Submitted:
04 July 2023
Posted:
07 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
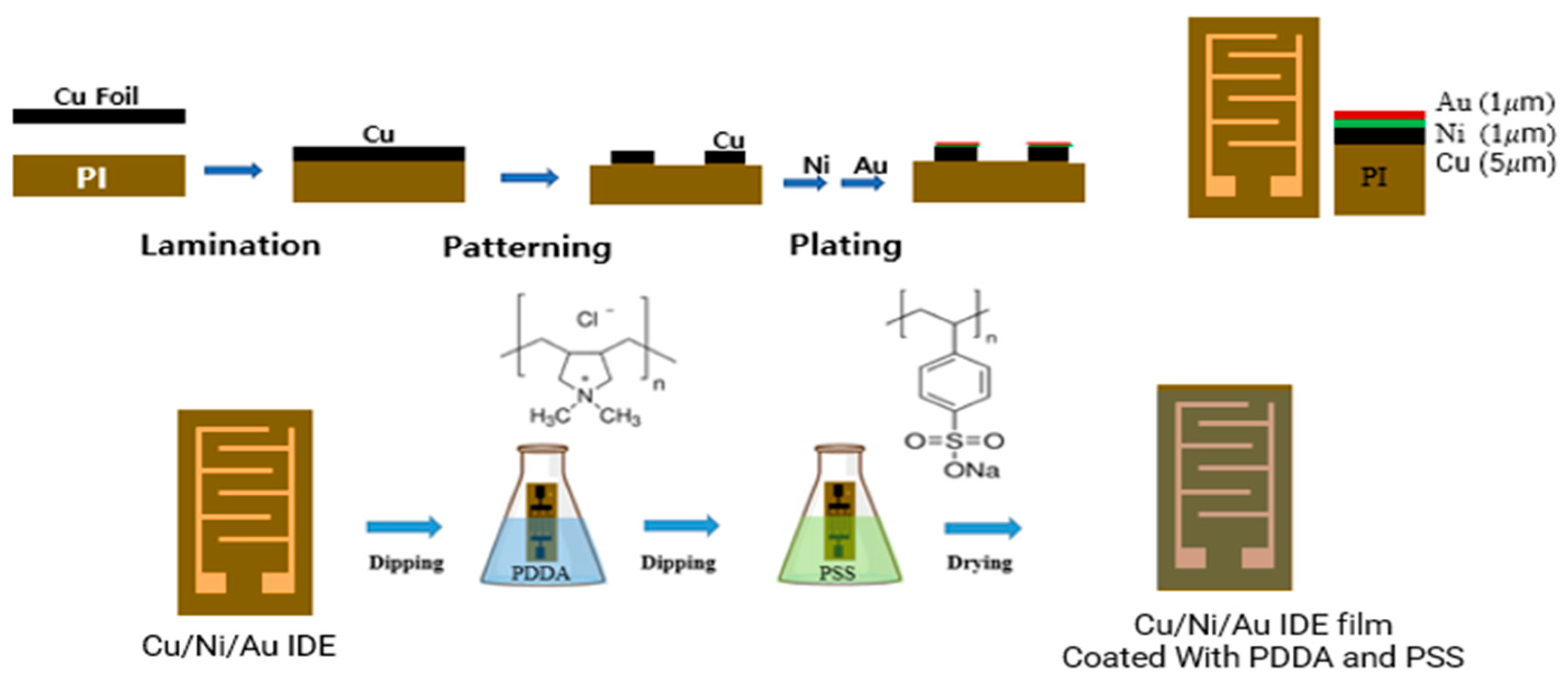
2.1. Fabrication of PI film interdigitated with Cu/Ni/Au electrodes: IDE substrate
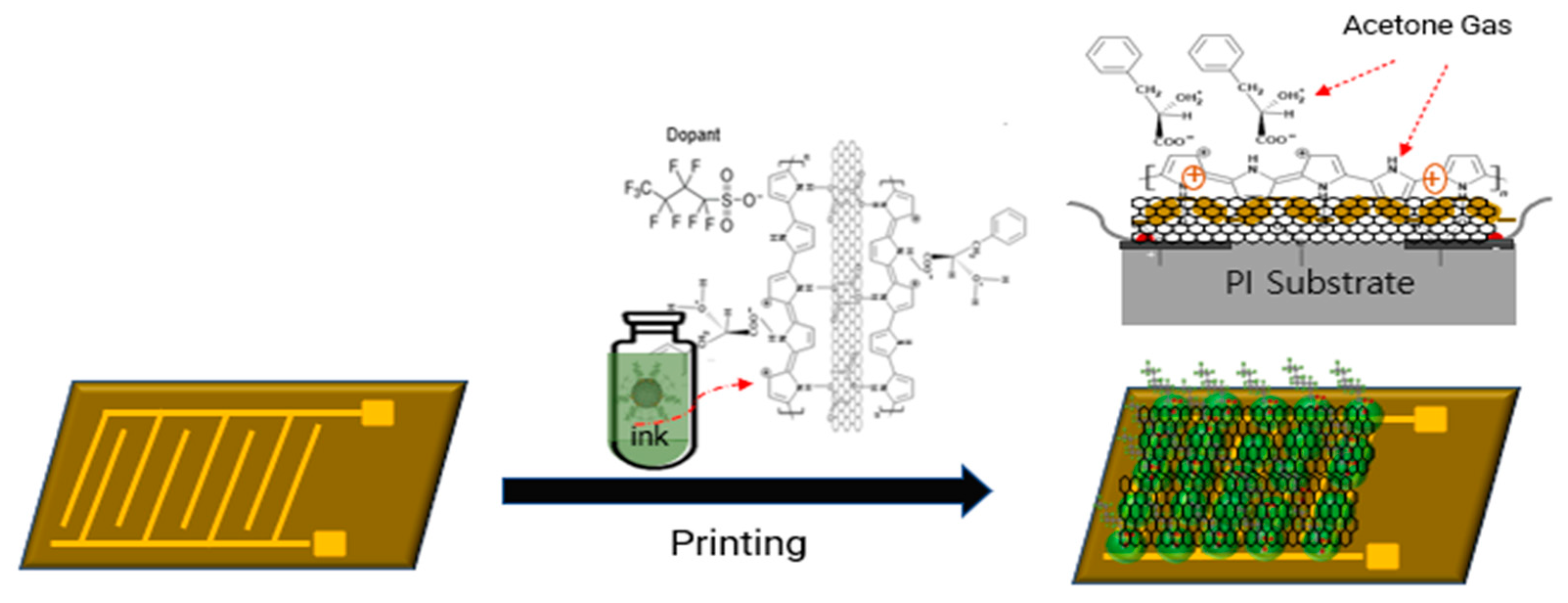
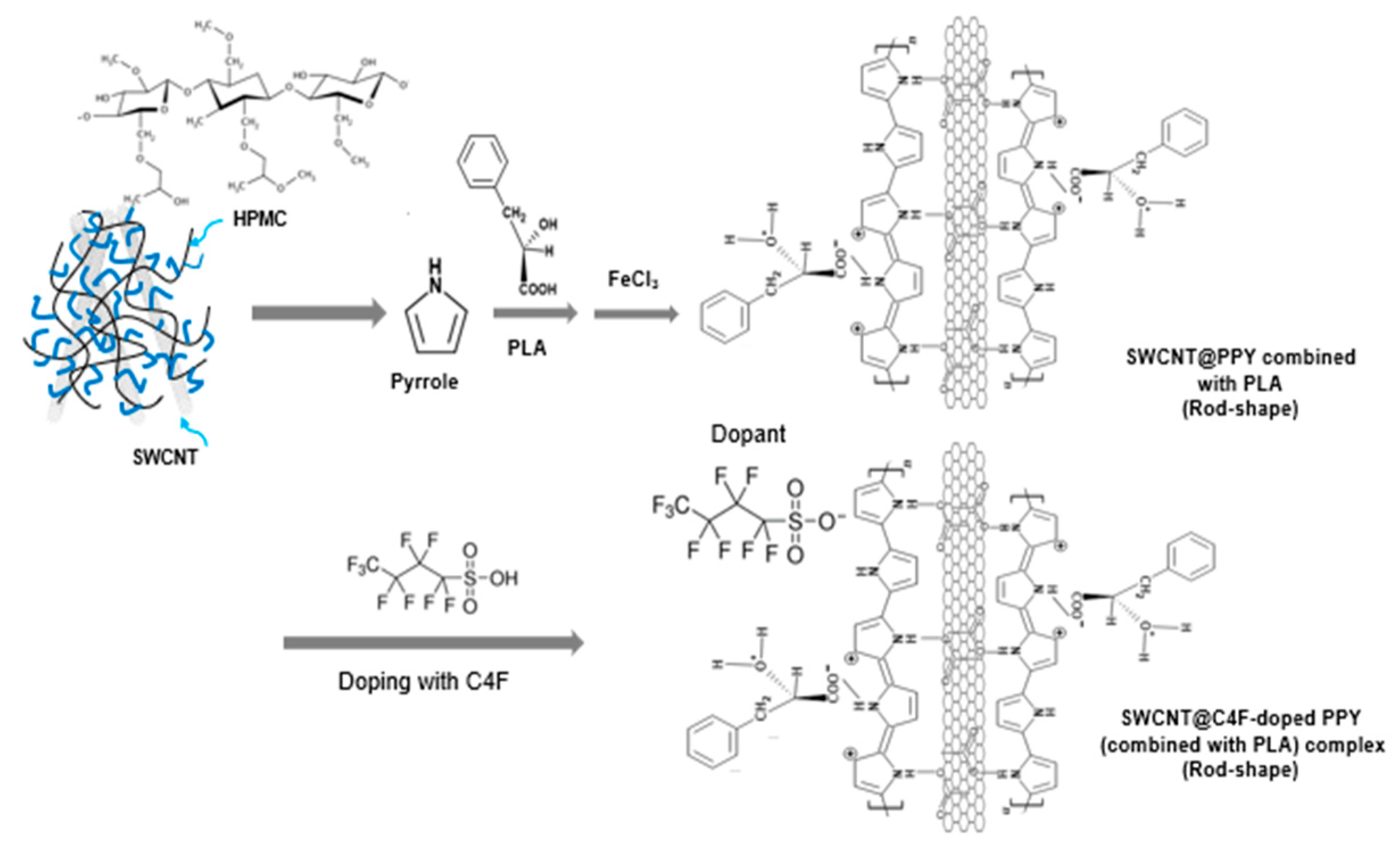
2.2. Synthesis of SWCNT/C4F-PPy nanocomposite
2.3. Performance evaluation of gas sensors
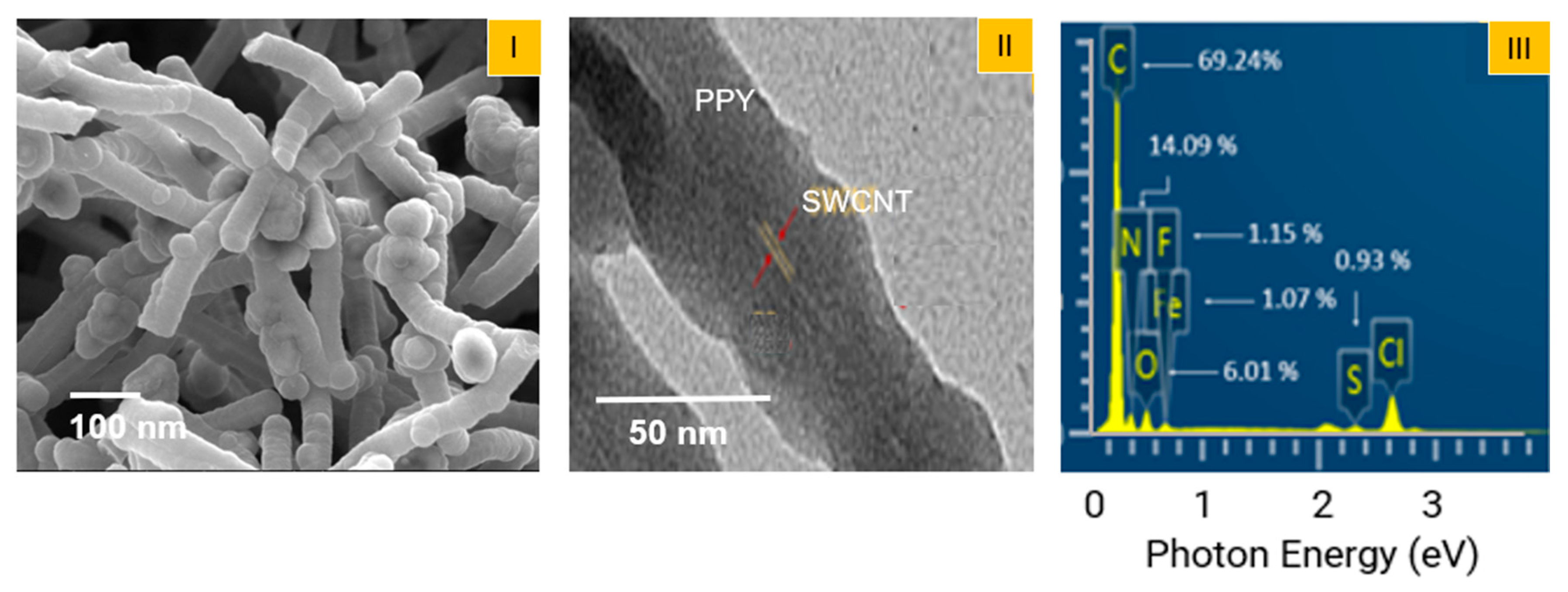
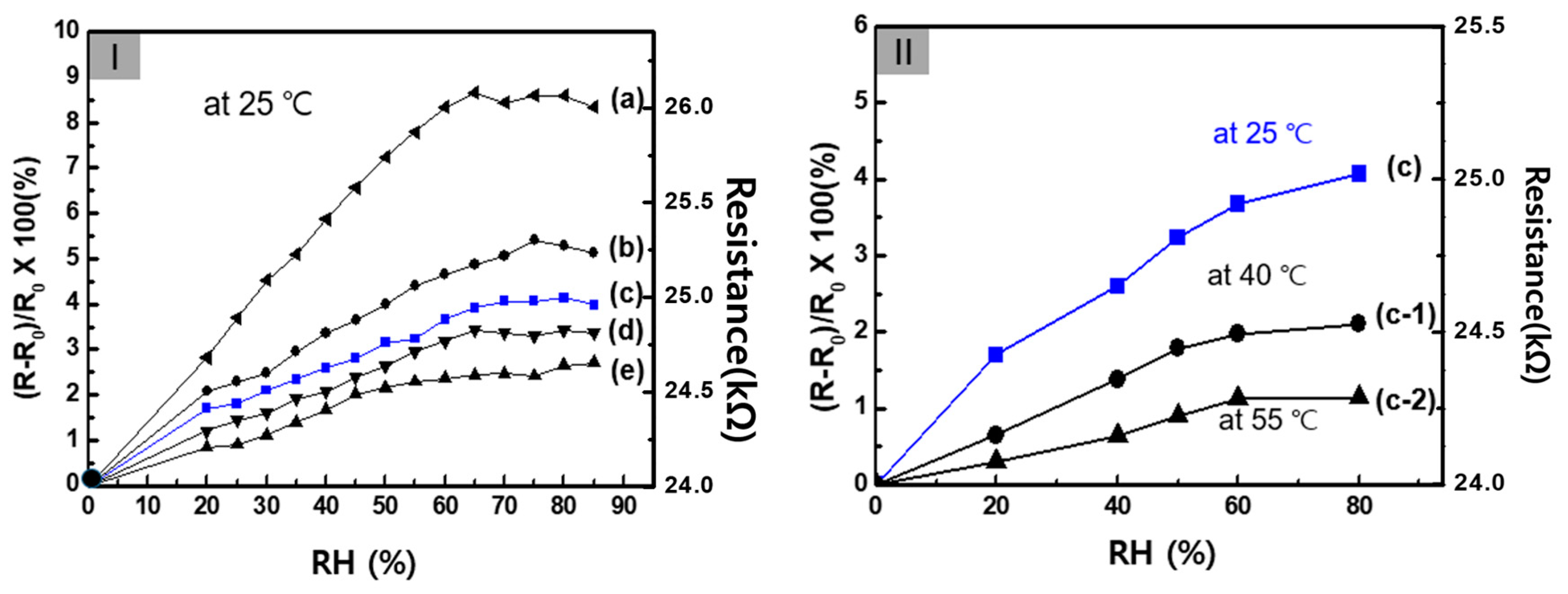
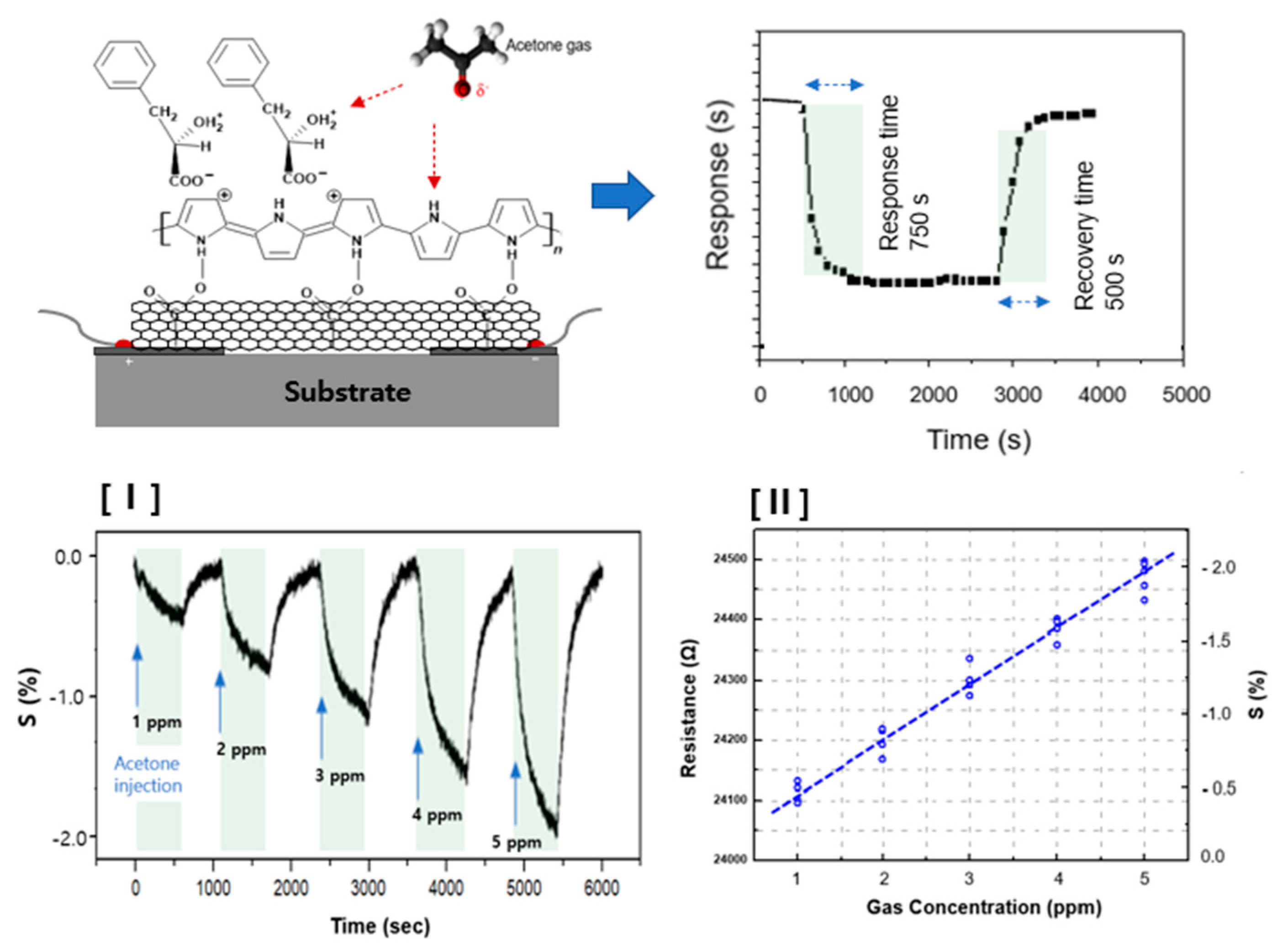
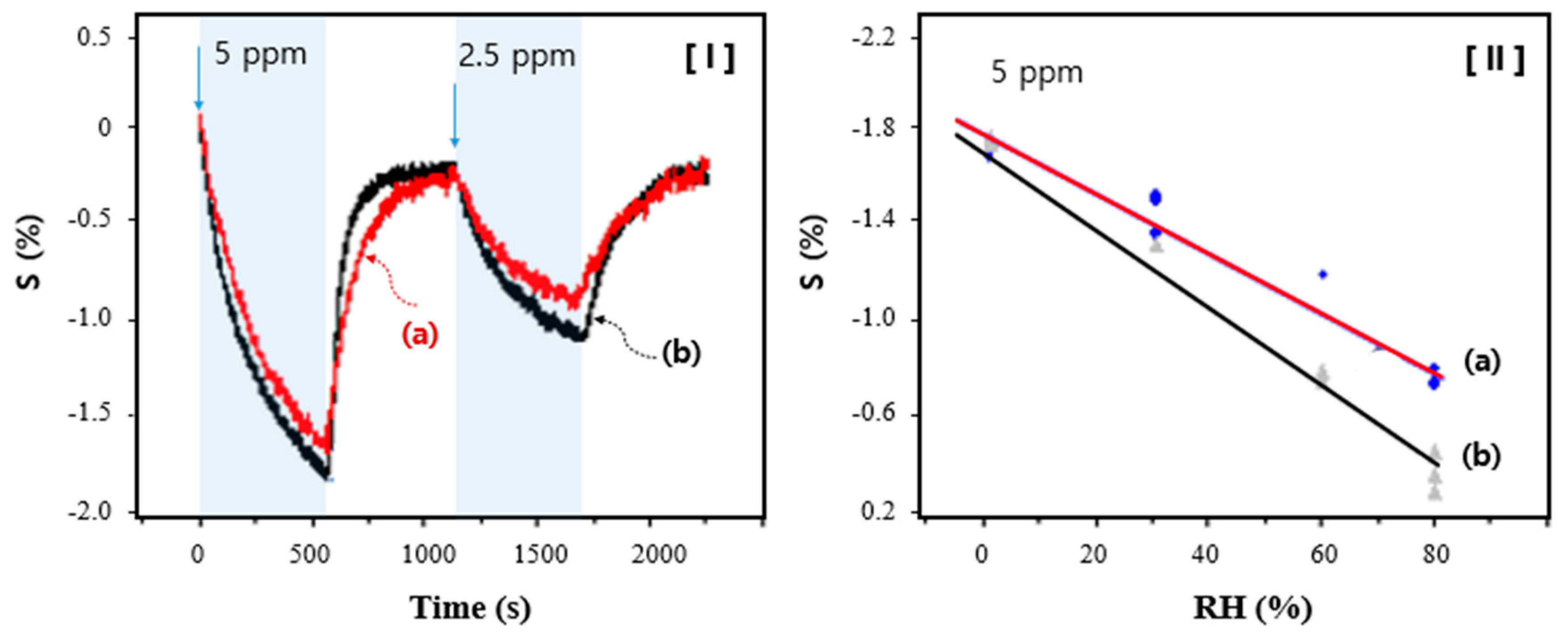
3. Results and Discussion
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Tille, T. Automotive suitability of air quality gas sensor. Sens. Actuators B 2012, 170, 40–44. [Google Scholar] [CrossRef]
- Mohankumar, P.; Ajayan, J.; Yasodharan, R.; Devendran, P.; Sambasivam, R. A review of micromachined sensors for automotive applications. Measurement 2019, 140, 305–322. [Google Scholar] [CrossRef]
- Rydosz, A. Sensors for Enhanced Detection of Acetone as a Potential Tool for Noninvasive Diabetes Monitoring. Sensors 2018, 18, 2298. [Google Scholar] [CrossRef] [PubMed]
- Saidi, T.; Zaim, O.; Moufid, M.; Bari, N.E.; Ionescu, R.; Bouchikhi, B. Exhaled breath analysis using electronic nose and gas chromatography–mass spectrometry for non-invasive diagnosis of chronic kidney disease, diabetes mellitus and healthy subjects. Sens. Actuators B 2018, 257, 178–188. [Google Scholar] [CrossRef]
- Kita, J.; Schubert, F.; Retting, F.; Engelbrecht, A.; Gross, A.; Moos, R. Ceramic alumina substrates for high-temperature ga sensors-implications for applicatbility. Proc. Eng. 2017, 87, 1505–1508. [Google Scholar] [CrossRef]
- Rydosz, A.; Szkudlarek, A.; Ziabka, M.; Momanski, K.; Maziarz, W.; Pisarkiewicz, T. Performance of Si-Doped WO3Thin Films for Acetone Sensing Prepared by Glancing Angle DC Magnetron Sputtering. IEEE Sens. J. 2016, 16, 1004–1012. [Google Scholar] [CrossRef]
- Ozdemir, S.; Gole, J.L. The potential of porous silicon gas sensors. Curr. Opin. Solid State Mater. Sci. 2007, 11, 92–100. [Google Scholar] [CrossRef]
- Lee, Y.M.; Zheng, M.R. Preparation of high-aspect-ratio ZnO nanorod arrays for the detection of several organic solvents at room working temperature. Appl. Surf. Sci. 2013, 285, 241–248. [Google Scholar] [CrossRef]
- Xing, R.; Li, Q.; Xia, L.; Song, J.; Xu, L.; Zhang, J.; Xie, Y.; Song, H. Au-modified three-dimensional In2O3 inverse opals: synthesis and improved performance for acetone sensing toward diagnosis of diabetes. Nanoscale 2015, 7, 13051–13060. [Google Scholar] [CrossRef]
- Khun, K.K.; Mahajan, A.; Bedi, R.K. SnO2 thick films for room temperature gas sensing applications. J. Appl. Phys. 2009, 106, 124509. [Google Scholar] [CrossRef]
- Shi, J.; Hu, G.; Sun, Y.; Geng, M.; Wu, J.; Liu, Y.; Ge, M.; Tao, J.; Cao, M.; Dai, N. WO3 nanocrystals: Synthesis and application in highly sensitive detection of acetone. Sens. Actuators B 2011, 156, 820–824. [Google Scholar] [CrossRef]
- Liu, C.; Tai, H.; Zhang, P.; Yuan, Z.; Du, X.; Xie, G.; Jiang, Y. A high-performance flexible gas sensor based on self-assembled PANI-CeO2 nanocomposite thin film for trace-level NH3 detection at room temperature. Sens. Actuators B 2018, 261, 587–597. [Google Scholar] [CrossRef]
- Liu, B.; Liu, X.; Yuan, Z.; Jiang, Y.; Su, Y.; Ma, J.; Tai, H. A flexible NO2 gas sensor based on polypyrrole/nitrogen-doped multiwall carbon nanotube operating at room temperature. Sens. Actuators B. 2019, 295, 86–92. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, W.; Fu, J.; Zhu, Y.; Huang, Y.; Peng, P. Laser writing of Cu/CuxO integrated structure on flexible substrate for humidity sensing. Appl. Surf. Sci. 2019, 494, 684–690. [Google Scholar] [CrossRef]
- Kuberský, P.; Syrový, T.; Hamacek, A.; Nespurek, S.; Stejskal, J. Printed Flexible Gas Sensors based on Organic Materials. Procedia Eng. 2015, 120, 614–617. [Google Scholar] [CrossRef]
- Kumar, L.; Rawal, I.; Kaur, A.; Annapoorni, S. Flexible room temperature ammonia sensor based on polyaniline. Sens. Actuators B 2017, 240, 408–416. [Google Scholar] [CrossRef]
- Cherenack, K.; Zysset, C.; Kinkeldei, T.; Munzenrieder, N.; Troster, G. Woven Electronic Fibers with Sensing and Display Functions for Smart Textiles. Adv. Mater. 2010, 22, 5178–5182. [Google Scholar] [CrossRef]
- Zhang, R.; Deng, H.; Valenca, R.; Jin, J.; Fu, Q.; Bilotti, E.; Peijs, T. Strain sensing behaviour of elastomeric composite films containing carbon nanotubes under cyclic loading. Compos. Sci. Technol. 2013, 74, 1–5. [Google Scholar] [CrossRef]
- Hua, C.; Shang, Y.; Wang, Y.; Xu, J.; Zhang, Y.; Li, X.; Cao, A. A flexible gas sensor based on single-walled carbon nanotube-Fe2O3 composite film. Appl. Surf. Sci. 2017, 405, 405–411. [Google Scholar] [CrossRef]
- Du, W.X.; Lee, H.J.; Byeon, J.H.; Kim, J.S.; Cho, K.S.; Kang, S.M.; Takada, M.; Kim, J.Y. Highly sensitive single-walled carbon nonotube/polypyrrole/phenylalanine core-shell nanorods for ammonia gas sensing. J. Matter. Chem C. 2020, 8, 15609–15615. [Google Scholar] [CrossRef]
- Cho, S.; Leel, J.S.; Jun, J.; Jang, J. High-sensitivity hydrogen gas sensors based on Pd-decorated nanoporous poly(aniline-co-aniline-2-sulfonic acid):pss. J. Matter. Chem C 2014, 2, 1955–1966. [Google Scholar] [CrossRef]
- Byeon, J.H.; Kim, J.S.; Kang, H.K.; Kang, S.; Kim, J.Y. Acetone gas sensor based on SWCNT/polypyrrole/phenyllactic acid nanocomposite with high sensitivity and humidity stability. Biosensors 2022, 12, 354. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Kameoka, J. D.; Czaplewski, A.; Craighead, H. G. Polymeric Nanowire Chemical Sensor. Nano Lett. 2004, 4, 671–675. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Wang, K.; Wei, J.; Wu, D.; Cao, A.; Li, Z.; Cheng, Y.; Zheng, Q. Sci. Rep. 2012, 16, 87.
- Liu, W.; Xu, L.; Sheng,K.; Zhou, X.; Dong, B.; Lu, G.; Song, H. NPG Asia Matter. 2018, 10, 293–308.
- Kawashima, H.; Mayama, H.; Nakamura, Y.; Fujii, S. Hydrophobic polypyrroles synthesized by aqueous chemical oxidative polymerization and their use as light-responsive liquid marble stabilizers. Polym. Chem. 2017, 8, 2609–2618. [Google Scholar] [CrossRef]
- Hieu, N.; Dung, N.Q.; Tam, P.D.; Trung, T.; Chien, N.D. Thin film polypyrrole/SWCNTs nanocomposites-based NH3 sensor operated at room temperature. Sens. Actuators B 2009, 140, 500–507. [Google Scholar] [CrossRef]
- Jang, W.K.; Yun, J.M.; Kim, H.I.; Lee, Y.S. Improvement in ammonia gas sensing behavior by polypyrrole/ multi-walled carbon nanotubes composites. Carbon Lett. 2012, 13, 88–93. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




