (94.5-100) | 85.8 (79.1-92.5) | 74 (64.3-83.7) | 5.193e | 0.023 |
| Prezent | 95 (90-100) | 95 (90-100) | 88.7 (81.1-96.3) | 81.8 (72.2-91.4) | 96.7 (93.4-100) | 92.9 (88.1-97.7) | 77.4 (69.2-85.6) | 59.7 (48.7-70.7) | 11.639e | 0.001 |
| Lymph node metastases | ||||||||||
| Absent | 98.1 (96.3-100) | 98.1 (96.3-100) | 93.5 (89.8-97.2) | 93.5 (89.8-97,2) | 97.6 (95.2-100) | 97.5 (95.2-100) | 94.5 (90.7-98.3) | 85.3 (92.4-78.2) | 1.155f | 0.282 |
| Present | - | 66.7(39.5-93.9) | 66.7(39.5-93.9) | - | 95.8 (91.7-100) | 90.8 (84.6-97) | 58.1 (46.6-69.6) | 30.5 (17.2-43.8) | 23.84f | <0.001 |
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Factors | Event/N (total =127) |
HR | [95% CI] | p | HR | [95% CI] | p |
| Age ≥55 years | 12/48 | 2.74 | [1.12 - 6.73] | 0.027 | 2.39 | [0.82-6.96] | 0.109 |
| Sex, male | 6/17 | 3.91 | [1.4 - 10.21] | 0.005 | 3.80 | [1.30-11.06] | 0.014 |
| PTC, conventional | 12/88 | 0.82 | [0.33 - 2.01] | 0.620 | |||
| PTC, “tall cell” variant | 4/9 | 2.71 | [0.91 - 8.12] | 0.075 | |||
| Tumor size >40mm | 3/9 | 2.40 | [0.70 - 8.23] | 0.163 | |||
| Extrathyroidal extension | 9/24 | 2.96 | [1.22 - 7.15] | 0.016 | 1.02 | [0.31 – 3.47] | 0.905 |
| Multifocality | 13/51 | 2.81 | [1.12 - 7.05] | 0.027 | 3.11 | [0.97 - 9.95] | 0.055 |
| Lymph node metastasis | 12/26 | 9.14 | [3.63 - 22.97] | <0.001 | 6.71 | [2.29-19.69] | <0.001 |
| Positive resection margin | 5/18 | 2.79 | [0.99 - 7.89] | 0.05 | |||
| Positive BRAFV600E mutation | 16/67 | 3.74 | [1.25 - 11.21] | 0.018 | 1.02 | [0.27-3.61] | 0.998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





