Submitted:
07 July 2023
Posted:
10 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
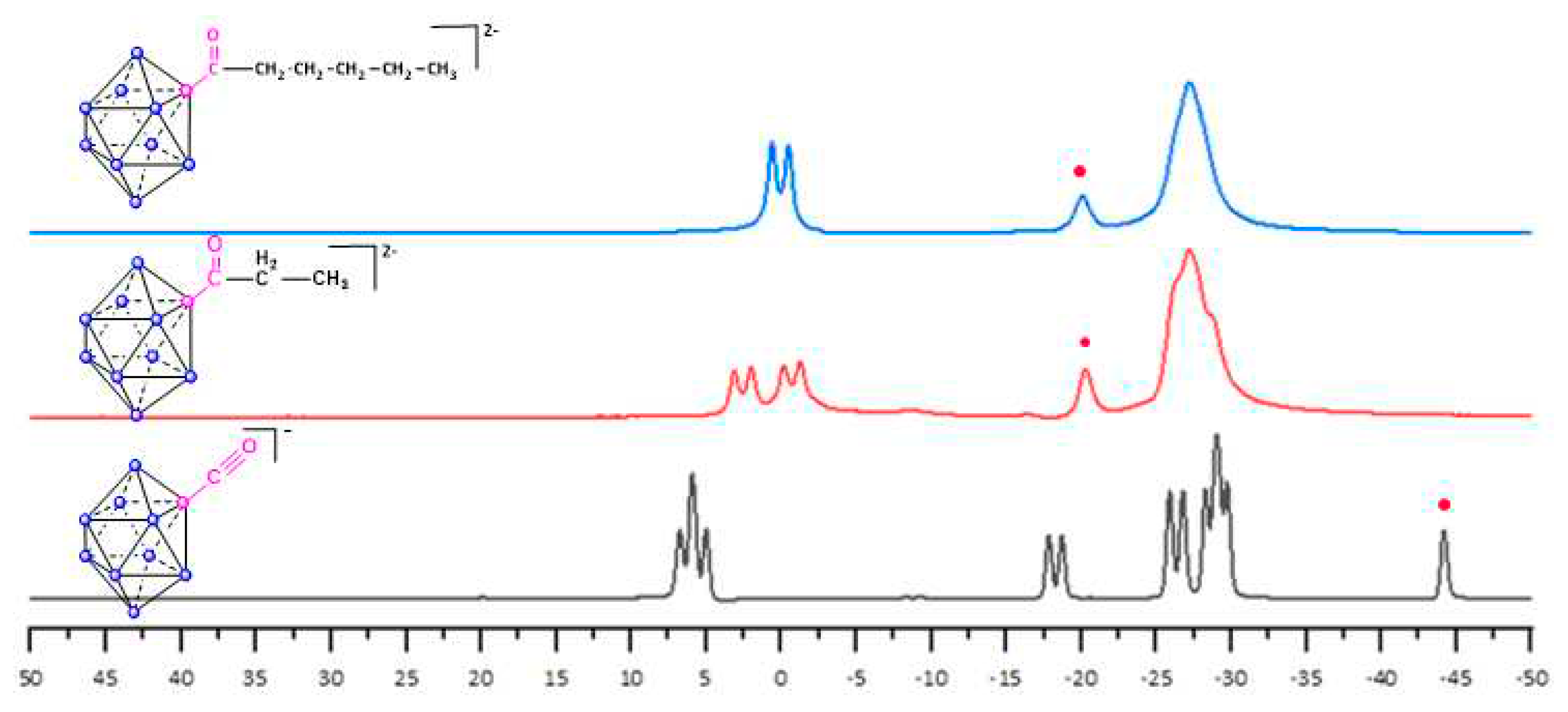
2. Results
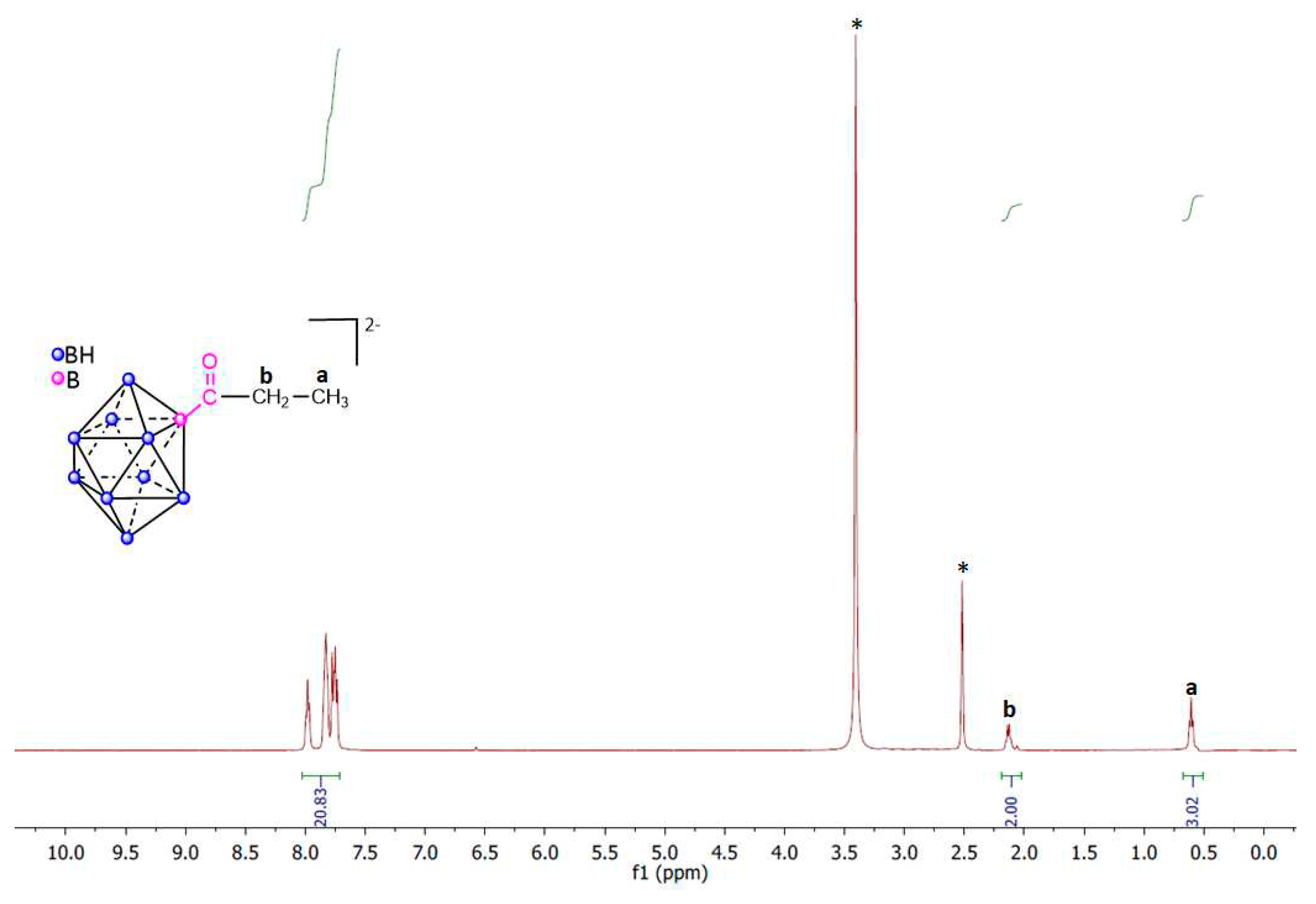
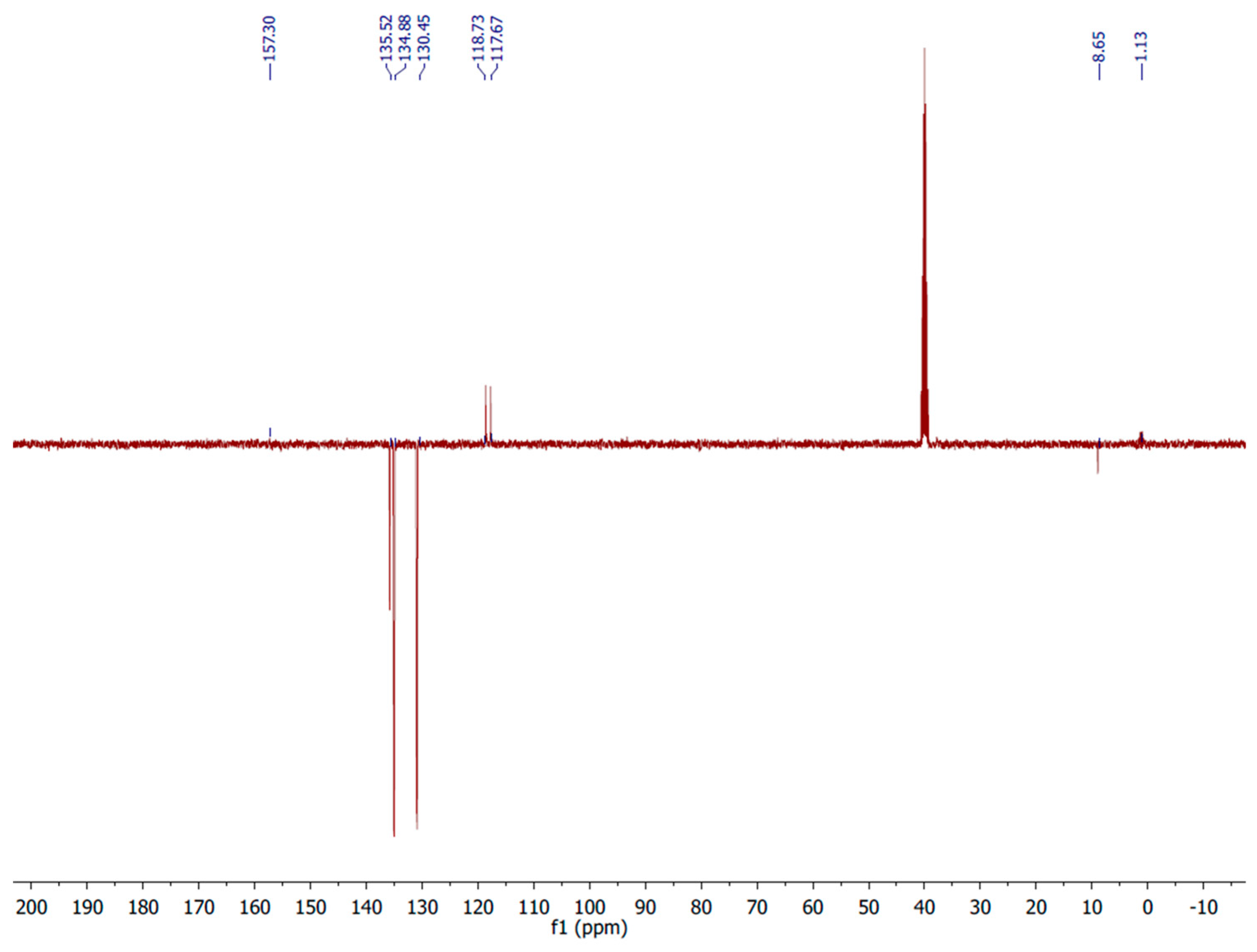
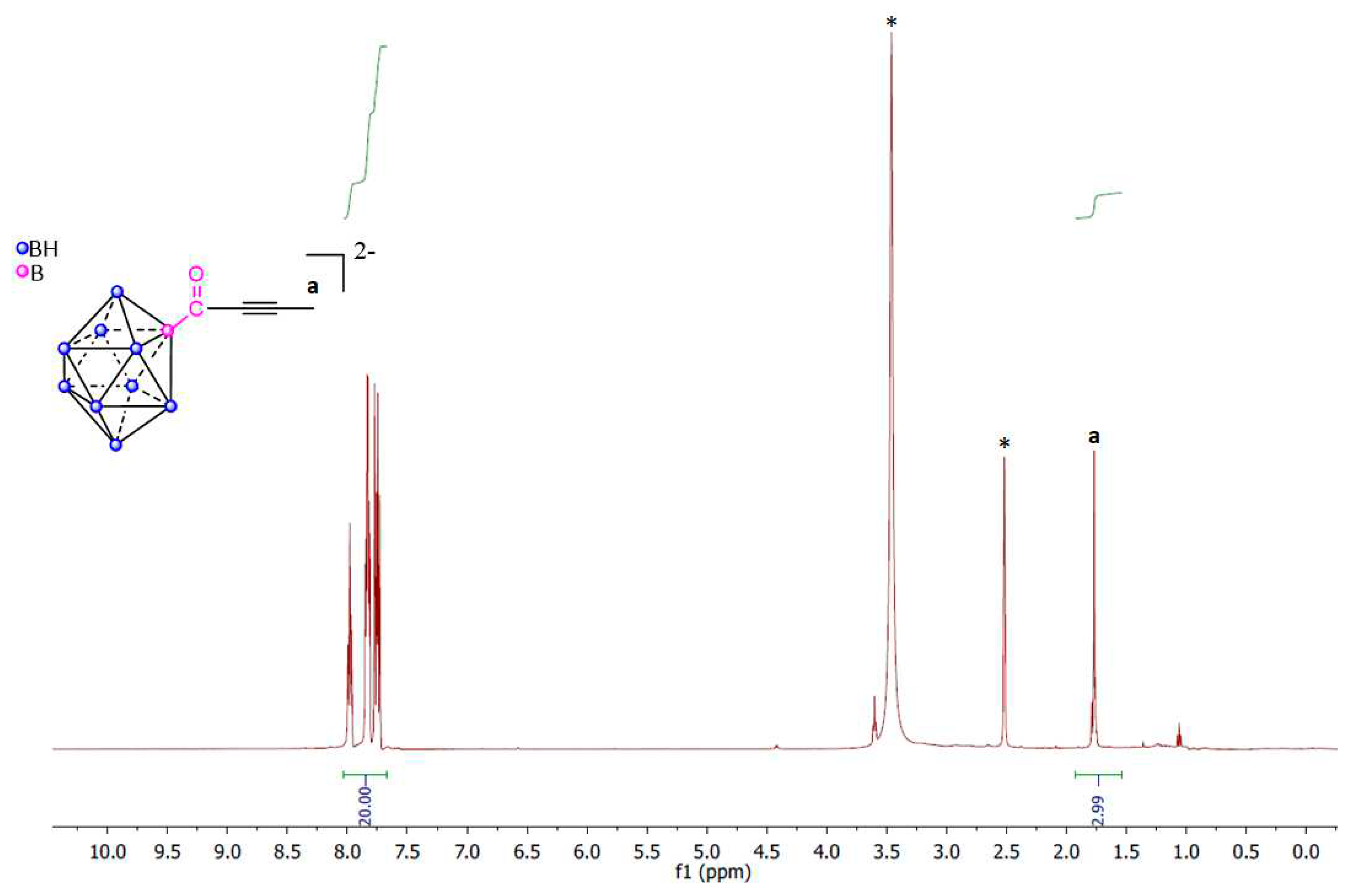
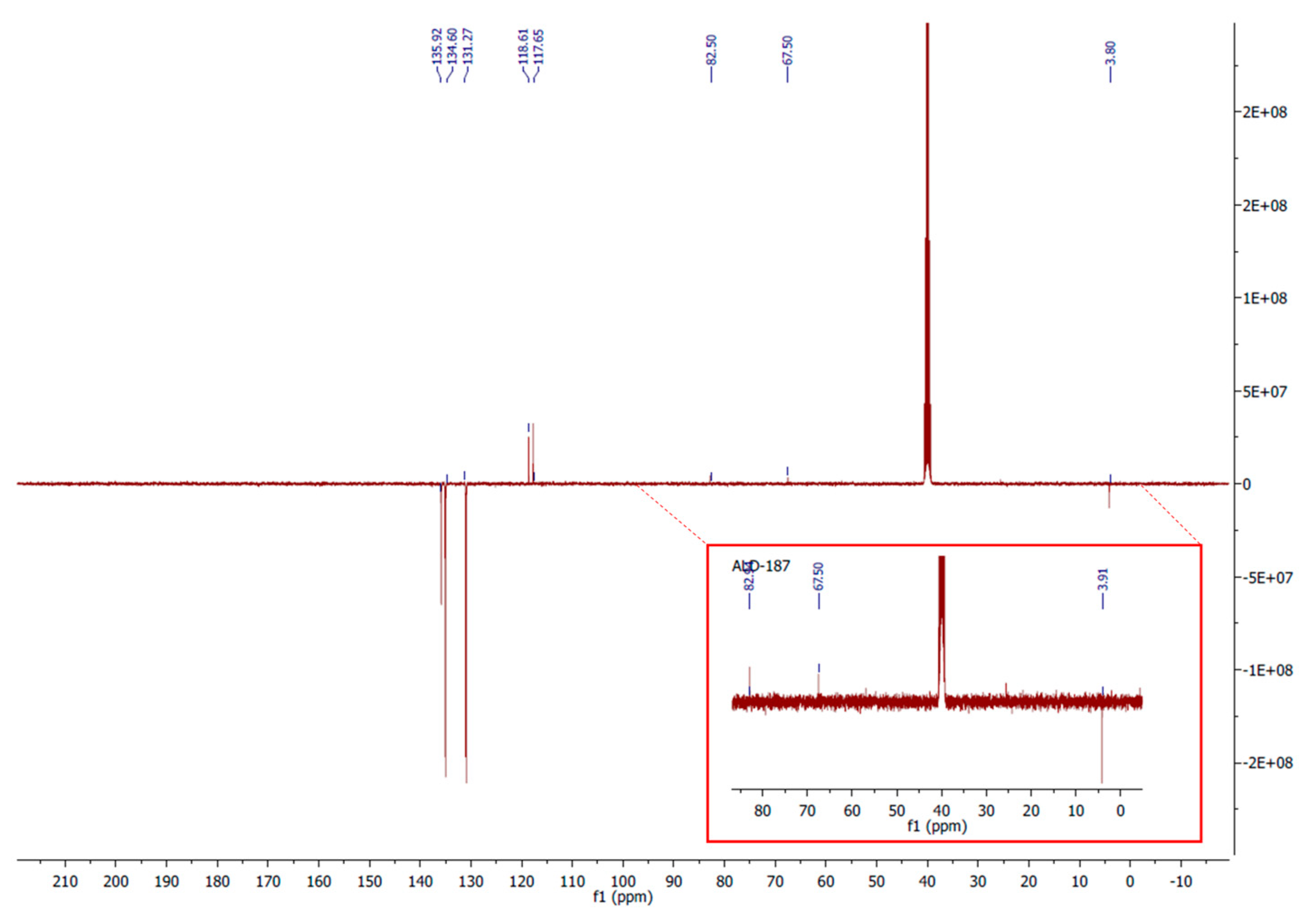
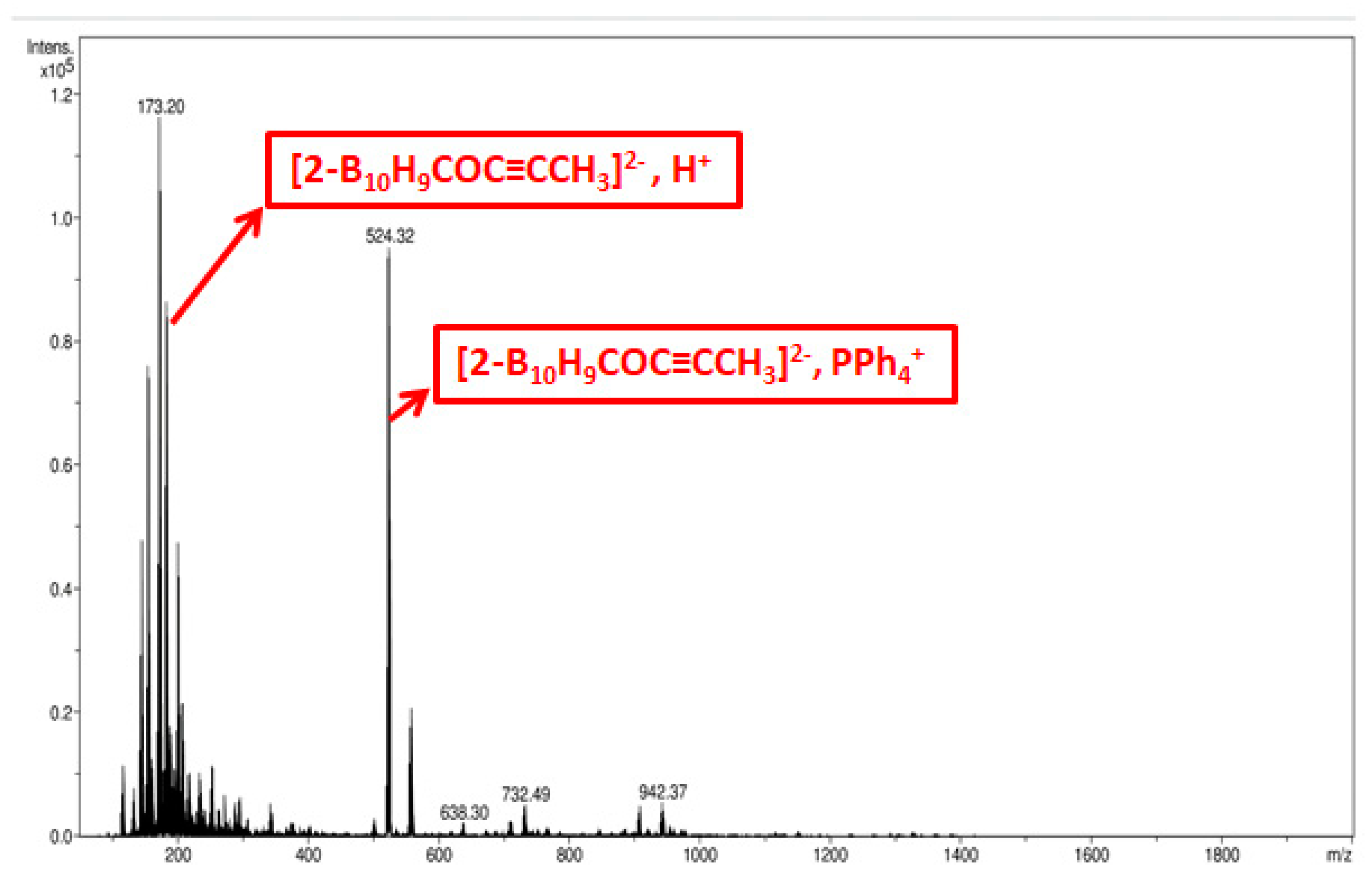
3. Materials and Methods
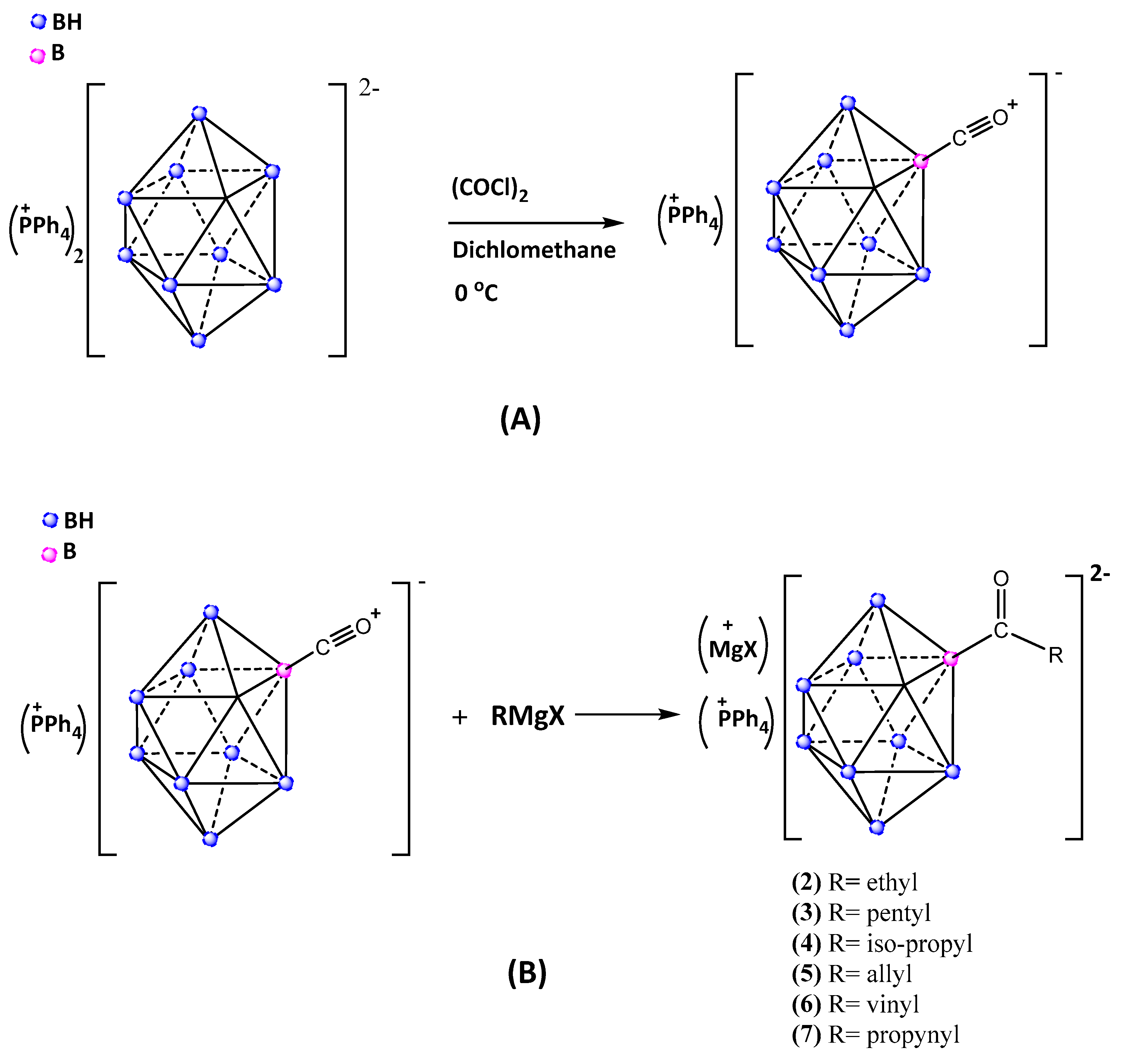
Synthesis of carbonyl-closo-decaborate (PPh4) [B10H9CO] (1)
Synthesis of closo-decaborate derivatives (PPh4)[B10H9COR]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
References
- Mingos, D.M.P. 50th Anniversary of Electron Counting Paradigms for Polyhedral Molecules: Historical and Recent Developments; Mingos, D.M.P., Ed. Springer International Publishing: 2022.
- Moury, R.; Łodziana, Z.; Remhof, A.; Duchêne, L.; Roedern, E.; Gigante, A.; Hagemann, H. Study of the Temperature- and Pressure-Dependent Structural Properties of Alkali Hydrido-closo-borate Compounds. Inorganic Chemistry 2022, 61, 5224–5233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, Z.; Chen, H.; Wang, Z.; Zhou, X.; Zhang, H. Progress in three-dimensional aromatic-like closo-dodecaborate. Coordination Chemistry Reviews 2021, 444, 214042. [Google Scholar] [CrossRef]
- Klyukin, I.N.; Vlasova, Y.S.; Novikov, A.S.; Zhdanov, A.P.; Zhizhin, K.Y.; Kuznetsov, N.T. Theoretical Study of closo-Borate Anions [BnHn]2− (n = 5–12): Bonding, Atomic Charges, and Reactivity Analysis. In Symmetry, 2021; Vol. 13.
- Voinova, V.V.; Klyukin, I.N.; Novikov, A.S.; Koz’menkova, A.Y.; Zhdanov, A.P.; Zhizhin, K.Y.; Kuznetsov, N.T. Electrochemical Properties of the closo-Decaborate Anion [B10H10]2– and a New Method for Preparation of the [B20H18]2– Anion. Russian Journal of Inorganic Chemistry 2021, 66, 295–304. [Google Scholar] [CrossRef]
- Green, M.; Kaydanik, K.; Orozco, M.; Hanna, L.; Marple, M.A.T.; Fessler, K.A.S.; Jones, W.B.; Stavila, V.; Ward, P.A.; Teprovich Jr, J.A. Closo-Borate Gel Polymer Electrolyte with Remarkable Electrochemical Stability and a Wide Operating Temperature Window. Advanced Science 2022, 9, 2106032. [Google Scholar] [CrossRef]
- Ali, F.; S Hosmane, N.; Zhu, Y. Boron Chemistry for Medical Applications. Molecules 2020, 25, 828. [Google Scholar] [CrossRef]
- Hu, K.; Yang, Z.; Zhang, L.; Xie, L.; Wang, L.; Xu, H.; Josephson, L.; Liang, S.H.; Zhang, M.-R. Boron agents for neutron capture therapy. Coordination Chemistry Reviews 2020, 405, 213139. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Malinina, E.A.; Kuznetsov, N.T. Boron cluster anions and their derivatives in complexation reactions. Coordination Chemistry Reviews 2022, 469, 214636. [Google Scholar] [CrossRef]
- Hosmane, N.S.; Eagling, R.D. Handbook Of Boron Science: With Applications In Organometallics, Catalysis, Materials And Medicine (In 4 Volumes); World Scientific Publishing Company: 2018.
- Abi-Ghaida, F.; Mehdi, A.; Naoufal, D. Boratosilane Precursors: Integration Into Hybrid Materials. 2: In Handbook of Boron Science, WORLD SCIENTIFIC (EUROPE), 2018. [Google Scholar] [CrossRef]
- Diab, M.; Mateo, A.; El Cheikh, J.; El Hajj, Z.; Haouas, M.; Ranjbari, A.; Guérineau, V.; Touboul, D.; Leclerc, N.; Cadot, E.; et al. Grafting of Anionic Decahydro-Closo-Decaborate Clusters on Keggin and Dawson-Type Polyoxometalates: Syntheses, Studies in Solution, DFT Calculations and Electrochemical Properties. In Molecules, 2022; Vol. 27.
- Diab, M.; Mateo, A.; Al Cheikh, J.; Haouas, M.; Ranjbari, A.; Bourdreux, F.; Naoufal, D.; Cadot, E.; Bo, C.; Floquet, S. Unprecedented coupling reaction between two anionic species of a closo-decahydrodecaborate cluster and an Anderson-type polyoxometalate. Dalton Transactions 2020, 49, 4685–4689. [Google Scholar] [CrossRef]
- Payandeh, S.; Asakura, R.; Avramidou, P.; Rentsch, D.; Łodziana, Z.; Černý, R.; Remhof, A.; Battaglia, C. Nido-Borate/Closo-Borate Mixed-Anion Electrolytes for All-Solid-State Batteries. Chemistry of Materials 2020, 32, 1101–1110. [Google Scholar] [CrossRef]
- Duchêne, L.; Kim, D.H.; Song, Y.B.; Jun, S.; Moury, R.; Remhof, A.; Hagemann, H.; Jung, Y.S.; Battaglia, C. Crystallization of closo-borate electrolytes from solution enabling infiltration into slurry-casted porous electrodes for all-solid-state batteries. Energy Storage Materials 2020, 26, 543–549. [Google Scholar] [CrossRef]
- Dobbins, T.A. Overview of the Structure-Dynamics-Function Relationships in Borohydrides for Use as Solid-State Electrolytes in Battery Applications. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Golub, I.E.; Filippov, O.A.; Kulikova, V.A.; Belkova, N.V.; Epstein, L.M.; Shubina, E.S. Thermodynamic Hydricity of Small Borane Clusters and Polyhedral closo-Boranes. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, S.; Dewhurst, R.D.; Ignat'ev, N.V.; Finze, M.; Braunschweig, H. Boron: Its Role in Energy-Related Processes and Applications. Angewandte Chemie International Edition 2020, 59, 8800–8816. [Google Scholar] [CrossRef] [PubMed]
- Avdeeva, V.V.; Garaev, T.M.; Malinina, E.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Physiologically Active Compounds Based on Membranotropic Cage Carriers–Derivatives of Adamantane and Polyhedral Boron Clusters (Review). Russian Journal of Inorganic Chemistry 2022, 67, 28–47. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Sivaev, I.B.; Bregadze, V.I. Nitrilium derivatives of polyhedral boron compounds (boranes, carboranes, metallocarboranes): Synthesis and reactivity. 2019, 194, 983–988. [Google Scholar] [CrossRef]
- Sedlák, D.; Wilson, T.A.; Tjarks, W.; Radomska, H.S.; Wang, H.; Kolla, J.N.; Leśnikowski, Z.J.; Špičáková, A.; Ali, T.; Ishita, K.; et al. Structure–Activity Relationship of para-Carborane Selective Estrogen Receptor β Agonists. Journal of Medicinal Chemistry 2021, 64, 9330–9353. [Google Scholar] [CrossRef]
- Jin, W.H.; Seldon, C.; Butkus, M.; Sauerwein, W.; Giap, H.B. A Review of Boron Neutron Capture Therapy: Its History and Current Challenges. Int J Part Ther 2022, 9, 71–82. [Google Scholar] [CrossRef]
- Yorov, K.E.; Zhdanov, A.P.; Kamilov, R.K.; Baranchikov, A.E.; Kopitsa, G.P.; Pokrovskiy, O.I.; Popov, A.L.; Ivanova, O.S.; Almásy, L.; Kolyagin, Y.G.; et al. [B10H10]2– Nanoclusters Covalently Immobilized to Hybrid SiO2 Aerogels for Slow Neutron Shielding Applications. ACS Applied Nano Materials 2022, 5, 11529–11538. [Google Scholar] [CrossRef]
- Abi-Ghaida, F.; Laila, Z.; Ibrahim, G.; Naoufal, D.; Mehdi, A. New triethoxysilylated 10-vertex closo-decaborate clusters. Synthesis and controlled immobilization into mesoporous silica. Dalton Transactions 2014, 43, 13087–13095. [Google Scholar] [CrossRef]
- Abi-Ghaida, F.; Clément, S.; Safa, A.; Naoufal, D.; Mehdi, A. Multifunctional Silica Nanoparticles Modified via Silylated-Decaborate Precursors. Journal of Nanomaterials 2015, 2015, 608432. [Google Scholar] [CrossRef]
- Stepanova, M.; Dobrodumov, A.; Averianov, I.; Gofman, I.; Nashchekina, J.; Guryanov, I.; Klyukin, I.; Zhdanov, A.; Korzhikova-Vlakh, E.; Zhizhin, K. Design, Fabrication and Characterization of Biodegradable Composites Containing Closo-Borates as Potential Materials for Boron Neutron Capture Therapy. Polymers 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, N.; Ghaida, F.A.; El Hajj, Z.; Diab, M.; Floquet, S.; Mehdi, A.; Naoufal, D. Recent Achievements on Functionalization within closo-Decahydrodecaborate [B10H10]2− Clusters. ChemistrySelect 2022, 7, e202200770. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Polyakova, I.N.; Churakov, A.V.; Vologzhanina, A.V.; Malinina, E.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Complexation and exopolyhedral substitution of the terminal hydrogen atoms in the decahydro-closo-decaborate anion in the presence of cobalt(II). Polyhedron 2019, 162, 65–70. [Google Scholar] [CrossRef]
- Kubasov, A.S.; Matveev, E.Y.; Turyshev, E.S.; Polyakova, I.N.; Bykov, A.Y.; Kopytin, A.V.; Zhizhin, K.Y.; Kuznetsov, N.T. Methods of Creating closo-Decaborate Anion Derivatives with Bridging and Terminal Exopolyhedral Cyclic Substituents of Sulfonium Type. Doklady Chemistry 2018, 483, 263–265. [Google Scholar] [CrossRef]
- Zhizhin, K.Y.; Zhdanov, A.P.; Kuznetsov, N.T. Derivatives of closo-decaborate anion [B10H10]2− with exo-polyhedral substituents. Russian Journal of Inorganic Chemistry 2010, 55, 2089–2127. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Prikaznov, A.V.; Naoufal, D. Fifty years of the closo -decaborate anion chemistry. Collection of Czechoslovak Chemical Communications 2010, 75, 1149–1199. [Google Scholar]
- Grimes, R.N. Carboranes; Academic Press: 2016.
- Marfavi, A.; Kavianpour, P.; Rendina, L.M. Carboranes in drug discovery, chemical biology and molecular imaging. Nature Reviews Chemistry 2022, 6, 486–504. [Google Scholar] [CrossRef]
- Flieger, S.; Takagaki, M.; Kondo, N.; Lutz, M.R.; Gupta, Y.; Ueda, H.; Sakurai, Y.; Moran, G.; Kempaiah, P.; Hosmane, N.; et al. Carborane-Containing Hydroxamate MMP Ligands for the Treatment of Tumors Using Boron Neutron Capture Therapy (BNCT): Efficacy without Tumor Cell Entry. International Journal of Molecular Sciences 2023, 24. [Google Scholar]
- Issa, F.; Kassiou, M.; Rendina, L.M. Boron in Drug Discovery: Carboranes as Unique Pharmacophores in Biologically Active Compounds. Chemical Reviews 2011, 111, 5701–5722. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, P.; Xu, J.; Ma, W.; Tu, D.; Lu, C.-s.; Yan, H. Direct B–H Functionalization of Icosahedral Carboranes via Hydrogen Atom Transfer. Journal of the American Chemical Society 2023, 145, 7638–7647. [Google Scholar] [CrossRef]
- Dziedzic, R.M.; Spokoyny, A.M. Metal-catalyzed cross-coupling chemistry with polyhedral boranes. Chem Commun (Camb) 2019, 55, 430–442. [Google Scholar] [CrossRef]
- Liu, F.; Chen, T.; Zhang, K.; Jiang, T.; Liu, J.; Duttwyler, S. Sonogashira coupling of the ethynyl monocarborane [CB11H11-12-CCH]−. Dalton Transactions 2022, 51, 10880–10886. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Winberg, K.J.; Claro, R.T.; Sjöberg, S. Palladium-Catalyzed Heck Reactions of Styrene Derivatives and 2-Iodo-p-carborane. The Journal of Organic Chemistry 2003, 68, 3569–3573. [Google Scholar] [CrossRef] [PubMed]
- Lennox, A.J.J.; Lloyd-Jones, G.C. Transmetalation in the Suzuki–Miyaura Coupling: The Fork in the Trail. Angewandte Chemie International Edition 2013, 52, 7362–7370. [Google Scholar] [CrossRef]
- Zheng, Z.; Jiang, W.; Zinn, A.A.; Knobler, C.B.; Hawthorne, M.F. Facile Electrophilic Iodination of Icosahedral Carboranes. Synthesis of Carborane Derivatives with Boron-Carbon Bonds via the Palladium-Catalyzed Reaction of Diiodocarboranes with Grignard Reagents. Inorganic Chemistry 1995, 34, 2095–2100. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, J.; Xie, Z. Direct Nucleophilic Substitution Reaction of Cage B−H Bonds by Grignard Reagents: A Route to Regioselective B4-Alkylation of o-Carboranes. Angewandte Chemie International Edition 2017, 56, 8642–8646. [Google Scholar] [CrossRef] [PubMed]
- El Anwar, S.; Laila, Z.; Ramsubhag, R.; Tlais, S.; Safa, A.; Dudley, G.; Naoufal, D. Synthesis and characterization of click-decahydrodecaborate derivatives by the copper(I) catalyzed [3+2] azide-alkyne cycloaddition reaction. Journal of Organometallic Chemistry 2018, 865, 89–94. [Google Scholar] [CrossRef]
- Laila, Z.; Yazbeck, O.; Ghaida, F.A.; Diab, M.; El Anwar, S.; Srour, M.; Mehdi, A.; Naoufal, D. Clean-activation of the B–H bond in closo-decahydrodecaborate [B10H10]2− anion via soft-route. Journal of Organometallic Chemistry 2020, 910, 121132. [Google Scholar] [CrossRef]
- Laila, Z.; Abi-Ghaida, F.; Al Anwar, S.; Yazbeck, O.; Jahjah, R.; Aoun, R.; Tlais, S.; Mehdi, A.; Naoufal, D. Study of the controlled temperature reaction between closo-decahydrodecaborate and alcohols in H 2SO 4 medium. Main Group Chemistry 2015, 14, 301–312. [Google Scholar] [CrossRef]
- Shelly, K.; Knobler, C.B.; Hawthorne, M.F. Synthesis of monosubstituted derivatives of closo-decahydrodecaborate (2-). X-ray crystal structures of [closo-2-B10H9CO]-and [closo-2-B10H9NCO] 2. Inorganic Chemistry 1992, 31, 2889–2892. [Google Scholar]
- Rzeszotarska, E.; Novozhilova, I.; Kaszyński, P. Convenient Synthesis of [closo-B10H9-1-I]2– and [closo-B10H8-1,10-I2]2– Anions. Inorganic Chemistry 2017, 56, 14351–14356. [Google Scholar] [CrossRef] [PubMed]
- Klyukin, I.N.; Kolbunova, A.V.; Selivanov, N.A.; Bykov, A.Y.; Zhdanov, A.P.; Zhizhin, K.Y.; Kuznetsov, N.T. Study of Protonation of the Monocarbonyl Derivative of the closo-Decaborate Anion [B10H9CO]–. Russian Journal of Inorganic Chemistry 2021, 66, 1798–1801. [Google Scholar] [CrossRef]
- Klyukin, I.N.; Selivanov, N.A.; Bykov, A.Y.; Zhdanov, A.P.; Zhizhin, K.Y.; Kuznetsov, N.T. Synthesis and Physicochemical Properties of C-Borylated Amides Based on the closo-Decaborate Anion. Russian Journal of Inorganic Chemistry 2019, 64, 1405–1409. [Google Scholar] [CrossRef]
- Fink, K.; Uchman, M. Boron cluster compounds as new chemical leads for antimicrobial therapy. Coordination Chemistry Reviews 2021, 431, 213684. [Google Scholar] [CrossRef]
- Li, J.; Sun, Q.; Lu, C.; Xiao, H.; Guo, Z.; Duan, D.; Zhang, Z.; Liu, T.; Liu, Z. Boron encapsulated in a liposome can be used for combinational neutron capture therapy. Nature Communications 2022, 13, 2143. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




