Submitted:
08 July 2023
Posted:
10 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals
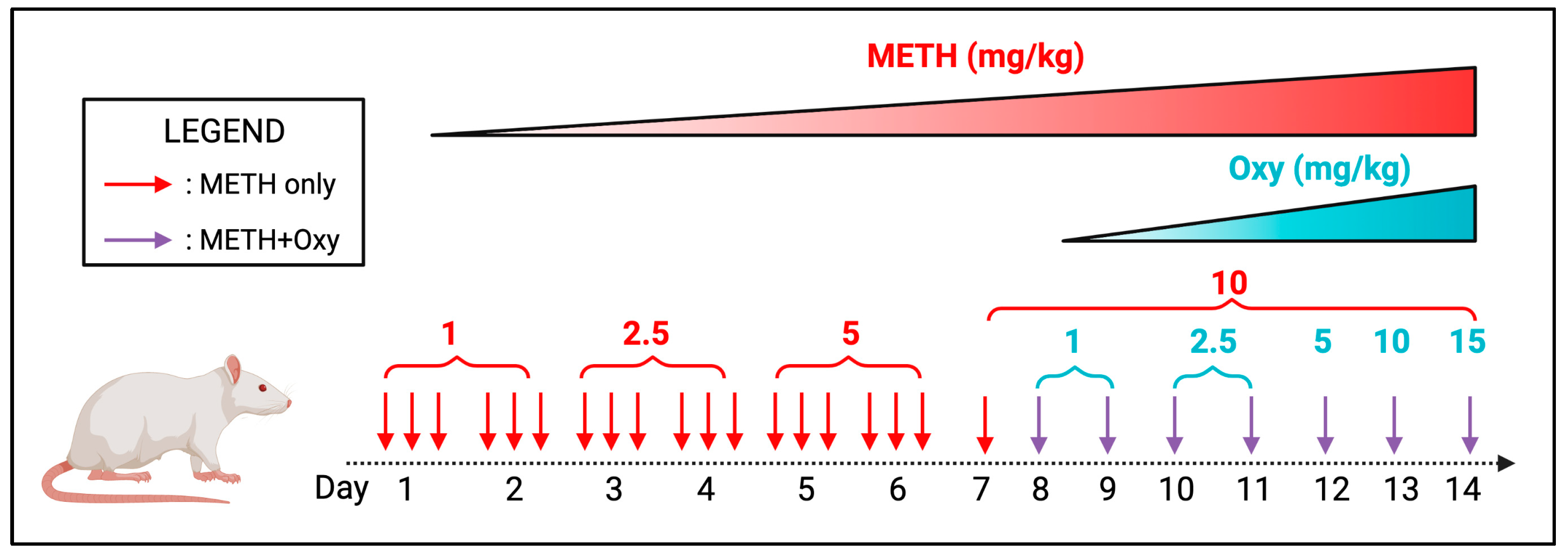
2.2. Polysubstance administration
2.3. Total RNA extraction, quality control, library preparation, and RNA-Sequencing
2.4. Bioinformatic data analysis
3. Results
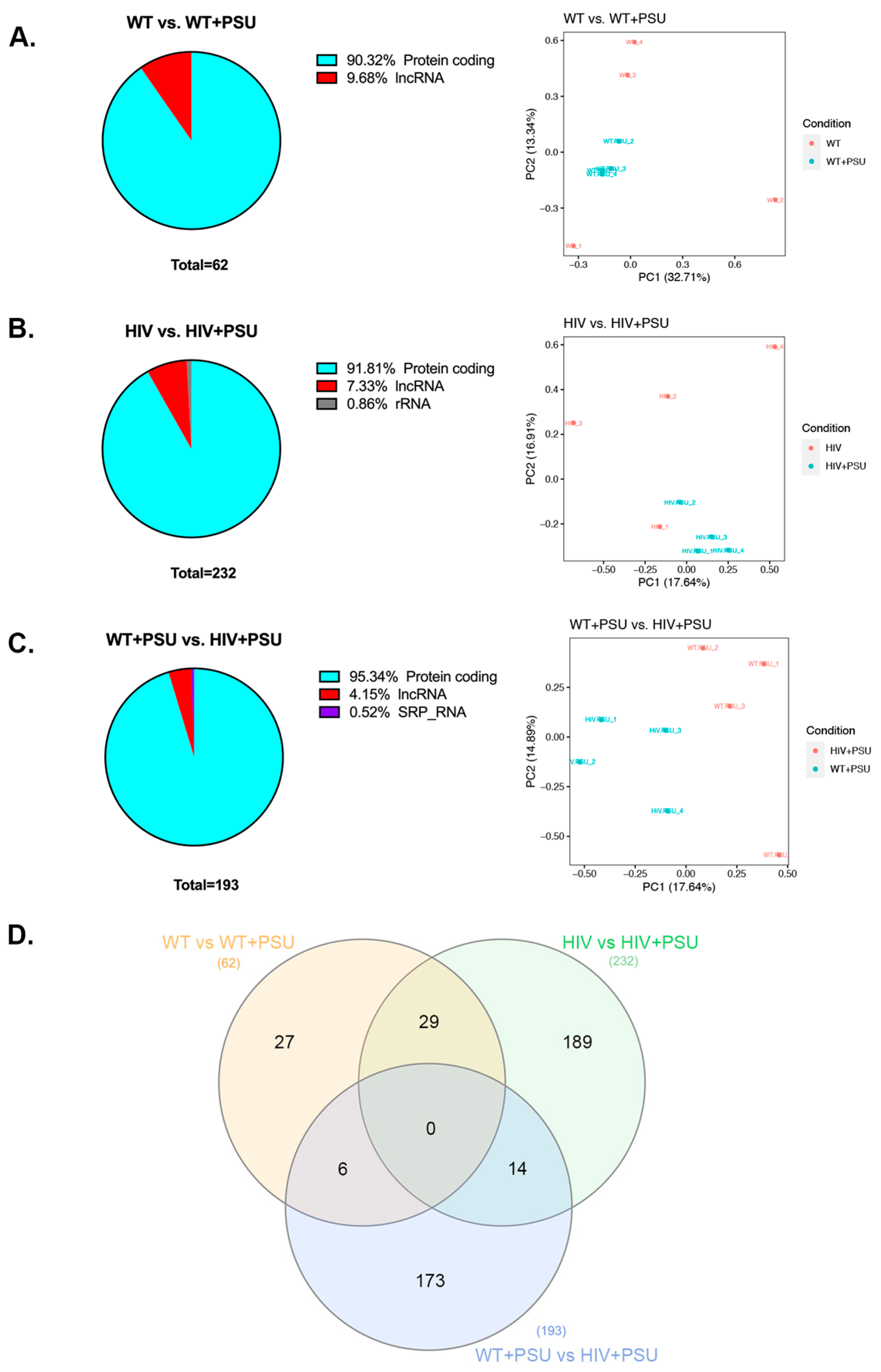
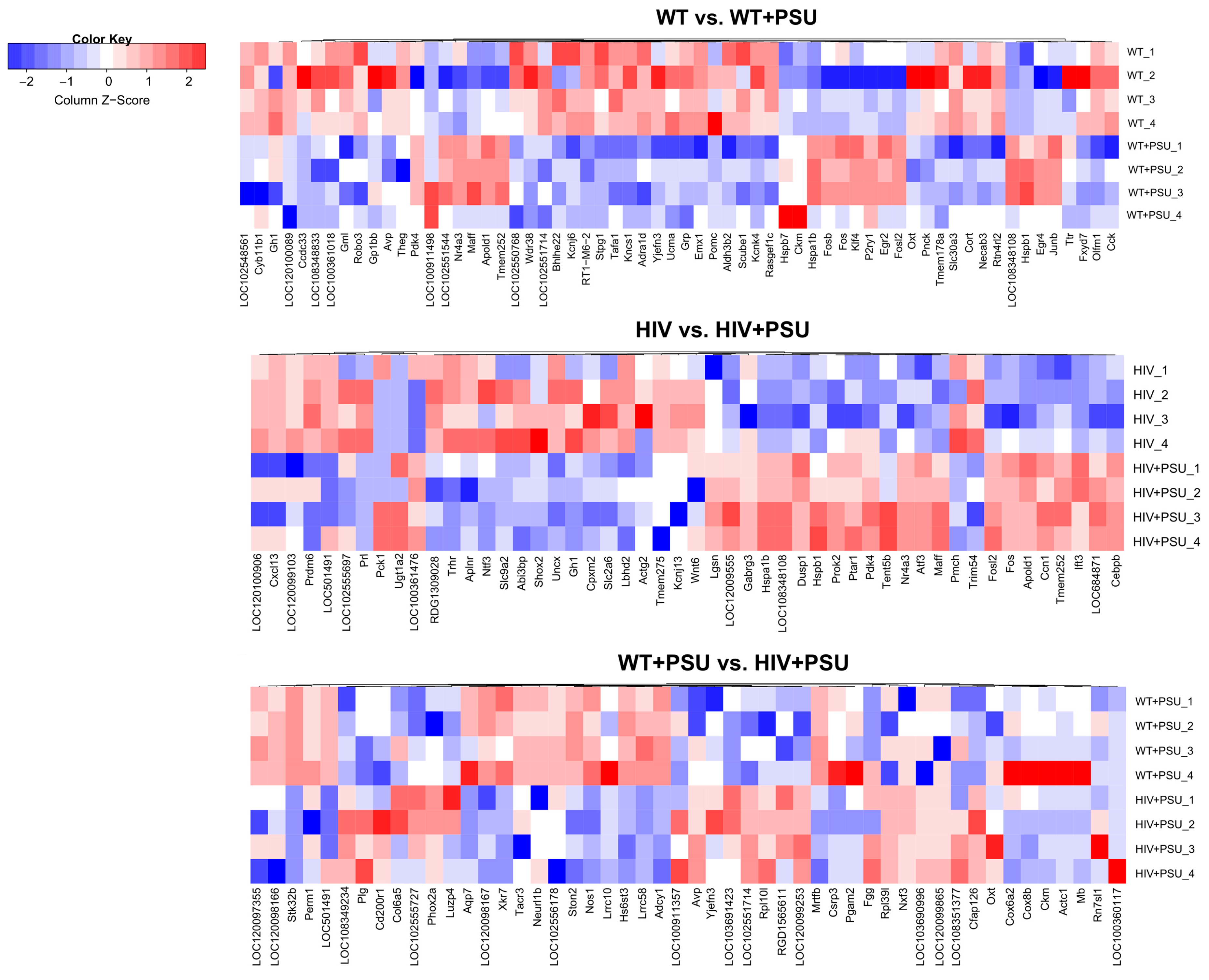
3.1. HIV and Chronic PSU Leads to Changes in Striatum Transcriptome
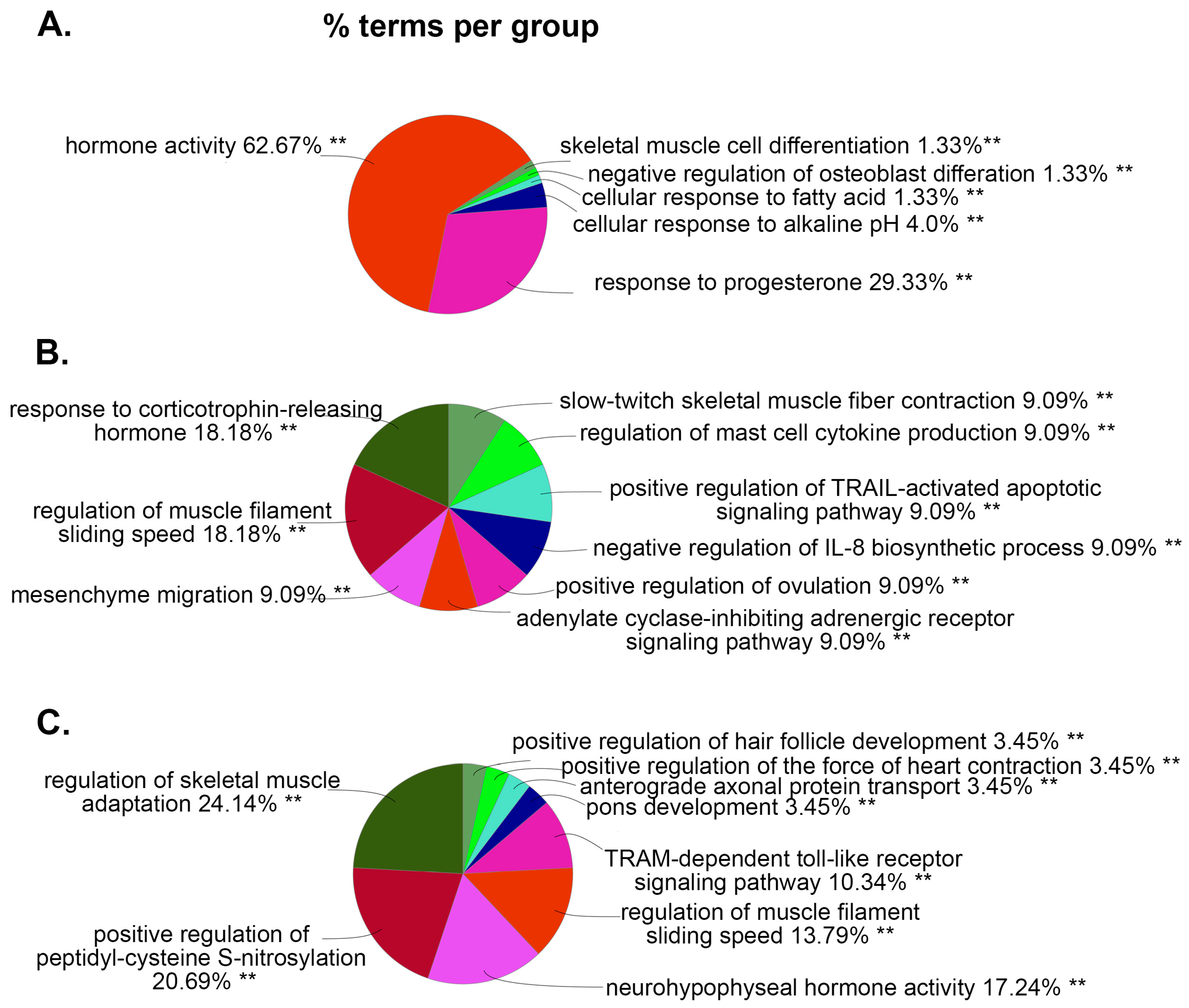
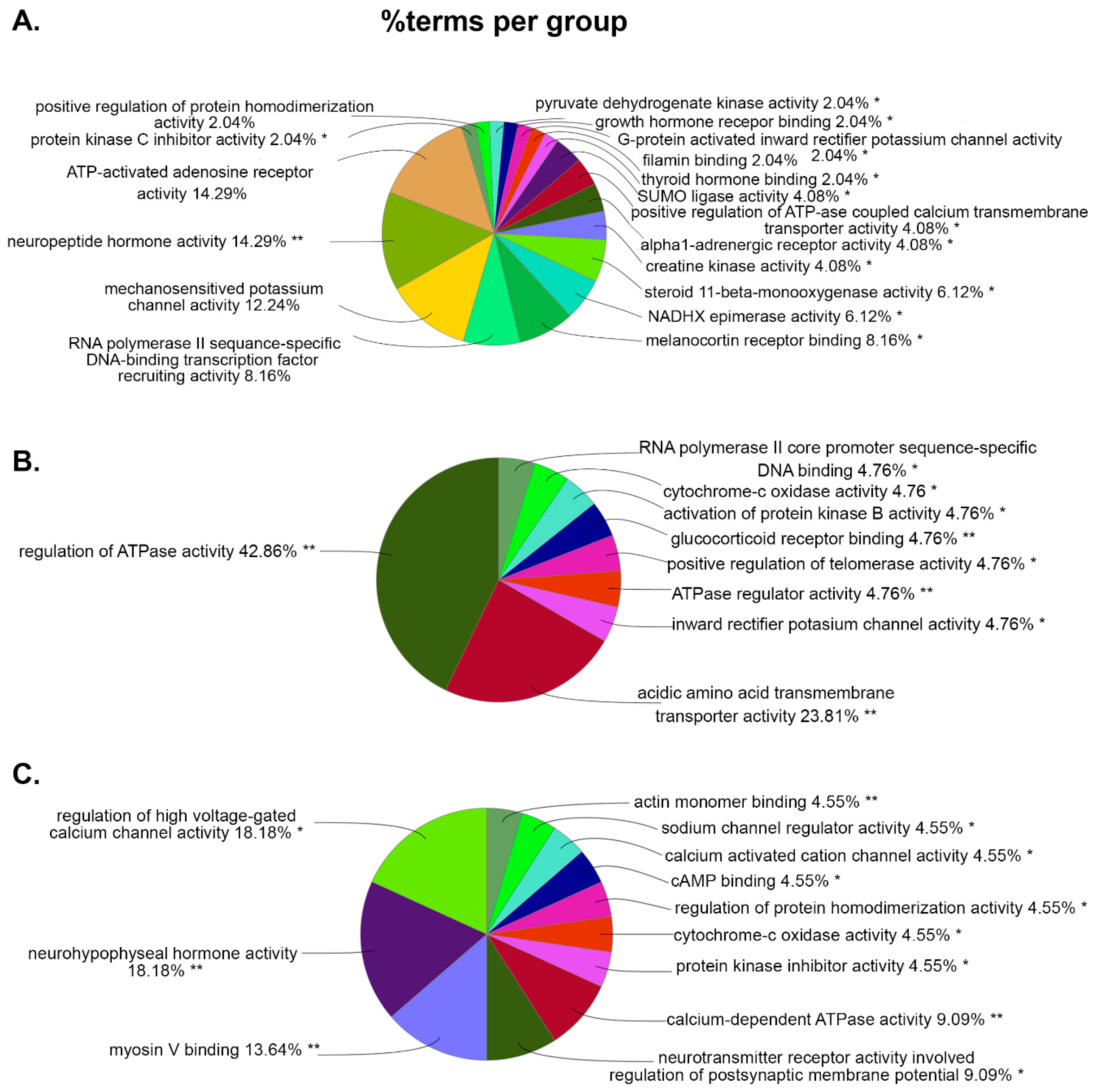
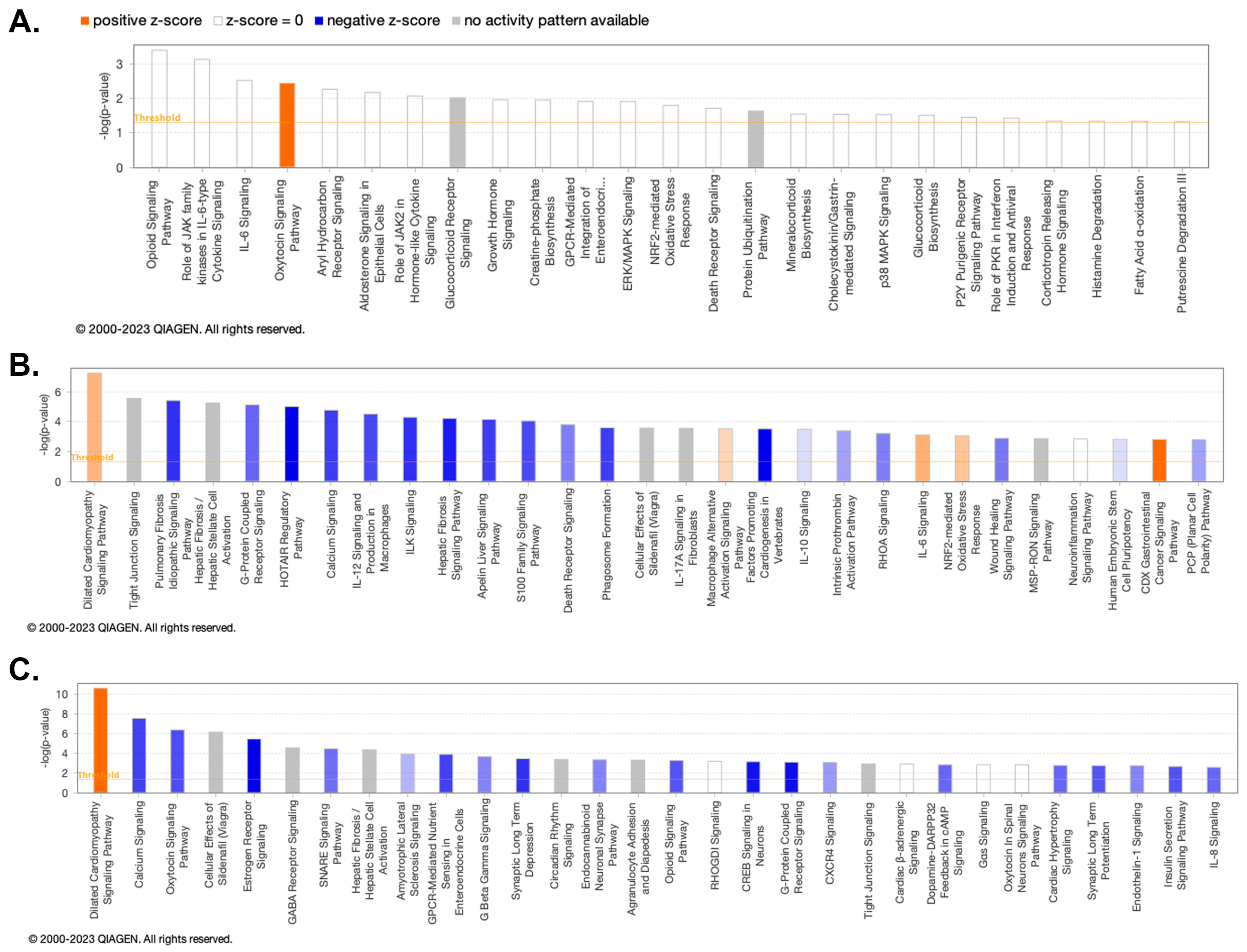
3.2. ClueGO and IPA Analysis Demonstrates Molecular Activities and Pathways Associated with HIV and Chronic PSU
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cicero, T.J.; Ellis, M.S.; Kasper, Z.A. Polysubstance Use: A Broader Understanding of Substance Use During the Opioid Crisis. Am J Public Health 2020, 110, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Crummy, E.A.; O'Neal, T.J.; Baskin, B.M.; Ferguson, S.M. One Is Not Enough: Understanding and Modeling Polysubstance Use. Front Neurosci 2020, 14, 569. [Google Scholar] [CrossRef] [PubMed]
- O'Donnell, J.; Gladden, R.M.; Mattson, C.L.; Hunter, C.T.; Davis, N.L. Vital Signs: Characteristics of Drug Overdose Deaths Involving Opioids and Stimulants - 24 States and the District of Columbia, January-June 2019. MMWR Morb Mortal Wkly Rep 2020, 69, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Compton, W.M.; Valentino, R.J.; DuPont, R.L. Polysubstance use in the U.S. opioid crisis. Molecular Psychiatry 2021, 26, 41–50. [Google Scholar] [CrossRef]
- Pimentel, E.; Sivalingam, K.; Doke, M.; Samikkannu, T. Effects of Drugs of Abuse on the Blood-Brain Barrier: A Brief Overview. Frontiers in Neuroscience 2020, 14. [Google Scholar] [CrossRef]
- CDC. Substance Use and Sexual Risk Behaviors. Available online: https://www.cdc.gov/healthyyouth/substance-use/dash-substance-use-fact-sheet.htm.
- Baskin-Sommers, A.; Sommers, I. The co-occurrence of substance use and high-risk behaviors. Journal of Adolescent Health 2006, 38, 609–611. [Google Scholar] [CrossRef]
- Wang, S.-C.; Maher, B. Substance Use Disorder, Intravenous Injection, and HIV Infection: A Review. Cell Transplantation 2019, 28, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Scutari, R.; Alteri, C.; Perno, C.F.; Svicher, V.; Aquaro, S. The Role of HIV Infection in Neurologic Injury. Brain Sci 2017, 7. [Google Scholar] [CrossRef]
- Lashomb, A.L.; Vigorito, M.; Chang, S.L. Further characterization of the spatial learning deficit in the human immunodeficiency virus-1 transgenic rat. Journal of NeuroVirology 2009, 15, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Kass, M.D.; Liu, X.; Vigorito, M.; Chang, L.; Chang, S.L. Methamphetamine-induced behavioral and physiological effects in adolescent and adult HIV-1 transgenic rats. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 2010, 5, 566–573. [Google Scholar] [CrossRef]
- Vigorito, M.; Cao, J.; Li, M.D.; Chang, S.L. Acquisition and long-term retention of spatial learning in the human immunodeficiency virus-1 transgenic rat: effects of repeated nicotine treatment. J Neurovirol 2013, 19, 157–165. [Google Scholar] [CrossRef]
- Fan, R.; Schrott, L.M.; Arnold, T.; Snelling, S.; Rao, M.; Graham, D.; Cornelius, A.; Korneeva, N.L. Chronic oxycodone induces axonal degeneration in rat brain. BMC Neuroscience 2018, 19, 15. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: a quality control tool for high throughput sequence data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol 2010, 11, R106. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J., Jr; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2013, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Williamson, A.; Darke, S.; Ross, J.; Teesson, M. The effect of persistence of cocaine use on 12-month outcomes for the treatment of heroin dependence. Drug Alcohol Depend 2006, 81, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Staiger, P.K.; Richardson, B.; Long, C.M.; Carr, V.; Marlatt, G.A. Overlooked and underestimated? Problematic alcohol use in clients recovering from drug dependence. Addiction 2013, 108, 1188–1193. [Google Scholar] [CrossRef]
- de la Fuente, L.; Molist, G.; Espelt, A.; Barrio, G.; Guitart, A.; Bravo, M.J.; Brugal, M.T. Mortality risk factors and excess mortality in a cohort of cocaine users admitted to drug treatment in Spain. J Subst Abuse Treat 2014, 46, 219–226. [Google Scholar] [CrossRef]
- CDC. HIV Surveillance Report. Available online: https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-33/index.html(accessed on Feb 20).
- Nweke, M.; Mshunqane, N.; Govender, N.; Akinpelu, A.O.; Ukwuoma, M. Impact of HIV-associated cognitive impairment on functional independence, frailty and quality of life in the modern era: a meta-analysis. Scientific Reports 2022, 12, 6470. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Sarkar, A.; Mitsuya, H. HIV-Associated Neurocognitive Disorder (HAND) and the Prospect of Brain-Penetrating Protease Inhibitors for Antiretroviral Treatment. Med Res Arch 2017, 5. [Google Scholar]
- Wang, Y.; Liu, M.; Lu, Q.; Farrell, M.; Lappin, J.M.; Shi, J.; Lu, L.; Bao, Y. Global prevalence and burden of HIV-associated neurocognitive disorder. A meta-analysis 2020, 95, e2610–e2621. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Sasaki, Y.; Ohsawa, K.; Imai, Y.; Nakamura, Y.; Inoue, K.; Kohsaka, S. Extracellular ATP or ADP Induce Chemotaxis of Cultured Microglia through G<sub>i/o</sub>-Coupled P2Y Receptors. The Journal of Neuroscience 2001, 21, 1975–1982. [Google Scholar] [CrossRef]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.-B. ATP mediates rapid microglial response to local brain injury in vivo. Nature Neuroscience 2005, 8, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Berríos-Cárcamo, P.; Quezada, M.; Quintanilla, M.E.; Morales, P.; Ezquer, M.; Herrera-Marschitz, M.; Israel, Y.; Ezquer, F. Oxidative Stress and Neuroinflammation as a Pivot in Drug Abuse. A Focus on the Therapeutic Potential of Antioxidant and Anti-Inflammatory Agents and Biomolecules. Antioxidants (Basel) 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Cuzzo, B.; Padala, S.A.; Lappin, S.L. Physiology, Vasopressin. In StatPearls; StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.: Treasure Island (FL), 2022. [Google Scholar]
- Caldwell, H.K.; Lee, H.J.; Macbeth, A.H.; Young, W.S. , 3rd. Vasopressin: behavioral roles of an "original" neuropeptide. Prog Neurobiol 2008, 84, 1–24. [Google Scholar] [CrossRef]
- Logrip, M.L.; Koob, G.F.; Zorrilla, E.P. Role of corticotropin-releasing factor in drug addiction: potential for pharmacological intervention. CNS Drugs 2011, 25, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Macbeth, A.H.; Pagani, J.H.; Young, W.S. , 3rd. Oxytocin: the great facilitator of life. Prog Neurobiol 2009, 88, 127–151. [Google Scholar] [CrossRef]
- Neumann, I.D.; Slattery, D.A. Oxytocin in General Anxiety and Social Fear: A Translational Approach. Biological Psychiatry 2016, 79, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lindenberg, A.; Domes, G.; Kirsch, P.; Heinrichs, M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience 2011, 12, 524–538. [Google Scholar] [CrossRef]
- Uhrig, S.; Hirth, N.; Broccoli, L.; von Wilmsdorff, M.; Bauer, M.; Sommer, C.; Zink, M.; Steiner, J.; Frodl, T.; Malchow, B.; et al. Reduced oxytocin receptor gene expression and binding sites in different brain regions in schizophrenia: A post-mortem study. Schizophrenia Research 2016, 177, 59–66. [Google Scholar] [CrossRef]
- Bartholomeusz, C.F.; Ganella, E.P.; Labuschagne, I.; Bousman, C.; Pantelis, C. Effects of oxytocin and genetic variants on brain and behaviour: Implications for treatment in schizophrenia. Schizophrenia Research 2015, 168, 614–627. [Google Scholar] [CrossRef] [PubMed]
- McGregor, I.S.; Bowen, M.T. Breaking the loop: oxytocin as a potential treatment for drug addiction. Horm Behav 2012, 61, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Sundar, M.; Patel, D.; Young, Z.; Leong, K.C. Oxytocin and Addiction: Potential Glutamatergic Mechanisms. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.C.; Zhou, L.; Ghee, S.M.; See, R.E.; Reichel, C.M. Oxytocin decreases cocaine taking, cocaine seeking, and locomotor activity in female rats. Exp Clin Psychopharmacol 2016, 24, 55–64. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Thompson, E.; Merriman, C.E.; Alvarez, M.R.; Alpaugh, E.S.; Cornett, E.M.; Murnane, K.S.; Kozinn, R.L.; Shah-Bruce, M.; Kaye, A.M.; et al. Oxytocin, a Novel Treatment for Methamphetamine Use Disorder. Neurol Int 2022, 14, 186–198. [Google Scholar] [CrossRef] [PubMed]
- King, C.E.; Gano, A.; Becker, H.C. The role of oxytocin in alcohol and drug abuse. Brain Res 2020, 1736, 146761. [Google Scholar] [CrossRef] [PubMed]
- Katrukha, I.A. Human cardiac troponin complex. Structure and functions. Biochemistry (Mosc) 2013, 78, 1447–1465. [Google Scholar] [CrossRef]
- Gomes, A.V.; Potter, J.D.; Szczesna-Cordary, D. The role of troponins in muscle contraction. IUBMB Life 2002, 54, 323–333. [Google Scholar] [CrossRef]
- Dominguez, R.; Holmes, K.C. Actin structure and function. Annual review of biophysics 2011, 40, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.; Picard, D.; Lo, R.; Beaulieu, J.; Remke, M.; Diaz, R.J. Expression of cell type incongruent alpha-cardiac actin 1 subunit in medulloblastoma reveals a novel mechanism for cancer cell survival and control of migration. Neuro-Oncology Advances 2021, 3. [Google Scholar] [CrossRef]
- Berridge, M.J. Dysregulation of neural calcium signaling in Alzheimer disease, bipolar disorder and schizophrenia. Prion 2013, 7, 2–13. [Google Scholar] [CrossRef]
- Hu, X.T. HIV-1 Tat-Mediated Calcium Dysregulation and Neuronal Dysfunction in Vulnerable Brain Regions. Curr Drug Targets 2016, 17, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.; Thiel, F.; Beutner, F.; Teren, A.; Frisch, S.; Ballarini, T.; Möller, H.E.; Ihle, K.; Thiery, J.; Schuler, G.; et al. Brain Damage With Heart Failure: Cardiac Biomarker Alterations and Gray Matter Decline. Circ Res 2020, 126, 750–764. [Google Scholar] [CrossRef]
- Rangel, I.; Amorim, M.; Gonçalves, A.; Sousa, C.; Bettencourt, P.; Maciel, M.J. Toxic dilated cardiomyopathy: recognizing a potentially reversible disease. Arq Bras Cardiol 2014, 102, e37. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tang, H.; Zhu, F.; Jiang, Z.; Shi, J.; Zhou, Y.; Garcia, E.V.; Li, D.; Zhou, W. Prognostic value of left-ventricular systolic and diastolic dyssynchrony measured from gated SPECT MPI in patients with dilated cardiomyopathy. Journal of Nuclear Cardiology 2020, 27, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, R.T.; Hedlin, H.; Greuenwald, P.; Wilson, D.M.; Segal, J.I.; Jorden, M.; Kudelko, K.; Liu, J.; Hsi, A.; Rupp, A.; et al. Features and Outcomes of Methamphetamine-associated Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 2018, 197, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, R.H.; Bloomfield, G.S. The Causes of HIV-Associated Cardiomyopathy: A Tale of Two Worlds. BioMed Research International 2016, 2016, 8196560. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, G. HIV-associated cardiomyopathy etiopathogenesis and clinical aspects. Herz 2005, 30, 486–492. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




