1. Introduction
Premature infants are observed to be at a higher risk of developing anemia due to iatrogenic blood loss and physiological responses, often necessitating multiple blood transfusions during hospitalization.[
1] Extremely low birth weight infants (ELBWIs) are particularly susceptible to iatrogenic anemia due to the relatively larger sampling volume required for frequent laboratory tests compared to their small total blood volume, shorter lifespan of blood cells, and immature erythropoietic response.[
2,
3] It is estimated that approximately 90% of ELBWIs will require at least one red blood cell transfusion; however, transfusions can lead to complications, mortality, and major neonatal morbidities, such as necrotizing enterocolitis and intraventricular hemorrhage in premature infants. Therefore, a reduction in the blood sampling frequency and volume is crucial for quality improvement in neonatal intensive care units (NICUs).[
4,
5]
In 2016, we implemented a change in our blood sampling protocol from routinely scheduled sampling to a need-based schedule with a lower sampling volume. The present study aimed to determine whether this protocol change successfully reduced iatrogenic blood loss, incidence of anemia, and frequency of blood transfusions in ELBWIs without negatively affecting the neonatal outcomes.
2. Materials and Methods
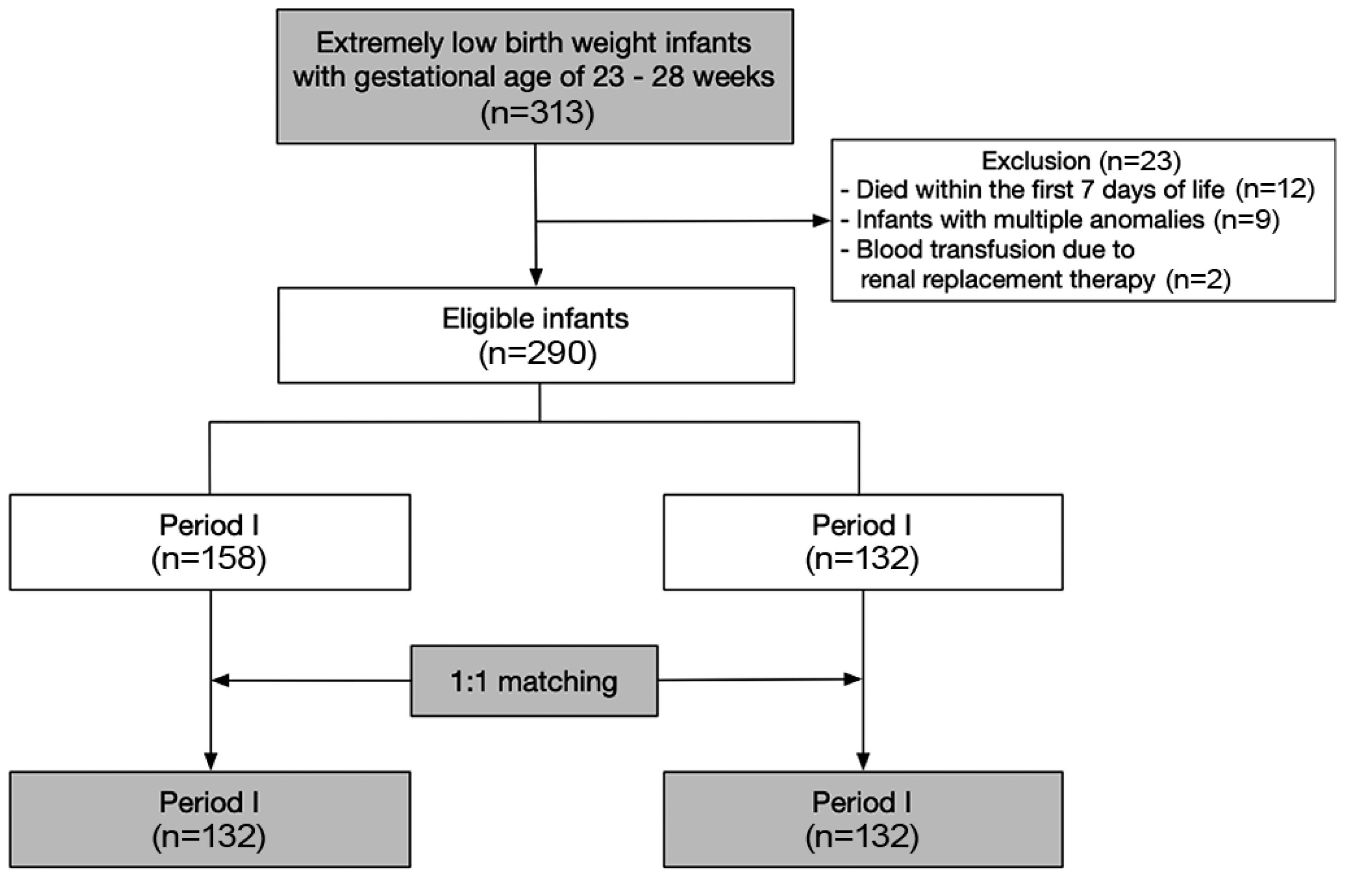
As part of a QI project, we implemented a modified blood sampling protocol in January 2016. To assess the impact of this change, we retrospectively reviewed the medical records of 313 ELBWIs of gestational age between 23 and 28 weeks, who were born and admitted to the Samsung Medical Center NICU between 2013 and 2019. The infants were divided into two groups based on their date of birth: before (period I) and after (period II) January 2016. Infants who died within the first seven days of life (n=12), those with multiple congenital anomalies (n=9), and those who received blood transfusions due to continuous renal replacement therapy (n=2), were excluded from the analysis. Of the remaining 290 infants, 264 (132 per period group) were selected using propensity score matching to minimize selection bias (
Figure 1).
During period I, the blood sampling protocol involved routine scheduling, which encompassed: (1) venous sampling for complete blood cell count (CBC), electrolytes, c-reactive protein (CRP), and chemistry profiles on postnatal days 1, 3, and 7 during the first week, and subsequently once per week for infants with central parenteral nutrition, and once in two weeks for infants without central parenteral nutrition, with a total blood volume of 1.2 mL per sampling; (2) heel puncture for capillary blood gas analysis (CBGA) on a daily basis for infants under mechanical ventilation, with 0.1 mL per sampling. In period II, the protocol was modified to focus on needs-based laboratory testing and minimize venous sampling by replacing it with point-of-care testing (POCT) via capillary puncture. The modified protocol included: (1) umbilical cord blood testing instead of venous sampling on day 1, excluding blood culture; (2) POCT-chemistry profile for blood urea nitrogen, creatinine, sodium, potassium, chloride, ionized calcium, lactate, total bilirubin, and micro-CRP, with a total volume of 0.1 mL per capillary puncture, performed once per one or two weeks; (3) venous sampling for CBC and other chemistry profiles not included in POCT-chemistry, such as liver enzymes, triglycerides, and serum magnesium, was performed based on the clinician’s decision; (4) CBGA was performed only when clinically necessary. In a typical case involving mechanical ventilation, the cumulative volume of blood samples collected on postnatal day 7 was approximately 4.3 mL during period I, ranging from 1.5 to 2.7 mL in period II.
We extracted various clinical data from medical records, including the frequency and amount of blood sampling, frequency and volume of red blood cell (RBC) transfusions, and clinical characteristics, such as gestational age, birth weight, Apgar scores, and perinatal maternal history. The neonatal outcomes data included the incidence of intraventricular hemorrhage grades 3 and 4 based on Papile’s classification, retinopathy of prematurity (ROP) stage III or higher, necrotizing enterocolitis (NEC) stage 2b or higher based on modified Bell’s criteria, moderate-to-severe bronchopulmonary dysplasia (BPD) defined as oxygen dependency at a corrected age of 36 weeks, and culture-proven sepsis. The demographic characteristics, sampling frequency and volume, hemoglobin (Hb) and hematocrit (Hct) levels on postnatal days 30 and 60, frequency of RBC transfusions, and major neonatal morbidities were compared between the two groups (periods I and II).
Among the eligible infants (n=290,
Figure 1), we selected 132 infants per period using a propensity score analysis with 1:1 matching using the nearest matching method to minimize the bias stemming from differences in the clinical characteristics of the infants in the different study periods. The variables included in the propensity score analysis were gestational age, sex, antenatal steroid use, incidence of intraventricular hemorrhage grade 3–4, and moderate-to-severe bronchopulmonary dysplasia. After propensity matching, we compared periods I and II using the chi-square test for categorical variables and the t-test for continuous variables. Linear regression was conducted to evaluate the correlation between the cumulative blood sampling volume and transfusion frequency during hospitalization. We conducted a data analysis using R, version 4.2 (R Project for Statistical Computing).
3. Results
Table 1 presents the demographic and clinical characteristics of patients during each study period. There were no significant differences in the antenatal corticosteroid use, maternal hypertension, or gestational age between the two periods. However, Apgar scores at 1 and 5 minutes were significantly higher in period II compared to period I (4.3 ± 1.4 vs. 4.9 ± 1.5 at 1 min,
P<0.001; 6.8 ± 1.4 versus 7.7 ± 1.2 at 5 min,
P<0.001). The cumulative sampling volume until postnatal day 30 and the total cumulative sampling volume during hospitalization were both significantly lower in period II compared to period I (16.7 ± 4.1 mL vs. 15.6 ± 4.4 mL,
P=0.03; 51.4 ± 29.7 mL vs. 44.3 ± 27.5 mL,
P=0.04, respectively). Although Hb and Hct levels on day 30 were slightly higher in period II (10.8 g/dL vs. 11.0 g/dL; 31.7 % vs. 32.7 %), the difference was not statistically significant (
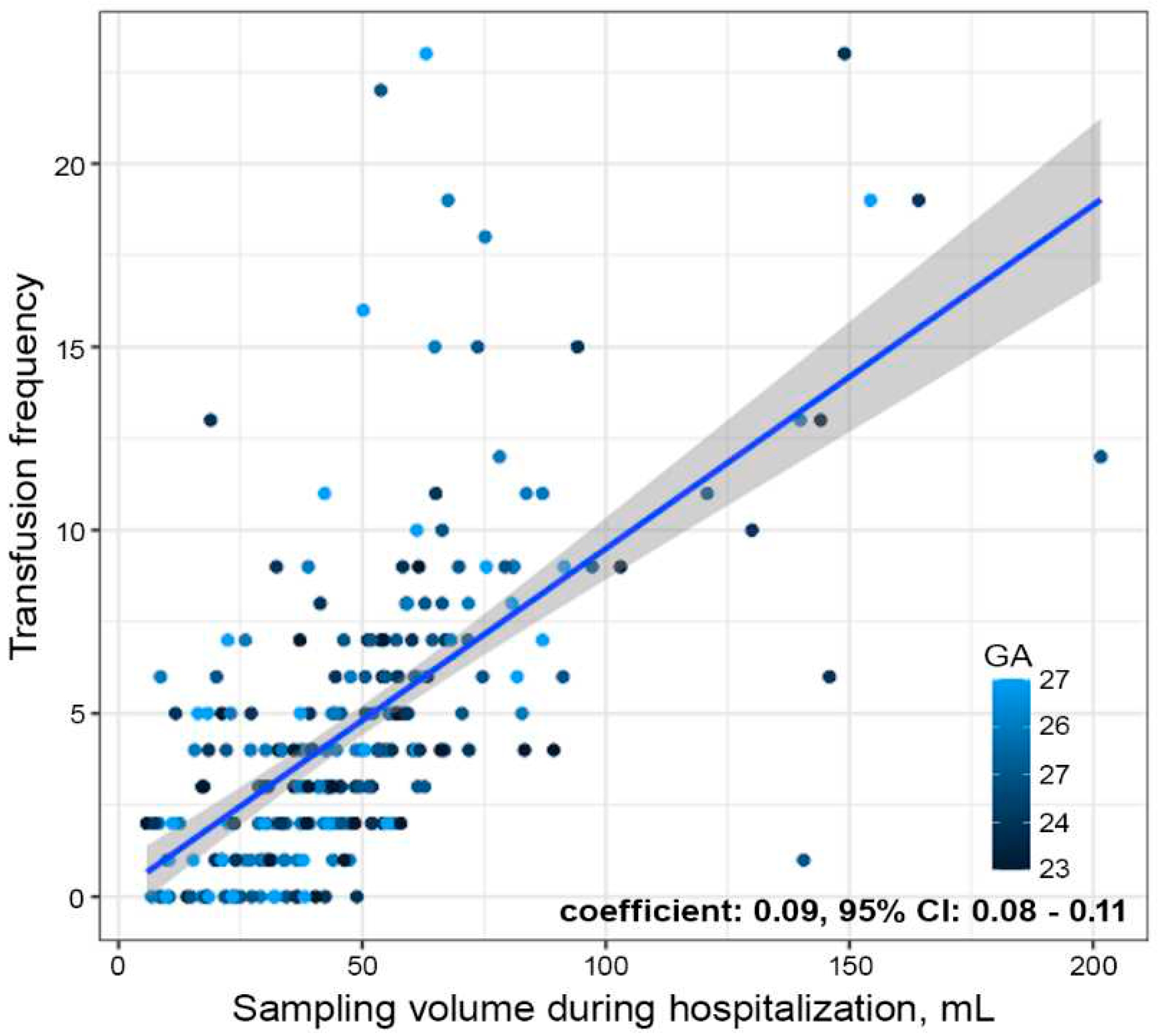
Table 2). There was no difference in the frequency or cumulative volume of RBC transfusions during hospitalization between the two periods. However, the linear regression model showed a statistically significant positive correlation between the cumulative blood sampling volume and transfusion frequency during hospitalization (
Figure 2), with every 11 mL of blood sampling leading to one blood transfusion (coefficient: 0.09, 95% CI: 0.08–0.11). Regarding neonatal outcome variables, there were no differences between the two periods with respect to mortality (18% vs. 22%,
P=0.54), intraventricular hemorrhage (IVH), retinopathy of prematurity (ROP), bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), and sepsis.
4. Discussion
In the present study, we were able to successfully reduce the iatrogenic blood loss in ELBWIs by implementing a new QI project. Although the reduction in blood loss did not result in an increase in the hemoglobin levels or a decrease in the frequency of transfusions, this may be due to the low statistical power resulting from the small sample size, considering the evidence of the positive correlation between the cumulative blood loss volume and the frequency of transfusions (
Figure 2). Additionally, because there were no differences in the neonatal outcome variables between the two periods, it can be inferred that the protocol change toward fewer laboratory tests did not have any adverse effects on patient safety.
Blood sampling for diagnostic purposes is the most common cause of anemia and a significant contributor to the need for RBC transfusion in preterm infants.[
2,
4,
6] According to a study by Blanchette and Zippursky, laboratory blood losses in the first six weeks of life were between 11 to 22 mL/kg per week, which is equivalent to 15–30% of the circulating blood volume in very low birth weight infants (<1,500 g).[
6] Another study by Aboalqez
et al. reported a median absolute amount of blood sampling of 16.5 mL (IQR 12.3–21.1 mL) during the initial four weeks in very low birth weight infants (<1,500 g).[
7] Compared to previous studies, our data showed a similar blood loss of 15.6 mL during the first four weeks in even smaller infants (<1,000 g), which could be achieved by the adoption of a need-based sampling protocol with active use of POCT.
Physiological anemia is a common phenomenon in term infants and is typically characterized by a drop in hemoglobin levels to 11.0 g/dL at the age of 2 months. However, preterm infants experience earlier and more substantial hemoglobin drops, resulting in a decrease from 9.5 mL/dL to 9.0 mL/dL.[
8] Anemia of prematurity is caused by premature birth, which occurs before placental iron transport and fetal erythropoiesis are complete, low plasma levels of erythropoietin due to reduced production and accelerated catabolism, and iatrogenic blood losses caused by laboratory testing.[
9] Thus, preterm infants require a larger number of RBC transfusions than mature infants.[
10] The study by Jansen
et al. showed that infants born at 24–28, 28–30, and 30–32 weeks of gestation experienced RBC transfusion rates of 94%, 62%, and 35%, respectively.[
11]
On the other hand, the relationship between RBC transfusion and mortality in ELBWIs is well-established.[
3,
12,
13,
14] The underlying mechanism of this association is believed to be related to transfusion-related immunomodulation with a massive release of proinflammatory cytokines.[
15] Furthermore, transfusions in ELBWIs are also known to be associated with an increased risk of several morbidities, including NEC, ROP, IVH, and BPD.[
16,
17,
18] In a study by Su
et al., they could reduce the incidence of NEC by the effort of reducing the cumulative blood sampling volume in very low birth weight infants.[
19] Taken together, considering the adverse effect of transfusion, it is crucial to reduce iatrogenic blood loss in preterm infants. Our data indicate that the new QI project aimed at reducing iatrogenic blood loss is effective and feasible even in extremely premature infants. This was achieved by carefully evaluating the necessity and timing of each laboratory blood draw, and actively using POCT.
5. Conclusions
In conclusion, despite the several limitations of the study, including the relatively small study population from a single center and the retrospective study design, our results show the effectiveness and feasibility of implementing the new QI project for reducing iatrogenic blood loss without compromising patient safety, possibly resulting from fewer diagnostic intervention strategies for ELBWIs. Our data also suggest that careful evaluation of the necessity and timing of laboratory tests is vital to reduce the risk of transfusion in these infants.
Author Contributions
Sung SI and Chang YS conceived the study. Jung, Kim, and Kim developed the theory and performed the analytic calculations. Seo and Hwang verified this analytical method. Jung N wrote the manuscript with support from Yang and Ahn. Sung SI and Chang YS helped interpret the results and worked on the manuscript. All the authors discussed the results and commented on the manuscript.
Funding
This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIT) (No. NRF-2021R1F1A1064592).
Institutional Review Board Statement
This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (IRB no. 2022-11-041-001).
Informed Consent Statement
Due to the retrospective nature of this study, the requirement for informed consent was waived by the IRB.
Data Availability Statement
Conflicts of Interest
The authors declare no conflict of interest.
References
- Widness, J.A. Pathophysiology of Anemia During the Neonatal Period, Including Anemia of Prematurity. Neoreviews 2008, 9, e520. [Google Scholar] [CrossRef] [PubMed]
- Carroll, P.D.; Widness, J.A. Nonpharmacological, blood conservation techniques for preventing neonatal anemia--effective and promising strategies for reducing transfusion. Semin. Perinatol. 2012, 36, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Widness, J.A.; Madan, A.; Grindeanu, L.A.; Zimmerman, M.B.; Wong, D.K.; Stevenson, D.K. Reduction in red blood cell transfusions among preterm infants: Results of a randomized trial with an in-line blood gas and chemistry monitor. Pediatrics 2005, 115, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Obladen, M.; Sachsenweger, M.; Stahnke, M. Blood sampling in very low birth weight infants receiving different levels of intensive care. Eur. J. Pediatr. 1988, 147, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.P.; Rasmussen, M.K.; Bjerregaard, L.L.; Nøhr, S.B.; Ebbesen, F. Impact of blood sampling in very preterm infants. Scand. J. Clin. Lab. Investig. 2000, 60, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, V.S.; Zipursky, A. Assessment of anemia in newborn infants. Clin. Perinatol. 1984, 11, 489–510. [Google Scholar] [CrossRef] [PubMed]
- Aboalqez, A.; Deindl, P.; Ebenebe, C.U.; Singer, D.; Blohm, M.E. Iatrogenic Blood Loss in Very Low Birth Weight Infants and Transfusion of Packed Red Blood Cells in a Tertiary Care Neonatal Intensive Care Unit. Children 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Dallman, P.R. Anemia of prematurity. Annu. Rev. Med. 1981, 32, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Strauss, R.G. Anaemia of prematurity: Pathophysiology and treatment. Blood Rev. 2010, 24, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Aher, S.M. Early erythropoiesis-stimulating agents in preterm or low birth weight infants. Cochrane Database Syst. Rev. 2017, 11, Cd004863. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.; Brand, A.; von Lindern, J.S.; Scherjon, S.; Walther, F.J. Potential use of autologous umbilical cord blood red blood cells for early transfusion needs of premature infants. Transfusion 2006, 46, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Chan, O.W.; Chiang, M.C.; Yang, P.H.; Chu, S.M.; Hsu, J.F.; Fu, R.H.; Lien, R. Red Blood Cell Transfusion and Clinical Outcomes in Extremely Low Birth Weight Preterm Infants. Pediatr. Neonatol. 2017, 58, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Crawford, T.M.; Andersen, C.C.; Hodyl, N.A.; Robertson, S.A.; Stark, M.J. The contribution of red blood cell transfusion to neonatal morbidity and mortality. J. Paediatr. Child. Health 2019, 55, 387–392. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.M.; Guinsburg, R.; de Almeida, M.F.; Procianoy, R.S.; Leone, C.R.; Marba, S.T.; Rugolo, L.M.; Fiori, H.H.; Lopes, J.M.; Martinez, F.E.; et al. Red blood cell transfusions are independently associated with intra-hospital mortality in very low birth weight preterm infants. J. Pediatr. 2011, 159, 371–376.e371–373. [Google Scholar] [CrossRef] [PubMed]
- Vamvakas, E.C.; Blajchman, M.A. Transfusion-related immunomodulation (TRIM): An update. Blood Rev. 2007, 21, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Reali, M.F.; Bertini, G.; Martelli, E.; Pezzati, M.; Rubaltelli, F.F. The role of blood transfusions and iron intake on retinopathy of prematurity. Early Hum. Dev. 2001, 62, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Mally, P.; Golombek, S.G.; Mishra, R.; Nigam, S.; Mohandas, K.; Depalhma, H.; LaGamma, E.F. Association of necrotizing enterocolitis with elective packed red blood cell transfusions in stable, growing, premature neonates. Am. J. Perinatol. 2006, 23, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R.W.; Drury, J.A.; Yoxall, C.W.; James, C. Blood transfusion and chronic lung disease in preterm infants. Eur. J. Pediatr. 1997, 156, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Su, P.C.; Chung, H.W.; Yang, S.T.; Chen, H.L. Effect of Small Volume Blood Sampling on the Outcomes of Very Low Birth Weight Preterm Infants. Children 2022, 9, 1190. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).