1. Introduction
Clinical decision-making is a complex process that involves numerous factors, including clinical evidence, patient values, and healthcare professionals’ expertise [
1]. Clinical decision support systems (CDSSs) have been developed to aid healthcare providers in making informed decisions. However, traditional CDSSs have limitations in considering patient values, which are crucial in clinical decision-making. These limitations can lead to a mismatch between the treatment plan and the patients’ values and preferences, leading to compromise health outcomes.
There is a growing trend towards patient values into CDSSs through various methods such as using PROMs and SDM [
2,
3]. PROMs are standardized questionnaires that assess the patients’ health status from their perspectives and can be incorporated into CDSSs to gain a better understanding of the patients’ values and preferences. Torenholt et al. proposed the concept of recontextualisation work, revealing the efforts of nurses in recontextualizing decontextualized PRO data [
4]. SDM is a collaborative process for the patients and healthcare providers to make informed decisions together by discussing treatment options, their risks and benefits, and patients’ values.
In the NICE clinical guidelines, some treatment options may mention associated side effects that can potentially challenge certain values held by patients. The impact of values is described ambiguously in the NICE clinical guidelines, lacking standardized quantitative analysis, which hinders the connection between values and treatment plans.
Incorporation of patient values into CDSSs through the use of PROMs and SDM [
2,
3] results in the development of more personalized treatment plans that align with patients’ values and preferences, ultimately leading to a higher patient satisfaction. However, patient-reported PROMs are scattered, and no patients’ value-based evidence is get available to support personalized treatment recommendation systematically.
There are challenges in realizing this vision. In particular, we need to answer three research questions:
Q1: What are patients’ values, exactly, that affect clinical decision-making, and how can we build a value-based evidence for it? (
Section 3.3)
Q2: Can we put forward a unified formalism for value-based evidence and clinical evidence already adopted, so that decision makers can be supported in a systematic manner? (
Section 3.4 to
Section 3.6)
The remaining parts of the paper is organised as follows:
1.
Section 2 provides an overview of the research background, covering conventional methods in clinical decision-making, integration of patient values, and the use of literature analysis.
2.
Section 3 explains the process and methodology used for data collection and analysis, including the clinical evidence format, value-based evidence extraction, and the design of an integrated format. It also introduces the operational mode of fused evidence, population values, and the recommendation algorithm.
3.
Section 4 presents a prototype system used for validation. It demonstrates the system’s functionality using real-life cases and includes a comparative experiment with GPT, patient reselection schemes, and the system.
2. Background and Related Works
2.1. Conventional Methods for Clinical Decision-Making
The recommendation of treatment plans has traditionally been explored in the realm of representing and interpreting clinical guidelines. John Fox and his colleagues proposed PROforma, a formalism that offers an evidence-based and objective tool for selecting treatment plans and providing decision support. Its main goal is to empower clinicians with optimal treatment recommendations [
5]. Systems based on this formalism have the capability to furnish doctors with tailored treatment recommendations, taking into account patients’ conditions, medical history, and examination results, through computer-interpretable representation.
In addition to PROforma, several studies have explored alternative methods and techniques for recommending treatment plans. Romina et al. examined the interpretation and utilization of clinical practice guidelines or recommendations [
6]. Some research focuses on recommending treatment plans through the utilization of clinical guidelines or expert knowledge. For instance, Parikh et al. developed guidelines for diagnosing, clinically assessing, treating, and managing patients with mitochondrial disease [
7]. Domain et al. proposed a decision support system for breast cancer treatment based on data mining technologies and clinical practice guidelines. They discussed the system’s implementation, application, and evaluation [
8]. In collaboration with John Fox, we have proposed a systematic approach for representing argumentation, recommendation, and explanation in clinical decision support [
9]. The foundation of this approach lies in a generic argumentation and recommendation scheme. Building upon this foundation, we represent argumentation rules for clinical guidelines using the Resource Description Framework (RDF). Additionally, an associated rule engine is developed for interpreting these rules, and recommendation rules are represented using the Semantic Web Rule Language (SWRL) [
10].
2.2. Studies on Patient Values and Their Relationships with Decision-Making
Patient values play a crucial role in medical decision-making, and numerous studies have focused on incorporating patient values to assist doctors in making personalized and patient-centered decisions. In their article, Berry et al. outlined six categories of patient values, including activities, abilities, possessions, principles, emotions, and relationships [
11]. These values reflect patients’ perspectives and evaluations of their preferred activities, functional abilities, material possessions, guiding principles, emotional well-being, and social relationships. These factors significantly impact medical decision-making and the selection of treatment plans, ensuring a more holistic and patient-centric approach to healthcare.
In addition to the aforementioned studies, Epstein and Street extensively discussed the significance of patient-centered care and its practical application in considering patient values [
12]. They argue that patient-centered care better addresses patients’ needs and values, enabling them to participate more effectively in medical decision-making. Furthermore, Curtis et al. proposed guidelines to assist healthcare providers in gaining a better understanding of patients’ values and needs, and incorporating them into the selection of treatment plans [
13]. These researches offer valuable insights and strategies for promoting patient-centered care and facilitating shared decision-making processes in clinical practice.
Furthermore, numerous studies have investigated the integration of values into medical decision support systems. For instance, Wherton et al. developed a value-based medical decision support system that aids doctors in comprehending patients’ values and needs, thereby providing personalized treatment recommendations [
14]. Liu et al. proposed a method to integrate clinical knowledge and patient preferences into an integrated knowledge graph. They extracted objective data from semi-structured online medical service interfaces and subjective emotional data from patient review pages. The system can recommend a ranked list of doctors based on the best alignment with both clinical background and patient preferences [
15]. Wu et al. proposed a method that utilizes patient experience data and clinical guidelines to construct evidence for patient-oriented clinical decision support. This method incorporates a weighted expression that balances patient symptoms and treatment plans [
16]. In our previous work, we presented a model that utilizes clinical experience data as evidence to support patient-oriented decision-making. This model combines the experience data of similar patients from social networks with argumentation rules derived from clinical guidelines [
17].
In summary, while certain studies have introduced the concept of patient values into clinical decision-making and developed models that incorporate patient values, there remains a lack of systematic utilization of values to establish concrete evidence of their influence on medical decision-making.
2.3. Literature Analysis Methods
Literature analysis is a widely adopted research method that facilitates the systematic collection, organization, evaluation, and synthesis of literature, enabling the exploration and examination of the current state, trends, and issues within a specific research field. This methodology finds applications in various domains, including medicine, social sciences, and education. For instance, Porr et al. employed literature analysis to investigate the ethical challenges confronted by nurses in long-term care facilities when providing care for individuals with dementia[
18] Hsieh et al. utilized literature analysis to compare and contrast three qualitative content analysis methods, thoroughly discussing their respective advantages, disadvantages, and appropriate usage scenarios [
19]. In another study, Taremwa et al. conducted a comprehensive literature analysis to quantitatively analyze the number of publications, themes, and authors concerning malaria vector control and drug resistance in malaria vectors [
20].
There may exist implicit mapping relationships between treatment plans and values, which are often not explicitly addressed in clinical guidelines. It is hypothesized that these relationships can be supplemented by analyzing clinical decision literature using a literature analysis approach, incorporating relevant references to inform the decision-making process. Patient values can be influenced by diverse factors, including cultural background, beliefs, social environment, educational background, and personal experiences. By systematically collecting, organizing, and analyzing relevant literature, a deeper understanding of patient needs and values can be attained, facilitating the development of treatment plans that better align with patient requirements. Through literature analysis, the objective is to establish a foundation of arguments centered on patient values.
Table 1 presents the key terms identified for different categories of values, while
Table 2 presents the key terms for candidate treatments. These two sets of keywords are integrated and utilized as filters to retrieve relevant literature from the database, thereby enhancing the retrieval of literature pertinent to the identified keywords.
3. Materials and Methods
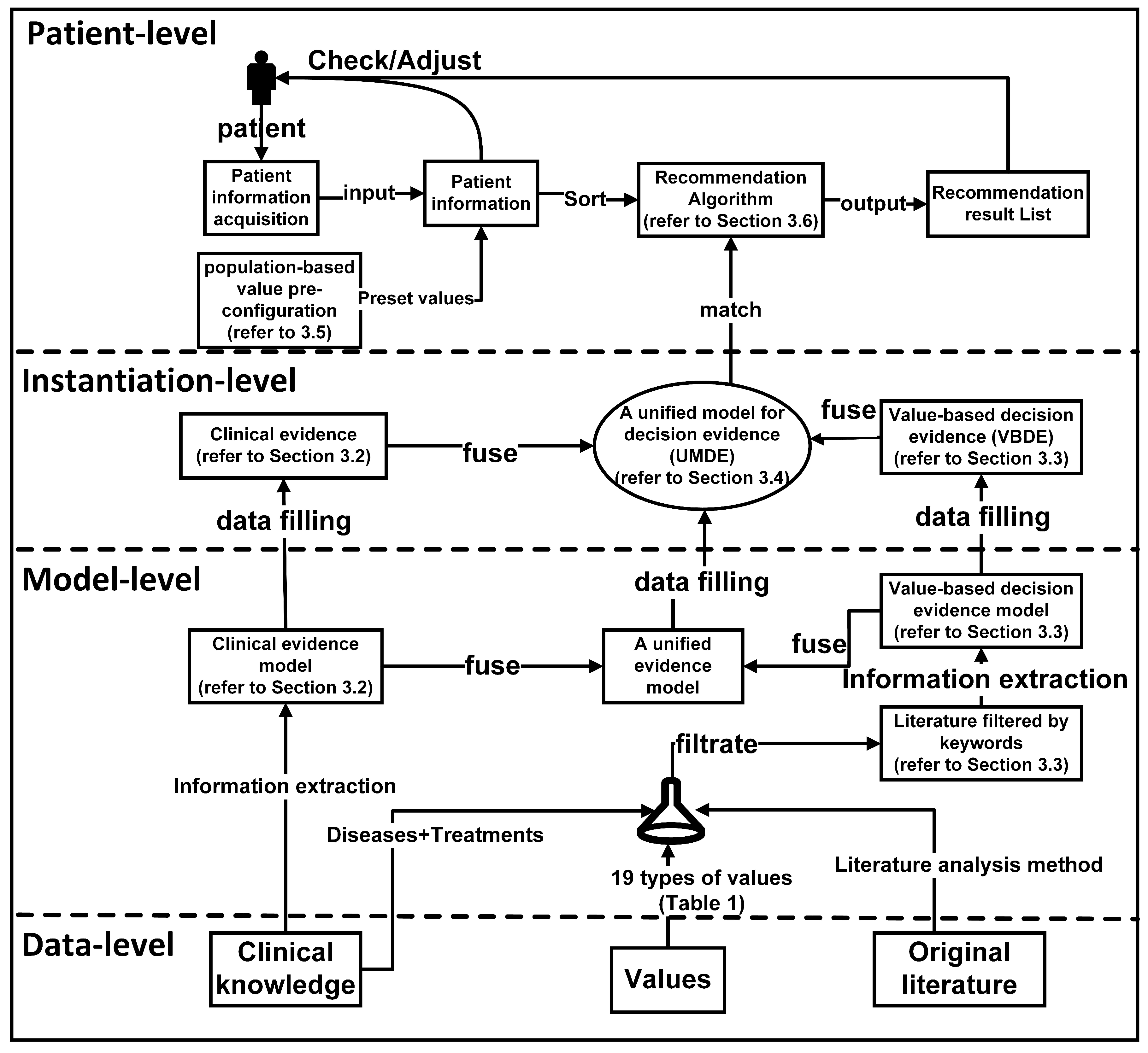
3.1. An Overview of the Model
The model overview, which encompasses the influence of values on decision-making, is depicted in
Figure 1, and the process unfolds as follows:
1. Collecting clinical evidence and conducting model analysis (detailed in
Section 3.2): In the initial phase, we gathered objective clinical knowledge from NICE clinical guidelines, encompassing disease names, associated symptoms, diagnoses, and treatment plans. The interrelationships among these entities are outlined in the NICE clinical guidelines. Based on the analysis of these entities and relationships, we formulated a model for clinical evidence, as depicted in
Figure 2. Subsequently, the clinical knowledge acquired from NICE underwent formatting and decomposition using the proposed approach. To facilitate computer recognition and processing of clinical evidence, we devised a hierarchical structure to represent the clinical evidence in the RDF format.
2. Collecting value evidence (literature) and developing a model (detailed in
Section 3.3): We obtained research papers pertaining to diseases and values from PubMed. To illustrate the process, we employed a combination of keywords, specifically ’breast cancer’, along with the 29 values listed in
Table 1, to retrieve 2506 relevant papers. After eliminating duplicates, we were left with 2487 unique papers. Subsequently, we augmented the screening process by incorporating the treatment plans provided by NICE, as presented in
Table 2, as additional screening keywords. This refinement resulted in a final set of 341 papers encompassing diseases, treatment plans, and values. These 341 papers were then categorized into 27 groups based on the combination of treatment plans and organized into a literature database. Utilizing a manual reading method, we meticulously reviewed the papers and extracted key content. To effectively capture the manifestation of value influence on decision-making within the literature and establish a hierarchical relationship among entities, we devised a model for value evidence.
3. Constructing a unified model for decision evidence (UMDE) (detailed in
Section 3.4): To ensure that the value evidence extracted from the literature is accessible for patient decision-making, it is crucial to integrate the clinical evidence model and the value evidence model into a unified framework. Hence, we propose the UMDE, depicted in
Figure 3. UMDE incorporates the structural characteristics of both the clinical evidence and value evidence models and leverages the extracted value influence from the literature as an incremental factor in clinical decision-making, thereby facilitating subsequent patient decision-making processes.
4. Establishing population-based value pre-configuration(detailed in
Section 3.5): During our investigation of patient values, we have observed a strong correlation between the characteristics of the patient population and their prioritized values. For instance, occupation plays a significant role in individuals’ lives, and the values associated with different occupations often align with those valued by patients. Moreover, most patients prefer treatment plans that do not disrupt their occupational routines. To capture this relationship, we have collected extensive occupational information through occupational classification. Leveraging statistical knowledge, as depicted in
Table 3, we have linked these occupations to medically relevant values and assigned them predefined weights. This approach aims to facilitate the model in providing essential values while exploring patient values in subsequent analyses.
5. Performing runtime clinical data and value elicitation, and VMDE application with weight adjustment (detailed in
Section 3.6 -
Section 4.1): Clinical symptoms and value information are acquired from patients through the utilization of a problem-guided interview method. The collected clinical information encompasses disease type, symptom severity, disease progression, treatment history, and other relevant details. Moreover, understanding the patients’ personal values is crucial, including their values pertaining to activities, possessions, principles, emotions, relationships, abilities, and the 29 values listed in
Table 1. The predefined values outlined are employed to map and analyze the patients’ objective conditions and value inputs. The resulting degree of patient value preference and objective situation, obtained by the current model, are then outputted and provided to the patients for data refinement and verification. The patients’ fine-tuning behavior regarding the data is recorded within the model to enable automatic adjustment of predefined values, rendering them more realistic. Clinical symptom information obtained from patients is compared with symptom details in treatment plans and ranked based on severity and impact, utilizing predefined weights. These weights are subsequently used for plan recommendation and weight calculation in subsequent stages. The ranking of objective symptom information, based on weight, is integrated with evidence-based decision-making rooted in values. Relevant information that aligns with the evidence is extracted, and objective weights are consolidated to determine the final weight for each recommended plan. Different treatment plans are then ranked and recommended based on the degree of influence and weight assigned to distinct values, thereby assisting doctors in formulating personalized treatment plans that align with patient requirements. For detailed algorithms, please refer to
Section 3.6.
Through these sequential steps and processes, patients’ personalized needs and value factors can be integrated into clinical decision-making and treatment plan selection, adhering to a value-based decision-making approach. This, in turn, assists doctors in comprehensively understanding patients’ needs and expectations, and developing highly personalized treatment plans to meet their individual requirements.
3.2. Collecting and Modeling Clinical Evidence
In this section, a method has been employed to extract objective knowledge evidence from clinical guidelines. The specific steps involved in this approach will now be provided in detail. By systematically extracting objective knowledge evidence from clinical guidelines, this section aims to contribute to the development of a comprehensive and reliable knowledge base.
Based on previous work, the treatment option analysis for breast cancer was specifically chosen from the NICE guidelines. In order to extract objective knowledge, a combination of manual reading and information extraction tools was utilized, as illustrated in
Figure 4. The extracted objective knowledge encompasses crucial information about the disease, treatment options, and associated symptoms. To ensure a structured representation of this knowledge, a hierarchical Resource Description Framework (RDF) format was meticulously designed, as depicted in
Figure 2. The RDF consists of two distinct parts: the first part focuses on the treatment options, while the second part elaborates on the weights assigned to objective symptoms for each treatment option. This structured representation enables a comprehensive and organized understanding of the extracted knowledge, facilitating subsequent analysis and decision-making processes.
Patient information is effectively matched with the symptoms stored in the RDF, thereby activating the corresponding arguments within the database. Each argument is equipped with a "support-type" attribute, which signifies its stance in relation to the treatment option. When the attribute value is "for", it indicates that the activated argument supports the corresponding treatment option, and the specific weight is specified within the "weight" attribute. Conversely, when the attribute value is "against", it signifies that the activated argument opposes the treatment option, and the weight is derived from the negation of the value provided in the "weight" attribute. Through the process of matching and activating the objective symptoms using the RDF file, the weights and argument information for each treatment option are effectively obtained, enabling a comprehensive evaluation and comparison of different treatment options based on their corresponding evidence.
This systematic approach serves as a valuable resource, empowering medical professionals and decision-makers to make well-informed choices when considering various breast cancer treatment options. The structured RDF representation of the extracted knowledge further enhances the clarity and comprehensibility of the information. For a more detailed visualization and understanding of the RDF structure, please refer to
Figure 2.
3.3. Value-based Medical Decision-Making and Treatment Plan Selection
We adopted a literature analysis method to investigate the impact of diverse values on medical decision-making and treatment plan selection. It is crucial to emphasize that the automatic keyword filtering from the repository is conducted using code, whereas the extraction of value evidence relies on a manual reading of the literature content. Before commencing the literature screening process, it is essential to establish a dedicated keyword database to identify relevant literature pertinent to our research objectives.
We have expanded on prior research [
11] by enhancing the six categories of values, specifically activities, abilities, relationships, emotions, principles, and possessions, through the introduction of more nuanced sub-values. These refined values can fall into multiple categories, and their interrelationships are outlined in
Table 1.
The classification and categorization of values aim to enhance our understanding of individuals’ experiences and needs in medical treatment, as well as the focus and priorities of medical institutions and practitioners when it comes to patient care. For the fine-grained values, we need to classify and sort them to facilitate literature screening and analysis. It should be noted that certain fine-grained values may fall into multiple categories. Thus, we need to organize and classify the relationships between these values to enhance literature screening and analysis.
As an illustrative example, our study specifically targeted breast cancer, and we extracted 14 treatment plans mentioned in the NICE guidelines to serve as keywords for subsequent literature screening. These keywords function as criteria for matching and identifying literature pertaining to breast cancer treatment plans. The specific keywords utilized in this context are provided in
Table 2. By employing these keywords as screening criteria, we can identify literature that specifically addresses breast cancer treatment plans, allowing for further analysis of the values and concerns associated with them.
It was observed that utilizing a combination of disease and a single value as search keywords had the potential to retrieve articles multiple times, especially if they contained multiple values. To mitigate this issue, a filtering process was implemented to eliminate articles with completely duplicated titles and abstracts. This meticulous filtering process resulted in a final selection of 2487 unique articles, ensuring the inclusion of diverse and distinct literature for analysis.
The primary objective of our research is to establish meaningful connections between values and relevant treatment plans. To achieve this, the initial pool of 2487 articles underwent a meticulous screening process, specifically targeting those articles that mentioned the specific treatment plan keywords listed in
Table 2. Through this rigorous screening, a subset of 341 articles was identified from the original 2506 articles, where these selected articles made explicit references to treatment plans. This refined subset of articles will serve as a valuable resource for our analysis and examination of the relationship between values and treatment plans.
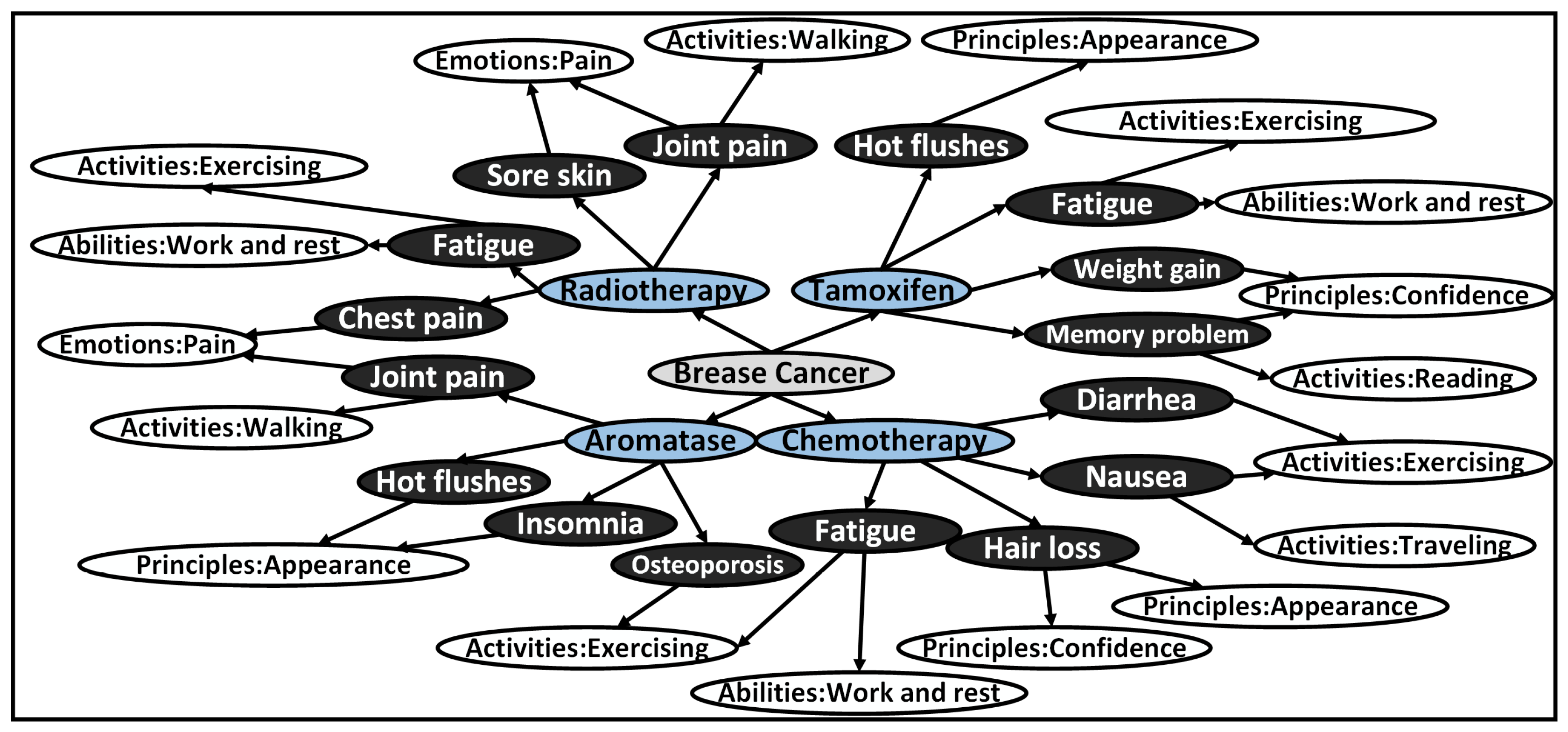
Through meticulous analysis, we categorized the treatment plans mentioned in the 341 selected articles, leading to the identification of 27 distinct combinations associated with specific values. This classification, visually represented in
Figure 5, provides a comprehensive overview of the relationships between treatment plans and their corresponding values. To support our research and analysis, we compiled a comprehensive literature database consisting of article titles, abstracts, links, treatment plans mentioned in the articles, and their associated values. This database serves as a crucial foundation for constructing our value-based decision-making model, as depicted in
Figure 3. By conducting manual reading of 100 papers from the selected subset, we extracted pertinent information that significantly influences decision-making based on values, which has been thoughtfully incorporated into
Table 4 as part of the literature database for values influencing decision-making. The amalgamation of these steps and resources has enabled us to establish meaningful connections between treatment plans and the relevant values, contributing to an enhanced understanding of the impact of values on medical decision-making.
3.4. A Unified Model for Decision Evidence
Through meticulous analysis of the extracted values from the decision literature database, as presented in
Table 4, numerous pieces of evidence have emerged concerning the impact of values on decision-making. Within the literature analysis process, it was revealed that the literature addresses the potential existence of single or multiple treatment options. Comparisons are drawn between these options, with a single value-based evidence potentially incorporating two treatment alternatives, one garnering support and the other facing opposition. The evidence that elucidates the influence of values on decision-making encompasses the clinical context or population characteristics, pertinent values, the treatment options advocated or contested, as well as the weight attributed to the evidence.
Table 5 offers a comprehensive overview of decision justifications influenced by values, thus serving as an indispensable resource to guide value-based clinical decisions.
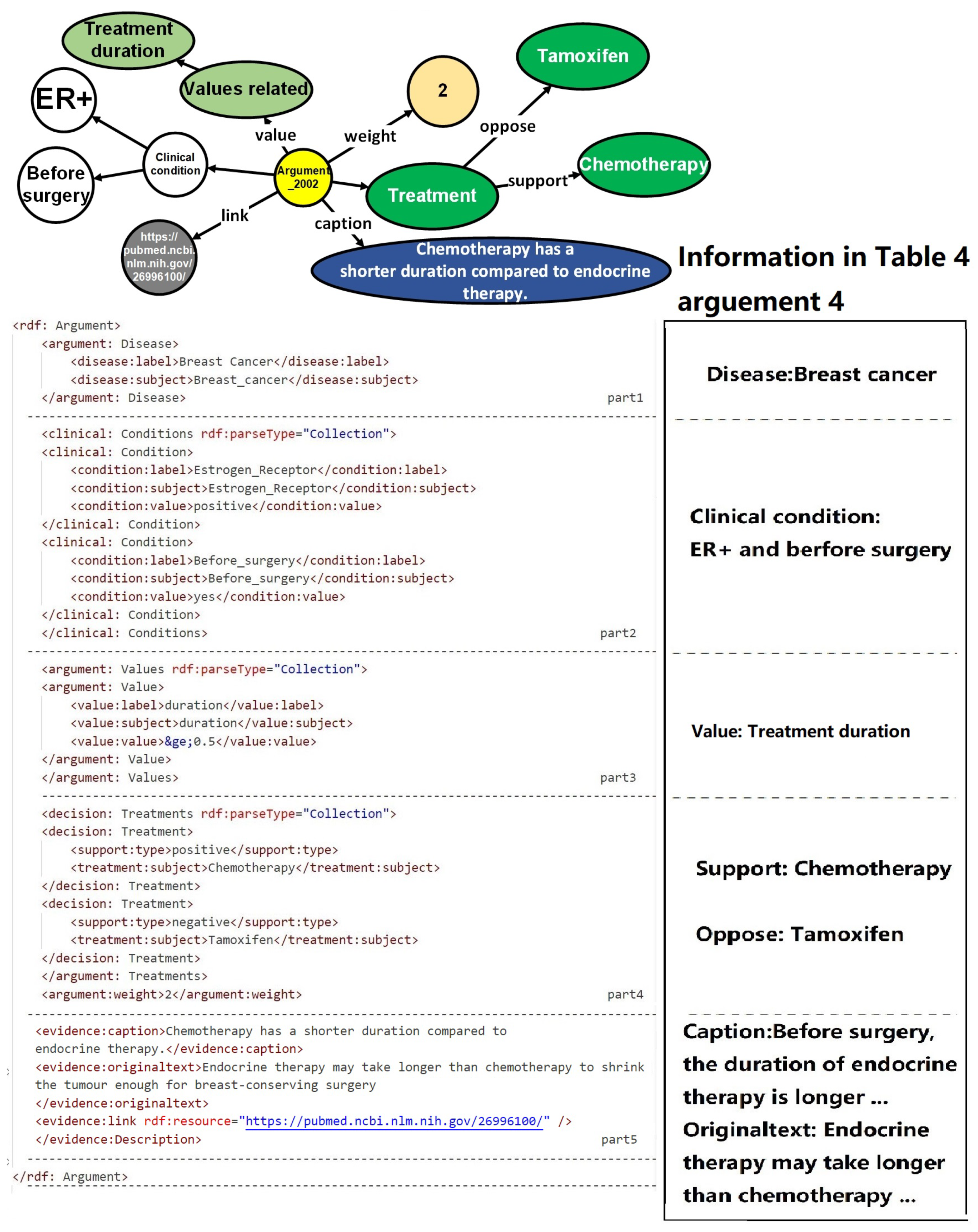
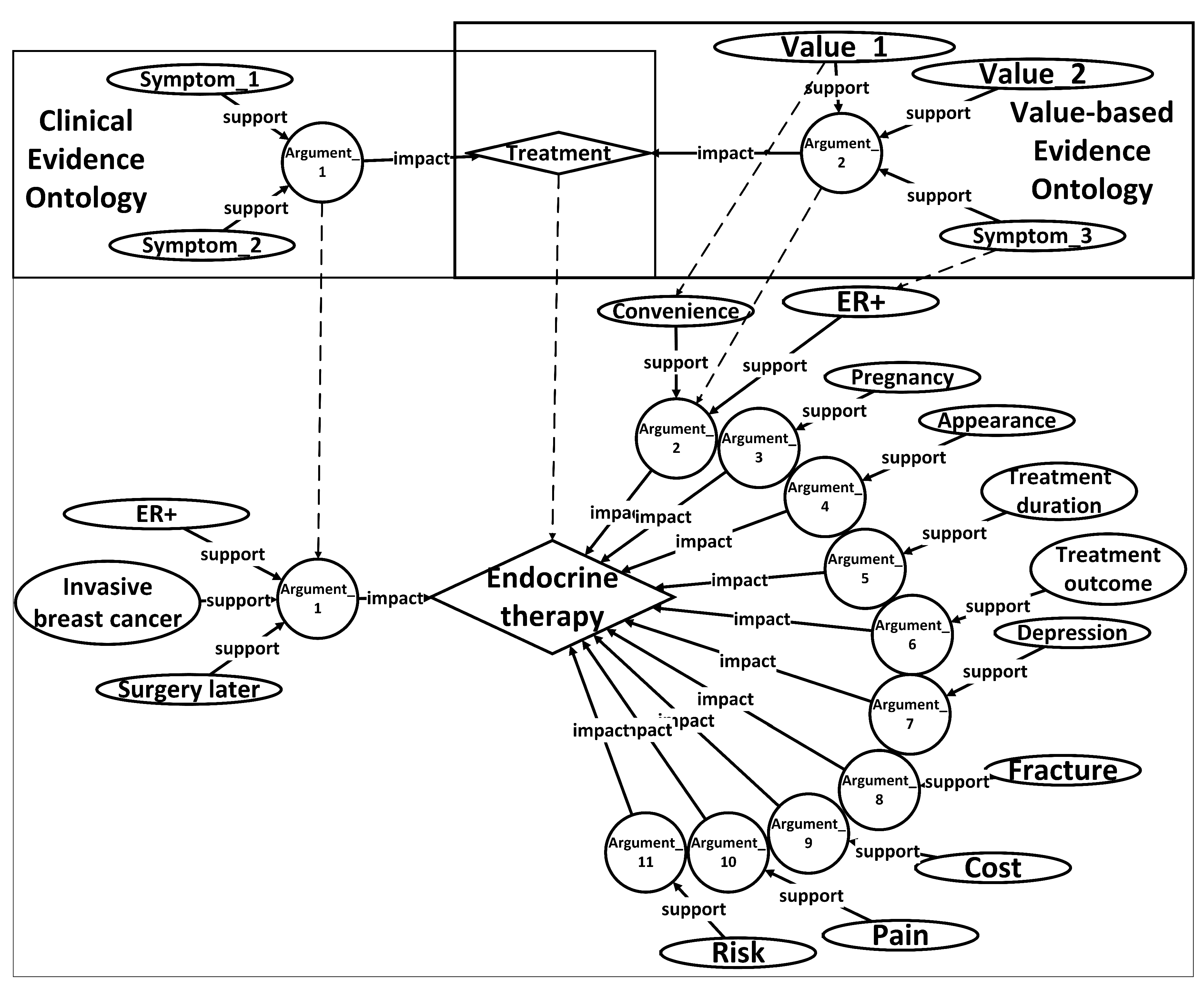
In order to facilitate the recognition and utilization of evidence by computers, surpassing the limitations imposed by tabular formats, an ontology named Unified Decision Evidences has been developed to organize the information. The upper of
Figure 6 illustrates the abstract ontology layer, wherein treatment options act as intermediate entities that establish connections between the clinical evidence ontology and the value-based evidence ontology. The relationships between these ontologies are exemplified in the lower part of
Figure 6, employing endocrine therapy as a case study, amalgamating clinical evidence and value-based evidence derived.
Upon the establishment of the ontology structure, an RDF framework has been devised to store and organize the argumentation structure showcased in the diagram. This RDF-based arrangement facilitates the seamless integration and linkage of information in a machine-readable format. The design of the argumentation structure aims to forge connections between arguments and diseases, thereby enabling a comprehensive representation of objective conditions and values. Furthermore, it offers a lucid depiction of treatment plans while furnishing supporting information to bolster the arguments.
Figure 3 portrays a prototypical argumentation structure encompassing five distinct parts. The first part establishes the correlation between the argumentation and the pertinent disease, thereby ensuring contextual relevance. The subsequent portion delineates the rules governing objective conditions, providing a framework for evaluating the patient’s objective medical status. The third component places emphasis on values, accentuating the pivotal subjective factors that influence the decision-making process. The fourth segment offers a detailed explication of the target treatment plan, specifying the viable courses of action to be considered. Finally, the fifth division supplies corroborative information, substantiating the arguments with pertinent evidence and references.
To activate these arguments, the patient’s information must align with the definitions of the disease, objective conditions, and values expounded in the argumentation. In the case of value-based arguments, specific criteria must be met with regard to the patient’s values. The activated arguments are subsequently compiled and subjected to statistical analysis to extract treatment support types and ascertain their impact. It is worth mentioning that the activation of value-based arguments requires the fulfillment of conditions based on the values possessed by the patient.
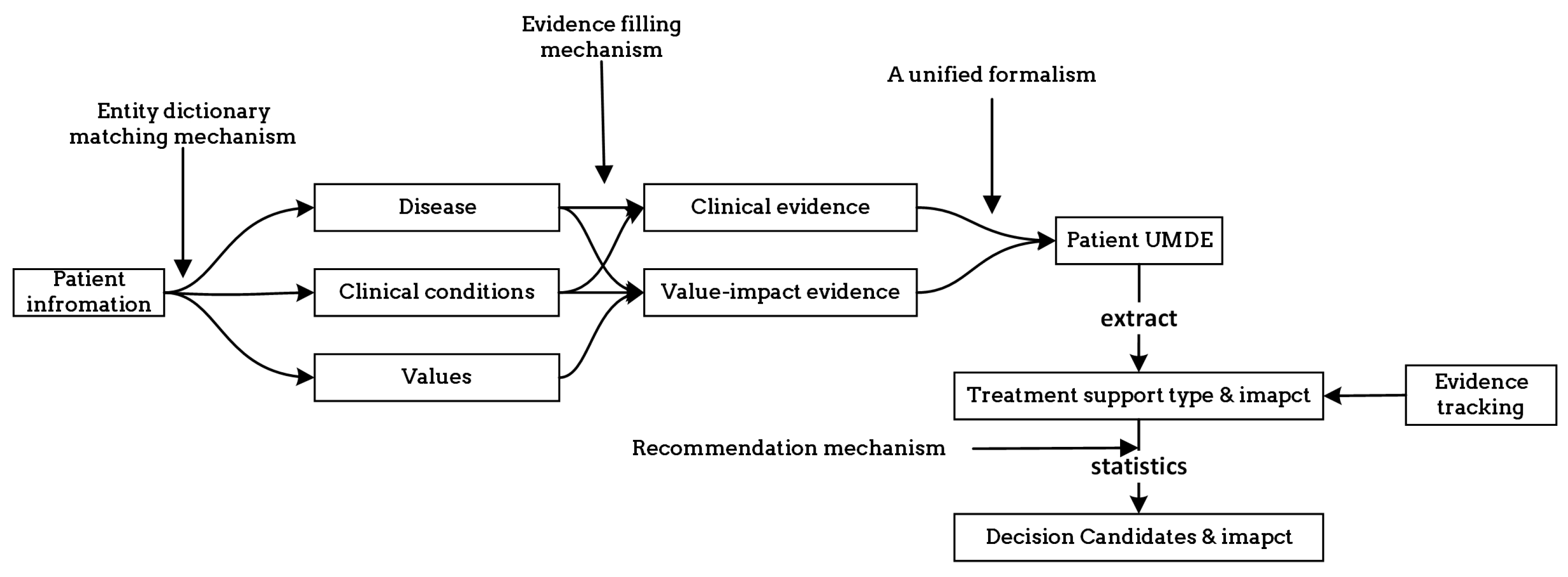
In order to enhance the clarity of unified decision evidences for use in patient healthcare decision-making, we have described the UMDE decision inference model in
Figure 7. The model takes the patient’s disease as input and invokes the corresponding knowledge base. The information is classified into clinical information and value-based information. By matching the clinical decision evidence with the evidence of values influencing the decision, we obtain an activated evidence library specific to the patient. Through the calculation of weights assigned to each treatment option mentioned in the evidence, we ultimately derive a ranked list of personalized treatment candidates for the patient, along with the corresponding evidence set as the basis for the decision-making process.
3.5. Population-Based Value Pre-Configuration
This correlated general medical knowledge plays a vital role in the formulation of personalized treatment plans that are aligned with patients’ needs and values, leading to improved satisfaction, enhanced recovery, and overall health benefits (refer to
Figure 8). The values of individuals are closely associated with their specific characteristics. For example, individuals in the public eye may prioritize appearance and charisma, while writers may highly value their creative abilities, and assembly line workers may prioritize rest. In our research, we specifically focus on exploring the relationship between occupation-related characteristics and values within the population. We employ statistical mapping techniques to identify the correlations between occupations and values, considering the impact of side effects on these values. Moreover, we provide preset values that represent the significance of such impact, contributing to a more comprehensive understanding of the values influencing decision-making processes.
The statistical preset values initially determined are further fine-tuned based on individualized values for each patient, thereby providing a more precise assessment of the influence of side effects on patients’ demographic characteristics. This personalized adjustment enables a better alignment of the preset values with the specific circumstances of each patient. For instance, personalized modifications can be made to accommodate patients in particular occupations, ensuring that the preset values are more tailored to their unique needs.
Table 3 exemplifies preset values for three distinct occupations, serving as a valuable reference for healthcare professionals to consider patients’ demographic characteristics and values during the formulation of personalized treatment plans. By incorporating these considerations, doctors can provide more targeted and effective care that accounts for the diverse needs and values of their patients.
In conclusion, our approach of correlating population and values enables a comprehensive understanding of patients’ unique requirements, allowing us to effectively address their individualized needs. By incorporating this knowledge, we can develop treatment plans that are closely aligned with their values, resulting in more personalized and patient-centered care. This approach provides robust support for enhancing the quality of medical interventions and promoting patient satisfaction and well-being.
3.6. Recommendation Algorithm and Solution Output
Referring to Algorithm 1 and
Table 5, we carefully consider both the clinical and value information provided by the patients. Initially, we match the patients’ set of clinical symptoms with the set of arguments to identify the arguments that meet the specified conditions. Subsequently, the weight assigned to each matching argument is incorporated into the corresponding treatment plan mentioned within that argument. In the case of a positive treatment plan type, the recorded impact value is directly aggregated. Conversely, for a treatment plan with negative type, the recorded impact value is subtracted. By diligently following this process, we obtain a set of weights for each treatment plan, which are determined based on the objective symptom evidence. The specific algorithm implementation is shown in Algorithm 1.
Following that, we proceed to traverse the arguments and assess the patients’ set of values, along with the objective symptom evidence that influences the weight of these values. To activate an argument, both the objective symptoms and values mentioned within the argument must be satisfied simultaneously. The information contained in the activated argument is then carefully analyzed. In the case of a treatment plan with a negative type, the weight impact is determined by multiplying the weight of the value involved in the corresponding argument within that treatment plan. Conversely, for a treatment plan with a positive type, the weight impact is directly added to the weight of the treatment plan. Finally, the weight ranking of the various treatment plans for the specific disease is established and presented as the outcome.
|
Algorithm 1 An algorithm for calculating the weight of treatment plans that integrates values through patient information and arguments. |
-
Require:
, , , ▹ Input data - 1:
▹ Initialize treatment plan weights - 2:
for each treatment plan in Disease do
- 3:
- 4:
end for - 5:
- 6:
▹ Match treatment plans with patient’s symptoms - 7:
for each symptom in do
- 8:
for each Argument in do
- 9:
if matches then
- 10:
if is positive then
- 11:
- 12:
else
- 13:
- 14:
end if
- 15:
end if
- 16:
end for
- 17:
end for - 18:
- 19:
▹ Match treatment plans with patient’s symptoms and values - 20:
for each value in do
- 21:
for each Argument in do
- 22:
if matches and matches then
- 23:
if is positive then
- 24:
- 25:
else
- 26:
- 27:
end if
- 28:
end if
- 29:
end for
- 30:
end for - 31:
- 32:
▹ Adjust treatment plan weights based on C:V ratio - 33:
for each treatment plan do
- 34:
- 35:
end for - 36:
- 37:
▹ Sort treatment plans by weight in descending order - 38:
Sort by in descending order - 39:
- 40:
▹ Output treatment plan weights and related arguments - 41:
for each treatment plan do
- 42:
output(treatment plan and active arguments) - 43:
end for |
Table 6.
The step description of algorithm 1.
Table 6.
The step description of algorithm 1.
| Row |
Step |
Description |
| 1-4 |
Step 1 |
Input data: Patient’s objective symptoms (Patient_obj_symptoms), Patient’s values (Patient_values), Arguments database (Arguments), C:V ratio |
| 6-17 |
Step 2 |
Match patient’s objective symptoms with arguments: For each symptom in Patient_obj_symptoms, and for each argument in Arguments, check if the symptom matches the argument’s symptom. If yes, update the treatment weight of the argument based on its type (positive or negative) |
| 19-30 |
Step 3 |
Match patient’s values with arguments: For each value in Patient_values, and for each argument in Arguments, check if the value matches the argument’s value and if the symptom matches the argument’s symptom. If yes, update the treatment weight of the argument based on its type |
| 32-35 |
Step 4 |
Adjust treatment plan weights based on C:V ratio: For each treatment plan in Arguments, calculate the adjusted weight by combining the clinical weight and value weight according to the C:V ratio |
| 37-38 |
Step 5 |
Sort treatment plans by weight in descending order: Sort the treatment plans by weight in descending order |
| 40-43 |
Step 6 |
Output treatment plan weights and related arguments: Output the sorted treatment plans and their associated clinical and value-based arguments |
4. Experiment and Evaluation
4.1. Prototype System Implementation
To evaluate the efficacy of our methods and models, we developed and implemented a Value-incorporated clinical decision system (VICDS). The system is designed to collect clinical information and values from patients, with clinical information stored in key-value pairs to create a comprehensive collection of patient clinical data. By employing a series of guided questions, we obtain patients’ clinical information, occupational and personalized values. The patients’ value list comprises a predefined set of occupational values, each assigned a weight ranging from 0 to 1. Both clinical and value information can be added or adjusted by the patients, ensuring that the system captures information that better reflects their specific circumstances, thereby enabling more tailored recommendations. In our recommendation module, we have introduced sliders for patients and doctors, enabling them to adjust the weights and proportions of clinical and value perspectives. This empowers them to tailor the overall balance according to their individual needs. The effectiveness of the model is demonstrated in
Figure 9 and
Figure 10, where we showcase information from two patients with identical clinical data but different value profiles.
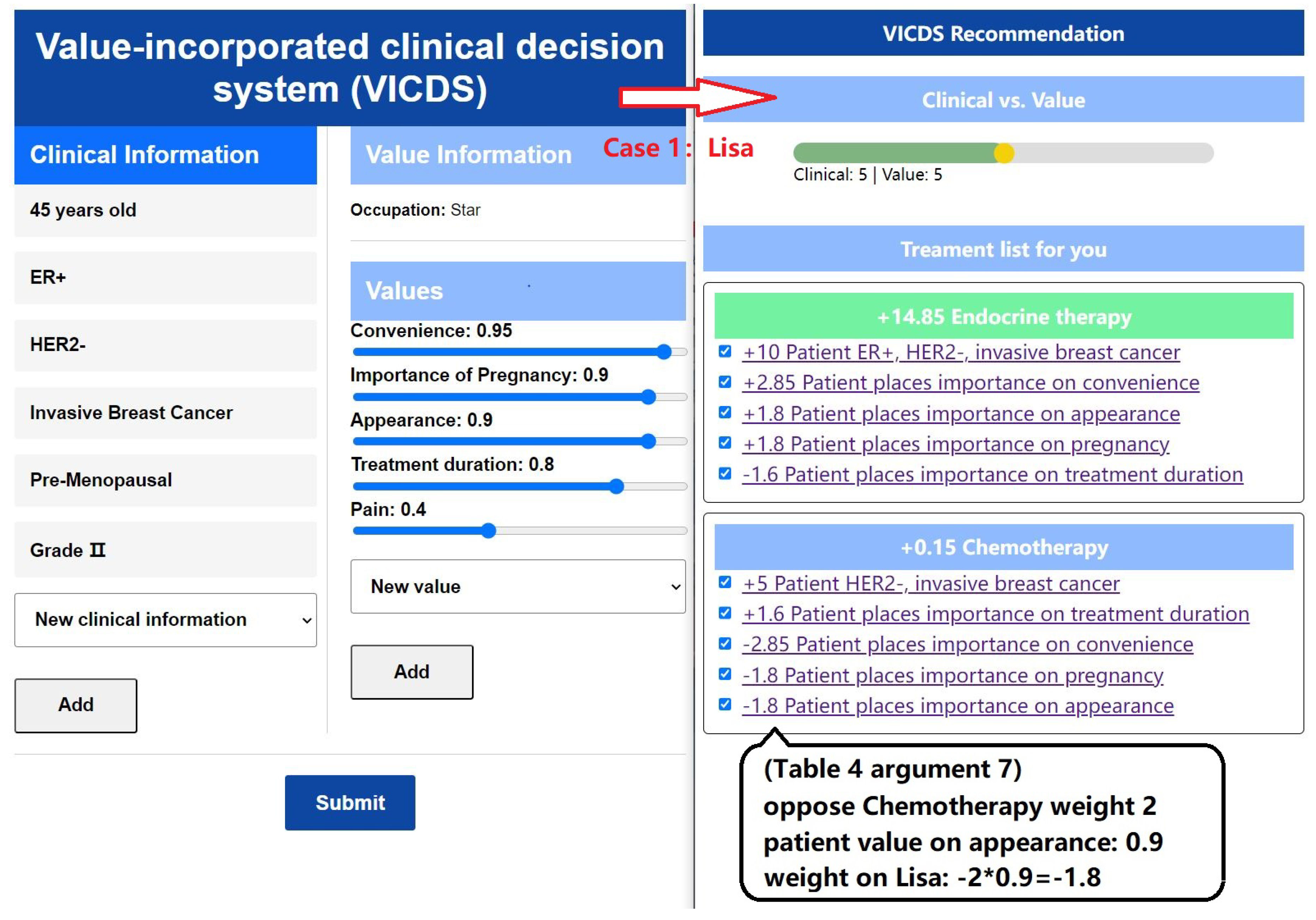
In the left part of
Figure 9, we present the input information of a patient named Lisa, who works as a star. Lisa highlights her busy schedule, emphasizing the limited time available for hospital treatments. Additionally, she expresses her desire for a future pregnancy and the importance of avoiding permanent infertility. Considering her occupation, Lisa places significant value on commercial performances and prioritizes her appearance. Moreover, Lisa demonstrates a higher threshold for pain compared to others and is willing to endure some discomfort in exchange for other values.
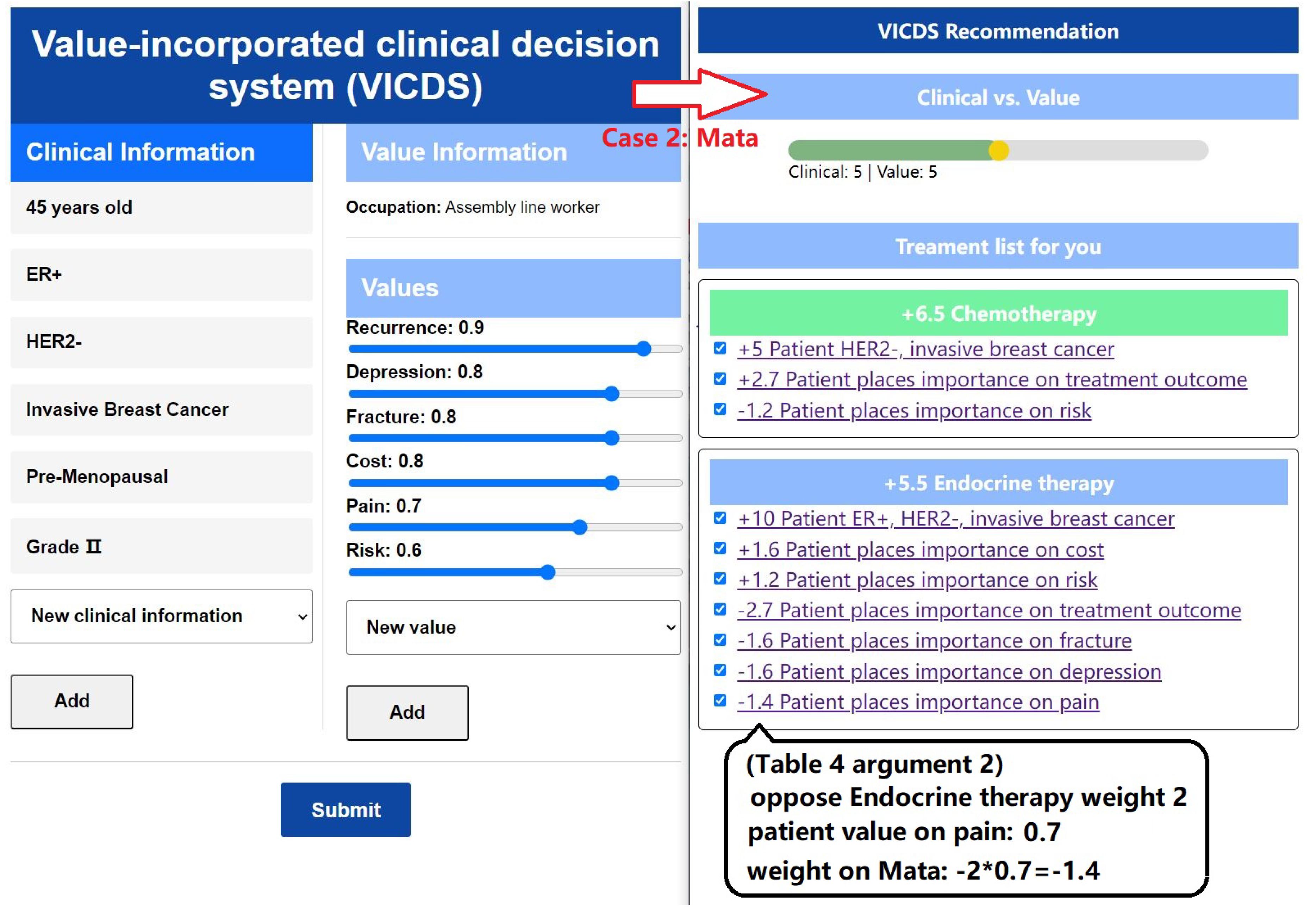
In the left part of
Figure 10, we present the input information of a patient named Mata, who works as an assembly worker. Mata emphasizes her preference for a favorable recovery outcome and is less concerned about the duration of the treatment period; her primary goal is to achieve a better recovery. She expresses significant fears regarding complications. Additionally, Mata hopes that the treatment plan will not incur excessive costs, as this would result in financial strain for her. Given her daily work involving prolonged movement and walking, it is crucial for Mata to avoid experiencing pain throughout the day, as it would have a substantial impact on both her personal and professional life.
Once the patients’ clinical and value information is collected, the matching of arguments is performed using Algorithm 1, resulting in sets of activated arguments and their corresponding weights. The process is illustrated in the right part of
Figure 9 and
Figure 10. The ratio between clinical weights and value weights can be adjusted through discussions between doctors and patients. Based on these adjustments, specific treatment recommendations are provided. The treatment plan is divided into three stages: pre-surgery, surgery, and post-surgery. Recommendations for each stage are derived from the arguments, with the default selection being the treatment plan with the highest weight. In the section explaining the treatment plan, the corresponding arguments and their weights are presented, providing doctors and patients with insights into the reasons behind the recommendations.
4.2. Model Validation and Evaluation
A series of controlled experiments were conducted to validate the rationality and effectiveness of the model. In the control experiments, the widely popular GPT model was utilized. Real-life information was collected from 28 patients, most of them had already completed their treatments. Follow-up conversations were held with these patients, posing the question, "If given another opportunity, what treatment option would you choose?"
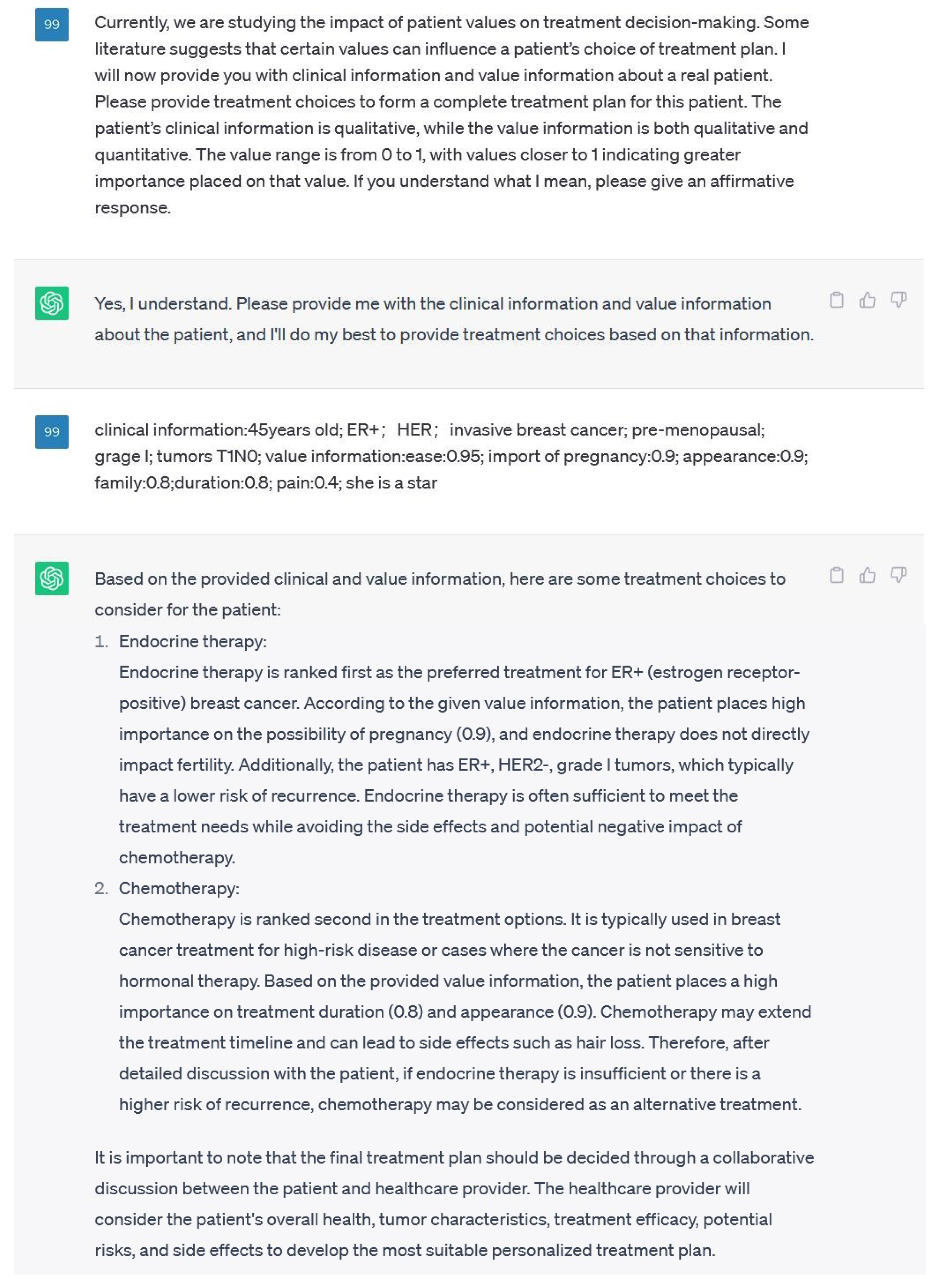
In the experiment, the patient dataset was utilized to simulate the input provided by real human users to the VICDS, with the objective of obtaining personalized treatment recommendations for the patients. The treatment option with the highest recommendation weight was recorded as the result. In the control group, the research purpose was explicitly communicated to GPT as follows: "We now need to generate a recommended list of treatment options, along with the reasons behind the recommendations, based on specific patients’ disease conditions and value preferences. The level of importance is represented by numerical values ranging from 0 to 1, with 1 indicating the highest level of importance. Each treatment option may have its own set of side effects, which could be influenced by the patients’ values. Consequently, in certain cases, the ranking order of treatment options can be influenced by the patients’ values. Furthermore, specific values can also impact the selection of treatment options, as suggested by relevant medical literature. The patients’ information will be provided in a key-value pair format." A dialogue with GPT was initiated using Lisa’s case study, as depicted in
Figure 11. GPT generated treatment plan recommendations along with the corresponding justifications.
The patient dataset was fed into the GPT model, and the treatment option with the highest weight recommended by GPT was recorded for each patient. Afterwards, the treatment options from the experimental group, control group, and patients’ inquiries were combined and summarized in
Table 7.
4.3. Experimental Results
To assess the feasibility and effectiveness of the model, real data from 28 patients was incorporated into VICDS and GPT, and the resulting treatment recommendations were examined.
Table 7 provides an overview of the recommendation outcomes. Additionally, follow-up inquiries were conducted for patients who had completed their treatment. The findings indicated that the treatment plans recommended by VICDS exhibited a higher degree of alignment with the actual needs of the patients compared to those suggested by GPT.
We compared VICDS, GPT, and the results obtained from patient inquiries to analyze the feasibility and effectiveness of the model. In comparison to patient inquiries, significant deviations were observed in the recommended plans generated by GPT. This disparity can be attributed to GPT’s sole reliance on the patients’ objective symptoms and value sets, without a systematic analysis of the arguments regarding the impact of values on decisions mentioned in the literature. As a result, GPT lacked sufficient data evidence, leading to recommended plans that did not fully align with the patients’ objective and value-based needs.
On the other hand, we found similarities between VICDS and the patient inquiry results, although some differences were also noted. After careful analysis, two potential reasons were identified for these disparities. Firstly, the impact of certain patients’ values on treatment plans had not undergone systematic validation and documentation in published papers. Consequently, this connection was not included in the VICDS knowledge repository, resulting in recommended plans that differed from the patient inquiry results. Secondly, while this study encompassed the analysis of all relevant papers in PubMed, it is possible that other databases may contain literature that was not considered in the analysis and argumentation, leading to deviations in the results.
In the process of using VICDS and GPT for comparison, we found that GPT may provide arguments from the literature that could influence decision-making due to underlying values. Our research also involves extracting evidence from the literature. When GPT provides a ranked list of recommended solutions, it also presents the reasoning behind the ranking. We discovered that some of the reasons provided by GPT are consistent. For example, the value that chemotherapy-induced hair loss affects appearance.
However, in the GPT example of
Figure 11, GPT indicates that chemotherapy may extend the treatment timeline, which contradicts the values argument we have obtained. We identified some inconsistencies within these reasons, which can be categorized into two types:
1. Contradictions between the reasons given by VICDS and GPT: It is well-known that GPT performs well in integrating information, but it may also include unfounded "claims" that do not align with facts, leading to errors. The methods presented by VICDS rely on literature-supported evidence, which increases their reliability compared to GPT. Therefore, in the field of decision-making influenced by values, the value-influenced decision papers obtained through literature analysis that we provide can serve as error-correction for GPT, enabling it to better serve humanity.
2. Currently, there are limitations in the evidence available in VICDS, resulting in knowledge gaps. When GPT mentions arguments not mentioned in VICDS and traces their verifiability, we can supplement VICDS’s evidence database with the arguments mentioned by GPT. This enhances the relevance of VICDS’s recommendations to the best treatment options needed by patients.
5. Discussion
This study makes four main contributions, with the aim of providing comprehensive and personalized support for decision-making in specific disease domains.
Firstly, medical objective factors such as diseases and treatment plans are acquired from medical clinical guidelines. An online literature database is screened and analyzed using disease names, treatment plans, and values as keywords. Through this process, a Value-Based Evidence Database (VBED) is constructed, encompassing relevant literature in the specific disease field where values influence decision-making. The importance of values in the decision-making process is considered, ensuring that the database covers not only objective medical guidelines but also the impact of values.
Secondly, the evidence database of how values influence decision-making is analyzed and formalized. The goal is to unify value-based evidence with objective evidence, establishing the Unified Model for Decision-Making with Values (UMDE). Through this unified model, healthcare professionals and patients can better understand and balance different factors when making treatment choices.
Thirdly, a prototype system called the VICDS is designed and implemented. VICDS matches patient information with the unified evidence and provides comprehensive and personalized treatment recommendations. Leveraging the information in the database and the structure of the model, VICDS considers individual patient needs and values, offering customized advice for each patient. This approach aims to enhance the treatment experience for patients and facilitate better treatment outcomes.
Fourthly, the identical clinical conditions and value systems of the patient are inputted into both VICDS and GPT for a comparative analysis based on their respective recommendation schemes. VICDS can issue declarations concerning errors in GPT, while the evidence-based components within GPT can complement VICDS, thereby facilitating the establishment of a virtuous information feedback loop.
In summary, this study make contributes by constructing a Value-Based Evidence Database, unifying objective evidence with value-based evidence, and designing a prototype system that provides comprehensive and personalized treatment recommendations for patients. These contributions aim to provide healthcare professionals and patients with more comprehensive and accurate information for decision-making in specific disease domains, thereby promoting better treatment decisions and outcomes.
6. Conclusion
This study uses disease names, treatment plans, and values as keywords for literature analysis to construct a value evidence database in a certain disease field where values influence decision-making. By analyzing the value evidence database and formalizing it, the goal is to unify it with objective evidence. The unified evidence is used to provide patients with comprehensive decision-making.
The contributions of this article are as follows:
1.The connection of the factors that influence values with treatment plans was made, and evidence of patient values was established based on literature analysis.
2.A unified formalism of value-based evidence and clinical evidence was developed.
3.A running CDSS prototype for breast cancer was built on top of the unified formalism, and its feasibility and effectiveness were evaluated.
4.A method for knowledge complementarity and error correction with GPT has been proposed.
In the future, we will utilize more practical cases to optimize our preset values. Optimize Q&A guidance through feedback from patients and doctors, allowing patient information to be fully discovered and improving user experience. The weight generated by the value-based decision-making evidence is incorporated into local treatment plans, potentially introducing fairness concerns in the overall ranking of treatment plans. Moving forward, we aim to explore alternative methods that mitigate the impact on fairness while seeking further improvements.
Author Contributions
Conceptualization, Z.L. and L.X.; methodology, Z.L. and L.X.; software, Z.L.; validation, Z.L. and L.X.; investigation, Z.L. and L.X.; writing—original draft preparation, Z.L. and L.X.; writing—review and editing, L.X.; visualization, Z.L. and L.X.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CDSS |
Clinical decision support systems |
| PROM |
Patient-reported outcome measures |
| SDM |
Shared decision making |
| NICE |
National institute for health and care excellence |
| VBDE |
Value-based decision evidence |
| UMDE |
Unified model for decision evidence |
| RDF |
Resource description framework |
| VICDS |
Value-incorporated clinical decision system |
References
- Hibbard, J.H.; Greene, J. What The Evidence Shows About Patient Activation: Better Health Outcomes And Care Experiences; Fewer Data On Costs. Health Affairs 2013, 32, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, J.; Long, A.F.; Flynn, R. The use of patient reported outcome measures in routine clinical practice: lack of impact or lack of theory? Social Science & Medicine 2005, 60, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Elwyn, G.; Frosch…, D. Shared Decision Making: A Model for Clinical Practice. Journal of General Internal Medicine 2012, 27, 1361–1367. [Google Scholar] [CrossRef]
- Torenholt, R.; Tjørnhøj-Thomsen, T. ‘Is this something I should be worried about?’: A study of nurses’ recontextualisation work when making clinical decisions based on patient reported outcome data. Social Science & Medicine 2022, 294, 114645. [Google Scholar]
- Sutton, D.R.; Fox, J. The syntax and semantics of the PROforma guideline modeling language. Journal of the American Medical Informatics Association 2003. [Google Scholar] [CrossRef] [PubMed]
- Brignardello-Petersen, R.; Carrasco-Labra, A.; Guyatt, G.H. How to Interpret and Use a Clinical Practice Guideline or Recommendation: Users’ Guides to the Medical Literature. JAMA 2021, 326, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.; Goldstein, A.; Koenig, M.K.; Scaglia, F.; Enns, G.M.; Saneto, R.; Anselm, I.; Cohen, B.H.; Falk, M.J.; Greene, C. Diagnosis and management of mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society. Genetics in Medicine Official Journal of the American College of Medical Genetics 2015. [Google Scholar] [CrossRef]
- Domain, M. decision support system for breast cancer treatment based on data mining technologies and clinical practice guidelines. 2005. [Google Scholar]
- Xiao, L.; Fox, J. Towards an Agent-Oriented Framework for Multidisciplinary Decision Support and Its Application to Triple Assessment of Breast Cancer. In Proceedings of the Computer Software & Applications Conference; 2017. [Google Scholar]
- Xiao, L.; Zhou, H.; Fox, J. Towards a systematic approach for argumentation, recommendation, and explanation in clinical decision support. Mathematical Biosciences and Engineering: MBE 2022, 19, 10445–10473. [Google Scholar] [CrossRef]
- Berry, A.B.; Lim, C.Y.; Hartzler, A.L.; Hirsch, T.; Ludman, E.; Wagner, E.H.; Ralston, J.D. "It’s good to know you’re not a stranger every time": Communication about Values Between Patients with Multiple Chronic Conditions and Healthcare Providers. Proceedings of the ACM on Human-Computer Interaction 2017, 1, 1–20. [Google Scholar] [CrossRef]
- Epstein, R.M.; Street, R.L. The values and value of patient-centered care. Annals of Family Medicine 2011, 9, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.R.; Patrick, D.L.; Engelberg, R.A.; Norris, K.; Asp, C.; Byock, I. A measure of the quality of dying and death. Initial validation using after-death interviews with family members. Journal of Pain & Symptom Management 2002, 24, 17–31. [Google Scholar] [CrossRef]
- Wherton, J.; Sugarhood, P.; Procter…, R. Designing assisted living technologies ‘in the wild’: preliminary experiences with cultural probe methodology. BMC Medical Research Methodology,12,1(2012-12-20) 2012, 12, 188–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xiao, L.; Chen, J.; Yu, H.; Ye, Y. An Emotion-fused Medical Knowledge Graph and its Application in Decision Support. In Proceedings of the 2022 IEEE 46th Annual Computers, , Software, and Applications Conference (COMPSAC); IEEE, 2022; pp. 1381–1388. [Google Scholar]
- Wu, C.; Xiao, L. Evidence based on patient’s experience data and clinical guidelines-for patient-oriented clinical decision support. In Proceedings of the 2021 International Conference on Public Health and Data Science (ICPHDS). IEEE; 2021; pp. 240–247. [Google Scholar]
- Yang, J.; Xiao, L.; Li, K. Modelling clinical experience data as an evidence for patient-oriented decision support. BMC Medical Informatics and Decision Making 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Porr, C.; Gaudine, A.; Woo, K.; Smith-Young, J.; Green, C. How community nurses manage ethical conflicts: A grounded theory study. Global Qualitative Nursing Research 2019, 6, 2333393619894958. [Google Scholar] [CrossRef]
- Hsieh, H.-F. Three Approaches to Qualitative Content Analysis. Qual Health Res 2005, 15, 1277–1288. [Google Scholar] [CrossRef]
- Sweileh, W.M.; Sawalha, A.F.; Al-Jabi, S.W.; Zyoud, S.H.; Shraim, N.Y.; Abu-Taha, A.S. A bibliometric analysis of literature on malaria vector resistance: (1996 – 2015). Globalization & Health 2016, 12, 76. [Google Scholar] [CrossRef]
- Durrani, S.; Heena, H. Controversies regarding ovarian suppression and infertility in early stage breast cancer. Cancer management and research 2020, 12, 813. [Google Scholar] [CrossRef]
- Castel, L.D.; Hartmann, K.E.; Mayer, I.A.; Saville, B.R.; Alvarez, J.; Boomershine, C.S.; Abramson, V.G.; Chakravarthy, A.B.; Friedman, D.L.; Cella, D.F. Time course of arthralgia among women initiating aromatase inhibitor therapy and a postmenopausal comparison group in a prospective cohort. Cancer 2013, 119, 2375–2382. [Google Scholar] [CrossRef]
- Rachner, T.D.; Coleman, R.; Hadji, P.; Hofbauer, L.C. Bone health during endocrine therapy for cancer. The Lancet Diabetes & Endocrinology 2018, 6, 901–910. [Google Scholar]
- Jankowitz, R.C.; McGuire, K.P.; Davidson, N.E. Optimal systemic therapy for premenopausal women with hormone receptor-positive breast cancer. The Breast 2013, 22, S165–S170. [Google Scholar] [CrossRef]
- Murray, J.; Miller, W.R.; Dixon, J.M. Neoadjuvant endocrine therapy models. Breast Cancer Research Protocols 2006, 489–502. [Google Scholar]
- New aromatase inhibitors for breast cancer. Drug and Therapeutics Bulletin 1997, 35, 55–56. [https://dtb.bmj.com/content/35/7/55.full.pdf]. [CrossRef] [PubMed]
- Kanti, V.; Nuwayhid, R.; Lindner, J.; Hillmann, K.; Bangemann, N.; Kleine-Tebbe, A.; Blume-Peytavi, U.; Garcia Bartels, N. Evaluation of trichodynia (hair pain) during chemotherapy or tamoxifen treatment in breast cancer patients. Journal of the European Academy of Dermatology and Venereology 2016, 30, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Reinert, T.; Gonçalves, R.; Ellis, M.J. Current status of neoadjuvant endocrine therapy in early stage breast cancer. Current Treatment Options in Oncology 2018, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.T.M.; de Carvalho, K.P.; Mazzutti, F.S.; de Almeida Maia, M.; Canto, P.P.L.; Paiva, C.E.; de Paiva Maia, Y.C. Temporal influence of endocrine therapy with tamoxifen and chemotherapy on nutritional risk and obesity in breast cancer patients. BMC cancer 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Desai, P.; Aggarwal, A. Breast cancer in women over 65 years-a review of screening and treatment options. Clinics in Geriatric Medicine 2021, 37, 611–623. [Google Scholar] [CrossRef]
- Brown, L.C.; Murphy, A.R.; Lalonde, C.S.; Subhedar, P.D.; Miller, A.H.; Stevens, J.S. Posttraumatic stress disorder and breast cancer: Risk factors and the role of inflammation and endocrine function. Cancer 2020, 126, 3181–3191. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, X.; Huang, X.; Lin, S.; Luo, S.; Gu, D.; Weng, X.; Xu, X. Cost-effectiveness analysis of ovarian function preservation with GnRH agonist during chemotherapy in premenopausal women with early breast cancer. Human Reproduction 2023, 38, 1099–1110. [Google Scholar] [CrossRef]
- Ellis, M.J.; Rigden, C.E. Initial versus sequential adjuvant aromatase inhibitor therapy: a review of the current data. Current medical research and opinion 2006, 22, 2479–2487. [Google Scholar] [CrossRef]
- Lee, E.; Nelson, O.L.; Puyana, C.; Takita, C.; Wright, J.L.; Zhao, W.; Reis, I.M.; Lin, R.Y.; Hlaing, W.M.; Bakalar, J.L.; et al. Association between C-reactive protein and radiotherapy-related pain in a tri-racial/ethnic population of breast cancer patients: a prospective cohort study. Breast Cancer Research 2019, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hodis, H.N.; Mack, W.J. Postmenopausal hormone therapy and cardiovascular disease in perspective. Clinical obstetrics and gynecology 2008, 51, 564. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Overall flowchart and main components of this article.
Figure 1.
Overall flowchart and main components of this article.
Figure 2.
Clinical evidence model using breast cancer as an example.
Figure 2.
Clinical evidence model using breast cancer as an example.
Figure 3.
An example of a normalized argument stored in an RDF file for subsequent matching of patient information and recommendation of treatment plans.
Figure 3.
An example of a normalized argument stored in an RDF file for subsequent matching of patient information and recommendation of treatment plans.
Figure 4.
Examples of conditions and treatment plans in NICE.
Figure 4.
Examples of conditions and treatment plans in NICE.
Figure 5.
The process and quantity of literature analysis based on keywords.
Figure 5.
The process and quantity of literature analysis based on keywords.
Figure 6.
Ontology of an unified decision evidences.
Figure 6.
Ontology of an unified decision evidences.
Figure 7.
Description of the process by which patient information is transformed into decision candidates.
Figure 7.
Description of the process by which patient information is transformed into decision candidates.
Figure 8.
Structure that takes breast cancer as an example to show the mapping of general medical knowledge and values.
Figure 8.
Structure that takes breast cancer as an example to show the mapping of general medical knowledge and values.
Figure 9.
Case1: Lisa’s information input and treatment plan recommendation display.
Figure 9.
Case1: Lisa’s information input and treatment plan recommendation display.
Figure 10.
Case2: Mata’s information input and treatment plan recommendation display.
Figure 10.
Case2: Mata’s information input and treatment plan recommendation display.
Figure 11.
A GPT dialogue for treatment plan recommendations using Lisa’s case as an example.
Figure 11.
A GPT dialogue for treatment plan recommendations using Lisa’s case as an example.
Table 1.
Six categories of values and 29 fine-grained values.
Table 1.
Six categories of values and 29 fine-grained values.
| Six categories values |
Fine-grained values |
Six categories values |
Fine-grained values |
| Activities |
fracture |
Principles |
independence |
| recurrence |
treatment duration |
| traveling |
confidence |
| reading |
appearance |
| walking |
weight |
| Abilities |
survival |
Emotions |
risk |
| work |
pregnancy |
| rest |
exhaustion |
| talking |
pain |
| vision |
unbearable |
| convenience |
depression |
| exercise |
|
| Possessions |
convenience |
Relationships |
family |
| transportation |
friend |
| expensive |
colleague |
| cost effective |
community |
Table 2.
Keywords of relevant treatment plans found in NICE, taking breast cancer as an example.
Table 2.
Keywords of relevant treatment plans found in NICE, taking breast cancer as an example.
| No. |
Category |
Keywords |
| 1 |
Endocrine therapy |
Hormone therapy |
| |
|
Tamoxifen |
| |
|
Aromatase inhibitors |
| |
|
Ovarian ablation/suppression |
| 2 |
Radiotherapy |
- |
| 3 |
Chemotherapy |
- |
| 4 |
Surgery |
Mastectomy |
| |
|
Breast reconstruction |
| |
|
Breast conservation |
| 5 |
Targeted Therapy |
- |
| 6 |
Immunotherapy |
- |
| 7 |
Bisphosphonates |
- |
| 8 |
Complementary Therapy |
- |
Table 3.
Population-based value pre-configuration.
Table 3.
Population-based value pre-configuration.
| Population features |
Related |
Side effects |
Weight |
| Actor |
Appearance,
Temperament |
Weight gain |
0.88 |
| Alopecia |
0.85 |
| Skin darkens |
0.74 |
| Diarrhea |
0.30 |
| Writer |
Creativity,
Spirit |
Memory loss |
0.94 |
| Tremor |
0.87 |
| Insomnia |
0.81 |
| Fatigue |
0.67 |
| Assembly line
worker |
Work and rest,
Repetitive work |
Joint pain |
0.86 |
| Insomnia |
0.73 |
| Numbness in limbs |
0.70 |
| Back pain |
0.65 |
Table 4.
Impact of values on decision-making, whcih is extracted or analyzed from literature.
Table 4.
Impact of values on decision-making, whcih is extracted or analyzed from literature.
| Index |
Author /Year |
Title |
Finding |
Link Start with
https://pubmed.ncbi.nlm.nih.gov/
|
| 1 |
Durrani /2020 |
Controversies Regarding Ovarian Suppression and Infertility in Early Stage Breast Cancer[21] |
The main concern after adjuvant chemotherapy is the risk of losing fertility, as chemotherapy can induce early menopause in most premenopausal breast cancer patients. Tamoxifen only slightly increases the risk of early menopause. |
32104064/ |
| 2 |
Castel / 2013 |
Time course of arthralgia among women initiating aromatase inhibitor therapy and a postmenopausal comparison group in a prospective cohort[22] |
Women undergoing endocrine therapy have more severe joint pain, and have more severe menopausal symptoms or existing joint-related diseases relative to before treatment. Joint pain is more severe than expected after menopause and often leads to reduced compliance. |
23575918/ |
| 3 |
Rachner / 2018 |
Bone health during endocrine therapy for cancer[23] |
Common osteoporosis guidelines are likely to have underestimated the fracture risk of patients receiving endocrine therapy—especially in patients on aromatase inhibitor therapy. |
29572126/ |
| 4 |
Jankowitz / 2013 |
Optimal systemic therapy for premenopausal women with hormone receptor-positive breast cancer[24] |
Chemotherapy has a shorter duration compared to endocrine therapy. |
26996100/ |
| 5 |
Murray /2006 |
Neoadjuvant endocrine therapy models[25] |
Chemotherapy is more effective than endocrine therapy at shrinking the tumour |
16491621/ |
| 6 |
Collier / 1997 |
New aromatase inhibitors for breast cancer[26] |
Endocrine therapy can provide self-administered oral medication, while chemotherapy requires injections at the hospital. |
9282426/ |
| 7 |
Kanti / 2015 |
Evaluation of trichodynia (hair pain) during chemotherapy or tamoxifen treatment in breast cancer patients[27] |
Chemotherapy has more severe hair loss and scalp pain compared to Tamoxifen, and the duration is also longer. |
26403680/ |
| 8 |
Reinert / 2018 |
Current Status of Neoadjuvant Endocrine Therapy in Early Stage Breast Cancer[28] |
Endocrine therapy is a practical, cost-effective treatment |
29663173/ |
| 9 |
Lima / 2017 |
Temporal influence of endocrine therapy with tamoxifen and chemotherapy on nutritional risk and obesity in breast cancer patients[29] |
Women on endocrine therapy with TMX are mostly overweighed and obese, most evidently in women who received CT, and who were at the beginning of treatment. |
28851304/ |
| 10 |
Desai / 2021 |
Breast Cancer in Women Over 65 years- a Review of Screening and Treatment Options[30] |
Primary endocrine therapy is a low-risk option for those with limited life expectancy. |
/34600726/ |
| 11 |
Brown / 2020 |
Posttraumatic stress disorder and breast cancer: Risk factors and the role of inflammation and endocrine function[31] |
Tamoxifen also has been shown to be involved in adverse mood reactions such as depression |
32374431/ |
| 12 |
Huang / 2023 |
Cost-effectiveness analysis of ovarian function preservation with GnRH agonist during chemotherapy in premenopausal women with early breast cancer[32] |
GnRHa plus Chemo was a cost-effective strategy for premenopausal women with BC in the USA. |
37075316/ |
| 13 |
Eills / 2006 |
Initial versus sequential adjuvant aromatase inhibitor therapy: a review of the current data[33] |
For those with positive nodes, initiation of treatment with aromatase inhibitors may be beneficial to avoid tamoxifen-associated early relapses after diagnosis. |
17257462/ |
| 14 |
Eills / 2006 |
Initial versus sequential adjuvant aromatase inhibitor therapy: a review of the current data[33] |
From an economic perspective, aromatase inhibitors are considered cost-effective compared to tamoxifen. |
17257462/ |
| 15 |
Lee / 2019 |
Association between C-reactive protein and radiotherapy-related pain in a tri-racial/ethnic population of breast cancer patients: a prospective cohort study[34] |
In the postoperative radiotherapy process of obese patients, pain occurs, which has a negative impact on the quality of life. |
31138314/ |
| 16 |
Hodis / 2008 |
Postmenopausal hormone therapy and cardiovascular disease in perspective[35] |
Hormone therapy after menopause can reduce the mortality rate and the risk of coronary heart disease. |
18677151/ |
Table 5.
Impact of values on decision-making, where the information is extracted and analyzed from literature.
Table 5.
Impact of values on decision-making, where the information is extracted and analyzed from literature.
| Index |
Clinical condition |
Value |
Support |
Oppose |
Weight |
Source in Table 3
|
| 1 |
premenopausal |
pregnancy later |
Endocrine therapy |
Chemotherapy |
2 |
1 |
| 2 |
- |
pain |
- |
Endocrine therapy |
2 |
2 |
| 3 |
- |
fracture |
- |
Endocrine therapy |
2 |
3 |
| 4 |
ER+,
pre-surgery |
treatment duration |
Chemotherapy |
Endocrine therapy |
2 |
4 |
| 5 |
premenopausal |
treatment outcome |
Chemotherapy |
Endocrine therapy |
3 |
5 |
| 6 |
ER+,
HER2- |
convenience |
Endocrine therapy |
Chemotherapy |
3 |
6 |
| 7 |
- |
appearance |
Endocrine therapy |
Chemotherapy |
2 |
7 |
| 8 |
ER+,
Grade 2 |
cost |
Endocrine therapy |
- |
2 |
8 |
| 9 |
- |
weight |
- |
Endocrine therapy |
2 |
9 |
| 10 |
ER+,
|
risk |
- |
Endocrine therapy |
2 |
10 |
| 11 |
ER+ |
depression |
- |
Endocrine therapy |
2 |
11 |
| 12 |
age 18-49,
premenopausal |
family
cost effective |
Chemotherapy+
GnRHa |
Chemotherapy |
3 |
12 |
| 13 |
node-positive |
recurrence |
Anastrozole |
Tamoxifen |
2 |
13 |
| 14 |
- |
cost effective |
Aromatase |
Tamoxifen |
2 |
14 |
| 15 |
, after surgery,
overweight |
pain |
- |
Radiotherapy |
2 |
15 |
| 16 |
postmenopausal |
mobility, survival |
- |
Hormone therapy |
2 |
16 |
| 17 |
ER+ |
risk |
Endocrine therapy |
Chemotherapy |
2 |
5 |
Table 7.
Comparing treatment plan recommendation methods: VICDS, GPT and follow-up questions.
Table 7.
Comparing treatment plan recommendation methods: VICDS, GPT and follow-up questions.
| Treatment |
VICDS |
GPT |
Follow-up questions |
| Chemotherapy |
8 |
10 |
8 |
| Radiotherapy |
6 |
7 |
5 |
| Tamoxifen |
4 |
4 |
5 |
| Aromatase |
4 |
3 |
4 |
| Mastectomy |
2 |
2 |
3 |
| Targeted therapy |
4 |
2 |
3 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).