1. Introduction
The circadian system (CS) plays an important role in ensuring the normal function of the human body and adaptation to life in an environment that changes over a period of 24 h [
1]. For the normal functioning of the CS, it must constantly receive external synchronizing signals for a period of 24 h. The main external synchronizing signal for the human CS is the daily rhythm of lighting [
2]. However, in modern society, a person spends most of his time indoors without access to sunlight and also actively uses artificial lighting sources that create "light pollution". In addition, the active use of shift work, frequent long-distance flights, and the introduction of time zones led to a mismatch between the daily rhythms of social life and lighting. As a result, the synchronizing role of illumination in the human CS has significantly decreased [
3]. In this regard, there are a number of CS function disorders that are characteristic only for modern human: desynchrony caused by shift work [
4,
5], rapid movement through several time zones (jetlag) [
4,
5], mismatch between the sleep phase on work/school days and weekends (social jetlag [SJL]) [
6]. SJL has a relatively mild effect on the human organism, but this form of circadian misalignment is extremely widespread, mainly among schoolchildren and university students. The incidence of SJL in schoolchildren and students varies from 40.1 % in Japan [
7] to 86.4 % in Russia [
8]. In people with SJL, there is a decrease in cognitive functions [
9], academic performance [
10], an increased risk of developing depression [
11], obesity [
12], and cardiovascular diseases [
13]. All this indicates that the human CS in modern society requires additional external synchronizing signals.
The second most important external synchronizing signal for the human CS is the circadian rhythm of food intake [
14]. The relationship between circadian eating disorders and the risk of obesity has been studied in the greatest detail. It has been shown that a shift in the phase of food intake to a later time leads to a delay in the phase of the circadian rhythm of melatonin production [
15]. Excessive shortening [
16] and an increase in the "eating window" [
17], the shift of the meal phase to a later time of day [
18,
19], as well as irregularity in meal timing [
20,
21] and food jetlag [
19,
22] are associated with an increase in body mass index (BMI). There are only a few publications devoted to the relationship of circadian eating disorders with the psychoemotional state, sleep function, and other functions. Irregularity in meal timing is associated with neuroticism, decreased physical activity, sleep quality, and subjective health assessment [
23], and eating jetlag
is associated with increased systolic blood pressure and glycemic levels [
21].
Another important factor that has both a positive and negative impact on the function of the human CS is the composition of the food consumed. It is known that the consumption of high-calorie, high-fat foods leads to a decrease in the amplitude of the circadian rhythms of the central oscillator of the CS [
24], which can lead to a mismatch of 24-h rhythms in individual organs and systems (internal desynchrony) and an increased risk of various diseases. At the same time, it is known that some foods are source of chronobiotics, biologically active substances such as melatonin (MT), which have a direct effect on the function of the CS and thus can increase the synchronization of the circadian rhythms [
25]. Convincing experimental data have now been obtained on the positive effect of food MT or its precursor tryptophan on sleep function [
26,
27,
28,
29,
30], well-being [
31], and health [
32] in young and elderly people.
Thus, SJL is one of the most common violations of the human CS function in modern society. Moreover, this mismatch in the CS is most common among young, socially active people, which reduces their ability to adapt to the social environment. Previous studies have not explored meal timing and diet disturbances in young people with SJL in sufficient detail. There is also insufficient experimental data on the potential possibility of using meal timing and diet modifications to prevent SJL and reduce the risk of its negative consequences. Therefore, more research is required to evaluate the relationship between meal timing, diet, sleep function, and CS state. Since these functions are closely interrelated, the most appropriate model for their study is a comprehensive study of young, healthy people in their natural habitat using subjective and non-invasive objective methods
The present study tests the hypothesis that meal timing, diet, and the consumption of FMT are associated with sleep function and the state of the CS.
2. Materials and Methods
2.1. Subjects
The studies were conducted from January to April 2018 in Syktyvkar (61.4°N, 50.5°E) and from October 2021 to April 2022 in Moscow (55.4°N, 37.4°E). Students from high schools, workers, and unemployed persons (n = 91) were recruited for the study. People with metabolic disorders, shift-work, acute and chronic inflammatory diseases, who use anti-inflammatory drugs, and with inflammation were excluded from the study. Thus, practically healthy persons (n = 83) of both sexes (female - 57.8 %) aged 26.7 ± 6.1 yrs were selected to participate in the study.
2.2. Instruments
The participants completed a questionnaire, which included personal information (sex, age, height, and weight), the Munich ChronoType Questionnaire (MCTQ) [
33] for evaluation chronotype and social jetlag, the Pittsburgh Sleep Quality Index (PSQI) [
34] for evaluation sleep quality, the Beck Depression Inventory (BDI) [
35] for evaluation depression, the Dutch Eating Behavior Questionnaire (DEBQ) [
36], the Checklist Individual Strength Questionnaire (CIS) [
37] for evaluation subjective fatigue, and the Yale Food Addiction Scale (YFAS) [
38].
2.2.1. MCTQ
The test contains questions about the time of sleep onset, sleep onset latency, time of awakening, the use of an alarm clock, and sleep inertia on weekdays and weekends. Based on these data, the following indicators were calculated: chronotype (MSF
SC), social jetlag (SJL), average weekly sleep duration, and sleep efficiency. The formulas and calculation methods for the characteristics listed above were described in Borisenkov et al. [
39].
Sleep debt across the week (SlDebt) was calculated as described in Roenneberg et al. [
12]:
where SlD
F: sleep duration on free days; SlD
W: sleep duration on weekdays; SlD: average weekly sleep duration; TiB: time in bed; FD: number of free days; SlE: sleep efficiency; MSF: mid-point of the sleep phase on free days; MSW: mid-point of the sleep phase on weekdays; SJL: social jetlag; and MSF
SC: mid-point of the sleep phase on free days, adjusted by the sleep debt accumulated on weekdays (chronotype).
2.2.2. PSQI
To assess sleep quality, we used the Russian version of the PSQI [
40]. This test consists of 19 questions related to sleep quality, including sleep latency, duration, efficiency, disturbance, use of sleep medication, and daytime sleepiness for a one-month period. Global PSQI scores range from 0 to 21 points. In our sample, the scores ranged from 2 to 11, with an overall group
M (SD) of 5.5 (2.1). According to the test authors, a PSQI score of ≤ 5 indicates good-quality sleep, and a PSQI score of > 5 indicates poor-quality sleep [
34].
2.2.3. BDI
The Beck Depression Inventory was used to assess the psychoemotional state of the study participants [
35]. The test lists 21 symptoms of depression and their severity, ranging from 0 to 3. The sum of the scores, which range from 0 to 63, is used to determine the subject's psychoemotional state. In our sample, the scores ranged from 0 to 26, with an overall group
M (SD) of 7.7 (5.6). Cronbach’s α for this sample was 0.85.
2.2.4. DEBQ
The Dutch Eating Behavior Questionnaire was used to assess eating behavior [
36]. The questionnaire consists of 33 statements and includes three subscales that evaluate three types of eating behavior: restraint (DEBQ
restr), which consists of 10 items; external (DEBQ
extern), 10 items; and emotional eating (DEBQ
emo), 13 items. A Likert-type scale ranging from 1 (seldom) to 5 (very often) is used. Scores are then summed separately for each subscale and divided by the total number of items on that subscale to derive a mean subscale score. Cronbach's α for DEBQ, DEDQ
restr, DEBQ
extern, and DEBQ
emo were 0.81, 0.92, 0.52, and 0.94, respectively.
2.2.5. CIS
The multidimensional checklist individual strength questionnaire (CIS) was used to measure chronic fatigue [
37]. The CIS consists of 20 statements on fatigue-related problems (fatigue severity, concentration, motivation, and activity) respondents might have experienced in the past 2 weeks. The items are scored on 7-point Likert scales (1 = "Yes, that is true" to 7 = "No, that is not true"). To assess the level of subjective fatigue, the sum of all scores was calculated. Higher scores indicate a higher degree of fatigue. In our sample, the scores ranged from 8 to 48, with an overall group
M (SD) of 26.1 (9.9). Cronbach’s α for this sample was 0.90.
2.2.6. YFAS
The scale consists of 25 questions and describes seven diagnostic criteria for drug addiction (such as tolerance, withdrawal, and loss of control) and clinically significant eating disorders [
38]. The results of the treatment are presented in the form of (a) a quantitative indicator equal to the sum of confirmed symptoms (SC, varying within the range of 0 to 7), and (b) a qualitative indicator corresponding to a clinically significant eating disorder in the presence of three or more symptoms of FA. Cronbach’s α for this sample was 0.91.
2.2.7. Dreem2 headband
The Dreem2 headband (D2H) (France) [
41] device is a wireless headband that records five types of physiological signals during sleep at home via three types of sensors embedded in the device: (1) brain cortical activity via five EEG dry electrodes yielding seven derivations; (2-4) movements, position, and breathing frequency via a 3D accelerometer located over the head; and (5) heart rate via a red-infrared pulse oximeter located in the frontal band. The following standard sleep variables were calculated: time in bed (TiB), as the number of minutes from lights-out to lights-on; total sleep time (TST) (min); SlE (%), as TST/TiB×100; sleep onset latency (SOL), as the number of minutes from lights-out to the first three consecutive epochs of any sleep stage; wake after sleep onset (WASO), as the number of minutes awake following the first three consecutive epochs of any sleep stage; and the time (min) and percentage of TST spent in each sleep stage (Non-REM: N1, N2, N3, and REM). The formulas and calculation methods for the characteristics listed above were described in Arnal et al. [
42]. In addition, objective MSFsc (oMSFsc) and SJL (oSJL) were calculated using data obtained by D2H and formulas (1-4) as described above. Participants were instructed on how to use the D2H. The D2H recorded every night for one week. After the study ended, the participant exported their data and sent it to the researcher.
2.2.8. Dietary intake
An online food diary was used to calculate dietary intake. Study participants were also instructed to download the free App "FatSecret" (Russia) [
43] on their own smart phones to self-report the food type and amount in the App for one week. After one week, the data from the app was collected and calculated [
44]. The Fat Secret application is a self-reported electronic food diary to collect data pertaining to calorie consumption, macronutrients, and the distribution of foods across three meal classifications. The classification of food into meals was based on app designations (breakfast, lunch, and dinner) over 24-h periods. Food-specific data (e.g., self-reported calories consumed and macronutrients based on app designation) from the electronic food diary were averaged for each participant: a 24-h work and 24-h non-work day food consumption average was calculated for each participant based on app data [
44]. From this data, we calculated the following variables: (a) average eating window. Eating window was defined as the length between the first and the last caloric event (in hours). To calculate the average eating window, first we estimated the eating window for weekdays and weekends as follows: Eating window (h) = Timing of the last meal −Timing of the first meal; (b) eating midpoint (EM), defined as the middle time point between the first and the last meal. This parameter was calculated for weekdays and weekends based on the methodology proposed to estimate the midpoint of sleep; (c) eating jetlag. This parameter was defined as the variability of the timing of the eating period and was estimated following the methodology proposed to calculate SJL [
6]. Accordingly, eating jetlag was calculated in hours as follows: EM on weekends – EM on weekdays. Additionally, the variability in the timing of breakfast, lunch, or dinner was calculated as follows: Breakfast or lunch or dinner jetlag (h) = Time on weekends − Time on weekdays. All analyses were conducted using the absolute value of the estimated eating, breakfast, lunch, or dinner jetlag. Also, for daily intake, we calculated the number of eating episodes per day. In order to verify consumption during the later hours of the day, we determined the total and the proportion (%) of calories consumed after 9 p.m. [
22,
45].
2.2.9. Food melatonin intake
The data in “FatSecret” was used to assess FMT consumption for the day (FMT
day) and for dinner (FMT
dinner) as described in
Supplementary Materials. The questionnaire included only products that, according to the literature, contain MT, as well as those that are used for food by residents of Russia. Descriptive statistics of FMT
day and FMT
dinner are present in
Tables S4 and S5.
2.3. Statistical analysis
The SJL was calculated as an absolute value. Participants were divided into three groups according to the results of the MCTQ: 1) SJL ≤ 1 h, 2) 1 < SJL ≤ 2 h, and 3) SJL > 2 h. All statistical analyses were performed with SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). The data were tested for normality using the Shapiro-Wilk test. We conducted a series of one-way ANCOVAs with SJL categories (1: “SJL ≤ 1 h”; 2: “1 < SJL ≤ 2 h”; 3: “SJL > 2 h”) specified as the independent variables and “General characteristics”, “Sleep pattern”, “Meal timing”, “Total calories and nutrient intake” and “ln(FMTday/dinner)”, (log-transformation was performed because the original sample is skewed) specified as the dependent variables. We included “sex”, “age”, and “BMI”, as covariates in the models (analyses). A Tukey test for post hoc comparisons, a Mann–Whitney U test for independent samples, and a Student’s t-test for dependent samples were performed to determine significant differences between groups.
A series of binary logistic regression analyses was performed (for details, see Table 4), in which in the first case "fatigue" (codes: low—0, high—1) was used as the dependent variable and sex (0—females, 1—males), age, BMI, eating window, eating midpoint, and eating jetlag were used as independent variables. In the second case, "sleep debt and sleep inertia" (codes: low—0, high—1) were used as dependent variables, and "meal timing" was used as an independent variable. A stepwise inclusion procedure was used to determine the final set of predictors in the model. The goodness of fit was evaluated by the Hosmer-Lemeshow test and Omnibus tests of model coefficients. The results were adjusted using the Bonferroni correction for multiple comparisons. Only significant factors were included in the final model.
3. Results
The study included 83 participants (58 % female; age 26.7 ± 6.1 yrs) (
Table 1). The mean chronotype and SJL were 4.4 ± 1.1 and 1.4 ± 0.9 h, respectively. Thirty (36 %), 35 (42 %), and 18 (22 %) participants were assigned to the SJL ≤ 1 h, 1 < SJL < 2 h, and SJL > 2 h groups, respectively. The group with SJL > 2 h was younger (p < 0.002). All participants, regardless of SJL, were of normal weight (BMI = 22.6 ± 3.1 kg/m
2) and reported similar depression and eating behaviors (external, emotional, restraint eating, and food addiction symptoms). The significant effect of SJL on chronotype and fatigue level was found (
Table 1). Individuals with SJL > 2 h had a significantly later chronotype and reported a 1.3-times higher subjective fatigue level than those with SJL < 1 h.
The sleep duration on weekdays in people with SJL > 2 h was 1.5 h less than in people with SJL < 1 h, which led to the formation of a sleep debt of 2.6 h. On weekends, participants with SJL > 2 h got up 118 min later and went to bed 65 min later than people without SJL (
Table 2).
3.1. Differences in Meal Timing Between Weekends and Weekdays among SJL Groups
The average number of eating episodes was 4.9 ± 1.2 and 4.7 ± 1.3 times on weekdays and weekends, respectively, and did not depend on the SJL. The meal timing for participants with SJL < 1 h did not differ on weekdays or weekends. Participants with 1 h < SJL < 2 h had breakfast 52 min later on weekends than weekdays (
Table 3). Participants with SJL > 2 h had breakfast 54 min later and dinner 79 min later on weekends than weekdays (
Table 3). However, differences between the SJL groups in the breakfast, lunch, and dinner jetlags were not found. The average eating jetlag was 0.8 ± 0.5 h and did not depend on the SJL
either.
The calories intake after 9 p.m. for participants with SJL < 1 h and 1 h < SJL < 2 h did not differ on weekdays or weekends. (
Table 3). Participants with SJL > 2 h consumed 1.9 times more calories after 9 p.m. on weekends than weekdays (
Table 3). In the group of SJL > 2 h, the weekly average calorie intake after 9 p.m. was 2.2 times more than that of the group SJL < 1 h (20 ± 16 vs. 9 ± 9 % of total energy intake;
F1,35 = 3.96;
p < 0.03;
η2 = 0.43), while weekend calorie intake after 9 p.m. was 3.3 times more than that of the group SJL < 1 h.
The time from waking up to breakfast did not differ on weekdays and weekends in the groups with SJL < 1 h and 1 h < SJL < 2 h. However, in the group with SJL > 2 h, the time from waking up to breakfast was reduced from 2 to 0.2 h on weekdays and weekends, respectively (
Table 3). It was found that participants had dinner for an average of 3.4 h before falling asleep, regardless of the day of the week and SJL.
Eating jetlag was significantly associated with sleep duration (
Table 4). Participants who slept less than 7 h had the most eating jetlag.
Participants with a late chronotype were found to have more pronounced dinner jetlag (
Table 5). In addition, late and intermediate chronotype participants consumed 2.3 times more calories after 9 p.m. than early chronotype participants.
Table 6 shows the logistic regression analyses examining associations between sleep pattern and meal timing. After adjusting for age and sex, the sleep debt was positively associated with breakfast jetlag and negatively associated with fatigue score and sleep inertia.
A series of binary logistic regression analyses were performed in which fatigue, depression, and sleep pattern characteristics (see
Table 2) were specified as the dependent variables, while age, sex (code: 0—female; 1—male), BMI, and meal timing characteristics (see
Table 4) were specified as independent variables (predictors); a stepwise inclusion of predictors in the model was performed, and only significant factors were included in the final model;
B: non-standardized regression coefficient;
OR: odds ratio;
CI: confidence interval; * Bonferroni-corrected significance of the regression coefficient; models' goodness of fit were tested using Omnibus and Hosmer–Lemeshow tests.
3.2. Differences in Food Intake Between Weekends and Weekdays among SJL Groups
The participants' average daily energy and macronutrient intake during the week was 2017 ± 200 kcal, 79.4 ± 10 g protein, 82.2 ± 10 g fat, 230.8 ± 30 g carbohydrates, 12175.8 ± 12296.0 ng/day, and 4744.9 ± 4214.9 ng/dinner FMT intake. No statistical difference was identified in weekday or weekend energy and macronutrient intake in any of the SJL groups (
Table 7).
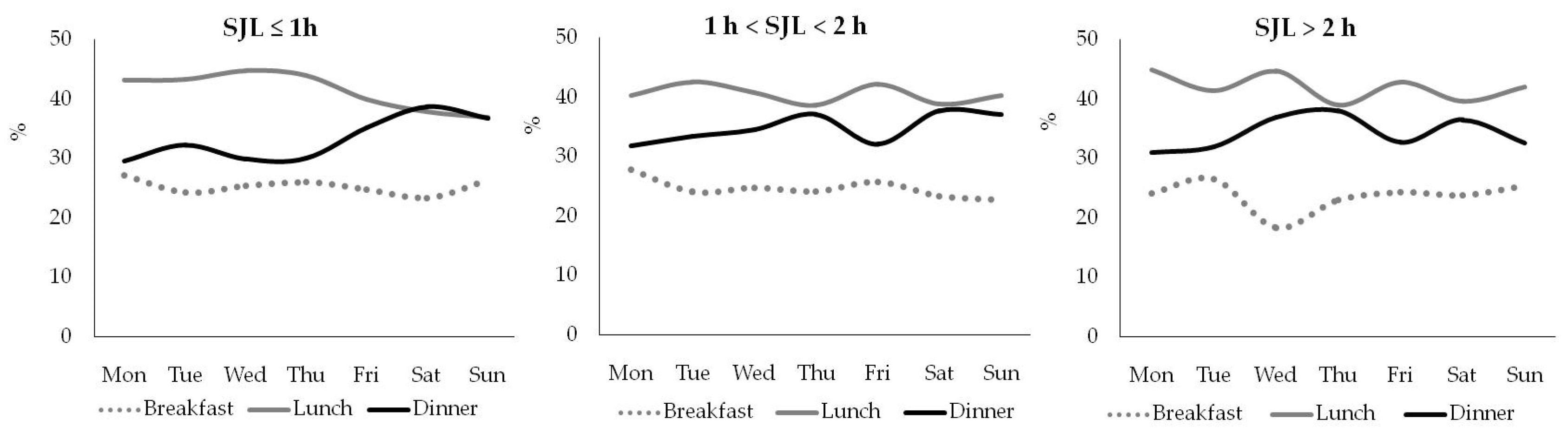
Breakfast, lunch, and dinner accounted for 25–30 %, 40–45 %, and 30–35 % of the daily energy intake, regardless of the day of the week and the SJL (
Figure 1).
Dietary fiber intake did not differ between weekdays and weekends in all SJL groups (
Table 7). However, participants with 1 h < SJL < 2 h and SJL > 2 h consumed less dietary fiber on average per week by 18.3 and 15.8 %, respectively, than those with SJL < 1 h (
F2,89 = 4.79;
p < 0.01
; η2 = 0.34). Participants with 1 h < SJL < 2 h consumed 15 and 16 % less dietary fiber than those with SJL < 1 h on weekdays (
p < 0.02) and weekends (
p < 0.001), respectively (
Table 7). Participants with SJL > 2 h consumed 10 and 22 % less dietary fiber than those with SJL < 1 h on weekdays (
p < 0.04) and weekends (
p < 0.05), respectively (
Table 7). The average daily decrease in dietary fiber intake in people with SJL appeared to be due to a decrease in dietary fiber intake for breakfast (
Figure 2). Participants with SJL < 1 h consumed 5.3 ± 3.8 g of dietary fiber for breakfast, while those with 1 h < SJL < 2 h, and SJL > 2 h consumed 3.3 ± 1.6 and 3.5 ± 1.8 g (
p < 0.01) of dietary fiber for breakfast, respectively.
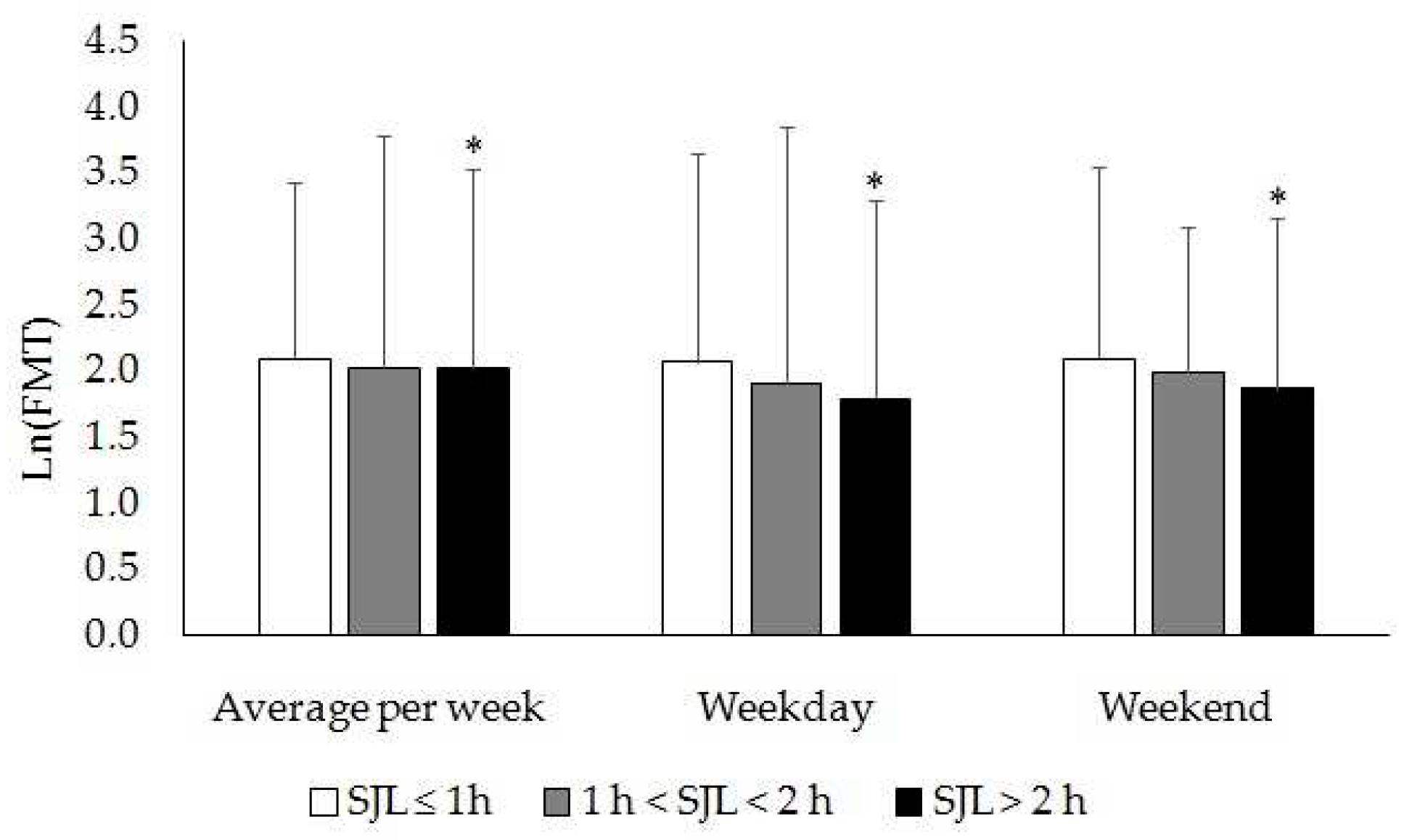
Melatonin-containing food intake for dinner did not differ between weekdays and weekends in groups with SJL < 1 h and 1 h < SJL < 2 h (
Table 7)
. However, food melatonin intake for dinner in the SJL > 2 h group was significantly less on weekends than weekdays. At the same time, participants with SJL > 2 h consumed 10 and 18 % less FMT
dinner than those with SJL < 1 h on weekdays and weekends, respectively (
Figure 3).
3.3. Association of SJL and Food Melatonin with Objectively Measured Sleep Characteristics
Sleep-related physiological signals (EEG, breathing, and heart rate) were monitored using D2H in 21 participants (
n = 147 measurements). There were no significant differences in the characteristics of sleep and sleep-wake rhythm assessed using MCTQ and D2H (
Table 8). In this section, we used the chronotype (oMSFsc) and SJL (oSJL) evaluated using D2H.
Then, the participants were divided into subgroups with oSJL ≤ 1 h (
N = 12 participants;
n = 84 measurements) and oSJL > 1 h (
N = 9 participants;
n = 63 measurements) based on D2H data. According to the D2H monitoring, the mean weekly sleep duration was 6.8 ± 0.5 and 6.9 ± 0.6 h/day in participants with oSJL > 1 h and oSJL ≤ 1 h, respectively (
Table 9). It was found that people with oSJL > 1 h slept 1.0 h less than those with oSJL ≤ 1 h on weekdays (
p = 0.01), but on weekends, they slept 1.1 h more (
p = 0.01). In the group of oSJL > 1 h, the average weekly REM sleep phase is 0.5 h longer (
p = 0.01) than in the group of oSJL ≤ 1 h (
Table 9). On work/school days, in the group of oSJL > 1 h, total sleep and the light sleep phase duration were 1.0 (
p = 0.01) and 0.6 h shorter (
p = 0.05) than in the group of oSJL ≤ 1 h, respectively. On weekends, total sleep and the REM sleep phase duration were 1.1 h (
p = 0.01) and 1 h longer (
p = 0.001), than in the group of oSJL ≤ 1 h, respectively (
Table 9). Among other indicators measured using D2H, differences in heart rate during sleep were noted: the average weekly heart rate in the group of oSJL > 1 h is 5 bmp higher (
p = 0.04) than in their peers from the group of oSJL ≤ 1 h. These differences were noted only on work/school days (
Table 9).
The relationship of the sleep function characteristics estimated using D2H with the FMT consumption for dinner was noted (
Table 10). In the group of persons with a consumption of FMT
dinner ≥ 4234.5 ng/dinner (tertile 3), compared with their peers from the group with a consumption of FMT
dinner ≤ 313.2 ng/dinner (tertile 1), there was a decrease in the level of oSJL and sleep debt by 0.9 and 1.5 h, respectively. In addition, there was an increase in average weekly sleep duration and deep sleep phase by 1.1 and 0.5 h, respectively (
Table 10). Moreover, the consumption of melatonin for dinner had a positive effect only on sleep characteristics during work/school days. Individuals from the group with the highest FMT
dinner consumption, compared with their peers from the group with the lowest FMT
dinner consumption, had an increase in total sleep, the light and deep sleep phase durations by 1.7, 1.1, and 0.6 h, respectively, on work/school days. At the same time, there was no significant association between sleep characteristics and the consumption of MT-containing products on weekends (
Table 10).
4. Discussion
The study showed that circadian eating disorders (such as food jetlag and delay in the phase of meal timing), as well as an increase in calorie intake after 9 p.m., were most often observed in young people with short sleep, late chronotype, and SJL. We have also shown that in people with SJL, there is a decrease in the intake of foods rich in dietary fiber and MT. Previously, numerous studies have noted the negative effects of SJL. It has been shown that people with a late chronotype and SJL skip breakfast more often [
22,
46], consume more high-calorie, fiber-poor foods [
22], consume the main part of the daily diet for dinner [
47], and have more pronounced eating jetlag [
48].
In this study, we noted an inverse relationship between SJL and FMT
dinner. To assess the consumption of MT-containing foods, we asked participants to fill out an online food diary for a week, in which they indicated all the foods eaten during the day and for dinner, as well as the size of the portion. The SJL value was assessed by MCTQ as well as using D2H-derived objective data on the sleep phases’ duration throughout the calendar week. The results obtained in the present study closely correspond to the theoretical expectations based on the mechanism of MT action on CS function. It is known that the risk of developing SJL is due to a combination of two main external and internal factors: (1) a biologically determined delay in the phase of the sleep-wake rhythm, steadily increasing from 10 to 20 yrs of age [
49], and (2) the need to get up early in the morning in order not to be late for work/school. Both of these factors are most pronounced in young, socially active people. That is why SJL is most often observed in young students [
8]. From a physiological point of view, SJL can be regarded as a periodic weekly change in the time of the onset of the mid-sleep phase due to a decrease in sleep duration during the work/school days and its compensatory increase on weekends. Therefore, potentially effective means of preventing SJL should increase the total sleep duration due to earlier falling asleep on work/school days. MT pills, used in the evening before going to bed, increasing drowsiness and speeding up falling asleep [
25]. The data obtained in this study on the relationship between SJL and FMT
dinner are completely consistent with the mechanism of action of MT on sleep function.
We have shown that young people who consume a large amount of MT-containing food for dinner (FMT
dinner ≥ 4234.5 ng/dinner) on work/school days have an increase in sleep duration of 1 h, mainly due to light and deep sleep phases. These data are consistent with the results presented in Duffy et al. [
50]. The authors showed that in older people (>55 yrs), high-dose MT intake (5 mg) significantly increased sleep efficiency during biological night, mainly by increasing the duration of Stage 2 non-REM sleep and slightly shortening awakenings. Moreover, this effect was weakly expressed at a dose of 3 mg [
50]. Based on this, it can be assumed that the consumption of foods containing MT ≥ 4234.5 ng for dinner creates an effective dose equivalent to 5 mg of pharmacologically pure MT. Previously, it was shown [
32] that an order of magnitude lower concentrations of MT consumed with food have biological effect similar to those observed when taking high pharmacological doses of the drug. Perhaps this difference in the effectiveness of the action of MT of different origins is due to the fact that MT contained in food products has a chronic effect on humans since human food preferences are quite stable and therefore a person, as a rule, consumes approximately the same set of products for many days. It is also possible that, in addition to MT, the products contain other chronobiotics, whose effects we did not take into account in this study. In particular, it is known that in addition to MT, cherry juice contains a significant amount of tryptophan, a precursor of serotonin and MT [
30].
The presented data indicate that not only the total sleep duration but also the ratio of sleep phases changes in people with SJL during the calendar week. During the work/school days, there is a shortening of total sleep duration mainly due to light and deep phases of sleep, that is, non-REM sleep phases, and on weekends, an increase in sleep duration occurs mainly due to REM sleep phases. Despite the fact that the average weekly sleep duration in persons with and without SJL does not differ, the structure of sleep phases in persons with SJL changes significantly, so an increase in sleep duration on weekends cannot be regarded as compensatory. From a physiological point of view, non-REM and REM sleep phases are fundamentally different. During the non-REM phase of sleep, the metabolic activity of brain cells decreases, the size of the cells decreases, and the volume of the intercellular space increases. All these functional and morphological changes facilitate the work of the glymphatic system of the brain, which ensures the removal of toxins accumulated during the day [
51,
52]. Whereas during the REM sleep phase, the metabolic activity of brain cells increases, their size increases, the volume of the intercellular space decreases, and the temperature of the brain increases [
52,
53,
54], which interferes with the functioning of the glymphatic system. Therefore, the decrease in non-REM sleep phase on work/school days and, at the same time, an increase in REM sleep on weekends observed in individuals with SJL leads to a deterioration in the functioning of the glymphatic system of the brain, accumulation of metabolic products in the brain, and deterioration of its function. It has previously been shown that the deterioration of cognitive functions in older people is due to age-related deterioration in the function of the glymphatic system [
52]. Patients with REM sleep behavior disorder often have cognitive impairments such as Parkinson's disease and dementia with Lewy body [
55]. It can be proposed that the deterioration of the glymphatic system in individuals with SJL is the cause of the previously described decreased level of intelligence [
9] and, as a consequence, low academic performance [
10].
Our study showed that people who consume more MT-containing foods for dinner have significantly longer non-REM sleep phases on work/school days than their peers who consume a minimum amount of MT-containing foods for dinner. These data indicate that the consumption of MT-containing products for dinner does not just increase the sleep duration but causes a change in the ratio of sleep phases, which contributes to the increase in glymphatic system function and thus can be used as a specific preventive means of restoring the physiological function of the glymphatic system of the brain caused by SJL.
The presented work has a number of advantages and limitations. The advantage of the work is that we conducted a comprehensive study using subjective and objective methods for assessing a number of interrelated indicators characterizing meal timing, diet, sleep function, and the state of the CS, which made it possible to identify the factors most closely associated with CS mismatch. This will allow us in the future to purpose fully conduct a search for effective means of preventing SJL and related negative consequences. Among the limitations of the study, we can single out a small sample size as well as a cross-sectional design, so the results presented in the study can be considered only preliminary, requiring additional research to confirm the conclusions drawn on their basis.
5. Conclusions
The study showed that when SJL occurs in young people, there is a change in meal timing, diet, and the ratio of sleep phases. The method of questionnaire survey has shown that SJL is associated with such disturbances in meal timing as food jetlag and a delay in the phase of the 24-h rhythm of eating. Social jetlag was found to be associated with dietary changes such as increased calorie intake after 9 p.m., reduced dietary fiber intake during the day and for breakfast, and reduced consumption of melatonin-containing foods at dinner. An objective method has shown that SJL is associated with changes in the total sleep duration and ratio of sleep phases during the calendar week. On work/school days, there was a reduction in the total sleep duration and the light sleep phase, and on weekends, there was an increase in the total sleep duration and the REM sleep phase. Young people who consume more MT-containing foods for dinner have lower SJL and sleep debt; they have a longer total sleep duration and deep sleep phases on work/school days. Moreover, these changes in sleep function were observed only on work/school days. Thus, the mismatch in circadian system function is accompanied by a violation of meal timing, a deterioration in the quality of the diet, and a decrease in sleep function. The consumption of foods containing MT is associated with a decrease in the mismatch of the circadian system's function and an improvement in the quality of sleep in young people.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: FMT concentration in food products, ng/g fresh weight; Table S2: Rounded values of FMT content in 5 product categories; Table S3: the sizes of food portions in grams; Table S4: Descriptive statistics of food melatonin consumption for day and dinner; Table S5: Descriptive statistics of FMTday and FMTdinner tertiles.
Author Contributions
Conceptualization: M.F.B. S.P; study design: M.F.B, S.P. and A.P.; supervision: S.P.; data collection: all authors; data analysis: M.F.B. and A.P.; data interpretation: M.F.B. and S.P.; manuscript preparation: M.F.B. and A.P.; drafting the manuscript: M.F.B, S.P, V.S. and A.P.; approval of the final version of the manuscript: all authors.
Funding
The research was carried out within the framework of the research project of the Institute of Physiology of the Federal Research Centre Komi Scientific Centre of the Ural Branch of the Russian Academy of Sciences FUUU-2022-0066 (No. 1021051201895-9).
Institutional Review Board Statement
This study was approved by the Ethics Committee of the Institute of Physiology of Komi Science Centre of the Ural Branch of the Russian Academy of Sciences (Protocol #6, 21.09.2020), and was conducted in accordance with the guidelines of the Declaration of Helsinki.
Informed Consent Statement
Verbal informed consent was obtained from all study participants. Additionally, the schoolchildren’s parents provided written informed consent.
Data Availability Statement
The data of this study are available on request from the corresponding author.
Conflicts of Interest
The authors report no conflict of interest.
References
- Aschoff, J.; Wever, R. In Biological rhythms. The circadian system of man. pp. 311-331. Springer, Boston, MA. 1981. [CrossRef]
- Duffy, J.F.; Wright, K.P. Jr. Entrainment of the human circadian system by light. J. Biol. Rhythms. 2005, 20(4), 326–338. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Pilz, L.K.; Zerbini, G.; Winnebeck, E.C. Chronotype and Social Jetlag: A (Self-) Critical Review. Biology (Basel). 2019, 8(3), 54. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, M.H.; Hermida, R.C.; Reinberg, A.; Sackett-Lundeen, L.; Portaluppi, F. Circadian disruption: New clinical perspective of disease pathology and basis for chronotherapeutic intervention. Chronobiol. Int. 2016, 33(8), 1101–1019. [Google Scholar] [CrossRef] [PubMed]
- Touitou, Y.; Touitou, D.; Reinberg, A. Disruption of adolescents' circadian clock: The vicious circle of media use, exposure to light at night, sleep loss and risk behaviors. J. Physiol. Paris. 2016, 110 (4 Pt B), 467–479. [Google Scholar] [CrossRef]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social jetlag: Misalignment of biological and social time. Chronobiol. Int. 2006, 23(1-2), 497-509. [CrossRef]
- Komada, Y.; Okajima, I.; Kitamura, S.; Inoue, Y. A survey on social jetlag in Japan: A nationwide, cross-sectional internet survey. Sleep Biol. Rhythms. 2019, 17, 417–422. [Google Scholar] [CrossRef]
- Borisenkov, M.F.; Tserne, T.A.; Panev, A.S.; Kuznetsova, E.S.; Petrova, N.B.; Timonin, V.D.; et al. Seven-year survey of sleep timing in Russian children and adolescents: chronic 1-h forward transition of social clock is associated with increased social jetlag and winter pattern of mood seasonality. Biol. Rhythm Res. 2017, 48, 3–12. [Google Scholar] [CrossRef]
- Panev, A.S.; Tserne, T.A.; Polugrudov, A.S.; Bakutova, L.A.; Petrova, N.B.; Tatarinova, O.V.; Kolosova, O.N.; Borisenkov, M.F. Association of chronotype and social jetlag with human non-verbal intelligence. Chronobiol. Int. 2017, 34(7), 977–980. [Google Scholar] [CrossRef]
- Haraszti, R.Á.; Ella, K.; Gyöngyösi, N.; Roenneberg, T.; Káldi, K. Social jetlag negatively correlates with academic performance in undergraduates. Chronobiol. Int. 2014, 31(5), 603–612. [Google Scholar] [CrossRef]
- Levandovski, R.; Dantas, G.; Fernandes, L.C.; Caumo, W.; Torres, I.; Roenneberg, T.; Hidalgo, M.P.; Allebrandt, K.V. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol. Int. 2011, 28(9), 771–778. [Google Scholar] [CrossRef]
- Roenneberg, T.; Allebrandt, K.V.; Merrow, M.; Vetter, C. Social jetlag and obesity. Curr. Biol. 2012, 22(10), 939–943. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.M.; Hasler, B.P.; Kamarck, T.W.; Muldoon, M.F.; Manuck, S.B. Social jetlag, chronotype, and cardiometabolic risk. J. Clin. Endocrinol. Metab. 2015, 100(12), 4612–4620. [Google Scholar] [CrossRef] [PubMed]
- Stephan, F.K. The "other" circadian system: Food as a Zeitgeber. J. Biol. Rhythms. 2002, 17(4), 284–292. [Google Scholar] [CrossRef] [PubMed]
- Wehrens, S.M.T.; Christou, S.; Isherwood, C.; Middleton, B.; Gibbs, M.A.; Archer, S.N.; Skene, D.J.; Johnston, J.D. Meal timing regulates the human circadian system. Curr. Biol. 2017, 27(12), 1768–1775. [Google Scholar] [CrossRef]
- O'Connor, S.G.; Reedy, J.; Graubard, B.I.; Kant, A.K.; Czajkowski, S.M.; Berrigan, D. Circadian timing of eating and BMI among adults in the American Time Use Survey. Int. J. Obes. (Lond). 2022, 46(2), 287–296. [Google Scholar] [CrossRef]
- Popp, C.J.; Curran, M.; Wang, C.; Prasad, M.; Fine, K, Gee, A. ; Nair, N.; Perdomo, K.; Chen, S.; Hu, L.; St-Jules, D.E.; Manoogian, E.N.C.; Panda, S.; Sevick, M.A.; Laferrère, B. Temporal eating patterns and eating windows among adults with overweight or obesity. Nutrients. 2021, 13(12), 4485. [Google Scholar] [CrossRef]
- McHill, A.W.; Phillips, A.J.; Czeisler, C.A.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.; Klerman, E.B. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 2017, 106(5), 1213–1219. [Google Scholar] [CrossRef]
- Kaur, S.; Ng, C.M.; Tang, S.Y.; Kok, E.Y. Weight status of working adults: The effects of eating misalignment, chronotype, and eating jetlag during mandatory confinement. Chronobiol. Int. 2023, 8, 1–10. [Google Scholar] [CrossRef]
- Alsayid, M.; Khan, M.O.; Adnan, D.; Rasmussen, H.E.; Keshavarzian, A.; Bishehsari, F. ; Behavioral circadian phenotypes are associated with the risk of elevated body mass index. Eat. Weight Disord. 2022, 27(4), 1395–1403. [Google Scholar] [CrossRef]
- Makarem, N.; Sears, D.D.; St-Onge, M.P.; Zuraikat, F.M.; Gallo, L.C.; Talavera, G.A.; Castaneda, S.F.; Lai, Y.; Aggarwal, B. Variability in daily eating patterns and eating jetlag are associated with worsened cardiometabolic risk profiles in the American Heart Association Go Red for Women Strategically Focused Research Network. J. Am. Heart Assoc. 2021, 10(18), e022024. [Google Scholar] [CrossRef]
- Zerón-Rugerio, M.F.; Hernáez, Á.; Porras-Loaiza, A.P.; Cambras, T.; Izquierdo-Pulido, M. Eating jet lag: A marker of the variability in meal timing and its association with body mass index. Nutrients. 2019, 11(12), 2980. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Makino, S.; Suiko, T.; Nagamori, Y.; Iwai, T.; Aono, M.; Shibata, S. Association between irregular meal timing and the mental health of Japanese workers. Nutrients. 2021, 13(8), 2775. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, J.S.; Branecky, K.L.; Yang, W.; Ellacott, K.L.; Niswender, K.D.; Yamazaki, S. High-fat diet acutely affects circadian organisation and eating behavior. Eur. J. Neurosci. 2013, 37(8), 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Arendt, J.; Skene, D.J. Melatonin as a chronobiotic. Sleep Med. Rev. 2005, 9(1), 25–39. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.; Paredes, S.D.; Cubero, J.; Lozano, M.; Toribio-Delgado, A.F.; Muñoz, J.L.; Reiter, R.J.; Barriga, C.; Rodríguez, A.B. Jerte Valley cherry-enriched diets improve nocturnal rest and increase 6-sulfatoxymelatonin and total antioxidant capacity in the urine of middle-aged and elderly humans. J. Gerontol. A. Biol. Sci. Med. Sci. 2010, 65(9), 909–914. [Google Scholar] [CrossRef]
- Pigeon, W.R.; Carr, M.; Gorman, C.; Perlis, M.L. Effects of a tart cherry juice beverage on the sleep of older adults with insomnia: A pilot study. J. Med. Food. 2010, 13(3), 579–683. [Google Scholar] [CrossRef]
- Lin, H.H.; Tsai, P.S.; Fang, S.C.; Liu, J.F. Effect of kiwifruit consumption on sleep quality in adults with sleep problems. Asia Pac. J. Clin. Nutr. 2011, 20(2), 169–174. [Google Scholar]
- Howatson, G.; Bell, P.G.; Tallent, J.; Middleton, B.; McHugh, M.P.; Ellis, J. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur. J. Nutr. 2012, 51(8), 909–916. [Google Scholar] [CrossRef]
- Losso, J.N.; Finley, J.W.; Karki, N.; Liu, A.G.; Prudente, A.; Tipton, R.; Yu, Y.; Greenway, F.L. Pilot study of the tart cherry juice for the treatment of insomnia and investigation of mechanisms. Am. J. Ther. 2018, 25(2), e194–e201. [Google Scholar] [CrossRef]
- Bravo, R.; Matito, S.; Cubero, J.; Paredes, S.D.; Franco, L.; Rivero, M.; Rodríguez, A.B.; Barriga, C. Tryptophan-enriched cereal intake improves nocturnal sleep, melatonin, serotonin, and total antioxidant capacity levels and mood in elderly humans. Age (Dordr). 2013, 35(4), 1277–1285. [Google Scholar] [CrossRef]
- Nagata, C.; Wada, K.; Yamakawa, M.; Nakashima, Y.; Koda, S.; Uji, T.; Onuma, S.; Oba, S.; Maruyama, Y.; Hattori, A. Associations between dietary melatonin intake and total and cause-specific mortality among Japanese adults in the Takayama Study. Am. J. Epidemiol. 2021, 190(12), 2639–2646. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Wirz-Justice, A.; Merrow, M. Life between clocks: Daily temporal patterns of human chronotypes. J. Biol. Rhythms. 2003, 18(1), 80–90. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F. 3rd.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28(2), 193–213. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- van Strien, T.; Frijters, J.E.; Bergers, G.P.; Defares, P.B. The Dutch eating behavior questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int. J. Eat. Disord. 1986, 5(2), 295–315. [Google Scholar] [CrossRef]
- Vercoulen, J.H.; Swanink, C.M.; Fennis, J.F.; Galama, J.M.; van der Meer, J.W.; Bleijenberg, G. Dimensional assessment of chronic fatigue syndrome. J. Psychosom. Res. 1994, 38(5), 383–392. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Preliminary validation of the Yale food addiction scale. Appetite. 2009, 52, 430–436. [Google Scholar] [CrossRef]
- Borisenkov, M.F.; Petrova, N.B.; Timonin, V.D.; Fradkova, L.I.; Kolomeichuk, S.N.; Kosova, A.L.; Kasyanova, O.N. Sleep characteristics, chronotype, and winter depression in 10–20-year-olds in northern European Russia. J. Sleep Res. 2015, 24, 288–295. [Google Scholar] [CrossRef]
- Semenova, E.A.; Danilenko, K.V. Russian version of Pittsburg Sleep Quality Index. 2009. https://newpsyhelp.ru/wp-content/uploads/2021/01/PSQI-rus.pdf.
- Dreem2 https://dreem.com/ . Available online: https://dreem.com/ (accessed on 7 June 2023).
- Arnal, P.; Thorey, V.; Debellemaniere, E.; Ballard, M.; Hernandez, A.; Guillot, A.; Jourde, H.; Harris, M.; Guillard, M.; Van Beers, P.; Chennaoui, M.; Sauvet, F. The Dreem Headband compared to polysomnography for electroencephalographic signal acquisition and sleep staging. Sleep. 2020, 43(11), zsaa097. [Google Scholar] [CrossRef]
- FatSecret Russia. Calorie counter and diet tracker. Available online: http://www.fatsecret.ru (accessed on 7 June 2023).
- Chen, C.; Taha Aslani, V.; Rosen, G.; Anderson, L.; Jungquist, C. Healthcare shift workers' temporal habits for eating, sleeping, and light exposure: A multi-instrument pilot study. J. Circad. Rhythms. 2020, 18(6). [Google Scholar] [CrossRef]
- Mota, M.; Silva, C.; Balieiro, L.; Gonçalves, B.; Fahmy, W.; Crispim, C. Association between social jetlag food consumption and meal times in patients with obesity-related chronic diseases. PLoS One. 2019, 14(2), e0212126. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Hood, M.M.; Crowley, S.J.; Morgan, M.K.; Teodori, M.; Knutson, K.L. The relationship between breakfast skipping, chronotype, and glycemic control in type 2 diabetes. Chronobiol. Int. 2014, 31(1), 64–71. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Hood, M.M.; Crowley, S.J.; Morgan, M.K.; Teodori, M.; Knutson, K.L.; Van Cauter, E. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care. 2013, 36(9), 2523–2529. [Google Scholar] [CrossRef] [PubMed]
- Stutz, B.; Buyken, A.E.; Schadow, A.M.; Jankovic, N.; Alexy, U.; Krueger, B. Associations of chronotype and social jetlag with eating jetlag and their changes among German students during the first COVID-19 lockdown. The Chronotype and Nutrition study. Appetite. 2023, 180, 106333. [Google Scholar] [CrossRef]
- Roenneberg, T.; Kuehnle, T.; Pramstaller, P.P.; Ricken, J.; Havel, M.; Guth, A.; Merrow, M. A marker for the end of adolescence. Curr. Biol. 2004, 14(24), R1038–R1039. [Google Scholar] [CrossRef]
- Duffy, J.F.; Wang, W.; Ronda, J.M.; Czeisler, C.A. High dose melatonin increases sleep duration during nighttime and daytime sleep episodes in older adults. J. Pineal Res. 2022, 73(1), e12801. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O'Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; Takano, T.; Deane, R.; Nedergaard, M. Sleep drives metabolite clearance from the adult brain. Science. 2013, 342(6156), 373–377. [Google Scholar] [CrossRef]
- Hablitz, L.M.; Nedergaard, M. The glymphatic system. Curr. Biol. 2021, 31(20), R1371–R1375. [Google Scholar] [CrossRef]
- Peever, J.; Fuller, P.M. The biology of REM sleep. Curr. Biol. 2017, 27(22), R1237–R1248. [Google Scholar] [CrossRef]
- Lee, D.A.; Lee, H.J.; Park, K.M. Glymphatic dysfunction in isolated REM sleep behavior disorder. Acta Neurol. Scand. 2022, 145(4), 464–470. [Google Scholar] [CrossRef]
- Leitner, C.; D'Este, G.; Verga, L.; Rahayel, S.; Mombelli, S.; Sforza, M.; Casoni, F.; Zucconi, M.; Ferini-Strambi, L.; Galbiati, A. Neuropsychological changes in isolated REM sleep behavior disorder: A systematic review and meta-analysis of cross-sectional and longitudinal studies. Neuropsychol. Rev. 2023. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Percentage of daily energy intake for breakfast, lunch, and dinner for participants with different SJL.
Figure 1.
Percentage of daily energy intake for breakfast, lunch, and dinner for participants with different SJL.
Figure 2.
Dietary fiber intake for breakfast in participants with different SJL. * Significant differences between SJL ≤ 1 h and SJL > 2 h groups, p < (0.02–0.04); ** Between SJL ≤ 1 h and 1 < SJL ≤ 2 h, p < (0.00-0.05), ANCOVA, post hoc comparisons, Tukey test.
Figure 2.
Dietary fiber intake for breakfast in participants with different SJL. * Significant differences between SJL ≤ 1 h and SJL > 2 h groups, p < (0.02–0.04); ** Between SJL ≤ 1 h and 1 < SJL ≤ 2 h, p < (0.00-0.05), ANCOVA, post hoc comparisons, Tukey test.
Figure 3.
Food melatonin intake for dinner in participants with different SJL. * Significant differences between SJL ≤ 1 h and SJL > 2 h groups, p < (0.02–0.04), ANCOVA, post hoc comparisons, Tukey test.
Figure 3.
Food melatonin intake for dinner in participants with different SJL. * Significant differences between SJL ≤ 1 h and SJL > 2 h groups, p < (0.02–0.04), ANCOVA, post hoc comparisons, Tukey test.
Table 1.
General characteristics of participants according to SJL group assignment.
Table 1.
General characteristics of participants according to SJL group assignment.
| Parameter |
Total (n=83) |
SJL≤1h
(n=30) |
1<SJL≤2 h
(n = 35) |
SJL>2 h
(n = 18) |
F |
P* |
η2
|
| Male/Female, n/n |
35/48 |
13/17 |
13/22 |
9/9 |
|
|
|
| Age, yrs |
26.7±6.1 |
29.7±5.8a
|
26.4±6.1b
|
22.4±3.8 |
9.78 |
0.00 |
0.44 |
| Weight, kg |
65.8±13.9 |
63.7±12.1 |
65.2±12.7 |
70.6±18.1 |
1.07 |
0.35 |
0.07 |
| Height, cm |
170.0±0.1 |
171.0±0.1 |
168.0±0.1 |
172.0±0.1 |
1.01 |
0.37 |
0.13 |
| BMI, kg/m2
|
22.6±3.1 |
21.7±2.2 |
22.8±3.1 |
23.7±4.1 |
2.62 |
0.08 |
0.13 |
| MSFsc, h |
4.4±1.1 |
4.0±0.9 |
4.2±1.0 |
5.3±1.0a,b
|
5.78 |
0.01 |
0.28 |
| SJL, h#
|
1.4±0.9 |
0.5±0.3 |
1.5±0.3c
|
2.8±0.6a,b
|
195.24 |
0.00 |
0.87 |
| Depression, scores |
7.7±5.6 |
6.6±5.8 |
7.8±5.5 |
9.3±5.1 |
0.91 |
0.41 |
0.11 |
| Fatigue, scores |
26.1±9.9 |
21.6±7.7 |
28.2±10.1c
|
28.6±10.8a
|
3.19 |
0.05 |
0.33 |
| DEBQrestr , scores |
2.2±0.9 |
2.1±0.9 |
2.2±0.8 |
2.2±1.0 |
0.04 |
0.96 |
0.01 |
| DEBQemo , scores |
2.0±0.8 |
1.9±0.7 |
2.1±0.9 |
2.1±0.7 |
0.24 |
0.79 |
0.09 |
| DEBQextern , scores |
3.3±0.5 |
3.1±0.5 |
3.4±0.5 |
3.3±0.6 |
1.47 |
0.24 |
0.15 |
| FA, symptoms |
2.0±1.4 |
2.0±1.8 |
2.0±1.0 |
2.3±0.6 |
0.07 |
0.93 |
0.05 |
Table 2.
Sleep pattern of participants according to SJL group assignment.
Table 2.
Sleep pattern of participants according to SJL group assignment.
| Parameter |
Total
(n = 83) |
SJL≤1h
(n = 30) |
1<SJL≤ 2 h
(n = 35) |
SJL>2 h
(n = 18) |
F |
P* |
η2
|
| Waketime weekday, hh:mm |
06:58±01:14 |
07:05±01:17 |
07:02±01:09 |
06:20±01:14 |
0.59 |
0.56 |
0.15 |
| Waketime weekend, hh:mm |
08:19±01:33 |
07:47±01:16 |
08:53±01:34 |
09:45±01:42a
|
4.47 |
0.02 |
0.47 |
| Bedtime weekday, hh:mm |
23:56±01:07 |
23:45±01:11 |
00:06±01:02 |
00:34±00:50 |
0.91 |
0.41 |
0.23 |
| Bedtime weekend, hh:mm |
00:20±01:29 |
23:57±01:14 |
00:43±01:20 |
01:25±02:23a
|
2.54 |
0.05 |
0.36 |
| Sleep duration weekday, h |
7.0±1.0 |
7.3±0.9a
|
6.9±0.8 |
5.8±1.3 |
4.42 |
0.02 |
0.42 |
| Sleep duration weekend, h |
8.0±0.9 |
7.8±1.0 |
8.2±0.8 |
8.3±0.9 |
0.69 |
0.51 |
0.20 |
| Sleep quality, scores |
5.5±2.1 |
5.1±2.1 |
5.7±2.1 |
5.6±2.3 |
0.93 |
0.40 |
0.16 |
| Sleep debt, h |
1.0±1.0 |
0.6±0.6 |
1.3±0.7c
|
2.6±1.5a,b
|
11.76 |
0.00 |
0.59 |
| Sleep latency, min |
24.1±21.8 |
29.8±25.6 |
14.3±9.3 |
17.5±9.2 |
2.12 |
0.13 |
0.27 |
| Sleep inertia, min |
14.5±10.0 |
14.3±11.2 |
17.0±8.6 |
10.6±4.3 |
0.98 |
0.39 |
0.10 |
| Sleep efficiency, % |
90.2±3.6 |
89.9±5.6 |
92.2±2.4 |
88.3±2.8 |
0.72 |
0.48 |
0.18 |
Table 3.
Meal timing of participants with different SJL.
Table 3.
Meal timing of participants with different SJL.
| Parameter* |
SJL ≤ 1 h
(n = 30) |
1 < SJL ≤ 2 h
(n = 35) |
SJL > 2 h
(n = 18) |
| Number of eating episodes weekday |
4.9±1.2 |
5.1±1.2 |
4.2±1.1 |
| Number of eating episodes weekend |
4.9±1.5 |
4.8±1.3 |
3.9±0.9 |
|
p-value b
|
0.98 |
0.45 |
0.75 |
| Breakfast weekday, hh:mm |
08:39±01:05 |
08:53±01:14 |
08:40±00:33 |
| Breakfast weekend, hh:mm |
08:43±01:33 |
09:45±01:10 |
09:34±01:01 |
|
p-value b
|
0.52 |
0.05 |
0.04 |
| Breakfast jetlag, h |
1.3±1.0 |
1.7±1.4 |
1.1±0.6 |
| Lunch weekday, hh:mm |
14:11±01:09 |
13:59±01:08 |
13:33±00:46 |
| Lunch weekend, hh:mm |
14:10±01:05 |
14:28±00:53 |
13:59±01:26 |
|
p-value b
|
0.97 |
0.22 |
0.80 |
| Lunch jetlag, h |
1.2±0.8 |
1.2±1.1 |
0.6±0.6 |
| Dinner weekday, hh:mm |
20:12±00:57 |
19:52±01:03 |
20:19±01:23 |
| Dinner weekend, hh:mm |
19:53±00:58 |
19:59±01:14 |
21:38±00:12 |
|
p-value b
|
0.21 |
0.66 |
0.01 |
| Dinner jetlag, h |
0.6±0.6 |
0.8±0.7 |
0.9±0.4 |
| Eating window weekday, h |
1.6±0.9 |
1.8±1.1 |
1.9±1.6 |
| Eating window weekend, h |
1.2±1.0 |
1.6±1.0 |
1.4±0.5 |
|
p-value b
|
0.23 |
0.53 |
0.16 |
| Eating midpoint weekday, h |
14.6±0.7 |
14.5±0.9 |
14.5±0.7 |
| Eating midpoint weekend, h |
14.6±1.0 |
14.9±0.9 |
14.4±0.8 |
|
p-value b
|
0.74 |
0.10 |
0.89 |
| Eating jetlag, h |
0.7±0.4 |
0.9±0.6 |
1.0±0.7 |
| Calories after 9 p.m. weekday (% of total EI) |
10±11 |
12±10 |
12±7 |
| Calories after 9 p.m. weekend (% of total EI) |
7±8 |
13±17 |
23±6a
|
|
p-value b
|
0.23 |
0.83 |
0.01 |
| Time from wake up to breakfast weekday, h |
1.3±0.9 |
1.3±1.5 |
2.0±0.9 |
| Time from wake up to breakfast weekend, h |
0.7±0.8 |
0.6±0.6 |
0.2±0.5 |
|
p-value b
|
0.12 |
0.08 |
0.01 |
| Time from dinner to sleep weekday, h |
3.1±1.5 |
3.0±1.3 |
3.6±0.4 |
| Time from dinner to sleep weekend, h |
3.1±1.4 |
3.8±2.1 |
3.9±2.9 |
|
p-value b
|
0.93 |
0.11 |
0.76 |
Table 4.
Association between eating jetlag and sleep duration.
Table 4.
Association between eating jetlag and sleep duration.
| Parameter |
Sleep duration |
F |
P* |
η2
|
| <7h |
7-8h |
>8h |
| Breakfast jetlag, h |
2.2±1.4 |
1.0±0.8 |
1.3±1.1 |
1.44 |
0.27 |
0.31 |
| Lunch jetlag, h |
1.1±0.8 |
1.0±1.0 |
1.4±1.0 |
0.76 |
0.49 |
0.14 |
| Dinner jetlag, h |
0.7±0.6 |
0.8±0.6 |
0.5±0.8 |
1.58 |
0.24 |
0.24 |
| Eating jetlag, h |
1.1±0.6a,b
|
0.6±0.4 |
0.8±0.5 |
2.74 |
0.05 |
0.57 |
| Calories after 9 p.m. (% of total EI) |
12±11 |
11±11 |
11±12 |
1.80 |
0.20 |
0.06 |
| Eating window, h |
1.6±0.9 |
1.7±0.8 |
1.5±0.5 |
0.51 |
0.61 |
0.25 |
| Eating midpoint, h |
14.8±0.8 |
14.4±0.6 |
14.8±0.7 |
0.69 |
0.52 |
0.31 |
| Number of eating episodes |
4.3±1.2 |
5.2±1.2 |
4.8±1.0 |
0.21 |
0.82 |
0.15 |
Table 5.
Association between eating jetlag and chronotype.
Table 5.
Association between eating jetlag and chronotype.
| Parameter |
Chronotype |
F |
P* |
η2
|
Early
(tertile 1) |
Intermediate
(tertile 2) |
Late
(tertile 3) |
| Breakfast jetlag, h |
1.5±1.8 |
1.5±0.7 |
1.3±1.0 |
1.26 |
0.32 |
0.09 |
| Lunch jetlag, h |
1.0±0.8 |
0.8±0.6 |
1.6±1.2 |
2.56 |
0.12 |
0.20 |
| Dinner jetlag, h |
0.6±0.5 |
0.7±0.6 |
0.9±0.8a,b
|
5.13 |
0.02 |
0.53 |
| Eating jetlag, h |
0.6±0.5 |
0.8±0.4 |
1.0±0.6 |
0.10 |
0.91 |
0.12 |
| Calories after 9 p.m. (% of total EI) |
6±9 |
14±10с
|
14±12a
|
4.09 |
0.04 |
0.47 |
| Eating window, h |
1.8±0.7 |
1.7±±0.9 |
1.4±0.7 |
1.08 |
0.37 |
0.30 |
| Eating midpoint, h |
14.6±0.8 |
14.7± 0.5 |
14.7±0.7 |
0.16 |
0.85 |
0.09 |
| Number of eating episodes |
4.9±1.3 |
4.9±1.0 |
4.7±1.3 |
0.24 |
0.79 |
0.16 |
Table 6.
Results of binary logistic regression analyses.
Table 6.
Results of binary logistic regression analyses.
| # |
Dependent variables |
Predictors |
B |
OR |
95% CI |
P* |
Omnibus test |
Hosmer–
Lemeshow
test |
|
χ2
|
P |
χ2
|
P |
| 1 |
Fatigue |
Eating midpoint |
-0.96 |
0.39 |
0.15-1.02 |
0.05 |
4.25 |
0.04 |
6.01 |
0.65 |
| 2 |
Sleep debt |
Breakfast jetlag |
0.71 |
2.03 |
0.96-4.29 |
0.05 |
4.52 |
0.03 |
6.40 |
0.60 |
| 3 |
Sleep inertia |
Eating jetlag |
-1.93 |
0.15 |
0.02-0.90 |
0.04 |
5.31 |
0.02 |
9.59 |
0.30 |
Table 7.
Total calories and nutrient intake of participants with different SJL.
Table 7.
Total calories and nutrient intake of participants with different SJL.
| Parameter* |
SJL ≤1 h
(n = 30) |
1 < SJL ≤ 2 h
(n = 35) |
SJL > 2 h
(n = 18) |
| Calories weekday, kcal/day |
1987.9±478.3 |
1804.1±560.8 |
2005.9±922.8 |
| Calories weekend, kcal/day |
2076.3±504.4 |
1753.2±655.5 |
1904.9±961.1 |
|
p-value b
|
0.16 |
0.65 |
0.43 |
| Protein weekday, kcal/day |
79.9±22.3 |
66.9±25.2 |
82.5±44.2 |
| Protein weekend, kcal/day |
76.1±26.3 |
60.6±21.9 |
84.5±62.1 |
|
p-value b
|
0.46 |
0.08 |
0.74 |
| Fat weekday, kcal/day |
83.1±27.9 |
72.1±25.6 |
82.2±35.5 |
| Fat weekend, kcal/day |
83.0±26.3 |
74.5±33.0 |
72.1±32.7 |
|
p-value b
|
0.99 |
0.45 |
0.09 |
| Dietary fiber weekday, g/day |
19.1±6.8a,c
|
16.3±7.4 |
17.2±3.9 |
| Dietary fiber weekend, g/day |
20.1±8.5a,c
|
16.8±7.3 |
15.7±5.8 |
|
p-value b
|
0.24 |
0.55 |
0.85 |
|
Ln(FMTdinner) weekday, ng/dinner |
9.4±1.1a
|
8.8±1.4 |
8.5±1.6 |
|
Ln(FMTdinner) weekend, ng/dinner |
9.6±1.2a,c
|
8.4±1.7 |
7.9±1.7 |
|
p-value b
|
0.69 |
0.30 |
0.05 |
Table 8.
Association between sleep and sleep-wake rhythm characteristics derived from MCTQ and D2H.
Table 8.
Association between sleep and sleep-wake rhythm characteristics derived from MCTQ and D2H.
| Parameter |
MCTQ (n=21) |
D2H (n=21) |
P* |
r2 |
| SJL, h |
1.01 ± 0.62 |
0.95 ± 0.73 |
0.40 |
0.84# |
| Chronotype, h |
3.80 ± 0.54 |
3.39 ± 0.44 |
0.15 |
0.84# |
| Sleep debt, h |
1.63 ± 1.02 |
1.34 ± 0.99 |
0.40 |
0.84# |
| Sleep latency, h |
0.16 ± 0.12 |
0.23 ± 0.13 |
0.09 |
0.10 |
| Waketime weekday, hh:mm |
06:34± 00:56 |
06:34 ± 00:58 |
0.99 |
0.88# |
| Waketime weekend, hh:mm |
08:53 ± 01:03 |
08:45 ± 01:08 |
0.69 |
0.72# |
| Bedtime weekday, hh:mm |
23:47 ± 01:13 |
00:03 ± 00:55 |
0.48 |
0.74# |
| Bedtime weekend, hh:mm |
00:29 ± 01:08 |
00:48 ± 01:17 |
0.45 |
0.74# |
| Sleep duration weekday, h |
6.62 ± 1.15 |
6.23 ± 0.87 |
0.27 |
0.71# |
| Sleep duration weekend, h |
8.15 ± 0.82 |
7.80 ± 0.56 |
0.14 |
0.50# |
| Sleep efficiency (average weekly), % |
90.5 ± 4.7 |
91.6 ± 4.2 |
0.39 |
0.19 |
| Sleep efficiency weekday, % |
91.4 ± 4.9 |
90.8 ± 4.7 |
0.66 |
0.30 |
| Sleep efficiency weekend, % |
89.9 ± 5.4 |
92.4 ± 4.4 |
0.07 |
0.03 |
Table 9.
Sleep pattern of participants monitored using D2H.
Table 9.
Sleep pattern of participants monitored using D2H.
| Variables |
oSJL ≤ 1 h
(N = 12; n = 84) |
oSJL > 1 h
(N = 9; n = 63) |
F |
P |
η2
|
| Weekly average |
| Sleep duration, h |
6.8 ± 0.5 |
6.9 ± 0.6 |
0.12 |
0.73 |
0.08 |
| Sleep onset duration, h |
0.3 ± 0.2 |
0.3 ± 0.1 |
0.04 |
0.85 |
0.05 |
| Light sleep duration, h |
3.4 ± 0.6 |
3.1 ± 0.5 |
1.74 |
0.20 |
0.30 |
| Deep sleep duration, h |
1.5 ± 0.4 |
1.5 ± 0.4 |
0.02 |
0.90 |
0.03 |
| REM sleep duration, h |
1.8 ± 0.3 |
2.3 ± 0.5a
|
7.64 |
0.01 |
0.55 |
| Wake after sleep onset duration, h |
0.2 ± 0.2 |
0.2 ± 0.2 |
0.04 |
0.84 |
0.05 |
| Number of awakening |
2.9 ± 2.5 |
2.3 ± 1.3 |
0.42 |
0.53 |
0.15 |
| Position changes |
23.4 ± 10.8 |
18.5 ± 6.7 |
1.53 |
0.23 |
0.11 |
| Mean heart rate, bpm |
57.8 ± 5.2 |
62.8 ± 5.0a
|
4.67 |
0.04 |
0.45 |
| Mean respiration, cpm |
15.4 ± 1.4 |
16.0 ± 2.1 |
0.56 |
0.46 |
0.17 |
| Sleep efficiency, % |
90.7 ± 5.4 |
92.7 ± 1.8 |
1.12 |
0.30 |
0.24 |
| Weekday |
| Sleep duration, h |
6.6 ± 0.7a
|
5.6 ± 0.8 |
7.60 |
0.01 |
0.54 |
| Sleep onset duration, h |
0.3 ± 0.2 |
0.3 ± 0.2 |
0.00 |
0.96 |
0.01 |
| Light sleep duration, h |
3.2 ± 0.6a
|
2.6 ± 0.7 |
4.29 |
0.05 |
0.44 |
| Deep sleep duration, h |
1.5 ± 0.4 |
1.5 ± 0.4 |
0.06 |
0.81 |
0.06 |
| REM sleep duration, h |
1.7 ± 0.3 |
1.7 ± 0.4 |
0.04 |
0.85 |
0.05 |
| Wake after sleep onset duration, h |
0.1 ± 0.1 |
0.2 ± 0.3 |
0.63 |
0.44 |
0.18 |
| Number of awakening |
2.5 ± 1.9 |
2.2 ± 1.3 |
0.15 |
0.70 |
0.09 |
| Position changes |
21.8 ± 11.1 |
18.4 ± 4.2 |
0.76 |
0.40 |
0.02 |
| Mean heart rate, bpm |
57.3 ± 4.9 |
63.1 ± 4.9a
|
6.98 |
0.02 |
0.53 |
| Mean respiration, cpm |
15.4 ± 1.5 |
16.0 ± 2.1 |
0.53 |
0.47 |
0.17 |
| Sleep efficiency, % |
90.0 ± 5.9 |
91.8 ± 2.6 |
0.73 |
0.40 |
0.20 |
| Weekend |
| Sleep duration, h |
7.0 ± 0.8 |
8.1 ± 0.1a
|
8.79 |
0.01 |
0.57 |
| Sleep onset duration, h |
0.2 ± 0.2 |
0.2 ± 0.1 |
0.12 |
0.74 |
0.08 |
| Light sleep duration, h |
3.7 ± 0.9 |
3.6 ± 0.4 |
0.05 |
0.82 |
0.05 |
| Deep sleep duration, h |
1.5 ± 0.5 |
1.5 ± 0.5 |
0.00 |
0.99 |
0.00 |
| REM sleep duration, h |
1.9 ± 0.5 |
2.9 ± 0.7a |
11.89 |
0.00 |
0.63 |
| Wake after sleep onset duration, h |
0.3 ± 0.4 |
0.1 ± 0.1 |
0.89 |
0.36 |
0.22 |
| Number of awakening |
3.3 ± 3.4 |
2.4 ± 1.9 |
0.48 |
0.50 |
0.16 |
| Position changes |
25.1 ± 12.4 |
18.6 ± 11.0 |
1.62 |
0.22 |
0.15 |
| Mean heart rate, bpm |
58.3 ± 6.2 |
62.4 ± 5.8 |
2.29 |
0.15 |
0.34 |
| Mean respiration, cpm |
15.4 ± 1.4 |
15.9 ± 2.1 |
0.56 |
0.46 |
0.17 |
| Sleep efficiency, % |
91.4 ± 5.6 |
93.6 ± 1.9 |
1.19 |
0.29 |
0.25 |
Table 10.
Association of sleep and sleep-wake rhythm characteristics derived from D2H with consumption of melatonin-containing food.
Table 10.
Association of sleep and sleep-wake rhythm characteristics derived from D2H with consumption of melatonin-containing food.
Variables
|
Ln(FMTdinner), ng/dinner |
F |
P |
η2
|
Low&
(tertile 1) |
Average
(tertile 2) |
High
(tertile 3) |
| Weekly average |
| oMSFsc, hh:mm |
03:12 ± 00:21 |
03:35 ± 00:17 |
03:33 ± 00:14 |
2.66 |
0.11 |
0.67 |
| oSJL, h |
1.4 ± 0.3a,b
|
0.6 ± 0.2 |
0.5 ± 0.2 |
14.15 |
0.00 |
0.98 |
| Sleep debt, h |
2.3 ± 0.5a
|
1.1 ± 0.4 |
0.8 ± 0.3 |
7.68 |
0.01 |
0.84 |
| Sleep duration, h |
6.0 ± 0.3 |
6.8 ± 0.5 |
7.1 ± 0.6a
|
4.94 |
0.02 |
0.76 |
| Sleep latency, h |
0.2 ± 0.1 |
0.2 ± 0.1 |
0.3 ± 0.2 |
0.89 |
0.43 |
0.09 |
| Light sleep duration, h |
3.1 ± 0.3 |
3.6 ± 0.4 |
3.5 ± 0.6 |
1.37 |
0.29 |
0.52 |
| Deep sleep duration, h |
1.1 ± 0.2 |
1.6 ± 0.5 |
1.6 ± 0.2a
|
3.77 |
0.05 |
0.75 |
| REM sleep duration, h |
2.3 ± 0.5 |
1.8 ± 0.3 |
2.0 ± 0.3 |
3.04 |
0.08 |
0.64 |
| WASO, h |
0.2 ± 0.1 |
0.1 ± 0.1 |
0.4 ± 0.3 |
2.90 |
0.09 |
0.14 |
| Number of awakening |
2.8 ± 0.7 |
2.4 ± 0.8 |
3.4 ± 0.8 |
2.80 |
0.08 |
0.21 |
| Position changes |
26.0 ± 6.8 |
25.5 ± 9.9 |
21.0 ± 6.7 |
0.76 |
0.49 |
0.20 |
| Mean heart rate, bpm |
63.3 ± 5.7 |
56.8 ± 4.9 |
60.6 ± 4.8 |
1.92 |
0.18 |
0.48 |
| Mean respiration, cpm |
16.2 ± 0.7 |
15.3 ± 1.0 |
15.4 ± 1.3 |
0.91 |
0.43 |
0.43 |
| Sleep efficiency, % |
90.6 ± 4.3 |
94.0 ± 1.1 |
87.6 ± 6.3 |
2.59 |
0.11 |
0.02 |
| Weekdays |
| Sleep duration, h |
5.1 ± 0.5 |
6.6 ± 0.6 |
6.8 ± 0.3a
|
3.69 |
0.05 |
0.71 |
| Sleep latency, h |
0.2 ± 0.1 |
0.2 ± 0.1 |
0.3 ± 0.2 |
0.73 |
0.50 |
0.24 |
| Light sleep duration, h |
2.3 ± 0.3 |
3.1 ± 0.9 |
3.4 ± 0.6a
|
3.00 |
0.05 |
0.64 |
| Deep sleep duration, h |
1.2 ± 0.2 |
1.7 ± 0.4 |
1.8 ± 0.3a
|
4.89 |
0.02 |
0.80 |
| REM sleep duration, h |
1.7 ± 0.5 |
1.7 ± 0.3 |
1.7 ± 0.4 |
0.06 |
0.94 |
0.02 |
| WASO, h |
0.1 ± 0.0 |
0.1 ± 0.1 |
0.3 ± 0.3 |
1.42 |
0.27 |
0.35 |
| Number of awakening |
1.3 ± 0.1 |
2.1 ± 1.2 |
3.3 ± 2.1 |
2.35 |
0.13 |
0.46 |
| Position changes |
18.3 ± 3.0 |
26.8 ± 12.2 |
18.2 ± 8.1 |
1.69 |
0.22 |
0.25 |
| Mean heart rate, bpm |
61.8 ± 7.9 |
57.5 ± 6.3 |
60.2 ± 5.3 |
0.56 |
0.58 |
0.27 |
| Mean respiration, cpm |
16.1 ± 2.1 |
15.7 ± 1.5 |
15.2 ± 2.2 |
0.29 |
0.75 |
0.18 |
| Sleep efficiency, % |
89.5 ± 7.9 |
92.1 ± 3.2 |
89.6 ± 7.2 |
0.28 |
0.76 |
0.12 |
| Weekends |
| Sleep duration, h |
7.0 ± 0.9 |
7.0 ± 0.9 |
7.4 ± 1.1 |
0.42 |
0.67 |
0.15 |
| Sleep latency, h |
0.3 ± 0.1 |
0.2 ± 0.1 |
0.2 ± 0.1 |
0.78 |
0.48 |
0.25 |
| Light sleep duration, h |
3.0 ± 0.4 |
3.4 ± 1.0 |
3.6 ± 0.9 |
0.35 |
0.71 |
0.04 |
| Deep sleep duration, h |
1.1 ± 0.4 |
1.5 ± 0.7 |
1.5 ± 0.4 |
1.08 |
0.37 |
0.47 |
| REM sleep duration, h |
3.0 ± 0.7 |
1.9 ± .0.4 |
2.4 ± 0.6 |
3.46 |
0.06 |
0.66 |
| WASO, h |
0.3 ± 0.2 |
0.0 ± 0.0 |
0.4 ± 0.4 |
2.01 |
0.17 |
0.12 |
| Number of awakening |
4.4 ± 1.5 |
2.7 ± 0.6 |
3.3 ± 2.1 |
2.80 |
0.08 |
0.21 |
| Position changes |
33.6 ± 11.9 |
24.1 ± 13.5 |
23.9 ± 10.5 |
1.00 |
0.40 |
0.45 |
| Mean heart rate, bpm |
64.9 ± 6.9 |
56.1 ± 6.4 |
59.9 ± 4.5 |
2.60 |
0.11 |
0.61 |
| Mean respiration, cpm |
16.3 ± 1.6 |
14.8 ± 2.2 |
15.4 ± 1.7 |
0.70 |
0.52 |
0.36 |
| Sleep efficiency, % |
91.8 ± 4.3 |
95.8 ± 1.6 |
87.2 ± 8.6 |
2.75 |
0.10 |
0.02 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).