1. Introduction
The mammal-specific mini-organ, the hair follicle (HF), plays a crucial role in skin homeostasis not least via its life-long cyclical tissue transformations characterized by distinct phases of active hair growth (anagen), partial involution (catagen), and relative rest (telogen) [
1]. Such dramatic tissue remodeling impacts not only hair fiber production but, more generally in the skin vasculature, innervation, immune status, etc. [
2,
3]. During re-entry into a new anagen phase of the asynchronous human hair growth cycle, the HF ‘reconstructs’ its so-called Follicular-Melanin-Unit (FMU) to facilitate new pigmented hair shaft formation [
4]. However, unlike epidermal melanocytes, the activity of some follicular melanocytes (e.g., melanogenically competent HF melanocytes) is tightly coupled to the hair growth cycle [
2,
5,
6]. In this way, melanin produced by this highly differentiated and post-mitotic subpopulation of follicular melanocytes is transferred to proliferative and differentiating pre-cortical keratinocytes in the anagen hair bulb, during the formation of the pigmented hair shaft. Although melanocyte subpopulations in the FMU and epidermal melanin unit (EMU) are derived from the same pluripotent cells of the neural crest during embryonic development, these pigment cell compartments remain distinct (but open) in adult human skin [
7]. Another striking difference between the cell biology of the epidermis and HF pigmentation processes is the lack of post-biogenic change to melanin when transferred to cortical keratinocytes that make up the bulk of the hair fiber. In this way, the distal tip of often exceptionally long scalp fibers retains the same melaninization as the most recently formed proximal segments of the hair emerging from the scalp [
4].
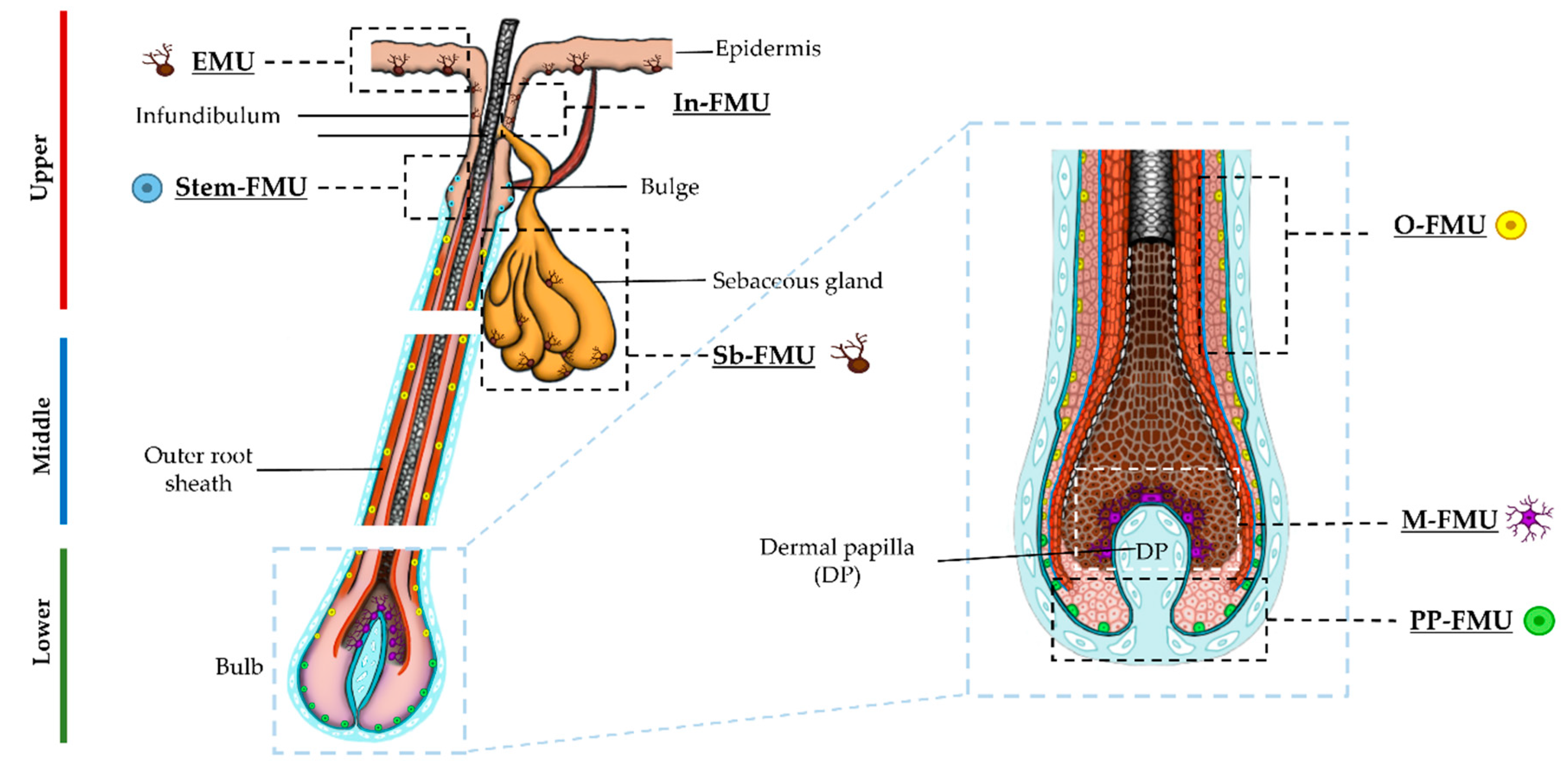
Melanocytes in the HF are extraordinarily diverse and can be largely defined by their tightly restricted anatomic location (
Figure 1), level of differentiation (wide morphologic variation), and melanogenic activity (amelanotic, eu/pheomelanic) [
2,
8]. During the first three to four decades of human life (i.e
., before the onset of hair graying), most pilosebaceous units in the adult scalp contain a phenomenally active group of melanocytes, in terms of their melanin synthesis, located in the anagen hair bulb (M-FMU) [
9,
10].
There, a relatively small number of post-mitotic and terminally differentiated melanocytes crosstalk with neighboring keratinocytes and dermal papilla fibroblasts to produce extraordinary amounts of melanin, year after year, to pigment scalp hair fibers that can be over a meter long. In this way, these M-FMU melanocytes experience a very different reality to similarly melanogenically active melanocytes in the HF infundibulum (In-FMU) or basal layer of the sebaceous gland (Sb-FMU); likely due to very different relationships with their partner keratinocytes and adjacent mesenchyme [
8]. At the other end of the pigmentary spectrum resides a diversity of amelanotic or poorly differentiated melanocytes and/or melanoblasts. The latter cells reside in the upper hair follicle close to the insertion site of the arrector pili muscle (euphemistically referred to as the HF ‘bulge’ region, although human HFs (unlike those in mice) do not have an anatomically distinct bulge. This region contains HF melanocyte stem cells (Stem-FMU). Amelanotic melanocytes are also located 1-3 mm more proximally in the skin, in the mid-to-lower outer root sheath (O-FMU), as well as in the most peripheral proximal bulb region (PP-FMU) of the growing anagen HF (
Figure 1) [
2,
8]. The nature of the relationships between the three ‘immature’ melanocyte subpopulations
(i.e., Stem-FMU, O-FMU, PP-FMU), between the four ‘mature’ melanocytes subpopulations (i.e., EMU, In-FMU, Sb-FMU, and M-FMU), and between both ‘mature’ and ‘immature’ pigment cell populations remains enigmatic. For example, it is not yet clear whether at least some of these pigment cell subpopulations can move or migrate throughout the HF during the anagen hair fibre growth phase of the hair growth cycle.
Much of our knowledge of melanocyte development and subpopulation heterogeneity has been obtained and deduced from inbred laboratory mice. These studies have been crucial for discovering genes underlying hair cycle control and general pigmentation. Inevitably however, distinct hair growth cycle (mouse synchronous versus human mosaic) and another cell biological and enzymological features of HFs between the nocturnal mouse and diurnal human, particularly around the regulation of skin and HF pigmentation, limit the accurate translation of findings from mouse models to human. Still, markers of murine HF melanocyte lineages have provided at least a starting point for the exploration and identification of melanocyte plasticity in human skin, and include markers for melanogenesis/melanin synthesis, (e.g
., MITF, DCT/Trp2, Trp1) and melanocyte development, differentiation, and survival (e.g
., SOX10, c-KIT, MITF) among others [
2,
6]. While much of current cutaneous pigmentation research continues to reside in the mouse domain, the identity and characterization of heterogeneous subpopulations of melanocytes of human scalp HFs is a fertile area of research where many important questions in human HF pigmentation science remain to be addressed.
This study addresses whether melanocytes in the growing human (anagen) scalp HF bulb exist along a differentiation/maturation spectrum, characterized here by the expression of so-called ‘early’ and ‘late’ maturity markers
in vivo. Additionally, using the receptor tyrosine kinase cell surface marker protein c-KIT (CD117), which in human skin identifies a population of ‘precursory’ melanocytes [
11,
12], we attempt to isolate from the lower portion of human anagen HFs, melanocyte subpopulations of the three distinct follicular units, namely O-, PP- and M-FMUs (
Figure 1), which reflect variably levels of cell differentiation. The SCF/KIT pathway plays a critical role in the control of normal human melanocyte homeostasis [
12] and c-KIT expression can demarcate a population of precursor melanocytes in human HF.
3. Discussion
The trajectories encountered by pigment cells in mammalian skin from their first commitment in the neural crest during development [
22] to their proliferation and differentiation as fully-committed cutaneous melanocytes of the skin and hair follicle [
23], ultimately their demise episodically during hair follicle regression (Catagen) or essentially permanently during canities (hair graying) has been best described in the mouse [
24], though some data for humans has begun to emerge [
25,
26]. We recently reviewed the multiplicity of melanocytes in the adult human scalp hair follicle [
8] and in the present study re-examined whether some of this multiplicity is also extended to the anagen hair bulb, which we conventionally see predominantly as the melanogenic centre of the growing HF [
4].
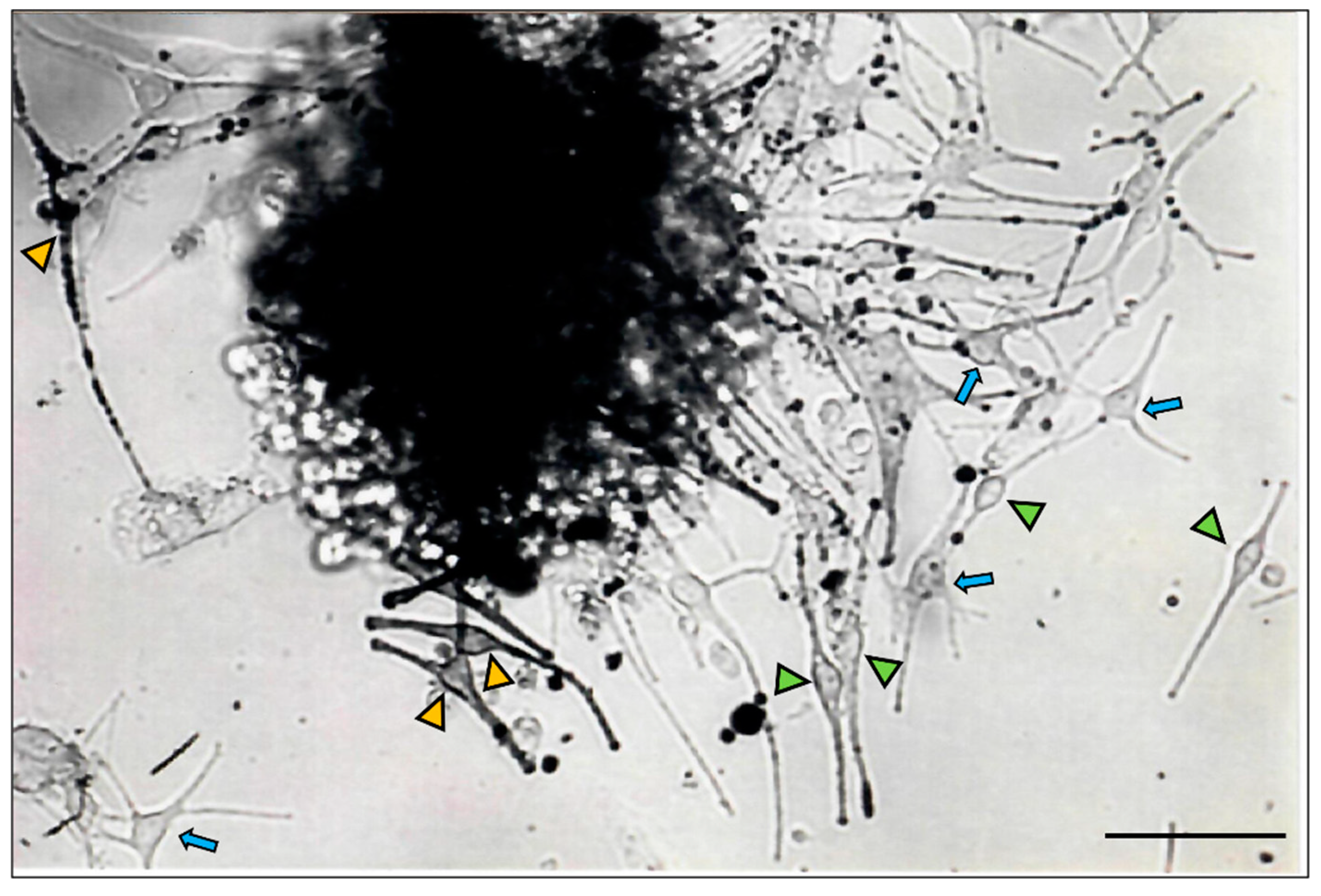
Initiation of HF melanocyte primary cultures from human scalp follicular tissue, limited to the anagen hair bulb region, revealed the explanation of a remarkable diversity of pigment cell morphologies (
Figure 2). The characteristic appearance of these explants before the proliferation of some cells occurred attests to the pre-existence of a range of pigment cell maturity states that is both tissue-restricted and importantly only transient, i.e., is dramatically remodeled during the hair growth cycle that sees the loss of the anagen hair bulb during catagen [
27].
One of the challenges of this field of melanocyte heterogeneity in adult human skin and HF is the scarcity of non-pigment cell differentiation-associated markers, and associated reagents, that can detect immature melanocytes. Specifically, amelanotic and non-dendritic melanocytes/melanoblasts are difficult to detect given the lack of specific markers, whereas the fully differentiated melanocytes of the bulb region (M-FMU) have been identified in HFs by their expression of melanocytic markers such as MITF, tyrosinase and tyrosinase-related proteins 1 and 2 (TRP1 and DCT/TRP2, respectively) [
2,
6]. Additionally, human HF melanocytes have been identified, isolated, and cultured [
13,
14]. However, it is our view that using these methods, we can expand selectively the immature melanocytes of the ORS (O-FMU). Thus, still more robust methods are needed to isolate and propagate HF melanocytes that are representative of all subpopulations in HF, including hair bulb
in vitro, to better functional characterize these cells. Recently, c-KIT was used to isolate and enrich melanocyte populations derived from human corneal limbus [
28] and melanocyte precursor cells present in the human limbal stroma [
29]. C-KIT, a receptor tyrosine kinase protein, and its ligand stem cell factor (SCF) are required for cyclic regeneration of the hair pigmentary unit and migration of melanoblasts in developing murine hair follicles [
12,
30,
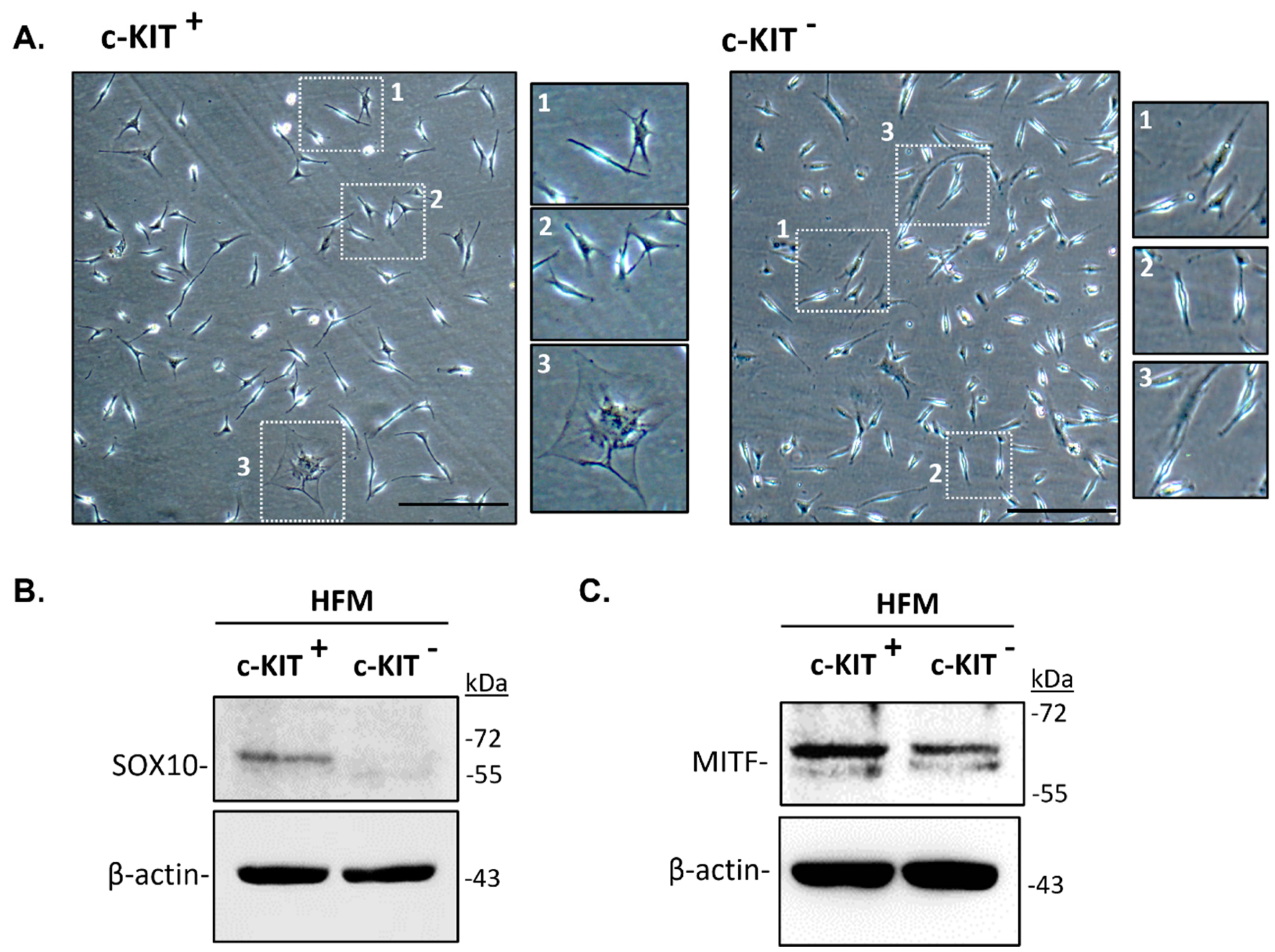
31]. In this study, we successfully isolated and expanded c-KIT-positive and c-KIT-negative populations of bulbar melanocytes of relatively similar morphologies. The melanocytic identity of the latter c-KIT-negative subpopulation was confirmed by the expression of MITF, while only the c-KIT-positive subpopulation additionally expressed readily detectable levels of the transcription factor SOX10.
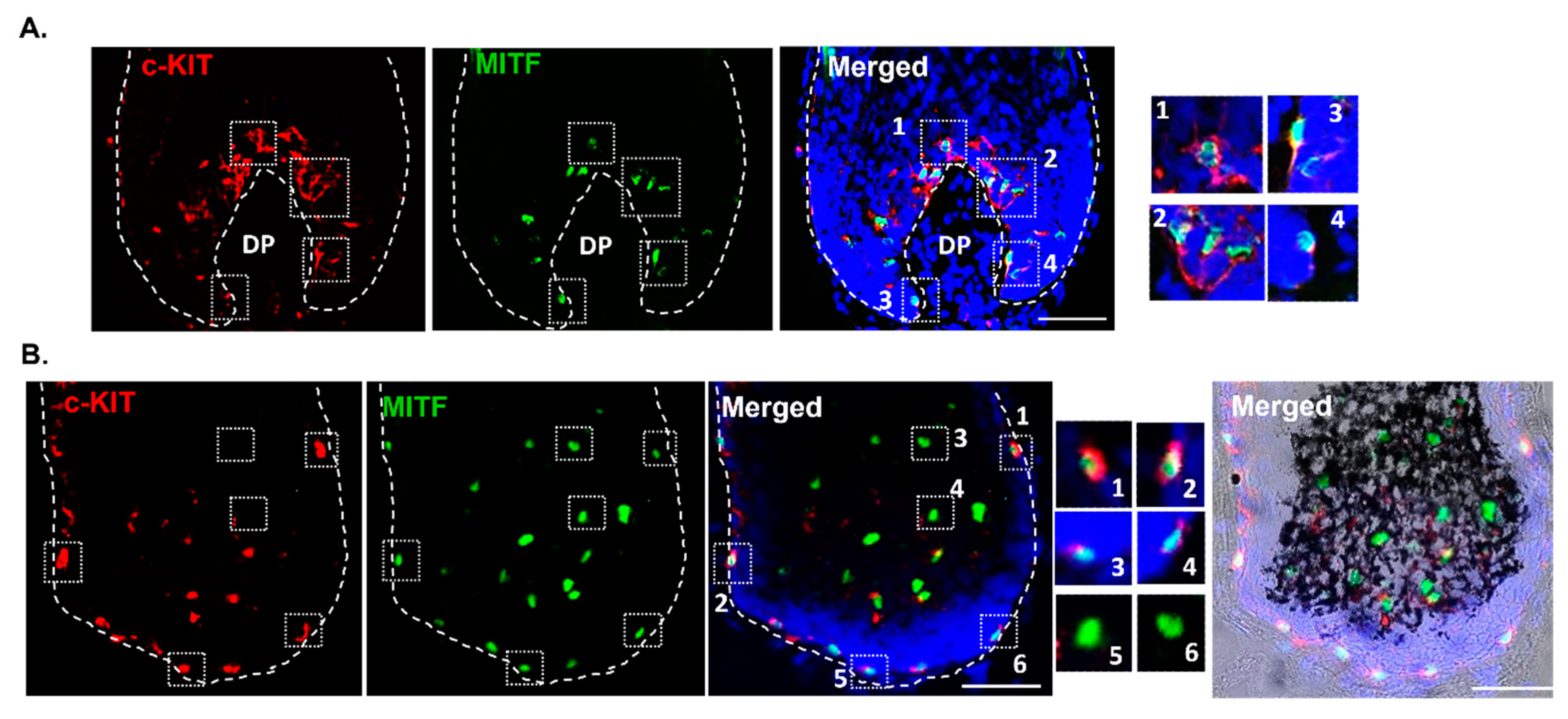
We have previously shown in developing mouse HFs that melanoblasts express c-KIT as a prerequisite for migration into the SCF-supplying HF epithelium [
32]. Importantly, differentiated c-KIT-positive melanocytes target the HF bulb, while c-KIT-negative melanoblasts invade both the ORS and bulge in the fully developed HF. Thus, we confirm here for the human hair bulb, the presence of melanocytes expressing markers of early pigment cell differentiation, such as c-KIT and additionally SOX10, together with melanocytes positive for more mature markers like MITF, gp100, and Melan-A. SOX10 as a neural crest marker can be considered an early differentiation melanocyte marker [
33] and in melanoma cells has been associated with melanoma cell proliferation, tumor formation, and growth [
34,
35]. SOX10 has been shown to heavily influence melanocyte development in adults, while SOX9 has been implicated in melanogenesis [
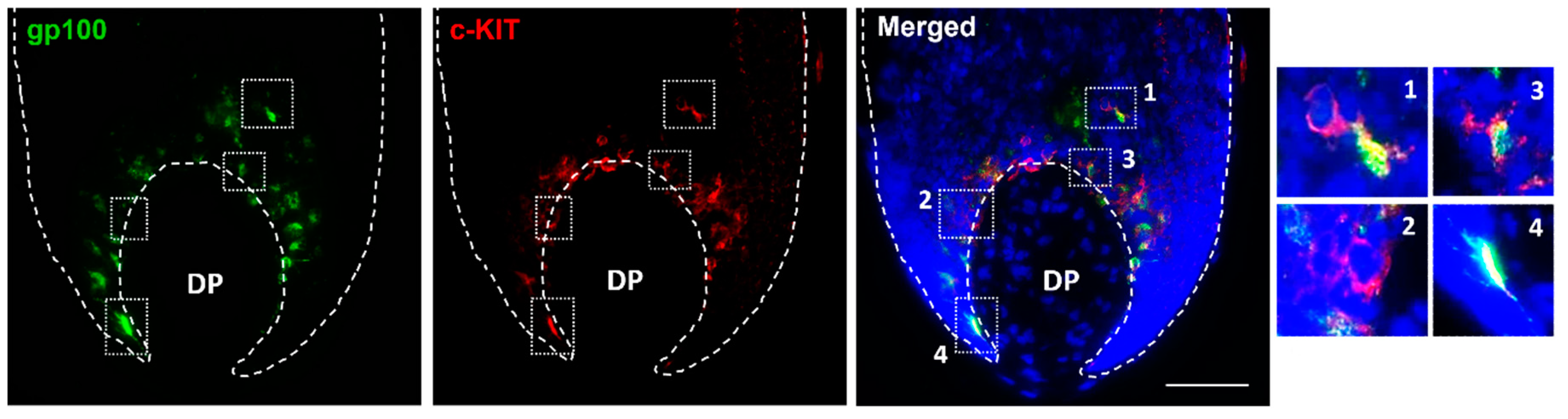
36]. This is supported by our finding that some SOX10-positive melanocytes in the anagen hair bulb appeared to be relatively immature (non-melanic) as evidenced by the absence of the melanosome marker gp100.
Importantly, variable expression of c-KIT in hair bulb melanocytes indicates that some pigment cells are more mature than others. Specifically, the sole expression of c-KIT (i.e
., without additional expression of gp100) in melanocytes suggested that these cells are not (yet) competent for melanosome biogenesis and subsequent melanogenesis. In this way, these melanocytes are positioned differently on the spectrum of pigment cell differentiation to closely located melanocytes that exhibit a co-expression of c-KIT with gp100. To our knowledge, no other cell types in this epithelial region of the human adult HF have been reported to express c-KIT. This suggests the existence of at least two distinct subpopulations of melanocytes in the proximal bulb of human HFs, characterized by the differential expression of markers of melanocyte development and differentiation (i.e., SOX10, c-KIT, and MITF). Support for this interpretation can be found in other expression pathways in the hair follicle pigmentary unit, including for hypothalamus-pituitary-adrenal axis components (e.g., α-MSH, ACTH, or CRF and their respective receptors), which are expressed in melanocytes of the PP-FMU but not the M-FMU [
37].
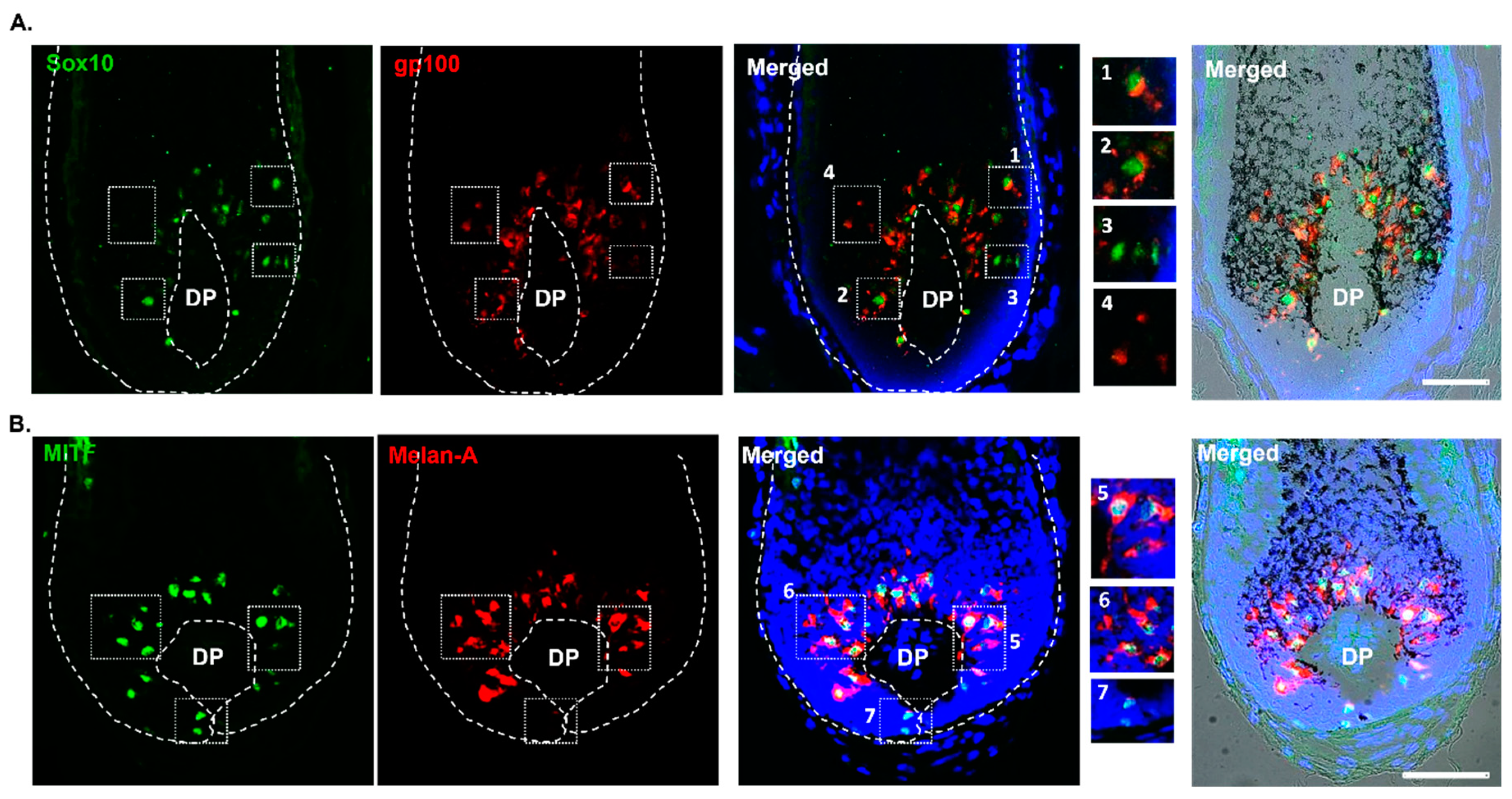
Melanocyte differentiation is largely dependent on the expression of SOX10 and MITF, the master regulator of melanocyte development, function, and survival. The best-characterized transcriptional target of SOX10 in melanocytes appears to be MITF and numerous studies demonstrated that SOX10 directly activates MIFT transcription [
38]. The expression of SOX10 in melanocytes localized in the melanogenic zone above the DP of adult scalp human HFs and in the c-KIT positive fraction (but not/much less in the c-KIT-negative fraction) of isolated hair bulb melanocytes indicates the presence of melanocytes of distinct differentiation stages in this region. We suggest that c-KIT expression per se does not itself confer permissiveness or capacity for melanogenic potential in hair bulb melanocytes, as c-KIT-positive melanocytes may or may not lack co-expression of the (pre)-melanosomal marker gp100, though all c-KIT-positive melanocytes expressed MITF. By contrast, the corollary does not appear to be true i.e
., while all hair bulb melanocytes are defined by their intrinsic MITF expression, not all MITF-positive melanocytes additionally express c-KIT, Melan-A, or gp100. Thus, distinct hair bulb melanocyte populations can be distinguished by the expression of the so-called ‘early’ pigment cell differentiation markers SOX10 and c-KIT in the melanogenically-active bulb region of HF, together with the expression of the master pigment cell transcription factor MITF, and down-stream differentiation-associated melanosome markers gp100 and Melan-A.
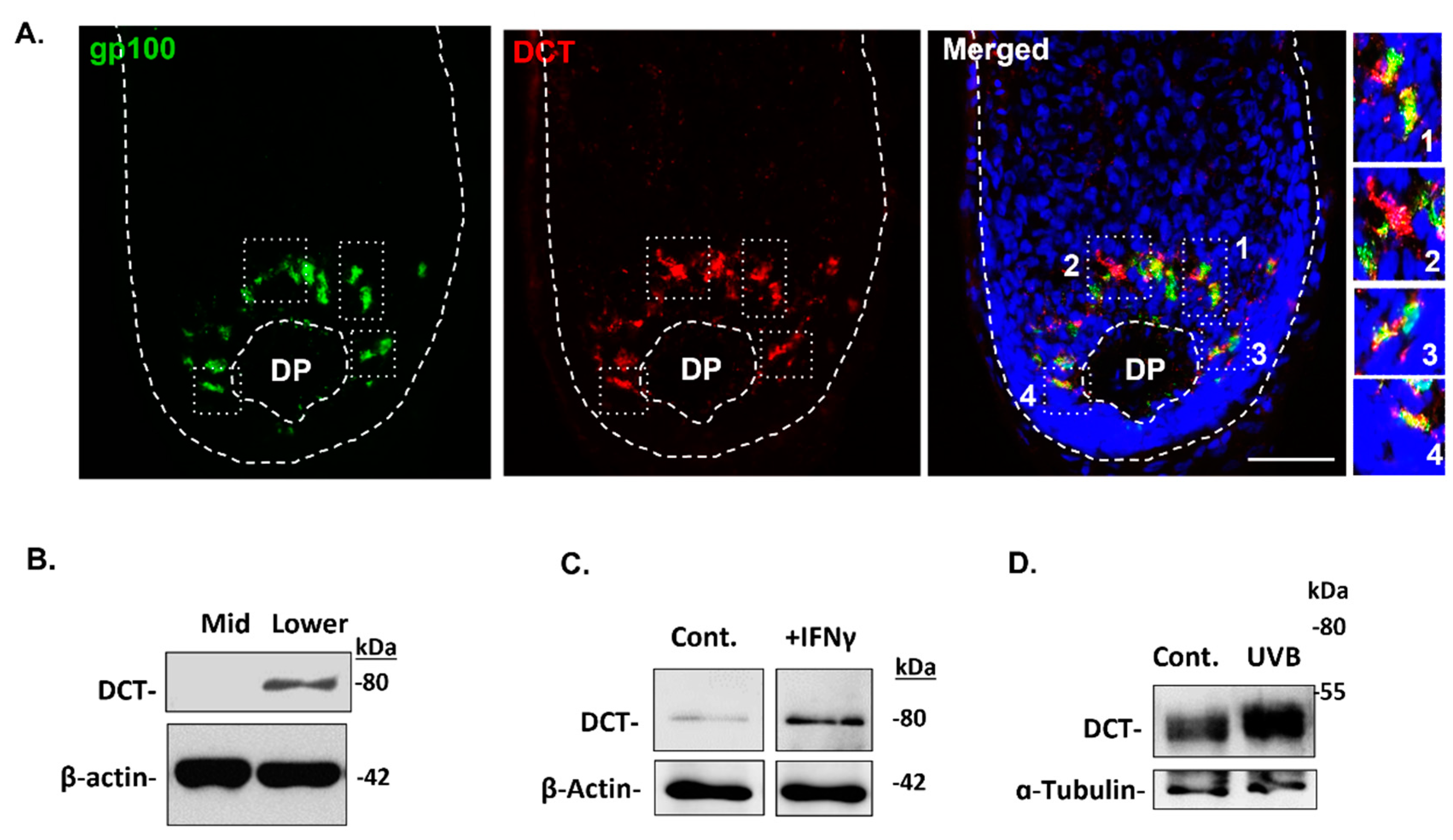
Despite the key role of dopachrome tautomerase (DCT) in melanin biosynthesis, this protein is also one of the markers of the earliest melanocyte differentiation reported in mouse HF [
18,
40]. Indeed, Wegner
et al. first reported that melanocyte-specific expression of DCT, a direct target of SOX10, was dependent on synergistic gene activation by both SOX10 and MITF transcription factors, in mouse HF melanocytes [
39]. Moreover, MITF synergistically enhances SOX10-dependent activation of the DCT promoter. Thus, it appears anomalous that DCT expression in the melanogenic bulb HF region would be lacking for human scalp anagen hair bulb melanocytes, as previously reported using a non-commercial antibody [
20]. Here, using two anti-DCT commercial antibodies, we localized DCT protein expression (by IHC and WB) to melanocytes of the human proximal bulb region, including to melanocytes that appear to not yet be actively engaged in melanosome biogenesis, given the lack of expression of the melanosome antigen gp100. We further confirmed the expression of this key component of the enzymatic pathway for melanogenesis in ex vivo HF organ cultures and found that this protein could be upregulated in the proximal bulb region of human HFs after exposure to stress signal mediators, like pro-inflammatory IFNg and UVB. This supports the previously reported view of DCT as a multi-functional protein, exerting functions beyond basal melanogenesis, including as a stem cell, and oxidative stress response modifier [
41,
42]. It additionally supports the view that stimulation of DCT levels (and activity) in melanocyte subpopulations of the human scalp may enhance local stress handling (e.g., oxidative stress/DNA damage/inflammation) to protect against melanocyte apoptosis, during canities-associated hair greying. By the same token, less mature gp100-negative but DCT-positive melanocytes in the hair bulb, as detected in the current study, may reflect an enzymatically-inactive DCT subpopulation that may be more vulnerable to stress handling.
In summary, our study provides new evidence that within the human anagen hair bulb in vivo melanocyte-lineage cells exhibiting immature or mature melanocyte marker expression co-exist. This implies a functional diversity in this region of the human scalp HF, beyond our traditional view of melanocyte functionality expressed as the capacity to make melanosomes (evidenced by gp100 and or Melan-A expression) and subsequently melanin production. What these immature bulbar melanocytes are doing in this part of the HF remains to be elucidated. Could they represent an in-transit migratory population destined to mature and synthesize melanin, or could they contribute in other ways to melanocyte biology, i.e., as non-melanogenic pigment cells?
The role of SOX10 in the life history of adult bulbar melanocytes is also intriguing, given that the expression of this transcription factor is required for melanocyte/melanoblast commitment in utero [
43]. Is SOX10 expression dispensable for some melanocytes in the adult hair bulb? Our study shows in vitro a subpopulation of c-KIT-negative but MITF-positive melanocytes that lack SOX10 protein expression. Lastly, the expression of the multi-functional protein DCT (i.e., as melanogenic enzyme, oxidative stress modulator, etc.) does appear to be an obligate feature of melanocytes in the human anagen hair bulb, regardless of their differentiation status. Much remains to be explored in the multi-FMU landscape of the human scalp HF, but it is our view that we now need to move beyond the mouse-centric view of HF melanocyte biology.
4. Materials and Methods
Human HF collection, organ histo-culture and follicular melanocyte isolation
Individual anagen HFs were isolated from the human occipital scalp from routine elective hair restoration surgeries, after informed patient consent, adhering to the declaration of Helsinki principles, following ethical approval granted by the UCD ethics committee (#LS-19-44). HFs were microdissected from scalp pieces of approximately 1 cm
2 under a stereomicroscope and cultured for 24 hours at 37°C with 5% CO
2 in minimal William’s media E (Gibco, #32557020), supplemented with 10mg/mL insulin (Sigma-Aldrich, #I6634),
10 ng/ml hydrocortisone (Sigma-Aldrich, #H0888), and 1% penicillin/streptomycin (Gibco, #15070063). After quality control, growing HFs were allocated randomly to the different experimental groups. At 24 h after isolation, supplemented William’s E media was replaced and the cultured hair follicles were treated with 50 IU/ml of IFN-γ (Stemcell technology #78020.1) for 24 h at 37°C, 5% CO
2. Furthermore, 24 h after isolation HFs were exposed to UVB (280-320 nm) irradiation (dosage 402 mJ/cm
2), by using a commercial UVB bulb. Treated HFs were collected for OCT preservation and Protein extraction. For follicular melanocyte isolation, individual hair follicles (at least 20 HFs) were treated according to methods described by Tobin
et al. [
13,
14]. Briefly, after microdissection and treatment of the HF sample with collagenase (Gibco, #17104019) for 2 hours at 37ºC, a cell suspension was obtained by 3 cycles of 0.05% trypsin/EDTA digestion (Gibco, 2530054). Single-cell suspensions were magnetically labeled with CD117 Human microbeads, according to the manufacturer’s instructions (Miltnyi Biotec, # 130091332). Both CD117
+ and CD117
- fractions were collected and cells were cultured in melanocyte media (2:1 media; MEM supplemented with 2% FBS, 1% non-essential aminoacids, 5 ngml
-1 of endothelin-1 and bFGF,
1% penicillin/streptomycin and complete keratinocyte growth medium; Promocell), at 37°C, 5% CO
2.
4.1. Frozen Hair Sample Processing and Immunohistochemistry
Hair follicle samples embedded in an OCT matrix were processed for longitudinal sections of 8-10 µm, (Leica cryostat). After air-dried for 10 minutes, cryosections were fixed with acetone at -20ºC for 10 minutes and preincubated with blocking buffer (PBS + 5% donkey serum + 0.5% BSA) for one hour at room temperature prior to incubation with primary antibodies overnight at 4ºC (see Table 1). Secondary antibody incubation was performed for one hour at 37°C with fluorescent-conjugated antibodies (see Table 1) and slides were mounted with antifade VectorShield mounting medium with DAPI (Vector laboratories, H-1500). Immunostaining visualization was performed using an IX83 Inverted Fluorescent Microscope (Olympus, Germany). Images were taken using Olympus cellSens Software and processed with ImageJ software. For DCT/TRP2 immunodetection, the fixation step was performed with 7% paraformaldehyde for 10 minutes.
4.2. Protein Extraction and Western-Blotting
To prepare HF protein extracts, the selected regions of HFs (Mid- Lower;
Figure 1) were collected and lysed with 8M urea buffer (8M urea; tris-Hcl pH 7.5) supplemented with protease and phosphatase inhibitors (Roche: #11836170001; 04906837001) and kept for 4 hours at 4ºC. Total protein extracts were obtained by using an ultrasonic homogenizer (Syclon SKL-150w; 2 cycles of 2 seconds on/off), followed by centrifugation at 10000
g for 10 minutes at 4°C. Protein concentration was determined using the DC protein Assay Kit (Bio-Rad) and an equal amount of each sample (40µg) was boiled at 95° C for 5 minutes. Total proteins were separated on SDS-PAGE gels, transferred onto nitrocellulose membranes in transfer buffer (25mM Tris; 192mM glycine; 0.5% SDS; 20% Ethanol) for 120 mins at 100V and processed for immunoblot analysis. Membranes were blocked with 5% skimmed milk in TBST (Tris Buffered Saline containing 0.1% Tween-20) for 1h at room temperature and incubated with primary antibodies (Table 1) overnight at 4ºC (supplementary
Table S1). After the washing steps, blots were incubated with the secondary antibodies HRP-conjugated secondary antibodies (supplementary
Table S1) for 1h at room temperature. Supersignal West Pico/Femto chemiluminescence substrate (Thermo Fisher Scientific #34577; #34095) and G:Box XRQ gel doc system (SynGene) were used for image capturing. Band intensities were quantified using ImageJ software. Quantitative normalization was achieved using β-actin or GAPDH antibodies.