1. Introduction
Hemophilia A and B are congenital bleeding disorders characterized by missing or defective factor VIII (FVIII) or factor IX, respectively [
1,
2]. Regular replacement treatment with FVIII concentrate is preferred to prevent bleeding and joint damage in children with severe hemophilia [
1,
2,
3,
4]. Extended half-life FVIII (EHL-FVIII), which reduces the number of injections, would substantially improve the treatment options for hemophilia A patients [
4,
5]. Recently, efanesoctocog alfa, a von Willebrand factor (VWF) independent, recombinant DNA-derived FVIII concentrate, was developed by Bioverativ Therapeutics, Inc (a Sanofi company; Paris, France) and Swedish Orphan Biovitrum AB (Sobi, Stockholm, Sweden) [
6,
7]. This FVIII concentrate may elevate FVIII activity to more than 100% in hemophilic patients.
Emicizumab (Chugai Pharmaceutical Co., Ltd., Tokyo, Japan) [
8,
9], which is a bispecific antibody for clotting factor X (FX) and FIX, is useful for life-threatening hemophilic patients with inhibitors for FVIII and it can reduce the injection frequency for FVIII products in the treatment of hemophilia A. However, measuring the FVIII activity and inhibitors for FVIII is difficult to carry out in hemophilic patients being treated with emicizumab. Although hemophilic patients with inhibitors should be treated with bypass therapy at the time of major surgery or severe bleeding, FVIII inhibitors cannot be evaluated in patients treated with emicizumab. Therefore, anti-idiotype monoclonal antibodies for emicizumab have been established, and the possibility of measuring the FVIII activity and inhibitor titer in the presence of emicizumab has been reported [
10].
FVIII activity and hemostatic ability have been analyzed in several reports using a routine APTT assay based on the peak times of a clot waveform analysis (CWA)-activated partial thromboplastin time (APTT) and chromogenic substrate assay [
11,
12,
13]. However, few reports have described this relationship between the FVIII activity assessed using the peak time and height of CWA-APTT, including a small amount of tissue factor-induced activated FIX (sTF/FIXa) assay [
14]. A CWA-small amount of thrombin time (CWA-TT) also reflects thrombin burst and FVIII activity [
15] and can be used to measure the FVIII activity independent of the presence of emicizumab [
16].
In the present study, the hemostatic ability and FVIII activity were evaluated in 25 patients with hemophilia-related diseases using a CWA-APTT, CWA-TT and chromogenic assays and we discuss thrombotic risk in hemophilic patients treated with FVIII concentrate.
2. Materials and Methods
Twenty-eight plasma samples were obtained from 23 patients with hemophilia, 1 patient who was a carrier of hemophilia A and 1 patient with acquired hemophilia A who were managed at Mie university Hospital from January 1, 2022 to December 31, 2022. (Table 1). The study protocol was approved by the Human Ethics Review Committee of Mie University Hospital, and signed informed consent was obtained from each participant. This study was carried out in accordance with the principles of the Declaration of Helsinki.
The CWA-TT was measured using 0.5 IU thrombin (Thrombin 500 units; Mochida Pharmaceutical Co., Ltd., Tokyo, Japan) with an ACL-TOP
® system (Instrumentation Laboratory, Bedford, MA, USA) [
15,
16]. Three types of curves are shown on this system monitor [
15,
16]. One shows the changes in the absorbance observed while measuring the TT, corresponding to the fibrin formation curve (FFC). The second is the first derivative peak of the absorbance (1st DP), corresponding to the coagulation velocity. The third is the second derivative peak of the absorbance (2nd DP), corresponding to the coagulation acceleration. FⅧ-deficient plasma (Instrumentation Laboratory), and calibration plasma (Instrumentation Laboratory) were used as normal plasma. Emicizumab was kindly provided by Chugai Pharmaceutical CO., Ltd.
The CWA-APTT of platelet poor plasma (PPP) was measured using a HemosIL APTT-SP (Instrumentation Laboratory) as previously reported [20]. PRP was prepared by centrifugation at 900 rpm for 15 minutes (platelet count, 40 x 10
10 /L), and PPP was prepared by centrifugation at 3,000 rpm for 15 minutes (platelet count, <0.5 x 10
10 /L) [
17]. The sTF/FIX assay was performed using PRP, 10 IU/ml of FIX (Nonacog Alfa; Pfizer Japan, Tokyo Japan) and 2,000-fold diluted HemosIL RecombiPlasTin 2G, (Instrumentation Laboratory) with an ACL-TOP
® system [
18].
The FVIII activity was measured by the one-stage clotting method of APTT peak time using APTT-SP in an ACL-TOP system, with the chromogenic substrate method using a Revohem
TM FVIII chromogenics system (HYPHEN BioMed, Neuville-sur-Oise, France) using a CS-5100 device (Sysmex Corporation, Kobe, Japan), or with the CWA-TT method with an ACL-TOP system [
16].
Statistical Analyses
The data are expressed as the median (25th-75th percentiles). The significance of differences between groups was examined using the Mann-Whitney U-test. P values of <0.05 were considered to indicate statistical significance. All of the statistical analyses were performed using the Stat-Flex software program (version 6; Artec Co., Ltd., Osaka, Japan).
3. Results
Of the 23 patients with hemophilia, 15 were treated with regular replacement therapy, and 7 were treated with EHL-FVIII and 5 were treated with emicizumab (
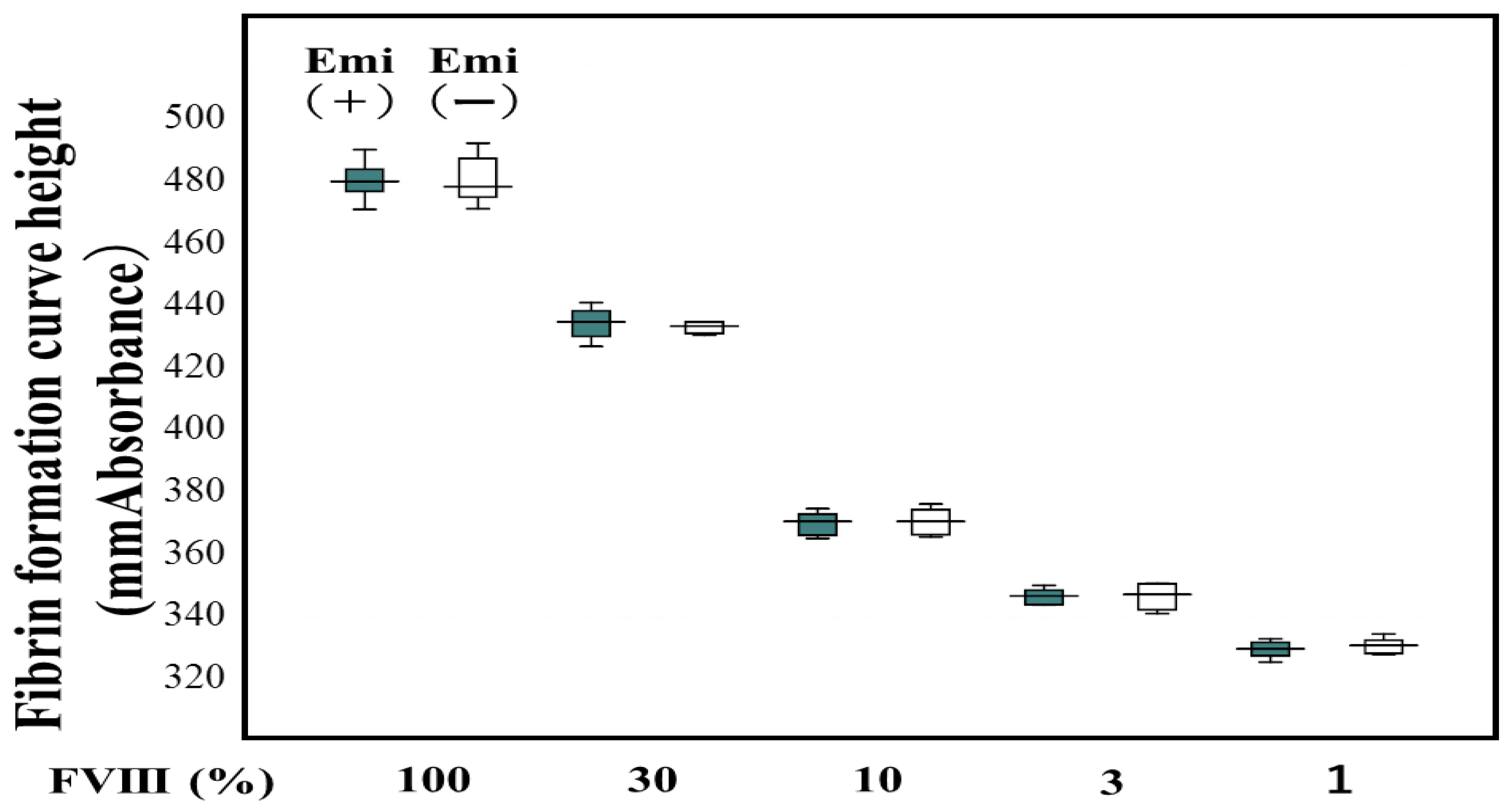
Table 1). Two standard curves for FVIII activity using the CWA-TT in plasma with and without emicizumab were almost similar to be able to determine FVIII activity in plasma with emicizumab using a standard curve in plasma without emicizumab (
Figure 1).
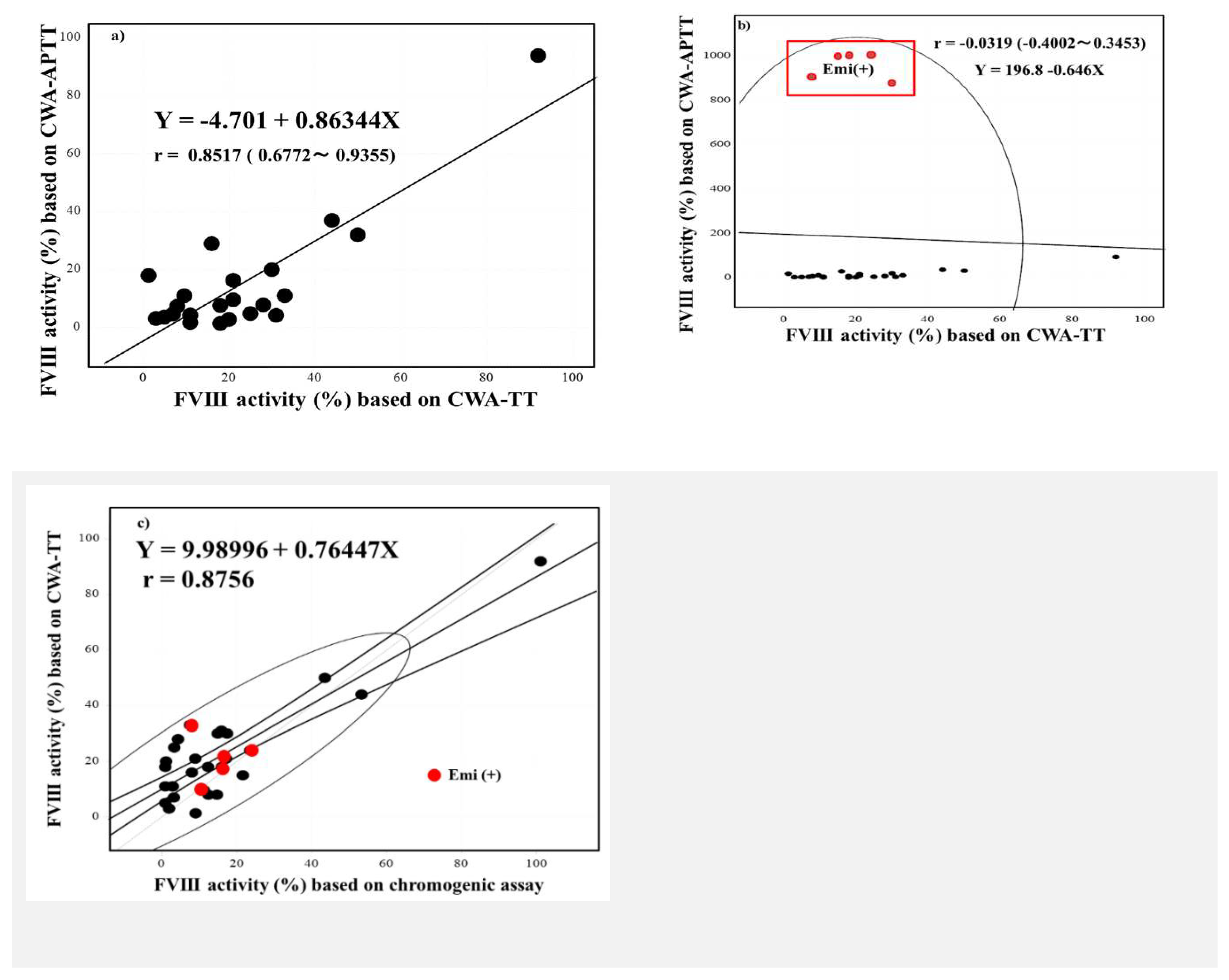
FVIII activities based on the one-stage clotting assay using the CWA-TT were significantly higher than those based on the one-stage clotting assay using the CWA-APTT or chromogenic assay (
Figure 2). Although the FVIII activity using the CWA-APTT peak time was scaled over in plasm with emicizumab, the activities based on a CWA-APTT and CWA-TT in plasma without emicizumab were closely correlated, and those based on a CWA-TT and chromogenic assay in plasma with and without emicizumab were also closely correlated (
Figure 3a-c).
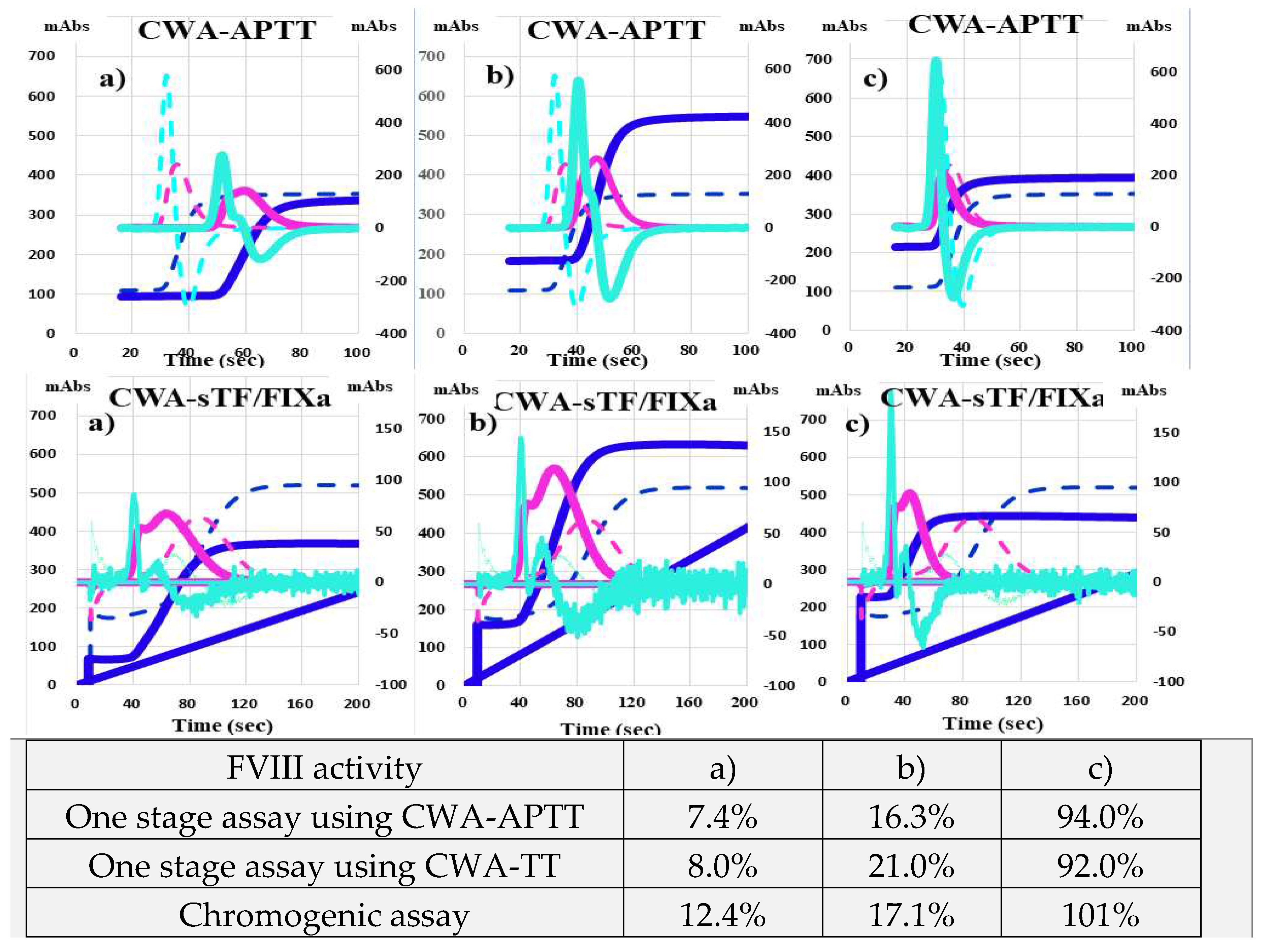
The CWA-APTT in HA-2 treated with FVIII concentrate (efraloctocog alfa; Sanofi K.K., Tokyo, Japan) showed that the peak time was prolonged and the peak height was similar in comparison with the normal control (
Figure 4a), whereas the CWA-APTT in HA-22 treated with emicizumab was showed that the peak time was shortened and the peak height was relatively low in comparison with the normal control (Figure 4b). HA-3 treated with FVIII concentrate (rurioctocog alfa pegol; Takeda Pharmaceuticals, Osaka, Japan) showed that FVIII activities were 7.4%-12.4%, with a prolonged peak time and relatively low peak height on CWA-APTT and shortened peak time and normal peak height of 1st DP on the CWA-TT (Figure 5a). HA-2 treated with FVIII concentrate (efraloctocog alfa) showed that FVIII activities were 16.3%-21.0%, with a slightly prolonged peak time and normal peak height on the CWA-APTT and shortened peak time and elevated peak height of 1st DP on the CWA-TT (
Figure 5b). HA-10 treated with FVIII concentrate (lonoctocog alfa; CSL Behring K.K., Tokyo, Japan) showed that FVIII
4. Discussion
Although there was not sufficiently high frequency of regular replacement ther apy in the present study, regular replacement therapy including emicizumab is a common therapy [
19,
20] that is being administered with increasing frequency in Japan. In particular, treatment with emicizumab has spread [
21,
22]. Emicizumab can decrease the injection time and its injection procedure is easy. However, FVIII activity and inhibitors cannot be measured using routine APTT assays in patients treated with emicizumab [
23]. Therefore, we developed an FVIII assay based on a CWA-TT independent of the presence of emicizumab [
16]. Although three FVIII activities in plasma without emicizumab based on a CWA-APTT, CWA-TT or chromogenic assay were well correlated, FVIII activities based on a CWA-TT were higher than those based on a CWA-APTT or chromogenic assay, suggesting that thrombin burst [
17,
24] may increase FVIII activity based on a CWA-TT.
The CWA-APTT in patients treated with emicizumab shows shortened peak time, however this shortness on CWA-APTT does not reflect the p
hysiological hemostatic ability. Although combination therapy with emicizumab and activated plasma prothrombin complex concentrate was reported to be associated with thrombosis [
8]
, single therapy with emicizumab cannot cause thrombotic complications. The
peak height on the CWA-APTT may reflect the p
hysiological hemostatic ability, and a low peak height on the CWA-APTT was reported to be associated with major bleeding [
25]
. In addition, the peak height on the CWA-APTT may well reflect FVIII activity [
26,
27]
. Elevated peak heights on the CWA-APTT and CWA-sTF/FIXa were reported to be associated with thrombosis [
28,
29]
, suggesting that markedly high peak heights on the CWA-APTT and CWA-sTF/FIXa may indicate a risk of thrombosis. This increase in peak height on the CWA-APTT and CWA-sTF/FIXa may ne due to thrombin burst [
17]
.
Regarding hemophilic patients treated with FVIII concentrate, patient HA-2 showed a prolonged peak time on the CWA-APTT but a normal peak height on the CWA-APTT, suggesting that the peak time and peak height may have different hemostatic abilities in the same CWA-APTT. At approximately 8% FVIII activity based on APTT, the peak height on the CWA-APTT showed a relatively low hemostatic ability and the peak height on the CWA-sTF/FIXa showed a normal hemostatic ability, suggesting that the evaluation of the hemostatic ability may differ between these assays. At approximately 16% of FVIII activity based on APTT, the peak height on the CWA-APTT showed a normal hemostatic ability and the peak height on the CWA-sTF/FIXa showed an elevated hemostatic ability, suggesting that the thrombotic risk in this state may be temporarily similar or higher than that in healthy persons. At approximately 100% of FVIII activity based on APTT, both the CWA-APTT and CWA-sTF/FIXa suggest that the thrombotic risk may be similar or higher than that in healthy persons.
In addition, the peak height on the CWA-TT was significantly higher in PRP than in PPP, suggesting that hemophilic patients treated FVIII concentrate, especially EHL-FVIII concentrate, were affected by thrombin burst-dependent platelets. These findings suggest that hemophilic patients treated with FVIII concentrate may temporarily have the same or higher risk of thrombosis than healthy individuals. This raises the question of whether or not this temporal effect of high-dose FVIII concentrate is problematic for thrombotic risk in hemophilic patients.
5. Conclusions
The FVIII activity in the present study was evaluated by several methods, including the APTT, CWA-TT and chromogenic assays, and the one stage method did not show the physiological activity. The peak height on the CWA-TT or CWA-sTF/FIXa showed higher coagulability than routine APTT. Hemophilic patients treated with FVIII concentrate are affected by thrombin burst. This raises the question of whether or not does the transient increase in FVIII activity leads to a thrombotic risk.
Author Contributions
Conceptualization, H.W.; methodology, T.M.; validation, K.S., formal analysis, Y.Y.; investigation, I.T; data curation, M.T.; writing—original draft preparation, M.T. and H.W.; writing—review and editing, M.S.; visualization, H.W.; supervision, H.S.; project administration, K.S.; funding acquisition, H.W. All authors have read and agreed to the published version of the manuscript.”
Funding
This research was funded by a Grant-in-Aid from the Ministry of Health, Labor and Welfare of Japan (21FC1008) and by the grant from the Japan Agency for Medical Research and Development (AMED) (JP22fk0410037)
Data Availability Statement
The data presented in this study are available on request to the corresponding author. The data are not publicly available due to privacy restrictions.
Acknowledgments
The authors thank Ms Nisii H and Sakano Y for their kind support in performing the assay for the CWA.
Conflicts of Interest
The measurements of CWA were partially supported by Instrumentation Laboratory Japan. In the other points, the authors declare no conflict of interest.
References
- Páramo, J.A. Treatment of haemophilia: From replacement to gene therapy. Med Clin (Barc). 2021, 157, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Carcao, M.; Srivastava, A. Factor VIII/factor IX prophylaxis for severe hemophilia. Semin Hematol. 2016, 53, 3–9. [Google Scholar] [CrossRef]
- National Hemophilia Foundation. MASAC document 267- MASAC recommendation concerning prophylaxis for hemophilia A and B with and without inhibitors. Available online: http://www.hemophilia.org/NHFWeb/MainPgs/MainNHF.aspx?menuid=57&contentid=1007 (accessed on 22 June 2023).
- Manco-Johnson, M.J.; Abshire, T.C.; Shapiro, A.D.; et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med 2007, 357, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Di Minno, G.; Cerbone, A.M.; Coppola, A.; et al. Longer-acting factor VIII to overcome limitations in haemophilia management: the PEGylated liposomes formulation issue. Haemophilia 2010, 16 (Suppl. 1), 2–6. [Google Scholar] [CrossRef] [PubMed]
- von Drygalski, A.; Chowdary, P.; Kulkarni, R.; Susen, S.; Konkle, B.A.; Oldenburg, J.; Matino, D.; Klamroth, R.; Weyand, A.C.; Jimenez-Yuste, V.; Nogami, K.; Poloskey, S.; Winding, B.; Willemze, A.; Knobe, K. XTEND-1 Trial Group. : Efanesoctocog Alfa Prophylaxis for Patients with Severe Hemophilia A. N Engl J Med. 2023, 388, 310–318. [Google Scholar] [PubMed]
- Keam, S.J. Efanesoctocog Alfa: First Approval. Drugs. 2023, 83, 633–638. [Google Scholar] [CrossRef]
- Oldenburg, J.; Mahlangu, J.N.; Kim, B.; Schmitt, C.; Callaghan, M.U.; Young, G.; Santagostino, E.; Kruse-Jarres, R.; Negrier, C.; Kessler, C.; Valente, N.; Asikanius, E.; Levy, G.G.; Windyga, J.; Shima, M. Emicizumab Prophylaxis in Hemophilia A with Inhibito. N Engl J Med. 2017, 377, 809–818. [Google Scholar] [CrossRef]
- Mahlangu, J.; Oldenburg, J.; Paz-Priel, I.; Negrier, C.; Niggli, M.; Mancuso, M.E.; Schmitt, C.; Jiménez-Yuste, V.; Kempton, C.; Dhalluin, C.; Callaghan, M.U.; Bujan, W.; Shima, M.; Adamkewicz, J.I.; Asikanius, E.; Levy, G.G.; Kruse-Jarres, R. Emicizumab Prophylaxis in Patients Who Have Hemophilia A without Inhibitors. N Engl J Med. 2018, 379, 811–822. [Google Scholar] [CrossRef]
- Nogami, K.; Soeda, T.; Matsumoto, T.; Kawabe, Y.; Kitazawa, T.; Shima, M. Routine measurements of factor VIII activity and inhibitor titer in the presence of emicizumab utilizing anti-idiotype monoclonal antibodies. J Thromb Haemost. 2018, 16, 1383–1390. [Google Scholar] [CrossRef]
- Peyvandi, F.; Kenet, G.; Pekrul, I.; Pruthi, R.K.; Ramge, P.; Spannagl, M.J. Laboratory testing in hemophilia: Impact of factor and non-factor replacement therapy on coagulation assays. Thromb Haemost. 2020, 18, 1242–1255. [Google Scholar] [CrossRef]
- Gray, E.; Kitchen, S.; Bowyer, A.; Chowdary, P.; Jenkins, P.V.; Murphy, P.; Platton, S.; Riddell, A.; Lester, W. Laboratory measurement of factor replacement therapies in the treatment of congenital haemophilia: A United Kingdom Haemophilia Centre Doctors' Organisation guideline. Haemophilia. 2020, 26, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Jeanpierre, E.; Pouplard, C.; Lasne, D.; Le Cam Duchez, V.; Eschwege, V.; Flaujac, C.; Galinat, H.; Harzallah, I.; Proulle, V.; Smahi, M.; Sobas, F.; Stepina, N.; Toulon, P.; Voisin, S.; Ternisien, C.; Nougier, C. French Study Group on the Biology of Hemorrhagic Diseases (the BIMHO group). Factor VIII and IX assays for post-infusion monitoring in hemophilia patients: Guidelines from the French BIMHO group (GFHT). Eur J Haematol. 2020, 105, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Matsumoto, T.; Ohishi, K.; Shiraki, K.; Shimaoka, M. Update on the Clot Waveform Analysis. Clin Appl Thromb Hemost; 26:1076029620912027.
- Wada, H.; Ichikawa, Y.; Ezaki, E.; Matsumoto, T.; Yamashita, Y.; Shiraki, K.; Shimaoka, M.; Shimpo, H. The reevaluation of thrombin time using a clot waveform analysis, J. Clin. Med. 2021, 10, 4840. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Shiraki, K.; Matsumoto, T.; Suzuki, K.; Yamashita, Y.; Tawara, I.; Shimpo, H.; Shimaoka, M. A Clot Waveform Analysis of Thrombin Time Using a Small Amount of Thrombin Is Useful for Evaluating the Clotting Activity of Plasma Independent of the Presence of Emicizumab. J Clin Med. 2022, 11, 6142. [Google Scholar] [CrossRef]
- Wada, H.; Shiraki, K.; Matsumoto, T.; Ohishi, K.; Shimpo, H.; Shimaoka, M. Effects of platelet and phospholipids on clot formation activated by a small amount of tissue factor. Thromb Res 2020, 193, 146–153. [Google Scholar] [CrossRef]
- Matsumoto, T.; Wada, H.; Toyoda, H.; Hirayama, M.; Yamashita, Y.; Katayama, N. Modified clot waveform analysis to measure plasma coagulation potential in the presence of the anti-factor IXa/factor X bispecific antibody emicizumab: comment. J Thromb Haemost. 2018, 16, 1665–1666. [Google Scholar] [CrossRef]
- Iorio, A.; Königs, C.; Reding, M.T.; Rotellini, D.; Skinner, M.W.; Mancuso, M.E.; Berntorp, E. Prophylaxis use of clotting factor replacement products in people with non-severe haemophilia: A review of the literature. Haemophilia. 2023, 29, 33–44. [Google Scholar] [CrossRef]
- Schmitt, C.; Mancuso, M.E.; Chang, T.; Podolak-Dawidziak, M.; Petry, C.; Sidonio Jr, R.; Yoneyama, K.; Key, N.S.; Niggli, M.; Lehle, M.; Peyvandi, F.; Oldenburg, J. Emicizumab dose up-titration in case of suboptimal bleeding control in people with haemophilia, A. Haemophilia. 2023, 29, 90–99. [Google Scholar] [CrossRef]
- Nogami, K.; Shima, M. Current and future therapies for haemophilia-Beyond factor replacement therapies. Br J Haematol. 2023, 200, 23–34. [Google Scholar] [CrossRef]
- López-Jaime, F.J.; Benítez, O.; Díaz Jordán, B.L.; Montaño, A.; Coll, J.; Quintana París, L.; Gómez-Del Castillo Solano, M.D.C. Expert opinion paper on the treatment of hemophilia a with emicizumab. Hematology. 2023, 28, 2166334. [Google Scholar] [CrossRef]
- Nardi, M.A. Hemophilia A: Emicizumab monitoring and impact on coagulation testing. Adv Clin Chem. 2023, 113, 273–315. [Google Scholar] [PubMed]
- Dev, P.; Ekhlak, M.; Dash, D.; Pathak, A. Platelet function suggests cardioembolic aetiology in cryptogenic stroke. Sci Rep. 2023, 13, 7615. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Wada, H.; Shinkai, T.; Tanemura, A.; Matsumoto, T.; Mizuno, S. Evaluation of hemostatic abnormalities in patients who underwent major hepatobiliary pancreatic surgery using activated partial thromboplastin time-clot waveform analysis. Thromb Res. 2021, 201, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Nogami, K.; Shima, M. A combined approach using global coagulation assays quickly differentiates coagulation disorders with prolonged aPTT and low levels of FVIII activity. Int J Hematol. 2017, 105, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Nogami, K. Semin Thromb Hemost.: Clot Waveform Analysis for Monitoring Hemostasis. 2023 (in press).
- Kobayashi, M.; Wada, H.; Fukui, S.; Mizutani, H.; Ichikawa, Y.; Shiraki, K.; Moritani, I.; Inoue, H.; Shimaoka, M.; Shimpo, H. A Clot Waveform Analysis Showing a Hypercoagulable State in Patients with Malignant Neoplasms. J Clin Med. 2021, 10, 5352. [Google Scholar] [CrossRef]
- Kamon, T.; Horie, S.; Inaba, T.; Ito, N.; Shiraki, K.; Ichikawa, Y.; Ezaki, M.; Shimpo, H.; Shimaoka, M.; Nishigaki, A.; Shindo, A.; Wada, H. The Detection of Hypercoagulability in Patients with Acute Cerebral Infarction Using a Clot Waveform Analysis. Clin Appl Thromb Hemost. 2023, 29, 10760296231161591. [Google Scholar] [CrossRef]
Figure 1.
Standard curve for the FVIII activity with and without emicizumab. Emi, emicizumab; FVIII, coagulation factor FVIII; closed box, FVIII activity with emicizumab; open box, FVIII activity without emicizumab.
Figure 1.
Standard curve for the FVIII activity with and without emicizumab. Emi, emicizumab; FVIII, coagulation factor FVIII; closed box, FVIII activity with emicizumab; open box, FVIII activity without emicizumab.
Figure 2.
The FVIII activity based on a one-stage clotting assay using the clot waveform analysis (CWA)-APTT or CWA-thrombin time or a chromogenic assay. FVIII, coagulation factor FVIII; APTT, activated partial thromboplastin time; TT, thrombin time; FVIII activity without emicizumab; NS, not significant; Plasma with emicizumab was excluded for measurement of FVIII activity.
Figure 2.
The FVIII activity based on a one-stage clotting assay using the clot waveform analysis (CWA)-APTT or CWA-thrombin time or a chromogenic assay. FVIII, coagulation factor FVIII; APTT, activated partial thromboplastin time; TT, thrombin time; FVIII activity without emicizumab; NS, not significant; Plasma with emicizumab was excluded for measurement of FVIII activity.
Figure 3.
Correlation between the FVIII activity based on a one-stage clotting assay using the CWA-APTT and CWA-thrombin time (a, without emicizumab and b, with emicizumab) and between the FVIII activity based on a one stage clotting assay using the CWA-thrombin time and a chromogenic assay. FVIII, coagulation factor FVIII; CWA, clot waveform analysis; APTT, activated partial thromboplastin time; TT, thrombin time; Emi, emicizumab; red symbol, plasma with emicizumab.
Figure 3.
Correlation between the FVIII activity based on a one-stage clotting assay using the CWA-APTT and CWA-thrombin time (a, without emicizumab and b, with emicizumab) and between the FVIII activity based on a one stage clotting assay using the CWA-thrombin time and a chromogenic assay. FVIII, coagulation factor FVIII; CWA, clot waveform analysis; APTT, activated partial thromboplastin time; TT, thrombin time; Emi, emicizumab; red symbol, plasma with emicizumab.
Figure 4.
CWA-APTT in a hemophilia patient treated with FVIII concentrate (a) and a hemophilia patient treated with emicizumab (b). CWA, clot waveform analysis; APTT, activated partial thromboplastin time; navy line, fibrin formation curve; FFH, fibrin formation height; pink line, 1st derivative curve (velocity); 1
stDPH, first derivative peak height; light blue, 2nd derivative curve (acceleration); 2nd DPH, second derivative peak height; solid line, patient; dotted line, healthy volunteer activities were 92.0%-101% with a normal peak time and normal peak height on the CWA-APTT and a markedly shortened peak time and elevated peak height of the 1st DP on the CWA-TT (
Figure 5c). Regarding the CWA-TT, HA-6 treated with emicizumab showed a low peak height of the 1st DP and no significant difference between PPP and PRP (
Figure 6a,b), whereas HA-2 treated with FVIII concentrate showed a low peak height and second peak of the 1st DP in PPP and a markedly high peak height and combined first and second peaks of the 1st DP in PRP (
Figure 6c,d).
Figure 4.
CWA-APTT in a hemophilia patient treated with FVIII concentrate (a) and a hemophilia patient treated with emicizumab (b). CWA, clot waveform analysis; APTT, activated partial thromboplastin time; navy line, fibrin formation curve; FFH, fibrin formation height; pink line, 1st derivative curve (velocity); 1
stDPH, first derivative peak height; light blue, 2nd derivative curve (acceleration); 2nd DPH, second derivative peak height; solid line, patient; dotted line, healthy volunteer activities were 92.0%-101% with a normal peak time and normal peak height on the CWA-APTT and a markedly shortened peak time and elevated peak height of the 1st DP on the CWA-TT (
Figure 5c). Regarding the CWA-TT, HA-6 treated with emicizumab showed a low peak height of the 1st DP and no significant difference between PPP and PRP (
Figure 6a,b), whereas HA-2 treated with FVIII concentrate showed a low peak height and second peak of the 1st DP in PPP and a markedly high peak height and combined first and second peaks of the 1st DP in PRP (
Figure 6c,d).
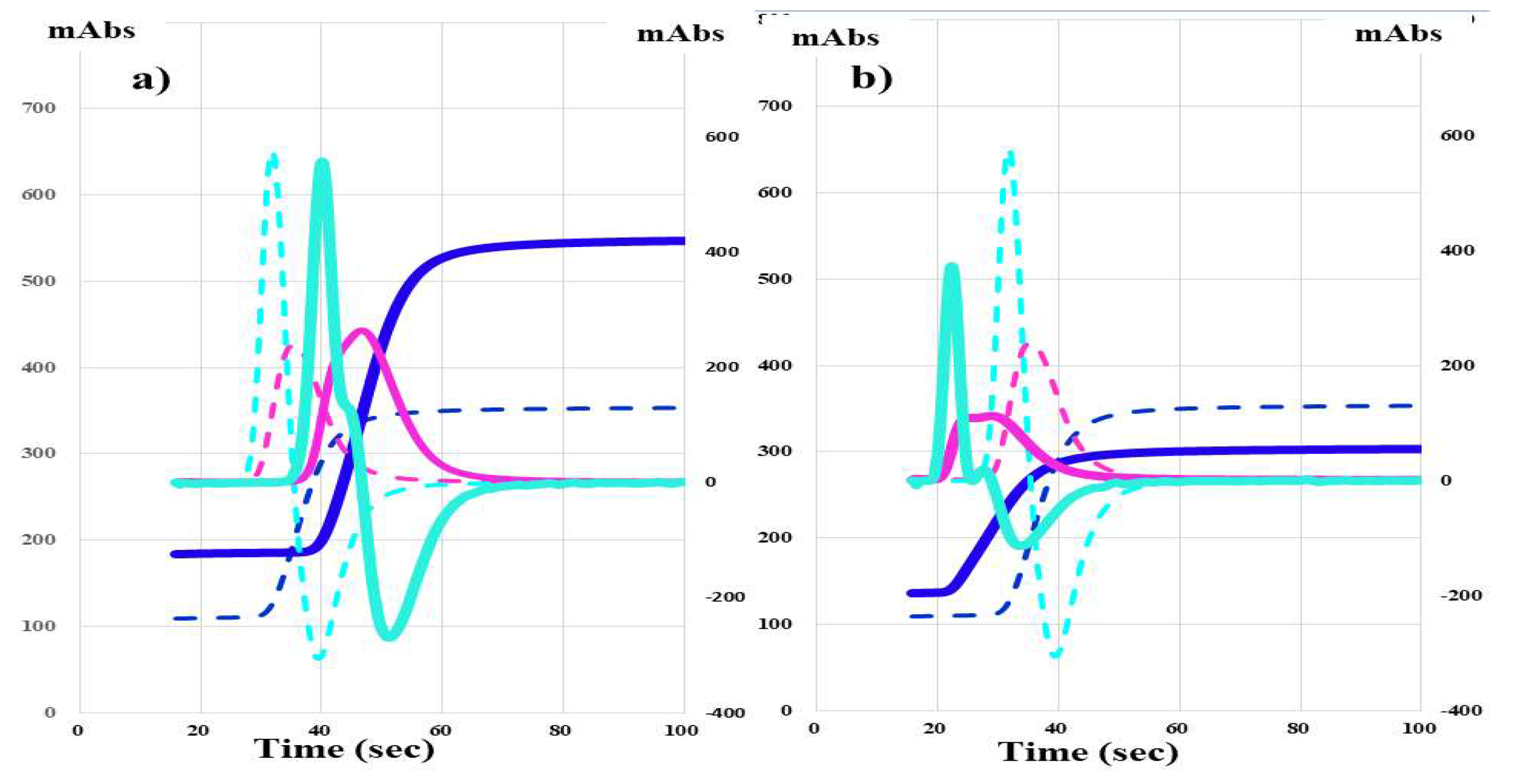
Figure 5.
CWA-APTT and CWA-sTF/FIXa in a hemophilia patient treated with FVIII concentrate. CWA, clot waveform analysis; APTT, activated partial thromboplastin time; sTF/FIXa, small amount of tissue factor induced FIX activation assay; navy line, fibrin formation curve; FFH, fibrin formation height; pink line, 1st derivative curve (velocity); 1stDPH, first derivative peak height; light blue, 2nd derivative curve (acceleration); 2nd DPH, second derivative peak height; solid line, patient; dotted line, healthy volunteer .
Figure 5.
CWA-APTT and CWA-sTF/FIXa in a hemophilia patient treated with FVIII concentrate. CWA, clot waveform analysis; APTT, activated partial thromboplastin time; sTF/FIXa, small amount of tissue factor induced FIX activation assay; navy line, fibrin formation curve; FFH, fibrin formation height; pink line, 1st derivative curve (velocity); 1stDPH, first derivative peak height; light blue, 2nd derivative curve (acceleration); 2nd DPH, second derivative peak height; solid line, patient; dotted line, healthy volunteer .
Figure 6.
CWA-TT in a hemophilia patient treated with emicizumab or FVIII concentrate. CWA, clot waveform analysis; TT, thrombin time; navy line, fibrin formation curve; FFH, fibrin formation height; pink line, 1st derivative curve (velocity); 1stDPH, first derivative peak height; light blue, 2nd derivative curve (acceleration); 2nd DPH, second derivative peak height; PPP, platelet poor plasma; PRP, platelet rich plasma; EMI. Emicizumab; FVIII, coagulation factor FVIII reagent.
Figure 6.
CWA-TT in a hemophilia patient treated with emicizumab or FVIII concentrate. CWA, clot waveform analysis; TT, thrombin time; navy line, fibrin formation curve; FFH, fibrin formation height; pink line, 1st derivative curve (velocity); 1stDPH, first derivative peak height; light blue, 2nd derivative curve (acceleration); 2nd DPH, second derivative peak height; PPP, platelet poor plasma; PRP, platelet rich plasma; EMI. Emicizumab; FVIII, coagulation factor FVIII reagent.
Table 1.
Subjects.
| No |
Disease |
Severity |
FVIII activity |
Inhibitor |
RRT |
Drug |
| HA-1 |
HA |
Severe |
≤1.0% |
Negative |
Yes |
Rurioctocog alfa pegol |
| HA-2 |
HA |
Moderate |
1.8% |
Negative |
Yes |
Efraloctocog alfa |
| HA-3 |
HA |
Severe |
≤1.0% |
Negative |
Yes |
Rurioctocog alfa pegol |
| HA-4 |
HA |
Severe |
≤1.0% |
Negative |
Yes |
Rurioctocog alfa pegol |
| HA-5 |
HA |
Mild |
23.3% |
Negative |
No |
Octocog beta |
| HA-6 |
HA |
Severe |
≤1.0% |
Positive |
Yes |
Emicizumab |
| HA-7 |
HA |
Severe |
≤1.0% |
Negative |
Yes |
Octocog beta |
| HA-8 |
HA |
Moderate |
≤1.0% |
Negative |
No |
Rurioctocog alfa pegol |
| HA-9 |
HA |
Severe |
≤1.0% |
Negative |
Yes |
Emicizumab |
| HA-10 |
HA |
Severe |
≤1.0% |
Negative |
Yes |
Lonoctocog alfa |
| HA-11 |
HA |
Severe |
1.0% |
Negative |
Yes |
Octocog beta |
| HA-12 |
HA |
Mild |
14.1% |
Negative |
No |
FDCHB-FVIII |
| HA-13 |
HA |
Severe |
≤1.0% |
Negative |
Yes |
Rurioctocog alfa pegol |
| HA-14 |
HA |
Moderate |
1.6% |
Negative |
No |
― |
| HA-15 |
HA |
Moderate |
3.9% |
Negative |
No |
Rurioctocog alfa pegol |
| HA-16 |
HA |
Mild |
6.7% |
Negative |
No |
Rurioctocog alfa |
| HA-17 |
HA |
Mild |
5.4% |
Negative |
No |
Rurioctocog alfa |
| HA-18 |
HA |
Moderate |
2.2% |
Negative |
No |
― |
| HA-19 |
HA |
Severe |
≤1.0% |
Negative |
Yes |
Rurioctocog alfa pegol |
| HA-20 |
HA |
Severe |
≤1.0% |
Negative |
Yes |
Emicizumab |
| HA-21 |
HA |
Moderate |
1.0% |
Negative |
Yes |
Emicizumab |
| HA-22 |
HA |
Severe |
≤1.0% |
Negative |
Yes |
Emicizumab |
| HA-23 |
HA |
Severe |
≤1.0% |
Negative |
Yes |
Efraloctocog alfa |
| 24 |
HA* |
Mild |
20.4% |
Negative |
No |
― |
| 25-1 |
AHA |
Severe |
≤1.0% |
Positive |
No |
APCC |
| 25-2 |
AHA |
Severe |
≤1.0% |
Positive |
No |
APCC |
| 25-3 |
AHA |
Severe |
≤1.0% |
Positive |
No |
APCC |
| 25-4 |
AHA |
Severe |
≤1.0% |
Positive |
No |
― |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).