1. Introduction
Chronic graft-versus-host disease (GVHD) is one of the major complications of allogeneic hematopoietic stem cell transplantation (HSCT). It occurs in up to 70% of patients who underwent allogeneic HSTC, representing a leading cause of non-relapse morbidity and mortality [
1]. Systemic corticosteroids have been the standard first-line treatment for chronic GVHD. However, approximately 50% of patients may develop a corticosteroid refractory chronic GVHD, requiring second-line systemic treatments [
2]. Calcineurin inhibitors, mycophenolate mofetil, mTOR and Janus kinase (JAK) inhibitors, and extracorporeal photopheresis have shown promising efficacy with an adequate safety profile [
3]. Among the adverse effects of these drugs, metabolic syndrome has frequently been documented in the literature [
4]. Particularly, severe hypertriglyceridemia in patients treated with ruxolitinib (JAK1/2 inhibitor) in combination with sirolimus (mTOR inhibitor) has been reported both in adult and in pediatric populations, requiring a dose adjustment or drug withdrawal alone or in association with insulin drip or therapeutic plasma exchange [
5,
6]. In adult patients with severe hypertriglyceridemia who require a pharmacologic intervention, fibrates represent the first-line treatment. Omega-3 fatty acids, niacin, and statins in high doses have shown modest effectiveness [
7]. Recently, novel lipid-lowering medications addressing new targets have been tested in adults. However, none has been approved for commercial use in the pediatric population [
8]. In adult patients, particularly promising results have been shown by evinacumab. This is a first-in-class recombinant human monoclonal antibody that inhibits angiopoietin-like protein 3 (ANGPTL3) developed for treating homozygous familial hypercholesterolemia (HoFH), refractory hypercholesterolemia, both familial and non-familial, and severe hypertriglyceridemia. An intravenous formulation of evinacumab received U.S. Food and Drug Administration (FDA) approval in 2021 as an add-on therapy in HoFH for patients aged 12 years or older [
9]. Regarding safety profile and pharmacological toxicity, the drug is usually well tolerated. Thus, in phase 1, a single-dose study, up to 80% reduction in triglyceride levels has been reported in evinacumab-treated adult patients with moderate hypertriglyceridemia [
10], prompting the effectiveness of evinacumab as a therapeutic option to lower elevated triglycerides. In June 2021evinacumab obtained authorization for clinical use by the European Medicines Agency (EMA) under exceptional circumstances because comprehensive data has not been provided on the efficacy and safety of the medicine under normal conditions of use. This approval is reserved for treating rare diseases or because collecting complete information is impossible or unethical [
11].
In this paper, we report the case of a 10-year-old boy who presented life-threatening refractory hypertriglyceridemia associated with the concomitant use of ruxolitinib and sirolimus for chronic GVHD. After the failure of the insulin treatment and due to the technical impossibility of performing lipid apheresis, we underwent the child to evinacumab treatment, obtaining a rapid and dramatic reduction in triglyceride and cholesterol levels. To our knowledge, this is the first-ever description of a pediatric patient younger than 12 years with life-threatening hypertriglyceridemia treated in Europa with evinacumab on a compassionate-use basis.
2. Case presentation
A 10-year-old Caucasian boy affected by T-cell acute lymphoblastic leukemia underwent an allogenic HSCT from a matched unrelated donor in May 2020. His post-transplant course was complicated by the chronic hepatic and diffuse sclerotic form of skin GVHD, well controlled on prednisone, tacrolimus, and ruxolitinib. Due to prolonged high-dose corticosteroid treatment associated with ruxolitinib e tacrolimus, the boy has developed a metabolic syndrome featured by dyslipidemia, increased insulin resistance, and severe refractory hypertension. During routine periodic assessments, his cholesterol and triglyceride levels were between 300 and 400 mg/dL over the last several months. A liver ultrasound and liver biopsy confirmed the presence of steatosis. No family history of dyslipidemia.
In addition, immediately after HSCT, the high-level BK virus (BKV) blood and urine loads were documented. Subsequently, BK viremia stabilized between 2000 - 4000 copies/mL, while viruria fluctuated between 50000 and 100000 copies/mL without any clinical signs.
Two years after transplantation, a routine outpatient follow-up noted an acute kidney injury requiring hospitalization. At the admission, the child’s immunosuppressive therapy consisted of low-dose prednisone, tacrolimus, and ruxolitinib. Laboratory investigation revealed elevated values of creatinine (1.63 mg/dL, range 0.26 – 0.55 mg/dL), blood urea nitrogen (92 mg/dL, range 19 - 47 mg/dL), and hyperuricemia (8.9 mg/dL, range 1.8 – 4.9 mg/dL). The state of well-known immunodeficiency was confirmed: the number of CD3 was 402 cells/µL, CD4 168 cells/µL, and the absence of CD19. In addition, moderate hypertriglyceridemia was reported (394 mg/dL, acceptable value for age < 90 mg/dL).
PCR for BKV was performed, showing a marked increase in virus load with more than 100 million copies/mL in the urine and more than 25 million copies/mL in the blood. Clinical and laboratory findings were consistent with the diagnosis of BKV-associated nephropathy, which typically occurs in tacrolimus treatment [
12]. According to the literature data, a reduction in immunosuppression was performed [
13]. The dosage of cortisone and ruxolitinib was halved, while tacrolimus was replaced with sirolimus. Intravenous immunoglobulin was also administered.
As renal function continued to deteriorate with no trend of decreasing BKV load, a single dose of cidofovir (3 mg/kg) was administrated. Cidofovir led to a progressive reduction of BKV blood load, down to 33.000 copies/mL, but caused significant worsening of kidney function, with a creatinine clearance reduction of up to 32 mL/min. Furthermore, the boy developed severe trilineage hematologic toxicity, rapidly leading to profound neutropenia, thrombocytopenia, and anemia.
Two weeks later, he was diagnosed with invasive Scedosporium apiospermum osteomyelitis which he requested surgical debridement with resection of surgically amenable lesions. The clinical picture was rapidly complicated by life-threatening chronic norovirus enteritis and hepatitis, beyond systemic HSV-1 and CMV infections with pulmonary and central nervous system involvement. Because impossibility of starting antiviral therapy due to kidney failure, the cortisone was stopped in the expectancy of obtaining an immune recovery. The boy remained in combined therapy with sirolimus and ruxolitinib.
An already clinically difficult situation was further complicated by acute pancreatitis. Excluding the various causes, the child's metabolic profile was studied. Triglyceride levels became extremely high, up to 6000 mg/dL, while cholesterol increased to 2900 mg/dL. In the suspicion that hypertriglyceridemia is due to the combination of sirolimus and ruxolitinib, the immunosuppression was discontinued. Considering that the severity of dyslipidemia required urgent intervention beyond the simultaneous presence of renal and hepatic insufficiency, we excluded the possibility of using fibrates and statins. Therapeutic apheresis was excluded because of the absence of central line accesses that guarantee sufficient flow during apheresis. Given the child's diffuse scleroderma, placing a single central venous catheter of a small gauge was possible. The insulin-dextrose infusion was a unique suitable therapeutic option. The boy was started on a continuous insulin infusion with substantially reduced triglyceride levels, which dropped to 1300 mg/dL in one week. Unfortunately, the patient has demonstrated poor tolerance of insulin treatment due to frequent episodes of hypoglycemia and the impossibility of monitoring glucose levels with the FreeStyle Libre (intermittently scanned continuous glucose monitoring) through fibrotic skin.
Having obtained the favorable opinion of the Regional Committee for Bioethics for using evinacumab, we asked Ultragenyx Pharmaceutical Inc. for the medicine supply on a compassionate basis. Within a few days, we received evinacumab (evinacumab-dgnb, EVKEEZATM) from Regeneron Pharmaceuticals, Inc. (777 Old Saw Mill River Rd, Tarrytown, NY 10591 USA). The child started therapy with evinacumab at a dose of 15 mg/kg every four weeks, for a total of 450 mg per dose, with a pre-treatment triglyceride level of 1341 mg/dL. We documented a dramatic reduction of blood triglycerides, reaching 154 mg/dL in 24 hours. The drug was well-tolerated, and the boy continued the inpatient treatment for another three months, maintaining triglyceride levels below 350 mg/dL. However, they remained higher than the normal range for age.
In the meantime, his clinical conditions have progressively improved, and he has been discharged with a complete immunological reconstruction. Eight months later, continuing evinacumab outpatient treatment without any adverse events and maintaining triglyceride levels markedly reduced (167 mg/dL at the last follow-up).
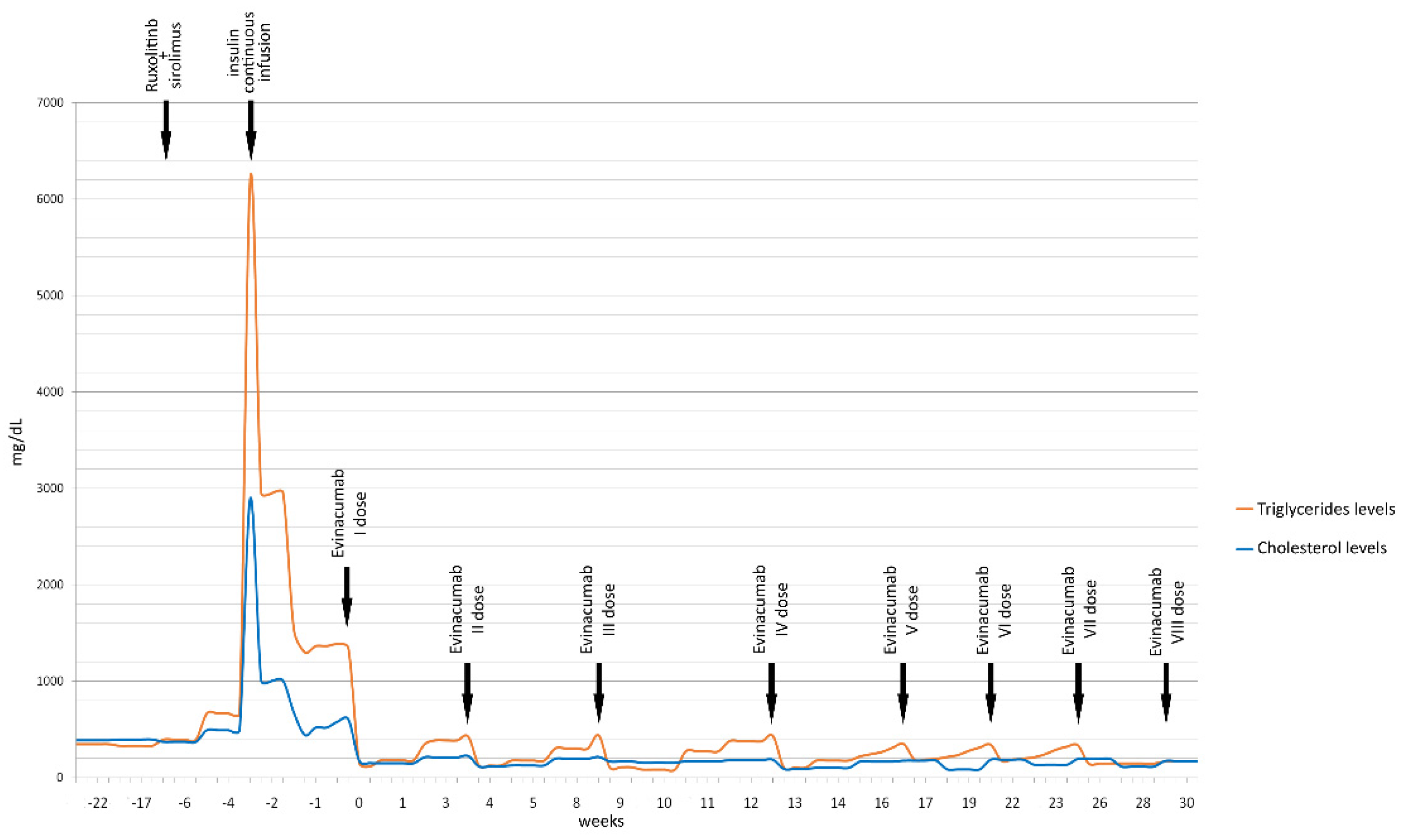
Figure 1 displays the reduction of triglyceride and total cholesterol values over time.
2.1. Figures, Tables and Schemes
Figure 1.
Timeline of patient’s triglyceride and cholesterol levels (mg/dL) before and after the start of evinacumab treatment.
Figure 1.
Timeline of patient’s triglyceride and cholesterol levels (mg/dL) before and after the start of evinacumab treatment.
3. Discussion
In our patient, we assumed that markedly high triglyceride levels were derived from the combined therapy with sirolimus and ruxolitinib. Dyslipidemia, particularly hypertriglyceridemia, is a major adverse effect of sirolimus described in the literature [
15]. Thus, a mild increment in triglyceride levels without any significant clinical outcome has been described in ruxolitinib use [
16]. The association between these two immunosuppressors increases the risk of hypertriglyceridemia and metabolic syndrome exponentially, as previously reported in the literature in adult and pediatric patients [
5,
6].
The 2018 American College of Cardiology/American Heart Association cholesterol-levels guidelines define “severe hypertriglyceridemia” as triglyceride levels are ≥ 500 mg/dL [
14]. The Endocrine Society specifying further defines2000 mg/dL values L as very severe hypertriglyceridemia [
17].
The guidelines for managing severe hypertriglyceridemia in adults recommended using fibrates as the first-line treatment if creatinine clearance is > 30 ml/min and liver transaminases values < 2,5 x upper limit of the normal. Furthermore, omega-3 fatty acids are recommended, alone or with statins [
17]. However, hypolipidemic drugs fail to reduce triglyceride-rich lipoproteins in patients with hypertriglyceridemia-induced pancreatitis or severe hyperviscosity symptoms. In these cases, insulin-dextrose infusion or therapeutic apheresis should be recommended [
18].
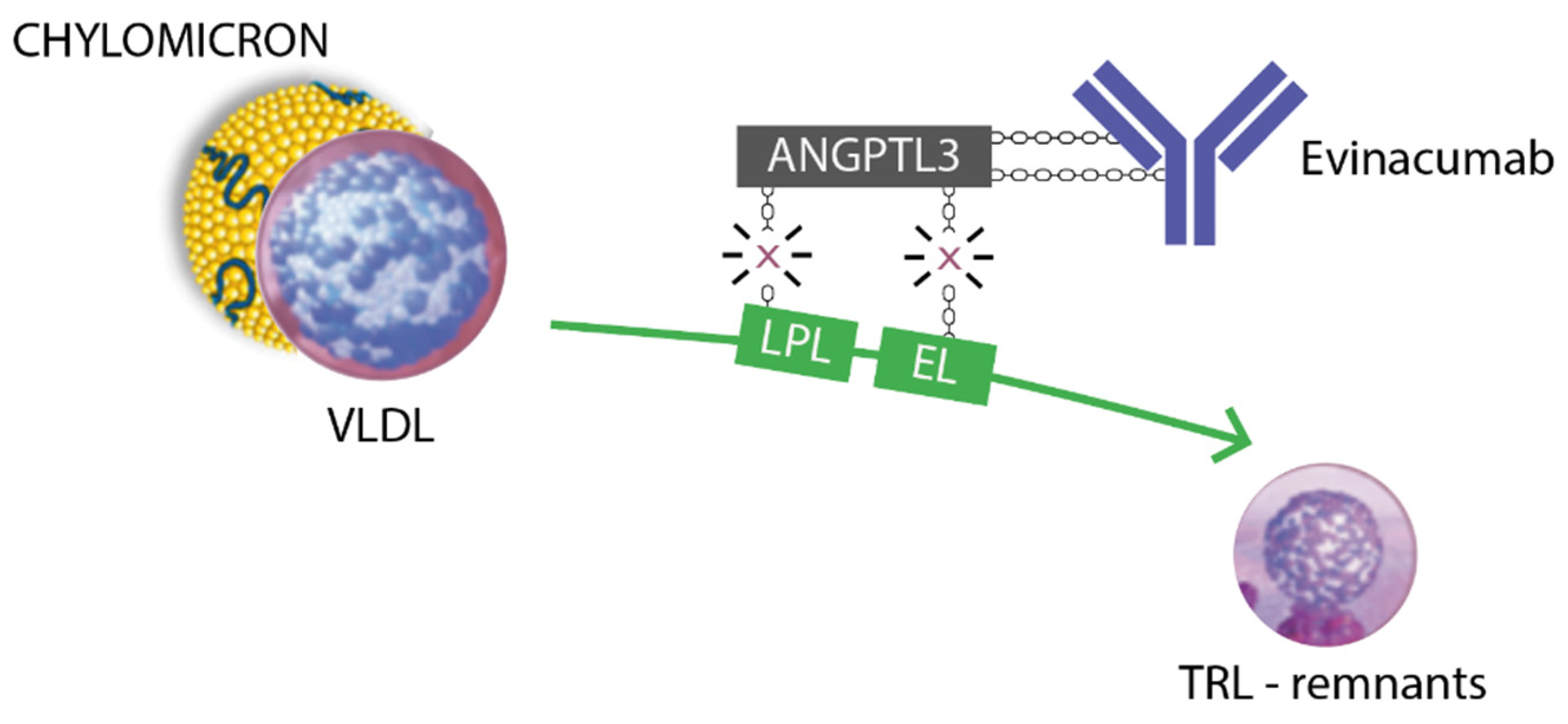
Managing severe hypertriglyceridemia in children is challenging because of the lack of evidence-based guidelines. Furthermore, no FDA/EMA-approved triglycerides-lowering drugs have been approved for commercial use in pediatrics. Some novel lipid-lowering medications acting on the lipoprotein lipase (LPL) complex have recently emerged in the adult population. These include drugs targeting ANGPTL3, a glycoprotein secreted by the liver that represents an important regulator of lipoprotein metabolism and other angiopoietin-like proteins. These proteins inhibit LPL and endothelial lipase (EL), increasing plasmatic levels of triglycerides, LDL cholesterol, and HDL cholesterol [
19]. Thus, Dewey et al. documented that triglyceride levels were notably reduced in carriers of loss-of-function mutations in the ANGPTL3 encoding gene, suggesting it could be a possible therapeutic target for lowering elevated triglycerides [
20].
Evinacumab, a monoclonal antibody that acts as an ANGPTL3-inhibitor, received FDA approval in 2021 as an add-on therapy in HoFH for patients aged 12 years or older [
9]. Currently, a three-part, single-arm, open-label clinical trial is evaluating the efficacy and safety of evinacumab in children with HoFH between the ages of 5 and 11 years (NCT04233918) [
21]. Despite the data collected from two phase-1, single-dose studies, which have demonstrated a dose-dependent reduction of up to 80% in triglyceride levels in evinacumab-treated adults, ANGPTL3-inhibitors have not received FDA approval as triglyceride-lowering drugs [
22]. In HoFH, the FDA-recommended dosage of evinacumab is 15 mg/kg, administered intravenously for one hour once every four weeks [
22].
Treatment is generally well-tolerated [
23]. In an eight-month follow-up, our patient did not present any of the most common side effects previously described, such as hypersensitivity reactions, infusion site reactions, flu-like symptoms, nasal congestion, abdominal pain, diarrhea, and elevation of transaminases [
24].
3.1. Figures, Tables and Schemes
4. Conclusions
To our knowledge, we describe the first pediatric patient younger than 12 years receiving evinacumab to treat severe hypertriglyceridemia on a compassionate-use basis. In our experience, therapy with evinacumab was effective, safe, and well-tolerated. It allowed us to reduce triglyceride levels quickly, avoiding more invasive and unsafe therapeutic options, such as prolonged continuous insulin infusion or therapeutic plasma exchange. There are no current FDA/EMA-approved triglyceride-lowering drugs in pediatric patients. We hope our case report could represent a breakthrough in treating life-threatening hypertriglyceridemia in children affected by hematological malignancies, with numerous severe complications due to the treatments.
Furthermore, we would like to stress the importance of treating and preventing the underlying causes leading to secondary hypertriglyceridemia. In a transplantation setting, in agreement with the reports of Watson and Bauters [
5,
6], we emphasize the need to monitor severe side effects in immunosuppressive drugs combination, especially regarding the patient’s metabolic status.
Author Contributions
AF was involved in writing the manuscript. CDC and NM were involved in the patient’s clinical care, conceptualizing the study, and editing relevant sections of the manuscript. AM and DZ were involved in patient data collection, creating figures, and editing the manuscript. EB critically revised the manuscript. All authors contributed to revising the manuscript. All authors read and approved the final manuscript. .
Funding
The study did not provide any funding source to the Sponsor.
Institutional Review Board Statement
The study was approved by the ethical committee of the Institute of Maternal and Child Health, IRCCS Burlo Garofolo, Trieste (Italy). All methods were carried out in accordance with relevant guidelines and regulations.
Informed Consent Statement
Written informed consent has been obtained from the participant(s) to publish this case report.
Data Availability Statement
The original contributions presented in this case report are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank Regeneron and Ultragenyx pharmaceutical companies for the evinacumab supply. This work was supported by the Ministry of Health, Rome - Italy, in collaboration with the Institute for Maternal and Child Health IRCCS Burlo Garofolo, Trieste – Italy.
Conflicts of Interest
The authors declare that they have no competing interests.
Abbreviations
GVHD = Graft-Versus-Host Disease, HSCT = Hematopoietic Stem Cell Transplantation, ANGPTL3 = Angiopoietin-like protein 3, HoFH = Homozygous Familial Hypercholesterolemia, IV = Intra-venous, FDA = Food and Drug Administration, T-ALL = T-cell Acute Lymphoblastic Leukemia, CMV = Cytomegalovirus, BKV = BK virus, IVIG = Intravenous immunoglobulin, LPL = Lipoprotein lipase, EL = Endothelial lipase.
References
- Zeiser R, Blazar BR. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N Engl J Med. 2017;377(26):2565-2579. [CrossRef]
- Pagliuca S, Prata PH, Xhaard A, Frieri C, Giannoni L, Sutra Del Galy A, Brignier A, Sicre de Fontbrune F, Michonneau D, Dhedin N, Peffault de Latour R, Socié G, Robin M. Long-term outcomes and risk factor analysis of steroid-refractory graft versus host disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2021;56(1):38-49. [CrossRef]
- Hill L, Alousi A, Kebriaei P, Mehta R, Rezvani K, Shpall E. New and emerging therapies for acute and chronic graft versus host disease. Ther Adv Hematol. 2018;9(1):21-46. [CrossRef]
- Hoosain J, Hamad E. Adverse Effects of Immunosuppression: Nephrotoxicity, Hypertension, and Metabolic Disease. Handb Exp Pharmacol. 2022;272:337-348. [CrossRef]
- Watson AP, Brunstein CG, Holtan SG. Life-Threatening Hypertriglyceridemia in a Patient on Ruxolitinib and Sirolimus for Chronic Graft-versus-Host Disease. Case Rep Transplant. 2018;2018:4539757. [CrossRef]
- Bauters T, Bordon V, Laureys G, Dhooge C. Combined use of ruxolitinib and sirolimus: increased monitoring of triglycerides required. Bone Marrow Transplant. 2019;54(8):1372-1373. [CrossRef]
- Elkins C, Friedrich D. Hypertriglyceridemia: A review of the evidence. Nurse Pract. 2018;43(10):22-29.
- Sunil B, Ashraf AP. Childhood Hypertriglyceridemia: Is It Time for a New Approach? Curr Atheroscler Rep. 2022;24(4):265-275.
- Markham A. Evinacumab: First Approval. Drugs. 2021;81(9):1101-1105. [CrossRef]
- Ahmad Z, Banerjee P, Hamon S, Chan KC, Bouzelmat A, Sasiela WJ, Pordy R, Mellis S, Dansky H, Gipe DA, Dunbar RL. Inhibition of Angiopoietin-Like Protein 3 With a Monoclonal Antibody Reduces Triglycerides in Hypertriglyceridemia. Circulation. 2019;140(6):470-486. [CrossRef]
- 11. https://www.ema.europa.eu/en/medicines/human/EPAR/evkeeza#authorisation-details-section.
- Chen XT, Li J, Deng RH, Yang SC, Chen YY, Chen PS, Wang ZY, Huang Y, Wang CX, Huang G. The therapeutic effect of switching from tacrolimus to low-dose cyclosporine A in renal transplant recipients with BK virus nephropathy. Biosci Rep. 2019;39(2):BSR20182058. [CrossRef]
- Kotla SK, Kadambi PV, Hendricks AR, Rojas R. BK polyomavirus-pathogen, paradigm and puzzle. Nephrol Dial Transplant. 2021;36(4):587-593.
- Grundy SM et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):3168-3209. [CrossRef]
- Hakeam HA, Al-Jedai AH, Raza SM, Hamawi K. Sirolimus induced dyslipidemia in tacrolimus based vs. tacrolimus free immunosuppressive regimens in renal transplant recipients. Ann Transplant. 2008;13(2):46-53.
- Mesa, R. A., Verstovsek, S., Gupta, V., Mascarenhas, J. O., Atallah, E., Burn, T., Sun, W., Sandor, V., & Gotlib, J. Effects of ruxolitinib treatment on metabolic and nutritional parameters in patients with Myelofibrosis from COMFORT-I. Clinical Lymphoma, Myeloma and Leukemia. 2015;15(4), 214-221.e1. [CrossRef]
- Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Sacks F, Murad MH, et al. Evaluation and treatment of hypertriglyceridemia: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969–89. [CrossRef]
- Filippatos TD, Elisaf MS. Recommendations for severe hypertriglyceridemia treatment, are there new strategies? Curr Vasc Pharmacol. 2014;12(4):598-616. [CrossRef]
- Tikka A, Jauhiainen M. The role of ANGPTL3 in controlling lipoprotein metabolism. Endocrine. 2016;52(2):187-93. [CrossRef]
- Dewey FE, Gusarova V, Dunbar RL, et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med. 2017;377(3):211–221. [CrossRef]
- ClinicalTrials.gov Identifier: NCT04233918. A three-part, single-arm, open-label study to evaluate the efficacy, safety, and pharmacokinetics of evinacumab in pediatric patients with homozygous familial hypercholesterolemia [available from: https://clinicaltrials.gov/ct2/show/NCT04233918, accessed March 13, 2022].
- Regeneron Pharmaceuticals. EVKEEZATM (evinacumab-dgnb) injection: US prescribing information. 2021. https://www.regen eron.com/sites/default/files/Evkeeza_PI.pdf. Accessed 15 Feb 2021.
- Rosenson RS, Gaudet D, Ballantyne CM, Baum SJ, Bergeron J, Kershaw EE, Moriarty PM, Rubba P, Whitcomb DC, Banerjee P, Gewitz A, Gonzaga-Jauregui C, McGinniss J, Ponda MP, Pordy R, Zhao J, Rader DJ. Evinacumab in severe hypertriglyceridemia with or without lipoprotein lipase pathway mutations: a phase 2 randomized trial. Nat Med. 2023;29(3):729-737. [CrossRef]
- Stefanutti C, Chan DC, Di Giacomo S, Morozzi C, Watts GF. Long-Term Efficacy and Safety of Evinacumab in Patients with Homozygous Familial Hypercholesterolemia: Real-World Clinical Experience. Pharmaceuticals (Basel). 2022;15(11):1389. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).