Submitted:
09 July 2023
Posted:
10 July 2023
You are already at the latest version
Abstract
Keywords:
Introduction
The Use of Antimicrobial Agents and its Affects Resistance
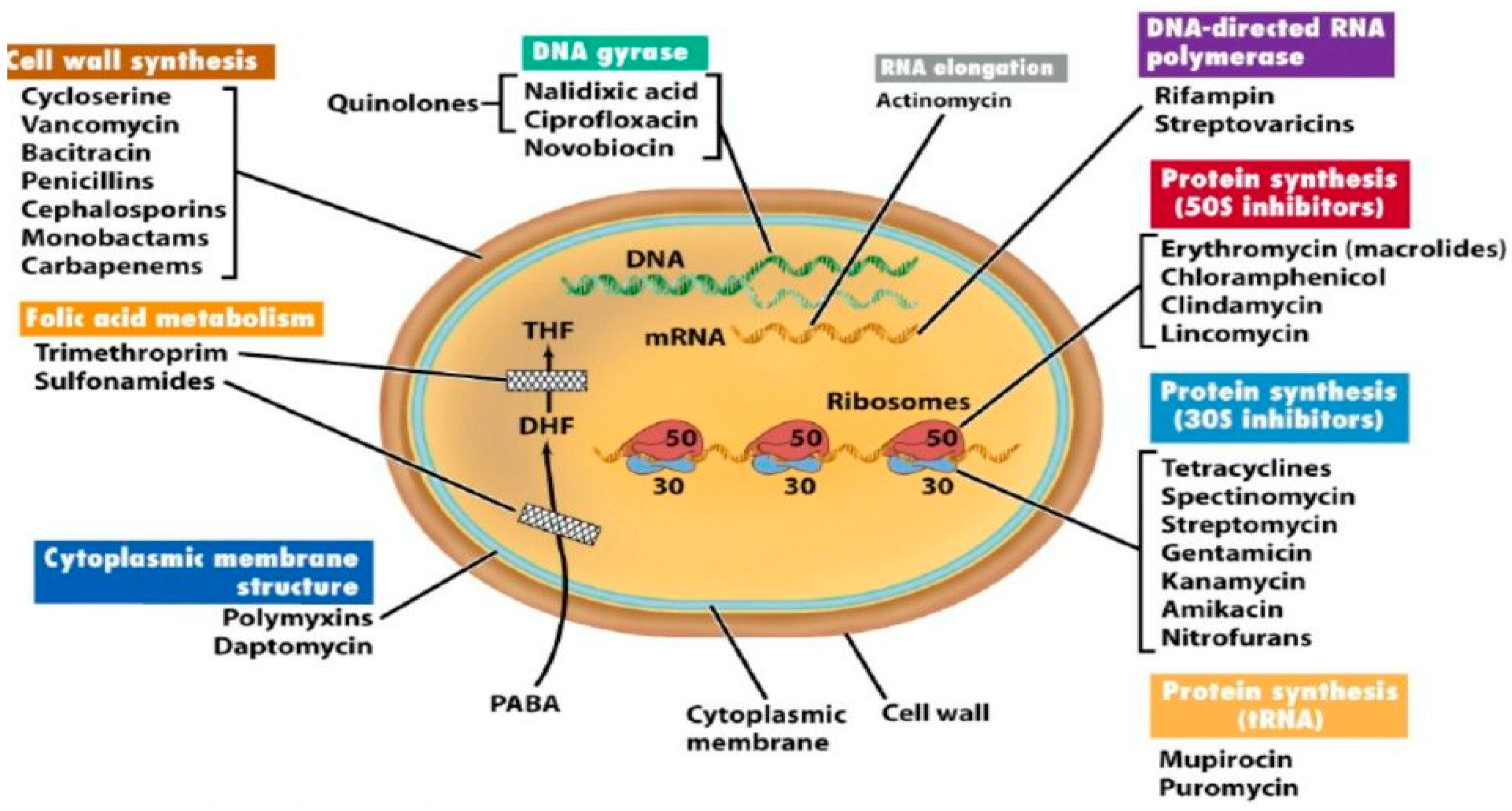
- Enzyme inactivation
- b.
- Enzyme modification
- c.
- Modification of the antibiotic target site
- d.
- Replacement of the target site
- e.
- Overproduction of the target
- f.
- Efflux and reduced permeability
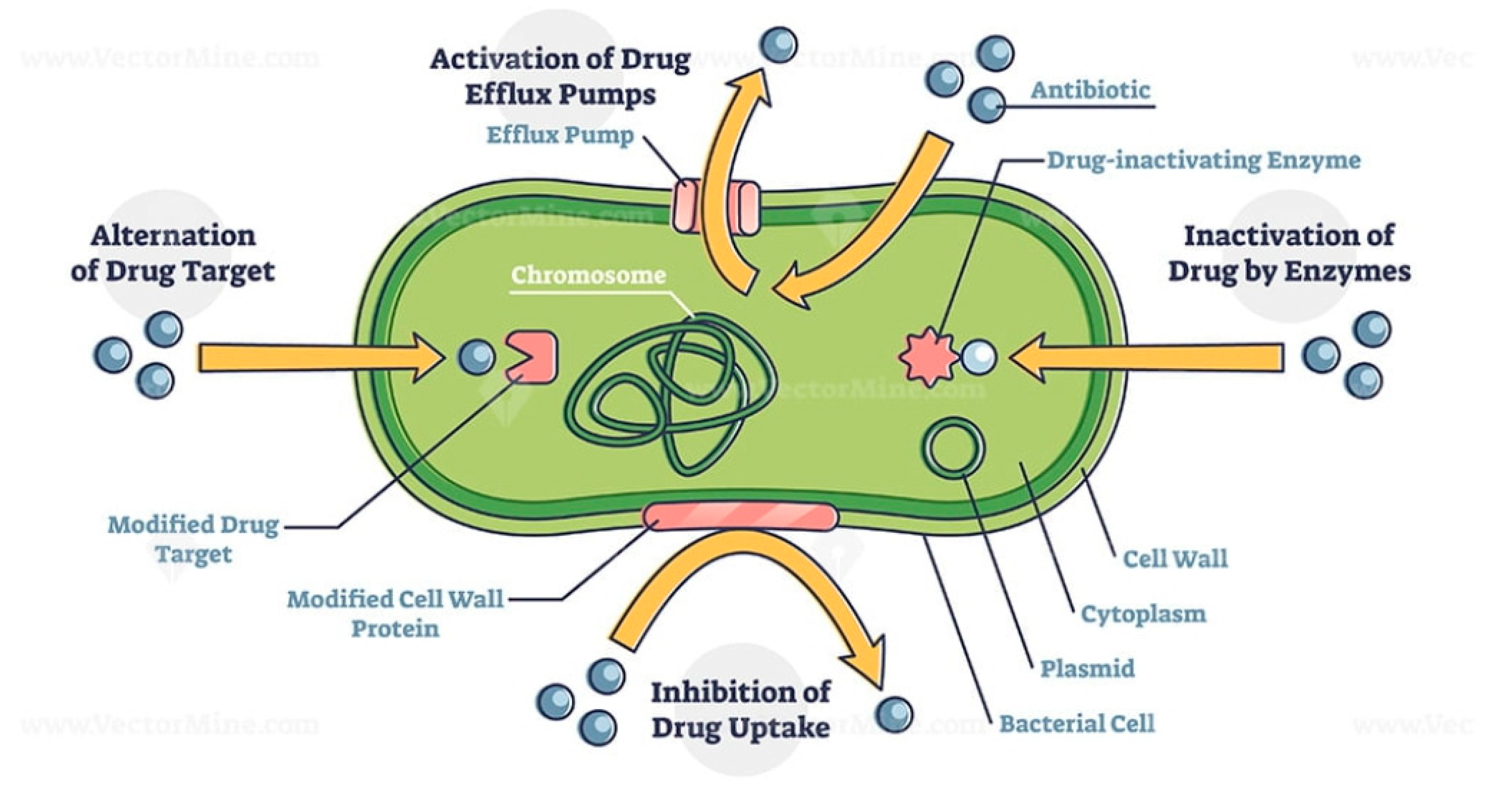
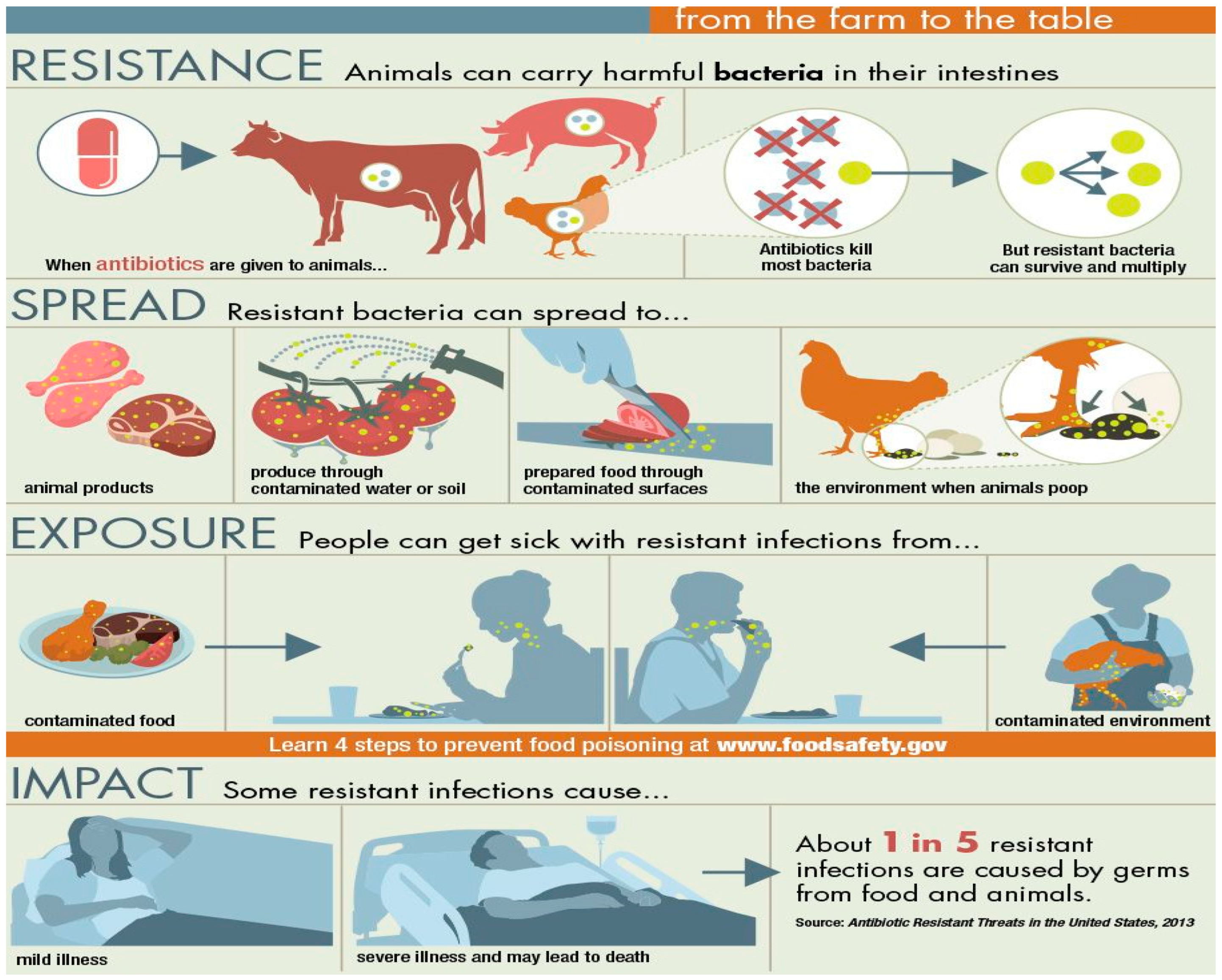

- i.
- Utilize current antibiotics wisely
- ii.
- Control the use of antibiotics in crops, food, animals, and people.
- iii.
- Creation of vaccines in advance to reduce antibiotic use.
- iv.
- Improve diagnostic testing speed.
- v.
- Support programs for antibiotic stewardship.
Conclusion
Disclaimer
References
- Samtiya, M.; Matthews, K.R.; Dhewa, T.; Puniya, A.K. Antimicrobial Resistance in the Food Chain: Trends, Mechanisms, Pathways, and Possible Regulation Strategies. Foods 2022, 11, 2966. [CrossRef]
- Tanvir, M. U.; Arka, J.; Chakraborty, A. K.; Redwan, M. Z.; Saikat, M. T. B. E.; Kuldeep, D. M.D.; Kamal, H. R.; Márió, G.; Muhammad, U. K.; Sahibzada, Md. J.; Hossain, N. K. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and prospects, Journal of Infection and Public Health, 2021, 14, 12, 1750-1766. [CrossRef]
- Thomas, E.; Wolfgang, K; Leo, M. Pathogenic microbes in water and food: changes and challenges, FEMS Microbiology Reviews, 2002, 26(2), 111–112. [CrossRef]
- Jans, C.; Sarno, E.; Collineau, L.; Meile, L.; Stärk, K.D.C.; Stephan, R. Consumer Exposure to Antimicrobial Resistant Bacteria from Food at Swiss Retail Level. Front. Microbiol. 2018, 9:362. [CrossRef]
- David, W. N; John E. M.; Juluri, R. R. Antimicrobial resistance (AMR): significance to food quality and safety, Food Quality and Safety, 2019, 3, 15–22. [CrossRef]
- Jonathan, G. F.; Charlene R. J. Genetic mechanisms of antimicrobial resistance identified in Salmonella enterica, Escherichia Coli, and Enterococcus spp. Isolated from U.S. food animals. Frontier in Microbiology, 2013, 4, 35 1 - 22. [CrossRef]
- FOA 2017www.fao.org/food-chain-crisis; http://www.fao.org/antimicrobial-resistance, Retrieved 0n 24th June, 2023.
- Vikesland, P.; Garner, E.; Gupta, S.; Kang, S.; Maile-Moskowitz, A., Zhu, N. Differential Drivers of Antimicrobial Resistance across the World. Acc Chem Res. 2019 Apr 16;52(4):916-924. [CrossRef]
- https://www.pinterest.com/pin/325596248054170021/.
- Djordjevic, S. P.; Stokes, H. W.; Chowdhury, P. R. Mobile elements, zoonotic pathogens and commensal bacteria: conduits for the delivery of resistance genes into humans, production animals and soil microbiota. Front Microbiol. 2013; 4:86. [CrossRef]
- Peterson, E.; Kaur, P. Antibiotic Resistance Mechanisms in Bacteria: Relationships Between Resistance Determinants of Antibiotic Producers, Environmental Bacteria, and Clinical Pathogens. Frontiers in Microbiology, 2018; 9. DOI=10.3389/fmicb.2018.02928.
- Hallsworth, M.; Sallis, C. T. Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomised controlled trial. Lancet. 2016; 387:1743–1752. [CrossRef]
- Kmietowicz, Z. New antibiotics: NHS will test “pay for usefulness” model to stimulate research. BMJ. 2019; 366:l4610. [CrossRef]
- García-Quintanilla, M.; Pulido, M. R.; Carretero-Ledesma, M. Vaccines for antibiotic-resistant bacteria: possibility or pipe dream? Trends Pharmacol Sci. 2016; 37:143–152. [CrossRef]
- Fletcher, S. Understanding the contribution of environmental factors in the spread of antimicrobial resistance. Environ Health Prev Med. 2015 20(4):243-52. [CrossRef]
- Hu, Y.; Yang, X.; Li, J.; Lv, N.; Liu, F.; Wu, J.; Lin, I. Y.; Wu, N.; Weimer, B. C.; Gao, G. F.; Liu, Y.; Zhu, B. The Bacterial Mobile Resistome Transfer Network Connecting the Animal and Human Microbiomes. Appl Environ Microbiol. 2016, 27, 82(22):6672-6681. https://doi: 10.1128/AEM.01802-16.
- Abdelhamid, A. G.; El-Dougdoug, N. K. Controlling foodborne pathogens with natural antimicrobials by biological control and antivirulence strategies. Heliyon. 2020; 6(9):e05020. [CrossRef]
- Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Foodborne, Waterborne, and Environmental Diseases (DFWED), 2022.
- Ryding, Sara. (2019). Phylogenies of Pathogenic Microorganisms. News-Medical. Retrieved on June 25, 2023, from https://www.news-medical.net/life-sciences/Phylogenies-of-Pathogenic-Microorganisms.aspx.
- Piña-Iturbe, A.; Ulloa-Allendes, D.; Pardo-Roa, C. Comparative and phylogenetic analysis of a novel family of Enterobacteriaceae-associated genomic islands that share a conserved excision /integration module. Sci Rep 2018, 8, 1029. [CrossRef]
- Fouts, D. E.; Matthias, M. A.; Adhikarla, H.; Adler, B.; Amorim-Santos, L.; Berg, D. E. What Makes a Bacterial Species Pathogenic? Comparative Genomic Analysis of the Genus Leptospira. PLoS Negl Trop Dis. 2016 10(2): e0004403. [CrossRef]
- Prasanna, A. N.; Mehra, S. Comparative Phylogenomics of Pathogenic and Non-Pathogenic Mycobacterium. PLOS ONE, 2013, 8(8), e71248. [CrossRef]
- https://www.pinterest.com/pin/72128031523410933/.
- Skandalis, N.; Maeusli, M.; Papafotis, D.; Miller, S.; Lee, B.; Theologidis, I.; Luna, B. Environmental Spread of Antibiotic Resistance. Antibiotics (Basel). 2021 27;10(6):640. [CrossRef]
- Možina, S.; Klančnik, A.; Kovac, J.; Jeršek, B.; Bucar, F. Antimicrobial Natural Products Against Campylobacter. In: Mérillon, JM., Riviere, C. (eds) Natural Antimicrobial Agents. Sustainable Development and Biodiversity, 2018, 19. doi.org/10.1007/978-3-319-67045-4_1.
- Mathela, C. S.; Kumar, V. (2018). Antifungal Activities of Essential Oils from Himalayan Plants. In: Mérillon, JM., Riviere, C. (eds) Natural Antimicrobial Agents. Sustainable Development and Biodiversity, 2018, 19. doi.org/10.1007/978-3-319-67045-4_4.
- Rabin G.; Salam A. I. Natural products as antimicrobial agents, Food Control, 2014; 46, 412-429, doi.org/10.1016/j.foodcont.2014.05.047.
- Quinto, E.J.; Caro, I.; Villalobos-Delgado, L. H.; Mateo, J; De-Mateo-Silleras, B.; Redondo-Del-Río, M. P. Food Safety through Natural Antimicrobials. Antibiotics (Basel). 2019; 31;8(4):208. [CrossRef]
- Holley, R. A.; Patel, D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005; 22:273–292. [CrossRef]
- Davidson, P. M.; Critzer, F. J.; Taylor, T. M.; Naturally occurring antimicrobials for minimally processed foods. Annu Rev Food Sci Technol. 2013; 4:163-90. [CrossRef]
- Teshome, E.; Forsido, S.F.; Rupasinghe, H. P. V.; Olika, K. E. Potentials of Natural Preservatives to Enhance Food Safety and Shelf Life: A Review. Scientific World Journal. 2022; 9901018. [CrossRef]
- https://www.fightbac.org/food-poisoning/foodborne-pathogens/.
- Verraes, C.; Van, B. S.; Van, M. E.; Van, C. E.; Butaye, P.; Catry. B.; de Schaetzen, M. A.; Van, H. X.; Imberechts, H.; Dierick, K.; Daube, G.; Saegerman, C.; De Block, J.; Dewulf, J.; Herman L. Antimicrobial resistance in the food chain: a review. Int J Environ Res Public Health. 2013 28;10(7):2643-69. [CrossRef]
- Reygaert, W. C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018 26;4(3):482-501. [CrossRef]
- Willers, C.; Wentzel, J. F.; du-Plessis, L. H.; Gouws, C.; Hamman, J. H. Efflux as a mechanism of antimicrobial drug resistance in clinical relevant microorganisms: the role of efflux inhibitors. Expert Opin Ther Targets. 2017 21(1):23-36. [CrossRef]
- Munita, J. M.; Arias, C. A.; Mechanisms of Antibiotic Resistance. Microbiol Spectr. 2016 4(2):10.1128/microbiolspec.VMBF-0016-2015. [CrossRef]
- Tegos, G. P.; Haynes, M.; Strouse, J. J.; Khan, M. M.; Bologa, C. G.; Oprea, T. I.; Sklar, L. A.; Microbial efflux pump inhibition: tactics and strategies. Curr Pharm Des. 2011; 17(13):1291-302. [CrossRef]
- Medina, E.; Pieper, D. H. Tackling Threats and Future Problems of Multidrug-Resistant Bacteria. Curr Top Microbiol Immunol. 2016; 398:3-33. [CrossRef]
- Kaye, K. S.; Fraimow, H. S.; Abrutyn E. Pathogens resistant to antimicrobial agents. Epidemiology, molecular mechanisms, and clinical management. Infect Dis Clin North Am. 2000; 14(2):293-319. [CrossRef]
- Williams, J. D.; Sefton, A. M. The prevention of antibiotic resistance during treatment. Infection. 1999; 27 Suppl 2:S29-31. [CrossRef]
- Editorial. NICE antimicrobial stewardship: right drug, dose, and time? Lancet. 2015; 386:717. [CrossRef]
- Annunziato, G.; Strategies to Overcome Antimicrobial Resistance (AMR) Making Use of Non-Essential Target Inhibitors: A Review. Int J Mol Sci. 2019;20(23):5844. [CrossRef]
- Uchil, R. R.; Kohli, G. S.; Katekhaye, V. M.; Swami, O. C.; Strategies to combat antimicrobial resistance. J Clin Diagn Res. 2014; 8(7):ME01-4. [CrossRef]
- Sarfraz, A.; Muhammad, Z. A.; Safa, R.; Seham, E. A.; Mohibullah, S.; Nur, A. C. J.; Suvash C. S. "Recent Approaches for Downplaying Antibiotic Resistance: Molecular Mechanisms", BioMed Research International, 2023, Article ID 5250040, 27 pages. [CrossRef]
- https://www.gavi.org/vaccineswork/what-antimicrobial-resistance-and-how-can-we-tackle-it?gclid=Cj0KCQjwnf-kBhCnARIsAFlg492wec2dzban88CsvAozlXO3gd_tHJJRl1-Q9rprteF-9Gy9XjNouTAaApN8EALw_wcB.
- Wall, S. Prevention of antibiotic resistance - an epidemiological scoping review to identify research categories and knowledge gaps. Glob Health Action. 2019; 12(1):1756191. [CrossRef]
- Aryee, A. Price, N. Antimicrobial stewardship – can we afford to do without it? Br J Clin Pharmacol. 2014; 79:173–181. [CrossRef]
- Dyar, O. J.; Huttner, B.; Schouten, J. What is antimicrobial stewardship? Clin Microbiol Infect. 2017; 23:793–798.
- Bertollo, L. G.; Lutkemeyer, D. S.; Levin, A. S. Are antimicrobial and stewardship programs effective strategies for preventing antibiotic resistance? A systematic review. Am J Infect Control. 2018; 46: 824–836. [CrossRef]
- Van Dijck, C.; Vlieghe, E.; Cox, J. A. Antibiotic stewardship interventions in hospitals in low-and middle- income countries: a systematic review. Bull World Health Organ. 2018; 96:266–280. [CrossRef]
- McCullough, A. R.; Rathbone, J.; Parekh, S. Not in my backyard: a systematic review of clinicians’ knowledge and beliefs about antibiotic resistance. J Antimicrob Chemother. 2015; 70: 2465–2473. [CrossRef]
- Piacenti, F. J.; Leuthner, K. D. Antimicrobial stewardship and clostridium difficile–associated diarrhoea. J Pharm Pract. 2013; 26:506–513. [CrossRef]
- Baur, D.; Gladstone, B. P.; Burkert, F. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017; 17:990–1001. [CrossRef]
- Principi, N. Esposito S. Antimicrobial stewardship in paediatrics. BMC Infect Dis. 2016; 16:424.
- McNulty, C. A. M. European Antibiotic Awareness Day 2012: general practitioners encouraged to target antibiotics through guidance, education and tools. J Antimicrob Chemother. 2012; 67: 2543–2546. [CrossRef]
- Pires, D.; deKraker, M. E. A.; Tartari, E.; ‘Fight Antibiotic Resistance—It’s in Your Hands’: call from the World Health Organization for 5th May 2017. Clinl Infect Dis. 2017; 64:1780–1783. [CrossRef]
- Cox, J. A.; Vlieghe, E.; Mendelson, M. Antibiotic stewardship in low- and middle-income countries: the same but different? Clin Microbiol Infect. 2017; 23:812–818. [CrossRef]
- Gould, I. M.; Lawes, T.; Antibiotic stewardship: prescribing social norms. Lancet (Editorial). NICE antimicrobial. 2016; 387:1699–1701. [CrossRef]
- Yoshinaga, M.; Niu, G.; Yoshinaga-Sakurai, K.; Nadar, V.S.; Wang, X.; Rosen, B.P.; Li, J. Arsinothricin Inhibits Plasmodium falciparum Proliferation in Blood and Blocks Parasite Transmission to Mosquitoes. Microorganisms 2023, 11, 1195. [CrossRef]
- Nichol, D. Jeavons, P.; Fletcher, A. G. Steering evolution with sequential therapy to prevent the emergence of bacterial antibiotic resistance. PLoS Comput Biol. 2015, 11:1004493. [CrossRef]
- Ojala, V.; Laitalainen, J.; Jalasvuori, M. Fight evolution with evolution: plasmid-dependent phages with a wide host range prevent the spread of antibiotic resistance. Evol Appl. 2013; 6:925–932.
- Guiton, P.S.; Sagawa, J.M.; Fritz, H.M.; Boothroyd, J.C. An in vitro model of intestinal infection reveals a developmentally regulated transcriptome of Toxoplasma sporozoites and a NF-κB-like signature in infected host cells. PLoS ONE 2017, 12, e0173018. [CrossRef]
- Hendaus, M.A.; Jomha, F.A.; Ehlayel, M. Allergic diseases among children: nutritional prevention and intervention. Ther Clin Risk Manag. 2016, 12:361-72. [CrossRef]
- Mack, D.R. Probiotics-mixed messages. Can Fam Physician. 2005, 51(11):1455-7, 1462-4.
- Holst, H.; Breves, G.P. Erfahrungsmedizin zum therapeutischen Standard [Probiotics--from empirical medicine to therapeutic standard]. Z Gastroenterol. 2005, 43(6):601-6. German. [CrossRef]
- Megha, S.; Shalini, G,; Varsha, S.A.; Abhishek, D.; Neetu, J. Effect of Short-Term Placebo-Controlled Consumption of Probiotic Yoghurt and Indian Curd on the Streptococcus mutanTs Level in Children Undergoing Fixed Interceptive Orthodontic Therapy. Turk J Orthod. 2019, 32(1):16-21. [CrossRef]
- Cabana, M.D.; Shane, A.L.; Chao, C.; Oliva-Hemker, M. Probiotics in primary care pediatrics. Clin Pediatr (Phila). 2006, 45(5):405-10. [CrossRef]
- Kahbazi, M.; Ebrahimi, M.; Zarinfar, N.; Arjomandzadegan, M.; Fereydouni, T.; Karimi, F.; Najmi, A.R. Efficacy of Synbiotics for Treatment of Bacillary Dysentery in Children: A Double-Blind, Randomized, Placebo-Controlled Study. Adv Med. 2016; 2016:3194010. [CrossRef]
- Buhl, M.R. Probiotika til behandling af infektiøs diaré [Probiotics in the treatment of infectious diarrhoea]. Ugeskr Laeger. 2005, 167(22):2427.
- Ouwehand, A.; Vesterlund, S. Health aspects of probiotics. I Drugs. 2003, 6(6):573-80.
- Manzanares, W.; Lemieux, M.; Langlois, P.L.; Wischmeyer, P.E. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Crit Care. 2016, 19:262. [CrossRef]
- Baştürk, A.; Artan, R.; Yılmaz, A. Efficacy of synbiotic, probiotic, and prebiotic treatments for irritable bowel syndrome in children: A randomized controlled trial. Turk J Gastroenterol. 2016, 27(5):439-443. [CrossRef]
- Hendaus, M.A.; Jomha, F.A.; Ehlayel, M. Allergic diseases among children: nutritional prevention and intervention. Ther Clin Risk Manag. 2016, 12:361-72. [CrossRef]
- Jiang, Z.; Yuping, Z.; Gang, S. Effect of Probiotic Yogurt Supplementation(Bifidobacterium animalis ssp. lactis BB-12) on Gut Microbiota of Female Taekwondo Athletes and Its Relationship with Exercise-Related Psychological Fatigue, Microorganisms 2023, 11(6), 1403. [CrossRef]
- Weili, S.; Hongpeng, S.; Chengyan, G.; Keyuan. L.; Guangyu, L. Effects of Different Yeast Selenium Levels on Rumen Fermentation Parameters, Digestive Enzyme Activity and Gastrointestinal Microflora of Sika Deer during Antler Growth, Microorganisms 2023, 11(6), 1444. [CrossRef]
- Jang Z.; Yuping, Z.; Gang, S. Effect of Probiotic Yogurt Supplementation(Bifidobacterium animalis ssp. lactis BB-12) on Gut Microbiota of Female Taekwondo Athletes and Its Relationship with Exercise-Related Psychological Fatigue. Microorganisms 2023, 11(6), 1403. [CrossRef]
- Krista, S.; Johanna, H.; Heli, A.; Ashley, A.H.; Ilmari Ahonen, M.T.; Saarinen, J.M.; Arthur C. O. The Effect of Human Milk Oligosaccharides and Bifidobacterium longum subspecies infantis Bi-26 on Simulated Infant Gut Microbiome and Metabolites. Microorganisms 2023, 11(6), 1553. [CrossRef]
| Type | Bacteria and associated infections |
|---|---|
|
Obligate aerobic (Gram-negative cocci) |
Moraxella catarrhalis is a gram-negative diplococcus that causes ear and upper and lower respiratory infections. M. catarrhalis was formerly known as Branhamella catarrhalis. Neisseria gonorrhoeae is caused by the bacterium Neisseria gonorrhoeae. It typically infects the epithelia of the urethra, cervix, rectum, pharynx, or conjunctivae, causing irritation or pain and purulence. N. meningitidis Meningococcal (Neisseria meningitidis) are gram-negative diplococci that cause meningitis and meningococcemia. Symptoms, usually severe, include headache, nausea, vomiting, photophobia, and Meningococcal diseases. |
| Gram-positive bacilli | Corynebacterium jeikeium |
| Acid-fast bacilli | Mycobacterium avium complex, M. kansasii, M. leprae, M. tuberculosis, Nocardia species |
| Nonfermentative, non-Enterobacterales (formerly Enterobacteriaceae) | Acinetobacter calcoaceticus, Elizabethkingia meningoseptica (formerly Flavobacterium meningosepticum), Pseudomonas aeruginosa Pseudomonas and Related Infections Pseudomonas aeruginosa and other members of this group of gram-negative bacilli are opportunistic pathogens that frequently cause hospital-acquired infections, particularly in ventilator, P. alcaligenes, others are Pseudomonas species, and Stenotrophomonas maltophilia |
| Fastidious gram-negative coccobacilli and bacilli | Brucella Brucellosis is caused by Brucella species, which are gram-negative bacteria. Symptoms begin as an acute febrile illness with few or no localized signs and may progress to a chronic stage. Bordetella, Francisella, Legionella, and Legionella pneumophila are gram-negative bacillus that most often cause pneumonia with extrapulmonary features. Diagnosis requires specific growth media, serologic or urine antigen. |
| Leptospiraceae (spiral bacteria) | Leptospira Leptospirosis is an infection caused by one of several pathogenic serotypes of the spirochete Leptospira. Symptoms are biphasic. Both phases involve acute febrile episodes. |
| Obligate anaerobic | |
| Gram-negative bacilli | Bacteroides fragilis, other Bacteroides Mixed Anaerobic Infections Anaerobes can infect normal hosts and hosts with compromised resistance or damaged tissues. Mixed anaerobic infections can include both single anaerobic species or multiple anaerobic species... read more species, Fusobacterium species, Prevotella species. |
| Gram-negative cocci | Veillonella species |
| Gram-positive cocci | Peptococcus niger, Peptostreptococcus Mixed Anaerobic Infections Anaerobes can infect normal hosts and hosts with compromised resistance or damaged tissues. Mixed anaerobic infections can include both single anaerobic species or multiple anaerobic species. |
| Non–spore-forming gram-positive bacilli | Actinomyces, Bifidobacterium, Eubacterium, and Cutibacterium (formerly Propionibacterium) species. |
| Endospore-forming gram-positive bacilli | Clostridium botulinum, Botulism is poisoning that is due to Clostridium botulinum toxin and that affects the peripheral nerves. Botulism may occur without infection if the toxin is ingested, injected, or inhaled. C. perfringens Clostridium perfringens is a Food Poisoning Clostridium perfringens Food poisoning is acute gastroenteritis caused by ingestion of contaminated food. Symptoms are watery diarrhoea and abdominal cramps. Diagnosis is by identifying. C. tetani, others are Clostridium species. |
| Facultative anaerobic | |
| Gram-positive cocci, catalase-positive | Staphylococcus aureus is a gram-positive aerobic organism. Staphylococcus aureus is the most pathogenic; it typically causes skin infections and sometimes pneumonia, endocarditis, and osteomyelitis. Staphylococcal Infections(coagulase-positive), S. epidermidis (coagulase-negative), and others are coagulase-negative staphylococci. |
| Gram-positive cocci, catalase-negative | Enterococcus faecalis are gram-positive, facultative anaerobic organisms. Enterococcus faecalis and E. faecium cause a variety of infections, including endocarditis, and urinary tract infections. E. faecium, Streptococcus agalactiae (group B streptococcus), S. bovis, S. pneumoniae Streptococcal Infections Streptococci are gram-positive aerobic organisms that cause many disorders, including pharyngitis, pneumonia, wound and skin infections, sepsis, and endocarditis. S. pyogenes (group A streptococcus), viridans group streptococci (S. mutans, S. mitis, S. salivarius, S. sanguis), S. anginosus group (S. anginosus, S. milleri, S. constellatus), Gemella morbillorum |
| Gram-positive bacilli | Bacillus anthracis Anthrax is caused by the gram-positive Bacillus anthracis, which are toxin-producing, encapsulated, facultative anaerobic organisms. Anthrax is a fatal disease of animals transmitted by Anthrax, Erysipelothrix rhusiopathiae, Gardnerella vaginalis (gram-variable) |
| Gram-negative bacilli | Enterobacterales (Citrobacter species, Enterobacter Klebsiella, Enterobacter, and Serratia Infections). The gram-negative bacteria Klebsiella, Enterobacter, and Serratia are closely related to normal intestinal flora that rarely cause disease in normal hosts. The gram-negative bacterium Escherichia coli is the most numerous aerobic commensal inhabitants of the large intestine. Certain strains cause diarrhoea, and all can cause infection when Klebsiella species, Morganella morganii, Proteus species, Plesiomonas shigelloides, Providencia rettgeri, Salmonella typhi whose genus Salmonella is divided into two species, S. enterica and S. bongori, which include over 2500 known serotypes. Some of these serotypes are named. In such cases, other Salmonella species, Serratia marcescens, Shigella Shigellosis Shigellosis is an acute infection of the intestine caused by the gram-negative Shigella species. Yersinia enterocolitica Plague and Other Yersinia Infections Plague is caused by the gram-negative bacterium Yersinia pestis. |
| Fermentative, non-Enterobacterales | Aeromonas hydrophila, Chromobacterium violaceum, and Pasteurella multocida |
| Fastidious gram-negative coccobacilli and bacilli | Aggregatibacter (formerly Actinobacillus) actinomycetemcomitans, Eikenella corrodens, Haemophilus influenzae Haemophilus Infections. The gram-negative bacteria Haemophilus species cause numerous mild and serious infections, including bacteremia, meningitis, pneumonia, sinusitis, otitis media, cellulitis, and epiglottitis and, other Haemophilus species. |
| Mycoplasma Mycoplasmas | Mycoplasma pneumoniae, Mycoplasmas Mycoplasmas are ubiquitous bacteria that differ from other prokaryotes in that they lack a cell wall. Mycoplasma pneumoniae is a common cause of pneumonia, particularly community-acquired. |
| Microaerophilic | |
| Curved bacilli | Campylobacter jejuni, Helicobacter pylori Infection Helicobacter pylori is a common gastric pathogen that causes gastritis, peptic ulcer disease, gastric adenocarcinoma, and low-grade gastric lymphoma. Infection may be asymptomatic or Vibrio cholerae, and V. vulnificus |
| Spirochaetaceae (spiral bacteria) | Borrelia burgdorferi Lyme is a tick-transmitted infection caused by the spirochete Borrelia species. Treponema pallidum Syphilis is caused by the spirochete Treponema pallidum and is characterized by three sequential symptomatic stages separated by periods of asymptomatic latent infection. |
| Obligate intracellular parasitic | |
| Chlamydiaceae | Chlamydia trachomatis Three species of Chlamydia cause human disease, including sexually transmitted infections and respiratory infections. |
| Coxiellaceae | Coxiella burnetii is an acute or chronic disease caused by the rickettsial-like bacillus Coxiella burnetii. Acute disease causes sudden onset of fever, headache, malaise, and interstitial pneumonitis. |
| Rickettsiales | Rickettsia prowazekii, R. rickettsii, R. typhi, Orientia tsutsugamushi Scrub Typhus Scrub typhus is a mite-borne disease caused by Orientia tsutsugamushi (formerly Rickettsia tsutsugamushi). Scrub Typhus, Ehrlichia chaffeensis Ehrlichiosis and Anaplasmosis Ehrlichiosis and anaplasmosis are caused by rickettsial-like bacteria. Ehrlichiosis is caused mainly by Ehrlichia chaffeensis; anaplasmosis is caused by Anaplasma phagocytophilum and Ehrlichiosis and Anaplasmosis, Anaplasma phagocytophilum. |
| Types of organisms | Sources Foods |
|---|---|
| Vibrio vulnificus | raw or undercooked seafood, particularly shellfish. |
| Staphylococcus aureus: | cooked foods high in protein (e.g. cooked ham, salads, bakery products, dairy products) that are held too long at room temperature. |
| Shigella | salads, unclean water, and any food handled by someone who is infected with the bacterium |
| Toxoplasma gondii | raw or undercooked pork. |
| Salmonella | raw and undercooked eggs, undercooked poultry and meat, fresh fruits and vegetables, and unpasteurized dairy products |
| Listeria monocytogenes | unpasteurized dairy products, including soft cheeses; sliced deli meats; smoked fish; hot dogs; pate’; and deli-prepared salads (i.e. egg, ham, seafood, and chicken salads). |
| Norovirus | Any food contaminated by someone who is infected with this virus. |
| Clostridium botulinum | improperly prepared home-canned foods; honey should not be fed to children less than 12 months old. |
| E. coli O157:H7 | beef, especially undercooked or raw hamburger; produce; raw milk; and unpasteurized juices and ciders. |
| Campylobacter | raw and undercooked poultry and other meat, raw milk and untreated water. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




