Submitted:
06 July 2023
Posted:
07 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
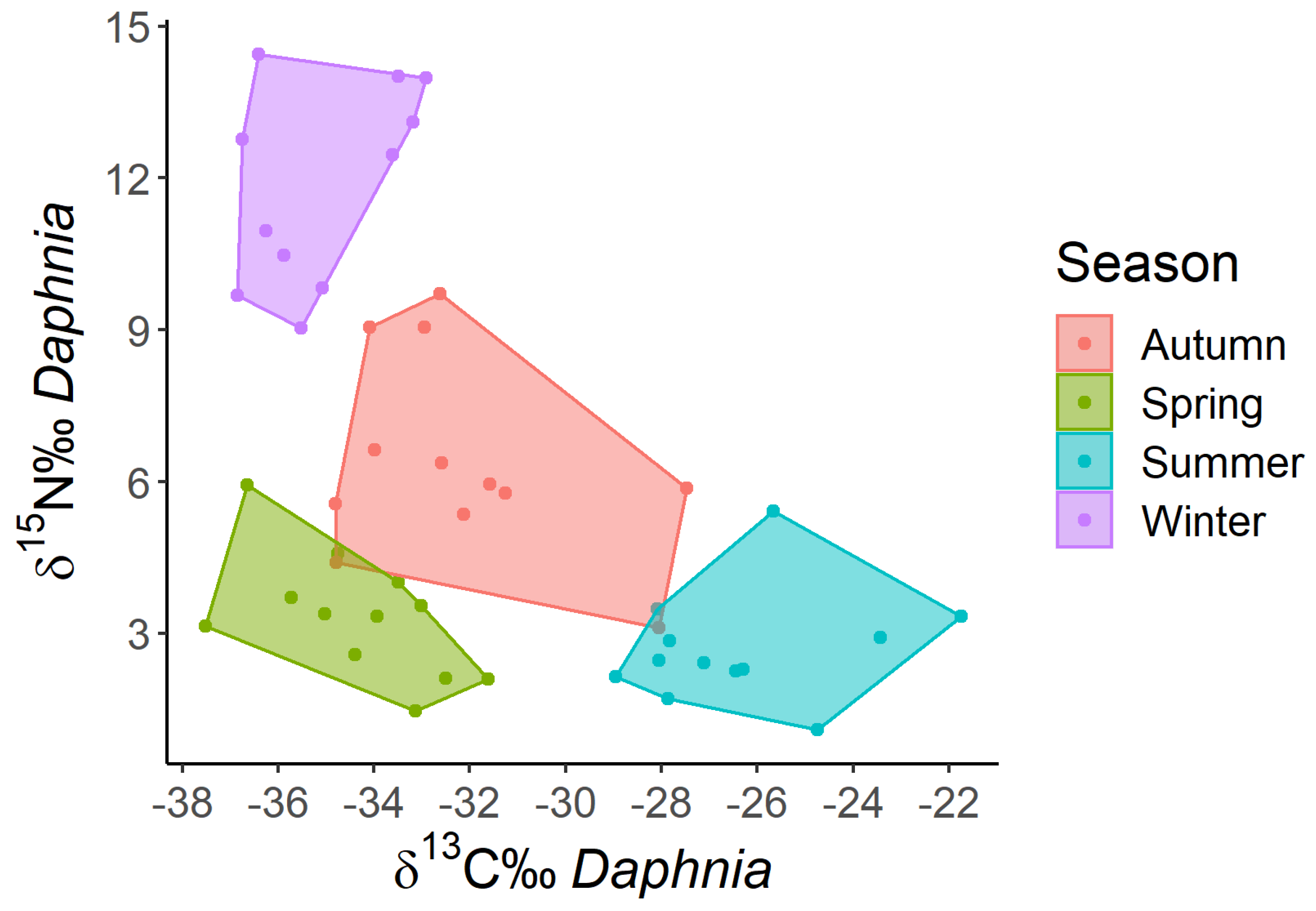
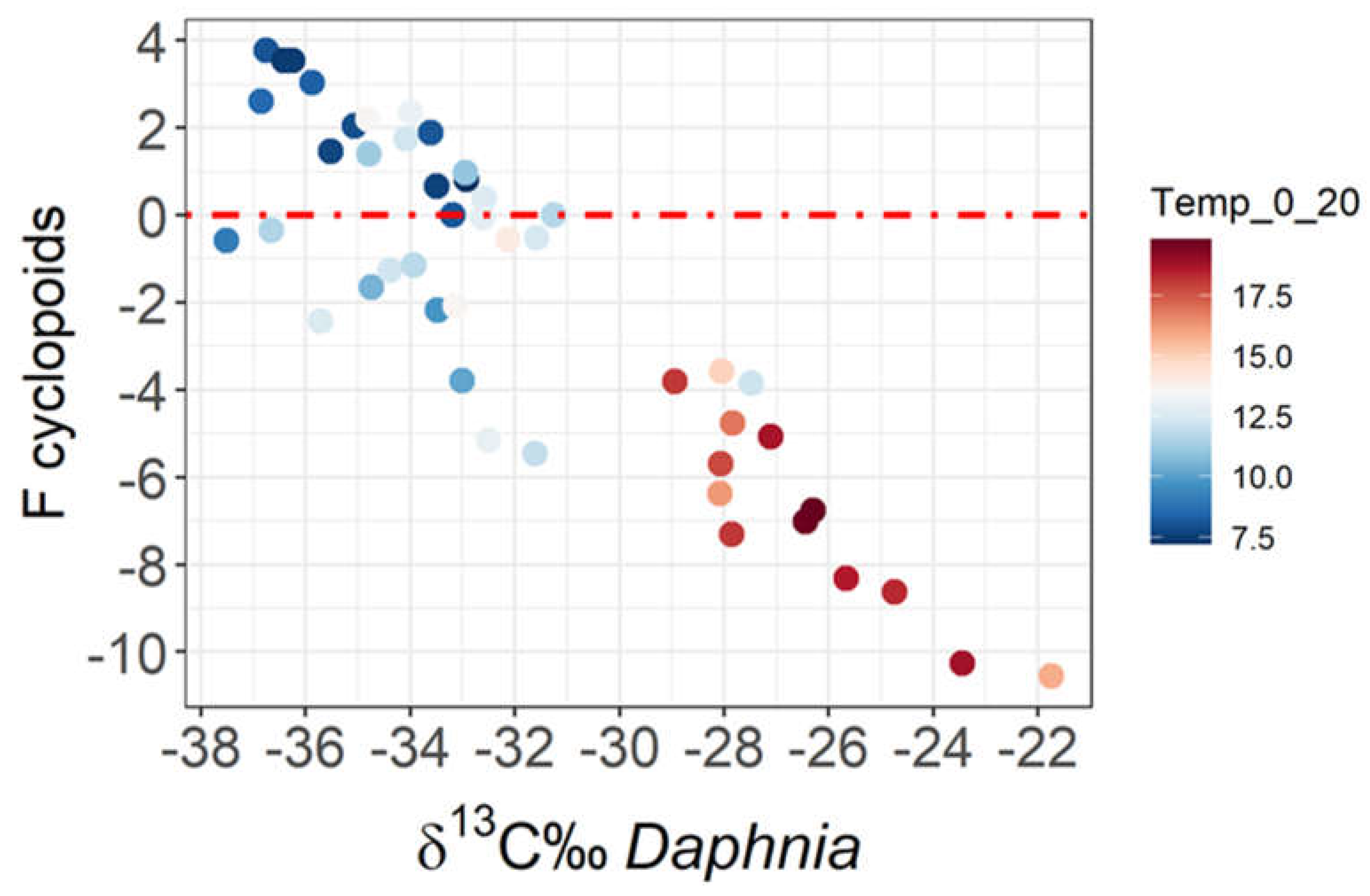
3. Results
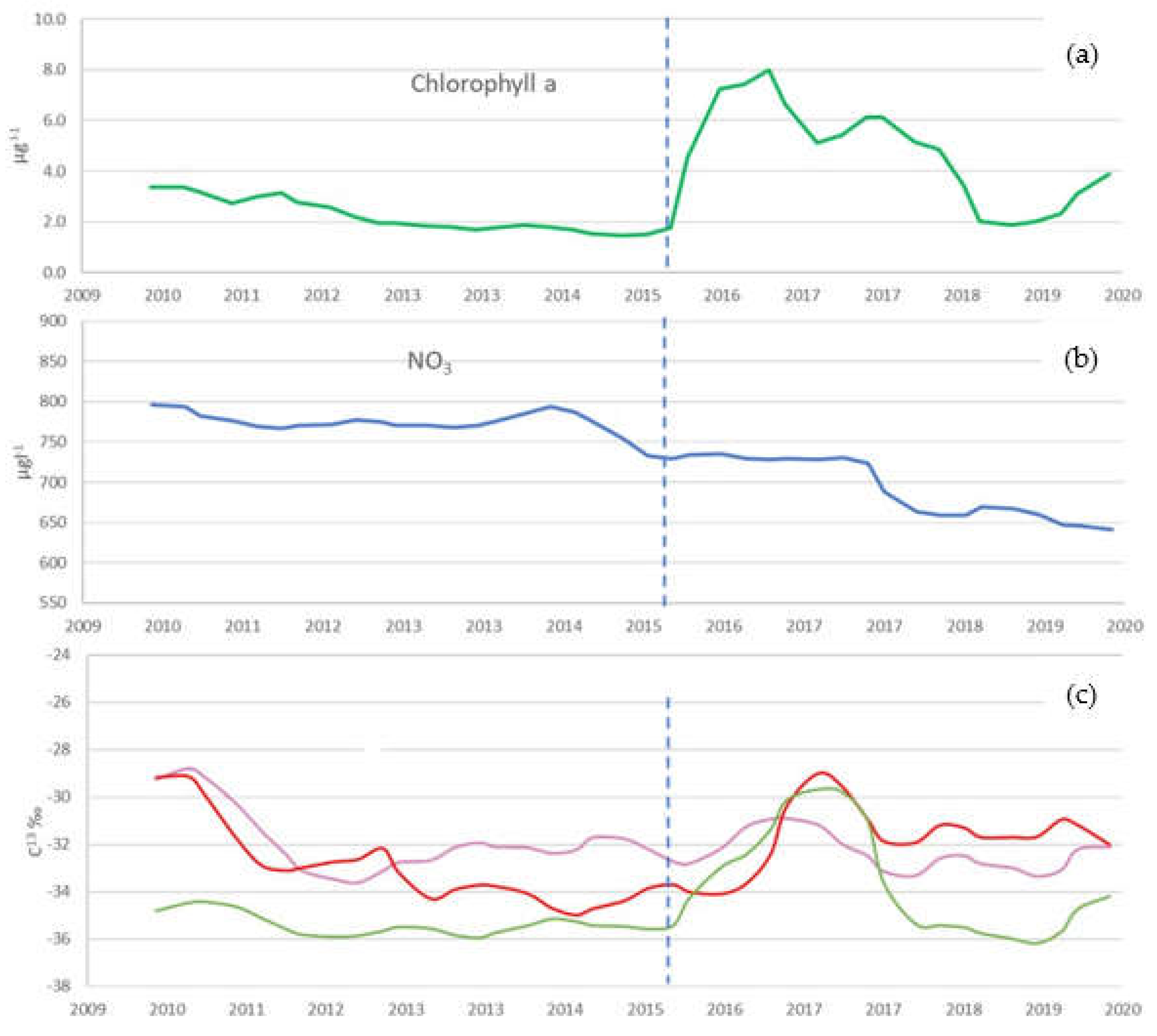
4. Discussion
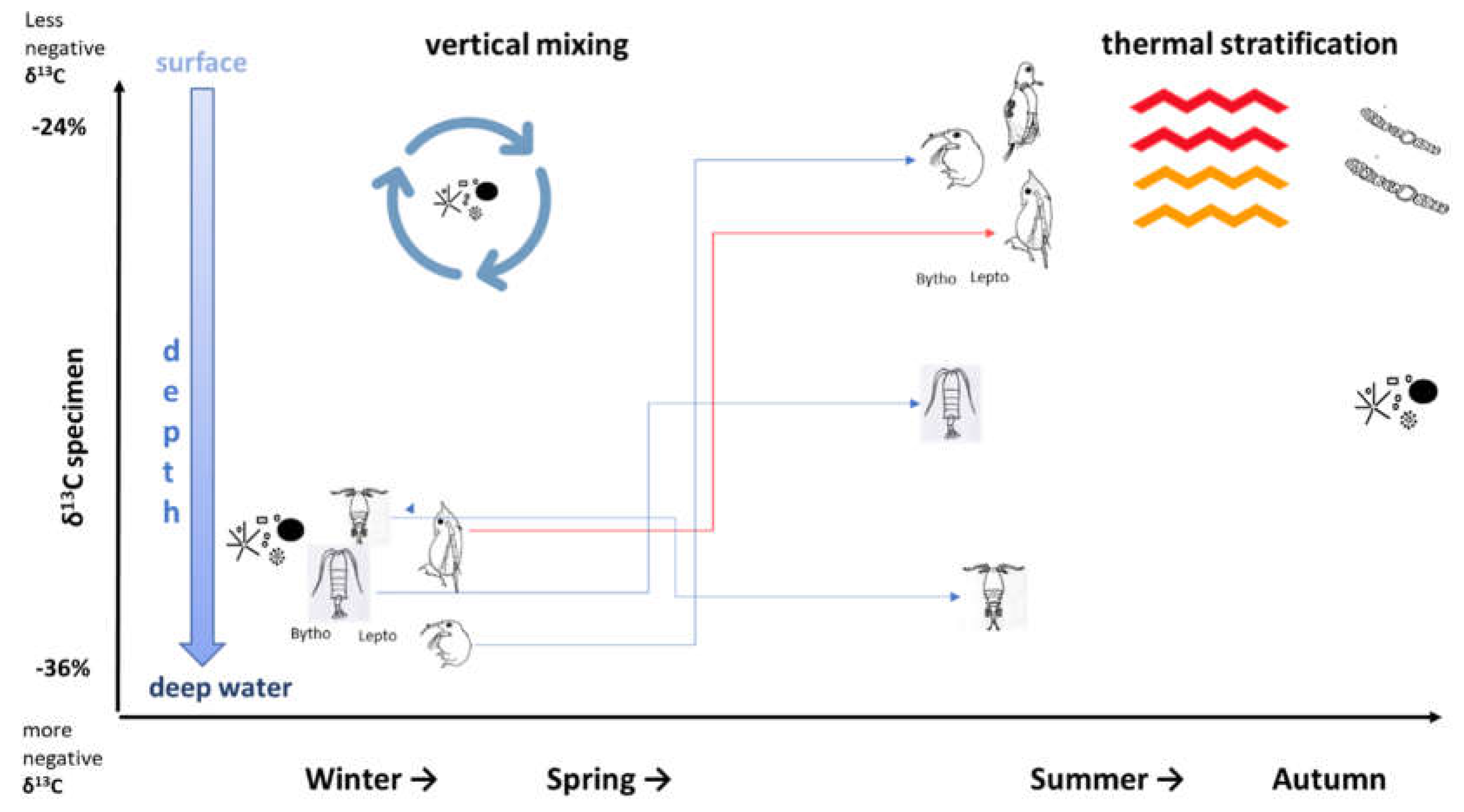
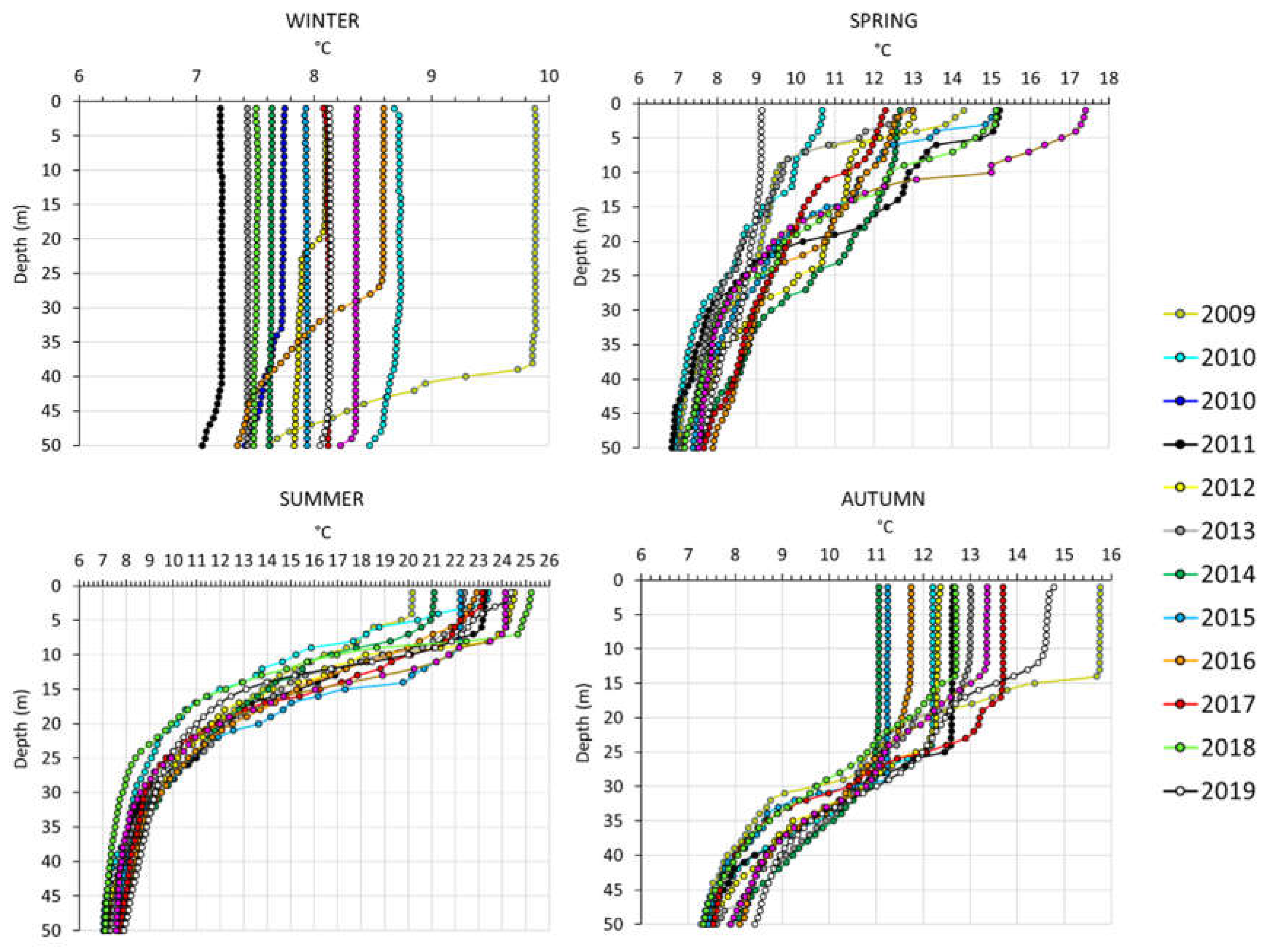
4.1. δ13 C and temperature
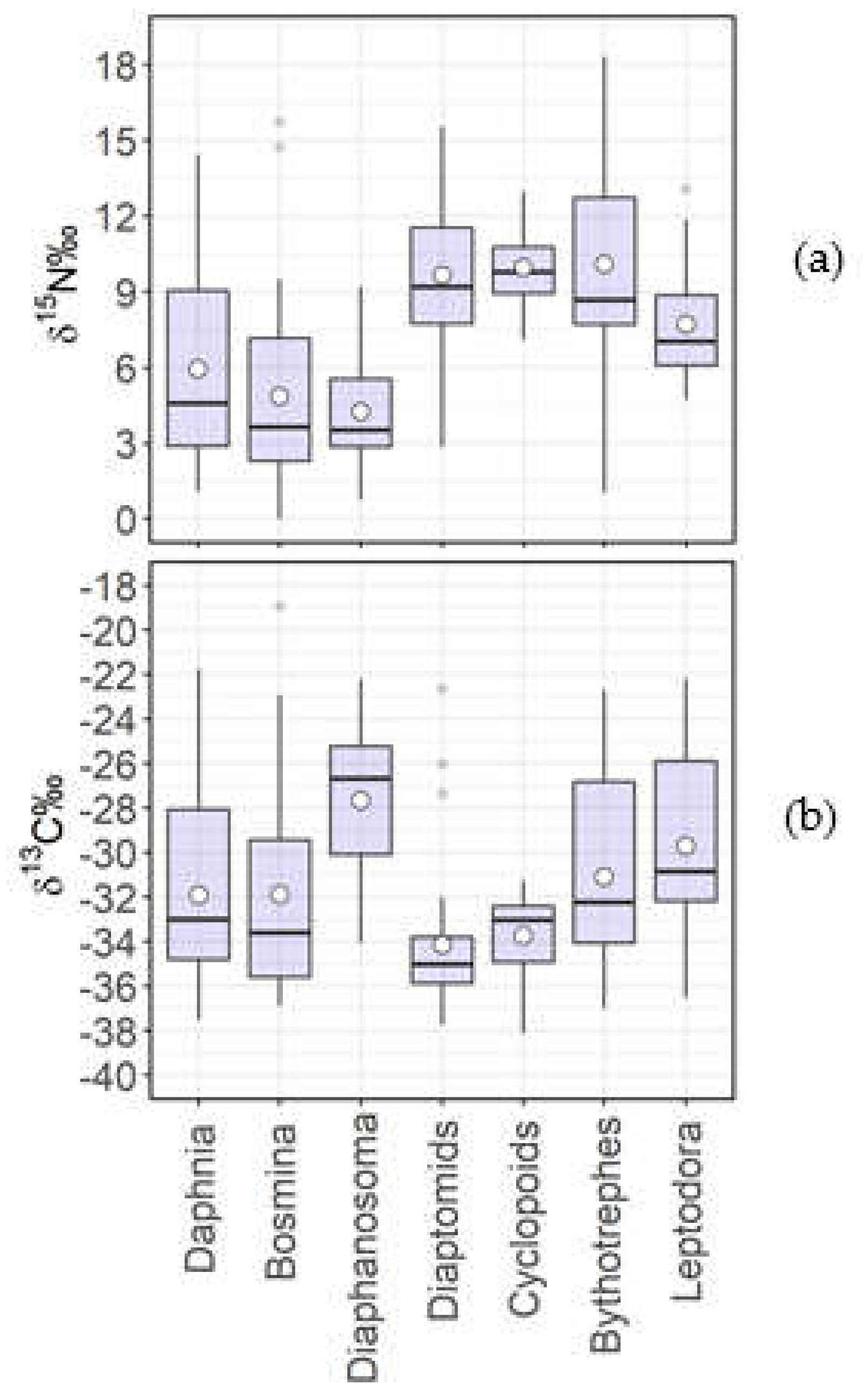
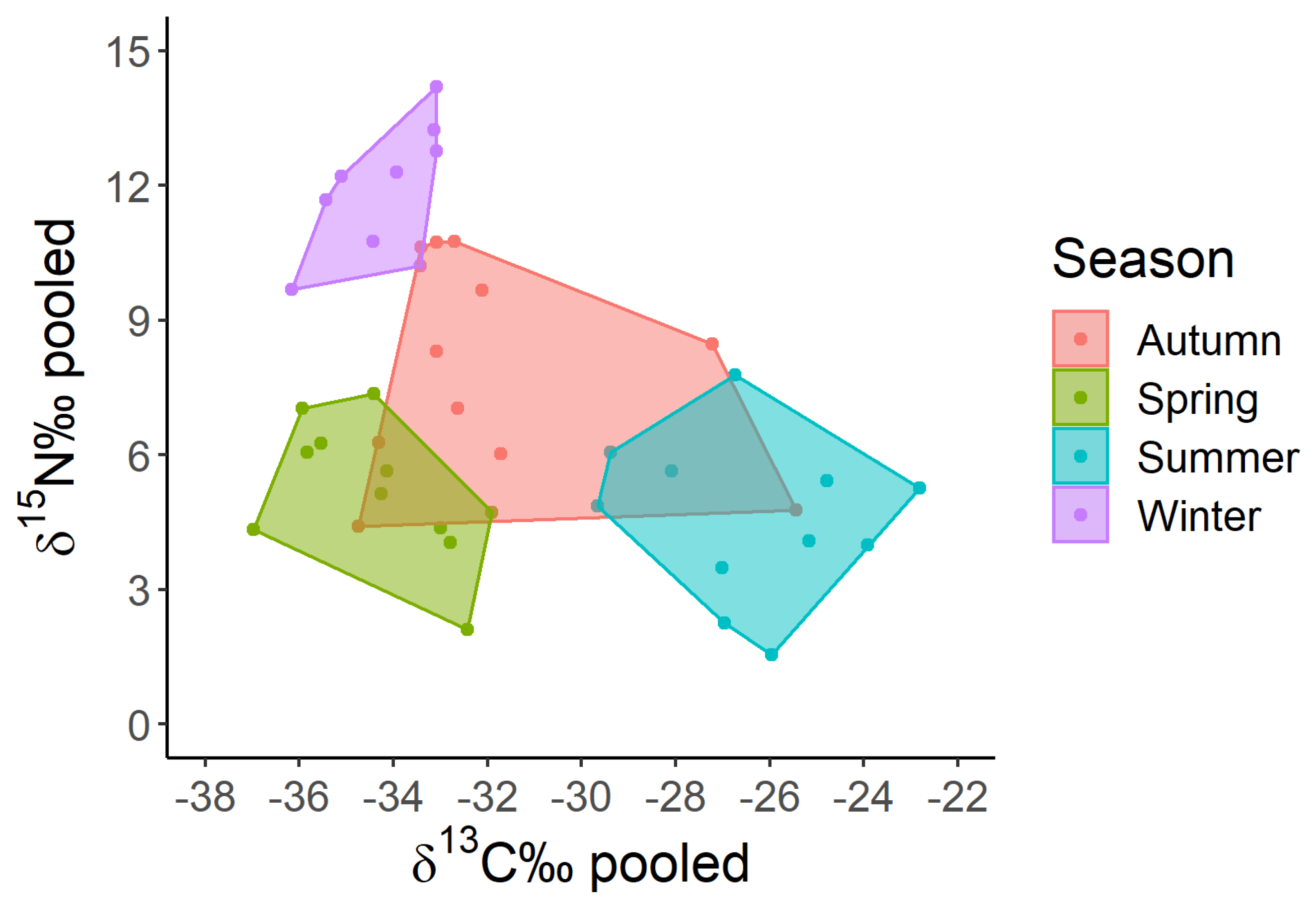
4.2. Carbon isotopic signature in different taxa/groups as niche indicators
4.3. Zooplankton (Daphnia) δ13C and chlorophyll-a: indication of food quality and availability
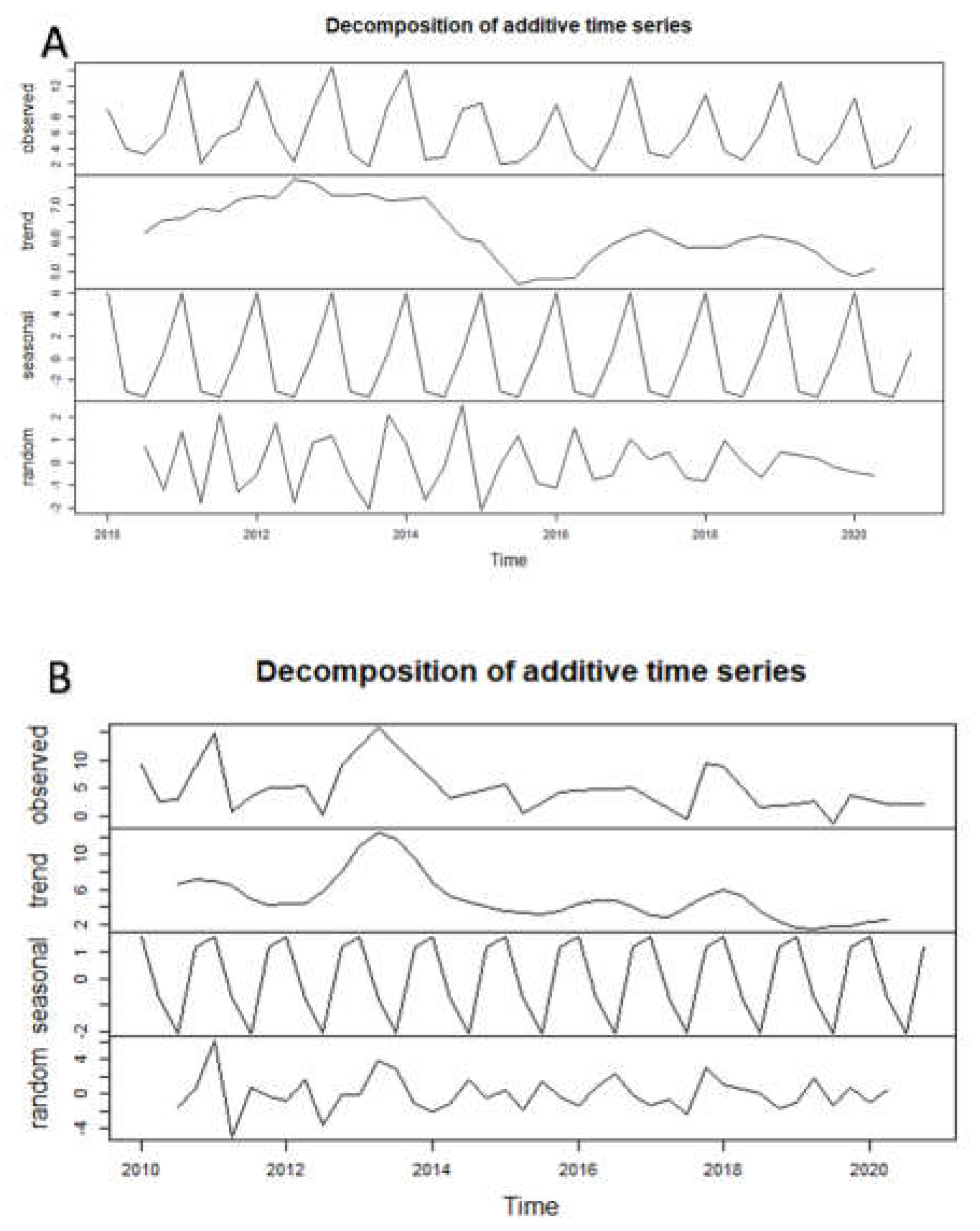
4.4. δ13C and δ15N time series and environmental variables
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
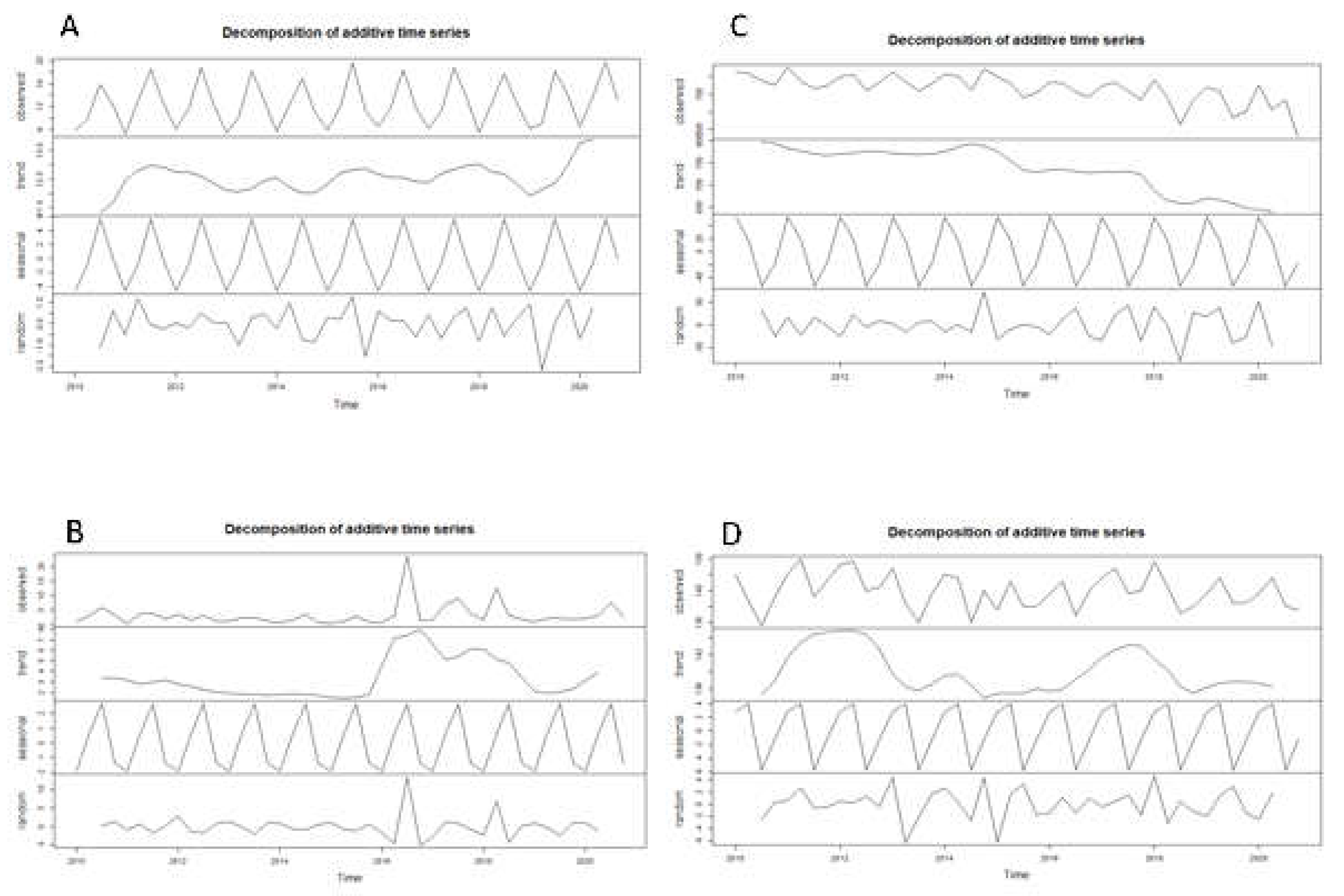
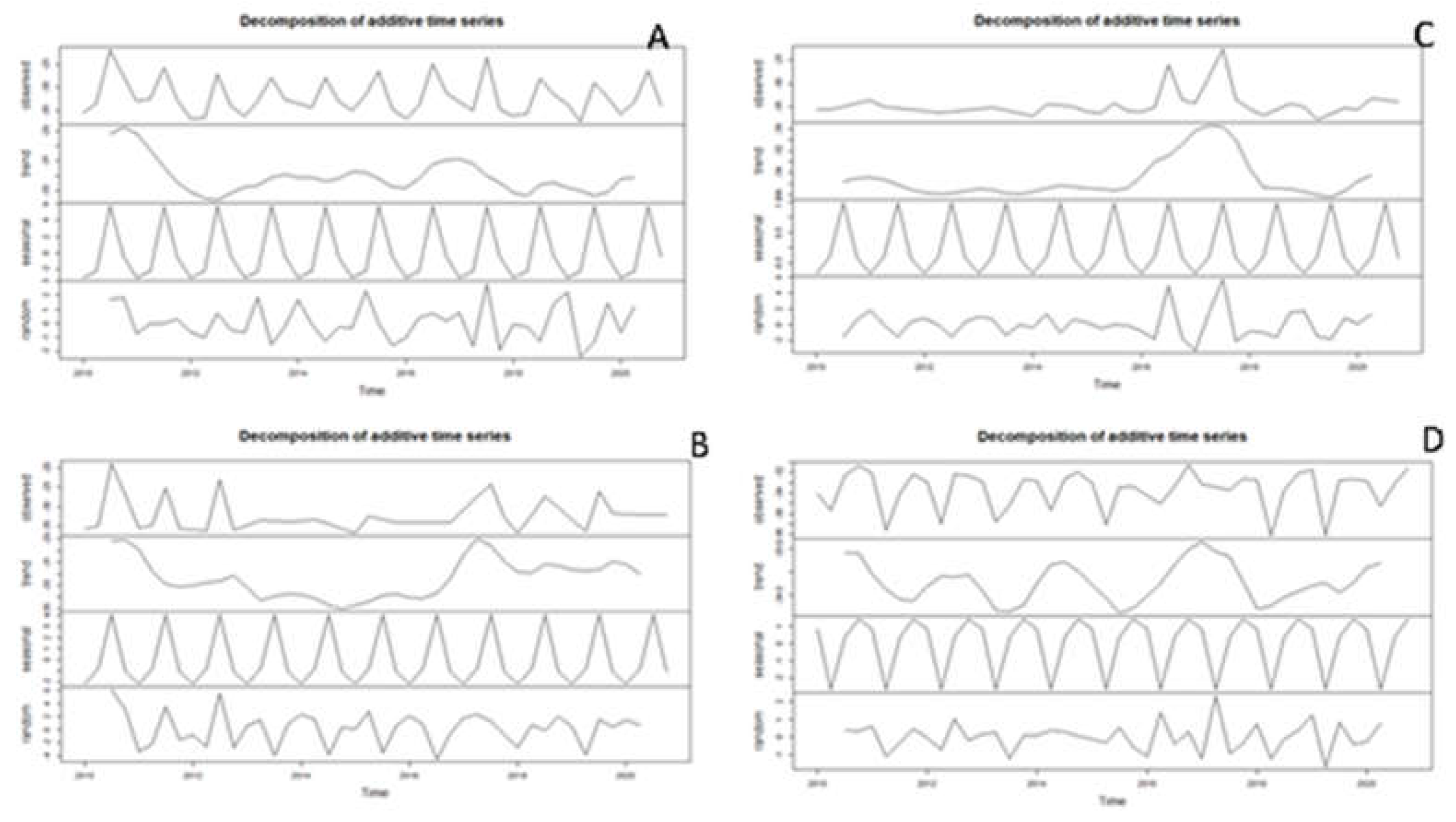
References
- Moss, B. Cogs in the endless machine: lakes, climate change and nutrient cycles: a review. STOTEN, 2012, 434, 130-142. [CrossRef]
- Kagalou, I., Papastergiadou, E., Leonardos, I. Long term changes in the eutrophication process in a shallow Mediterranean lake ecosystem of W. Greece: Response after the reduction of external load. J. Environ. Manage., 2008, 87(3), 497-506. [CrossRef]
- Foley, B., Jones, I.D., Maberly, S.C., Rippey, B. Long-term changes in oxygen depletion in a small temperate lake: effects of climate change and eutrophication. Freshwat. Biol., 2012, 57(2), 278-289. [CrossRef]
- Arfè, A., Quatto, P., Zambon, A., MacIsaac, H. J., Manca, M. Long-term changes in the zooplankton community of Lake Maggiore in response to multiple stressors: A functional principal components analysis. Water, 2019, 11(5), 962. [CrossRef]
- Rogora, M., Austoni, M., Caroni, R., Giacomotti, P., Kamburska, L., Marchetto, A., Mosello, R., Orrù, A., Tartari, G., Dresti, C. Temporal changes in nutrients in a deep oligomictic lake: the role of external loads versus climate change. J. Limnol., 2021, 80(3), 2051. [CrossRef]
- Chang, C.W., Miki, T., Ye, H., Souissi, S., Adrian, R., Anneville, O., Agasild, H., Ban, S., Be’eri-Shlevin, Y., Chiang, Y.R., Feuchtmayr, H., Gal, G., Ichise, S., Kagami, M., Kumagai, M., Liu, X., Matsuzaki, S.-I. S., Manca, M. M., Nõges, P., Piscia, R., Rogora, M., Shiah, F.K., Thackeray, S.J., Widdicombe, C. E., Wu, J.T., Zohary, T., Hsieh, C.-H. Causal networks of phytoplankton diversity and biomass are modulated by environmental context. Nat. Commun., 2022, 13(1), 1-11. [CrossRef]
- Poff, N.L., Brinson, M.M., Day, J.W. Aquatic ecosystems and global climate change. Pew Center on Global Climate Change, Arlington, VA 2002, 44, 1-36. https://www.c2es.org/wp-content/uploads/2002/01/aquatic.pdf.
- Mooij, W.M., Hülsmann, S., De Senerpont Domis, L.N., Nolet, B.A., Bodelier, P.L., Boers, P., Pires, L.M.D., Gons, H.J., Ibelings, B.W., Noordhuis, R., Portielje, R., Wolfstein, K., Lammens, E.H.R.R. The impact of climate change on lakes in the Netherlands: a review. Aquat. Ecol., 2005, 39(4), 381-400. [CrossRef]
- Tylianakis, J.M., Didham, R.K., Bascompte, J., Wardle, D.A. Global change and species interactions in terrestrial ecosystems. Ecol. Lett., 2008, 11(12), 1351-1363. [CrossRef]
- Williamson, C.E., Saros, J.E., Vincent, W.F., Smol, J.P. Lakes and reservoirs as sentinels, integrators, and regulators of climate change. Limnol. Oceanogr., 2009, 54(6), 2273-2282. [CrossRef]
- Fenocchi, A., Rogora, M., Sibilla, S., Dresti, C. Relevance of inflows on the thermodynamic structure and on the modeling of a deep subalpine lake (Lake Maggiore, Northern Italy/Southern Switzerland). Limnologica, 2017, 63, 42-56. [CrossRef]
- Tanentzap, A.J., Morabito, G., Volta, P., Rogora, M., Yan, N.D., Manca, M. Climate warming restructures an aquatic food web over 28 years. Glob. Change Biol., 2020, 26(12), 6852-6866. [CrossRef]
- Jeppesen, E., Meerhoff, M., Holmgren, K., González-Bergonzoni, I., Teixeira-de Mello, F., Declerck, S. A., De Meester, L., Søndergaard, M., Lauridsen, T.L., Bjerring, R., Conde-Porcuna, J.M., Mazzeo, N., Iglesias, C., Reizenstein, M., Malmquist, I.J., Liu, Z., Balayla, D., Lazzaro, X. Impacts of climate warming on lake fish community structure and potential effects on ecosystem function. Hydrobiologia, 2010, 646(1), 73-90. [CrossRef]
- Jeppesen, E., Brucet, S., Naselli-Flores, L., Papastergiadou, E., Stefanidis, K., Noges, T., Noges, P., Attayde, J.L., Zohary, T., Coppens, J., Bucak, T., Menezes, R.F., Freitas, F.R.S., Kernan, M., Søndergaard, M., Beklioğlu, M. Ecological impacts of global warming and water abstraction on lakes and reservoirs due to changes in water level and related changes in salinity. Hydrobiologia, 2015, 750(1), 201-227. [CrossRef]
- Lin, Q., Xu, L., Hou, J., Liu, Z., Jeppesen, E., Han, B.P. Responses of trophic structure and zooplankton community to salinity and temperature in Tibetan lakes: Implication for the effect of climate warming. Wat. Res., 2017, 124, 618-629. [CrossRef]
- Rantala, M.V., Luoto, T.P., Weckström, J., Rautio, M., Nevalainen, L. Climate drivers of diatom distribution in shallow subarctic lakes. Freshwat. Biol., 2017, 62(12), 1971-1985. [CrossRef]
- Woolway, R.I., Merchant, C.J. Worldwide alteration of lake mixing regimes in response to climate change. Nat. Geosci., 2019, 12(4), 271-276. [CrossRef]
- Woolway, R.I., Kraemer, B.M., Lenters, J.D., Merchant, C.J., O’Reilly, C.M., Sharma, S. Global lake responses to climate change. Nat. Rev. Earth Environ., 2020, 1(8), 388-403. [CrossRef]
- Sommer, U., Adrian, R., De Senerpont Domis, L., Elser, J.J., Gaedke, U., Ibelings, B., Jeppesen, E., Lurling, M., Molinero, J. C., Mooij, W.M., van Donk, E., Winder, M. Beyond the Plankton Ecology Group(PEG) Model: Mechanisms Driving Plankton Succession. Annu. Rev. Ecol. Evol. Syst., 2012, 43, 429-448. 2012. [CrossRef]
- Varpe, Ø. Fitness and phenology: annual routines and zooplankton adaptations to seasonal cycles. J. Plank. Res., 2012, 34(4), 267-276. [CrossRef]
- Kürten, B., Painting, S.J., Struck, U., Polunin, N.V., Middelburg, J.J. Tracking seasonal changes in North Sea zooplankton trophic dynamics using stable isotopes. Biogeochemistry, 2013, 113(1), 167-187. [CrossRef]
- Bănaru, D., Carlotti, F., Barani, A., Grégori, G., Neffati, N., Harmelin-Vivien, M. Seasonal variation of stable isotope ratios of size-fractionated zooplankton in the Bay of Marseille (NW Mediterranean Sea). J. Plank. Res., 2014, 36(1), 145-156. [CrossRef]
- Moss, B.R. Ecology of fresh waters: man and medium, past to future. John Wiley & Sons Pub., 2009, 482 pp ISBN-10:1405113324.
- Visconti, A., Caroni, R., Rawcliffe, R., Fadda, A., Piscia, R., Manca, M. Defining seasonal functional traits of a freshwater zooplankton community using δ13C and δ15N stable isotope analysis. Water, 2018, 10(2), 108. [CrossRef]
- Cicala, D., Polgar, G., Mor, J. R., Piscia, R., Brignone, S., Zaupa, S., Volta, P. Trophic niches, trophic positions, and niche overlaps between non-native and native fish species in a subalpine lake. Water, 2020, 12(12), 3475. [CrossRef]
- Pel, R., Hoogveld, H., Floris, V. Using the hidden isotopic heterogeneity in phyto-and zooplankton to unmask disparity in trophic carbon transfer. Limnol. Oceanogr., 2003, 48(6), 2200-2207. [CrossRef]
- Matthews, B., Mazumder, A. Temporal variation in body composition (C:N) helps explain seasonal patterns of zooplankton δ13C. Freshwat. Biol., 2005, 50, 502–515. [CrossRef]
- Perga M.E., Gerdaux, D. Seasonal variability in the δ13C and δ15N values of the zooplankton taxa in two alpine lakes. Acta Oecol., 2006, 30, 69-77. [CrossRef]
- Gearing, J.N., Gearing, P.J., Rudnick, D.T., Requejo, A.G., Hutchins, M.J. Isotopic variability of organic carbon in a phytoplankton-based, temperate estuary. Geochim. Cosmochim. Acta, 1984, 48(5), 1089-1098. [CrossRef]
- Rau, G.H., Takahashi, T., Marais, D.J.D. Latitudinal variations in plankton δ13C: implications for CO2 and productivity in past oceans. Nature, 1989, 341(6242), 516-518. [CrossRef]
- Goericke, R., Fry, B. (1994). Variations of marine plankton δ13C with latitude, temperature, and dissolved CO2 in the world ocean. Glob. Biogeochem. Cycles, 1994, 8(1), 85-90. [CrossRef]
- Miller, T.W., Brodeur, R.D., Rau, G.H. Carbon stable isotopes reveal relative contribution of shelf-slope production to the Northern California Current pelagic community. Limnol. Oceanogr., 2008, 53(4), 1493-1503. [CrossRef]
- Lara, R.J., Alder, V., Franzosi, C.A., Kattner, G. Characteristics of suspended particulate organic matter in the southwestern Atlantic: influence of temperature, nutrient and phytoplankton features on the stable isotope signature. J. Mar. Systems, 2010, 79(1-2), 199-209. [CrossRef]
- Papiol, V., Cartes, J.E., Fanelli, E., Rumolo, P. Food web structure and seasonality of slope megafauna in the NW Mediterranean elucidated by stable isotopes: relationship with available food sources. J. Sea Res., 2013, 77, 53-69. [CrossRef]
- Kürten, B., Al-Aidaroos, A.M., Struck, U., Khomayis, H.S., Gharbawi, W.Y., Sommer, U. Influence of environmental gradients on C and N stable isotope ratios in coral reef biota of the Red Sea, Saudi Arabia. J. Sea Res., 2014, 85, 379-394. [CrossRef]
- Gu, B., Schelske, C.L., Waters, M.N. Patterns and controls of seasonal variability of carbon stable isotopes of particulate organic matter in lakes. Oecologia, 2011, 165(4), 1083-1094. [CrossRef]
- Hou W., Gu B., Zhang, H., Gu, J, Han, B.P. The relationship between carbon and nitrogen stable isotopes of zooplankton and select environmental variables in low-latitude reservoirs. Limnology, 2013, 14(I), 97-104. [CrossRef]
- Ruggiu, D., Morabito, G., Panzani, P., Pugnetti, A. Trends and relations among basic phytoplankton characteristics in the course of the long-term oligotrophication of Lake Maggiore (Italy). Hydrobiologia, 1998, 369/370, 243–257. [CrossRef]
- Salmaso, N., Mosello, R. Limnological research in the deep southern subalpine lakes: synthesis, directions and perspectives. Adv. Oceanogr.Limnol., 2010, 1(1), 29-66. [CrossRef]
- Tapolczai, K., Anneville, O., Padisák, J., Salmaso, N., Morabito, G., Zohary, T., Tadonléké, R.D., Rimet, F. Occurrence and mass development of Mougeotia spp. (Zygnemataceae) in large, deep lakes. Hydrobiologia, 2015, 745(1), 17-29. [CrossRef]
- Bresciani, M., Cazzaniga, I., Austoni, M., Sforzi, T., Buzzi, F., Morabito, G., Giardino, C. Mapping phytoplankton blooms in deep subalpine lakes from Sentinel-2A and Landsat-8. Hydrobiologia, 2018, 824(1), 197-214. [CrossRef]
- Morabito, G., Oggioni, A., Austoni, M. Resource ratio and human impact: how diatom assemblages in Lake Maggiore responded to oligotrophication and climatic variability. In: Phytoplankton responses to human impacts at different scales. Salmaso, N., Naselli-Flores, L., Cerasino, L., Flaim, G., Tolotti, M., Padisák, J. (eds), Develop. Hydrobiol., 2012, 221, 47-60. [CrossRef]
- Tanentzap, A.J., Fitch, A., Orland, C., Emilson, E.J., Yakimovich, K.M., Osterholz, H., Dittmar, T. Chemical and microbial diversity covary in fresh water to influence ecosystem functioning. PNAS, 2019, 116(49), 24689-24695. [CrossRef]
- Fenocchi, A., Rogora, M., Sibilla, S., Ciampittiello, M., Dresti, C. Forecasting the evolution in the mixing regime of a deep subalpine lake under climate change scenarios through numerical modelling (Lake Maggiore, Northern Italy/Southern Switzerland). Clim. Dyn., 2018, 51(9), 3521-3536. [CrossRef]
- Rogora, M., Buzzi, F., Dresti, C., Leoni, B., Lepori, F., Mosello, R., Patelli, M., Salmaso, N. Climatic effects on vertical mixing and deep-water oxygen content in the subalpine lakes in Italy. Hydrobiologia, 2018, 824(1), 33-50. [CrossRef]
- Zohary T., Erez J., Gophen M., Berman-Frank I., Stiller M. Seasonality of stable carbon isotopes within the pelagic food web of Lake Kinneret. Limnol. Oceanogr., 1994, 39(5), 1030-1043. [CrossRef]
- Schindler, D.W. The cumulative effects of climate warming and other human stresses on Canadian freshwaters in the new millennium. Can. J. Fish. Aquat. Sci., 2001, 58, 18-29. [CrossRef]
- Visconti, A., Manca, M., de Bernardi, R. Eutrophication-like response to climate warming: an analysis of Lago Maggiore (N. Italy) zooplankton in contrasting years. J. Limnol., 2008, 67(2), 87-92. [CrossRef]
- Takahashi, K., Yoshioka, T., Wada, E., Sakamoto, M. Temporal variations in carbon isotope ratio of phytoplankton in a eutrophic lake. J. Plank. Res., 1990, 12(4), 799-808. [CrossRef]
- Visconti, A., Manca, M. Seasonal changes in the δ13C and δ15N signatures of the Lago Maggiore pelagic food web. J. Limnol., 2011, 70(2), 263–271. [CrossRef]
- Matthews, B., Mazumder, A. (2003). Compositional and interlake variability of zooplankton affect baseline stable isotope signatures. Limnol. Oceanogr., 2003, 48(5), 1977–1987. [CrossRef]
- Wetzel, R.G. Limnology: lake and river ecosystems. Academic Press, London, 2001, 1024 pp. eBook ISBN: 9780080574394.
- Post, D.M., Pace, M.L., Hairston Jr, N.G. Ecosystem size determines food-chain length in lakes. Nature, 2000, 405(6790), 1047-1049. [CrossRef]
- DeNiro, M. J., Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta, 1978, 42, 495-506. [CrossRef]
- Ambrosetti, W., Barbanti, L., Carrara, E.A. (2010). Mechanisms of hypolimnion erosion in a deep lake (Lago Maggiore, N. Italy). J. Limnol., 2010, 69(1), 3-14. [CrossRef]
- APHA, AWWA and WEF. Standard Methods for the Examination of Water & Wastewater, 22nd Edition. 2012, Washington DC: American Public Health Association.
- P. A. T. IRSA-CNR. Metodi analitici per le acque. Manuali e Linee guida, 2003, 29, 1153. ISBN 88-448-0083-7.
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis. Springer Cham Pubb., 2006, 260pp. [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing (version 3.6.1). Vienna, Austria: R Foundation for Statistical Computing, 2019. https://www.R-project.org/.
- Piscia, R., Mazzoni, M., Bettinetti, R., Caroni, R., Cicala, D., Manca, M.M. Stable Isotope Analysis and persistent organic pollutants in crustacean zooplankton: The role of size and seasonality. Water, 2019, 11(7), 1490. [CrossRef]
- Lehmann, M.F., Bernasconi, S.M., McKenzie, J.A. Seasonal variation of the δ13C and δ15N of particulate and dissolved carbon and nitrogen in Lake Lugano: Constraints on biogeochemical cycling in a eutrophic lake. Limnol. Oceanogr., 2004, 49(2), 415-429. [CrossRef]
- Grey, J., Jones R.I. Seasonal changes in the importance of the source of organic matter to the diet of zooplankton in Loch Ness, as indicated by stable isotope analysis. Limnol. Oceanogr., 2001, 46(3), 505–513. [CrossRef]
- Caroni, R., Free, G., Visconti, A., Manca, M. Phytoplankton functional traits and seston stable isotopes signature: a functional-based approach in a deep, subalpine lake, Lake Maggiore (N. Italy). J. Limnol., 2012, 71(1), 84-94. [CrossRef]
- Fadda, A., Rawcliffe, R., Padedda, B. M., Luglie, A., Sechi, N., Camin, F., Ziller, L., Manca, M. Spatiotemporal dynamics of C and N isotopic signature of zooplankton: a seasonal study on a man-made lake in the Mediterranean region. Ann. Limnol.-Int. J. Limnol., 2014; 50(4), 279-287. [CrossRef]
- Rau, G.H., Takahashi, T., Des Marais, D.J., Repeta, D.J., Martin, J.L. The relationship between δ13C of organic matter and [CO2 (aq)] in ocean surface water: data from a JGOFS site in the northeast Atlantic Ocean and a model. Geochim. Cosmochim. Acta, 1992, 56(3), 1413-1419. [CrossRef]
- Yoshioka, T., Wada, E., Hayashi, H. A stable isotope study on seasonal food web dynamics in a eutrophic lake. Ecology, 1994, 75(3), 835-846. [CrossRef]
- Gu, B., Alexander, V., Schell, D.M. Seasonal and interannual variability of plankton carbon isotope ratios in a subarctic lake. Freshwat. Biol., 1999, 42(3), 417-426. [CrossRef]
- Masclaux, H., Richoux, N.B. Effects of temperature and food quality on isotopic turnover and discrimination in a cladoceran. Aquat. Ecol., 2017, 51(1), 33-44. [CrossRef]
- Power, M., Guiguer, K.R.R.A., Barton, D.R. Effects of temperature on isotopic enrichment in Daphnia magna: implications for aquatic food-web studies. RCM, 2003, 17(14), 1619-1625. [CrossRef]
- Gu, B. Schelske, D.M. Temporal and spatial variations in phytoplankton carbon isotopes in a polymictic subtropical lake. J. Plank. Res., 1996, 18, 2081-2092. [CrossRef]
- Vander Zanden, M.J., Rasmussen, J.B. Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology, 1999, 1395-1404. [CrossRef]
- Grey, J., Jones, R. I., Sleep, D. Stable isotope analysis of the origins of zooplankton carbon in lakes of differing trophic state. Oecologia, 2000, 123, 232-240. [CrossRef]
- Cattaneo, A., Manca, M., Rasmussen, J.B. Peculiarities in the stable isotope composition of organisms from an alpine lake. Aquat. Sci., 2004, 66(4), 440-445. [CrossRef]
- Leoni, B. Zooplankton predators and preys: Body size and stable isotope to investigate the pelagic food in a deep lake (Lake Iseo, Northern Italy). J. Limnol., 2017, 76(1), 85-93. [CrossRef]
- Makino, W., Yoshida, T., Sakano, H., Ban, S. Stay cool: habitat selection of a cyclopoid copepod in a north temperate oligotrophic lake. Freshwat. Biol., 2003, 48(9), 1551-1562. [CrossRef]
- Helland, I.P., Freyhof, J., Kasprzak, P., Mehner, T. Temperature sensitivity of vertical distributions of zooplankton and planktivorous fish in a stratified lake. Oecologia, 2007, 151(2), 322-330. [CrossRef]
- Thackeray, S.J., Elliott, J.A., Fielding, R.F., Swinburne, K. Effects of onset of thermal stratification on vertical distribution of phytoplankton and zooplankton species. Verh. Int. Ver. Theor. Angew. Limnol., 2005, 29(1), 555-559. [CrossRef]
- Smyntek, P.M., Maberly, S.C., Grey, J. Dissolved carbon dioxide concentration controls baseline stable carbon isotope signatures of a lake food web. Limnol. Oceanogr., 2012, 57(5), 1292-1302. [CrossRef]
- Smyntek, P.M., Teece, M.A., Schulz, K.L., Storch, A.J. Taxonomic differences in the essential fatty acid composition of groups of freshwater zooplankton relate to reproductive demands and generation time. Freshwat. Biol., 2008, 53(9), 1768-1782. [CrossRef]
- Manca, M., Cavicchioni, N., Morabito, G. First observations on the effect of a complete, exceptional overturn of Lake Maggiore on plankton and primary productivity. Int. Rev. Hydrobiol., 2000, 85(2-3), 209-222. [CrossRef]
- France, R.L. Differentiation between littoral and pelagic food webs in lakes using stable carbon isotopes. Limnol. Oceanogr., 1995, 40(7), 1310-1313. [CrossRef]
- Croteau, M.N., Luoma, S.N., Stewart, A.R. Trophic transfer of metals along freshwater food webs: evidence of cadmium biomagnification in nature. Limnol. Oceanogr., 2005, 50(5), 1511-1519. [CrossRef]
- Visconti, A., Volta, P., Fadda, A., Manca, M. Roach in Lake Maggiore: A Peaceful Invasion Detected with C, N Stable Isotope Analysis. GJSFR, 2013, 13, 1-8.
- Buhan, E., Kaymak, N., Akin, S., Turan, H. Trophic Pathways from Pelagic and Littoral Sources Supports Food Web in A Eutrophic Natural Lake (Lake Zinav, Turkey). Turk. J. Fish. Aquat. Sci., 2018, 19(2), 99-107. [CrossRef]
- Geller, W., Müller, H. The filtration apparatus of Cladocera: filter mesh-sizes and their implications on food selectivity. Oecologia, 1981, 49(3), 316-321. [CrossRef]
- DeMott, W.R., Kerfoot, C.W. Competition among cladocerans: nature of the interaction between Bosmina and Daphnia. Ecology, 1982, 63(6), 1949-1966. [CrossRef]
- Deng, D., Xie, P., Zhou, O., Yang, H., Guo, L., Geng, H. Field and experimental studies on the combined impacts of cyanobacterial blooms and small algae on crustacean zooplankton in a large, eutrophic, subtropical, Chinese lake. Limnology, 2008, 9(1), 1-11. [CrossRef]
- Carpenter, E.J., Montoya, J.P., Burns, J., Mulholland, M.R., Subramaniam, A., Capone, D.G. Extensive bloom of a N2-fixing diatom/cyanobacterial association in the tropical Atlantic Ocean. Mar. Ecol. Progr. Series, 1999, 185, 273-283. [CrossRef]
- Bauersachs, T., Schouten, S., Compaoré, J., Wollenzien, U., Stal, L.J., Damsteé, J.N.S. Nitrogen isotopic fractionation associated with growth on dinitrogen gas and nitrate by cyanobacteria. Limnol Oceanog., 2009, 54(4), 1403-1411. [CrossRef]
- Gu, B., Chapman, A.D., Schelske, C.L. Factors controlling seasonal variations in stable isotope composition of particulate organic matter in a softwater eutrophic lake. Limnol. Oceanogr., 2006, 51(6), 2837-2848. [CrossRef]
- Jones, R.I., Grey, J., Quarmby, C., Sleep, D. An assessment using stable isotopes of the importance of allochthonous organic carbon sources to the pelagic food web in Loch Ness. Proc. R. Soc. Lond. B, 1998, 265, 105–111. [CrossRef]
- Winder, M., Hunter, D.A. Temporal organization of phytoplankton communities linked to physical forcing. Oecologia, 2008, 156(1), 179-192. [CrossRef]
- Lampert, W. The adaptive significance of diel vertical migration of zooplankton. Func. Ecol., 1989, 3(1), 21-27. [CrossRef]
- Hanazato, T. Direct and indirect effects of low-oxygen layers on lake zooplankton communities. Ergeb. Limnol., 1992, 35, 87-98.
- Dini, M.L., Soranno, P.A., Scheuerell, M., Carpenter, S.R. Effects of predators and food supply on diel vertical migration of Daphnia. In: The trophic cascade in lakes; Carpenter, S.R., Kitchell, J.F. Eds., Cambridge University Press, 1993, 153-171. [CrossRef]
- Persaud, A.D., Williamson, C.E. Ultraviolet and temperature effects on planktonic rotifers and crustaceans in northern temperate lakes. Freshwat. Biol., 2005, 50(3), 467-476. doi:10.1111/j.1365-2427.2005.01334.x. [CrossRef]
- Thackeray, S.J., George, D.G., Jones, R.I., Winfield, I.J. Statistical quantification of the effect of thermal stratification on patterns of dispersion in a freshwater zooplankton community. Aquat. Ecol., 2006, 40(1), 23-32. [CrossRef]
- Gelinas, M., Pinel-Alloul, B. Summer depth selection in crustacean zooplankton in nutrient-poor boreal lakes is affected by recent residential development. Freshwat. Biol., 2008, 53(12), 2438-2454. [CrossRef]
- Williamson, C.E., Rose, K.C. Ultraviolet insights: Attempting to resolve enigmatic patterns in pelagic freshwaters–The historical context and a view to the future. Int. Rev. Hydrobiol., 2009, 94(2), 129-142. [CrossRef]
- Kratz, T.K., MacIntyre, S., Webster, K.E. Causes and consequences of spatial heterogeneity in lakes. In Ecosystem function in heterogeneous landscapes. Springer, New York, NY, 2005, 329-347 pp.
- Borrelli, J.J., Relyea, R.A. A review of spatial structure of freshwater food webs: Issues and opportunities modeling within-lake meta-ecosystems. Limnol. Oceanogr., 2022, 67, 1746–1759. [CrossRef]
- Bertoni, R., Callieri, C. Organic carbon trend during the oligotrophication of Lago Maggiore. In: Strategies for lake ecosystems beyond 2000; de Bernardi, R., Pagnotta, R., Pugnetti, A. Eds., Mem. Ist. Ital. Idrobiol., 1993, 52, 191-205.
- Smith, V.H. Nutrient dependence of primary productivity in lakes 1. Limnol. Oceanogr., 1979, 24(6), 1051-1064. [CrossRef]
- Beaver, J.R., Crisman, T.L. Temporal variability in algal biomass and primary productivity in Florida lakes relative to latitudinal gradients, organic color and trophic state. Hydrobiologia, 1991, 224(2), 89-97. [CrossRef]
- Cifuentes, L.A., Sharp, J.H., Fogel, M.L. Stable carbon and nitrogen isotope biogeochemistry in the Delaware estuary. Limnol. Oceanogr., 1998, 33(5), 1102-1115. [CrossRef]
- 105 Fry, B., Wainright, S.C. Diatom sources of 13C-rich carbon in marine food webs. Mar. Ecol. Progr. Series, 1991, 76(2), 149-157. [CrossRef]
- Nakatsuka, T., Handa, N., Wada, E., Wong, C.S. The dynamic changes of stable isotopic ratios of carbon and nitrogen in suspended and sedimented particulate organic matter during a phytoplankton bloom. J. Mar. Res., 1992, 50(2), 267-296. [CrossRef]
- Takahashi, A., Takeda, K., Ohnishi, T. Light-induced anthocyanin reduces the extent of damage to DNA in UV-irradiated Centaurea cyanus cells in culture. Plant Cell Physiol., 1991, 32(4), 541-547. [CrossRef]
- Laws, E.A., Popp, B.N., Bidigare, R.R., Kennicutt, M.C., Macko, S.A. Dependence of phytoplankton carbon isotopic composition on growth rate and [CO2) aq: theoretical considerations and experimental results. Geochim. Cosmochim. Acta, 1995, 59(6), 1131-1138. [CrossRef]
- Francois, R., Altabet, M.A., Goericke, R., McCorkle, D.C., Brunet, C., Poisson, A. Changes in the δ13C of surface water particulate organic matter across the subtropical convergence in the SW Indian Ocean. Glob. Biogeochem. Cycles, 1993, 7(3), 627-644. [CrossRef]
- France, R.L., Del Giorgio, P.A., Westcott, K.A. Productivity and heterotrophy influences on zooplankton delta13C in northern temperate lakes. Aquat. Microb. Ecol., 1997, 12(1), 85-93. [CrossRef]
- Leggett, M.F., Servos, M.R., Hesslein, R., Johannsson, O., Millard, E.S., Dixon, D.G. Biogeochemical influences on the carbon isotope signatures of Lake Ontario biota. Can. J. Fish. Aquat. Sci., 1999, 56, 2211-2218. [CrossRef]
- Leggett, M.F., Johannsson, O., Hesslein, R., Dixon, D.G., Taylor, W.D., Servos, M.R. Influence of inorganic nitrogen cycling on the δ15N of Lake Ontario biota. Can. J. Fish. Aquat. Sci., 2000, 57, 1489-1496. [CrossRef]
- Vuorio, K., Meili, M., Sarvala, J. Taxon-specific variation in the stable isotopic signatures (δ13C and δ15N) of lake phytoplankton. Freshwat. Biol., 2006, 51(5), 807-822. [CrossRef]
- Free, G., Bresciani, M., Pinardi, M., Ghirardi, N., Luciani, G., Caroni, R., Giardino, C. Detecting climate driven changes in chlorophyll-a in deep subalpine lakes using long term satellite data. Water, 2021, 13(6), 866. [CrossRef]
- Gu, B., Ma, L. Smoak, D., Ewe, S., Zhu, Y., Irick, D., Ross, M., Li, Y. Mercury and sulfur environmental assessment for the Everglades. South Florida Environmental Report. South Florida Water Management District, West Palm Beach, 2013 3B18–3B20.
- Gu, B., Schell, D.M., Alexander, V. Stable carbon and nitrogen isotopic analysis of the plankton food web in a subarctic lake. Can. J. Fish. Aquat. Sci., 1994, 51(6), 1338-1344. [CrossRef]
- Gulati, R.D., Bronkhorst, M., Van Donk, E. Feeding in Daphnia galeata on Oscillatoria limnetica and on detritus derived from it. J. Plank. Res., 2001, 23(7), 705-718. [CrossRef]
- Rogora, M., Steingruber, S., Marchetto, A., Mosello, R., Giacomotti, P., Orru, A., Tartari, G.A., Tiberti, R. Response of atmospheric deposition and surface water chemistry to the COVID-19 lockdown in an alpine area. Environ. Sci. Pollut. Res., 2022, 1-18. [CrossRef]
- Webb, B.W., Hannah, D.M., Moore, R.D., Brown, L.E., Nobilis, F. Recent advances in stream and river temperature research. Hydrol. Process., 2008, 22(7), 902-918. [CrossRef]
- Woodward, G, Perkins, D.M., Brown, L.E. Climate change and freshwater ecosystems: impacts across multiple levels of organization. Philos. Trans. R. Soc. Lond., B, Biol. Sci., 2010, 365(1549), 2093-2106. [CrossRef]
- McQueen, D.J., Johannes, M.R.S., Post, J.R., Stewart, T.J., Lean, D.R.S. Bottom-up and top-down impacts on freshwater pelagic community structure. Ecol. Monogr., 1989, 59(3), 289-309. [CrossRef]
- Brett, M.T., Goldman, C.R. A meta-analysis of the freshwater trophic cascade. PNAS, 1996, 93(15), 7723-7726. [CrossRef]
- Brett, M.T., Goldman, C.R. Consumer versus resource control in freshwater pelagic food webs. Science, 1997, 275(5298), 384-386. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



