Submitted:
07 July 2023
Posted:
10 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study design and mining contexts
2.2. Participants and data collection
2.3. Pesticides in blood and urine
2.4. Element mixtures in hair
2.5. Citogenetic analysis
2.6. Statistical methods
3. Results
3.1. Mean characteristics of participants
3.2. Pesticide exposure
3.2. Chemical element mixtures in hair
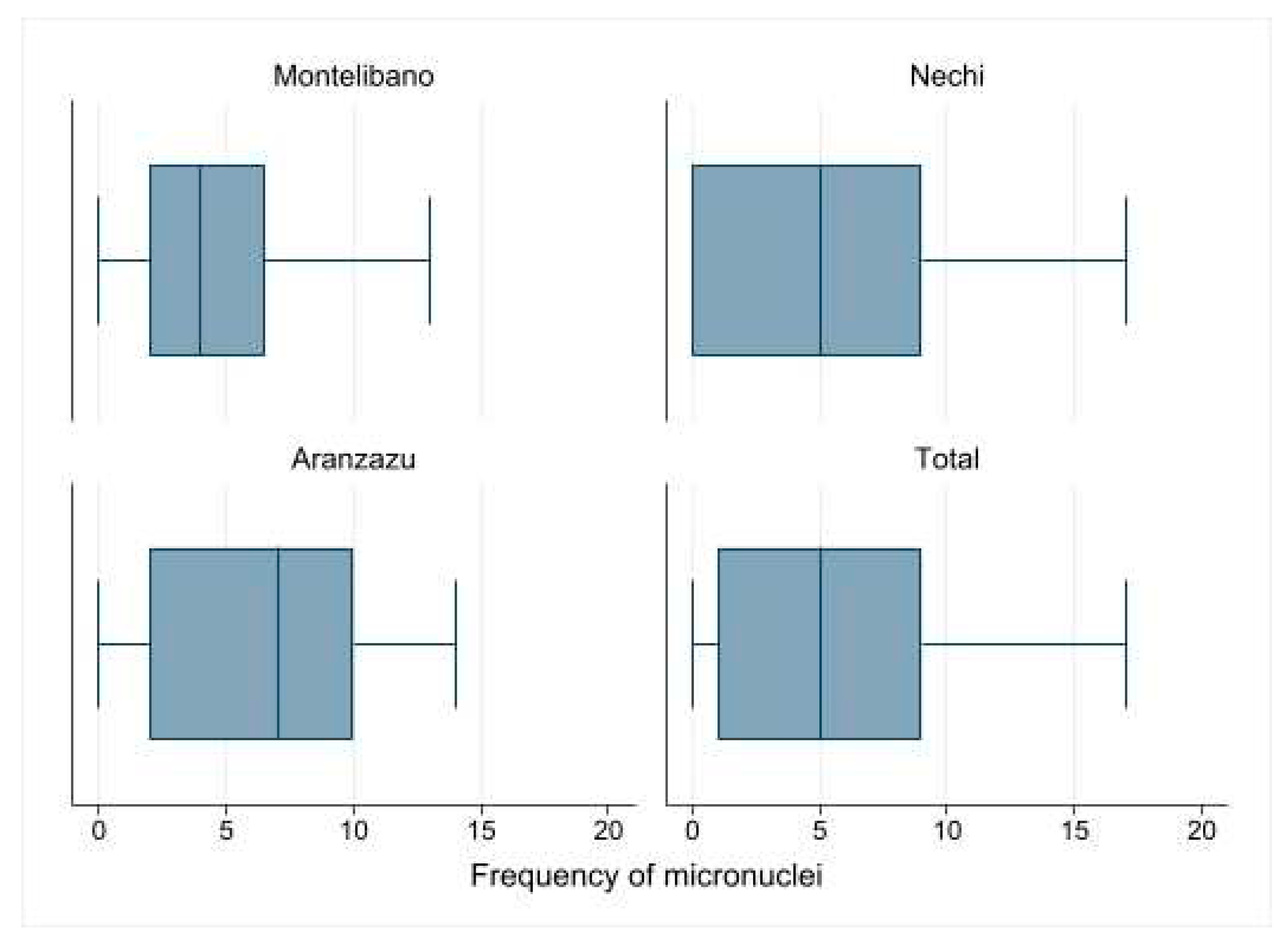
3.3. Micronuclei frequency
3.4. Bivariate analysis
3.5. Multiple analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Kaur, K.; Kaur, R. Occupational Pesticide Exposure, Impaired DNA Repair, and Diseases. Indian J Occup Environ Med 2018, 22, 74. [Google Scholar] [CrossRef]
- Stanaway, J.D.; Afshin, A.; Gakidou, E.; Lim, S.S.; Abate, D.; Abate, K.H.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; et al. Global, Regional, and National Comparative Risk Assessment of 84 Behavioural, Environmental and Occupational, and Metabolic Risks or Clusters of Risks for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. The Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef]
- Damalas, C.; Koutroubas, S. Farmers’ Exposure to Pesticides: Toxicity Types and Ways of Prevention. Toxics 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to Pesticides and the Associated Human Health Effects. Sci Total Environ 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An Extensive Review on the Consequences of Chemical Pesticides on Human Health and Environment. Journal of Cleaner Production 2021, 283, 124657. [Google Scholar] [CrossRef]
- Cotton, J.; Edwards, J.; Rahman, M.A.; Brumby, S. Cholinesterase Research Outreach Project (CROP): Point of Care Cholinesterase Measurement in an Australian Agricultural Community. Environ Health 2018, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Imran, I.; Ansari, A.; Saleem, S.; Azhar, A.; Zehra, S. Insights of OPs and PYR Cytotoxic Potential Invitro and Genotoxic Impact on PON1 Genetic Variant among Exposed Workers in Pakistan. Sci Rep 2022, 12, 9498. [Google Scholar] [CrossRef]
- Marcelino, A.F.; Wachtel, C.C.; Ghisi, N. de C. Are Our Farm Workers in Danger? Genetic Damage in Farmers Exposed to Pesticides. Int J Environ Res Public Health 2019, 16, 358. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Pesticides: An Update of Human Exposure and Toxicity. Arch Toxicol 2017, 91, 549–599. [Google Scholar] [CrossRef]
- Ofosu, G.; Dittmann, A.; Sarpong, D.; Botchie, D. Socio-Economic and Environmental Implications of Artisanal and Small-Scale Mining (ASM) on Agriculture and Livelihoods. Environmental Science & Policy 2020, 106, 210–220. [Google Scholar] [CrossRef]
- Valbuena, D.; Cely-Santos, M.; Obregón, D. Agrochemical Pesticide Production, Trade, and Hazard: Narrowing the Information Gap in Colombia. Journal of Environmental Management 2021, 286, 112141. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, A.; Chaparro-Narváez, P.; Morales-Plaza, C.D.; Alzate, A.; Padilla, J.; Arévalo, M.; Herrera, S. Malaria in Gold-Mining Areas in Colombia. Mem Inst Oswaldo Cruz 2016, 111, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza-Bastida, A.; Valladares-Carranza, B.; Ortega-Santana, C.; Zamora-Espinosa, J.; Velázquez-Ordoñez, V.; Aparicio-Burgos, J. Repercusiones del uso de los organoclorados sobre el ambiente y salud pública. AbanicoVet 2016, 6, 43–55. [Google Scholar]
- Varona, M.E.; Díaz-Criollo, S.M.; Lancheros-Bernal, A.R.; Murcia-Orjuela, A.M.; Henao-Londoño, G.L.; Idrovo, A.J. Organochlorine Pesticide Exposure among Agricultural Workers in Colombian Regions with Illegal Crops: An Exploration in a Hidden and Dangerous World. Int J Environ Health Res 2010, 20, 407–414. [Google Scholar] [CrossRef] [PubMed]
- García Ubaque, C.A.; García Ubaque, J.C.; Vaca Bohórquez, M.L. Compuestos orgánicos persistentes en Colombia: cuantificacióny diagnóstico para pesticidas organoclorados. RT 2015, 19, 163. [Google Scholar] [CrossRef]
- Ancizar-Sordo, J. Occurrence of Selenium in Soils and Plants of Colombia, South America. Soil Science 1947, 63, 437–438. [Google Scholar] [CrossRef]
- Spiller, H.A.; Hays, H.L.; Casavant, M.J. Rethinking Treatment of Mercury Poisoning: The Roles of Selenium, Acetylcysteine, and Thiol Chelators in the Treatment of Mercury Poisoning: A Narrative Review. Toxicology Communications 2021, 5, 19–59. [Google Scholar] [CrossRef]
- Rodriguez-Villamizar, L.A.; Medina, O.M.; Flórez-Vargas, O.; Vilanova, E.; Idrovo, A.J.; Araque-Rodriguez, S.A.; Henao, J.A.; Sánchez-Rodríguez, L.H. Chemical Element Mixtures and Kidney Function in Mining and Non-Mining Settings in Northern Colombia. Int J Environ Res Public Health 2023, 20, 2321. [Google Scholar] [CrossRef]
- Braun, J.M.; Gennings, C.; Hauser, R.; Webster, T.F. What Can Epidemiological Studies Tell Us about the Impact of Chemical Mixtures on Human Health? Environmental Health Perspectives 2016, 124, A6–A9. [Google Scholar] [CrossRef]
- Martin-Reina, J.; Casanova, A.G.; Dahiri, B.; Fernández, I.; Fernández-Palacín, A.; Bautista, J.; Morales, A.I.; Moreno, I. Adverse Health Effects in Women Farmers Indirectly Exposed to Pesticides. Int J Environ Res Public Health 2021, 18, 5909. [Google Scholar] [CrossRef]
- Cordy, P.; Veiga, M.M.; Salih, I.; Al-Saadi, S.; Console, S.; Garcia, O.; Mesa, L.A.; Velásquez-López, P.C.; Roeser, M. Mercury Contamination from Artisanal Gold Mining in Antioquia, Colombia: The World’s Highest per Capita Mercury Pollution. Science of The Total Environment 2011, 410–411, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Idrovo, A.J.; Rivero-Rubio, C.; Amaya-Castellanos, C.; Idrovo, A.J.; Rivero-Rubio, C.; Amaya-Castellanos, C. Perception of Pollution and Arsenic in Hair of Indigenous Living near a Ferronickel Open-Pit Mine (Córdoba, Colombia): Public Health Case Report. Revista de la Universidad Industrial de Santander. Salud 2017, 49, 115–123. [Google Scholar] [CrossRef]
- Idrovo, A.J. Cerro Matoso Mine, Chemical Mixtures, and Environmental Justice in Colombia. The Lancet 2018, 391, 2320. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Niño, J.A.; Idrovo, A.J.; Cucunubá, Z.M.; Reyes-Harker, P.; Guerra, Á.P.; Moncada, L.I.; López, M.C.; Barrera, S.M.; Cortés, L.J.; Olivera, M.; et al. Paradoxical Associations between Soil-Transmitted Helminths and Plasmodium Falciparum Infection. Trans R Soc Trop Med Hyg 2012, 106, 701–708. [Google Scholar] [CrossRef]
- Bonilla Ayala, S.; Rojas, A.; Rojas Velandia, C.; Figueroa Salamanca, H.; Idrovo, A. Dimercaprol and Penicillamine in Occupational Hydrargyrism in Aranzazu: Birth of Occupational Toxicology in Colombia (Submitted).
- Word Health Organization Guidance for Identifying Populations at Risk from Mercury Exposure. Available online: https://www.who.int/publications/m/item/guidance-for-identifying-populations-at-risk-from-mercury-exposure (accessed on 28 June 2023).
- Shin, Y.; Lee, J.; Park, E.; Lee, J.; Lee, H.S.; Kim, J.-H. A Quantitative Tandem Mass Spectrometry and Scaled-Down QuEChERS Approach for Simultaneous Analysis of Pesticide Multiresidues in Human Urine. Molecules 2019, 24, 1330. [Google Scholar] [CrossRef] [PubMed]
- Kales, S.; Christiani, D. Hair and Metal Toxicity. In Hair in Toxicology; Tobin, D.J., Ed.; The Royal Society of Chemistry, 2005; pp. 125–158. ISBN 978-0-85404-587-7. [Google Scholar]
- Nunes, J.A.; Batista, B.L.; Rodrigues, J.L.; Caldas, N.M.; Neto, J.A.G.; Barbosa, F. A Simple Method Based on ICP-MS for Estimation of Background Levels of Arsenic, Cadmium, Copper, Manganese, Nickel, Lead, and Selenium in Blood of the Brazilian Population. J Toxicol Environ Health A 2010, 73, 878–887. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-Block Micronucleus Cytome Assay. Nat Protoc 2007, 2, 1084–1104. [Google Scholar] [CrossRef]
- Widaman, K.F. Common Factor Analysis Versus Principal Component Analysis: Differential Bias in Representing Model Parameters? Multivariate Behav Res 1993, 28, 263–311. [Google Scholar] [CrossRef]
- Barros, A.J.D.; Hirakata, V.N. Alternatives for Logistic Regression in Cross-Sectional Studies: An Empirical Comparison of Models That Directly Estimate the Prevalence Ratio. BMC Med Res Methodol 2003, 3, 21. [Google Scholar] [CrossRef]
- Stafoggia, M.; Breitner, S.; Hampel, R.; Basagaña, X. Statistical Approaches to Address Multi-Pollutant Mixtures and Multiple Exposures: The State of the Science. Curr Environ Health Rep 2017, 4, 481–490. [Google Scholar] [CrossRef]
- Ramos, S.J.; Dinali, G.S.; Oliveira, C.; Martins, G.C.; Moreira, C.G.; Siqueira, J.O.; Guilherme, L.R.G. Rare Earth Elements in the Soil Environment. Curr Pollution Rep 2016, 2, 28–50. [Google Scholar] [CrossRef]
- Gwenzi, W.; Mangori, L.; Danha, C.; Chaukura, N.; Dunjana, N.; Sanganyado, E. Sources, Behaviour, and Environmental and Human Health Risks of High-Technology Rare Earth Elements as Emerging Contaminants. Science of The Total Environment 2018, 636, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. Inorganics and Hormesis. Critical Reviews in Toxicology 2003, 33, 215–304. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rincón, M.P.; Peralta-Ardila, M.; Vélez-Torres, I.; Méndez, F. Conflicto Armado Interno y Ambiente en Colombia: Análisis desde los Conflictos Ecológicos, 1960-2016. J Pol Ecol 2022, 29, 672–703. [Google Scholar] [CrossRef]
- Albarracín, J.; Milanese, J.P.; Valencia, I.H.; Wolff, J. Local Competitive Authoritarianism and Post-Conflict Violence. An Analysis of the Assassination of Social Leaders in Colombia. Int Interact 2022, 49, 237–267. [Google Scholar] [CrossRef]
- Bedoya, G.; García, J.; Montoya, P.; Rojas, W.; Amézquita, M.E.; Soto, I.; López, M.C.; Ospina-Duque, J.; Ruiz-Linares, A. Análisis de isonimia entre poblaciones del noroeste de Colombia. Biomedica 2006, 26, 538. [Google Scholar] [CrossRef] [PubMed]
- Bedoya P, S.; García R, A.; Londoño F, Á.L.; Restrepo C, B. Determinación de residuos de plaguicidas organoclorados en suero sanguíneo de trabajadores de cultivo de Café y plátano en el departamento del quindío por gc-µecd. Revista Colombiana de Química 2014, 43, 11–16. [Google Scholar] [CrossRef]
- Espitia-Pérez, L.; Sosa, M.Q.; Salcedo-Arteaga, S.; León-Mejía, G.; Hoyos-Giraldo, L.S.; Brango, H.; Kvitko, K.; da Silva, J.; Henriques, J.A.P. Polymorphisms in Metabolism and Repair Genes Affects DNA Damage Caused by Open-Cast Coal Mining Exposure. Mutat Res Genet Toxicol Environ Mutagen 2016, 808, 38–51. [Google Scholar] [CrossRef]
- Guerrero-Castilla, A.; Olivero-Verbel, J.; Marrugo-Negrete, J. Heavy Metals in Wild House Mice from Coal-Mining Areas of Colombia and Expression of Genes Related to Oxidative Stress, DNA Damage and Exposure to Metals. Mutat Res Genet Toxicol Environ Mutagen 2014, 762, 24–29. [Google Scholar] [CrossRef]
- Vélez-Torres, I.; Vanegas, D. Contentious Environmental Governance in Polluted Gold Mining Geographies: The Case of La Toma, Colombia. World Development 2022, 157, 105953. [Google Scholar] [CrossRef]
| Site(Minerals) | Montelibano (Fe/Ni) | Nechí (Au) | Aranzazu (Closed Hg) | |
|---|---|---|---|---|
| Variables | (n=76) % / median (min-max) | (n=84)% / median (min-max) | (n=87)% / median (min-max) | P value |
| Sex (female) | 80.26 | 72.62 | 60.92 | 0.023 |
| Age (years) | 54 (21 – 81) | 48 (21 – 83) | 40 (19 – 83) | <0.001a |
| Civil status | ||||
| Married / Free union | 85.53 | 65.48 | 70.11 | 0.029 |
| Divorced / Widower | 2.63 | 7.14 | 10.34 | |
| Single | 11.84 | 27.38 | 19.54 | |
| Education | ||||
| Illiteracy | 2.63 | 11.90 | 5.75 | <0.001 |
| Elementary (partial or full) | 13.16 | 39.29 | 39.08 | |
| Secondary (partial or full) | 31.58 | 22.62 | 37.93 | |
| Technic (partial or full) | 35.53 | 19.05 | 4.60 | |
| University (partial or full) | 17.11 | 7.14 | 12.64 | |
| Occupation | ||||
| In mining activities | 52.63 | 44.05 | 0 | <0.001 |
| In agricultural activities | 6.58 | 22.62 | 45.98 | <0.001 |
| Cigarette consumption (any moment) | 21.05 | 39.29 | 43.68 | 0.007 |
| Alcohol consumption (any moment) | 61.84 | 44.05 | 40.23 | 0.015 |
| Food consumption | ||||
| Fish | 97.37 | 91.67 | 71.26 | <0.001 |
| Canned food | 56.58 | 48.81 | 60.92 | 0.274 |
| White meat | 97.37 | 92.86 | 96.55 | 0.328 |
| Red meat | 94.74 | 94.05 | 96.55 | 0.734 |
| Fruits | 96.05 | 94.05 | 94.25 | 0.825 |
| Vegetables | 98.68 | 97.62 | 94.25 | 0.242 |
| Carcinogenic elements* (IARC 1) | ||||
| Arsenic | 0.14 (0.05 – 4.28) | 0.18 (0.06 – 1.64) | 0.12 (0.05 – 0.36) | <0.001a |
| Beryllium | 0 (0 – 16.06) | 0 (0 – 0.07) | 0 (0 – 0.02) | <0.001a |
| Cadmium | 0.05 (0 – 1.69) | 0.04 (0.01 – 0.48) | 0.05 (0.01 – 0.94) | 0.395a |
| Chromium | 0.51 (0.18 – 19.06) | 0.33 (0.18 – 7.25) | 0.33 (0.20 – 3.53) | <0.001a |
| Strontium | 2.09 (0.1 – 55.42) | 3.49 (0.33 – 46.21) | 2.97 (0.19 – 46.13) | 0.011a |
| Nickel | 0.45 (0.05 – 17.77) | 0.25 (0.05 – 2.07) | 0.14 (0.02 – 3.92) | <0.001a |
| Thorium | 0 (0 – 0.27) | 0 (0 – 0.04) | 0 (0 – 0.01) | <0.001a |
| Probably carcinogenic* (IARC 2A) | ||||
| Antimony | 0.03 (0.00 – 0.35) | 0.02 (0.00 – 0.35) | 0.01 (0.00 – 0.29) | 0.040a |
| Cobalt | 0.04 (0.00 – 4.46) | 0.03 (0.01 – 1.14) | 0.01 (0.00 – 0.13) | <0.001a |
| Indium | 0 (0 – 0.002) | 0 | 0 (0 – 0.004) | 0.3484 |
| Magnesium | 77.90 (12.97 – 1449) | 105.81 (19.44 – 2131) | 49.73 (9.51 – 608.74) | <0.001a |
| Manganese | 1.66 (0.06 – 92.63) | 3.09 (0.15 – 46.54) | 0.80 (0.10 – 11.50) | <0.001a |
| Silica | 0 (0 -7598) | 0 (0 – 4495) | 1201 (0 – 3913) | <0.001a |
| Possibly carcinogenic* (IARC 2B) | ||||
| Mercury | 1.89 (0.06 – 12.29) | 2.48 (0.17 – 17.14) | 0.08 (0 – 1.63) | <0.001a |
| Molybdenum | 0.05 (0.01 – 1.40) | 0.06 (0.02 – 0.36) | 0.04 (0.02 – 0.24) | <0.001a |
| Lead | 0.92 (0.04 – 89.01) | 0.64 (0.08 – 75.17) | 0.55 (0.06 – 163.37) | 0.761a |
| Potassium | 1.83 (0 – 155.47) | 3.98 (0 – 106.10) | 1.08 (0 – 129.28) | <0.001a |
| Sodium | 3.12 (0.47 – 238.26) | 3.94 (0.49 – 701.25) | 1.44 (0.30 – 379.54) | <0.001a |
| Titanium | 0.13 (0 – 3.16) | 0.24 (0.09 – 1.24) | 0.18 (0.08 – 0.60) | <0.001a |
| Vanadium | 0.05 (0.00 – 1.41) | 0.12 (0.03 – 0.85) | 0.05 (0.02 – 0.45) | <0.001a |
| Other elements | ||||
| Phosphorus | 219 (66 – 2883) | 218 (111 – 342) | 205 (148 – 297) | 0.114a |
| Selenium | 1.35 (0.23 – 14.70) | 1.40 (0.46 – 60.69) | 1.01 (0.41 – 1.44) | <0.001a |
| Montelibano(Fe/Ni open-pit mine) | Concentrations (ppm) | ||||||||
| Positives | Percentiles | ||||||||
| Pesticide | Sample | % | Min | 25 | 90 | 95 | 99 | Max | |
| Paraoxon-methyl | Blood | 27.55 | ND | ND | 0.24 | 0.29 | 37.26 | 37.26 | |
| Parathion-ethyl | Blood | 1.02 | ND | ND | ND | ND | ND | 1.02 | |
| Paraoxon-ethyl | Urine | 2.04 | ND | ND | ND | ND | 0.06 | 0.06 | |
| Parathion-ethyl | Urine | 2.04 | ND | ND | ND | ND | 0.07 | 0.07 | |
| Parathion-methyl | Urine | 2.04 | ND | ND | ND | ND | 0.07 | 0.07 | |
| Propoxur | Urine | 2.04 | ND | ND | ND | ND | 0.13 | 0.44 | |
| Nechí(Au fluvial mines) | Concentrations (ppm) | ||||||||
| Positives | Percentiles | ||||||||
| Pesticide | Sample | % | Min | 25 | 90 | 95 | 99 | Max | |
| Paraoxon-ethyl | Blood | 0.99 | ND | ND | ND | ND | ND | 0.75 | |
| Parathion-ethyl | Urine | 0.99 | ND | ND | ND | ND | ND | 0.05 | |
| Aranzazu(Hg closed mine) | Concentrations (ppm) | ||||||||
| Positives | Percentiles | ||||||||
| Pesticide | Sample | % | Min | 25 | 90 | 95 | 99 | Max | |
| Paraoxon-ethyl | Blood | 18.81 | ND | ND | 0.08 | 0.09 | 0.10 | 0.13 | |
| Paraoxon-methyl | Urine | 0.99 | ND | ND | ND | ND | ND | 0.38 | |
| Site | Montelibano (n=76) | Nechí (n=84) | Aranzazu (n=87) | |||
|---|---|---|---|---|---|---|
| (Type of mine) | Fe/Ni | Au | Closed Hg | |||
| Variables | PR | 95% CI | PR | 95% CI | PR | 95% CI |
| Sex (female) | 0.68 | 0.47 – 0.99 | 0.82 | 0.56 – 1.21 | 0.86 | 0.63 – 1.17 |
| Age (years) | 1.01 | 0.99 – 1.02 | 1.00 | 0.99 – 1.01 | 1.00 | 0.99 – 1.01 |
| Civil status | ||||||
| Married / Free union | 1 | 1 | 1 | |||
| Divorced / Widower | 1.26 | 0.51 – 3.11 | 1.31 | 0.64 – 2.68 | 1.14 | 0.68 – 1.91 |
| Single | 1.17 | 0.68 – 2.02 | 1.26 | 0.85 -1.87 | 1.01 | 0.70 – 1.45 |
| Education | ||||||
| Illiteracy | 1 | 1 | 1 | |||
| Elementary (partial or full) | 0.38 | 0.24 – 0.60 | 1.02 | 0.56 – 1.86 | 0.63 | 0.42 – 0.94 |
| Secondary (partial or full) | 0.48 | 0.34 – 0.66 | 0.85 | 0.46 -1.59 | 0.67 | 0.43 – 1.03 |
| Technic (partial or full) | 0.41 | 0.31 – 0.53 | 1.00 | 0.53 – 1.89 | 0.78 | 0.40 – 1.54 |
| University (partial or full) | 0.45 | 0.31 – 0.64 | 1.29 | 0.65 – 2.55 | 0.78 | 0.50 – 1.22 |
| Occupation | ||||||
| In mining activities | 0.90 | 0.66 – 1.23 | 1.12 | 0.77 – 1.64 | NA | |
| In agricultural activities | 1.13 | 0.71 – 1.79 | 0.58 | 0.33 – 1.02 | 0.99 | 0.72 – 1.35 |
| Consumption (any moment) | ||||||
| Cigarette | 1.33 | 0.94 – 1.87 | 0.88 | 0.58 – 1.33 | 1.15 | 0.84 – 1.57 |
| Alcohol | 0.81 | 0.59 – 1.12 | 1.18 | 0.81 – 1.71 | 0.86 | 0.62 – 1.17 |
| Food consumption | ||||||
| Fish | 2.27 | 1.11 – 4.64 | 1.74 | 0.53 – 5.74 | 1.33 | 0.91 – 1.97 |
| Canned food | 1.14 | 0.84 – 1.54 | 1.44 | 0.99 – 2.08 | 1.09 | 0.78 – 1.54 |
| White meat | 1.29 | 1.00 – 1.66 | 1.93 | 0.80 – 4.65 | 1.05 | 0.67 – 1.64 |
| Red meat | 0.89 | 0.62 – 1.26 | 1.34 | 0.45 – 4.01 | 0.84 | 0.35 – 2.04 |
| Fruits | 1.12 | 0.38 – 3.30 | 0.48 | 0.32 – 0.71 | 0.73 | 0.52 – 1.01 |
| Vegetables | 1.50 | 1.28 – 1.75 | 0.52 | 0.19 – 1.39 | 0.75 | 0.58 – 0.96 |
| Pesticides (ppb) | ||||||
| Paraoxon-methyl in blood | 1.00 | 0.99 – 1.01 | ||||
| Paraoxon-ethyl in blood | 18.43 | 0.71 – 476.02 | ||||
| Element mixtures in hair | ||||||
| FC1 | 0.97 | 0.94 – 1.00 | 0.90 | 0.83 – 0.98 | 1.04 | 1.00 – 1.07 |
| FC2 | 0.98 | 0.94 – 1.03 | 1.04 | 0.99 – 1.09 | 1.02 | 0.99 – 1.05 |
| FC3 | NA | 0.96 | 0.90 – 1.03 | NA | ||
| Selected elements in hair | ||||||
| Mercury | 0.97 | 0.93 – 1.02 | 0.98 | 0.92 – 1.03 | 1.76 | 1.06 – 2.93 |
| Selenium | NA | 1.02 | 1.01 – 1.02 | 0.58 | 0.25 – 1.34 | |
| Lead | 1.01 | 1.00 – 1.01 | 0.94 | 0.89 – 0.98 | 1.00 | 0.99 – 1.00 |
| Berylium | 0.77 | 0.66 – 0.89 | NA | NA | ||
| Site | Montelibano (n=76) | Nechí (n=84) | Aranzazu (n=87) | |||
|---|---|---|---|---|---|---|
| (Type of mine) | (Open-pit Fe/Ni) | (Fluvial Au) | (Closed Hg) | |||
| Variables | Adj. PR | 95% CI | Adj. PR | 95% CI | Adj. PR | 95% CI |
| Pesticides (ppb) | ||||||
| Parathion ethyl (blood) | 0.04 | 0.01 – 0.14 | ||||
| Paraoxon ethyl (urine) | 0.01 | 0.00 – 0.04 | ||||
| Element mixtures in hair (scores) | ||||||
| Factor 1 | 0.97 | 0.94 – 0.99 | 0.88 | 0.79 – 0.98 | ||
| Factor 2 | 1.05 | 1.00 – 1.09 | ||||
| Hair concentration (ppm) | ||||||
| Beryllium | 0.67 | 0.59 – 0.76 | ||||
| Lead | 1.01 | 1.00 – 1.01 | 1.37 | 1.06 – 1.77 | ||
| Mercury | 36.02 | 2.69 – 380.55 | ||||
| Selenium | 1.05 | 1.03 – 1.08 | 0.86 | 0.32 – 2.33 | ||
| Nickel | 1.28 | 1.13 – 1.45 | ||||
| Interactions | ||||||
| Selenium*Lead | 0.78 | 0.64 – 0.95 | ||||
| Selenium*Mercury | 0.11 | 0.02 – 0.77 | ||||
| Diet consumption | ||||||
| Fish | 4.73 | 3.94 – 5.68 | ||||
| Vegetables | 0.47 | 0.38 – 0.59 | ||||
| Red meat | 0.67 | 0.54 – 0.83 | ||||
| Fruits | 0.55 | 0.38 – 0.80 | 0.66 | 0.46 – 0.95 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





