1. Introduction
Lung cancer is the second most prevalent neoplastic disease globally, accounting for 2,206,771 new cases in 2020 (11.4% of all new cancer diagnoses) and is the leading cause of cancer-related deaths, with 1,796,144 deaths in 2020 (18% of all cancer deaths)1. It predominantly affects males2, and tobacco smoking is the primary etiological factor3, with the incidence correlating with smoking prevalence in different countries4. Other contributing factors include exposure to radon5, a history of chronic obstructive pulmonary disease (COPD), interstitial lung diseases6, and the consumption of red meat7. Non-small cell lung cancer (NSCLC), comprising adenocarcinoma, squamous cell carcinoma, and large cell carcinoma, is the most common histological subtype, constituting 85% of cases8. NSCLC is often diagnosed only when symptoms like hemoptysis, chest pain, and dyspnea manifest9.
This study presents a case of metastatic NSCLC achieving complete remission (CR) and extremely long Progression Free Survival (PFS) trough immunotherapy.
2. Case Report
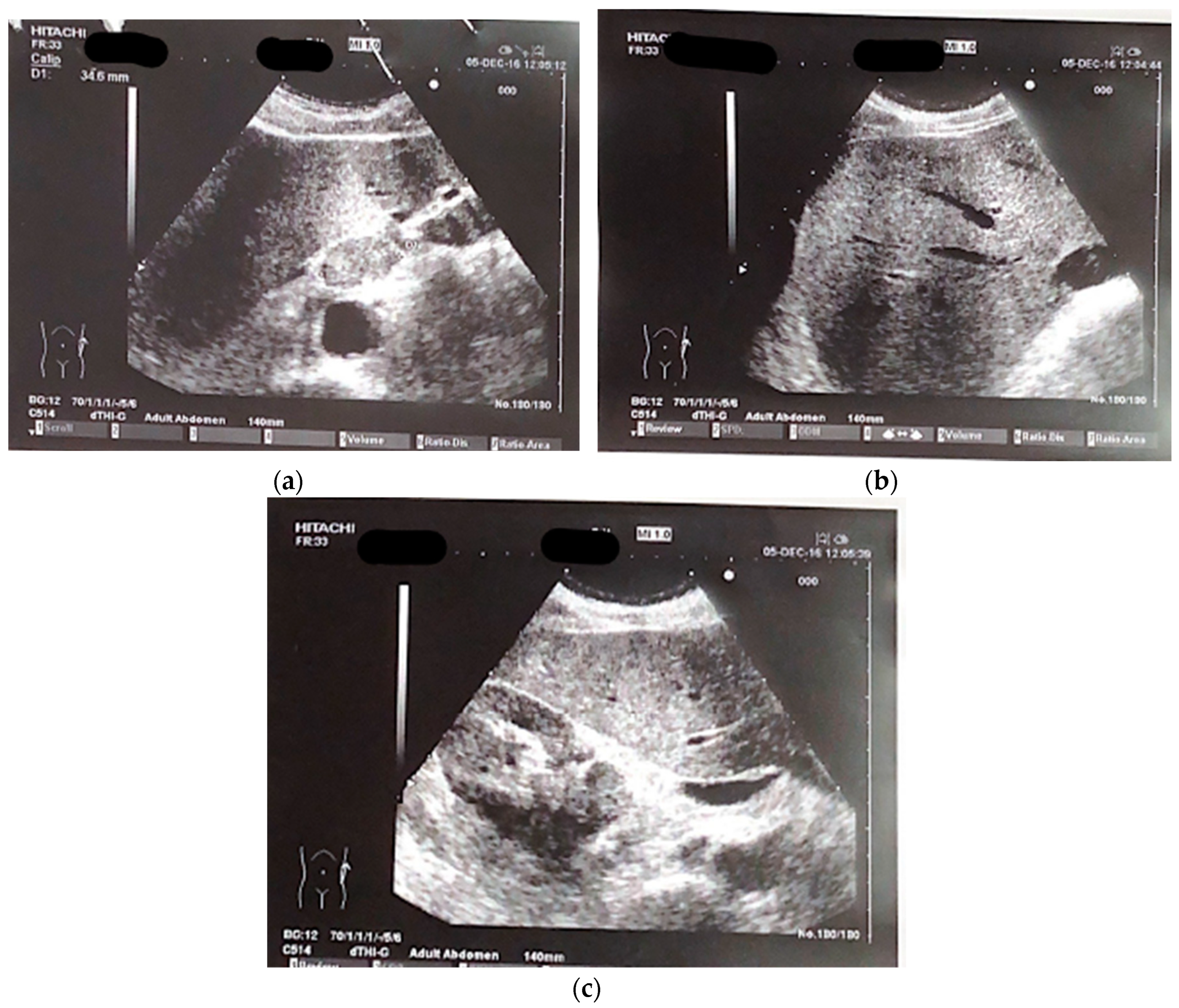
A 65-year-old male patient presented at our hospital in 2016 with worsening non-productive night cough but no dyspnea. He had a history of hypertension and a recent diagnosis of gastroesophageal reflux. A former smoker who quit 10 years ago, he had not experienced weight loss in recent months. An abdominal ultrasound revealed an area near the hepatic hilum with lobulated contours, roughly oval, displaying iso-hypoechogenic echo structure, with a maximum diameter of 43 mm, indicating the presence of malignant lymph nodes (
Figure 1a–c).
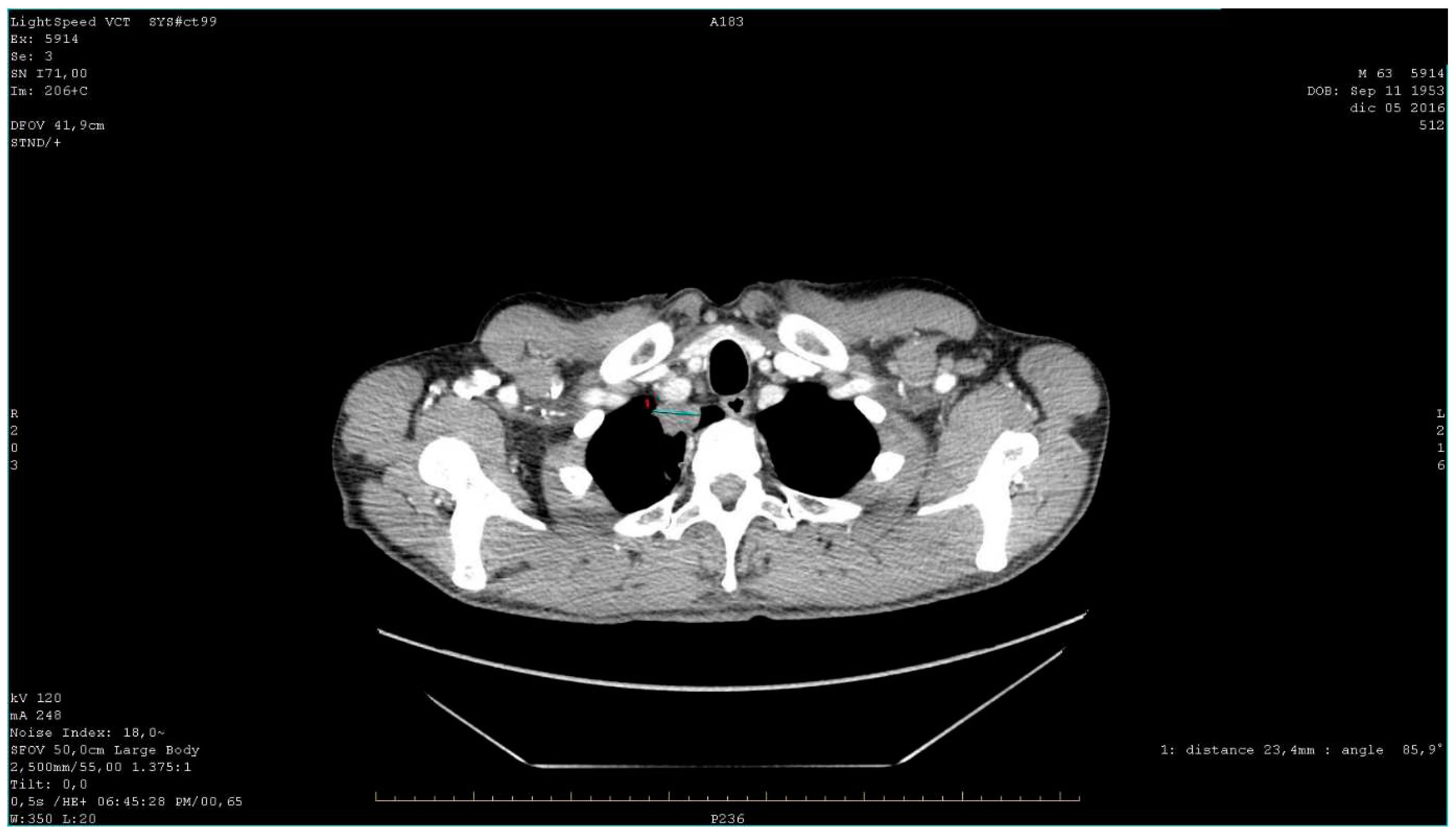
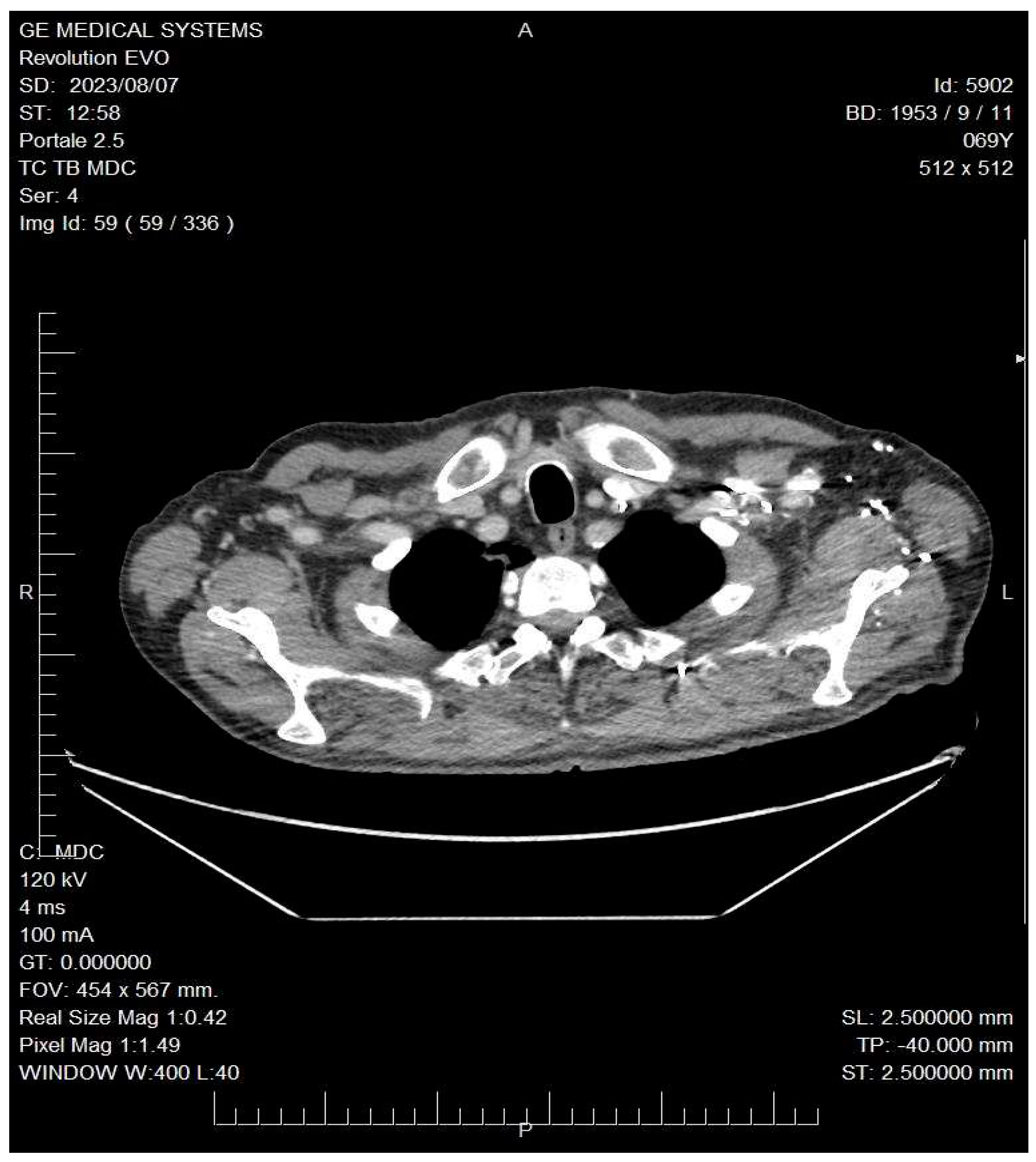
A total body CT (Computed Tomography) scan was performed which confirmed the suspect and revealed the presence of a solid nodular-like expansive process in the right upper lobe of the lung (21x29mm, adjacent to the subclavian artery) and multiple lymphadenopathies in the mediastinum (paratracheal and subcarinal) and abdomen (near the origin of the celiac trunk and periaortic lymph nodes) (
Figure 2).
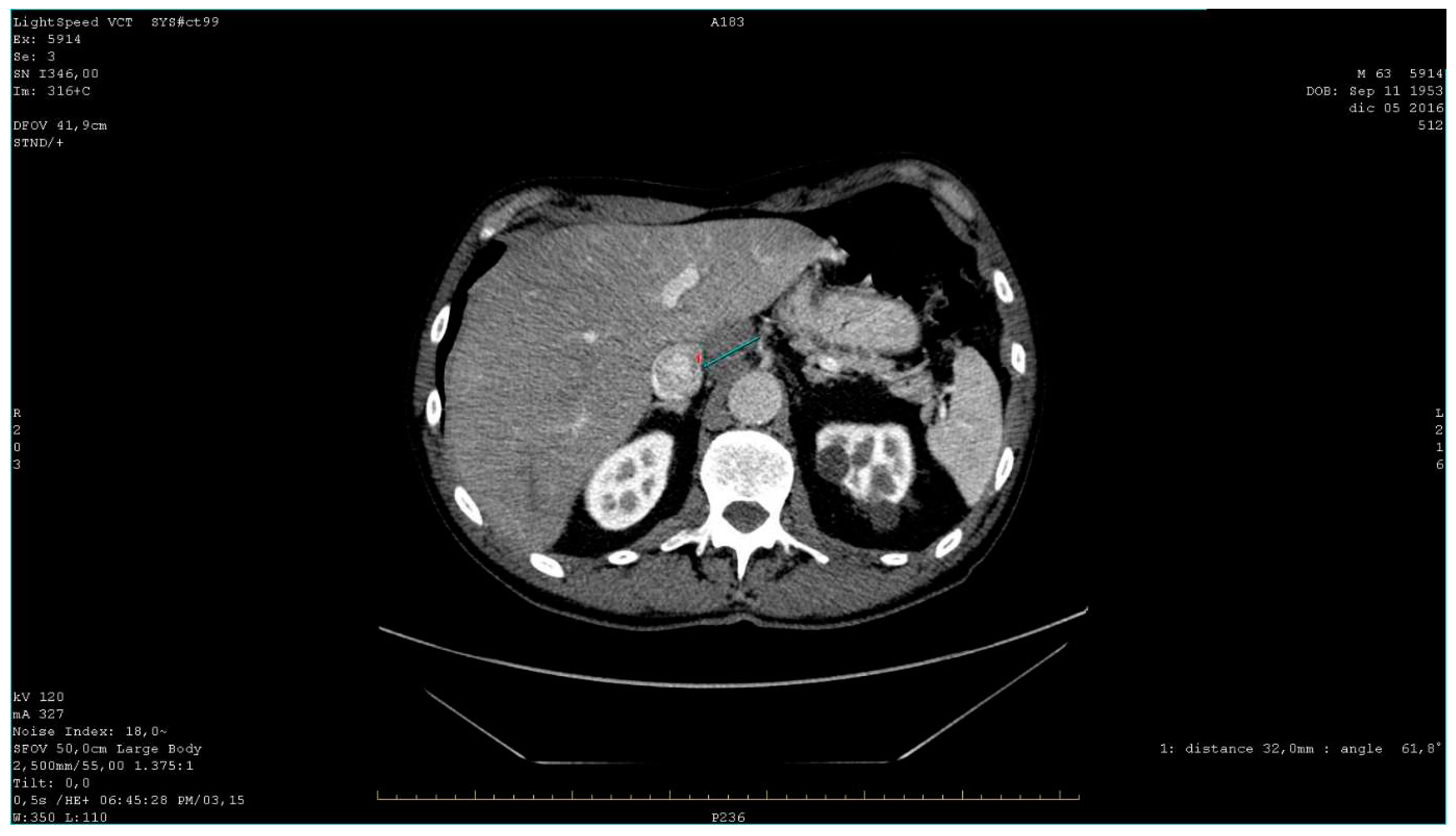
Bronchoscopy and cytology on bronchial lavage returned negative results, while FDG PET (fluorodeoxyglucose-positron emission tomography) confirmed the presence of multiple hypercapturing lesions consistent with the CT findings. Subsequently, an ultrasound-guided biopsy was performed on lymph nodes near the celiac tripod(
Figure 3).
The histological examination described a poorly differentiated neoplasm suggestive of primary lung cancer. Immunohistochemistry results were positive for CKAE1/AE3, CK7, EMA, and focally for TTF1 and p63, while negative for CK5/6, CD56, Chromogranin A, and Synaptophysin. The Ki67 value was 50%. The study on EGFR for the mutation of exons 18, 19, 20, and 21 gave a negative result.
Various tumor markers were measured, indicating a primary Non-Small Cells Lung Carcinoma (NSCLC): CEA was 1021.2 ng/ml (<6), Ca19.9 was 9.0 UI/ml (<37), and NSE was 6.6 UI/ml (<10).
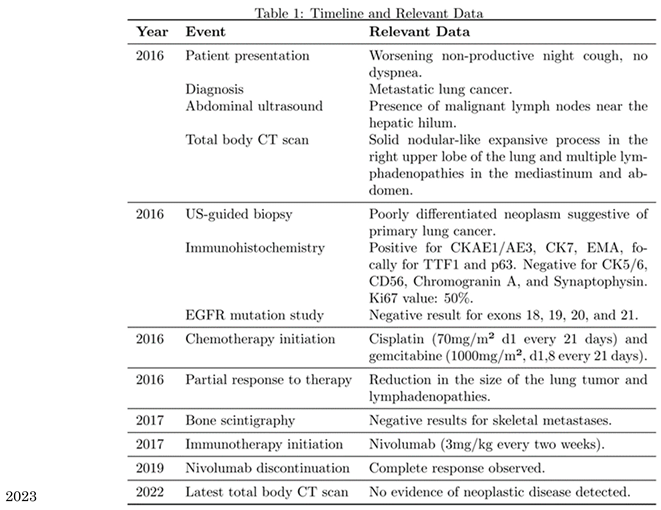
Given the metastatic stage of the disease, surgical removal of the primary lesion was not pursued. The patient initiated a chemotherapy regimen consisting of cisplatin (70mg/m² on day 1 every 21 days) and gemcitabine (1000mg/m² on days 1 and 8 every 21 days). After three cycles, blood tests showed hyponatremia (Na+ 127mEq/ml, successfully treated) and a favorable response in cancer markers: CEA decreased from 1021.2 ng/ml to 116.2 ng/ml, and Ca15.3 decreased to 27.5 UI/ml (<30 UI/ml). Subsequent CT scans revealed a partial response to therapy with a reduction in the size of the lung tumor (from 21x29mm to 10x15mm) as well as thoracic and abdominal lymphadenopathies. Three additional cycles were planned, but the patient could only complete two cycles due to bone marrow suppression and subsequent anemia, which required two blood transfusions.The patient underwent a bone scintigraphy, which yielded negative results for metastases in the skeletal system. Following another CT scan which showed a progression of the disease in the subdiaphragmatic lymph nodes, the patient started immunotherapy with Nivolumab (3mg/kg every two weeks) (PD-L1 expression<10%) .
This treatment showed a positive response with a progressive decrease in CEA levels (reaching 3ng/ml after six months) and Ca 15.3 (22, ng/ml) and a reduction in the size of the lesions observed in subsequent CT scans (no new lymphadenopathies, and shrinkage of existing ones). After 18 months, Nivolumab therapy was discontinued. The patient developed pruritus unresponsive to antihistamines, which was managed with glucocorticoids. Over the next 3 years, regular monitoring with CEA, Ca15.3, and CT-PET scans indicated a complete response. CEA levels remained stable below 6ng/ml, and subsequent CT-PET scans revealed the absence of areas exhibiting FDG uptake in the thoracic and abdominal regions. In the latest total body CT scan performed in 2023, no evidence of neoplastic disease was detected in the lung parenchyma or in the subdiaphragmatic, paratracheal, subcarinal and periceliac lymph nodes (
Figure 4).
3. Discussion and Conclusions
The patient in our case presents with nonspecific symptoms, a history of heavy smoking (although currently not a smoker), and no previous access to cancer screening programs. The diagnosis of cancer is therefore incidental, although already at an advanced stage (T1c-N3-M1c Stage IV B according to the eighth edition of the AJCC TNM classification10).
It is quite common for many patients with NSCLC to be diagnosed with symptomatic disease or even at metastatic stage (37.9%)11. However, this poses a problem because several studies have shown that in patients with a heavy smoking history, participation in screening programs (typically performed using low-dose CT techniques12) significantly reduces mortality from this condition through early detection13,14. Despite this, only about the 4% of high-risk patients (people with a ≥ 20 pack-year smoking history and currently smoking or quint within the past 15 years and are between 50 and 80 year old15) participate in screening programs16.
During the case management, we found that CEA (carcinoembryonic antigen) was a reliable predictor of disease progression and regression, consistent with other studies17.
Given the absence of suitable mutations for targeted therapy, the initial line of treatment was chemotherapy with cisplatin and gemcitabine, as recommended by guidelines at the time18. However, this treatment regimen resulted in toxicity, including bone marrow depression leading to the need for two blood transfusions, as well as severe hyponatremia. Despite an initial partial response, the disease progressed after treatment suspension due to the mentioned side effects.
Subsequently, the patient was started on immunotherapy using Nivolumab at a dose of 3mg/kg every 3 weeks. This led to a complete response, and the patient has remained disease-free since the treatment was discontinued in mid-2018, which is now five years ago. Over time, immunotherapy has demonstrated good efficacy in the treatment of metastatic NSCLC, as supported by another works19.
It is important to state that cases like this, with patients experiencing a complete response to treatment and maintaining a disease-free state for over 5 years, are still rare in the context of NSCLC. Despite ongoing advancements in research and the increasingly detailed molecular characterization, tumor-related mortality and the rates of overall survival (OS), recurrence-free survival (RFS) and Progression Free Survival remain suboptimal20.
Indeed, achieving such extended overall survival (OS) times (at the time of writing the article, 75 months, calculated from the date of diagnosis of the disease) in a patient with stage IVB non-small cell lung cancer (NSCLC) and negative for EGFR/ALK mutations who could not undergo radical tumor resection is exceedingly rare. A retrospective Austrian study conducted in 201521, which examined 2993 patients treated between 1989 and 2009, reported a median OS of 7.2 months for patients treated prior to 2000 and 8.4 months for patients treated after 2000, considering recurrence-free survival (RFS) for patients receiving chemotherapy (up to 5 different therapeutic regimens) in this patient cohort. A recent American article that considered various treatment options with chemotherapy (carboplatin in combination with paclitaxel or pemetrexed) reported similar overall survival (OS) data, with a median OS of 6.8 months for patients treated with first-line chemotherapy22.
Higher values have been achieved with the use of immunotherapy. In the KEYNOTE-024 trial, the use of Pembrolizumab was evaluated in patients with stage IV NSCLC and a PD-L1 Tumor Proportion Score of 50% or greater, without EGFR/ALK mutations. In this patient pool, the median OS was reported to be 30 months23,24.
Other trials have evaluated the response to immunotherapy with Pembrolizumab in combination with Carboplatin and Paclitaxel chemotherapy in patients with non-small cell squamous carcinoma, regardless of PD-L1 expression. These trials reported a median OS of 15.9 months in patients treated with the combination compared to 11.3 months in patients treated with chemotherapy alone. The hazard ratio for death was 0.64 in patients with PD-L1 expression greater than 50%, 0.57 in patients with PD-L1 expression between 1% and 49%, and 0.61 in patients with PD-L1 expression less than 1%25.
Similar results were also obtained in the KEYNOTE-042 trial. In this trial, the response to Pembrolizumab monotherapy was evaluated in patients with non-small cell lung cancer, regardless of PD-L1 expression. The trial demonstrated a comparable median overall survival (OS) of 16.7 months in patients treated with Pembrolizumab compared to 12.1 months in patients treated with standard chemotherapy26,27.
Other studies have evaluated the combination of immunotherapy with stereotactic body radiation. In a 2020 study, the response was assessed in patients with metastatic NSCLC using either Pembrolizumab or Ipilimumab (anti-CTLA-4) combined with radiation treatment of 50 Gy at the site of the primary lesion. The median overall survival (OS) of patients treated with Ipilimumab was reported to be 10.7 months, while it was not reached during the study for patients treated with Pembrolizumab (with a survival rate of 66% at 18 months). These findings suggest that combining immunotherapy with stereotactic body radiation may provide favorable outcomes for patients with metastatic NSCLC28.
In the literature, cases with overall survival (OS) exceeding 36 months are rare. A Chinese case report from 202129 describes a 78-year-old male patient with a history of prolonged tobacco abuse (20 cigarettes per day) and tuberculosis, diagnosed with NSCLC stage T1N0M0, who underwent surgery and radiation therapy. After one year of radiation treatment, the patient developed lung, liver, cardiac, and bone metastases. Subsequently, the patient received radiation therapy for the bone lesions and was administered pembrolizumab (200 mg every 21 days). The patient showed symptomatic improvement with a partial response (PR) as evidenced by CT scans. The authors report a 7-year overall survival (OS) from the time of diagnosis, similar to the presented case, with the difference being that the patient developed metastases after 3 years from the initial diagnosis, rather than at the time of initial diagnosis.
Another similar case is reported in a French study from 202230, describing a 61-year-old female patient (with a heavy smoking history) with non-small cell lung cancer (NSCLC) and bone, liver, and adrenal gland metastases. Molecular screening with Next-Generation Sequencing (NGS) of the primary lesion identified EGFR mutations (V843I, a mutation not associated with a treatment benefit from targeted therapy) and KRAS G13D. Subsequently, the patient received systemic treatment with Pembrolizumab (PD-L1>50%), resulting in a partial response in all tumor locations. After 30 months of progression-free survival (PFS), the patient experienced progression at the previous metastatic sites and underwent chemotherapy with pemetrexed and carboplatin, with a reported overall survival (OS) of 48 months.
Another Chinese study published in 202331 reported a partial response duration of 45 months in a 66-year-old male patient undergoing systemic therapy with pembrolizumab (200mg every 21 days). The management of the patient was challenging due to the presence of chronic renal insufficiency and the absence of immune-related adverse events (irAEs).
Another exceptional case of overall survival (OS) is described in a 2019 Japanese article32, involving a 71-year-old male patient diagnosed with lung adenocarcinoma. Initially, the patient underwent chemotherapy with carboplatin and docetaxel, achieving a partial response that lasted for 8 months. However, the disease eventually progressed, leading to repeated cycles of re-challenging chemotherapy, which successfully controlled the disease for 6 years. Subsequently, the patient received treatment with erlotinib, resulting in a partial response. With a survival time exceeding 11 years from the time of diagnosis, this case is noteworthy. However, it differs from the case previously reported as it lacks a complete response to therapy.
A similar story is that of a 73-year-old American patient33 who underwent immunotherapy with Nivolumab and achieved a survival time of approximately five years from the time of diagnosis. However, it should be noted that there was no complete response to the treatment.
Lastly, noteworthy is the case reported in a 2019 Chinese study34 of a patient with NSCLC and brain metastases who was treated with Temozolomide (150 mg/m2/d for 5 days every 28-day cycle) and achieved an overall survival (OS) of 55 months.
It remains evident that long-term survival for patients with metastatic NSCLC remains nowadays short despite the use of increasingly advanced medical and pharmaceutical technologies. In this scenario, the collection and study of patients and exceptional cases with long-term survival become increasingly crucial in the research for better treatment options for this patient population.
Author Contributions
Conceptualization, C.D.I. and P.I.; methodology, S.I..; validation, L.I., M.M.; formal analysis, M.C.P.; investigation, M.C., S.S.; resources, M.C.P.; data curation, M.C.P..; writing—original draft preparation, C.D.I., P.I.; writing—review and editing, S.I., L.I.; visualization, D.M.; supervision, S.L.; project administration, M.C.P, C.D.I., P.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Local Institutional Review Board deemed the study exempt from review.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Lung cancer. Available at: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed June 06,2023.
- Bade BC, Dela Cruz CS. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin Chest Med. 2020 Mar;41(1):1-24. [CrossRef]
- U.S. Public Health Service, Office of the Surgeon General: the health consequences of smoking. 72-7516 PNH. Washington, DC: National Clearinghouse for Smoking Health.; 1972.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68(6): 394–424.
- Ajrouche R, Ielsch G, Cle’ro E, et al. Quantitative health risk assessment of indoor radon: a systematic review. Radiat Prot Dosimetry 2017;177(1–2):69–77. [CrossRef]
- Alberg AJ, Brock MV, Ford JG, et al. Epidemiology of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143(5 Suppl):e1S–29S.
- Gnagnarella P, Caini S, Maisonneuve P, et al. Carcinogenicity of high consumption of meat and lung cancer risk among non-smokers: a comprehensive meta-analysis. Nutr Cancer 2018;70(1):1–13. [CrossRef]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243-1260.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer.
- J Clin. 2018;68(1):7-30Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The Eighth Edition Lung Cancer Stage Classification. Chest. 2017 Jan;151(1):193-203. [CrossRef]
- Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193-200. [CrossRef]
- Jemal A, Fedewa SA. Lung Cancer Screening With Low-Dose Computed Tomography in the United States-2010 to 2015. JAMA Oncol. 2017 Sep 1;3(9):1278-1281. [CrossRef]
- National Lung Screening Trial Research Team. et al.Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 365, 395–409 2011).
- Walter, J. E. et al. New subsolid pulmonary nodules in lung cancer screening: the NELSON trial. J. Thorac.Oncol. 13, 1410–1414 (2018). [CrossRef]
- Jonas DE, Reuland DS, Reddy SM, et al. Screening for Lung Cancer With Low-Dose Computed Tomography: An Evidence Review for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2021 Mar. (Evidence Synthesis, No. 198.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK568573/.
- Doria-Rose VP, White MC, Klabunde CN, et al.. Use of lung cancer screening tests in the United States: results from the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1049-1059. [CrossRef]
- Dal Bello MG, Filiberti RA, Alama A, Orengo AM, Mussap M, Coco S, Vanni I, Boccardo S, Rijavec E, Genova C, Biello F, Barletta G, Rossi G, Tagliamento M, Maggioni C, Grossi F. The role of CEA, CYFRA21-1 and NSE in monitoring tumor response to Nivolumab in advanced non-small cell lung cancer (NSCLC) patients. J Transl Med. 2019 Mar 8;17(1):74. [CrossRef]
- Linee guida AIOM. Neoplasie del polmone- Edizione 2016. Available online: https://www.aiom.it/neoplasie-del-polmone-5/.
- Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018 Oct;52(Pt 1):103-109. [CrossRef]
- Alexander M, Kim SY, Cheng H. Update 2020: Management of Non-Small Cell Lung Cancer. Lung. 2020 Dec;198(6):897-907. [CrossRef]
- Kocher F, Hilbe W, Seeber A, Pircher A, Schmid T, Greil R, Auberger J, Nevinny-Stickel M, Sterlacci W, Tzankov A, Jamnig H, Kohler K, Zabernigg A, Frötscher J, Oberaigner W, Fiegl M. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015 Feb;87(2):193-200. [CrossRef]
- Simeone JC, Nordstrom BL, Patel K, Klein AB. Treatment patterns and overall survival in metastatic non-small-cell lung cancer in a real-world, US setting. Future Oncol. 2019 Oct;15(30):3491-3502. [CrossRef]
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. New Engl J Med 375(19):1823–1833. [CrossRef]
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Vandormael K, Riccio A, Yang J, Pietanza MC, Brahmer JR (2019) Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol 37(7):537–546. [CrossRef]
- Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, Hermes B, Cay Senler F, Csoszi T, Fulop A, Rodriguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM (2018) Pembrolizumab plus Chemotherapy for Squamous Non-Small- Cell Lung Cancer. N Engl J Med 379(21):2040–2051. [CrossRef]
- Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush D, Lopes G; KEYNOTE-042 Investigators. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019 May 4;393(10183):1819-1830. [CrossRef]
- Wu YL, Zhang L, Fan Y, Zhou J, Zhang L, Zhou Q, Li W, Hu C, Chen G, Zhang X, Zhou C, Dang T, Sadowski S, Kush DA, Zhou Y, Li B, Mok T. Randomized clinical trial of pembrolizumab vs chemotherapy for previously untreated Chinese patients with PD-L1-positive locally advanced or metastatic non-small-cell lung cancer: KEYNOTE-042 China Study. Int J Cancer. 2021 May 1;148(9):2313-2320. [CrossRef]
- Chen D, Menon H, Verma V, Guo C, Ramapriyan R, Barsoumian H, Younes A, Hu Y, Wasley M, Cortez MA, Welsh J. Response and outcomes after anti-CTLA4 versus anti-PD1 combined with stereotactic body radiation therapy for metastatic non-small cell lung cancer: retrospective analysis of two single-institution prospective trials. J Immunother Cancer. 2020 Jan;8(1):e000492. doi: 10.1136/jitc-2019-000492. Erratum in: J Immunother Cancer. 2020 Apr;8(1): PMID: 31996395; PMCID: PMC7057428.
- Ni J, Yang L, Zhu H, Chu M, Zhang C, Zhao W, Yang M, Xu X, Zheng E, Jiang X, Li R, Zhao G. A patient with metastatic non-small cell lung cancer who received pembrolizumab monotherapy after stereotactic body radiotherapy had progression-free survival of nearly 5 years: a case report. Ann Palliat Med. 2021 Apr;10(4):4999-5009. [CrossRef]
- Trabelsi Grati O, Zemoura L, Nhy C, Daniel C, Melaabi S, Callens C, Gauthier Villars M, Bièche I, Girard N. Long response to immune checkpoint inhibitors in metastatic NSCLC despite EGFR germline mutation. A case report. Lung Cancer. 2022 Dec;174:186-187. [CrossRef]
- Yun JW, Kwon J, Lim T. Long-Term Response of Pembrolizumab in a Patient with Metastatic Squamous Non-Small Cell Lung Cancer on Hemodialysis: Case Report and Review of the Literature. Medicina (Kaunas). 2023 Feb 9;59(2):325. [CrossRef]
- Matsuzaki T, Iwami E, Sasahara K, Kuroda A, Nakajima T, Terashima T. A case report of metastatic lung adenocarcinoma with long-term survival for over 11 years. Medicine (Baltimore). 2019 Jan;98(4):e14100. [CrossRef]
- Baseri B, Samra B, Tam E, Chiu E, Leaf A. An Exceptional Responder to Nivolumab in Metastatic Non-Small-Cell Lung Cancer: A Case Report and Literature Review of Long-Term Survivors. Case Rep Oncol Med. 2019 Dec 5;2019:1816472. [CrossRef]
- Yang Y, Pu Y, Dai N, Wang D, Xu M. Complete response of radioresistant brain metastases from non-small cell lung cancer with temozolomide: A case report and literature review. Medicine (Baltimore). 2020 Dec 18;99(51):e23592. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).