1. Introduction
The clustered regularly interspaced short palindromic repeats (CRISPR) - CRISPR-associated protein (Cas) system is an adaptive immune system widely present in bacteria and archaea [
1,
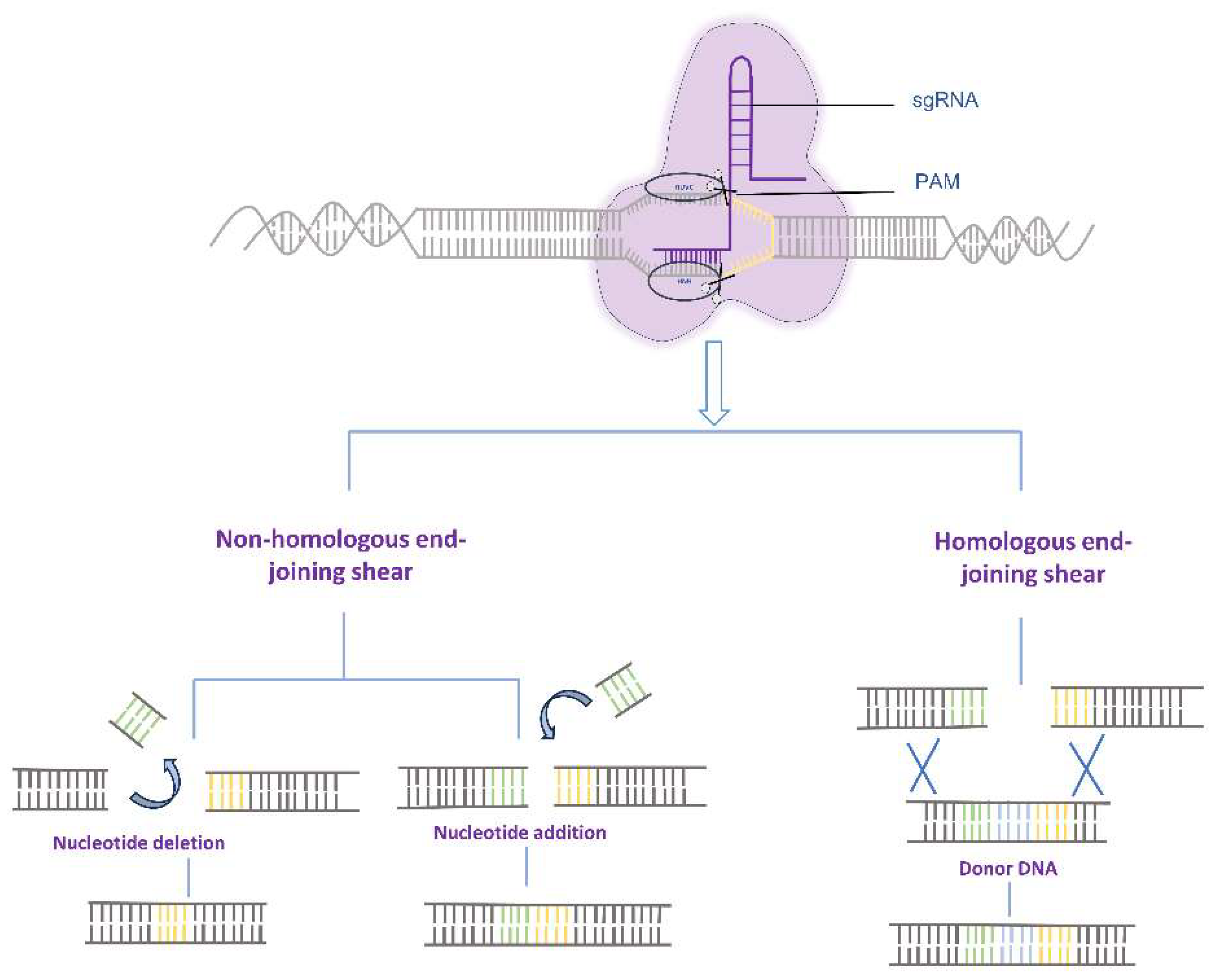
2]. This system has been harnessed by researchers for precise genome editing by inducing targeted double-strand breaks (DSBs) at specific sites, followed by repair through non-homologous end joining (NHEJ) or homology-directed repair (HDR) mechanisms, enabling the insertion of desired DNA fragments [
3]. Among the CRISPR/Cas systems, the type II system is the most commonly used, consisting of the DNA endonuclease Cas9, CRISPR RNA (crRNAs), and trans-activating crRNAs (tracrRNA). Upon application, crRNA and tracrRNA are fused to form a single guide RNA (sgRNA) [
4,
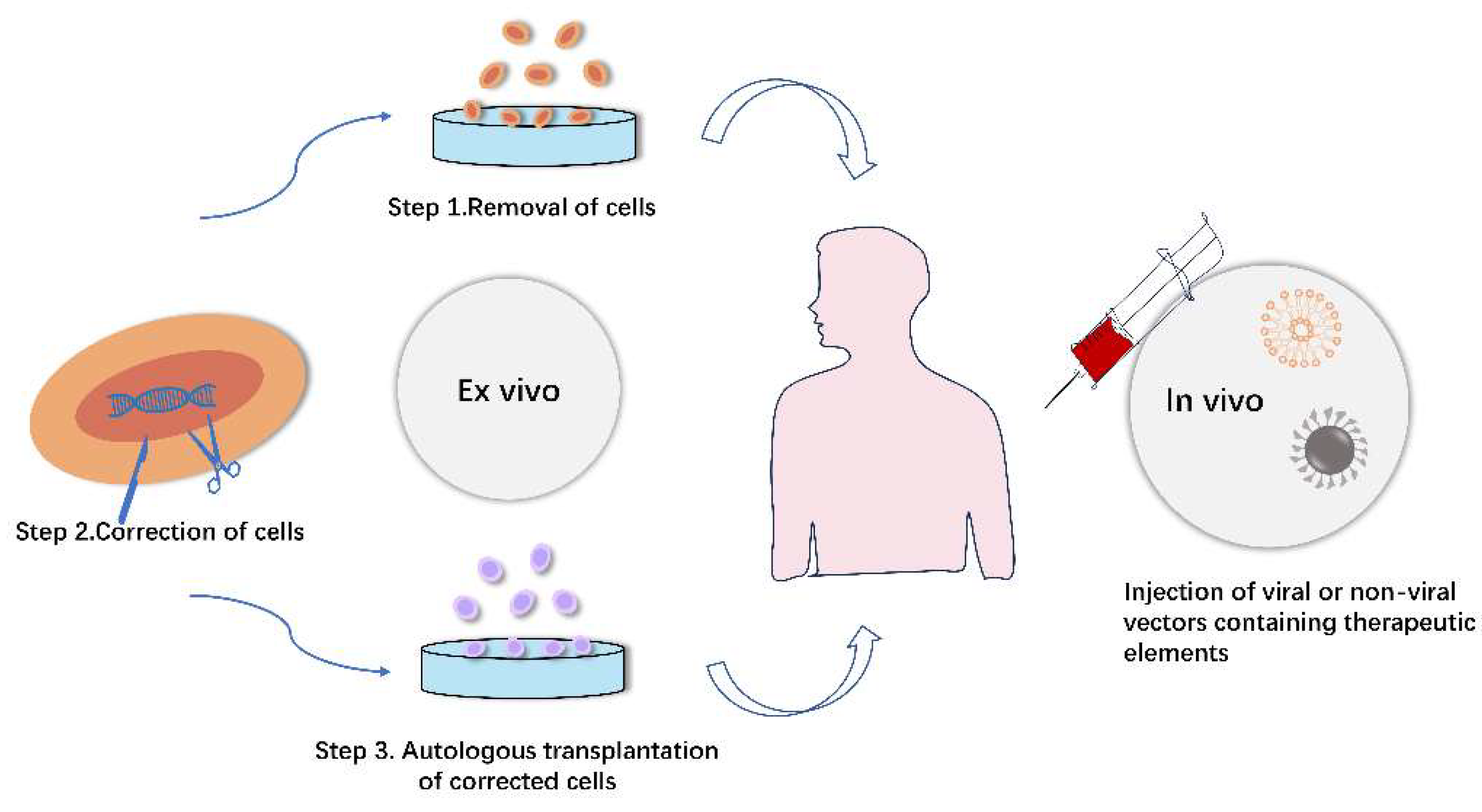
5]. Although the CRISPR-Cas9 system has been extensively applied for genome editing in diverse organisms, including humans, animals, plants, and microorganisms, it faces challenges such as limited efficiency of homologous recombination and the risk of DSB-induced genetic abnormalities, such as chromosome translocations and rearrangements, which hinder its therapeutic utility in disease treatment (
Figure 1) [
6,
7].
In 2016, David Liu et al. introduced the base editor (BE) system, representing a significant advancement in precise gene editing without the requirement for DNA double-strand breaks. This breakthrough garnered substantial attention and widespread adoption [
8]. Subsequently, in 2019, the Liu research group further expanded on their work by fusing nCas9 with the reverse transcriptase MMLV-RT, leading to the development of prime editing (PE). Through the utilization of an engineered guide RNA, PE enables efficient conversion of all 12 single nucleotides, independent of DNA templates. Furthermore, it facilitates precise insertion and deletion of multiple nucleotides [
9]. However, both BE and PE are limited to single-base editing capabilities. Therefore, the development of tools capable of site-specific integration of foreign DNA fragments without the need for DSB continues to be a prominent research focus in the field of genome editing.
Subsequently, David Liu et al. introduced a novel genome editing system known as TwinPE, which represents an advancement of the previous PE system through the incorporation of a site-specific serine integrase enzyme. TwinPE is designed to enhance the efficiency and capabilities of PE. This system employs a prime editing guide RNA (pegRNA) to introduce the integrase recognition site and utilizes a serine integrase to precisely insert specific DNA sequences at targeted site. Notably, TwinPE enables the insertion of DNA fragments as long as 40 kb with an efficiency of approximately 9.6%. It is important to acknowledge that the TwinPE system requires the artificially introduction of the integrase Subsequently, David Liu et al. introduced a novel genome editing system known as TwinPE, which represents an advancement of the previous PE system through the incorporation of a site-specific serine integrase enzyme. TwinPE is designed to enhance the efficiency and capabilities of PE. This system employs a prime editing guide RNA (pegRNA) to introduce the integrase recognition site and utilizes a serine integrase to precisely insert specific DNA sequences at targeted site. Notably, TwinPE enables the insertion of DNA fragments as long as 40 kb with an efficiency of approximately 9.6%. It is important to acknowledge that the TwinPE system requires the artificially introduction of the integrase fragment using this system is currently relatively low and requires further optimization and improvement [
10]. Subsequently, David Liu et al. introduced a novel genome editing system known as TwinPE, which represents an advancement of the previous PE system through the incorporation of a site-specific serine integrase enzyme. TwinPE is designed to enhance the efficiency and capabilities of PE. This system employs a prime editing guide RNA (pegRNA) to introduce the integrase recognition site and utilizes a serine integrase to precisely insert specific DNA sequences at targeted site. Notably, TwinPE enables the insertion of DNA fragments as long as 40 kb with an efficiency of approximately 9.6%. It is important to acknowledge that the TwinPE system requires the artificially introduction of the integrase attachment site into the genome. However, due to the two-step process involved, the overall insertion efficiency remains below 10% [
11].
Meanwhile, the Yin Lab has developed the GRAND editing system, which utilizes a pair of pegRNAs to facilitate efficient gene fragment insertion. Notably, the reverse transcriptase template within the pegRNAs is non-homologous to the target site but complementary, allowing for precise targeting of gene fragments ranging in size from 20 to 250 bp through the process of DNA reverse transcription. However, the efficiency of inserting a 400 bp fragment using this system is currently relatively low and requires further optimization and improvement [
12,
13].
CRISPR/Cas9, a cutting-edge gene editing technology, has shown great promise for gene therapy. However, the safe and efficient delivery of CRISPR/Cas9 remains a significant challenge in clinical applications [
14]. Nanoparticles, including lipid-based nanoparticles, polymeric nanoparticles, gold nanoparticles, and virus-like nanoparticles, have emerged as potential delivery systems for CRISPR/Cas9-based gene therapy due to their biocompatibility, safety, and tunable designability. These nanoparticles offer new opportunities to address the delivery challenges associated with CRISPR/Cas9-based gene therapy. This review provides a concise overview of the application of various delivery vehicles in gene therapy [
15].
2. CRISPR-Associated Transposase
Transposons, also known as transposable elements, are widespread in prokaryotic and eukaryotic genomes. These genetic elements have the capability to relocate within the chromosome, leading to an increase in their copy number through a process known as transposition [
16]. In various diseases resulting from gene mutations or deletions, transposon-based targeted insertion systems can be used for gene repair. Such systems enable precise integration and deletion of genetic material within the genome, independently of the host cell’s repair mechanism. This approach significantly expands the scope of genome editing in diverse cell types. Moreover, in comparison to current editing methods relying on homologous recombination, transposon-based strategies offer inherent advantages in terms of safety and efficiency [
17].
To enhance the specificity of transposon systems, several research groups have explored the fusion of site-specific DNA-binding proteins with transposases. Among these proteins, Cas9, with its high affinity and prolonged residence time at target sites, has garnered significant attention compared to DNA-binding zinc finger or transcriptional activator-like effector proteins. Bhatt et al. used catalytically dead Cas9 (dCas9) to precisely target the mariner transposon Hsmar1 for DNA integration [
18]. While this model system demonstrated high efficacy in vitro, it exhibited a relatively high frequency of random integration in E. coli. Likewise, when the Himar1 transposase was fused with the dCas9 nuclease, the engineered Himar1-dCas9 system achieved site-specific insertion of plasmids in E. coli with an efficiency of up to 80%.
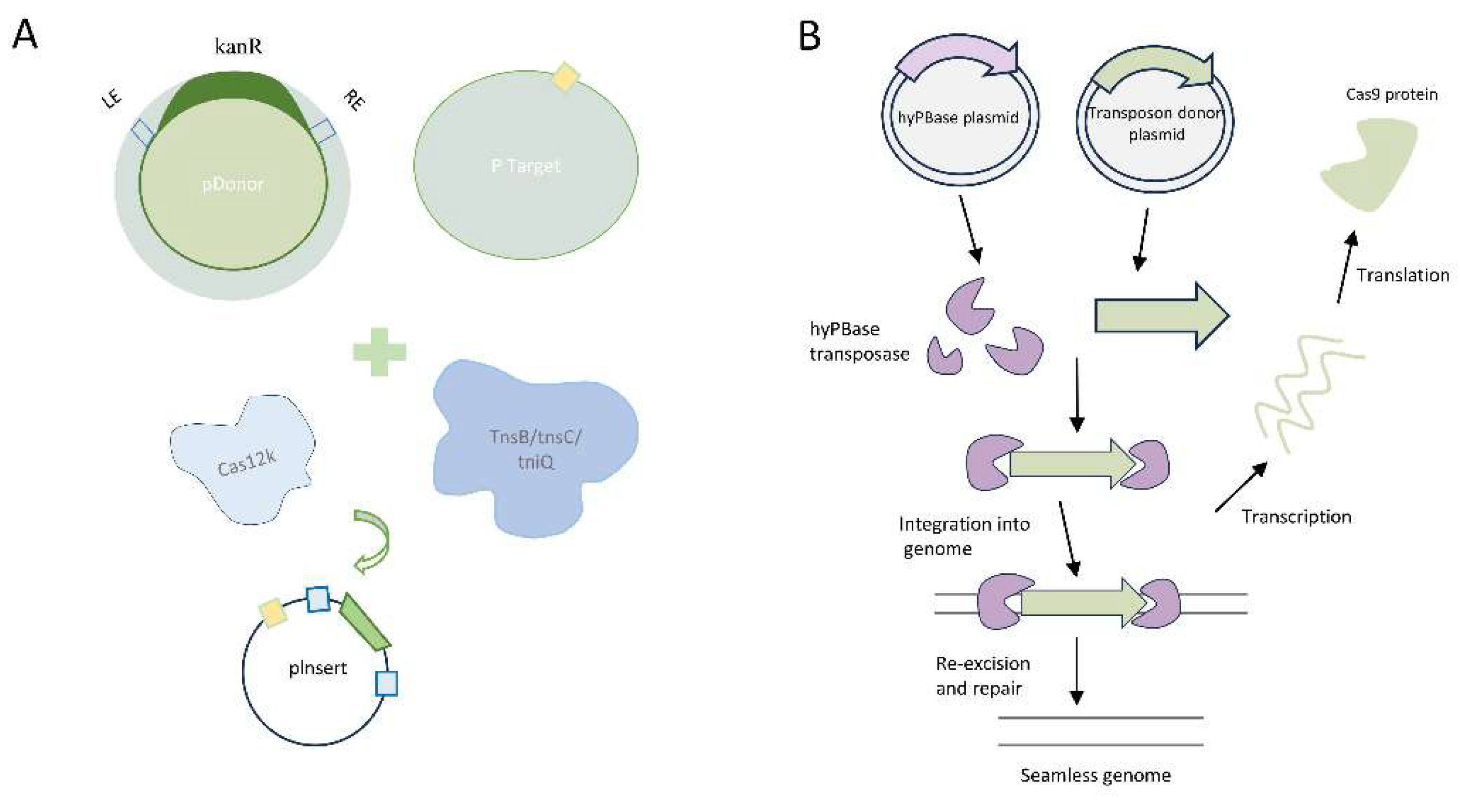
Strecker et al. have developed a CRISPR-associated transposase (CAST) system derived from cyanobacteria, which enables targeted DNA insertion. The CAST system utilizes Cas12k and effectively targets the supplementary region of the 24 bp spaced adjacent motif (PAM), but lacks cutting activity. Within the transposase-related components, TnsB and TnsC recognize and cleave specific sequences within the donor DNA, while TniQ is responsible for guiding DNA insertion. The CAST-mediated DNA insertion exhibits a directional preference; however, the efficiency of transposition is influenced by both the size of the DNA fragment and the target site.It is important to note that the off-target insertion rate, approximately 50%, should not be disregarded. In contrast, Klompe et al. have established the INTEGRATE system for targeted insertion of transposons facilitated by gRNA [
19]. Compared to the CAST system, the INTEGRATE system is better suited for direct insertion of the same DNA fragment into multiple genome targets. Transposition in this system predominantly occurs approximately 80-82 bp downstream of the 5'-CC PAM, and the off-target insertion rate is lower (<5%). However, it is worth noting that the DNA insertion in the INTEGRATE system is non-directional, and the efficiency of transposition for large DNA fragments ranging from 3 to 10 kb is lower compared to the CAST system (
Figure 2A).
The aforementioned transposon-related CRISPR-Cas systems provide efficient and sizeable donor DNA insertion into specific sites, circumventing the limitations of host cell repair mechanisms. However, certain limitations of these systems need to be addressed. Firstly, the transposon end sequences introduced along with the loaded DNA into the genome may hinder precise gene editing or scarless insertion. Secondly, while these systems have demonstrated successful DNA insertion in a limited number of prokaryotic genomes, their optimization for DNA-targeted insertion in mammalian cells or model organisms is still required. Further refinement is necessary to make them truly applicable for disease therapy.
Studies have revealed that PiggyBac (PB) and Sleeping Beauty (SB) transposons exhibit robust transposition activity in mammalian cells [
20]. In comparison to the SB transposon, PB transposon excisions typically do not leave a CAG footprint and display a preference for local hopping, which limits the utility of SB. PB, on the other hand, demonstrates higher transposition activity and typically does not leave footprints after excision, presenting new opportunities for its application in gene editing systems [
21,
22]. During transposition, the PB transposase (PBase) recognizes the specific inverted terminal repeat sequences (ITRs) present at both ends of the transposon vector, facilitating the efficient relocation of the target gene from its original position and its integration into the TTAA chromosomal site. PB exhibits high efficiency, safety, and stability, making it a highly desirable tool. Yusa et al. discovered that a hyperactive variant of PBase (hyPBase), created by introducing seven amino acid substitutions, mediates even more efficient transposition, enhancing transposition efficiency by tenfold without compromising genome integrity (
Figure 2B) [
23].
Marc Guell et al. devised a gene delivery tool named FiCAT by integrating the targeting capability of CRISPR-Cas9 with the cutting and transferring functions of PiggyBac (PB) transposase [
15]. Notably, FiCAT incorporates a strategy that renders transposition irreversible by disrupting the transposase recognition site during insertion. This innovative technology enables precise and targeted insertion of large DNA fragments, ranging from 2.5 to 9.5 kb. Experimental findings demonstrated that the FiCAT system achieved a targeted insertion efficiency of approximately 25% in human Hek293 cells and mouse C2C12 cells. However, the efficiency of targeted insertion was reduced to 4% in mouse liver and germ cells.
3. Disease Modeling and Gene Therapy
3.1. Duchenne Muscular Dystrophy
Duchenne muscular dystrophy (DMD) is an X-linked recessive genetic disorder characterized by mutations in the dystrophin (Dys) gene, resulting in the absence of dystrophin protein expression and the destabilization of muscle membrane structure [
24,
25]. It is a highly lethal genetic disease, with an incidence of approximately 1/3000 to 1/5000 male infants [
26]. In China, there are around 400 to 500 DMD cases reported annually, with a total of 70,000 to 80,000 affected individuals, making it one of the countries with the highest prevalence of DMD [
27]. The Dys gene is located in the XP21.1 to XP21.2 region and spans approximately 14 kb in length, encoding the 427 kDa dystrophin protein [
28]. There are approximately 7000 known types of mutations in the DMD gene. Deletions and duplications are the most common mutations, with 70% occurring in the central hot region spanning exons 44 to 51, and the remaining 30% in the 5' end deletion hot region encompassing exons 2 to 20 [
29]. Point mutations are randomly distributed throughout the gene, without a specific pattern [
30].
DMD currently lacks a definitive therapeutic approach. Although steroid hormone therapy can delay the loss of ambulation and improve the quality of life in DMD patients, it is associated with adverse effects such as obesity, growth impairment, and osteoporosis, and does not offer a curative solution. With the rapid progress in genetic technology, the utilization of the CRISPR/Cas system for targeted insertion of the Dys gene has gained significant attention as a promising research avenue for DMD treatment [
31].
3.2. Hemophilia
Hemophilia is an inherited bleeding disorder characterized by the deficiency of clotting factor VIII (hemophilia A) or clotting factor IX (hemophilia B). This deficiency impairs the normal clotting process, leading to prolonged bleeding following injury. The severity of hemophilia varies based on the level of clotting factor in the blood. Severe hemophilia is associated with frequent spontaneous bleeding, whereas mild hemophilia typically manifests as bleeding following trauma or surgery [
32].
Matsui, H. et al. demonstrated the efficacy of a non-viral vector gene delivery system utilizing the piggyBac DNA transposon for transferring full-length FVIII cDNA in the treatment of hemophilia A. Through in vitro electroporation, the PB vector efficiently transfected HEK293T and iPS cells, resulting in stable and sustained expression of FVIII for up to 300 days in vivo when administered via tail vein injection in mouse models. The treated group exhibited a significant reduction in mean bleeding time (6 min and 13 s) compared to the control group (18 min and 24 s). The PB vector proved to be an effective tool for mediating efficient and durable expression of the full-length FVIII gene, thereby improving the hemostatic profile in hemophilia A mice [
33].
Verma et al. employed the LUNAR platform, a safe and reliable liposomal nanoparticles (LNP) mRNA delivery system, for the treatment of hemophilia B mice with factor IX (FIX) deficiency, which resulted in rapid correction of coagulation abnormalities and sustained therapeutic effects for 4-6 days. By delivering FIX variant R338A mRNA, LUNAR LNPs demonstrated a remarkable 8-10fold increase in therapeutic efficacy and coagulation activity compared to adenoviral vectors. These findings highlight the potential of LUNAR as an effective platform for protein replacement therapy in diseases such as hemophilia, offering enhanced therapeutic benefits [
34].
3.3. Cystic Fibrosis
Cystic fibrosis (CF) is an autosomal recessive monogenic disorder that affects a significant number of individuals in the United States and Europe, with an estimated population of over 70,000 [
35].The underlying cause of CF is the mutation of the Transmembrane Conductance Regulator (CFTR) gene, which results in dysfunctional chloride ion transport. Given the genetic basis of the disease, genome intervention approaches hold potential for targeting and correcting specific alterations, offering the possibility of long-term solutions for CF. The emergence of CRISPR/Cas9, as a gene editing tool, coupled with advancements in nuclease technology, has provided new opportunities for precise genome correction and holds tremendous promise for the treatment of CF and other genetic disorders [
36,
37].
Anna Cereseto et al. employed an adenine base editing (ABE) approach using CRISPR technology to correct mutations without inducing DNA double-strand breaks (DSBs). Their study demonstrated up to 70% editing efficiency at the cellular level [
38].In a separate study, Katarina et al. aimed to enhance the delivery efficiency of Cas9-RNP. They developed a strategy based on the non-covalent binding of ABE to the amphiphilic S10 shuttle peptide. Furthermore, they derived a penetrating peptide, S315, to facilitate the formation of ABE8e-Cas9 RNP and S315 vectors for efficient delivery in rhesus airway epithelia. This approach achieved a gene editing efficiency of up to 5.3% [
39].
3.4. Thalassemia
Beta-thalassemia is a genetic disorder primarily affecting children, leading to growth failure and various complications such as chronic hepatitis, cirrhosis, and osteoporosis. Historically, the prognosis for patients with beta-thalassemia has been poor, with high mortality rates, particularly due to heart disease [
40]. However, with the advent of gene therapy, there is renewed hope for a potential cure for transfusion-dependent beta-thalassemia (TDT) through genetic interventions. TDT and sickle cell disease (SCD) are the most prevalent monogenic disorders globally, with approximately 60,000 cases of TDT and 300,000 cases of SCD diagnosed annually [
41]. These diseases are characterized by mutations in the hemoglobin beta subunit gene (HBB), which encodes adult hemoglobin, the primary oxygen-carrying protein in red blood cells[
42]. Hemoglobin is composed of α-globin and β-globin chains [
43] with alterations in hemoglobin synthesis leading to thalassemia disorders [
44].
BCL11A is a transcription factor that plays a key role in regulating the expression of γ-globin and fetal hemoglobin in erythroid cells. Researchers have employed CRISPR-Cas9 technology to target the BCL11A erythroid-specific enhancer, resulting in efficient modification of approximately 80% of the alleles at this specific genomic locus. Importantly, there were no observed off-target editing events, indicating the high specificity of the CRISPR-Cas9 approach [
45]. In a study conducted by Bin Fu et al., autologous hematopoietic stem and progenitor cells with edited BCL11A enhancers were transferred into the body. This approach demonstrated remarkable results, with patients achieving more than 18 months of transfusion independence. Furthermore, the edited cells showed high levels of gene editing efficiency, with 85.46% and 89.48% of the cells exhibiting the desired modifications, respectively. Notably, no significant adverse effects were observed, highlighting the potential safety and efficacy of this gene editing strategy [
46].
3.5. Familial Hypercholesterolemia
Familial hypercholesterolemia (FH) is a common inherited disorder characterized by elevated levels of low-density lipoprotein (LDL) cholesterol in the bloodstream, beginning from birth. This condition poses a significant risk for the development of cardiovascular disease (CVD) over time [
47]. FH is a prevalent hereditary hyperlipidemia in childhood and represents one of the most severe lipid disorders, associated with various cardiovascular complications and serving as a significant risk factor for coronary artery disease [
48]. Previous studies have estimated the frequency of heterozygous FH to be approximately 1:500, although recent data suggest that the prevalence may range from 1:200 to 1:300 [
49,
50]. In 2003, the role of the PCSK9 protein in lipid metabolism was discovered [
51]. PCSK9 interacts with the LDL receptor expressed on hepatocyte surfaces, thereby impeding receptor recycling and preventing its re-emergence on the cell surface [
52]. Normally, LDL particles are removed from the bloodstream through binding to LDL receptors on hepatocytes [
53].
Julius L.Katzmann et al. utilized an adenoviral vector-based CRISPR system for targeted gene editing of the PCSK9 gene, aiming to disrupt specific sites and reduce PSCK9 protein levels. Their study demonstrated a 9% editing efficiency, highlighting the potential of gene therapy for FH [
54]. FJ Real et al. identified Evinacumab, an anti-ANGPTL3 monoclonal antibody, as a promising therapeutic option for individuals with homozygous FH. Extensive experiments have shown that Evinacumab can induce a substantial relative reduction of LDL cholesterol by 47.1% in patients with the disease [
55]. Familial hyperlipoproteinemia (FHBL), often associated with a premature stop codon in the APOB gene, was investigated by Vanhoyo et al. using CRISPR-Cas9 technology. They successfully generated APOB knockout (KO) knock-in Huh9 cells and quantified APOB expression through ELISA and PCR. The heterozygous APOB-KO cells exhibited a 70% reduction, while the homozygous-KO cells showed near-complete eradication of APOB expression. This study highlights the potential utility of CRISPR/Cas9 in treating FHBL [
56].
3.6. Diabetic Retinopathy
The global prevalence of diabetes is projected to increase significantly, leading to a rise in the number of individuals affected by Diabetic Retinopathy (DR) and associated visual impairment worldwide [
57]. DR is a microvascular disease that contributes substantially to global visual impairment [
58]. Despite extensive research on its etiology, treatment options for DR remain limited, with up to 50% of patients showing inadequate response to current therapies [
59].
In the context of DR treatment, Haicheng Ao et al. investigated the involvement of autophagy and apoptosis in DR by employing the AAV vector-delivered CRISPR/Cas9 system to knock down the thioredoxin interacting protein (TXNIP) gene. Their study revealed the positive regulatory role of TXNIP in autophagy in rat Müller cells under hyperglycemic conditions, shedding light on the pathogenesis of DR [
60]. Additionally, Haicheng Li's team demonstrated that downregulating CCN1 using small interfering RNA (siRNA) and the CRISPR/Cas9 system led to increased levels of VE-calmodulin and reduced NADPH oxidase 4 (NOX4) levels. Elevated CCN1 expression was found to stimulate oxidative stress and disrupt the integrity of tight junctions in endothelial cells through NOX4 induction. Targeting the CCN1/NOX4 gene pathway to mitigate endothelial cell damage offers a potential therapeutic strategy for DR treatment [
61].
Figure 3.
Gene editing strategies utilizing viral and non-viral vectors in vitro and in vivo.
Figure 3.
Gene editing strategies utilizing viral and non-viral vectors in vitro and in vivo.
4. Delivery of Biomacromolecules
4.1. Duchenne Muscular Dystrophy
4.1.1. Adeno-Associated Virus (AAV)
Adeno-associated virus (AAV) has shown specific targeting capability for muscle cells, making it a potential carrier for the Dys gene. However, the limited packaging capacity of AAV vectors (up to 4.7 kb) restricts the delivery of full-length Dys gene, and only truncated micro-Dys that can produce partial proteins for gene replacement therapy can be accommodated [
62]. While micro-Dys has demonstrated improved muscle function in mouse models of Duchenne muscular dystrophy (mdx mice) and Golden Retriever Muscular Dystrophy (GRMD), it is important to consider the differences in muscle mass and atrophy severity between animal models and human patients. Consequently, the efficacy of micro-Dys in improving muscle function in humans remains uncertain. Additionally, challenges such as potential cellular toxicity, immunogenicity, and long-term expression of viral vectors hinder their clinical application [
63]. AAV vectors have the propensity to integrate the carried genes into the host DNA, often in close proximity to genes involved in cell growth regulation, which raises concerns about potential genomic alterations that could contribute to liver cancer and pose a risk for oncogenesis [
62].
Due to the heterogeneous nature of DMD gene mutations, a single CRISPR/Cas9 gene editing approach is insufficient to fully address all types of DMD patients [
66]. Therefore, it is essential to explore more precise and efficient endonuclease tools to enhance the safety and effectiveness of gene editing, expediting the progress towards comprehensive treatment of DMD patients. Tabebordbar et al. identified a group of AAV capsids, known as MyoAAV, that carry a 7-mer RGD motif. These capsids demonstrated muscle-specific gene delivery through integrin heterodimers after intravenous administration in both mouse and non-human primate models. Additionally, Weinman et al. reported the discovery of muscle-tropic capsid variants containing RGD motifs in mice [
64].
4.1.2. Lentiviral Vector (LV Vector)
LV vectors, with a size ranging from 80 to 120 nm, are enveloped viruses containing two single-stranded genomic RNAs of approximately 9 kb each. These RNAs undergo reverse transcription, resulting in the generation of double-stranded DNA that integrates into the host chromosomes [
65]. LV vectors have a higher capacity to accommodate gene fragments of up to 8 kb compared to AAV vectors, as they are enveloped by a cellular lipid bilayer rather than a viral capsid. This characteristic reduces their immunogenicity in comparison to AAV vectors [
66]. However, the integration of LV vectors into the host genome and their persistent gene expression pose challenges for the delivery of CRISPR-Cas9, as the random integration of viral DNA can activate oncogenes and increase the risk of off-target mutagenesis by Cas9 [
67].
To address the genotoxicity associated with LV vectors, researchers have developed strategies to improve their safety profile. One such approach is the use of integrase-deficient lentiviral vectors (IDLV) for gene editing delivery [
68]. Uchida et al. utilized IDLV vectors to deliver CRISPR-Cas9 components into human cord blood-derived erythroid progenitor cells (HUDEP-2), successfully correcting mutations associated with sickle cell disease in the β-globin gene. However, it was noted that the risk of viral DNA integration still exists with IDLV vectors [
69].
4.2. Non-Viral Delivery of Genome-Editing Systems
4.2.1. Exosomes
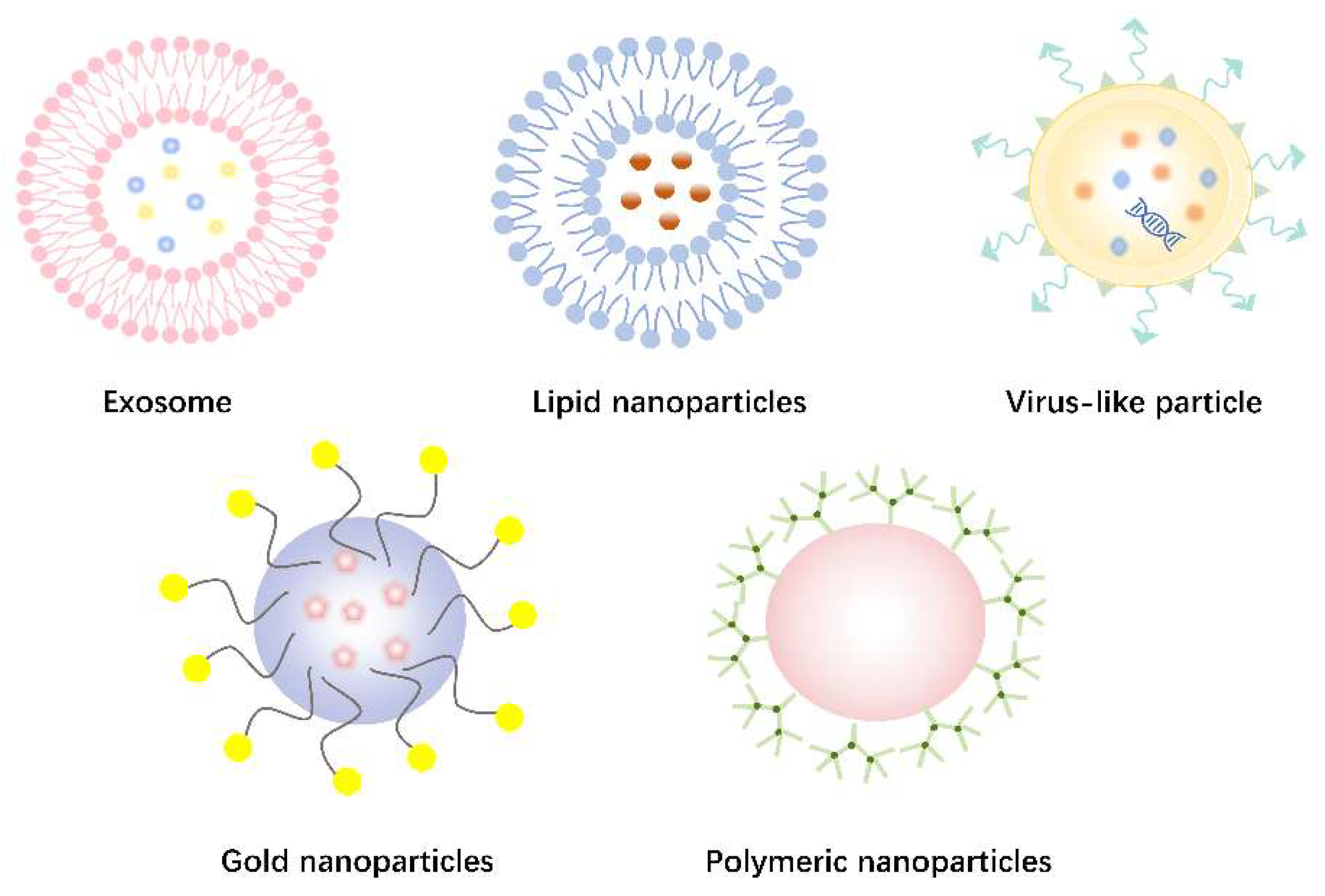
Exosomes are small lipid membrane-bound nanovesicles, ranging in diameter from 30 to 150 nm, that are released by cells [
70]. They can be isolated from various extracellular fluids, such as blood, saliva, amniotic fluid, urine, and cell culture supernatants. Exosomes play a crucial role in intercellular communication and have garnered significant attention in the field of clinical diagnostics and therapeutics [
71]. In recent years, exosomes have emerged as promising vehicles for targeted drug delivery. However, their efficiency in encapsulating large nucleic acid fragments is relatively low [
72]. To overcome this limitation, researchers have explored the use of exosome-liposome hybrid nanoparticles, which exhibit enhanced capability in encapsulating larger plasmids, including CRISPR/Cas systems associated with transposons [
73]. Consequently, exosomes-liposomes hybrid nanoparticles hold great potential for in vivo gene editing applications (
Figure 4A).
4.2.2. Lipid Nanoparticles
In recent years, numerous non-viral delivery methods have been developed for efficient in vitro delivery of CRISPR/Cas9. Among these methods, lipid nanoparticles (LNPs) have gained significant attention as effective carriers for delivering large nucleic acid and protein cargoes [
74]. LNPs have the capacity to encapsulate various genome editing systems, such as Cas9 mRNA, Cas9 protein, CRISPR/Cas9 RNPs, and base editors, allowing for targeted gene editing within cells [
75]. These LNPs provide several advantages, including biodegradability, biocompatibility, and protection of the genome-editing systems from degradation [
76]. Moreover, LNPs can be readily modified to enhance delivery efficiency and achieve cell- or tissue-specific targeting (
Figure 4B).
4.2.3. Virus-like Particle
In the context of lentivirus-based delivery systems for gene-editing therapies, researchers have recognized the associated risks and have developed Virus-like particles (VLPs) as an alternative approach (
Figure 4C). VLPs are self-assembled structures composed of structural proteins, resembling natural viruses but lacking a viral genome, thereby preventing replication within host cells [
77]. VLPs have gained significant attention in recent years, particularly in vaccine development [
78]. For instance, Liu Qi et al. developed a virus-like vector for co-delivery of the CRISPR-Cas9 system and small molecule drugs for the treatment of malignant tumors. Their VLP construct demonstrated structural stability in the bloodstream, and western blot analysis revealed a gene editing efficiency of 45.1%, offering valuable insights for utilizing VLPs in the delivery of CRISPR systems for disease treatment [
79].
4.2.4. Gold Nanoparticles
Gold nanoparticles (AuNPs) with sizes ranging from 1 to 100 nm have gained significant attention as delivery vehicles for gene editing systems due to their chemical inertness and immunological compatibility, distinguishing them from viral vectors (
Figure 4D) [
80]. Their low toxicity and high efficiency make them a promising alternative for safe gene delivery [
81]. The size of AuNPs and their intracellular retention time are critical factors influencing their efficacy as in vivo delivery vehicles for therapeutics [
82,
83]. Shahbaz et al. developed colloidal AuNPs for delivering the CRISPR/Cas9 plasmid DNA system, demonstrating both low toxicity and high editing efficiency in hematopoietic stem cells [
84].
Wang Peng et al. reported a strategy for delivering the Cas9-sgPIk-1 plasmid (CP) for tumor therapy using a multifunctional vector. They employed electrostatic interactions to assemble CP onto TAT peptide-modified AuNPs, forming a complex termed LACP. The TAT peptide was utilized for nuclear targeting to achieve gene knockdown in tumors. This approach effectively knocked down the melanoma target gene PIK-1, resulting in a 65% reduction in its protein expression, and exhibited significant tumor suppression in experimental models
4.2.5. Polymeric Nanoparticles [85]
Polymeric nanoparticles offer several advantages as delivery vehicles, including their small size, controlled drug release, biodegradability, and lower immunogenicity (
Figure 4E) [
86]. CRISPR/Cas9 systems encapsulated within polymeric nanoparticles form highly adaptable molecular complexes that can be functionally modified to enhance cell targeting and cellular uptake [
87,
88]. Polyethyleneimine (PEI) is a hydrophilic cationic polymer that can electrostatically bind to negatively charged DNA, facilitating endosomal escape [
89]. Ryu et al. utilized PEI for delivering CRISPR/Cas9 into Neuro2a cells, achieving transfection efficiencies of over 70% and insertional deletion efficiencies in the range of 20%. However, the high cationic charge of PEI can lead to high toxicity, limiting its application in vivo and in clinical settings [
90]. Moreover, Abbasi et al. developed poly (ethylene glycol) polymer micelles encapsulating Cas9 mRNA and sgRNA for efficient cellular delivery, providing protection against enzymatic degradation of sgRNA. They demonstrated in vivo gene editing using these multimeric micelles in animal models [
91].
5. Current Status of Genome-Editing Clinical Trials
The CRISPR system has emerged as a prominent gene editing tool in clinical research, demonstrating its potential in various applications such as key gene screening, cancer immunotherapy, and treatment of genetic diseases [
92]. CRISPR/Cas technology has been extensively employed for genome editing in bacteria, plants, animals, and humans, enabling rapid generation of disease models and therapeutic strategies for human genetic disorders [
93,
94]. Gene editing studies using sgRNA and Cas9 or mRNA have been conducted in animal models including mice, rats, pigs, and non-human primates, both in vivo and in vitro [
95]. However, challenges arise from the vulnerability of free sgRNA to nucleases, leading to limited therapeutic efficacy [
96]. Consequently, researchers have focused on developing innovative sgRNA delivery vectors, including viral and non-viral vectors, to enhance gene delivery efficiency for both in vivo and in vitro applications, thereby improving the therapeutic potential of gene editing [
97,
98]
In recent years, significant progress has been made in gene therapy for Leber's congenital amaurosis (LCA), a rare inherited retinal disease. Luxturna, the first gene therapy drug for an inherited retinal disease, received FDA approval for marketing in December 2017 [
99]. Notably, in 2020, an 8-year-old child with LCA treated with Luxturna demonstrated significant improvement in vision [
100]. Currently, a clinical trial utilizing CRISPR gene editing technology for the treatment of LCA10 is underway, and preliminary results have been recently published. Furthermore, there are over 40 genetic drugs in clinical trials for in vivo treatment of ocular genetic diseases [
101].
In the clinical management of β-thalassemia, two primary strategies are commonly employed for gene editing therapies: induction of fetal hemoglobin (HbF) expression and precise repair of mutations in the HBB gene [
102]. In August 2022, Wu Yuxuan's team at East China Normal University conducted a Phase I/II clinical trial involving two children who achieved transfusion independence for up to 16 months after transplantation. Notably, no significant side effects were observed [
103]. Ongoing research endeavors are further enhancing the adaptability of the CRISPR system, and investigations into gene editing tools for the treatment of other diseases are also underway, including liver diseases [
104]. Although clinical trials for gene editing in inherited diseases are still in early stages, the distinctive pathogenesis of these conditions renders CRISPR/Cas9 a promising and potentially available strategy for the treatment of inherited diseases in the near future.
6. Conclusions and Future Directions
The emergence of CRISPR/Cas gene editing technology has significantly propelled the advancement of gene editing therapies. Extensive preclinical investigations have demonstrated the precise editing capabilities of CRISPR/Cas9, as well as its derivative tools such as single-base editors and bootstrap editors, in disrupting or correcting disease-causing mutations in individual genes. Additionally, these technologies can be employed to modulate disease-associated genes, offering potential therapeutic benefits. Numerous clinical trials utilizing CRISPR/Cas-based genome editing have been approved for the treatment of various genetic diseases and cancer, primarily focusing on in vitro gene editing, with limited progress in in vivo applications. However, ongoing exploration holds promise for the potential cure of certain genetic diseases through gene editing therapies within the next 5 to 10 years.
In the future, CRISPR systems are anticipated to find wider applications in the treatment of diverse diseases, particularly cancer, where the development of engineered nucleases, such as AFN/TALENs and CRISPR/Cas9, has paved the way for the translation of gene editing from theoretical concepts to clinical practice. Furthermore, gene editing technologies have stimulated advancements in cellular imaging, genetic diagnostics, and the development of therapeutic drugs. Although off-target effects remain a concern in gene editing, innovations in gene delivery vectors have enhanced the efficiency of delivery and reduced toxicity, bringing gene editing closer to clinical implementation. Through continued exploration and global scientific collaboration, genome editing holds the potential to unravel the underlying biological mechanisms driving disease development and progression, thereby offering novel therapeutic approaches and advancing the field of life sciences.
Author Contributions
Writing—original draft preparation, Xinyue Lu; writing—review and editing, Miaomiao Zhang; software and validation, Ge Li; investigation, Jingbo Zhang; resources and visualization; Xiaoge Fu; supervision and project administration, Xinyue Lu author have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wada, N.; Osakabe, K.; Osakabe, Y. Expanding the plant genome editing toolbox with recently developed CRISPR-Cas systems. Plant Physiology 2022, 188, 1825–1837. [Google Scholar] [CrossRef]
- Kostyusheva, A.; Brezgin, S.; Babin, Y.; Vasilyeva, I.; Glebe, D.; Kostyushev, D.; Chulanov, V. CRISPR-Cas systems for diagnosing infectious diseases. Methods 2022, 203, 431–446. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Jiang, F.G.; Doudna, J.A. CRISPR-Cas9 Structures and Mechanisms. In Annual Review of Biophysics, Vol 46, Dill, K.A., Ed.; Annual Review of Biophysics; 2017; Volume 46, pp. 505-529.
- Zhang, F.; Wen, Y.; Guo, X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Human Molecular Genetics 2014, 23, R40–R46. [Google Scholar] [CrossRef]
- Zhang, F.; Wen, Y.; Guo, X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum Mol Genet 2014, 23, R40–46. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef]
- Yin, K.; Gao, C.; Qiu, J.L. Progress and prospects in plant genome editing. Nat Plants 2017, 3, 17107. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 2016, 529, 490–495. [Google Scholar] [CrossRef]
- Wang, J.; He, Z.; Wang, G.; Zhang, R.; Duan, J.; Gao, P.; Lei, X.; Qiu, H.; Zhang, C.; Zhang, Y.; et al. Efficient targeted insertion of large DNA fragments without DNA donors. Nat Methods 2022, 19, 331–340. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, F.; Ding, Y. CRISPR/Cas9 Delivery System Engineering for Genome Editing in Therapeutic Applications. Pharmaceutics 2021, 13. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, A.R.; Bhattacharya, M.; Lee, S.S.; Chakraborty, C. CRISPR-Cas9: A Preclinical and Clinical Perspective for the Treatment of Human Diseases. Mol Ther 2021, 29, 571–586. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, R.; Dai, Z. Key considerations in designing CRISPR/Cas9-carrying nanoparticles for therapeutic genome editing. Nanoscale 2020, 12, 21001–21014. [Google Scholar] [CrossRef]
- Ma, Y.; Deng, L.; Li, S. Application of nanoparticles in CRISPR/Cas9-based gene therapy. Sheng wu gong cheng xue bao = Chinese journal of biotechnology 2022, 38, 2087–2104. [Google Scholar] [CrossRef]
- Gainetdinov, I.; Vega-Badillo, J.; Cecchini, K.; Bagci, A.; Colpan, C.; De, D.; Bailey, S.; Arif, A.; Wu, P.H.; MacRae, I.J.; et al. Relaxed targeting rules help PIWI proteins silence transposons. Nature 2023. [Google Scholar] [CrossRef]
- Kovač, A.; Miskey, C.; Menzel, M.; Grueso, E.; Gogol-Döring, A.; Ivics, Z. RNA-guided retargeting of Sleeping Beauty transposition in human cells. Elife 2020, 9. [Google Scholar] [CrossRef]
- Bhatt, S.; Chalmers, R. Targeted DNA transposition in vitro using a dCas9-transposase fusion protein. Nucleic Acids Res 2019, 47, 8126–8135. [Google Scholar] [CrossRef]
- Strecker, J.; Ladha, A.; Gardner, Z.; Schmid-Burgk, J.L.; Makarova, K.S.; Koonin, E.V.; Zhang, F. RNA-guided DNA insertion with CRISPR-associated transposases. Science 2019, 365, 48–53. [Google Scholar] [CrossRef]
- Muto, V.; Benigni, F.; Magliocca, V.; Borghi, R.; Flex, E.; Pallottini, V.; Rosa, A.; Compagnucci, C.; Tartaglia, M. CRISPR/Cas9 and piggyBac Transposon-Based Conversion of a Pathogenic Biallelic TBCD Variant in a Patient-Derived iPSC Line Allows Correction of PEBAT-Related Endophenotypes. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, E.; Chen, S.; Gu, Y.; Shangguan, A.J.; Lv, T.; Luo, L.; Yu, Z. PiggyBac transposon vectors: the tools of the human gene encoding. Transl Lung Cancer Res 2016, 5, 120–125. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, Y.; Zhao, S.; Guan, K.L.; Zhuang, Y.; Zhou, W.; Wu, X.; Xu, T. DNA-PK facilitates piggyBac transposition by promoting paired-end complex formation. Proc Natl Acad Sci U S A 2017, 114, 7408–7413. [Google Scholar] [CrossRef] [PubMed]
- Yusa, K.; Zhou, L.; Li, M.A.; Bradley, A.; Craig, N.L. A hyperactive piggyBac transposase for mammalian applications. Proc Natl Acad Sci U S A 2011, 108, 1531–1536. [Google Scholar] [CrossRef]
- Ousterout, D.G.; Kabadi, A.M.; Thakore, P.I.; Majoros, W.H.; Reddy, T.E.; Gersbach, C.A. Multiplex CRISPR/Cas9-based genome editing for correction of dystrophin mutations that cause Duchenne muscular dystrophy. Nat Commun 2015, 6, 6244. [Google Scholar] [CrossRef]
- Zhao, X.; Qu, K.; Curci, B.; Yang, H.; Bolund, L.; Lin, L.; Luo, Y. Comparison of In-Frame Deletion, Homology-Directed Repair, and Prime Editing-Based Correction of Duchenne Muscular Dystrophy Mutations. Biomolecules 2023, 13. [Google Scholar] [CrossRef]
- Choi, E.; Koo, T. CRISPR technologies for the treatment of Duchenne muscular dystrophy. Mol Ther 2021, 29, 3179–3191. [Google Scholar] [CrossRef]
- Erkut, E.; Yokota, T. CRISPR Therapeutics for Duchenne Muscular Dystrophy. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Aslesh, T.; Erkut, E.; Yokota, T. Restoration of dystrophin expression and correction of Duchenne muscular dystrophy by genome editing. Expert Opin Biol Ther 2021, 21, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wei, T.; Yang, H.; Li, G.; Li, H. CRISPR-Based Therapeutic Gene Editing for Duchenne Muscular Dystrophy: Advances, Challenges and Perspectives. Cells 2022, 11. [Google Scholar] [CrossRef]
- Tabebordbar, M.; Zhu, K.; Cheng, J.K.W.; Chew, W.L.; Widrick, J.J.; Yan, W.X.; Maesner, C.; Wu, E.Y.; Xiao, R.; Ran, F.A.; et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 2016, 351, 407–411. [Google Scholar] [CrossRef]
- Birch, S.M.; Lawlor, M.W.; Conlon, T.J.; Guo, L.J.; Crudele, J.M.; Hawkins, E.C.; Nghiem, P.P.; Ahn, M.; Meng, H.; Beatka, M.J.; et al. Assessment of systemic AAV-microdystrophin gene therapy in the GRMD model of Duchenne muscular dystrophy. Sci Transl Med 2023, 15, eabo1815. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Hu, Z.; Zhao, J.; Zhou, T.; Tang, S.; Wang, P.; Xiao, R.; Chen, Y.; Wu, L.; Zhou, M.; et al. CRISPR-Mediated In Situ Introduction or Integration of F9-Padua in Human iPSCs for Gene Therapy of Hemophilia B. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Matsui, H.; Fujimoto, N.; Sasakawa, N.; Ohinata, Y.; Shima, M.; Yamanaka, S.; Sugimoto, M.; Hotta, A. Delivery of full-length factor VIII using a piggyBac transposon vector to correct a mouse model of hemophilia A. PLoS One 2014, 9, e104957. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Tonnu, N.; Tachikawa, K.; Limphong, P.; Vega, J.B.; Karmali, P.P.; Chivukula, P.; Verma, I.M. Systemic delivery of factor IX messenger RNA for protein replacement therapy. Proc Natl Acad Sci U S A 2017, 114, E1941–e1950. [Google Scholar] [CrossRef]
- Yan, Z.; McCray, P.B., Jr.; Engelhardt, J.F. Advances in gene therapy for cystic fibrosis lung disease. Hum Mol Genet 2019, 28, R88–r94. [Google Scholar] [CrossRef]
- Suzuki, S.; Crane, A.M.; Anirudhan, V.; Barillà, C.; Matthias, N.; Randell, S.H.; Rab, A.; Sorscher, E.J.; Kerschner, J.L.; Yin, S.; et al. Highly Efficient Gene Editing of Cystic Fibrosis Patient-Derived Airway Basal Cells Results in Functional CFTR Correction. Mol Ther 2020, 28, 1684–1695. [Google Scholar] [CrossRef]
- Mehta, A. CFTR: more than just a chloride channel. Pediatr Pulmonol 2005, 39, 292–298. [Google Scholar] [CrossRef]
- Amistadi, S.; Maule, G.; Ciciani, M.; Ensinck, M.M.; De Keersmaecker, L.; Ramalho, A.S.; Guidone, D.; Buccirossi, M.; Galietta, L.J.V.; Carlon, M.S.; et al. Functional restoration of a CFTR splicing mutation through RNA delivery of CRISPR adenine base editor. Mol Ther 2023, 31, 1647–1660. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Wohlford-Lenane, C.; Kandimalla, S.; Sartre, G.; Meyerholz, D.K.; Théberge, V.; Hallée, S.; Duperré, A.M.; Del'Guidice, T.; Lepetit-Stoffaes, J.P.; et al. Engineered amphiphilic peptides enable delivery of proteins and CRISPR-associated nucleases to airway epithelia. Nat Commun 2019, 10, 4906. [Google Scholar] [CrossRef]
- Meisel, R. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N Engl J Med 2021, 384, e91. [Google Scholar] [CrossRef]
- Khosravi, M.A.; Abbasalipour, M.; Concordet, J.P.; Berg, J.V.; Zeinali, S.; Arashkia, A.; Azadmanesh, K.; Buch, T.; Karimipoor, M. Targeted deletion of BCL11A gene by CRISPR-Cas9 system for fetal hemoglobin reactivation: A promising approach for gene therapy of beta thalassemia disease. Eur J Pharmacol 2019, 854, 398–405. [Google Scholar] [CrossRef]
- Quagliano, A.; Acevedo, D.; Hardigan, P.; Prasad, S. Using Clustered Regularly Interspaced Short Palindromic Repeats gene editing to induce permanent expression of fetal hemoglobin in β-thalassemia and sickle cell disease: A comparative meta-analysis. Front Med (Lausanne) 2022, 9, 943631. [Google Scholar] [CrossRef] [PubMed]
- Pavani, G.; Fabiano, A.; Laurent, M.; Amor, F.; Cantelli, E.; Chalumeau, A.; Maule, G.; Tachtsidi, A.; Concordet, J.P.; Cereseto, A.; et al. Correction of β-thalassemia by CRISPR/Cas9 editing of the α-globin locus in human hematopoietic stem cells. Blood Adv 2021, 5, 1137–1153. [Google Scholar] [CrossRef] [PubMed]
- Mettananda, S.; Fisher, C.A.; Hay, D.; Badat, M.; Quek, L.; Clark, K.; Hublitz, P.; Downes, D.; Kerry, J.; Gosden, M.; et al. Editing an α-globin enhancer in primary human hematopoietic stem cells as a treatment for β-thalassemia. Nat Commun 2017, 8, 424. [Google Scholar] [CrossRef]
- Paschoudi, K.; Yannaki, E.; Psatha, N. Precision Editing as a Therapeutic Approach for β-Hemoglobinopathies. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.; Tariq, M.A.; Shahid, K.; Ahmad, F.J.; Akram, J. Advances in genome editing: the technology of choice for precise and efficient β-thalassemia treatment. Gene Ther 2021, 28, 6–15. [Google Scholar] [CrossRef]
- Yang, Z.; Ji, P.; Li, Z.; Zhang, R.; Wei, M.; Yang, Y.; Yuan, L.; Han, Y.; Yang, G. Improved extracellular vesicle-based mRNA delivery for familial hypercholesterolemia treatment. Theranostics 2023, 13, 3467–3479. [Google Scholar] [CrossRef]
- Langsted, A.; Kamstrup, P.R.; Benn, M.; Tybjærg-Hansen, A.; Nordestgaard, B.G. High lipoprotein(a) as a possible cause of clinical familial hypercholesterolaemia: a prospective cohort study. Lancet Diabetes Endocrinol 2016, 4, 577–587. [Google Scholar] [CrossRef]
- Hu, P.; Dharmayat, K.I.; Stevens, C.A.T.; Sharabiani, M.T.A.; Jones, R.S.; Watts, G.F.; Genest, J.; Ray, K.K.; Vallejo-Vaz, A.J. Prevalence of Familial Hypercholesterolemia Among the General Population and Patients With Atherosclerotic Cardiovascular Disease: A Systematic Review and Meta-Analysis. Circulation 2020, 141, 1742–1759. [Google Scholar] [CrossRef]
- Vallejo-Vaz, A.J.; De Marco, M.; Stevens, C.A.T.; Akram, A.; Freiberger, T.; Hovingh, G.K.; Kastelein, J.J.P.; Mata, P.; Raal, F.J.; Santos, R.D.; et al. Overview of the current status of familial hypercholesterolaemia care in over 60 countries - The EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Atherosclerosis 2018, 277, 234–255. [Google Scholar] [CrossRef]
- Abifadel, M.; Varret, M.; Rabès, J.P.; Allard, D.; Ouguerram, K.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickham, L.; Erlich, D.; et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003, 34, 154–156. [Google Scholar] [CrossRef]
- Li, Z.F.; Wu, N.Q. The Progression of Treatment for Refractory Hypercholesterolemia: Focus on the Prospect of Gene Therapy. Front Genet 2022, 13, 911429. [Google Scholar] [CrossRef]
- Ebenezer, O.; Comoglio, P.; Wong, G.K.; Tuszynski, J.A. Development of Novel siRNA Therapeutics: A Review with a Focus on Inclisiran for the Treatment of Hypercholesterolemia. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Asmamaw, M.; Zawdie, B. Mechanism and Applications of CRISPR/Cas-9-Mediated Genome Editing. Biologics 2021, 15, 353–361. [Google Scholar] [CrossRef]
- Sosnowska, B.; Adach, W.; Surma, S.; Rosenson, R.S.; Banach, M. Evinacumab, an ANGPTL3 Inhibitor, in the Treatment of Dyslipidemia. J Clin Med 2022, 12. [Google Scholar] [CrossRef]
- Vanhoye, X.; Janin, A.; Caillaud, A.; Rimbert, A.; Venet, F.; Gossez, M.; Dijk, W.; Marmontel, O.; Nony, S.; Chatelain, C.; et al. APOB CRISPR-Cas9 Engineering in Hypobetalipoproteinemia: A Promising Tool for Functional Studies of Novel Variants. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Walker, A.F.; Graham, S.; Maple-Brown, L.; Egede, L.E.; Campbell, J.A.; Walker, R.J.; Wade, A.N.; Mbanya, J.C.; Long, J.A.; Yajnik, C.; et al. Interventions to address global inequity in diabetes: international progress. Lancet 2023. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simó, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res 2016, 51, 156–186. [Google Scholar] [CrossRef]
- Benati, D.; Patrizi, C.; Recchia, A. Gene editing prospects for treating inherited retinal diseases. J Med Genet 2020, 57, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Ao, H.; Li, H.; Zhao, X.; Liu, B.; Lu, L. TXNIP positively regulates the autophagy and apoptosis in the rat müller cell of diabetic retinopathy. Life Sci 2021, 267, 118988. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, T.; Wang, H.; He, X.; Li, Y.; Wen, S.; Peng, R.; Nie, Y.; Lu, Y.; Yang, H.; et al. Diabetes Promotes Retinal Vascular Endothelial Cell Injury by Inducing CCN1 Expression. Front Cardiovasc Med 2021, 8, 689318. [Google Scholar] [CrossRef]
- Li, A.; Lee, C.M.; Hurley, A.E.; Jarrett, K.E.; De Giorgi, M.; Lu, W.; Balderrama, K.S.; Doerfler, A.M.; Deshmukh, H.; Ray, A.; et al. A Self-Deleting AAV-CRISPR System for In Vivo Genome Editing. Mol Ther Methods Clin Dev 2019, 12, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.E.; Wu, Y.; Gemberling, M.P.; Oliver, M.L.; Waller, M.A.; Bohning, J.D.; Robinson-Hamm, J.N.; Bulaklak, K.; Castellanos Rivera, R.M.; Collier, J.H.; et al. Long-term evaluation of AAV-CRISPR genome editing for Duchenne muscular dystrophy. Nat Med 2019, 25, 427–432. [Google Scholar] [CrossRef]
- Shen, W.; Liu, S.; Ou, L. rAAV immunogenicity, toxicity, and durability in 255 clinical trials: A meta-analysis. Front Immunol 2022, 13, 1001263. [Google Scholar] [CrossRef]
- Puentes-Tellez, M.A.; Sánchez, O.F.; Rojas-Rodriguez, F.; Benincore-Flórez, E.; Barbosa, H.; Alméciga Díaz, C.J. Evaluation of HIV-1 derived lentiviral vectors as transductors of Mucopolysaccharidosis type IV a fibroblasts. Gene 2021, 780, 145527. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Mitani, K. A new hybrid system capable of efficient lentiviral vector production and stable gene transfer mediated by a single helper-dependent adenoviral vector. J Virol 2003, 77, 2964–2971. [Google Scholar] [CrossRef]
- Fu, Y.; Foden, J.A.; Khayter, C.; Maeder, M.L.; Reyon, D.; Joung, J.K.; Sander, J.D. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol 2013, 31, 822–826. [Google Scholar] [CrossRef]
- Ortinski, P.I.; O'Donovan, B.; Dong, X.; Kantor, B. Integrase-Deficient Lentiviral Vector as an All-in-One Platform for Highly Efficient CRISPR/Cas9-Mediated Gene Editing. Mol Ther Methods Clin Dev 2017, 5, 153–164. [Google Scholar] [CrossRef]
- Uchida, N.; Drysdale, C.M.; Nassehi, T.; Gamer, J.; Yapundich, M.; DiNicola, J.; Shibata, Y.; Hinds, M.; Gudmundsdottir, B.; Haro-Mora, J.J.; et al. Cas9 protein delivery non-integrating lentiviral vectors for gene correction in sickle cell disease. Mol Ther Methods Clin Dev 2021, 21, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Farzanehpour, M.; Miri, A.; Alvanegh, A.G.; Gouvarchinghaleh, H.E. Viral Vectors, Exosomes, and Vexosomes: Potential armamentarium for delivering CRISPR/Cas to cancer cells. Biochem Pharmacol 2023, 212, 115555. [Google Scholar] [CrossRef]
- Alptekin, A.; Parvin, M.; Chowdhury, H.I.; Rashid, M.H.; Arbab, A.S. Engineered exosomes for studies in tumor immunology. Immunol Rev 2022, 312, 76–102. [Google Scholar] [CrossRef]
- Wan, T.; Zhong, J.; Pan, Q.; Zhou, T.; Ping, Y.; Liu, X. Exosome-mediated delivery of Cas9 ribonucleoprotein complexes for tissue-specific gene therapy of liver diseases. Sci Adv 2022, 8, eabp9435. [Google Scholar] [CrossRef] [PubMed]
- Alghuthaymi, M.A.; Ahmad, A.; Khan, Z.; Khan, S.H.; Ahmed, F.K.; Faiz, S.; Nepovimova, E.; Kuča, K.; Abd-Elsalam, K.A. Exosome/Liposome-like Nanoparticles: New Carriers for CRISPR Genome Editing in Plants. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Li, L.; Hu, S.; Chen, X. Non-viral delivery systems for CRISPR/Cas9-based genome editing: Challenges and opportunities. Biomaterials 2018, 171, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Mout, R.; Ray, M.; Yesilbag Tonga, G.; Lee, Y.W.; Tay, T.; Sasaki, K.; Rotello, V.M. Direct Cytosolic Delivery of CRISPR/Cas9-Ribonucleoprotein for Efficient Gene Editing. ACS Nano 2017, 11, 2452–2458. [Google Scholar] [CrossRef]
- Kazemian, P.; Yu, S.Y.; Thomson, S.B.; Birkenshaw, A.; Leavitt, B.R.; Ross, C.J.D. Lipid-Nanoparticle-Based Delivery of CRISPR/Cas9 Genome-Editing Components. Mol Pharm 2022, 19, 1669–1686. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Cai, H.; Steinmetz, N.F. Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv Drug Deliv Rev 2020, 156, 214–235. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, B.; Lateef, Z.; Walker, G.F.; Young, S.L.; Ward, V.K. Virus-like particle vaccines: immunology and formulation for clinical translation. Expert Rev Vaccines 2018, 17, 833–849. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Zheng, Y.; Zhao, Y.; Wang, Y.; Hao, J.; Zhao, X.; Yi, K.; Shi, L.; Kang, C.; et al. Virus-like nanoparticle as a co-delivery system to enhance efficacy of CRISPR/Cas9-based cancer immunotherapy. Biomaterials 2020, 258, 120275. [Google Scholar] [CrossRef]
- Kumar, D.; Saini, N.; Jain, N.; Sareen, R.; Pandit, V. Gold nanoparticles: an era in bionanotechnology. Expert Opin Drug Deliv 2013, 10, 397–409. [Google Scholar] [CrossRef]
- García-Fernández, A.; Vivo-Llorca, G.; Sancho, M.; García-Jareño, A.B.; Ramírez-Jiménez, L.; Barber-Cano, E.; Murguía, J.R.; Orzáez, M.; Sancenón, F.; Martínez-Máñez, R. Nanodevices for the Efficient Codelivery of CRISPR-Cas9 Editing Machinery and an Entrapped Cargo: A Proposal for Dual Anti-Inflammatory Therapy. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef]
- Ho, L.W.C.; Chan, C.K.W.; Han, R.; Lau, Y.F.Y.; Li, H.; Ho, Y.P.; Zhuang, X.; Choi, C.H.J. Mammalian Cells Exocytose Alkylated Gold Nanoparticles via Extracellular Vesicles. ACS Nano 2022, 16, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Lee, C.W.; Chiou, A.; Wei, P.K. Size-dependent endocytosis of gold nanoparticles studied by three-dimensional mapping of plasmonic scattering images. J Nanobiotechnology 2010, 8, 33. [Google Scholar] [CrossRef]
- Shahbazi, R.; Sghia-Hughes, G.; Reid, J.L.; Kubek, S.; Haworth, K.G.; Humbert, O.; Kiem, H.P.; Adair, J.E. Targeted homology-directed repair in blood stem and progenitor cells with CRISPR nanoformulations. Nat Mater 2019, 18, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, L.; Zheng, W.; Cong, L.; Guo, Z.; Xie, Y.; Wang, L.; Tang, R.; Feng, Q.; Hamada, Y.; et al. Thermo-triggered Release of CRISPR-Cas9 System by Lipid-Encapsulated Gold Nanoparticles for Tumor Therapy. Angew Chem Int Ed Engl 2018, 57, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int J Pharm 2019, 561, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, A.M.; Torchilin, V.P. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front Pharmacol 2014, 5, 77. [Google Scholar] [CrossRef]
- Mariano, A.; Lubrano, C.; Bruno, U.; Ausilio, C.; Dinger, N.B.; Santoro, F. Advances in Cell-Conductive Polymer Biointerfaces and Role of the Plasma Membrane. Chem Rev 2022, 122, 4552–4580. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Lächelt, U.; Bartek, J.; Wagner, E.; Moghimi, S.M. Polyplex Evolution: Understanding Biology, Optimizing Performance. Mol Ther 2017, 25, 1476–1490. [Google Scholar] [CrossRef] [PubMed]
- Ryu, N.; Kim, M.A.; Park, D.; Lee, B.; Kim, Y.R.; Kim, K.H.; Baek, J.I.; Kim, W.J.; Lee, K.Y.; Kim, U.K. Effective PEI-mediated delivery of CRISPR-Cas9 complex for targeted gene therapy. Nanomedicine 2018, 14, 2095–2102. [Google Scholar] [CrossRef]
- Abbasi, S.; Uchida, S.; Toh, K.; Tockary, T.A.; Dirisala, A.; Hayashi, K.; Fukushima, S.; Kataoka, K. Co-encapsulation of Cas9 mRNA and guide RNA in polyplex micelles enables genome editing in mouse brain. J Control Release 2021, 332, 260–268. [Google Scholar] [CrossRef]
- Liu, X.; Li, G.; Liu, Y.; Zhou, F.; Huang, X.; Li, K. Advances in CRISPR/Cas gene therapy for inborn errors of immunity. Front Immunol 2023, 14, 1111777. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Chen, Z.; Huang, J.; Ye, H.; Lu, T.; Lu, M.; Rao, Y. [Application of CRISPR-Cas9 gene editing technology in crop breeding]. Sheng Wu Gong Cheng Xue Bao 2023, 39, 399–424. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.F.; Chen, G.J.; Luo, Y.L.; Zhang, Y.; Zhao, G.; Lu, Z.D.; Czarna, A.; Gu, Z.; Wang, J. Rational designs of in vivo CRISPR-Cas delivery systems. Adv Drug Deliv Rev 2021, 168, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhi, S.; Wu, G.; Wu, G.; Cao, T.; Hao, H.; Songyang, Z.; Liang, P.; Huang, J. Cost-effective generation of A-to-G mutant mice by zygote electroporation of adenine base editor ribonucleoproteins. J Genet Genomics 2020, 47, 337–340. [Google Scholar] [CrossRef]
- Uddin, F.; Rudin, C.M.; Sen, T. CRISPR Gene Therapy: Applications, Limitations, and Implications for the Future. Front Oncol 2020, 10, 1387. [Google Scholar] [CrossRef]
- Yu, L.; Marchisio, M.A. CRISPR-associated type V proteins as a tool for controlling mRNA stability in S. cerevisiae synthetic gene circuits. Nucleic Acids Res 2023, 51, 1473–1487. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Han, Y.; Jin, Z.; Hang, W.; Shu, H.; Wen, Z.; Ni, L.; Wang, D.W. Homology-directed repair of an MYBPC3 gene mutation in a rat model of hypertrophic cardiomyopathy. Gene Ther 2023, 30, 520–527. [Google Scholar] [CrossRef]
- Darrow, J.J. Luxturna: FDA documents reveal the value of a costly gene therapy. Drug Discov Today 2019, 24, 949–954. [Google Scholar] [CrossRef]
- Maguire, A.M.; Russell, S.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Drack, A.V.; Simonelli, F.; Leroy, B.P.; Reape, K.Z.; High, K.A.; et al. Durability of Voretigene Neparvovec for Biallelic RPE65-Mediated Inherited Retinal Disease: Phase 3 Results at 3 and 4 Years. Ophthalmology 2021, 128, 1460–1468. [Google Scholar] [CrossRef]
- Bainbridge, J.W.; Mehat, M.S.; Sundaram, V.; Robbie, S.J.; Barker, S.E.; Ripamonti, C.; Georgiadis, A.; Mowat, F.M.; Beattie, S.G.; Gardner, P.J.; et al. Long-term effect of gene therapy on Leber's congenital amaurosis. N Engl J Med 2015, 372, 1887–1897. [Google Scholar] [CrossRef]
- Rodgers, G.P.; Dover, G.J.; Uyesaka, N.; Noguchi, C.T.; Schechter, A.N.; Nienhuis, A.W. Augmentation by erythropoietin of the fetal-hemoglobin response to hydroxyurea in sickle cell disease. N Engl J Med 1993, 328, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Couette, M.; Forté, S.; Oudin Doglioni, D.; Mekontso-Dessap, A.; Calvet, D.; Kuo, K.H.M.; Bartolucci, P. Early Strokes Are Associated with More Global Cognitive Deficits in Adults with Sickle Cell Disease. J Clin Med 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Witzigmann, D.; Kulkarni, J.A.; Leung, J.; Chen, S.; Cullis, P.R.; van der Meel, R. Lipid nanoparticle technology for therapeutic gene regulation in the liver. Adv Drug Deliv Rev 2020, 159, 344–363. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).