1. Introduction
Environmental pollution is a serious environmental problem. A large number of anthropogenic pollutants (pharmaceuticals, pesticides, mycotoxins) enter the environment from industrial, commercial, domestic and agricultural sources. This leads to pollution of surface waters, which, in turn, not only threatens aquatic life, but also affects human health. River water is an important source of drinking water, so quality control is necessary to detect pollutants. Dibutyl phthalate (DBP) phthalic acid ester (PAE) is one of the most common plasticizers used in the manufacture of plastic products, which is usually added to DBP from 10% to 60% by weight of the polymer. The manufacture of certain plastics, such as soft toys, may require a higher DBP content. Since phthalates are not chemically bound to plastic, DBP is easily released from it and enters food and the environment, which pollutes water, soil, air and food [
1,
2]. It has been shown that DBP is able to accumulate in the human body, which leads to a general hormonal failure, negatively affects the functioning of the kidneys and liver, and can provoke cancer [
3], destroy the endocrine system and reduce fertility [
4]. Since DBP poses a great threat to human health and also poses a danger to the environment, it was classified as a hazardous substance subject to priority control [
5].
Permissible levels of phthalates in water and food are regulated [
6]. In addition, due to the negative impact of PAE on the human endocrine system, environmental quality standards were defined based on average annual concentrations in freshwater environments, so the maximum permissible concentration of DBP in open water and groundwater in the EU countries is 10 µg/l, the maximum acceptable concentration for aquatic ecosystems is 35 μg/l and the maximum acceptable concentration for aquatic ecosystems of 0.430 mg/l [
7,
8]. The maximum acceptable concentration of dibutyl phthalate in water established in Russia is 0.2 mg/l [
9], and the US Environmental Protection Agency (USEPA) maximum contaminant level (MCL) for DBP are higher - 0.45 mg/l [
10].
Thus, the development of sensitive and selective analytical methods for the detection of PAE, in particular DBP, is of great importance for monitoring their content in the waters of open reservoirs. Specifically, there is a need for methods that be methods that allow these studies to be carried out outside the walls of the laboratory.
Currently, the analysis of EPA is mainly carried out by chromatographic methods, for example, high performance liquid chromatography (HPLC) [
11] or gas chromatography-mass spectrometry (GC-MS) [
12]. These analytical methods allow accurate and sensitive quantitative analysis; however, these methods require expensive instrumentation, highly skilled personnel and complex sample processing that cannot meet the requirements for rapid detection of toxicants outside the laboratory. Therefore, the development of more sensitive and selective analytical methods for the rapid and accurate detection of PAE in the field is necessary.
Immunoassay methods have great advantages over chromatographic methods due to their relatively low cost and ease of implementation. For example, to determine DBP, there are next commonly used immunological techniques such as enzyme-linked immunosorbent assay (ELISA) [
13,
14,
15], immunochromatographic (ICA) [
16] and polarization fluorescence immunoassay (FPIA) [
17,
18].
Among the immune methods of analysis, ELISA is most widely used in areas such as the diagnosis of diseases [
19], food safety [
20] and environmental monitoring [
21]. It has obvious advantages due to low cost, good specificity, automation and high performance. However, traditional colorimetric ELISA is often accompanied by false positive responses and has a long analysis time [
22]. The ICA method of analysis is fast and can be performed directly on site, but this method can give unreliable results in the presence of various phthalates in the sample, is sensitive to changes in pH and composition of the sample. In addition, ICA generally provides only a qualitative or semi-quantitative assessment of the content of the analyte. FPIA has several advantages over the above-mentioned immune methods: it is a homogeneous method, does not require separation of the free form from the bound one, is carried out quickly, provide the results in 5-10 minutes and can be carried out directly at the sampling site. The developed FPIA formats for the determination of DBP using immunoreagents of the conjugate of the amino derivative of fluorescein with succinate-dibutyl phthalate (DBP-EDF) and polyclonal antibodies obtained against DBP have a fairly high detection limit (350 ng/ml) [
17]. In another FPIA assay, instead of antibodies, the recombinant receptor protein mPPARα-LBD was used as a recognition element and fluorescently labeled nonanoic acid (C4-BODIPY-C9) [
18]. This fluorescence polarization assay can simultaneously detect multiple phthalic acid esters with different sensitivities and specificities. Given that real samples may contain more than one PAE, the proposed method is favorable for determining the total amount of PAE.
However, one of the most toxic esters of phthalic acid is DBP. Its content is currently strictly controlled in water bodies. Therefore, to meet the requirements for the detection of DBP, a more sensitive and accurate method of investigation is needed. Homogeneous FPIA allows quantitative, fast and specific determination of low-molecular pollutants in various objects both in the laboratory and directly at the detection site, thanks to the availability of portable analyzers. As any immune method, the specificity of the antigen/antibody interaction is important for the development of sensitive FPIA; therefore, the using of highly specific monoclonal antibodies and the obtaining of new highly purified antigenic conjugates with various fluorescein derivatives can solve this problem.
2. Materials and Methods
2.1. Reagents and materials
Dibutyl phthalate, dimethyl phthalate (DMP), diethyl phthalate (DEP), diisobutyl phthalate (DisoBP), diethylhexyl phthalate (DEHP), dioctyl phthalate (DOP), butyl benzyl phthalate (BBP), monomethyl phthalate (MMP), monobenzyl phthalate (MBP), monohexyl phthalate (MHP), 5- aminomethylfluorescein (AMF), N-hydroxysuccinimide (NHS), N,N’-dicyclohexylcarbodiimide (DCC) (Sigma Aldrich Corporation, USA). Dichlorotriazinylaminofluorescein (DTAF), sodium azide (Serva, USA). Triethylamine (Merck, Germany). Chloroform, methanol, dimethylformamide (DMF) (special purity grades, Khimmed, Russia).
Specific monoclonal antibodies against dibutyl phthalate Mab-DBP 2.4 mg/mL were provided by Prof. Chuanlai Xu (Jiangnan University, Wuhi, China).
During the experiments, a 50 mM borate buffer solution (pH 8.5) containing 0.1% NaN3 was used.
Stock solutions of phthalates were prepared at a concentration of 10 mg/ml in methanol, from which standard solutions were prepared in 10% methanol.
The DBP-AMF conjugate was purified by thin layer chromatography on pre-coated TLC sheets ALUGRAM®Xtra SIL G/UV254 (Germany). Fluorescence polarization measurements were carried out on a Sentry-200 portable device (Ellie, USA). The obtained data were processed using the Sigma Plot 11 software package (Systat Software Inc., USA).
2.2. Synthesis of the fluorescein labeled antigen (tracer)
Synthesis of conjugates of dibutyl phthalate with DTAF was carried out by dissolving 1 mg of 4-amino-dibutyl phthalate in 100 μl of methanol with 10 μl of triethylamine and adding 2 mg of DTAF. The mixture was incubated for 24 h in the dark.
The preparation of the DBP-AMF conjugate was carried out in two stages: 1) activation of the carboxyl group of DBP-Su: the molar ratio of DBP-Su : DCC : NHS during the synthesis was 1:2:2. DCC (1.3 mg) and NHS (0.75 mg) were dissolved in 200 µl of DMF. DBP-Su (1 mg) was then added and the solution was incubated for 18 h at room temperature. The reaction mixture was centrifuged at 10,000 g for 3 min and the supernatant was collected. 2) addition of fluorescent dye: AMF (2 mg) and 10 μl of trimethylamine were added to the supernatant and incubated for 24 hours in the dark.
2.2.1. Purification of the tracer
DBP-AMF and DBP-DTAF conjugates were purified from impurities by thin-layer chromatography using methanol : chloroform as an eluent in a ratio of 1:4 (v/v). The main yellow fluorescent band (Rf=0.9) was collected from the chromatographic plate and extracted with 1 ml of methanol. The concentration of DBP-AMF and DBP-DTAF conjugates was determined spectrophotometrically, by absorbance at 492 nm in 50 mM carbonate buffer solution, pH 9.0, using a fluorescein molar extinction coefficient of 8.78*10
4 (l/mol*cm) as described in [
24]. The DBP-AMF structure was checked by high-resolution tandem mass spectrometry coupled with high-performance liquid chromatography (see Supplementary).
2.3. FPIA for DBP
Monoclonal antibody against DBP were obtained using BALB/c mice (1.5–2 months) by hybridomic technology with antigen DBP-BSA as described [
25]. Animal studies were conducted in accordance with the EU Directive 2010/63/EU and are authorized by the Ethics Committee of the Biotechnology Research Center (Protocol N22-D of February 12, 2020).
2.3. FPIA for DBP
The standard solutions 500 μl of various concentrations of DBP in 5% methanol (0–10000 ng/ml) and 500 μl of a DBP-AMF conjugate solution with the selected optimal concentration 5 nM were added to glass tubes. Then, 50 µl of a solution of MAb-DBP antibodies with a concentration of 24 or 48 µg/ml was added, and after 60 min of incubation at 25°C, measurements were made on a Sentry-200 portable fluorimeter (Ellie LLC, USA). Each measurement was carried out in triplicate. Based on the measurement results, the dependence of the fluorescence polarization on the DBP concentration (calibration curve) was obtained using a semi-logarithmic scale for the DBP concentration, which was approximated by a four-parameter sigmoid function (1) using the Sigma Plot 11 software (Systat Software Inc., USA)
where mP is the measured fluorescence polarization signal, mP0 is the free tracer polarization, mPmax is the fluorescence polarization signal for the MAb-DBP*DBP-AMF complex in the absence of free DBP, IC50 is the DBP concentration at which a 50% change in FP occurs.
2.4. FPIA Specificity
The specificity of FPIA has been studied under optimal experiment conditions. Cross-reactivity (CR) was estimated using the following equation:
2.5. Preparation of water samples and recovery test
All water samples were obtained from Lake Onega and stored at 4°C. Water samples that did not contain DBP by GC-MS were used to check FPIA by the recovery test. Before FPIA, these water samples were spiked with DBP and analyzed without subsequent sample preparation. All experiments were duplicated.
2.6. Determination of DBP in water samples by GC-MS
Water samples were prepared for analysis in glass flasks with ground-in lids. Phthalates were extracted with n-hexane (10 ml sample + 1 ml n-hexane). The determination was carried out by gas chromatography-mass spectrometry in the mode of registration of isolated ions. [
26,
27]. Briefly, the column HP-5MS (30m×250 microns×0.25 microns) (“Agilent Technologies”, USA) was used, mobile phase – helium, eluent flow rate of 1 ml/min. The volume of the injected sample is 1 µl. Temperature gradient: from 40 to 130°C at a speed of 50° C/min, from 130 to 250 °C at a speed of 5°C/min, from 250 to 300°C at a speed of 10°C/min. Source temperature 230°C. Mass spectrometric detection was performed on the following ion: m/z 149 [
26].
3. Results and Discussion
3.1. Obtaining and characterization of the specific reagents
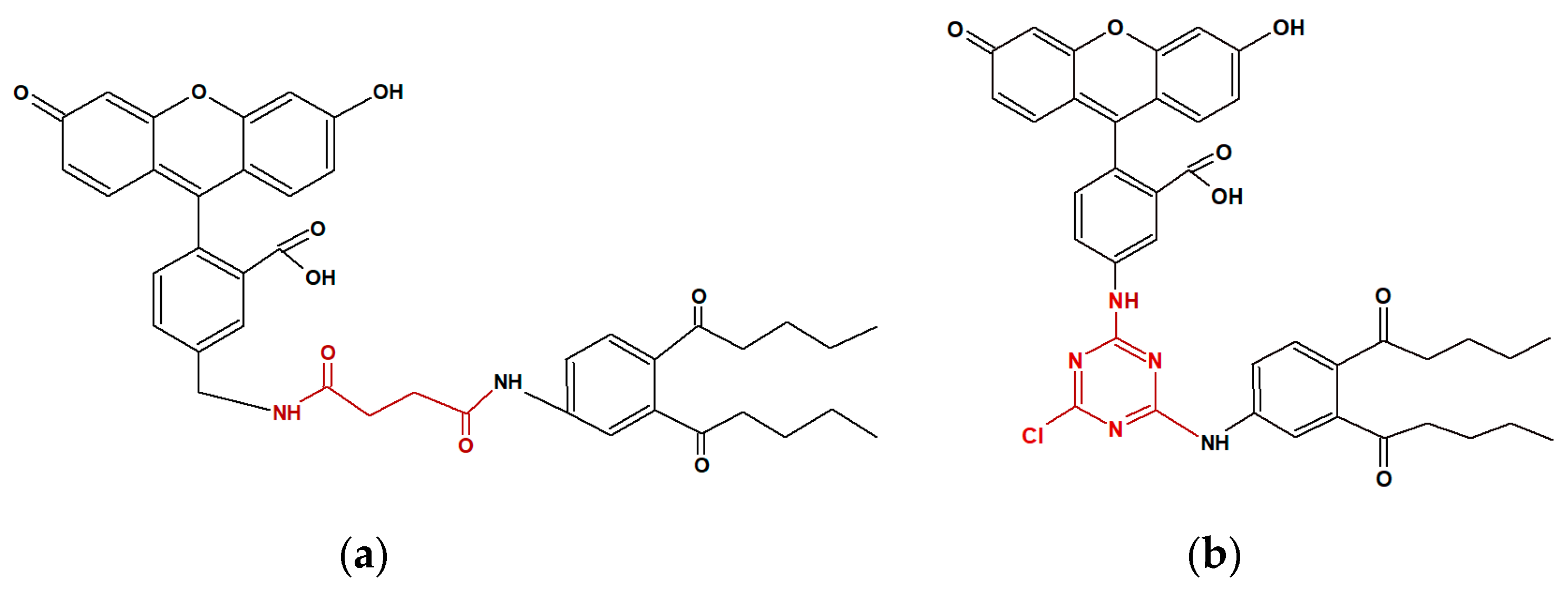
For the development of a highly sensitive FPIA assay, the quality of the immunoreagents plays an important role. A special role is assigned to the conjugate of a low molecular weight analyte with a fluorescent label - a tracer. It is important that the synthesized tracer effectively binds to antibodies and represents a highly purified preparation. In the previous study [
17], a fluorescently labeled conjugate with a carboxylated derivative of DBP (DBP-Su) with ethylenediamine fluoresceinthiocarbamate (EDF) was synthesized. However, the DBP detection limit using this tracer was quite high and amounted to 350 ng/mL. Therefore, in this work, we obtained DBP-Su conjugates with another fluorescein derivative, AMP and 4-NH2-DBP with DTAF. These DBP-AMP and DBP-DTAF tracers differ from EDF in the length of the spacer between fluorescein and DBP.
For the development of FPIA, the DBP-AMF and DBP-DTAF tracers were synthesized (see the structure shown in
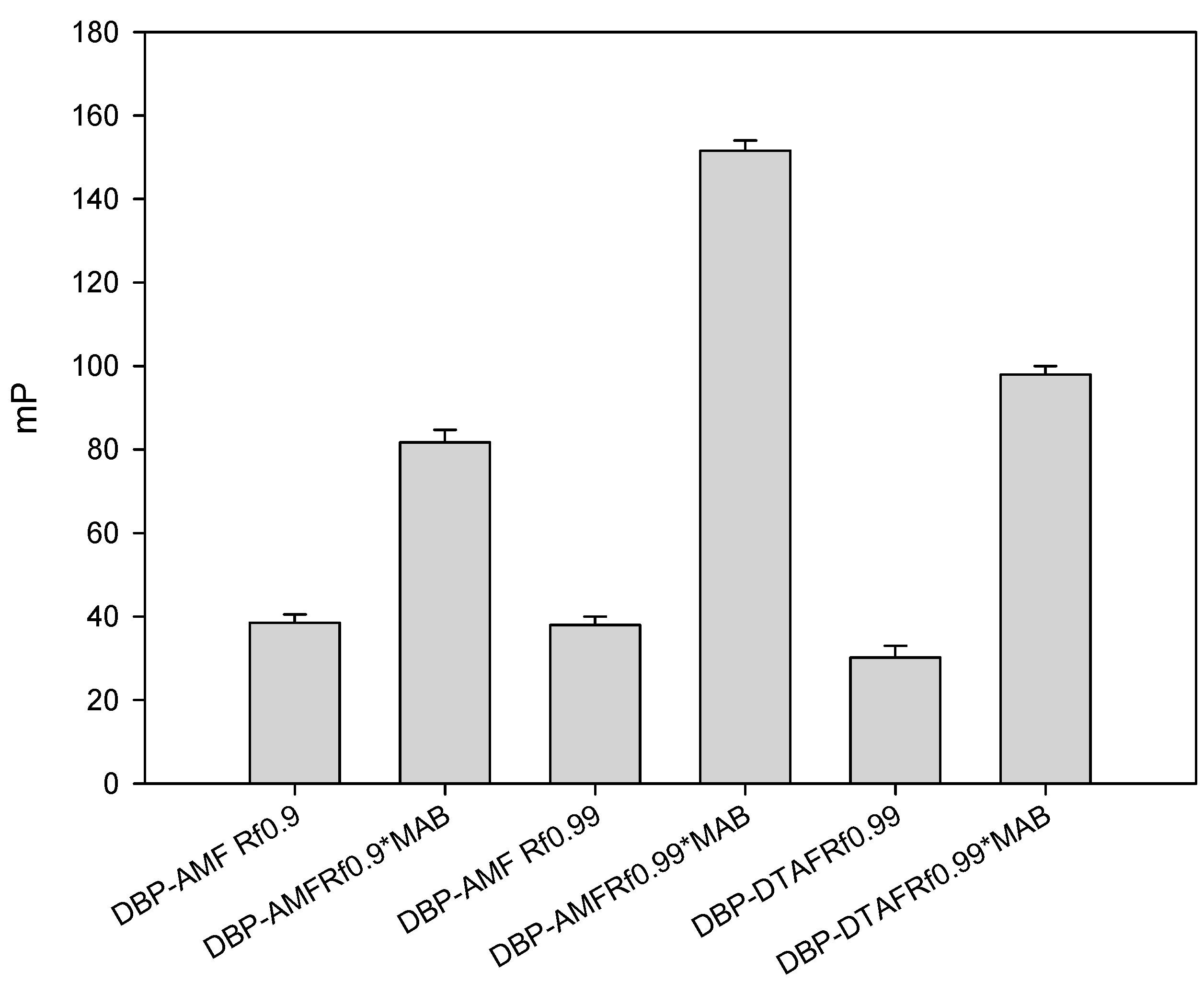
Figure 1). The obtained conjugates were purified by thin layer chromatography (TLC) and main fractions with a retention factor (Rf 0.9) were studied. To check the resulting conjugates, working solutions of each fraction were prepared, and dilutions of stock tracer solutions were selected, so that their fluorescence intensity exceeded the background signal of pure buffer by a factor of 20. Concentration of working tracers solutions was 5 nM. At a given concentration, the tracers gave the optimal signal/noise ratio and, consequently, a stable value of the fluorescence polarization. Then monoclonal antibody obtained against DBP (MAb-DBP) were added to the working solutions of tracers DBP-AMF and DBP-DTAF and their binding was studied (
Figure 2). Both tracers DBP-AMF and DBP-DTAP were shown to bind to MAb-DBP. It is known that the development of highly sensitive FPIA requires a high quality of immunoreagents. Therefore, we undertook additional purification of tracers by thin layer chromatography; the obtained conjugates DBP-AMF Rf 0.99 and DBP-DTAF Rf 0.99 were also tested for binding to the monoclonal antibody MAb-DBP. The results are shown in
Figure 2. As can be seen, the tracer fluorescence polarization signal after repeated purification increased markedly when bound to antibodies. However, it can be seen from the presented data that the increase in the FP signal upon binding to antibodies was higher for the DBP-AMF tracer than for DBP-DTAF, which is probably due to steric hindrances that may arise during the interaction of antibodies with DBP-DTAF.
3.2. Studying of binding kinetic DBP-AMF and DBP-DTAF with monoclonal antibody
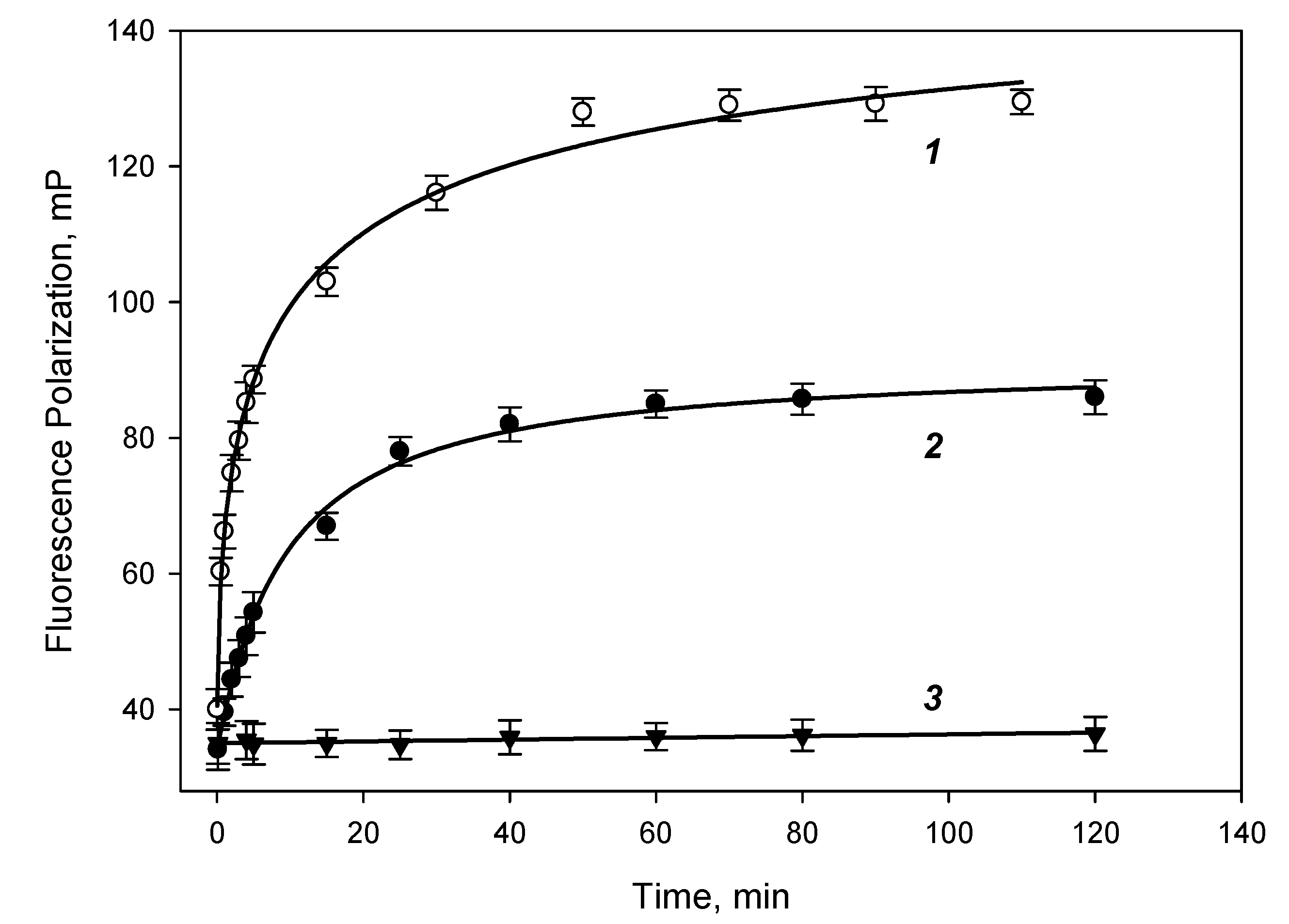
The study of the antibody binding kinetics to DBP-AMF and DBP-DTAF tracers was studied by measuring the fluorescence polarization signal (mP), 0.5 ml of borate buffer, 0.5 ml of the working solution of the DBP-AMF tracer and 0.05 ml of antibody solution were mixed in a test tube with a known concentration. The tube was placed in a Sentry-200 portable fluorimeter and the kinetics of binding of the DBP-AMF tracer to antibodies was studied.
Figure 3 shows the kinetic curves of the binding of the tracer DBP-AMF and DBP-MAb at two concentrations of 16 and 10 nM. Complete binding was achieved within 50-60 min of incubation at room temperature. Binding of the DBP-DTAF tracer took place almost immediately when the solutions were mixed, and the equilibrium was established within 1–2 min (data not shown). Then, while studying the binding of antibodies to DBP-AMF, we measured the fluorescence polarization signal after 60 min of incubation, and for the DBP-DTAF tracer after 2 min. We also studied the stability of the fluorescence polarization signal for 4 hours and showed that the change in the FP signal during this time did not exceed 1-2 units. In addition, the binding specificity of the tracers DBP-AMF and DBP-DTAF was tested with a monoclonal antibody that was obtained against progesterone: MAb-antiPG. The value of the polarization of the tracer fluorescence practically did not change (
Figure 3), which indicates a highly specific binding of this tracer only with antibodies obtained against DBP (
Figure 3 (curve 3).
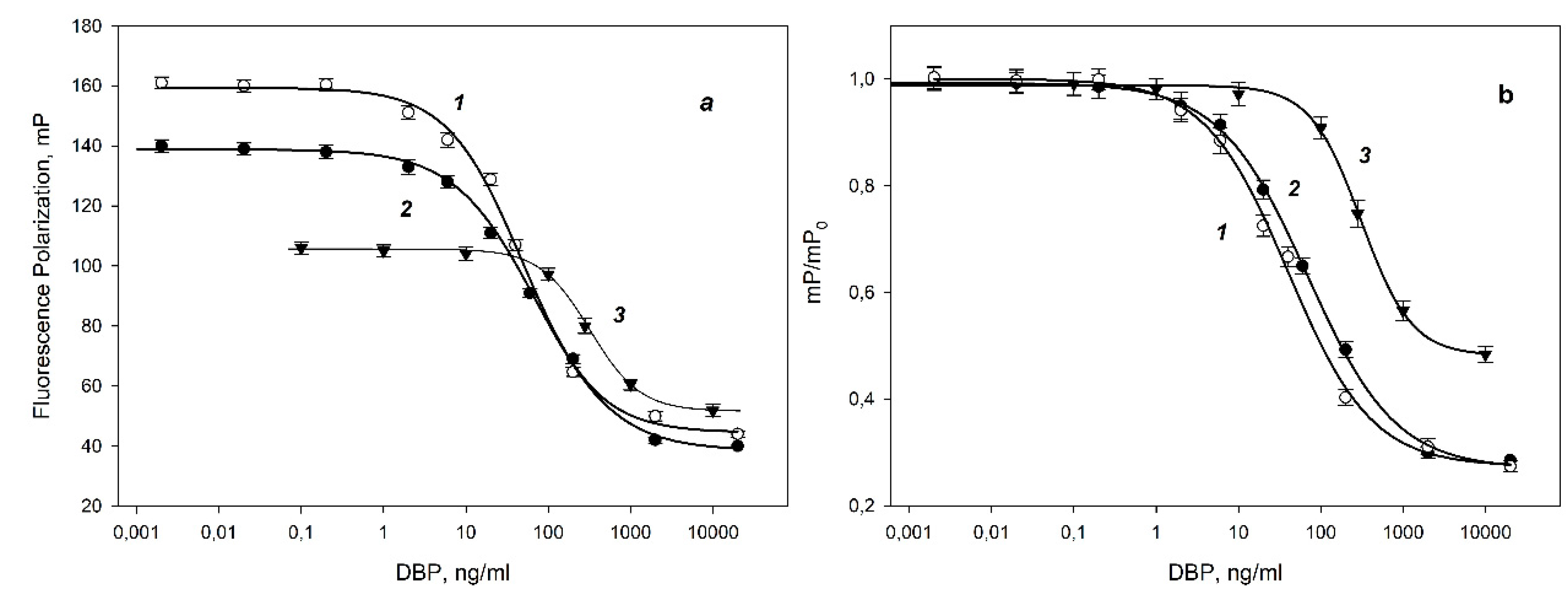
The combination of antibody/tracer has a significant impact on the specificity and sensitivity of competitive FPIA [
25]. For optimize the method, we also selected the concentration of antibodies. For the DBP-AMF tracer, two calibration dependences of the change in the fluorescence polarization signal on the DBP concentration were obtained at different concentrations of antibodies (10 and 16 nM) and analyzed their analytical characteristics (
Figure 4.). The DBP detection limit is defined as the concentration at which observed a 10% decrease in the fluorescence polarization signal (IC10), and the sensitivity of the IC50 analysis is the concentration causing a 50% decrease in the signal. Using antibodies at a concentration of 16 and 8 nM, the DBP detection limit was 10.0±0.2 ng/mL. The IC50 for these curves was 40±2 and 70±2 ng/mL for antibodies at 16 and 8 nM, respectively. For the DBP-DTAF tracer, at an antibody concentration of 16 nM, a higher detection limit (93 ng/ml) was obtained than for the DBP-AMF tracer. The structure of the best tracer DBP-AMP was confirmed by high-resolution tandem mass spectrometry coupled with high-performance liquid chromatography (see
Figure S1).
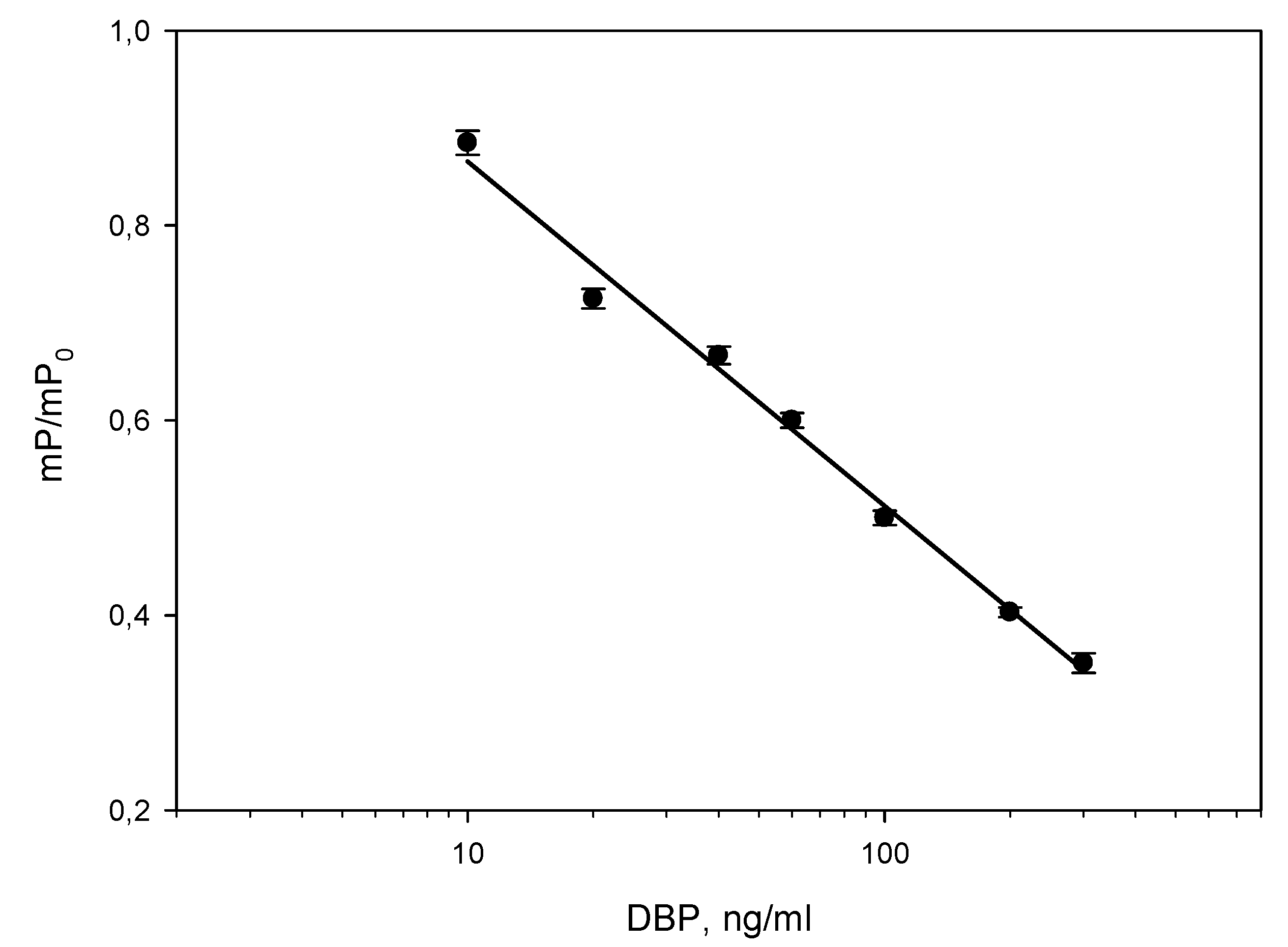
Thus, for optimal pair of immunoreagents MAb-DBP/DBP-AMF at concentrations of 16 nM / 2.5 nM, we obtained a more sensitive analysis with minimal limit of detection (10 ng/ml) (
Figure 4b curve 1). This curve was evaluated in accordance with the recommendations [
28] and linear range was 12-300 ng/ml. The linear range of the calibration curve for the determination of DBP (
Figure 5) was used this pair of immunoreagents. This calibration dependence was used to check the FPIA method by the recovery test and analysis of real water samples.
We have studied the cross-reactivity (CR) of other phthalates structurally similar to DBP. For this objective, other phthalic acid esters and their metabolites monophthalates were tested: DMP, DEP, DisoBP, DOP, DEHP, BBP, MMP, MBP, MHP. % CR was calculated according to equation (2).
However, the % CR for all checked di- and monoesters was less than 0.1%, which indicates a highly specific analysis for DBP.
After linearization of the curve section (
Figure 5), according to which the DBP determination was carried out, the equation of the straight line was:
Upon analysis of sixteen water samples from Lake Onega, which were checked by GC-MS, we selected two samples that did not contain phthalates (#1 and #2). Known concentrations of DBP were added to these water samples and mixed (finish concentration 30 and 90 ng/ml). The spiked samples were stored at room temperature overnight and analysed using the FPIA.
Table 2 demonstrates that the recovery percentage was 86-110%, which confirms that this method can be used to determine DBP in real water samples.
With our assistance for callibration, other water samples obtained from Lake Onega were also tested, in which the content of DBP was confirmed by GC-MS (
Table 3). Majority of samples we analyzed contained DBP below the detection limit of FPIA, or at the sensitivity limit of our method. Good correlation was obtained between the results of the FPIA and GC-MS methods (
Table 1). This result further confirmed the reliability of the FPIA for the detection of DBP.
Table 1.
Recoveries of DBP from environmental water (n = 3).
Table 1.
Recoveries of DBP from environmental water (n = 3).
| Water sample |
Sampling location |
Added DBP concentration, ng/mL |
Detected DBP concentration, ng/mL |
Recovery, % |
| 1 |
Sample 1 |
30 |
26 ± 1 |
86 ± 2 |
| 90 |
85 ± 3 |
94 ± 3 |
| 2 |
Sample 2 |
30 |
34 ± 2 |
113 ± 2 |
| 90 |
98 ± 2 |
109 ± 4 |
Table 2.
Detection of DBP from environmental water by FPIA and GC-MS (n = 3).
Table 2.
Detection of DBP from environmental water by FPIA and GC-MS (n = 3).
| Water sample |
DBP by GC-MS, ng/mL |
DBP by FPIA, ng/mL |
| 3 |
10 |
ND |
| 4 |
10.6 |
10.5 |
| 5 |
10.6 |
10.0 |
| 6 |
6.0 |
ND |
| 7 |
7.5 |
ND |
| 8 |
7.3 |
ND |
| 9 |
10.5 |
10.0 |
| 10 |
11.2 |
10.0 |
| 11 |
7.1 |
ND |
| 12 |
5.3 |
ND |
| 13 |
16.5 |
12.5 |
| 14 |
18.1 |
14.2 |
| 15 |
14.0 |
11.0 |
| 16 |
8.0 |
ND |
As we discussed, there are few immunoassay methods on DBP detection described in the literature.
Table 3 summarizes data on the sensitivity and types of matrices tested by the proposed analyses. As presented in the table below, immunochemical methods of analysis have good sensitivity and a low detection limit. In the literature, FPIA methods for determining DBP have been described and, as can be seen from the data presented, the use of monoclonal antibodies has reduced the detection limit of DBP compared to the previously described method [
17] by 35 times.
Table 3.
Studies on the detection of DBP by different immunoassays.
Table 3.
Studies on the detection of DBP by different immunoassays.
| Antibody |
Assay format |
Sample |
LOD ng/ml |
Range ng/mL |
Reference |
| Polyclonal |
icELISA |
White wine |
64.5 |
64.5–1606.2 |
[13] |
| Polyclonal |
BA-ELISA |
Drinking water |
5 |
0.02 - 8.97 |
[14] |
| Monoclonal |
ELISA |
Baverange |
3.6 |
|
[15] |
| Monoclonal |
ICA |
Water |
33.4 |
42.4 - 1500 |
[16] |
| Polyclonal |
FPIA |
Water |
350 |
500-7500 |
[17] |
| Receptor |
FPIA |
Spirite |
170 |
170-8700 |
[18] |
| Monoclonal |
FPIA |
Water |
10 |
12-300 |
This work |
4. Conclusions
In this work, a sensitive FPIA method for the determination of DBP was developed using a new pair of immunoreagents DBP-AMF and monoclonal antibodies MAb-DBP. It has been shown that the use of monoclonal antibodies and the production of a highly purified tracer makes it possible to develop a more sensitive and specific FPIA. The obtained analytical characteristics of the method allows to determine the content of DBP in the waters of open reservoirs, and the detection limit of phthalate by the FPIA method (10 ng / ml), which is 20 times lower than the maximum allowable concentration of dibutyl phthalate in water established in Russia (200 ng / ml). In this work, the FPIA method was used to test water samples obtained from Lake Onega, which were previously verified by GC-MS and showed that the content of phthalates in real water samples is lower than the MPC established in Russia, and a comparison of the results of determining DBP by two methods showed good convergence results. The FPIA method, due to its sensitivity and ease of analysis, can be used to determine the content of phthalates in real samples. In addition, the use of a portable Sentry-200 device allows analysis to be carried out directly at the sampling site.
Supplementary Materials
The following supporting information can be downloaded at Preprints.org.
Author Contributions
Conceptualization, S.E., L.M., and A.P.; methodology, S.E., L.M., and A.P; validation, L.M.; formal analysis, L.M., S.E., A.P.; investigation, M.R., O.Z., L.L., and V.L.; data curation, S.E. and L.M., E.C.; writing – original draft, L.M., S.E.; writing – review and editing, L.M., S.E.; and A.V.; supervision, S.E.; project administration, S.E., and C.X.; funding acquisition, S.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 21-14-00306
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors express their gratitude to Ph.D. Mikhail B. Zobkov for providing water samples from Onega Lake, collected from the RV “Poseidon” within the framework of the state assignment to the Northern Water Problems Institute of Karelian Research Centre of the Russian Academy of Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Berenstein, G., Hughes, E.A., Zalts, A., Basack, S., Bones,i S.M., Montserrat, J.M. Environmental fate of dibutylphthalate in agricultural plastics: photodegradation, migration and ecotoxicological impact on soil. Chemosphere 2022, 290, 133221. [CrossRef] [PubMed]
- Yan, Y., Zhu, F., Zhu, C., Chen, Z., Liu, S., Wang, C., Gu, C. Dibutyl phthalate release from polyvinyl chloride microplastics: Influence of plastic properties and environmental factors. Water Res. 2021, 204, 117597. [CrossRef] [PubMed]
- Li, E.H., Xu, B.H., Wei, H.B., Bai, Y.C., Zhang, Q., Yu, W.W., Xu, Z.H., Qi, X.L., Zhang, D.H., Wang H. Molecular mechanism of di-n-butyl phthalate promotion of bladder cancer development. Toxicol In Vitro. 2023, 86, 105508. [CrossRef] [PubMed]
- Xie, F., Chen, X., Weng, S., Xia, T., Sun, X., Luo, T., Li, P. Effects of two environmental endocrine disruptors di-n-butyl phthalate (DBP) and mono-n-butyl phthalate (MBP) on human sperm functions in vitro. Reprod Toxicol. 2019, 83, 1–7. [CrossRef]
- Jiang, N., Song, P., Li, X., Zhu, L., Wang, J., Yin, X., Wang, J. Dibutyl phthalate induced oxidative stress and genotoxicity on adult zebrafish (Danio rerio) brain. J. Hazard Mater. 2022, 424, 12774.
- Kortenkamp, A., Koch, H.M. Refined reference doses and new procedures for phthalate mixture risk assessment focused on male developmental toxicity. Int. J. Hyg. Environ. Health. 2020, 224, 113428. [CrossRef]
- Bodar, C.W.M. Environmental risk limits for dibutylphthalate (DBP). RIVM letter report 601782009/2008.
- National Research Council. Phthalates and cumulative risk assessment: the tasks ahead. 2009.
- SANPIN 1.2.3685-21. Hygienic standards and requirements for ensuring the safety and (or) harmlessness of environmental factors for humans. Federal Service for Supervision of Consumer Rights Protection and Human Well-Being, Registered with the Ministry of Justice of the Russian Federation on January 29, 2020, N 62296, 2021, P. 229.
- US EPA. United States Environmental Protection Agency (US). National primary drinking water regulations. Final rule. Fed. Regist. 1991, 56, 30266–81. [Google Scholar]
- Soheilifar, S., Rajabi-Moghaddam, M.-J., Karimi, G., Mohammadinejad, A., Motamedshariaty, V.S., Mohajeri, S.A. Application of molecularly imprinted polymer in solid-phase microextraction coupled with HPLC-UV for analysis of dibutyl phthalate in bottled water and soft drink samples. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 552–560.
- Cao, X.-L. Determination of phthalates and adipate in bottled water by headspace solid-phase microextraction and gas chromatography/mass spectrometry. J. Chromatogr. A. 2008, 1178, 231–238. [Google Scholar] [CrossRef]
- Sun, Q., Chen, Y., Li, F., Jia, M., Shi, G. A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor. Open Chemistry. 2019, 17, 392–400. [CrossRef]
- Xu, F., Wang, W., Jiang, H. et al. Indirect Competitive Enzyme-Linked Immunosorbent Assay for the Detection of Dibutyl Phthalate in White Wine, Compared With GC-MS. Food Anal. Methods 2014, 7, 1619–1626. [CrossRef]
- Sun, R., Zhuang, H. Biotin-Streptavidin Enzyme-linked Immunosorbent Assay for the Determination of Dibutyl Phthalate in Beverages and Drinking Water Using a Specific Polyclonal Antibody. Food Anal. Methods. 2015, 8, 1990–1999. [CrossRef]
- Berlina, A.N., Ragozina, M.Y., Komova, N.S., Serebrennikova, K.V., Zherdev, A.V., Dzantiev, B.B. Development of Lateral Flow Test-System for the Immunoassay of Dibutyl Phthalate in Natural Waters. Biosensors 2022, 1002.
- Baranovskaya, V.S., Berlina, A.N., Eremin, S.A. A Fluorescence Polarization Immunoassay Procedure for Determining Dibutyl Phthalate in Water. J. Anal. Chem. 2022, 77, 466–472. [CrossRef]
- Zhang, J., Li, T., Zhang, T. et al. Receptor-Based Fluorescence Polarization Assay to Detect Phthalate Esters in Chinese Spirits. Food Anal. Methods. 2017, 10, 1293–1300. [CrossRef]
- Iha, K., Inada, M., Kawada, N., Nakaishi, K., Watabe, S., Tan, Y.H., Shen, C., Ke, L.Y., Yoshimura, T., Ito, E. Ultrasensitive ELISA Developed for Diagnosis. Diagnostics 2019, 9, 78.
- Xiong, Y., Leng, Y., Li, X., Huang, X., Xiong, Y. Emerging strategies to enhance the sensitivity of competitive ELISA for detection of chemical contaminants in food samples. TrAC Trends Anal. Chem. 2020, 126, 115861. [CrossRef]
- Liu, Z., Zhang, B., Sun, J., Yi, Y., Li, M., Du, D., Zhu, F., Luan, J. Highly efficient detection of salbutamol in environmental water samples by an enzyme immunoassay. Sci. Total Environ. 2018, 613, 861–865.
- Liu, Y., Pan, M., Wang, W., Jiang, Q., Wang, F., Pang, D.-W., Liu, X. Plasmonic and photothermal immunoassay via Enzyme-Triggered Crystal Growth on gold nanostars. Anal. Chem. 2019, 91, 2086–2092. [CrossRef]
- Pourfarzaneh, M., White, G., Landon, J., Smith, D. Cortisol directly determined in serum by fluoroimmunoassay with magnetizable solid phase. Clin. Chem. 1980, 26, 730–733. [CrossRef]
- Smith, D.S., Eremin, S.A. Fluorescence polarization immunoassays and related methods for simple, high-throughput screening of small molecules. Anal. Bioanal. Chem. 2008, 394, 1499–1507.
- Kuang, H., Liu, L., Xu, L., Ma, W., Guo, L., Wang, L., Xu. C. Development of an Enzyme-Linked Immunosorbent Assay for Dibutyl Phthalate in Liquor. Sensors 2013, 13, 8331–8339. [CrossRef] [PubMed]
- Tolmacheva, N.G., Pirogov, A.V., Shpigun, O.A.. The use of microemulsions for the extraction of phtalic acid esters from soils with subsequent decomposition of microemulsions, simultaneous pre-concentration of dialkhylphthalates in organic phase and following GC-MS determination of target compounds. Moscow University Chemistry Bulletin 2017, 58, 83–88.
- Pirogov, A.V., Tolmacheva, N.G., Shpigun, O.A. Extraction and Subsequent Determination of Dialkyl Phthalates in the Soil Using Gas Chromatography Combined with Tandem Mass Spectrometry. Moscow University Chemistry Bulletin 2014, 69, 158–162. [CrossRef]
- International Union of Pure and Applied Chemistry (IUPAC), Compendium of Analytical Nomenclature (Orange Book). United Kingdom: Blackwell Science, 1998, P. 223.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).