1. Introduction
The intestinal microbiome has an important aspect of health and disease in a wide range of species [
1,
2,
3,
4,
5,
6,
7,
8], including the horse [
9,
10,
11,
12,
13,
14,
15]. As a hind-gut fermenter, the horse relies on the large intestinal microbiome for the breakdown of structural carbohydrates, including cellulose and fructans. The metabolism of these substances by the microbiome liberates energy substrates in the form of volatile fatty acids, predominantly butyrate, propionate and also lactate [
16]. Recent investigations have proposed derangements in the normal microbiome in horses in a variety of clinical presentations that have been reviewed elsewhere [
11,
13,
17]. While molecular techniques provide a unique insight into microbial populations, the role of these population changes is less clear, and causation is often hypothesized without evidence. There remains a lack of robust large-scale evidence base for therapeutic manipulation of the microbiome in any clinical presentation in the horse. Such studies are limited by the practicalities and expense of collection, storage and analysis [
18].
The intestinal metabolome has been more recently investigated alongside the microbiome to attempt to ascribe a functional significance to such changes [
19]. These rely upon the measurement of volatile fatty acids and other volatile organic compounds from the feces. Although sample collection is uncomplicated, the preservation requirements of such samples limit their usefulness in more extensive field trials of proposed interventions in the horse, where access to ultralow freezer capacity is not immediately accessible.
Fecal pH may represent a simple, cost-effective tool to document changes in the metabolome and can be measured patient side with limited equipment. Limited studies have evaluated fecal pH in horses undergoing attempts to manipulate the intestinal microbiome, although has been evaluated in responses to different diets [
20,
21,
22,
23]. However, if it can document the impact of manipulation of the microbiome, it would represent a valuable tool to generate large-scale data and thus be used to evaluate proposed therapeutic interventions.
Enzyme-rich malt-extract (ERME) is marketed as a pre-digestive supplement for a number of species and is proposed to promote pre-cecal digestion of starch, cellulose and fructans, to prevent excessive lactic acid production following their metabolism by the cecal microbiome [
24,
25]. ERME has been demonstrated to bring about changes in both the metabolome and microbiome of horses and this has been postulated to improve animal health and wellbeing, however investigations in larger populations of horses are limited by sample handling, storage and processing costs.
The hypothesis of this study was that ERME supplementation would bring about changes in the fecal metabolome, that would result in changes in fecal pH in racehorses being fed commercial diets. The aim was to determine both the rate and magnitude of change in fecal pH in Thoroughbred racehorses in training when supplemented with ERME.
2. Materials and Methods
2.1. Animal recruitment and data collection
Thoroughbred racehorses in flat race training were recruited from 9 training yards in Newmarket, UK. Horses were selected to enter the trial based on selection by the trainer, without any selection criteria. Fecal pH was determined at the start of the study and 2, 3 and 4 weeks after the initiation of supplementation, although the exact sampling period varied by 2-3 days based on availability and training / racing schedules. Fecal pH was determined by insertion of a handheld pH meter (HI-981030 soil pH tester; HANNA instruments, Woonsocket, RI. USA) into freshly produces feces. Recordings were made in triplicate from three areas of the feces and the average recorded for each animal at each time point. Where outliers were encountered, with individual recordings from a sample of feces differed by more than a pH of more than 0.2, further recordings were made and outliers excluded. The pH device was calibrated at the start of each day of data collection and washed between samples using the manufacturers instructions. All data were recorded using a digital record system (AppSheet; Solvebot Inc; WA, USA) and stored in a cloud database (Google Sheets; Google Inc, Mountain View, CA. USA).
2.2. Experimental design
All horses received enzyme-rich malt extract (Equinectar; Tharos, London. UK) in their normal feed at an inclusion rate of 150ml twice daily. There was no attempt to interfere with feeding patterns or to correct inclusion rates according to exact body weights. There was no control population. Horses that did not complete 4 weeks of supplementation from excluded from any subsequent analysis.
2.2. Statistical analysis
All pH data are presented as arithmetic mean (± 95% confidence intervals). Horses were classified into three categories of fecal pH, normal pH was considered above 6.40, subclinical acidosis was considered below 6.4 and severe acidosis below 6.0. Data were grouped to the nearest week period and grouped irrespective of actual day of sampling. Data were examined for normality using Shapiro-Wilk test and fecal pH were compared over the period of supplementation using mixed-effects analysis with the Geisser-Greenhouse correction. Tukey’s multiple comparisons tests were calculated between each time period. Comparisons between classification (normal, acidosis, severe acidosis) were performed using Chi-squared analysis. Significant differences were assumed when p<0.05 and all comparisons were performed using GraphPad Prism version 9.5.1 for Windows, GraphPad Software, San Diego, CA. USA)
3. Results
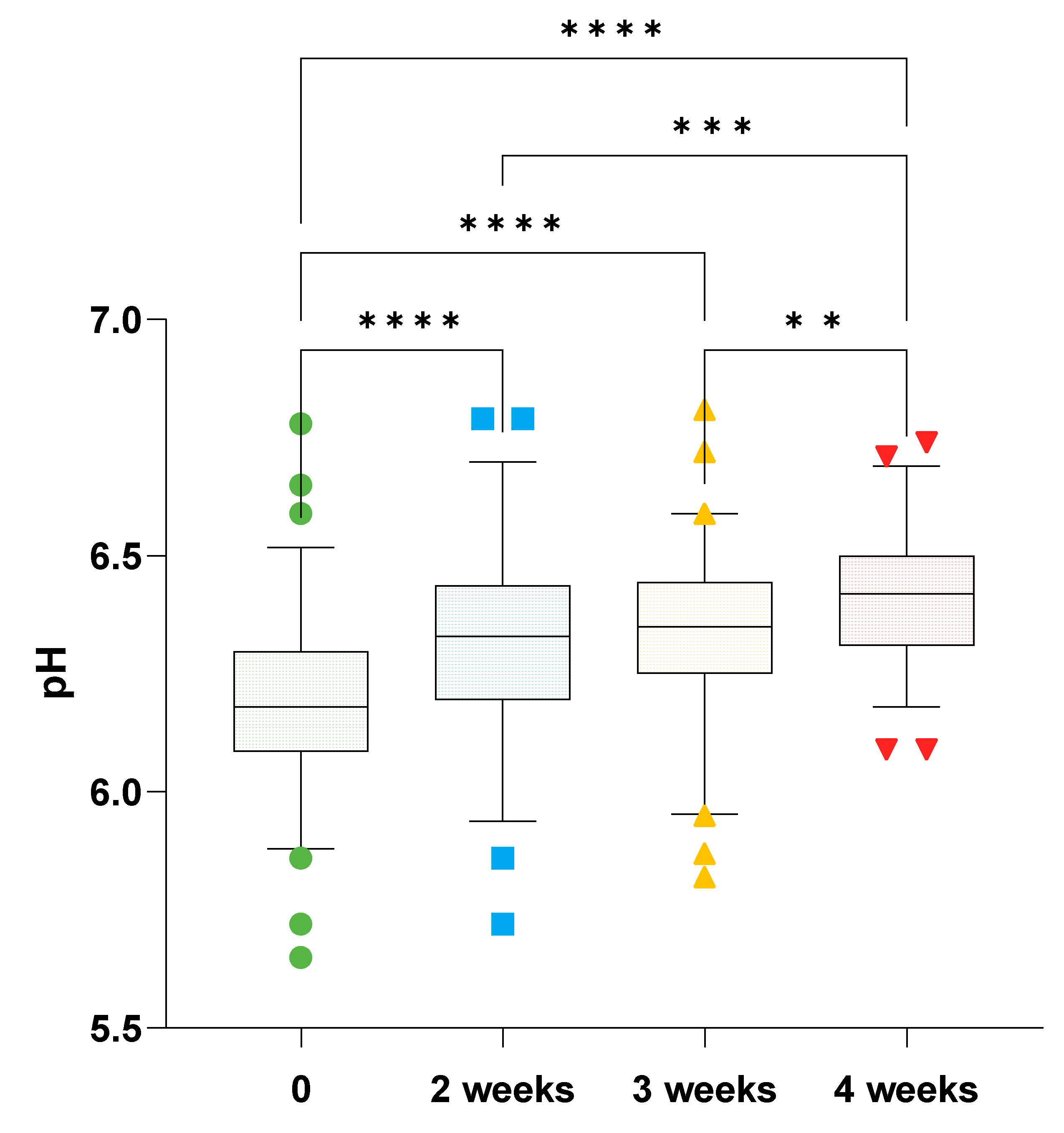
78 horses were recruited into the study across 8 trainers, with each trainer selected between 8 and 10 horses. Data from 6 horses was excluded from analysis since they did not remain in training at the same trainer during the course of the study. 52 horses were sampled at week 2, and 60 horses were sampled at week 3. All 72 horses were samples both at week 0 and week 4. Fecal pH was normally distributed (W=0.9). Fecal pH at the commencement of the study was 6.20 (6.15-6.24) and increased to 6.40 (6.38-6.44) after 4 weeks of treatment (p<0.0001). There was no difference between fecal pH when compared at one-week intervals, such that there were no differences between pH at week 3 compared to week 2 or between week 4 and week 3 (
Figure 1).
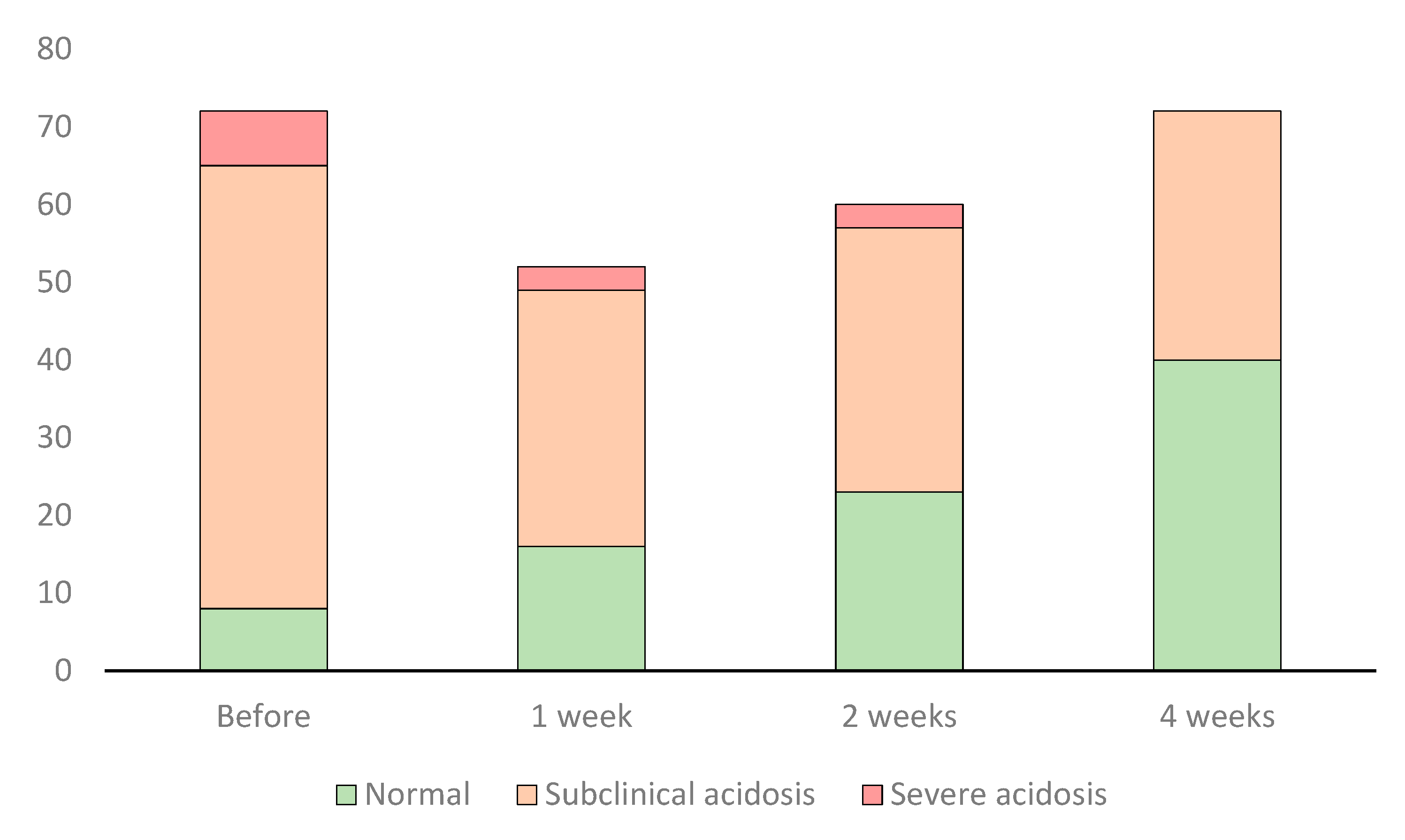
There were 8 horses (11%) with a normal fecal pH at the outset of the study, with a significant increase to 40 horses (56%) at the end of the study (
Figure 2; p<0.001), although a considerable number remained in the subclinical acidosis category. There were 7 horses in the severe acidosis category at the beginning of the study, but none at the end of the study.
4. Discussion
The study has demonstrated a significant reduction in fecal acidity in horses receiving enzyme rich malt extract. This demonstrates that fecal pH can be impacted by interventions that have been shown to impact the metabolome and microbiome. These changes correlate with the previous studies that have demonstrated changes in volatile organic compounds, including lactate [
24] when this supplement was included. While changes in pH were relatively slow, with no difference from week to week, these differences were observed after 2 weeks. The study ended after 4 weeks, and it is not clear whether pH would have continued to increase to the normal range had the study been continued for longer.
Most horses had relatively acidic feces compared to those of grazing horses at the start of the study [
22]. Previous studies have documented a normal pH in grazing horses in the range of 6.4-6.7 and that a pH between 6.0 and 6.4 of subclinical acidosis, while a pH below 6.0 has been associated with osmotic diarrhea[
23]. Clinical records about the fecal consistency were not made and it would have been useful to correlate these with clinical signs and performance in these horses. Many horses developed a pH that would be comparable to grazing horses during the course of the study, without any changes in diet. Again, whether more horses would have moved into the normal category of fecal pH had the study continued for longer is not clear, but worthy of future study.
Feeding of starch-based diets is a common feature of the diets in high-performance competition horses, and these factors can lead to overflow of water-soluble carbohydrates. Furthermore, fructans are relatively resistant to digestion in the small intestine [
26,
27,
28] and their breakdown to component parts within the large intestine. These factors can lead to change to rapid fermentation of these carbohydrates in the large intestine and accumulation of volatile organic compounds including lactate. Lactate is the primary driver of large intestinal acidosis that may subsequently impact the microbiome due to changes in the environment that alter bacterial proliferation [
10,
29]. It is assumed that the changes in fecal pH brought about by ERME dietary supplementation are related to these changes.
This pilot study did not consider a control population of horses, being fed a diet without supplementation and the validity of these conclusions would be greatly enhanced by such a study. Correlation between the pH changes and the microbiome and metabolome are warranted and a longer duration of intervention would determine whether the effect of ERME would be more pronounced in longer duration of supplementation.
5. Conclusions
Fecal pH could be a valuable tool for investigating interventions of the intestinal microbiome, although further validation is necessary. Enzyme rich malt extract supplementation reduces the presence of subclinical fecal acidosis in horses and may prove to be a valuable tool to improve morbidities associated with feeding the high performance athletic horse.
Author Contributions
Conceptualization, R.W. and B.N.; methodology, R.W.; software, B.N.; validation, M.B..; formal analysis, M.B..; investigation, T.J..; resources, B.N..; data curation, M.B..; writing—original draft preparation, M.B..; writing—review and editing, R.W..; visualization, M.B..; project administration, B.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Tharos Ltd.
Institutional Review Board Statement
Ethical review and approval were not required for this study due to the non-invasive nature of the sampling and routine use of the intervention in routine management of horses.
Data Availability Statement
Data available on request due to commercial restrictions
Acknowledgments
The authors acknowledge and thank the racehorse trainers for facilitating access to animals under their care.
Conflicts of Interest
The funders were involved in study design and the decision to publish the data including payment of APCs, but not in the analyses or interpretation of the data, nor in the preparation of the manuscript.
References
- Boerner, B.P.; Sarvetnick, N.E. Type 1 diabetes: role of intestinal microbiome in humans and mice. Ann. N. Y. Acad. Sci. 2011, 1243, 103–118. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- Moloney, R.D.; Desbonnet, L.; Clarke, G.; Dinan, T.G.; Cryan, J.F. The microbiome: stress, health and disease. Mamm. Genome 2014, 25, 49–74. [Google Scholar] [CrossRef]
- Nowland, T.L.; Plush, K.J.; Barton, M.; Kirkwood, R.N. Development and Function of the Intestinal Microbiome and Potential Implications for Pig Production. Animals 2019, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Ogunrinola, G.A.; Oyewale, J.O.; Oshamika, O.O.; Olasehinde, G.I. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol. 2020, 2020, e8045646. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef]
- Suchodolski, J.S. Analysis of the gut microbiome in dogs and cats. Vet. Clin. Pathol. 2022, 50, 6–17. [Google Scholar] [CrossRef]
- Wernimont, S. M.; et al. The Effects of Nutrition on the Gastrointestinal Microbiome of Cats and Dogs: Impact on Health and Disease. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Biddle, A.S.; Tomb, J.-F.; Fan, Z. Microbiome and Blood Analyte Differences Point to Community and Metabolic Signatures in Lean and Obese Horses. Front. Vet. Sci. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Biddle, A.S.; Black, S.J.; Blanchard, J.L. An In Vitro Model of the Horse Gut Microbiome Enables Identification of Lactate-Utilizing Bacteria That Differentially Respond to Starch Induction. PLOS ONE 2013, 8, e77599. [Google Scholar] [CrossRef]
- Garber, A.; Hastie, P.; Murray, J.-A. Factors Influencing Equine Gut Microbiota: Current Knowledge. J. Equine Vet. Sci. 2020, 88, 102943. [Google Scholar] [CrossRef]
- Gomez, A.; Sharma, A.K.; Grev, A.; Sheaffer, C.; Martinson, K. The Horse Gut Microbiome Responds in a Highly Individualized Manner to Forage Lignification. J. Equine Vet. Sci. 2021, 96, 103306. [Google Scholar] [CrossRef]
- Julliand, V.; Grimm, P. The Impact of Diet on the Hindgut Microbiome. J. Equine Vet. Sci. 2017, 52, 23–28. [Google Scholar] [CrossRef]
- Julliand, V.; Grimm, P. HORSE SPECIES SYMPOSIUM: The microbiome of the horse hindgut: History and current knowledge1. J. Anim. Sci. 2016, 94, 2262–2274. [Google Scholar] [CrossRef]
- Tuniyazi, M. Changes of microbial and metabolome of the equine hindgut during oligofructose-induced laminitis. BMC Vet. Res. 2021, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Leschine, S.B. Cellulose Degradation in Anaerobic Environments. Annu. Rev. Microbiol. 1995, 49, 399–426. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C. Alterations in the Fecal Microbiome and Metabolome of Horses with Antimicrobial-Associated Diarrhea Compared to Antibiotic-Treated and Non-Treated Healthy Case Controls. Animals 2021, 11, 1807. [Google Scholar] [CrossRef] [PubMed]
- Mullen, K.R.; Yasuda, K.; Divers, T.J.; Weese, J.S. Equine faecal microbiota transplant: Current knowledge, proposed guidelines and future directions. Equine Vet. Educ. 2018, 30, 151–160. [Google Scholar] [CrossRef]
- Muller, E.; Algavi, Y.M.; Borenstein, E. A meta-analysis study of the robustness and universality of gut microbiome-metabolome associations. Microbiome 2021, 9, 203. [Google Scholar] [CrossRef]
- Hydock, K.L.; Nissley, S.G.; Staniar, W.B. A standard protocol for fecal pH measurement in the horse. Prof. Anim. Sci. 2014, 30, 643–648. [Google Scholar] [CrossRef]
- Rowe, J.; Lees, M.; Pethick, D. Prevention of Acidosis and Laminitis Associated with Grain Feeding in Horses. J. Nutr. 1995, 124, 2742S–2744S. [Google Scholar] [CrossRef]
- van den Berg, M., Hoskin; Rogers, C. W.; Grinberg, A. Fecal pH and Microbial Populations in Thoroughbred Horses During Transition from Pasture to Concentrate Feeding. J. Equine Vet. Sci. 2013, 33, 215–222. [Google Scholar] [CrossRef]
- Kalantari, R.K.; Rouzbehan, Y.; Fazaeli, H.; Direkvandi, E.; Salem AZ, M. The Effect of Three Levels of Concentrate and Grain Processing on Feeding Behavior, Nutrient Digestibility, Blood Metabolites and Fecal pH Of Turkmen Horses. J. Equine Vet. Sci. 2021, 104, 103690. [Google Scholar] [CrossRef] [PubMed]
- Characterisation of the faecal metabolome and microbiome of Thoroughbred racehorses - Proudman - 2015 - Equine Veterinary Journal - Wiley Online Library. https://beva.onlinelibrary.wiley.com/doi/full/10.1111/evj.12324?casa_token=lVVziSuMwqsAAAAA%3AX mAO9-fVVITdKFmAGHLun13S6IBc8XJQZCBPkoPBCrqAsRSBsZPyNsMfSxkXFFgo_ea2ylXU1ExIN44_.
- Haworth, J.; Bloor, S.; Hobson, A. P230 Open label pilot study: an enzyme-rich malt extract (ERMETM) for the treatment of chronic constipation. Gut 2022, 71, A153–A153. [Google Scholar] [CrossRef]
- Crawford, C.; Sepulveda, M.F.; Elliott, J.; Harris, P.A.; Bailey, S.R. Dietary fructan carbohydrate increases amine production in the equine large intestine: Implications for pasture-associated laminitis1. J. Anim. Sci. 2007, 85, 2949–2958. [Google Scholar] [CrossRef]
- Strauch, S. Evaluation of an in vitro system to simulate equine foregut digestion and the influence of acidity on protein and fructan degradation in the horse′s stomach. J. Anim. Physiol. Anim. Nutr. 2017, 101, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Longland, A.C.; Byrd, B.M. Pasture Nonstructural Carbohydrates and Equine Laminitis. J. Nutr. 2006, 136, 2099S–2102S. [Google Scholar] [CrossRef] [PubMed]
- Al Jassim RA, M.; Andrews, F.M. The Bacterial Community of the Horse Gastrointestinal Tract and Its Relation to Fermentative Acidosis, Laminitis, Colic, and Stomach Ulcers. Vet. Clin. North Am. Equine Pract. 2009, 25, 199–215. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).