Thermodynamics is the most general qualitative theory for description of complex systems, dealing with simple mathematics in an elegant way. The Fist Law of thermodynamics recognizes the energy conservation
, where
are the system extensive variables, while
are the conjugated intensive parameters. The Zeroth Law of thermodynamics reflects transitivity of thermodynamic equilibrium and introduces temperature
accordingly as a unique intensive parameter, which becomes uniform in the entire system at equilibrium. Its conjugated extensive variable is the entropy
, being thermodynamically linked to temperature via the relation
. Entropy is ruled by the Second Law of thermodynamics, which governs the direction of spontaneous processes in Nature. The Third Law specifies temperature positively. Because thermodynamics is a macroscopic theory, the underlying microscopic dynamics is hidden from the observer. It is not lost, however, and the entire missing mechanical information builds up the entropy
and temperature
, which are not present in mechanics. Since the internal energy is a homogeneous function of first degree from the extensive variables, it equals to
according to the Euler theorem. Substituting this expression in the First Law of thermodynamics yields the important Gibbs-Duhem equation
, which relates the intensive parameters
via the intensive molar quantities
, where
is the number of particles in the system. These fundamental laws are the summit of thermodynamics, and the entire subject is application of the basic principles to solve particular problems. It was recently applied in socio-econophysics [
1], which is nowadays known as Thermodynamics 2.0 [
2].
Let us try to apply the thermodynamic approach to society. Because all underlying microscopic processes respect the energy conservation, it seems reasonable that this will be the case in the society as well, where the social internal energy
preserves. There is no doubt in the presence of social entropy
, because the society is even more complex than the simple molecular systems [
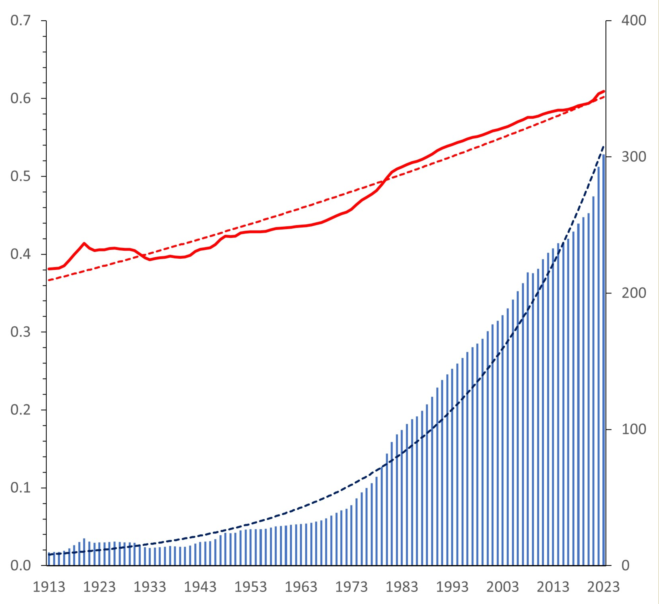
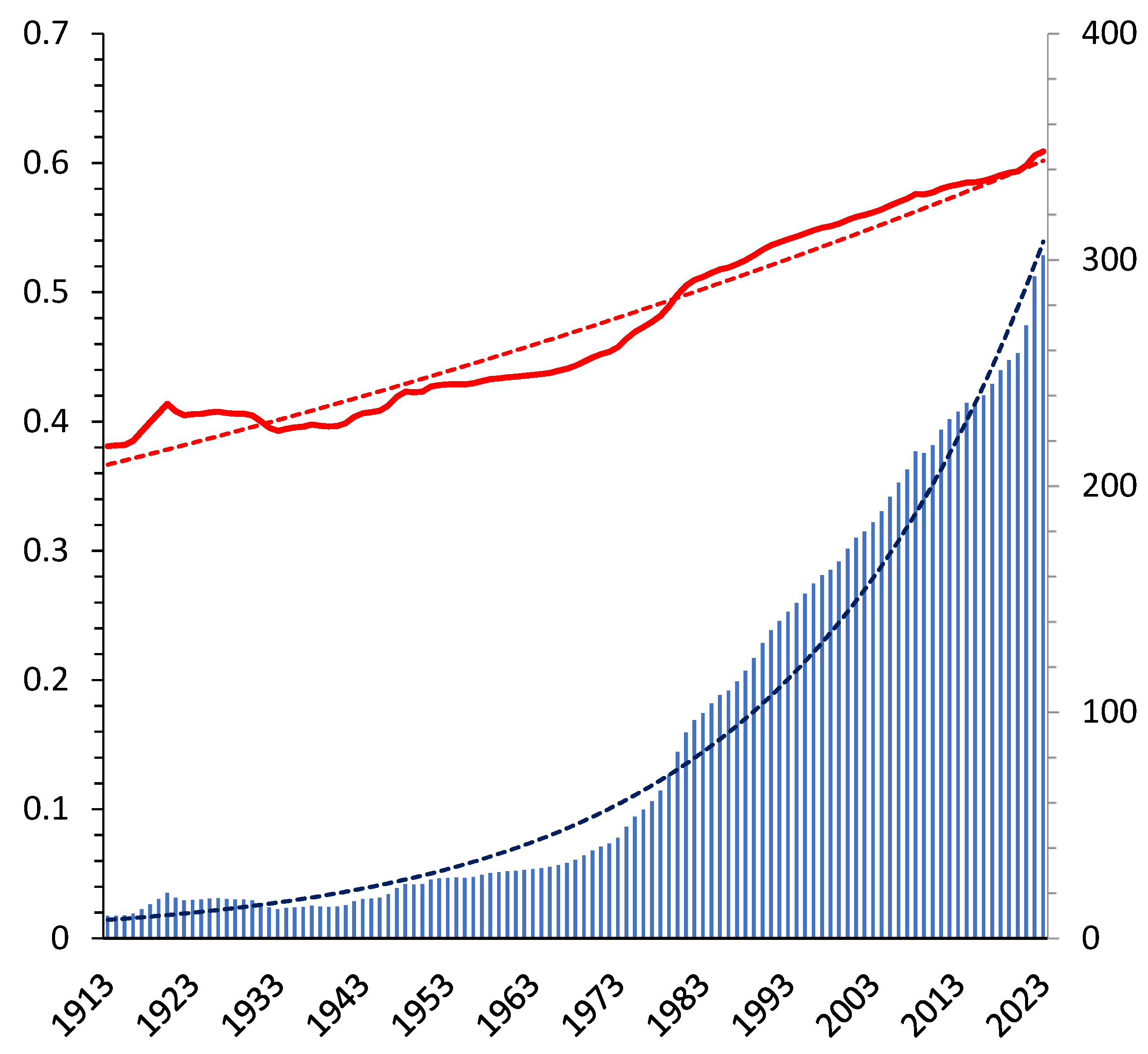
3]. Due to the well-known relation of entropy to information, one can sense the social entropy level in different countries via the Press Freedom Index of the mass media, for instance. From this perspective, it is obvious that the historical evolution of human society follows a permanent increase of the global social entropy. Therefore, the usual striving for liberty is simply a manifestation of the Second Law. Considering the social entropy
as dimensionless freedom, the social temperature
is the energy gain by unit liberalization. Temperature of living species is related to temperament [
4]. Economy is the second important element of the society, which is characterized by the dimensionless economic volume
. The conjugated intensive parameter
is the price pressure, which shows the relative energy cost for the economic growth. Finally, a minimalistic model requires number of people
with conjugated intensive quantity
. The latter is exploitation, which is measuring the contribution of a person to the increase of the social energy. Thus, following thermodynamics the social energy
obeys the First Law
. The exploitation
balances the social contribution
and the social reward
. Obviously, people generate either social energy
or economy
, and for this reason their contribution to the society equals to the molar social enthalpy
. The exploitation obeys the Gibbs-Duhem equation
, which shows that
and
. Therefore, the exploitation increases with inflation
but decreases with the social temperature rise
. Naturally, people migrate to lower exploitation. For instance,
is huge in the USA, which is evident from the highest economic standard, but obviously the reward
compensate enough the enthalpy to facilitate essential inflow of immigrants from countries with higher
.