1. Introduction
Lung cancer remains one of the most significant health concerns worldwide, with high morbidity and mortality rates. It is primarily associated with exposure to tobacco smoke, either through active or passive smoke. However, as mentioned in Dubinet.al. [
1], non-smokers may also develop lung cancer due to environmental pollutants, occupational hazards, genetic predisposition, and underlying lung diseases. The risk increases with prolonged exposure to carcinogens, such as asbestos, radon, and certain industrial chemicals. In recent years, there has been increasing emphasis on early detection and intervention of lung cancer. At early stages, the tumour is localized; hence the treatment options are more effective, and the chances of successful intervention and cure are significantly high. Unfortunately as sermonized in Selman et al. [
2], lung cancer often remains asymptomatic or presents nonspecific symptoms until it reaches an advanced stage, making early detection challenging.
Various detection methods have been employed to identify lung cancer early. Infante et al. [
3], utilized chest X-rays and Computed Tomography (CT) scan imaging techniques to detect suspicious lung nodules, masses, or other abnormalities that may indicate the presence of lung cancer. The Sputum Cytology method used in Thunnissenet al. [
4] analyzes the sputum sample for the presence of cancer cells. There are also procedures such as Bronchoscopy studied in Andolfiet al. [
5] that allow the collection of lung tissue samples and visualization of the airways using a thin, flexible tube and camera. All the above methods or procedures are conducted in the primary stages after the spread of lung cancer cells in the individual. However, Zhu et al. [
6] used method of Microarray-based gene expression analysis to detect lung cancer at its earliest stages, facilitating timely intervention and improving patient outcomes. The microarray analysis compares healthy lung tissue's gene expression profiles with cancerous tissue. The analysis aids the researchers in identifying distinct patterns and signatures associated with different lung cancer types.
1.1. Review of Previous Works
As mentioned, microarray based gene expression analysis is widely used for the detection and classification of various health conditions. A microarray is a tool used in molecular biology to study gene expression. As described by Churchill et al. [
7], microarray is a collection of microscopic spots containing DNA molecules representing individual genes. These spots are arranged in a grid pattern on a small glass slide or a silicon chip. Microarray gene expression analysis can investigate changes in gene expression patterns in cancer cells compared to normal cells, as sermonized in Ross et al. [
8]. By simultaneously measuring the expression levels of thousands of genes, microarrays can provide a comprehensive view of the changes in cancer cells. Also, as reported by Reis et al. [
9], microarray gene expression analysis can be utilized to classify different types of cancer, which can help and guide treatment decisions at early stages.
As indicated in Lapointe et al. [
10], different types of cancer have distinct gene expression profiles, which reflect the underlying molecular and genetic changes that drive the disease. In Dwivediet. al. [
11], researchers have used microarray analysis to identify gene expression patterns that distinguish between different types of leukaemia, such as acute lymphoblastic leukaemia (ALL) and acute myeloid leukaemia (AML). Similarly, Rody et al. [
12] used microarray analysis to distinguish between different breast cancer subtypes, such as estrogen receptor-positive and HER2-positive breast cancer.In Sanchez et al. [
13], microarray gene expression analysis is employed to predict the chances of tumour and lung cancer. Kerkentzes et al. [
14] classified Adenocarcinoma (Adeno) and Mesothelioma (Meso) lung cancer from microarray genes.Adeno and Meso are considered serious cancer conditions for diverse reasons mentioned in Wagner et al. [
15]. Adeno can exhibit rapid tumour growth and potentially spread to other body parts, including distant organs. Meso is extremely aggressive, making the treatment difficult when diagnosed at advanced stages. Both adeno and meso have limited treatment options compared to other forms of lung cancer. Although surgery, chemotherapy, and radiation therapy can be used, these treatments may not always be curative, especially if the cancer has spread all over the body.
Further, these cancer conditions develop resistance to standard cancer treatments due to genetic mutations in tumour cells, limiting the effectiveness of targeted therapies. Above all, the significance of this research investigation is the poor prognosis of adeno and meso conditions compared to other forms of lung cancer. The outcome of this research will help in timely detection, early intervention, and personalized treatment approaches to improve the conditions of cancer-diagnosed individuals. Microarray gene expression analysis-based lung cancer detection offers several advantages over traditional histopathology diagnostic methods as sermonized in Weigelt et al. [
16]. Unlike histopathology, gene expression analysis provides molecular insights into the underlying biology of lung cancer, which helps to understand lung cancer subtypes, disease progression, and treatment responses. The molecular information goes beyond the structural or morphological features captured by imaging or histopathology, providing a deeper understanding of the disease. Also, traditional diagnostic methods may only detect lung cancer when it has already reached a more advanced stage. Microarray gene expression analysis also brings up potential biomarkers with higher sensitivity and specificity than traditional diagnostic methods. All these benefits motivate us to use microarray gene expression analysis for early diagnosis of lung cancer.
1.2. Review of Feature extraction techniques
Feature extraction techniques are crucial in acquiring relevant features from microarray gene data. As described in Hiraet al. [
17], these methods aim to reduce the dimensionality of the data while retaining important information for subsequent analysis. The high dimensional data is reduced so that it can explain most of the variance in the dataset. The reduced data also contains the essential patterns and relationships between genes, entitling a more manageable and meaningful representation of the data. Feature extraction methods are advantageous in removing noise by extracting the underlying signal or patterns in the data by focusing on the most significant features.
In this paper, we employ Short-Time Fourier Transform (STFT) for analyzing and expressing the microarray gene expression data. The STFT provides a time-frequency representation of the signal, which can capture changes in gene expression over time. The STFT provides information about the temporal localization of signal events, allowing researchers to identify specific time points or intervals where gene expression changes occur as mentioned in Qi et al. [
18]. This representation can also reveal temporal patterns and relationships between genes, allowing for the identification of dynamic gene expression changes associated with specific conditions or biological processes. The STFT can also extract frequency-domain features from gene expression data. These features provide additional information about the distribution or characteristics of gene expression patterns that help to improve classification accuracy. Also, STFT identifies specific frequency bands representing relevant genes associated with biological processes and disease conditions.
1.3. Review of Feature selection techniques
After feature extraction, feature selection methods further improve lung cancer classification from microarray gene expression data. As described in Abdelwahab et al. [
19], these methods help identify a subset of relevant genes that are informative for distinguishing between different lung cancer types. In this paper, we employ Metaheuristic feature selection methods namely Particle Swarm Optimization (PSO) and Harmonic Search (HS) for selecting this subset of genes most discriminatory for lung cancer classification. As elaborated in Shukla et al. [
20], by focusing on the most discriminative genes, these metaheuristic methods can reduce the risk of overfitting and improve the generalization capability of the classifier, leading to better performance. Also, this is extremely useful for classifying the LH2 dataset, as the number of samples is limited. Metaheuristic feature selection methods can also handle correlations and dependencies between genes to remove redundant genes while maximizing their relevance to lung cancer classification. Overall, the metaheuristic feature selection methods identify a biologically relevant gene subset and can give insights into the underlying biological processes associated with lung cancer. But, the choice of metaheuristic algorithm for the feature selection method plays a vital role in selecting the relevant genes from the dataset. So it is important to evaluate different metaheuristic methods and consider their performance, computational complexity, and suitability for the specific problem.
1.4. Review of CNN Methodology
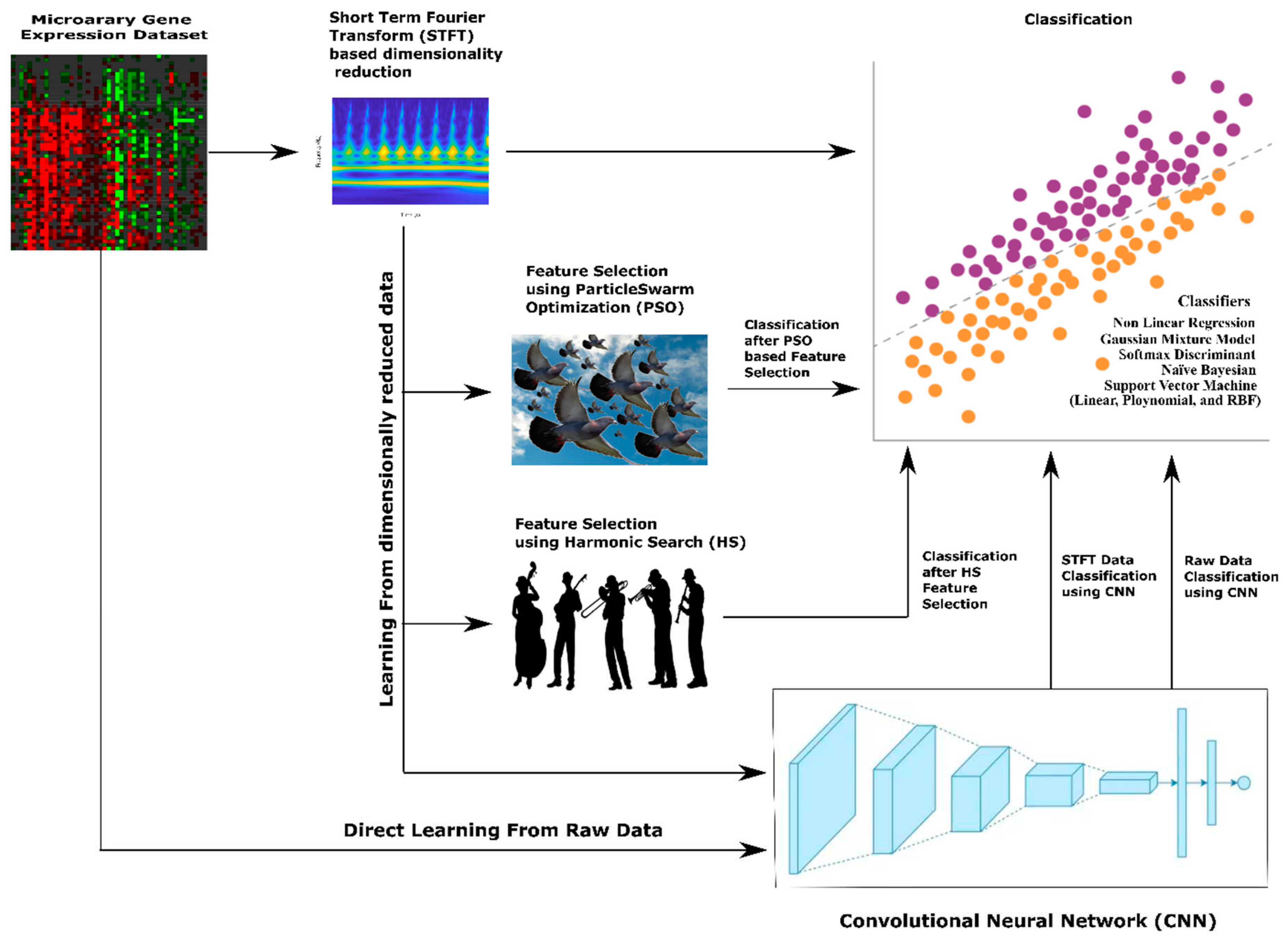
Apart from machine learning classifiers, Convolutional Neural Networks (CNNs) may make a significant difference in the classification of lung cancer from microarray gene expression data. Without manual feature extraction and selection methods, CNNs can automatically learn relevant features from raw input data. In the context of gene expression data, CNNs can extract meaningful patterns and relationships between genes. This methodology allows CNN to capture complex interactions that may be difficult to discern manually. For gene expression data, genes can be considered spatial entities, and their expressions across samples can be treated as spatial patterns. Here, CNNs excel at learning hierarchical representations, starting from low-level gene expression features and gradually building up to higher-level features that capture more abstract characteristics. Also, CNNs can identify gene expression patterns relevant to lung cancer, even if they occur in different regions or orders within the microarray.CNNs employ parameter optimisation and deduce conclusions better from limited training data like microarray gene expression datasets.Further, CNNs are robust to inherent noise, variability, and batch effects variations. CNNs can learn to identify important patterns despite these variations, making them suitable for analysing microarray gene expression datasets. Also, CNNs models inherently form non-linear relationships between features crucial for capturing the complex and non-linear interactions within gene expression data. So we also use experiment on employing CNN methodology to classify lung cancer from microarray gene expression data. The overall methodologies for dimensionality reduction and classification of Microarray gene expression data for lung cancer employed in this paper is shown in
Figure 1.
Despite the numerous benefits of using CNNs for classifying lung cancer from microarray gene expression data, they also have certain limitations.Even though CNNs are good at learning features, it is often challenging to interpret the physical significance behind the prediction and classification of gene expression patterns from the learned features due to their black-box nature. Further, CNNs are usually prone to overfitting due to the limited dataset availability; hence, it is important to carefully tune and select the parameters in the CNN model. The feature extraction and selection methods can solve the overfitting problem to a certain extent in CNN models. However, these stages also add computational complexity to CNN networks' inherent complexity. For the microarray gene expression dataset used in this paper (Lung Harvard 2 Data Set), the imbalanced class distributions in the lung cancer dataset can pose challenges. Due to class imbalance, CNNs may tend to bias towards the adeno class, leading to lower accuracy for the meso class. The performance of CNN can be improved by carefully choosing tuning parameters like learning rate, network architecture, and regularization strength. Also, suitable classifiers are integrated into the softmax layer to improve classification performance in CNN.
1.5. Description of the Data set
In this research, we have utilised using Lung Harvard 2 (LH2) medical imaging dataset [
21]. This dataset contains translated microarray data of patient samples affected by Adenocarcinoma (Adeno) and Mesothelioma (Meso). The gene expression ratio technique is the translation technique used in the dataset for a simpler representation. The technique can accurately differentiate between genetically disparate and normal tissues based on thresholds. The dataset contains a total of 150 Adeno and 31 Meso tissue samples. The 12533x 1 genes characterize each of the subject. The final row of the dataset is used for labelling Adenocarcinoma and malignant Mesothelioma samples. This microarray gene expression dataset is widely used for early and authentic diagnosis of lung cancer classification.
1.6. Relevance of this research
As mentioned earlier, lung cancer detection from microarray gene data helps to diagnose cancer at an earlier stage, which is crucial as the treatment options are limited in advanced stages. Thus early detection can significantly improve patient outcomes and survival rates. In this paper, we perform the lung cancer classification of adeno and meso cancer from the LH2 dataset. The dataset is of high volume and contains inherent non-linearity and class imbalance. Therefore it is important to adopt suitable preprocessing and classification methods to enhance the classification performance. Thus learning on microarray gene data analysis enables the identification of potential biomarkers associated with lung cancer to help in diagnosis, prognosis, and monitoring treatment response.
3. Classification using Machine Learning Techniques
This research employs various machine learning classifiers for lung cancer classification. The choice of the classifier is very significant in the classification methodology. Martín et al. [
23] employed a nonlinear regression classifier for heterogeneous and highly nonlinear datasets related to the manufacturing process. For this research problem, such a classifier can capture complex nonlinear dependencies between gene expression levels and class labels is suitable. Linear classifiers cannot effectively capture these complex patterns and nonlinear decision boundaries. Also, as discussed in Dalatu et al. [
24], Nonlinear regression models can be more robust to outliers than linear classifiers. Ficklin et al. [
25] have utilised Gaussian Mixture Models (GMMs) for the classification of microarray gene data for various cancer types, namelylower-grade glioma, thyroid, glioblastoma, ovarian, and bladder cancer.Since GMMs are probabilistic models, they can work effectively on microarray gene expression datasets that are combinations of multi-modal distributions. The GMMs can capture complex data distributions by representing them as a combination of Gaussian components. Further, each Gaussian component in the mixture model can represent a different class, and the combination of these components enables the modelling of nonlinear decision boundaries that can be particularly useful when large amounts of samples overlap among the classes.Shah et al. [
26] used the softmax discriminant classifier for lung classification from microarray gene expression. The classifier works on estimating probability for each class, and it produces a probability distribution over all possible classes. This property is useful and suitable for multi-class classification problems, such as microarray gene expression data. The softmax discriminant classifier is also computationally efficient as it incorporates regularisation techniques to prevent overfitting and improve generalisation. Ahmed et al. [
27] used Naive Bayesian Classifier (NBC) to classify head and neck cancer from microarray gene expression data. NBCs are based on probabilistic principles, specifically Bayes' theorem. The classifier computes the probabilities of diverse classes based on the feature values and allocates the class label with the highest probability. NBC framework also facilitates the integration of prior knowledge or expert beliefs through prior probabilities. Moreover, NBCs are computationally efficient, making them particularly suitable for large-volume and sparse data sets like microarray gene expression datasets. Vanitha et al. [
28] used a Support Vector Machine (SVM) for gene expression-based data classification. SVM classifiers work on Vapnik's statistical learning theory and thus handle the curse of dimensionality problem by finding the optimal hyperplanes in high-dimensional feature space. This process maximises the margin between classes and provides robustness to outliers. In SVM, the mapping of higher dimensional feature space is achieved through the kernel trick. SVMs often yield sparse solutions and select the most informative genes contributing to the classification task. The sparse solution reduces the computational burden and improves generalisation and classification accuracy. All the above features of SVM make it a suitable classifier for microarray gene expression datasets.The following sections discuss the classification methodology of each classifier mentioned above.
3.1. Nonlinear Regression (NLR)
The nonlinear regression classification is another variant of the linear regression classifier that associates variables in the form of y = mx + c. Unlike linear regression models, nonlinear regression models associate variables by randomly changing the variable y. NLR strives to minimise the MSE of the individual observations in the dataset. The data points are adapted to a nonlinear function through multiple iterations for building a classifier model. The Euclidean distance is initially calculated from the dataset's target and input data through the following expression as in Dai et.al. [
29]
Here T represents target data, and X represents input data. The Euclidean distance is then projected to a three-dimensional space using the following cuboid equation:
Later the minimum value of the three-dimensional space, f = min (z) is computed
The classification problem is now framed with the help of function ‘f’ and d
0 as follows:
The d0is the sum ofthe squares of mean deviation values. Since the calculation of d0 involves least squares method, the methodology employed here may be also called as a least square based NLR.
3.2. Gaussian Mixture Model
The Gaussian Mixture Model (GMM) is a probabilistic model for classification and clustering tasks. The model considers that the data points are generated from a mixture of Gaussian distributions. The probability Density Function (PDF) of the Gaussian distribution having mean μ and covariance Ԑ is given by Ge et.al [
30].
Where x is the data point, n hold the dimensionality of the data, and T indicates the transpose operation.The GMM assumes that the observed data is generated from a mixture of K Gaussian distributions. The posterior probability of thek
th Gaussian component with mixing coefficient π
k is given by:
µ
k and Ԑ
k are the covariance matrix and mean vector for the k
th component with k as total number of components in the mixture.In the next step, the parameters of the GMM, including the mixing coefficients, means, and covariances, will be estimated using the Maximum Likelihood Estimation (MLE) technique.In the next step, the parameters of the GMM, including the mixing coefficients, means, and covariances, will be estimated using the Maximum Likelihood Estimation (MLE) technique. The MLE estimation for the GMM is defined as the log-likelihood of the observed data.
Where θ represents the set of all parameters of the GMM and N is the total number of observed data points.In the next step, the expectation minimization is performed through an iterative approach by computing posterior probabilities
for each data point and updating parameters θ by maximizing the log-likelihood, considering the computed posterior probabilities.
3.3. Softmax Discriminant Classifier
Softmax discriminant classifier uses the Softmax function to transform the discriminant scores into class probabilities. At first, in SDC, the discriminant scores are calculated by taking the dot product between the feature vector x and the weight vector w
k for each class k, along with the bias term b
k. Mathematically, the discriminant score z
k for class k is given by Hastie et al. [
31]
For the discriminant scores z = [z
1, z
2... z
K] for K classes, the softmax function calculates the probability of the input x belonging to class k using the following expression
The loss function measures the discrepancy between the predicted class probabilities and the true class labels. The typically used loss function for softmax discriminant classification is the cross-entropy loss. For the training dataset with N samples, the cross-entropy loss ‘L’ is defined as
Where N is the number of samples in the training dataset, y
k(i) is the true label for sample i, which is 1 if the sample belongs to class k and 0 otherwise, and
is the predicted probability of class k for sample i. Finally, the weight vector
and bias term
are updated using gradient descent optimization method with learning rateα as follows.
3.4. Naive Bayesian Classifier (NBC)
The Naive Bayesian classifier (NBC) is efficient and straightforward and works on Bayes principles. NBC is a probabilistic classification algorithm based on the Bayes theorem and feature independence assumption. First, NBC calculates the posterior probability of a class using the prior probability and likelihood. For a given class C and features x
1, x
2… x
n, posterior probability
is expressed as Berrar [
32].
For the class C, represents the prior probability, is the likelihood, and is the evidence probability.
In Naive Bayes approach is that the features are conditionally independent for the class. This assumption that simplifies the calculation of the likelihood can be expressed as
Where
is the probability of feature
of the class C.The
is estimated from the fraction of class C training examples with the feature value
. Then the prior probability
can be estimated as the fraction of training examples belonging to class C.Finally, to predict the class label for the features
, the algorithm calculates the posterior probability for each class and assigns the instance to the class with the highest probability.
3.5. Support Vector Machine Classifier (Linear)
In machine learning, Support Vector Machine (SVM) is a robust learning algorithm used for analysing and classifying data.SVM with linear kernel model is the type of SVM that can solve linear problems by creating a line or hyperplane which separates the data into classes. The data that reside close to the hyperplane is considered the support vector. The expression for the hyperplane used in the binary classification problem can be written as Zhang et al. [
33].
Here f(x) is the objective function with x as the feature vector, w as the weight vector perpendicular to the hyper plane and b as the bias term, determining the offset of the hyper plane from the origin.SVM aims to find the hyperplane that maximizes the margin. The optimization problem for SVM linear classification withmargin
is given by:
Here
represents the class label (+1 or -1) for the training example with feature vector
From the above primal optimization problem, the dual optimization problem is derived by finding the Lagrange multipliers or dual variables for each training example. The dual problem reduces computational burden using optimal Lagrange multipliers
. Thus the SVMclassification with the decision function
is expressed as
If is positive, the instance is assigned to one class, and if it is negative, it is assigned to the other class.
3.6. Support Vector Machine Classifier (Polynomial)
SVM with a polynomial kernel is a variant of SVM that allows for non-linear classification by mapping the input features into a higher-dimensional space using a polynomial function. The polynomial kernel function calculates the dot product between the mapped feature vectors in the higher-dimensional space. The polynomial kernel function
with x and z are pair of feature vectors is given by Zhang et al. [
33].
Where γ is the coefficient scaling the dot product, r is the coefficient representing the independent term, and d is the degree of the polynomial.Further, the decision function in SVM polynomial classification is defined as the linear combination of the kernel evaluations between the support vectors and the input feature vector with the bias term. The decision function
. is given by:
Based on value, the class assignment is done in a similar way as performed in SVM (Linear) classifier.
3.7. Support Vector Machine Classifier (RBF)
The Radial Basis Function (RBF) can also be used as a kernel while using SVM classifier. Here, non-linear mapping of the input features into a higher-dimensional space is performed using an RBF.The RBF kernel
. that is used to compute the similarity between feature vectors in the input space is expressed as Zhang et al. [
33].
Where |x - z| is the Euclidean distance between x and z with σ as the kernel width parameter that controls the influence of each training sample.As computed before, the decision function for SVM RBF classification is also defined as a linear combination of the kernel evaluations between the support vectors and the input feature vector with the bias term. The decision function
for SVM RBF is given by:
Based on value, the class assignment is done in a similar way as performed in SVM linear and polynomial classifiers.
3.8. Selection of target
This research involves a binary classification, and hence two targets
and
for adeno and meso classification and mapping constraints must be selected.The target and mapping constraints are selected based on the distribution of classes in the dataset and the class imbalance problems involved in the microarray gene expression dataset. The target value,
is having the following constraint. For N number of features, with
as the average of input feature vector, the mapping constraint is framed as:
must have a target value greater than
and the average of input feature vectors
for meso case. The target value
∈[0,1], for meso case with M number of features, the mapping constraint is framed as
Also, the difference between the target values must follow the following constraint:
Thus, following all the above mentioned constraints, the targets,and for Adeno, andMeso output classes are chosen as 0.95, and 0.1, respectively. The performance of classifiers is will be evaluated based on the Mean Squared Error (MSE).
3.9. Training and Testing of Classifiers
The training data for the dataset is limited. So, we perform k-fold cross-validation. K-fold cross-validation is a popular method for estimating the performance of a machine-learning model. The process performed by Fushiki et al. [
34] for k-fold cross-validation is as follows. The first step is to divide the dataset into k equally sized subsets (or "folds"). For each fold, i, train the model on all the data except the i-th fold and test the model on the i-th fold. The process is repeated for all k folds so that each is used once for testing. At the end of the process, you will have k performance estimates (one for each fold). Now, calculate the average of the k performance estimates to get an overall estimate of the model's performance. Once the model has been trained and validated using k-fold cross-validation, you can retrain it on the full dataset and predict new, unseen data. The advantage of k-fold cross-validation is that it provides a more reliable estimate of a model's performance than a simple train-test split, as it uses all the available data. In this paper, the k-value is chosen as 10-fold.This research used a value of 2049 dimensionally reduced features per patient. This research is associated with 150 patients of Adenocarcinoma and 31 Meso cancer patients with multi trail training of classifiers required. The use of cross-validation removes any dependence on the choice of pattern for the test set. The training process is controlled by monitoring the Mean Square Error (MSE), which is defined by Wang et al. [
46] as,
Where Oj is the observed value at time j, Tj is the target value at model j;j=1and 2, and N is the total number of observations per epoch. In our case, it is 2049. The MSE value reached 1.0 E-10 within 1000 iterations as the training progressed.
In the case of lung cancer detection, the following terms can be defined:
True Positive (TP): A patient correctly identifies as having Adeno Carcinoma lung cancer.
True Negative (TN): A patient is correctly identified as having Meso cancer.
False Positive (FP): A patient is incorrectly identified as having Adeno Carcinoma lung cancer when they have Meso carcinoma cancer disease.
False Negative (FN): A patient is incorrectly identified as having Meso Carcinoma lung cancer when they do have the Adeno carcinoma Cancer disease.
Table 4 explores the training and testing MSE performance of the classifiers without and with feature selection (PSO and Harmonic Search) Methods for STFT Dimensionality reduction techniques. The training MSE always varies between 10E-07 to 10E-09, while the testing MSE varies from 10E-05 to 10E-08. GMM classifier without feature selection method settled at minimum training and Testing MSE of 3.6E-08 and 7.21E-07, respectively. PSO Feature Selection method SVM (RBF) Classifier scores minimum training and Testing MSE of 2.25E-09 and 3.6E-08, correspondingly. Like the Harmonic Search feature Selection method, the SVM (RBF) classifier once again attained minimum Training and Testing MSE of 2.56E-08 and 1.96E-07, respectively. The Minimum testing MSE is one of the indicators towards the attainment of better classifier performance. As shown in
Table 4, the higher the value of testing MSE leads to the poorer performance of the classifier, irrespective of the feature selection methods.
5. Results and Discussion
The research utilizes formal ten-fold testing for machine learning and CNN classification methodologies. The training uses 85% of the gene expression data, and the remaining 15% is employed for testing the model. Since this research is binary classification, the performance metrics are accordingly chosen. In binary classification problems, a confusion matrix is a valuable tool for evaluating the performance of a machine-learning model. As mentioned earlier, the confusion matrix summarizes the predictions made by the model against the true labels of the data. The performance metrics such as Accuracy, F1 score, MCC, Error Rate, and Kappa are derived by analyzing the values within the confusion matrix.Next, we discuss in detail the performance matrices employed in this research.
Accuracy
The accuracy of a classifier is a measure of how well it correctly identifies the class labels of a dataset. It is calculated by dividing the number of correctly classified instances by the total number of instances in the dataset.The equation for accuracy is given by Wilson et al. [
35].
F1 Score
The F1 score is a measure of a classifier's accuracy that combines precision and recall into a single metric. It is calculated as the harmonic mean of precision and recall, with values ranging from 0 to 1, where 1 indicates perfect precision and recall.The equation for F1 score is given by Koizumi et al. [
36].
Here, precision is the proportion of true positives among all instances classified as positive, and recall is the proportion of true positives among all positive instances. The F1 score is useful when the classes in the dataset are imbalanced, meaning there are more instances of one class than the other. In such cases, accuracy may be a bad metric, as a classifier that predicts the majority class would have high accuracy but low precision and recall. The F1 score provides a more balanced measure of a classifier's performance.
Matthews Correlation Coefficient (MCC)
MCC stands for "Matthews Correlation Coefficient," which measures the quality of binary (two-class) classification models. It considers true and false positives and negatives and is particularly useful in situations where the classes are imbalanced.
The MCC is defined by the following equation as given in Chicco et al. [
37]:
The MCC takes on values between -1 and 1, where a coefficient of 1 represents a perfect prediction, 0 represents a random prediction, and -1 represents a perfectly incorrect prediction.
Error Rate
As mentioned by Duda et al. [
38], the error rate of a classifier is the proportion of misclassified instances. It can be calculated using the following equation:
Kappa <!-- MathType@Translator@5@5@MathML2 (no namespace).tdl@MathML 2.0 (no namespace)@ -->
e kappa statistic, also known as Cohen's kappa, measures agreement between two raters or between a rater and a classifier. In the context of classification, it is used to evaluate the performance of a classifier on a binary or multi-class classification task. The kappa sta
istic measures the agreement between the predicted and true classes, considering the possibility of the agreement by chance. Cohen et al. [
39] defined kappa as follows:
Where
is the observed proportion of agreement, and
is the proportion of agreement expected by chance.
and
. are calculated as follows:
The kappa statistic takes on values between -1 and 1, where values greater than 0 indicate agreement better than chance, 0 indicate agreement by chance, and values less than 0 indicate agreement worse than chance. The results are tabulated in the following tables.
Table 8 indicates the performance analysis of the classifiers based on parameters such as Accuracy, F1 Score, MCC, Error Rate, and Kappa values for the STFT Dimensionality Reduction method without feature selection methods. It is explored from
Table 8 that the GMM Classifier is a high performing one with an accuracy of 80.66%, an F1 Score of 87.71%, with a low error rate of 19.34%. The GMM Classifier also demonstrates a moderate value of MCC 0.4419 and a Kappa value of 0.4285. The Softmax discriminant Classifier is a low-performing classifier with a low accuracy of 59.11%, with high Error Rate of 40.89% and an F1 Score of 69.67%. The MCC and Kappa values of the SD classifier are 0.2083 and 0.1609, respectively.
Table 9 depicts the performance analysis of the classifiers for the STFT Dimensionality Reduction method with PSO feature selection methods. It is identified from
Table 9 that the SVM(RBF) Classifier achieved high accuracy of 94.47%, an F1 Score of 96.62% with a low error rate of 5.52%. The SVM(RBF)Classifier has also reached a high value of MCC 0.81709 and Kappa value of 0.81485. The Non-linear Regression classifier is placed at the lower edge with a low accuracy of 59.91 %, a high Error Rate of 43.09% and an F1 Score of 68.03%. The MCC and Kappa values of the Non-Linear Regression classifier are at 0.1496 and 0.1156, correspondingly.
Table 10 exhibits the performance analysis of the classifiers for the STFT Dimensionality Reduction method with Harmonic Search feature selection methods. As shown in
Table 10, the SVM (RBF) Classifier achieved high accuracy of 90.05% F1 Score of 93.75% with a low error rate of 9.94%. The SVM (RBF) Classifier is also maintained at a high value of MCC 0.711 and Kappa value of 0.6963. Unfortunately, SVM (poly) classifier is placed at the low performing one with an accuracy of 59.11 %, a high Error Rate of 40.89% and an F1 Score of 70.4%. The MCC and Kappa value of the SVM (Poly) classifier is at 0.1512 and 0.1217, accordingly.
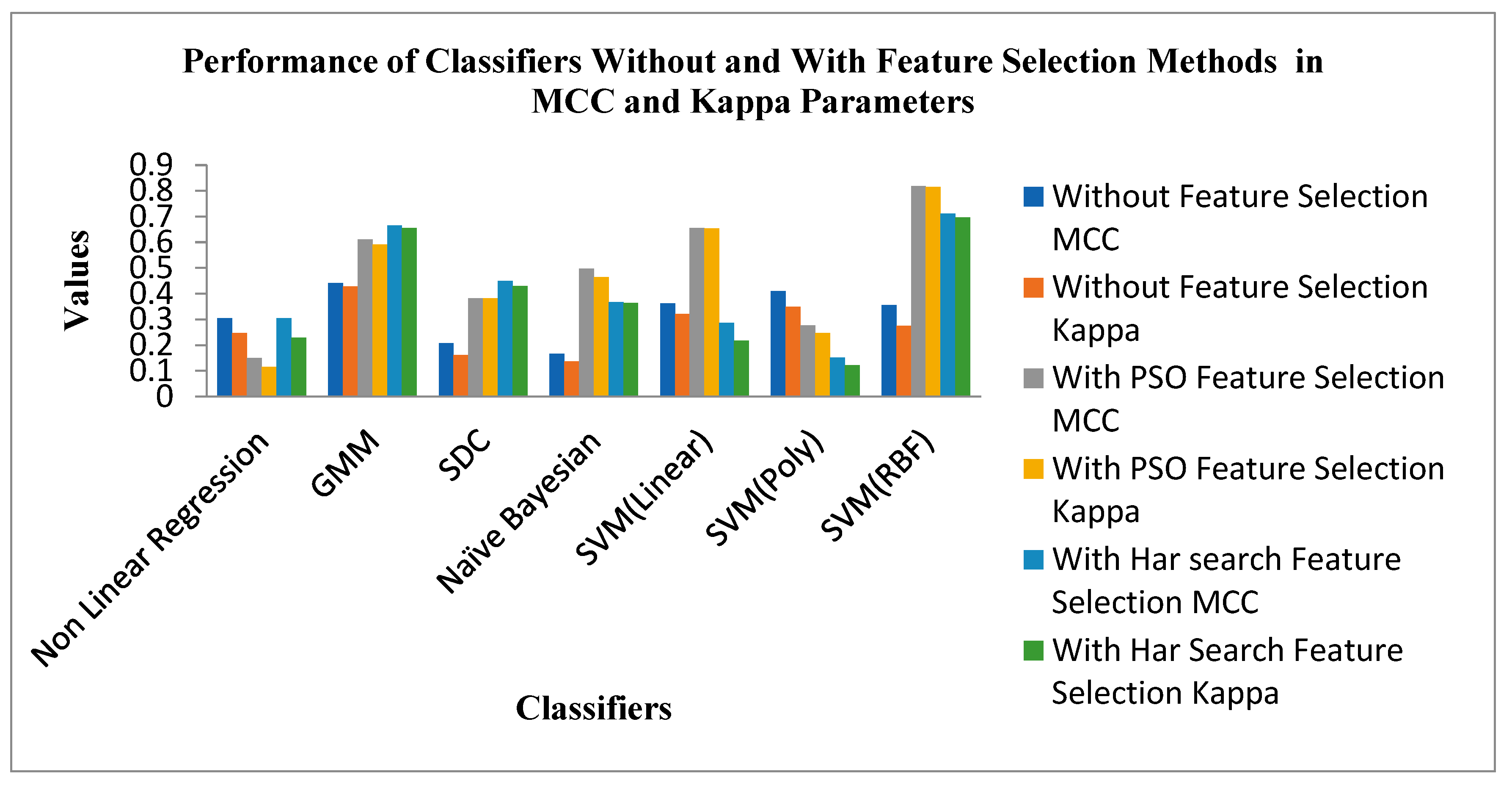
Figure 9 shows the Performance of Classifiers with and without Feature Selection regarding MCC and Kappa parameters. As indicated by
Figure 9 that the SVM (RBF) Classifier is reached a high value of MCC 0.81709 and Kappa value of 0.81485. The Non-Linear Regression classifier for the PSO feature selection method attains low MCC and Kappa values of 0.1496 and 0.1156. The average MCC and Kappa value across the classifiers for feature selection is 0.3212 and 0.27403, respectively. The average MCC and Kappa values across the classifiers for PSO and Harmonic Search Feature selection methods are settled at 0.4194, 0.3878 and 0.4841 and 0.4667. This signature effect of Feature selection shows the improvement of average MCC and Kappa values across the classifiers.
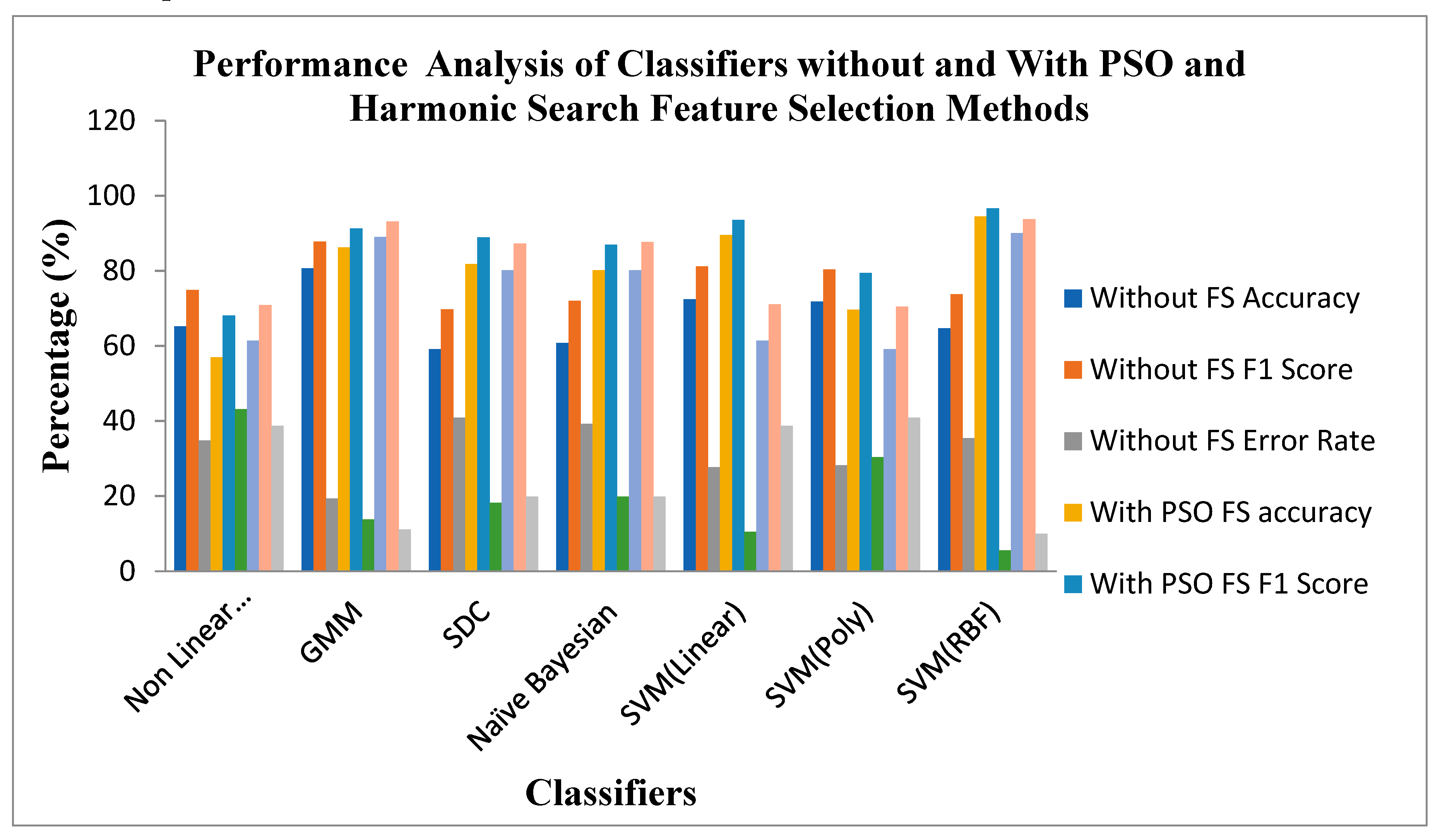
Figure 10 shows the Performance of Classifiers with and without Feature Selection regarding parameters like Accuracy, F1 Score and Error Rate. As identified by
Figure 10, the SVM (RBF) Classifier with the PSO feature selection method achieved higher values in Accuracy of 94.47%, an F1 Score of 96.62% and a lower Error Rate of 5.52% than all other categories of classifiers. In the case of the Harmonic Search feature selection method, once again SVM (RBF) classifier attained good values of Accuracy of 90.05%, an F1 Score of 93.75% and a low error Rate of 9.944% compared with all other six Classifiers. GMM Classifier attained an appreciable value of Accuracy of 80.66%, F1 Score of 87.71% and moderate Error Rate of 19.33% for the STFT inputs without any feature selection methods. The effect of feature selection improves the classifiers' benchmark parameters and overall performance. The PSO feature Selection retained its top position. It maintained its superiority over the harmonic search Feature selection method, reflected in the classical improvements of the classifier's performance.
Table 11 explores the performance analysis of the classifiers for raw micro array gene data with CNN methods.
As displayed in
Table 11, the SVM (RBF) Classifier achieved the highest accuracy of 90.607%, an F1 Score of 94.56%, with a low error rate of 9.39%. The SVM (RBF) Classifier is also maintained at a moderate value of MCC 0.6329 and Kappa value of 0.6031. For the CNN method, all seven classifiers are maintained at more than 83% accuracy and more than 90% F1 Score. The Softmax Discriminant Classifier attained minimum MCC and Kappa values of 0.5016 and 0.4616, respectively.
Table 12 exhibits the performance analysis of the classifiers for the STFT Dimensionality Reduction method with CNN methods.
Table 12 shows that Soft max Discriminant Classifier(SDC) achieved a high accuracy of 91.66%, an F1 Score of 95.08%, with a low error rate of 8.33%. The SD Classifier is also maintained at a high MCC value of 0.6825 and Kappa value of 0.6785. All seven classifiers are maintained at more than 86% of accuracy and more than 92% of F1 Score. The Naïve Bayesian Classifier achieved an accuracy of 91.66% and also attained MCC and Kappa values of 0.6742 and 0.625, respectively. It is also observed from
Table 12 that STFT input to the CNN method enhances the performance metrics of the classifiers when compared with raw input with CNN methods, as discussed in the paper.
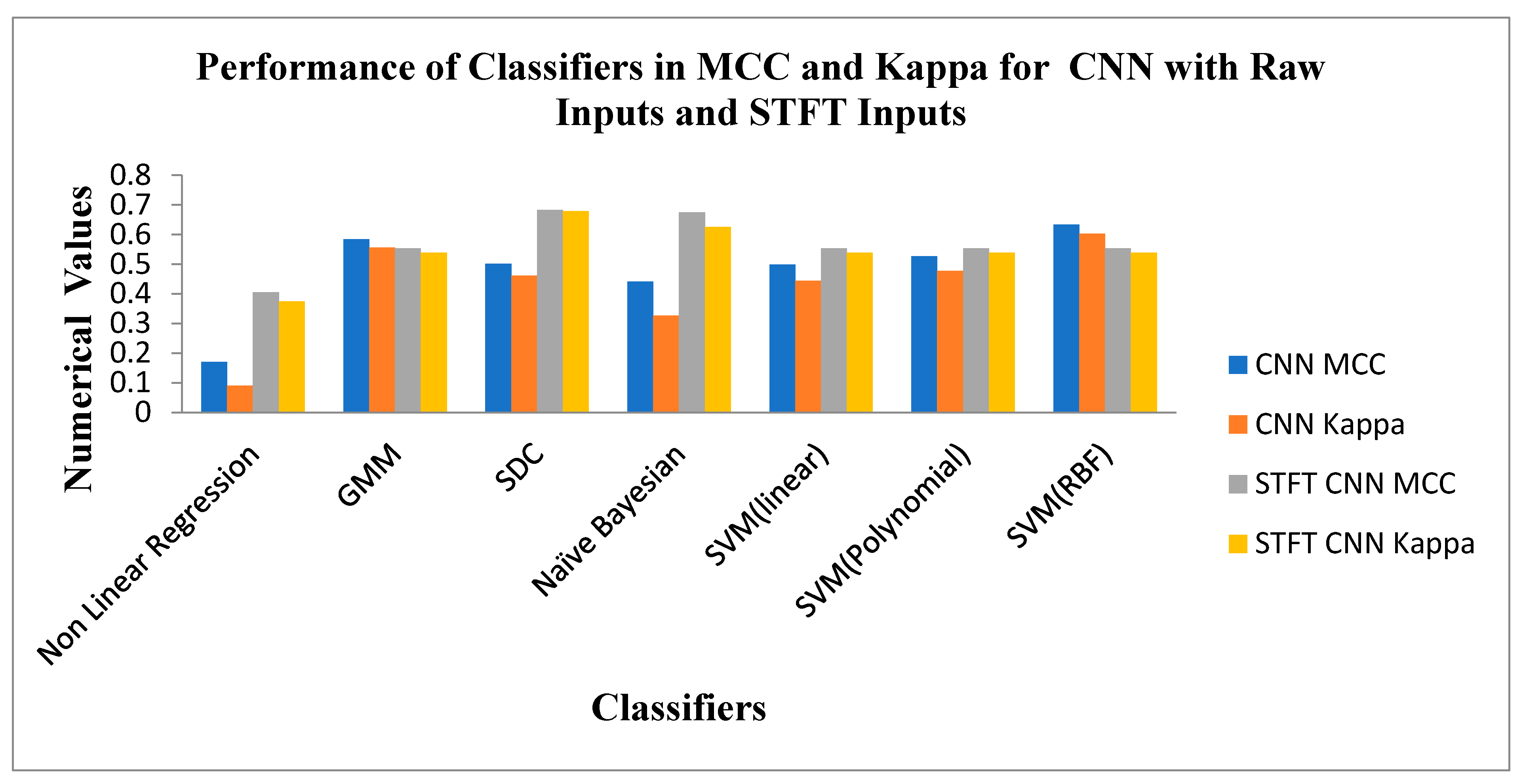
Figure 11 depicts the Performance of Classifiers regarding MCC and Kappa parameters for Raw and STFT inputs to the CNN method. As indicated by
Figure 11, the SD Classifier for the STFT feature method reached high MCC and Kappa values of 0.6825 and 0.6785, respectively. The average MCC and Kappa values across the classifiers are 0.5236 and 0.485. As shown by
Figure 11 that the SVM (RBF) Classifier is attained at good values of MCC 0.6329 and Kappa value of 0.6031 for the raw inputs to the CNN method. The average MCC and Kappa value across the classifiers for CNN is 0.4794 and 0.4226, respectively. The Scalogram effect of STFT inputs to the CNN methods exemplifies the enhancement of average MCC and Kappa values across the classifiers.
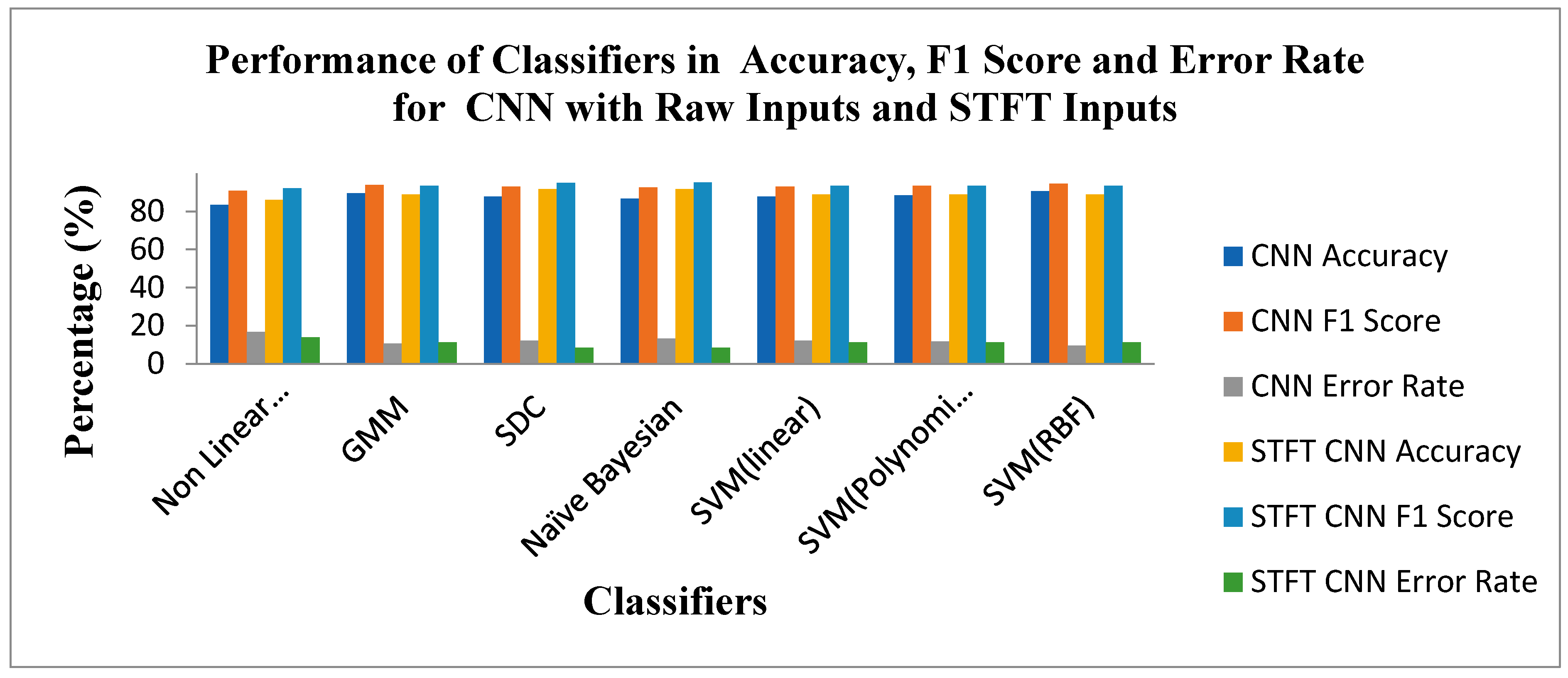
Figure 12 shows the Performance of Classifiers regarding Accuracy, F1 Score, and Error Rate parameters for raw and STFT inputs with the CNN method. As depicted by
Figure 12, the SVM(RBF) Classifier for raw input with the CNN method reached high accuracy, F1 Score and low error rates of 90.6%,94.56% and 9.39%, respectively. As displayed in
Figure 12 that the SD Classifier is attained at the high accuracy value, F1 Score and low Error Rate of91.66%, 95.08% and 8.33%, respectively, for STFT inputs with the CNN method. The yardstick effect of STFT inputs in CNN methods maketh the improvement in the accuracy, F1 Score and Error Rate values across the classifiers.
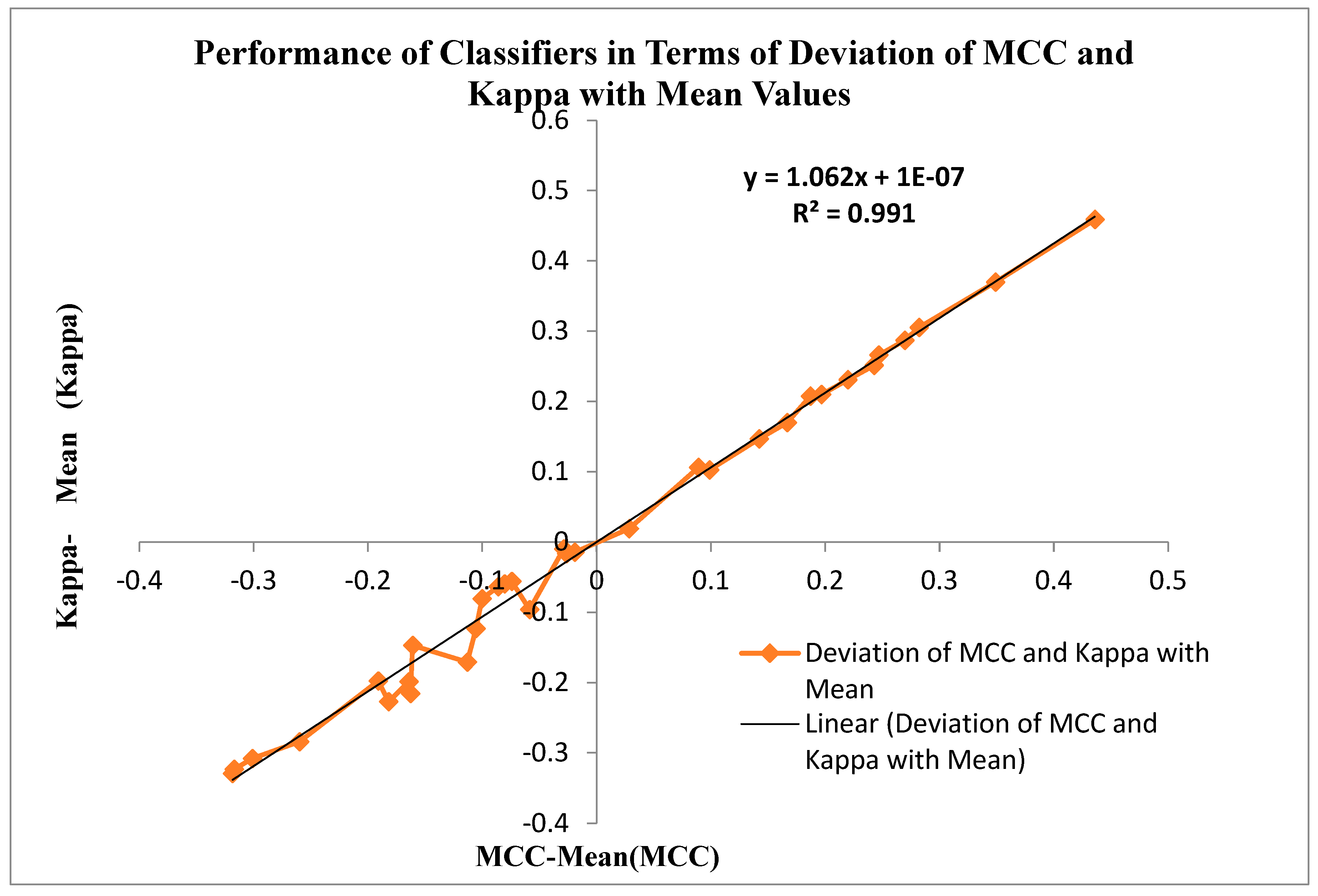
Figure 13 displays the Performance of Classifiers in terms of Deviation of MCC and Kappa Parameters with mean Values.The MCC and Kappa are the benchmark parameters that indicate the classifiers' outcomes for different inputs.As in this research, there are two categories of inputs like raw microarray genes, STFT, STFT with PSO and Harmonic Search Feature selection, STFT with Scalogram method and finally, CNN methods are provided to the classifiers. The classifier's performance is observed through the attained MCC and Kappa values for these inputs. The average MCC and Kappa values from the classifiers are 0.4681 and 0.44518, respectively. Now a methodology is devised to identify the deviation of MCC and Kappa values from their respective mean values to point out the classifier's performance. It is also observed from
Figure 13 that the MCC and Kappa values placed in the third quadrant of the graph depict the nonlinearoutcome of the classifiers with lower performance metrics. The MCC and Kappa values placed at the first quadrant show higher outcomes for classifiers with MCC and Kappa values more than the average value. This specifies the classifier's performance is improved for the STFT inputs along with the PSO Feature selection method.
Figure 13 is also denoted by the curve fitting of the linear line with the following equation y = 1.062x + 1E-07 and R² = 0.991 values
.
5.1. Computational Complexity
The computational complexity analyses the efficiency of the machine learning methods in terms of time and space complexity. In this research, the Big O notation represents the computational complexity of the dimensionality reduction, feature selection and classification methodologies. The computational complexity is represented by O(n),where n is the number of features. The O(log2n) means that the computational complexity increases 'log2n' times for any increase in 'n'. The classifiers considered in this research are integrated with either dimensionality reduction techniques, feature selection techniques, or a combination of both. Therefore, the computational complexity also is a combination of these hybrid methods. Overall, the choice of methodology should consider the trade-off between computational complexity and the desired level of performance in classification methodology.
Table 13 shows the computational complexity of the classifiers for the STFT dimensionality reduction method with and without feature selection methods, along with CNN Models.
As noted from
Table 13, the SVM(Linear), NBC, Nonlinear Regression, Soft max discriminant classifiers, and SVM(RBF) classifiers are at the level of low computational complexity for the STFT DR method without feature selection methods. GMM Classifier attains moderate complexity of O(2n
4 log2n) without feature selection methods and achieved good accuracy of 80.66%. For PSO and Harmonic search feature selection methods, the SVM(RBF) classifier with the computational complexity of O(2n
5 log 4n) placed at high accuracy of 94.47% and 90.05%, respectively.GMM and SVM(polynomial) classifiers are denoted by the high computational complexity of O(2n
7 log 2n) for the PSO and Harmonic Search Feature selection methods poorly performed in terms of accuracy score. These poor performances of classifiers are due to the presence of outlier problems in the STFT features. In order to enhance the performance of the classifiers, the STFT Scalogram features and CNN methods are included in this research. The SVM(RBF) Classifier attained good performance with moderate complexity of O(2n
3 log4n) and O(2n
3 log8n) for the methods, respectively.Next, through
Table 14, we compare the accuracy of our research work with various methodologies across diverse microarray gene datasets employed for Adenocarcinoma and mesothelioma lung cancer.
Overall, this research evaluates and explores machine learning and CNN classifiers for detecting lung cancer from microarray gene expressions. The research investigation shows that although CNNs are good at learning features, due to their black-box nature, the CNNs fail to analyse the physical meaning behind forecasting and categorising gene expression patterns from the learned features. Further, the LH2 dataset used for this research contains data imbalance, and many times during the analysis, CNNs were prone to overfitting and underfitting issues. The measures like the feature extraction step and replacement of the softmax layer with suitable classifiers were adopted to rectify these issues. But, the trade-off is that computational complexity will be increased while adding multiple stages to the CNN classification methodology.
Our research shows that microarray gene expression-based data is instrumental for disease classification. It contributes significantly to understanding gene expression patterns and their association with various biological processes and diseases. However, microarray genes have some limitations over general approaches like mRNA-Seq. The microarray gene expressions are selected from limited areas with specific genes or tissue regions. Therefore, the data generated may not capture the entire transcriptome and provide comprehensive information about gene expression. Also, microarray experiments may be prone to background noise and cross-hybridization problems due to non-specific binding, resulting in decreased sensitivity and specificity. Microarray experiments are expensive and sometimes have limited throughput compared to newer high-throughput sequencing technologies like mRNA-Seq.
Figure 1.
Methodology for Dimensionality Reduction and Classification of Microarray gene expression data for lung cancer.
Figure 1.
Methodology for Dimensionality Reduction and Classification of Microarray gene expression data for lung cancer.
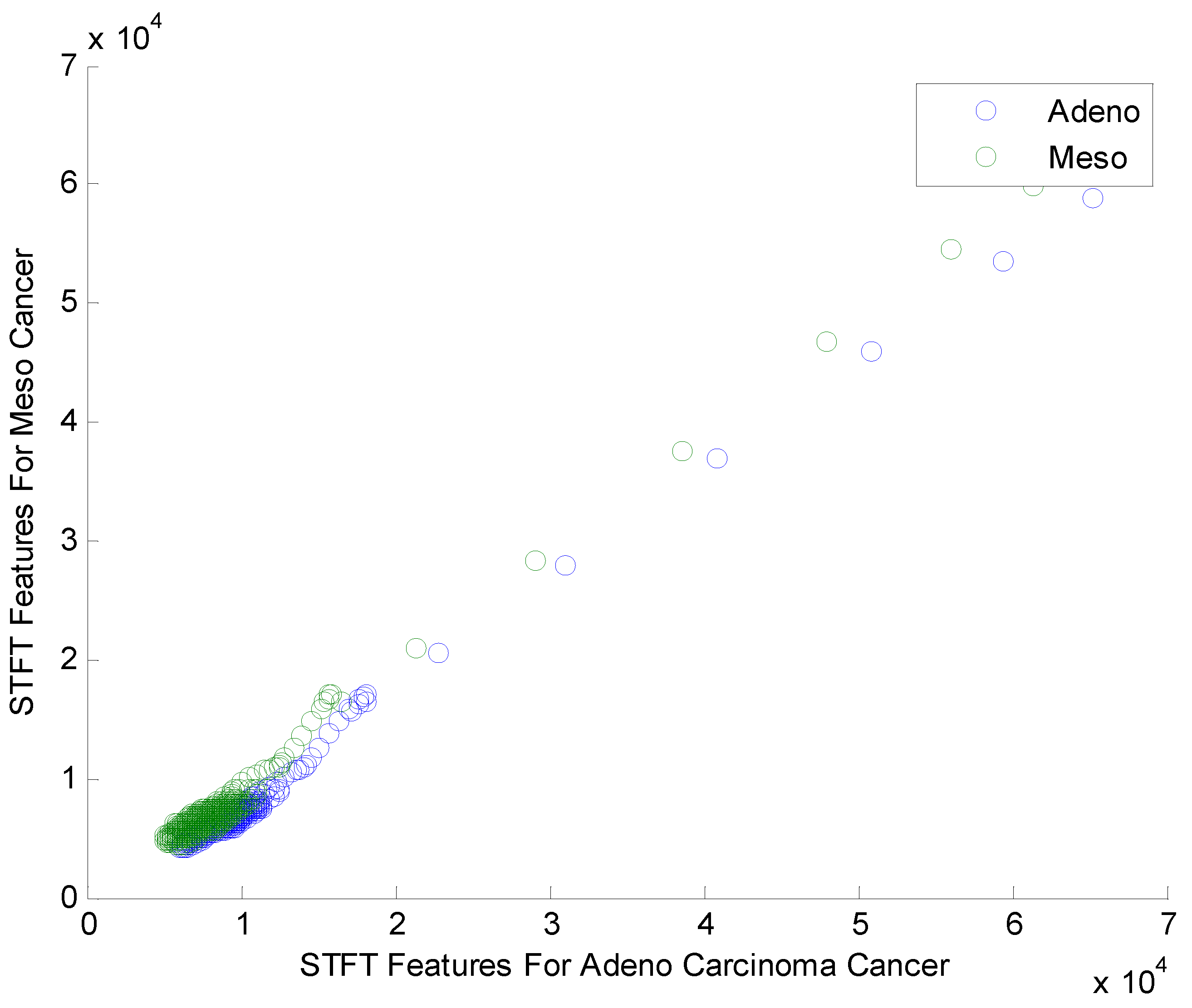
Figure 2.
Scatter plot for STFT based Dimensionality Reduction Method in Meso and Adeno carcinoma Cancer Classes.
Figure 2.
Scatter plot for STFT based Dimensionality Reduction Method in Meso and Adeno carcinoma Cancer Classes.
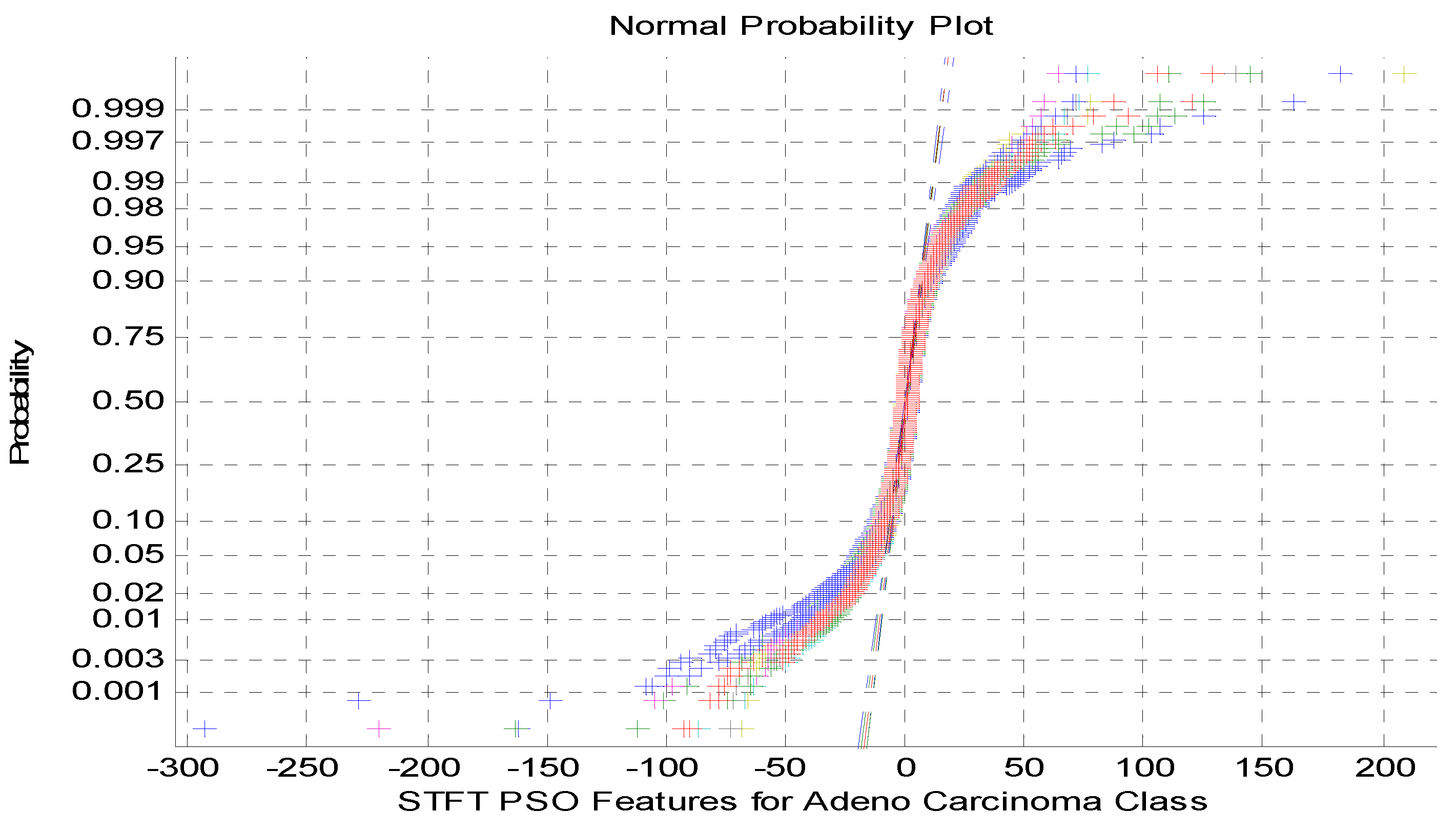
Figure 3.
NormalProbability plot for STFT Dimensionality Reduction Method with PSO Feature Selection in Adeno carcinoma Cancer Classes.
Figure 3.
NormalProbability plot for STFT Dimensionality Reduction Method with PSO Feature Selection in Adeno carcinoma Cancer Classes.
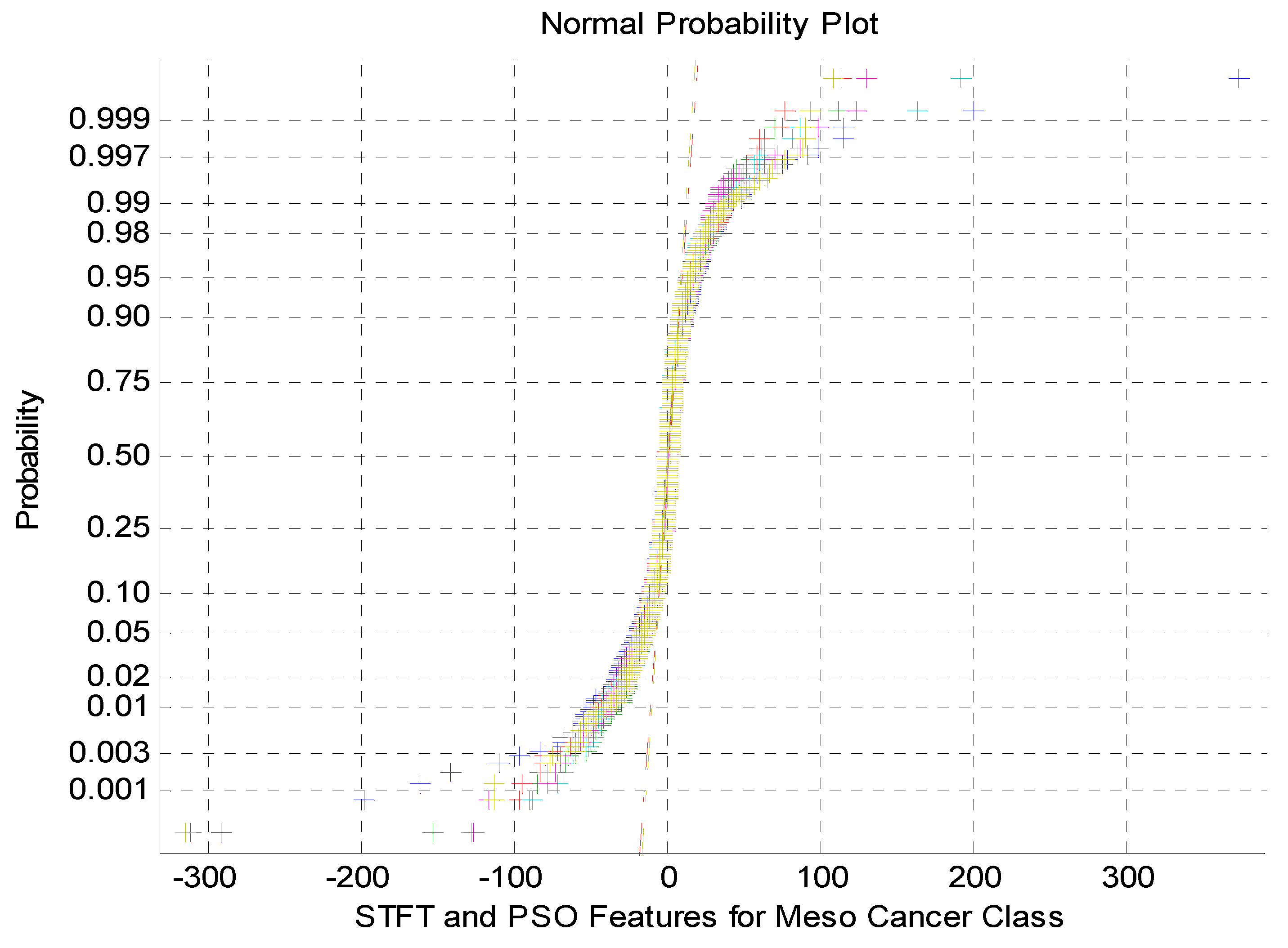
Figure 4.
Normalprobability plot for STFT Dimensionality Reduction Method with PSO Feature Selection in Meso carcinoma Cancer Classes.
Figure 4.
Normalprobability plot for STFT Dimensionality Reduction Method with PSO Feature Selection in Meso carcinoma Cancer Classes.
Figure 5.
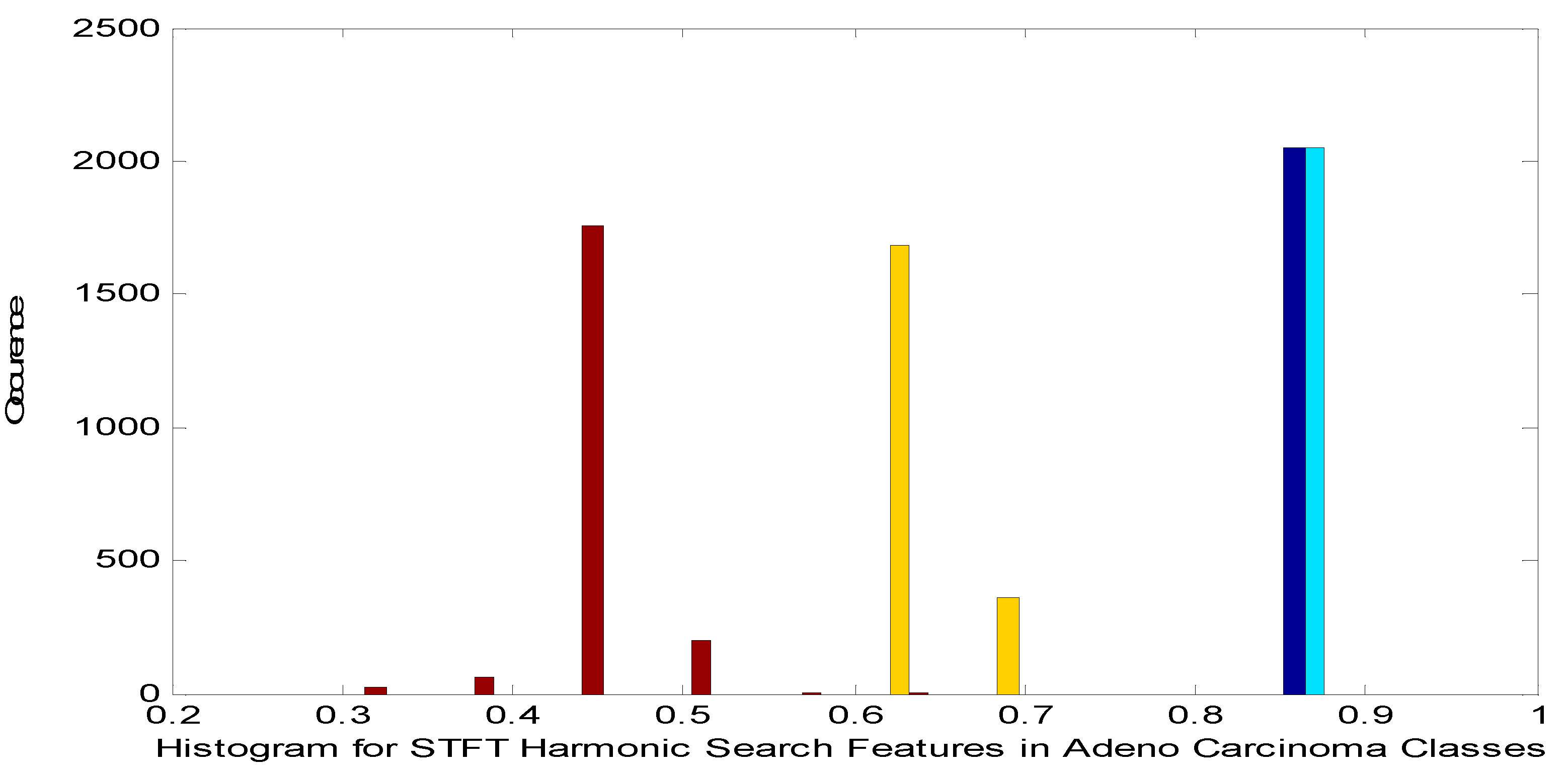
Histogram for STFT Dimensionality Reduction Method with Harmonic Search Feature Selection in Adeno carcinoma Cancer Classes.
Figure 5.
Histogram for STFT Dimensionality Reduction Method with Harmonic Search Feature Selection in Adeno carcinoma Cancer Classes.
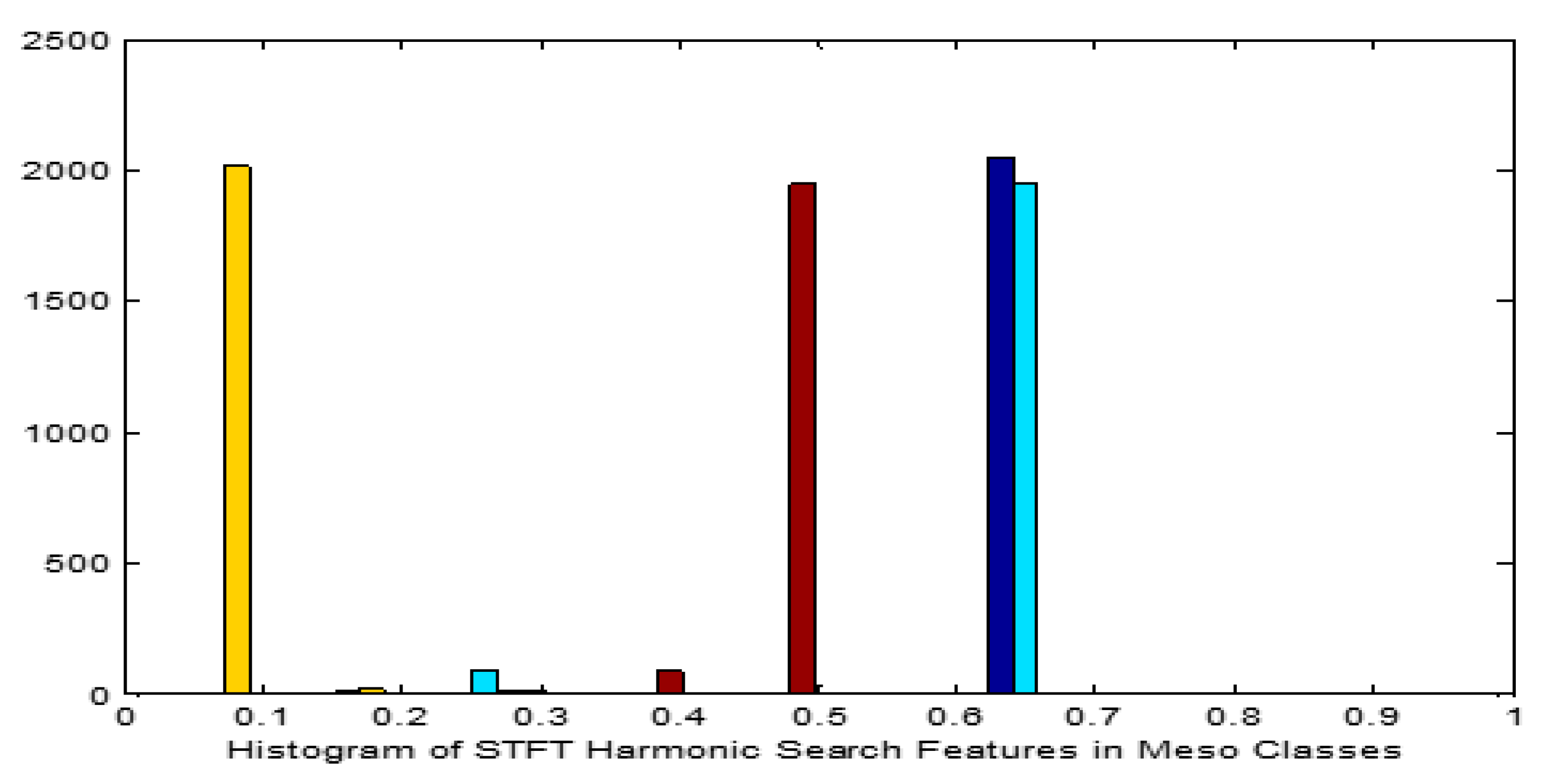
Figure 6.
Histogram for STFT Dimensionality Reduction Method with Harmonic Search Feature Selection in Meso carcinoma Cancer Classes.
Figure 6.
Histogram for STFT Dimensionality Reduction Method with Harmonic Search Feature Selection in Meso carcinoma Cancer Classes.
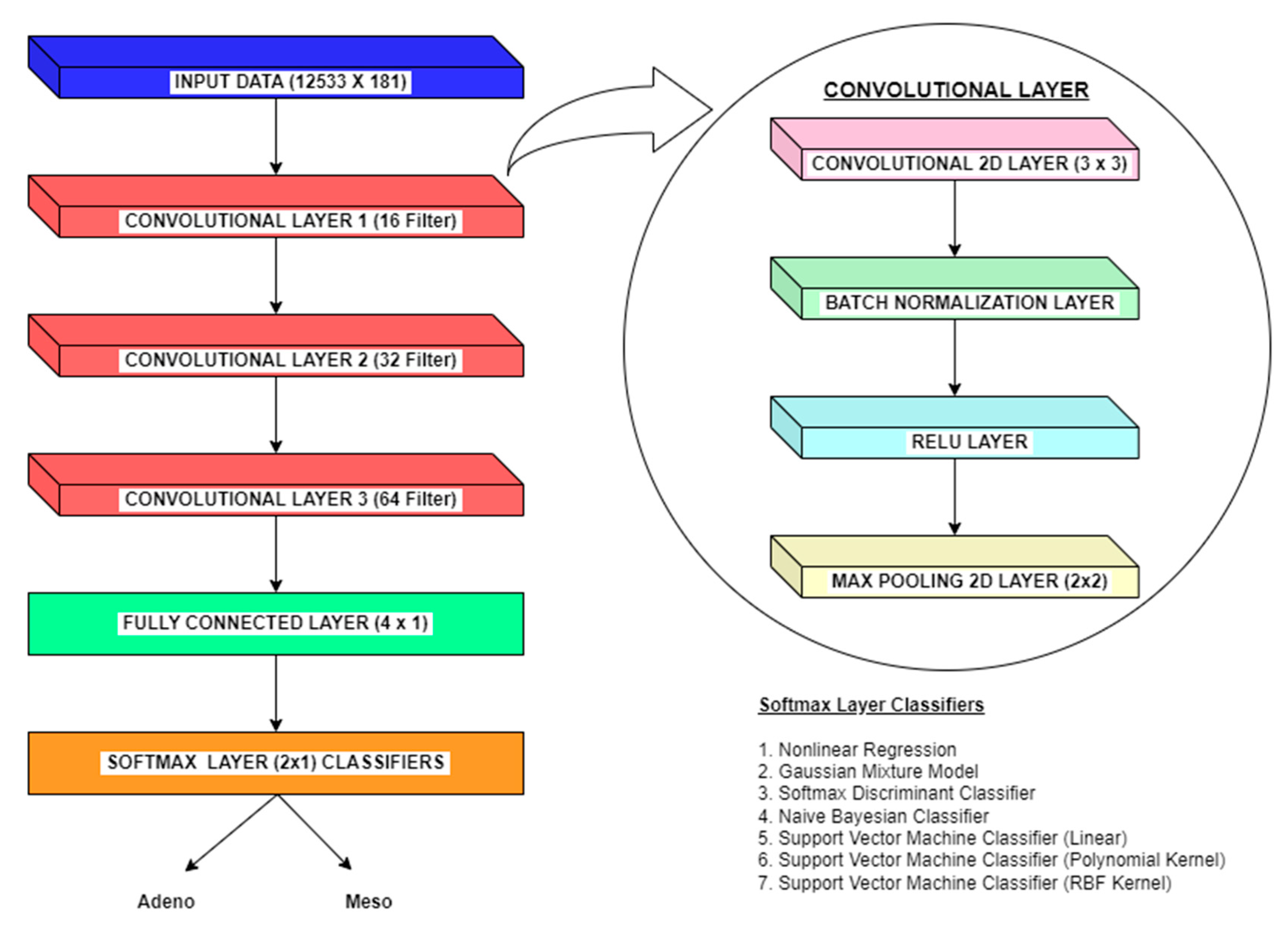
Figure 7.
CNN Classification Methodology.
Figure 7.
CNN Classification Methodology.
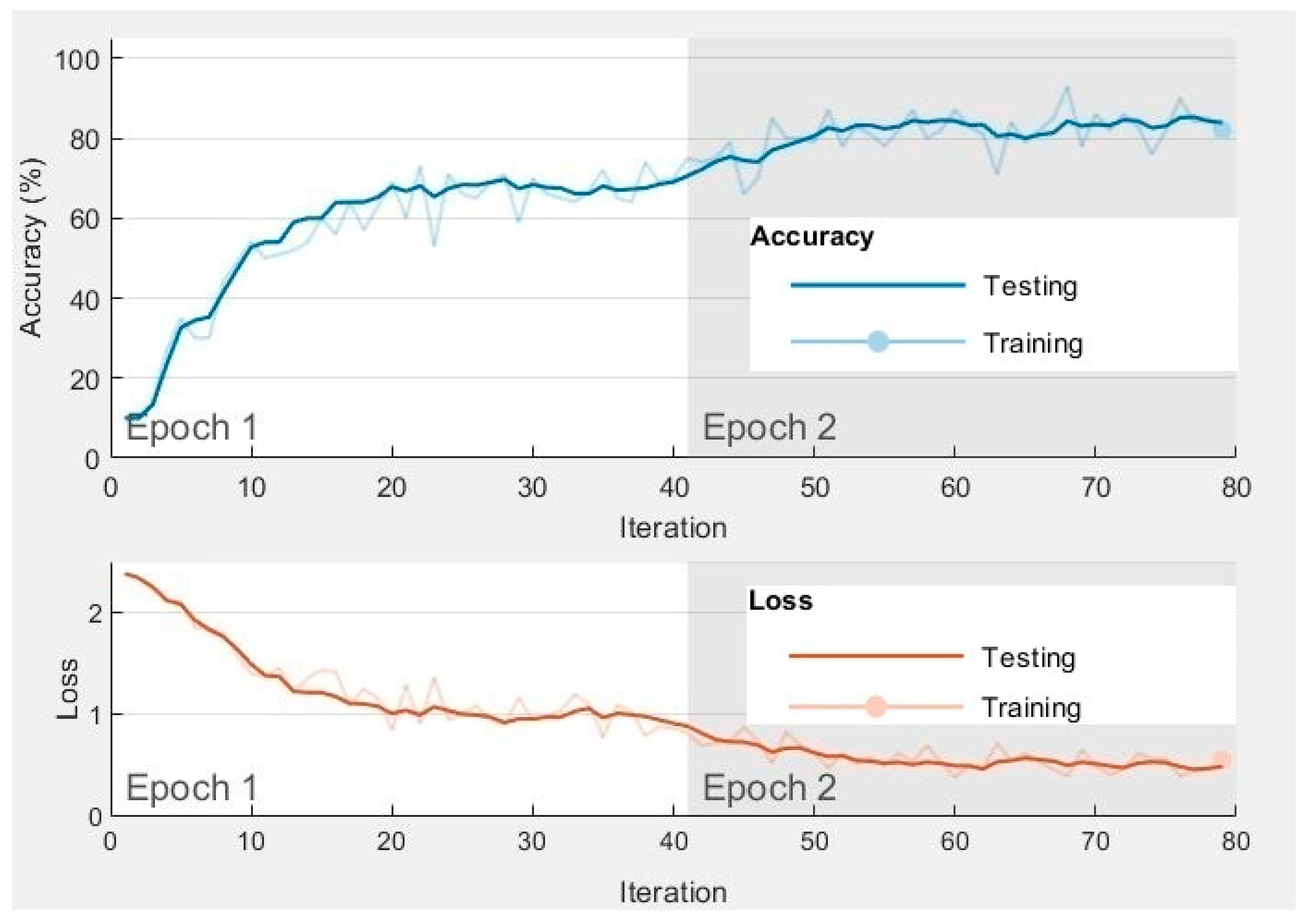
Figure 8.
Training and testingprogress of the first two epochs of SVM (RBF) Classifier in CNN Method with Raw Data.
Figure 8.
Training and testingprogress of the first two epochs of SVM (RBF) Classifier in CNN Method with Raw Data.
Figure 9.
Performance of Classifiers Without and With Feature Selection Methods in terms of MCC and Kappa Parameters.
Figure 9.
Performance of Classifiers Without and With Feature Selection Methods in terms of MCC and Kappa Parameters.
Figure 10.
Performance of Classifiers Without and With Feature Selection Methods in terms of Accuracy, F1 Score and Error Rate Parameters.
Figure 10.
Performance of Classifiers Without and With Feature Selection Methods in terms of Accuracy, F1 Score and Error Rate Parameters.
Figure 11.
Performance of Classifiers in terms of MCC and Kappa Parameters for Raw and STFT Inputs for CNN Methods.
Figure 11.
Performance of Classifiers in terms of MCC and Kappa Parameters for Raw and STFT Inputs for CNN Methods.
Figure 12.
Performance of Classifiers in terms of Accuracy, F1 Score and Error Rate Parameters for Raw and STFT Inputs in CNN Methods.
Figure 12.
Performance of Classifiers in terms of Accuracy, F1 Score and Error Rate Parameters for Raw and STFT Inputs in CNN Methods.
Figure 13.
Performance of Classifiers in terms of Deviation of MCC and Kappa Parameters with mean Values.
Figure 13.
Performance of Classifiers in terms of Deviation of MCC and Kappa Parameters with mean Values.
Table 1.
Average Statistical Features for STFT Dimensionally Reduced Adeno carcinoma and Meso Cancer Cases.
Table 1.
Average Statistical Features for STFT Dimensionally Reduced Adeno carcinoma and Meso Cancer Cases.
| Sl.No |
Statistical Features |
Cancer Classes |
| Adenocarcinoma |
Meso cancer |
| 1 |
Mean |
7527.993 |
9410.937 |
| 2 |
Variance |
7274816 |
8102763 |
| 3 |
Skewness |
14.7733 |
14.68975 |
| 4 |
Kurtosis |
272.0948 |
270.2702 |
| 5 |
Pearson Correlation Coefficient (PCC) |
0.993359 |
0.993084 |
| 6 |
F-test |
0.011166 |
0.390122 |
| 7 |
T-test |
0.264175 |
0.000004 |
| 8 |
p-value <0.01 |
0.4958 |
0.98 |
| 9 |
Permutation Entropy |
0.6931 |
0.6931 |
| 10 |
Sample Entropy |
11.0007 |
11.0007 |
| 11 |
Canonical Correlation Analysis (CCA) |
0.367333 |
Table 2.
Analysis of Friedman Test in Feature Selection Methods on STFT Data.
Table 2.
Analysis of Friedman Test in Feature Selection Methods on STFT Data.
| Sl.No |
Feature Selection Methods |
Parameters |
AdenoCarcinoma Cancer |
Meso Cancer |
| 1 |
PSO |
X2r statistic |
2.326 |
1.376 |
|
p-value |
0.67604. |
0.84836. |
|
p < .01 |
Not Significant |
Not Significant |
| 2 |
Harmonic Search |
X2r statistic |
6.39 |
1.18 |
|
p-value |
0.17185 |
0 .88138 |
|
p < .01 |
Not Significant |
Not Significant |
Table 3.
Confusion matrix for Lung Cancer Detection
Table 3.
Confusion matrix for Lung Cancer Detection
| Truth of Clinical Situation |
Predicted Values |
| Adeno Carcinoma |
Meso cancer |
| Actual Values |
Adeno Carcinoma |
TP |
FN |
| Meso cancer |
FP |
TN |
Table 4.
Training and Testing MSE Analysis of Classifiers for STFT Dimensionality Reduction Technique without and with PSO and Harmonic Search Feature Selection.
Table 4.
Training and Testing MSE Analysis of Classifiers for STFT Dimensionality Reduction Technique without and with PSO and Harmonic Search Feature Selection.
| Classifiers |
Without Feature Selection |
With PSO Feature Selection |
With Harmonic Search Feature Selection |
| Training MSE |
Testing MSE |
Training MSE |
Testing MSE |
Training MSE |
Testing MSE |
| Non Linear Regression |
1.68E-07 |
5.63E-06 |
4.9E-07 |
1.32E-05 |
6.25E-06 |
9.6E-06 |
| GMM |
3.6E-08 |
7.21E-07 |
6.76E-08 |
4.84E-06 |
9E-07 |
1.96E-06 |
| SDC |
3.25E-07 |
8.84E-05 |
2.89E-07 |
3.61E-06 |
2.35E-07 |
1.16E-06 |
| Naïve Bayesian |
5.48E-07 |
6.24E-05 |
1.09E-07 |
1.52E-05 |
1.32E-07 |
6.76E-06 |
| SVM(Linear) |
2.02E-07 |
2.51E-05 |
1.76E-07 |
1.1E-06 |
1.22E-07 |
8.1E-05 |
| SVM(Poly) |
9E-07 |
3.48E-05 |
4.9E-08 |
3.03E-05 |
6.4E-07 |
7.06E-05 |
| SVM(RBF) |
4.84E-07 |
6.56E-05 |
2.25E-09 |
3.6E-08 |
2.56E-08 |
1.96E-07 |
Table 5.
Selection of Classifier Parameters.
Table 5.
Selection of Classifier Parameters.
| Classifiers |
Description |
| NLR |
Class target T1= 0.85 and T2=0.65, k1, k2, and k3 is from equation (4), Convergence Criteria: MSE |
| GMM |
Mean, Covariance of the input samples, and tuning parameters with test point likelihood probability 0.1, cluster probability of 0.5, with convergence rate of 0.6, Convergence Criteria: MSE |
| SDC |
γ = 0.5, along with mean of each class target values as 0.65 and 0.85, Class Weights (w) : 0.4, Bias (b): 0.05, Convergence Criteria: MSE |
| NBC |
Smoothing parameter (alpha): 0.06, Prior Probabilities: 0.15, Convergence Criteria: MSE |
| SVM (Linear) |
α (Regularization Parameter): 0.85, w : 0.4, b: 0.01, Convergence Criteria: MSE |
| SVM (Polynomial) |
α: 0.76, Coefficient of the kernel function (γ): 10, w: 0.5, b: 0.01, Convergence Criteria: MSE |
| SVM (RBF) |
α: 1, Kernel width parameter (σ): 100, w: 0.86, b: 0.01, Convergence Criteria: MSE |
Table 6.
Training and Testing Parameters of CNN Methodologyfor Raw Data and STFT Dimensionally reduced inputs.
Table 6.
Training and Testing Parameters of CNN Methodologyfor Raw Data and STFT Dimensionally reduced inputs.
| Parameter |
Value |
| Number of cross-validation |
10 |
| Learning rate |
0.01 |
| Number of mini batch |
100 |
| Maximum number of Epochs |
10 |
| Number of convolutional layer |
3 |
| Number of fully connected layer |
4 |
| Number of filter in first convolutional layer |
16 |
| Size of filter in first convolutional layer |
3 x 3 |
| Number of filter in second convolutional layer |
32 |
| Size of filter in second convolutional layer |
3 x 3 |
| Number of filter in third convolutional layer |
64 |
| Size of filter in third convolutional layer |
3 x 3 |
| Loss Function |
Cross Entropy |
Table 7.
Training and Testing Accuracy Analysis of variousClassifiers in CNN Method with Raw Data and STFT features.
Table 7.
Training and Testing Accuracy Analysis of variousClassifiers in CNN Method with Raw Data and STFT features.
| Classifiers |
CNN methods with Raw Data |
CNN methods with STFT Features |
| |
|
| Training Accuracy |
Testing Accuracy |
Training Accuracy |
Testing Accuracy |
| Non Linear Regression |
87.235 |
83.42541 |
89.327 |
86.11111 |
| GMM |
92.752 |
89.50276 |
91.458 |
88.88889 |
| SDC |
90.516 |
87.8453 |
94.372 |
91.66667 |
| Naïve Bayesian |
90.023 |
86.74033 |
94.4527 |
91.66667 |
| SVM(Linear) |
91.358 |
87.8453 |
91.753 |
88.88889 |
| SVM(Poly) |
90.145 |
88.39779 |
91.991 |
88.88889 |
| SVM(RBF) |
93.631 |
90.60773 |
92.047 |
88.88889 |
Table 8.
Performance Analysis of Classifiers for STFT Dimensionality Reduction Technique without Feature Selection.
Table 8.
Performance Analysis of Classifiers for STFT Dimensionality Reduction Technique without Feature Selection.
| Classifiers |
Parameters |
| Accuracy |
F1 Score |
MCC |
Error Rate |
Kappa |
| Non Linear Regression |
65.19337 |
74.9004 |
0.304098 |
34.80663 |
0.246382 |
| GMM |
80.66298 |
87.7193 |
0.441969 |
19.33702 |
0.428507 |
| SDC |
59.11602 |
69.67213 |
0.208379 |
40.88398 |
0.160987 |
| Naïve Bayesian |
60.77348 |
71.93676 |
0.167045 |
39.22652 |
0.137111 |
| SVM(Linear) |
72.37569 |
81.20301 |
0.362762 |
27.62431 |
0.321894 |
| SVM(Poly) |
71.8232 |
80.30888 |
0.409538 |
28.1768 |
0.348967 |
| SVM(RBF) |
64.64088 |
73.77049 |
0.355135 |
35.35912 |
0.274367 |
Table 9.
Performance Analysis of Classifiers for STFT Dimensionality Reduction Technique with PSO Feature Selection.
Table 9.
Performance Analysis of Classifiers for STFT Dimensionality Reduction Technique with PSO Feature Selection.
| Classifiers |
Parameters |
| Accuracy |
F1 Score |
MCC |
Error Rate |
Kappa |
| Non Linear Regression |
56.90608 |
68.03279 |
0.149676 |
43.09392 |
0.115635 |
| GMM |
86.18785 |
91.22807 |
0.610382 |
13.81215 |
0.591791 |
| SDC |
81.76796 |
88.88889 |
0.382089 |
18.23204 |
0.381485 |
| Naïve Bayesian |
80.1105 |
86.95652 |
0.496771 |
19.8895 |
0.463968 |
| SVM(Linear) |
89.50276 |
93.55932 |
0.655197 |
10.49724 |
0.652451 |
| SVM(Poly) |
69.61326 |
79.40075 |
0.277252 |
30.38674 |
0.247373 |
| SVM(RBF) |
94.47514 |
96.62162 |
0.817097 |
5.524862 |
0.814853 |
Table 10.
Performance Analysis of Classifiers for STFT Dimensionality Reduction Technique with Harmonic Search Feature Selection.
Table 10.
Performance Analysis of Classifiers for STFT Dimensionality Reduction Technique with Harmonic Search Feature Selection.
| Classifiers |
Parameters |
| Accuracy |
F1 Score |
MCC |
Error Rate |
Kappa |
| Non Linear Regression |
61.32597 |
70.83333 |
0.305452 |
38.67403 |
0.229319 |
| GMM |
88.95028 |
93.10345 |
0.664882 |
11.04972 |
0.654909 |
| SDC |
80.1105 |
87.23404 |
0.44911 |
19.8895 |
0.430519 |
| Naïve Bayesian |
80.1105 |
87.67123 |
0.368107 |
19.8895 |
0.364417 |
| SVM(Linear) |
61.32597 |
71.07438 |
0.286204 |
38.67403 |
0.217998 |
| SVM(Poly) |
59.11602 |
70.4 |
0.151209 |
40.88398 |
0.121705 |
| SVM(RBF) |
90.05525 |
93.75 |
0.711034 |
9.944751 |
0.696309 |
Table 11.
Performance Analysis of Classifiers for Raw data Without Dimensionality Reduction with CNN Method.
Table 11.
Performance Analysis of Classifiers for Raw data Without Dimensionality Reduction with CNN Method.
| Classifiers |
Parameters |
| Accuracy |
F1 Score |
MCC |
Error Rate |
Kappa |
| Non Linear Regression |
83.42541 |
90.85366 |
0.170709 |
16.5745856 |
0.090147 |
| GMM |
89.50276 |
93.92971 |
0.583974 |
10.4972376 |
0.55643 |
| SDC |
87.8453 |
93.03797 |
0.501658 |
12.1546961 |
0.461601 |
| Naïve Bayesian |
86.74033 |
92.59259 |
0.441204 |
13.2596685 |
0.325885 |
| SVM(Linear) |
87.8453 |
93.08176 |
0.498308 |
12.1546961 |
0.443699 |
| SVM(Poly) |
88.39779 |
93.37539 |
0.52711 |
11.6022099 |
0.477669 |
| SVM(RBF) |
90.60773 |
94.56869 |
0.632977 |
9.39226519 |
0.603121 |
Table 12.
Performance Analysis of Classifiers for STFT Dimensionality Reduction Technique with CNN Method.
Table 12.
Performance Analysis of Classifiers for STFT Dimensionality Reduction Technique with CNN Method.
| Classifiers |
Parameters |
| Accuracy |
F1 Score |
MCC |
Error Rate |
Kappa |
| Non Linear Regression |
86.11111 |
92.06349 |
0.40452 |
13.88889 |
0.375 |
| GMM |
88.88889 |
93.54839 |
0.553399 |
11.11111 |
0.538462 |
| SDC |
91.66667 |
95.08197 |
0.6825 |
8.333333 |
0.678571 |
| Naïve Bayesian |
91.66667 |
95.2381 |
0.6742 |
8.333333 |
0.625 |
| SVM(Linear) |
88.88889 |
93.54839 |
0.553399 |
11.11111 |
0.538462 |
| SVM(Poly) |
88.88889 |
93.54839 |
0.553399 |
11.11111 |
0.538462 |
| SVM(RBF) |
88.88889 |
93.54839 |
0.553399 |
11.11111 |
0.538462 |
Table 13.
Computational Complexity of the Classifiers for STFT Dimensionality Reduction Method without and with Feature selection methods and CNN Models.
Table 13.
Computational Complexity of the Classifiers for STFT Dimensionality Reduction Method without and with Feature selection methods and CNN Models.
| Classifiers |
Without Feature Selection |
With PSO Feature Selection |
With Harmonic Search |
CNN With Raw Data |
CNN STFT DR Method |
| Non Linear Regression |
O(2n3 log2n) |
O(2n6 log 2n) |
O(2n6 log 2n) |
O(2n4 log2n) |
O(2n4 log4n) |
| GMM |
O(2n4 log2n) |
O(2n7 log 2n) |
O(2n7 log 2n) |
O(2n5log2n) |
O(2n5log4n) |
| Soft Max Discriminant |
O(2n3 log2n) |
O(2n6 log 2n) |
O(2n6 log 2n) |
O(2n4 log2n) |
O(2n4 log4n) |
| Naïve Bayesian |
O(2n3 log2n) |
O(2n6 log 2n) |
O(2n6 log 2n) |
O(2n4 log2n) |
O(2n4 log4n) |
| SVM(Linear) |
O(2n3 log2n) |
O(2n6 log 2n) |
O(2n6 log 2n) |
O(2n4 log2n) |
O(2n4 log4n) |
| SVM(Poly) |
O(2n4 log2n) |
O(2n7 log 2n) |
O(2n7 log 2n) |
O(2n5 log2n) |
O(2n5 log4n) |
| SVM(RBF) |
O(2n2 log4n) |
O(2n5 log 4n) |
O(2n5 log 4n) |
O(2n3 log4n) |
O(2n3 log8n) |
Table 14.
Comparison with Existing Works in Adenocarcinoma and mesothelioma lung cancer classification from microarray gene datasets.
Table 14.
Comparison with Existing Works in Adenocarcinoma and mesothelioma lung cancer classification from microarray gene datasets.
| Serial No. |
Author |
Dataset |
Methodology |
Accuracy |
| 1 |
As reported in this research work |
LH2 Dataset |
STFT with PSO feature selection and SVM (RBF) classifier |
94.47 |
| 2 |
Gupta et al. (2022) [40] |
Cancer Genome Atlas dataset |
Deep learning with CNN Methodology |
92 |
| 3 |
Lin Ke et.al. (2022) [41] |
LH2 Dataset |
Decision Tree C4.5 Classification |
93.2 |
| 4 |
Federica Morani et al. (2021) [42] |
Cancer Genome Atlas and curated gene expression datasets |
Multivariate approach using Cox |
90 |
| 5 |
Fathi et al. (2021) [43] |
LH2 Dataset |
Feature combination from different network layers using Decision tree |
85 |
| 6 |
Daniel Xia et al. (2020) [44] |
LH2 Dataset |
Minimalist approach for Classification |
90.6 |
| 7 |
Azzavi(2015) et al. [45] |
Kent Ridge Bio-medical Dataset |
multilayer perceptron (MLP), RBF and SVM |
91.3991.7289.82 |
| 8 |
Guan et al.(2009) [46] |
HG-U95 Dataset |
SVM with RBF Kernel |
94 |
| 9 |
Mramor et al. (2007) [47] |
Whitehead Institute Database |
SVM, NBC, k-nearest neighbour (KNN) , and Decision Tree |
94.6790.3575.2891.21 |
| 10 |
Gordon (2002) [48] |
LH2 Dataset |
Translation of microarray data to gene expressions |
90 |