Submitted:
10 July 2023
Posted:
11 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
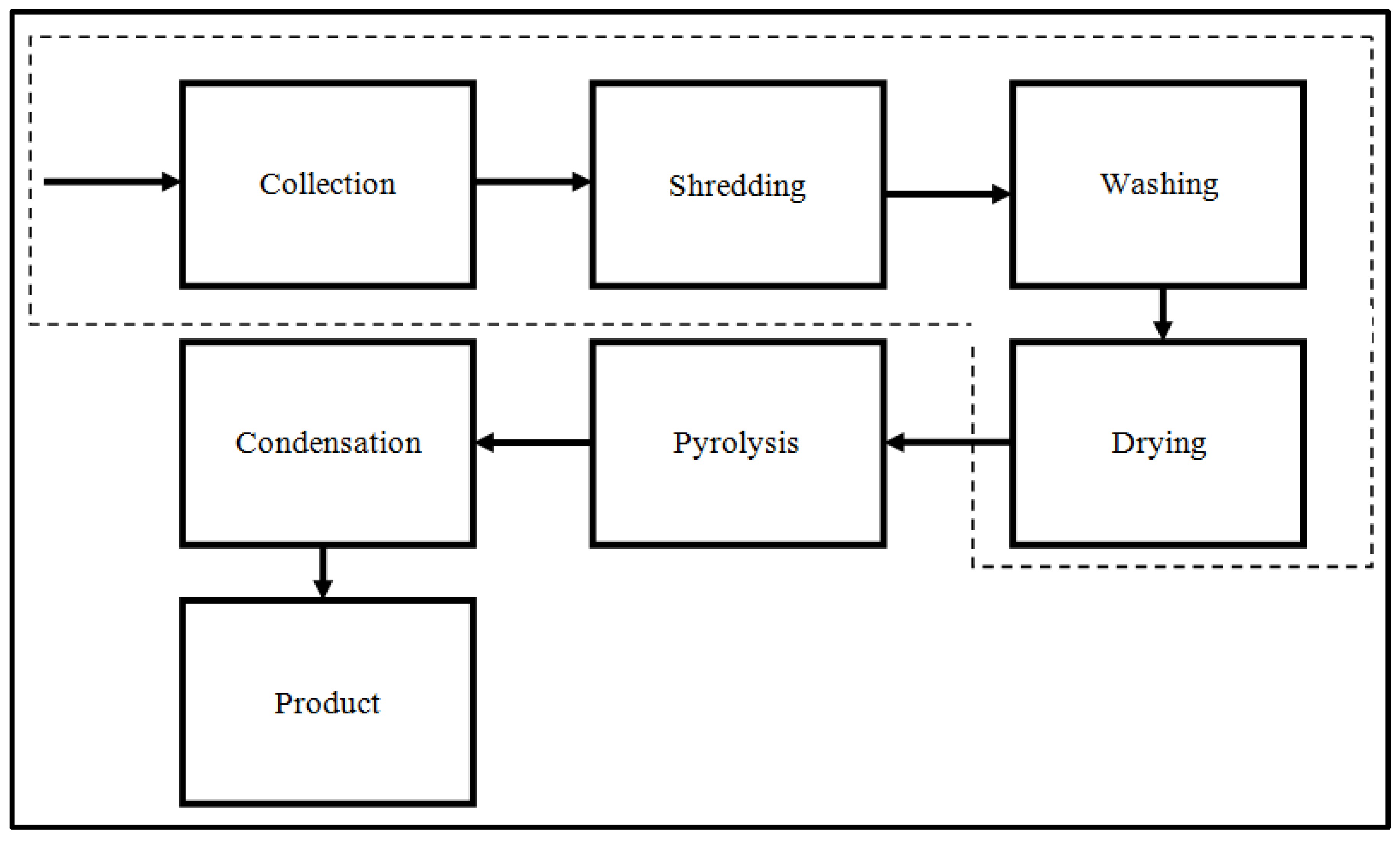
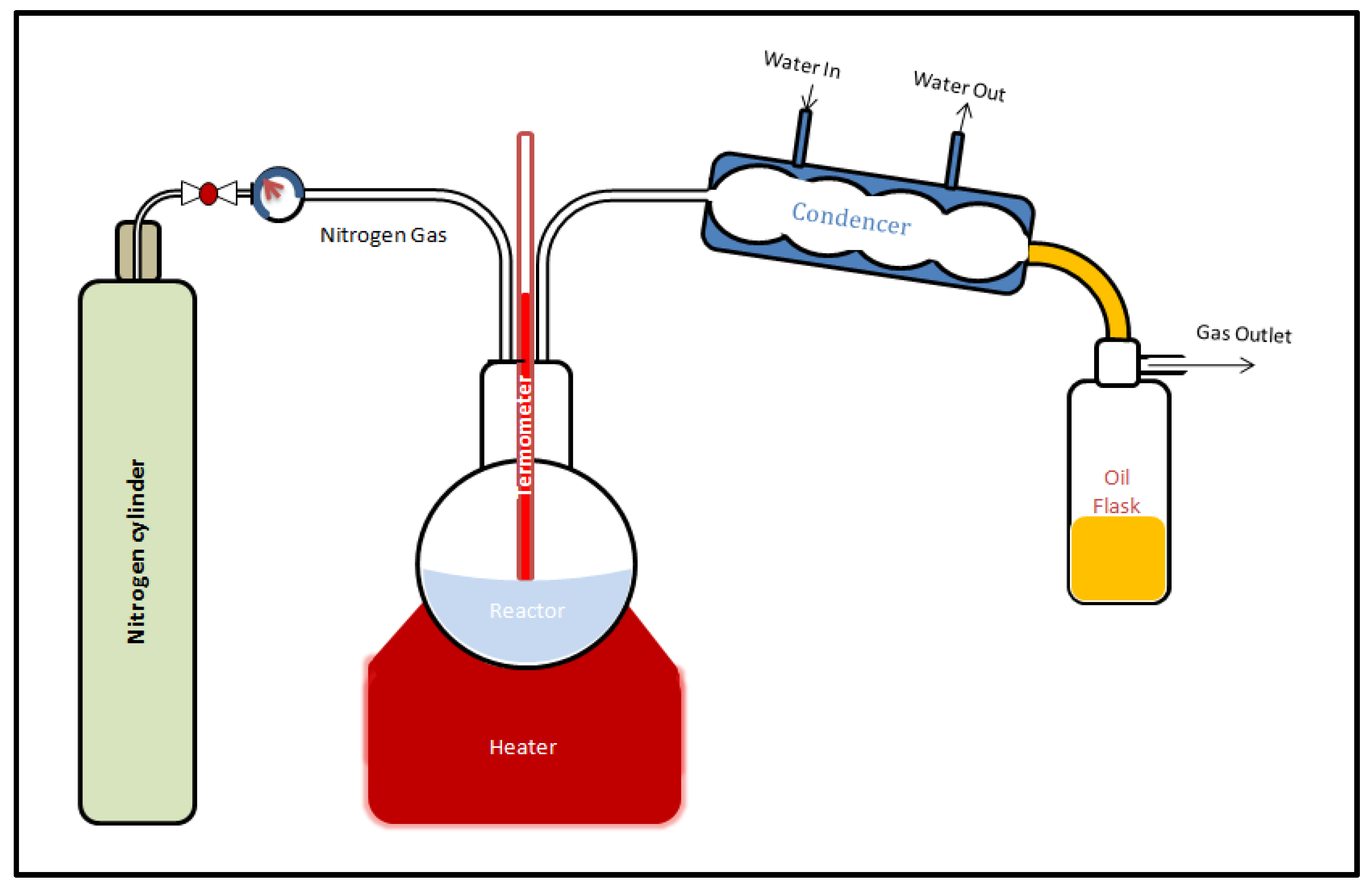
2. Experimental
2.1. Characterization
2.2. Exergy analysis
2.2.1. Physical exergy (
2.2.2. Chemical exergy ()
2.2.3. Process exergetic/thermodynamic efficiency
3. Results and discussion
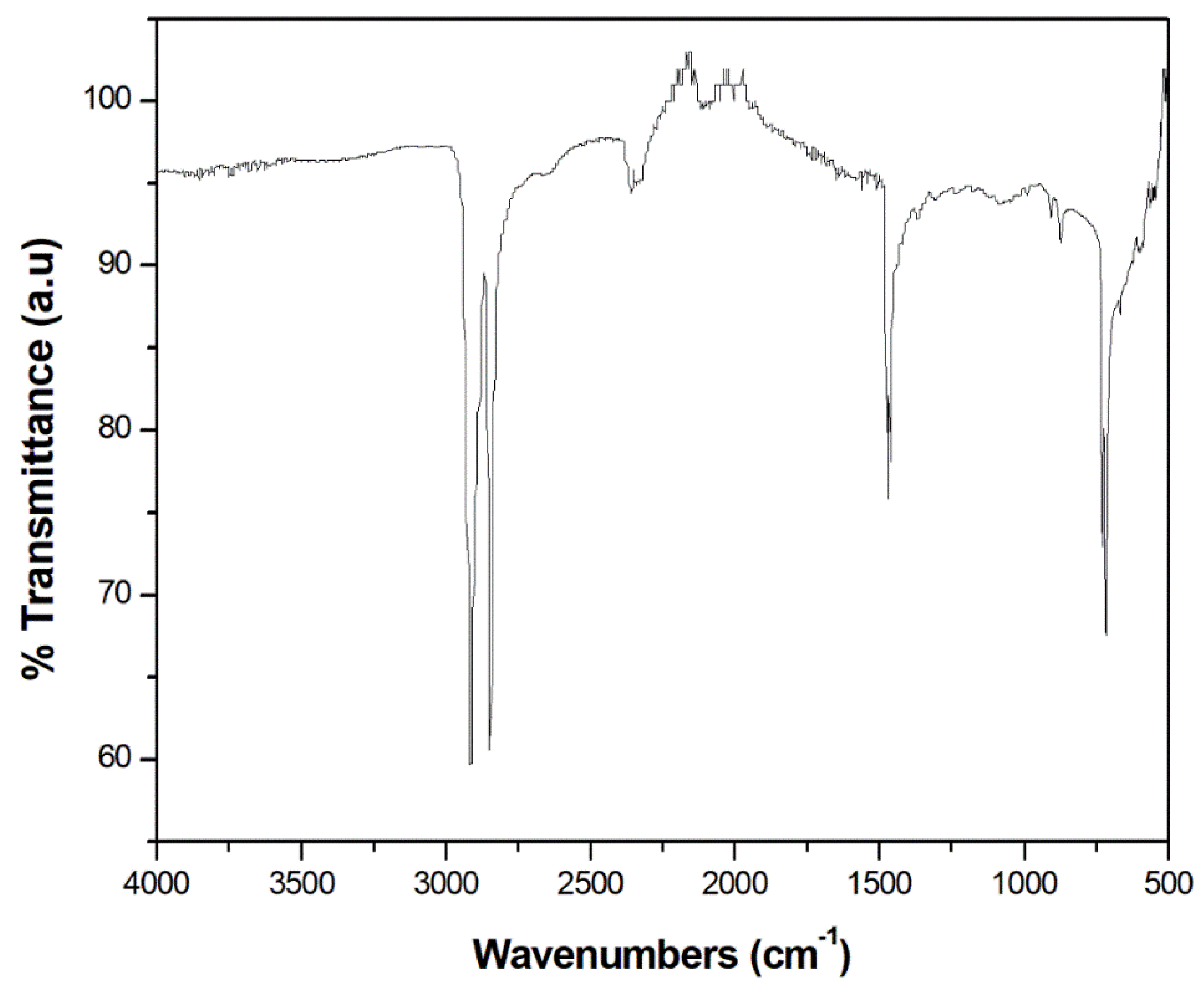
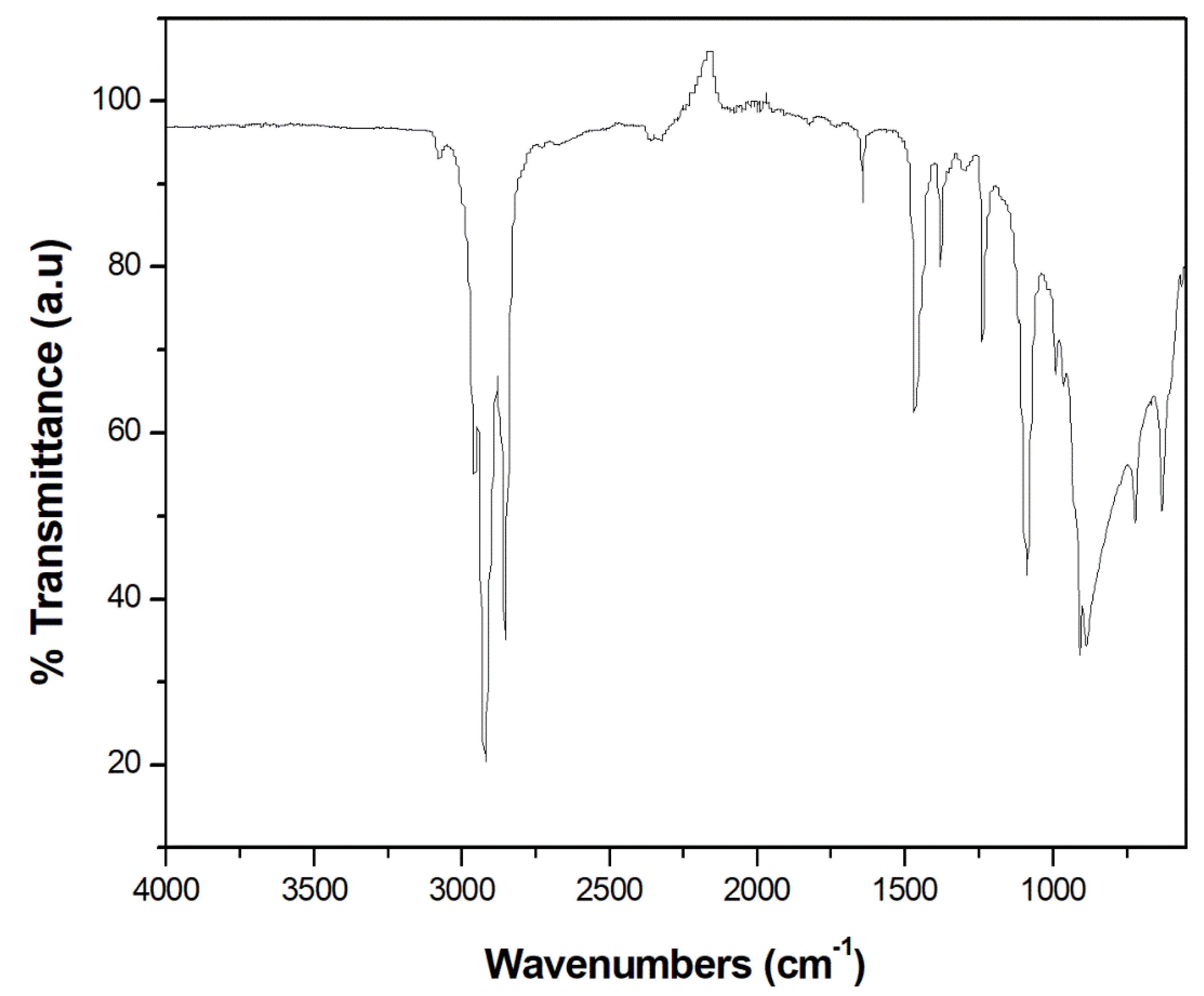
3.1. FTIR spectroscopy
3.2. Oil parameters
- 🠶
- Calorific Value: 43150 J/kg
- 🠶
- Carbon Ratio: 86.75 %
- 🠶
- Hydrogen Ratio: 13.25 %
- 🠶
- Research Octane No. : 80.41
- 🠶
- Motor Octane No. : 66.84
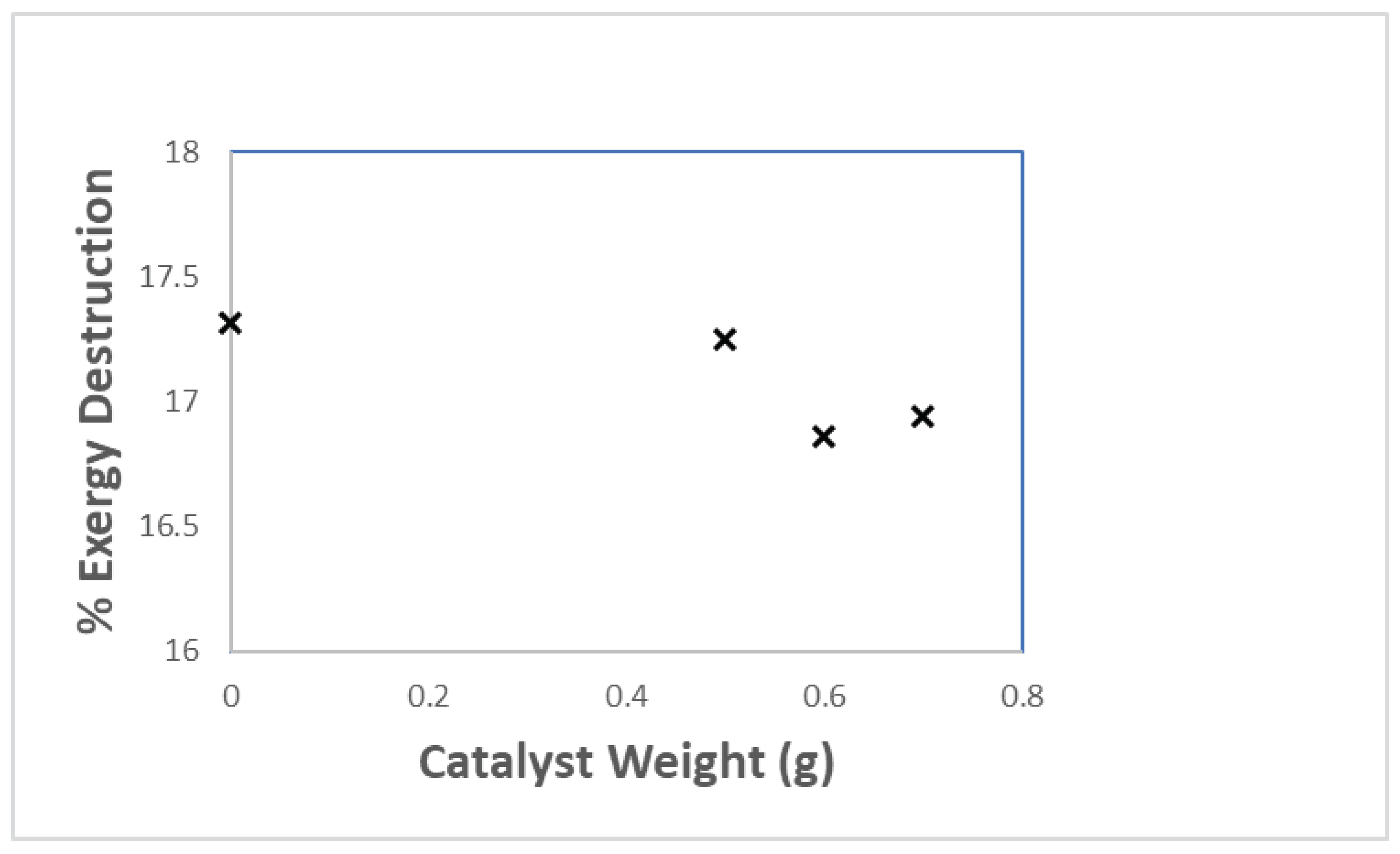
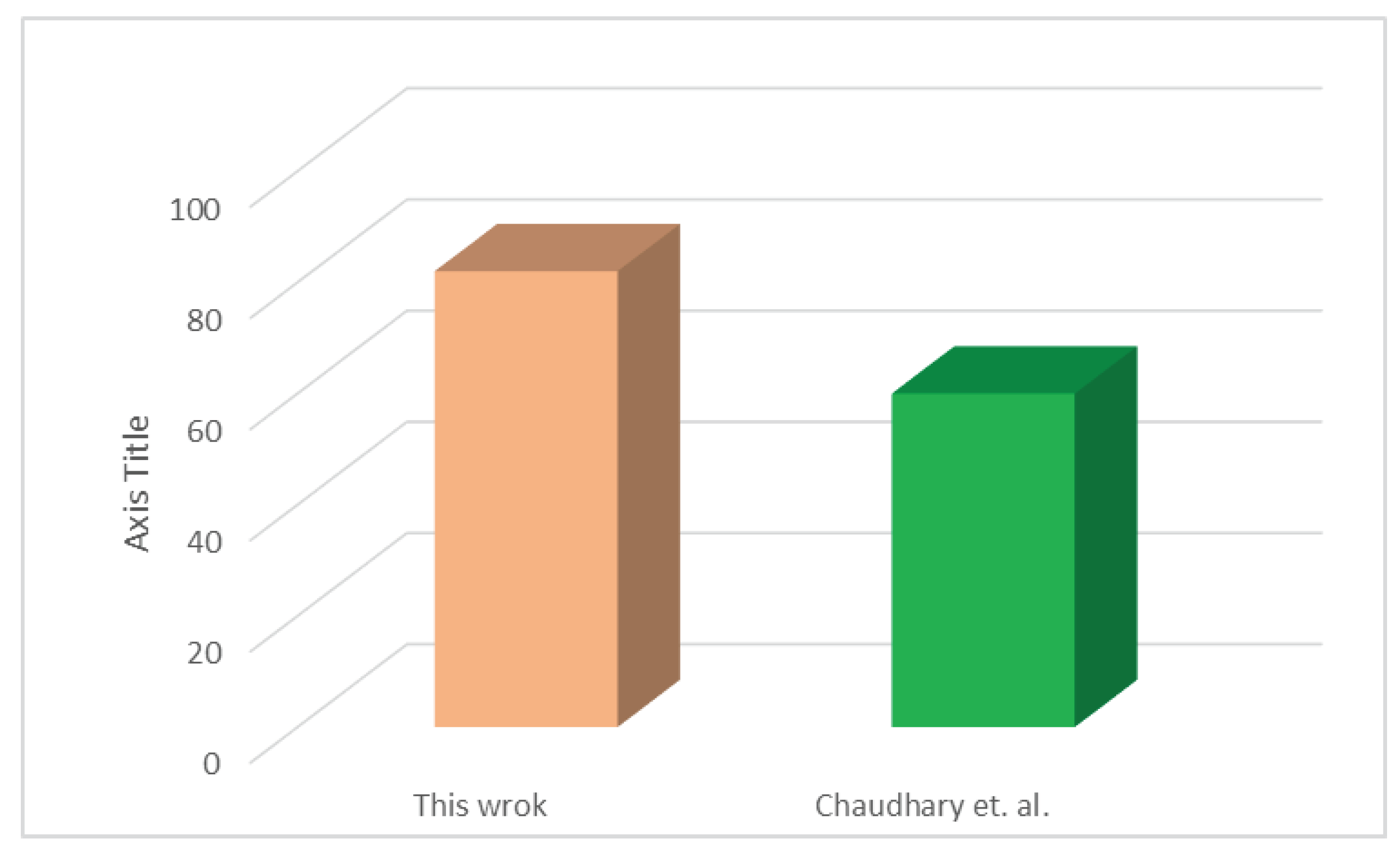
3.3. Exergy analysis
4. Conclusion
Funding
Conflicts of Interest
References
- Worldbank The world bank.
- Nanda, S.; Berruti, F. Municipal solid waste management and landfilling technologies: a review. Environ. Chem. Lett. 2021, 19, 1433–1456. [Google Scholar] [CrossRef]
- Battsetseg, B.; Sukhbaatar, S.; Oyunchimeg, T. CURRENT STATUS AND PROSPECTS OF PLASTIC AND PLASTIC BAG WASTE RECYCLING.
- Moon, Y.; Shim, W.J.; Han, G.M.; Jeong, J.; Cho, Y.; Kim, I.-H.; Kim, M.-S.; Lee, H.-R.; Hong, S.H. What type of plastic do sea turtles in Korean waters mainly ingest? Quantity, shape, color, size, polymer composition, and original usage. Environ. Pollut. 2022, 298, 118849. [Google Scholar] [CrossRef] [PubMed]
- Durak, S.G. Investigation and evaluation of the effect to environmental pollution of plastic shopping bags. Türk Bilim. Derlemeler Derg. 2016, 20–24.
- Suleman, R.; Amjad, A.; Ismail, A.; Javed, S.; Ghafoor, U.; Fahad, S. Impact of plastic bags usage in food commodities: An irreversible loss to environment. Environ. Sci. Pollut. Res. 2022, 29, 49483–49489. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.A.; Chowdhury, S.R.; Mitra, B.; Mozumder, M.S.; Elhaj, A.I.; Salami, B.A.; Rahman, M.M.; Rahman, S.M. Analysis of industrial symbiosis case studies and its potential in Saudi Arabia. J. Clean. Prod. 2023, 385, 135536. [Google Scholar] [CrossRef]
- AL-KALALI, A.; AL-AHMADI, M.A.; AL-BASSAM1, A.M.; RIYADH, S.A. MUNICIPAL SOLID WASTE MANAGEMENT IN SAUDI ARABIA: DRIVERS FOR IMPLEMENTING A CIRCULAR ECONOMY APPROACH. Integr. Waste Manag. Circ. Econ. 2023, 139. [Google Scholar]
- Tiwari, R.; Azad, N.; Dutta, D.; Yadav, B.R.; Kumar, S. A critical review and future perspective of plastic waste recycling. Sci. Total Environ. 2023, 163433. [Google Scholar] [CrossRef]
- Sasikumar, C.; Kannan, R.; Senthilkumar, C.; Sarweswaran, R.; Nagaraja, M.; Sundaresan, R. Pyrolysis of plastic waste for a better environmental system. Mater. Today Proc. 2022, 64, 1679–1684. [Google Scholar] [CrossRef]
- Mishra, R.; Kumar, A.; Singh, E.; Kumar, S. Recent research advancements in catalytic pyrolysis of plastic waste. ACS Sustain. Chem. Eng. 2023, 11, 2033–2049. [Google Scholar] [CrossRef]
- Yang, R.; Jan, K.; Chen, C.; Chen, W.; Wu, K.C. Thermochemical conversion of plastic waste into fuels, chemicals, and value-added materials: A critical review and outlooks. ChemSusChem 2022, 15, e202200171. [Google Scholar] [CrossRef]
- Gezginci, H. Pyrolysıs of polymers.
- Charitopoulou, M.A.; Alexopoulou, E.; Alexiou, P.; Achilias, D.S. Current Topics in Plastic Recycling. In Waste Material Recycling in the Circular Economy-Challenges and Developments; IntechOpen, 2021 ISBN 1839696818.
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Antelava, A.; Jablonska, N.; Constantinou, A.; Manos, G.; Salaudeen, S.A.; Dutta, A.; Al-Salem, S.M. Energy potential of plastic waste valorization: a short comparative assessment of pyrolysis versus gasification. Energy & Fuels 2021, 35, 3558–3571. [Google Scholar]
- Islam, M.K.; Khatun, M.S.; Arefin, M.A.; Islam, M.R.; Hassan, M. Waste to energy: An experimental study of utilizing the agricultural residue, MSW, and e-waste available in Bangladesh for pyrolysis conversion. Heliyon 2021, 7, e08530. [Google Scholar] [CrossRef] [PubMed]
- Jahirul, M.I.; Rasul, M.G.; Schaller, D.; Khan, M.M.K.; Hasan, M.M.; Hazrat, M.A. Transport fuel from waste plastics pyrolysis–A review on technologies, challenges and opportunities. Energy Convers. Manag. 2022, 258, 115451. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manage. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, Y.; Ke, L.; Dai, L.; Wu, Q.; Cobb, K.; Zeng, Y.; Zou, R.; Liu, Y.; Ruan, R. A review on catalytic pyrolysis of plastic wastes to high-value products. Energy Convers. Manag. 2022, 254, 115243. [Google Scholar] [CrossRef]
- Chaudhary, A.; Lakhani, J.; Dalsaniya, P.; Chaudhary, P.; Trada, A.; Shah, N.K.; Upadhyay, D.S. Slow pyrolysis of low-density Poly-Ethylene (LDPE): A batch experiment and thermodynamic analysis. Energy 2023, 263, 125810. [Google Scholar] [CrossRef]
- Wang, X.; Lv, W.; Guo, L.; Zhai, M.; Dong, P.; Qi, G. Energy and exergy analysis of rice husk high-temperature pyrolysis. Int. J. Hydrogen Energy 2016, 41, 21121–21130. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, G.; Ma, D.; Chen, C.; Wang, Y.; Wang, W.; Li, A. Exergy and energy analysis of pyrolysis of plastic wastes in rotary kiln with heat carrier. Process Saf. Environ. Prot. 2020, 142, 203–211. [Google Scholar] [CrossRef]
- Rajkumar, P.; Somasundaram, M. Pyrolysis of residual tyres: Exergy and kinetics of pyrogas. South African J. Chem. Eng. 2022, 42, 53–60. [Google Scholar] [CrossRef]
- Huang, Y.W.; Chen, M.Q.; Li, Q.H.; Xing, W. A critical evaluation on chemical exergy and its correlation with high heating value for single and multi-component typical plastic wastes. Energy 2018, 156, 548–554. [Google Scholar] [CrossRef]
- Hernandez, A.G.; Cullen, J.M. Exergy: A universal metric for measuring resource efficiency to address industrial decarbonisation. Sustain. Prod. Consum. 2019, 20, 151–164. [Google Scholar] [CrossRef]
- Szargut, J. Exergy method: technical and ecological applications; WIT press, 2005; Vol. 18; ISBN 1853127531.
- Bejan, A.; Tsatsaronis, G.; Moran, M.J. Thermal design and optimization; John Wiley & Sons, 1995; ISBN 0471584673.
- Kotas, T.J. The exergy method of thermal plant analysis; Paragon Publishing, 2012; ISBN 1908341890.
- Ahmed Al Ghamdi, I.M. Exergy analysis of a seawater reverse osmosis plant in Jeddah, Saudi Arabia.
- Sato, N. Chemical energy and exergy: an introduction to chemical thermodynamics for engineers; Elsevier, 2004; ISBN 044451645X.
- Michalakakis, C.; Fouillou, J.; Lupton, R.C.; Gonzalez Hernandez, A.; Cullen, J.M. Calculating the chemical exergy of materials. J. Ind. Ecol. 2021, 25, 274–287. [Google Scholar] [CrossRef]
- Rant, Z. Towards the estimation of specific exergy of fuels. Allg. Wärmetech 1961, 10, 172–176. [Google Scholar]
- Blanco-Marigorta, A.M.; Lozano-Medina, A.; Marcos, J.D. A critical review of definitions for exergetic efficiency in reverse osmosis desalination plants. Energy 2017, 137, 752–760. [Google Scholar] [CrossRef]
- Cengel, Y.A.; Boles, M.A.; Kanoğlu, M. Thermodynamics: an engineering approach; McGraw-hill New York, 2011; Vol. 5;
- Uthpalani, P.G.I.; De Silva, D.S.M.; Weerasinghe, V.P.A.; Premachandra, J.K.; Sarathchandra, T. V Pyrolysis of waste LDPE and waste PP plastics into fuel oil in a low-cost, lab-scale pyrolyzing unit. J. Sci. Univ. Kelaniya 2023, 16. [Google Scholar] [CrossRef]
- Abnisa, F. Enhanced Liquid Fuel Production from Pyrolysis of Plastic Waste Mixtures Using a Natural Mineral Catalyst. Energies 2023, 16, 1224. [Google Scholar] [CrossRef]
- Krieg, T.; Mazzon, C.; Gómez-Sánchez, E. Material analysis and a visual guide of degradation phenomena in historical synthetic polymers as tools to follow ageing processes in industrial heritage collections. Polymers (Basel). 2022, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, J.; Robinson, Y.; Ganapathi, P. Experimental investigation of performance, emission and combustion characteristics of waste plastic pyrolysis oil blended with diethyl ether used as fuel for diesel engine. Energy 2015, 85, 304–309. [Google Scholar] [CrossRef]
- Thahir, R.; Altway, A.; Juliastuti, S.R. Production of liquid fuel from plastic waste using integrated pyrolysis method with refinery distillation bubble cap plate column. Energy reports 2019, 5, 70–77. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Chandrasekaran, S.R.; Dutta, A.; Sharma, B.K. Study of the fuel properties of extracted oils obtained from low and linear low density polyethylene pyrolysis. Fuel 2021, 304, 121396. [Google Scholar] [CrossRef]
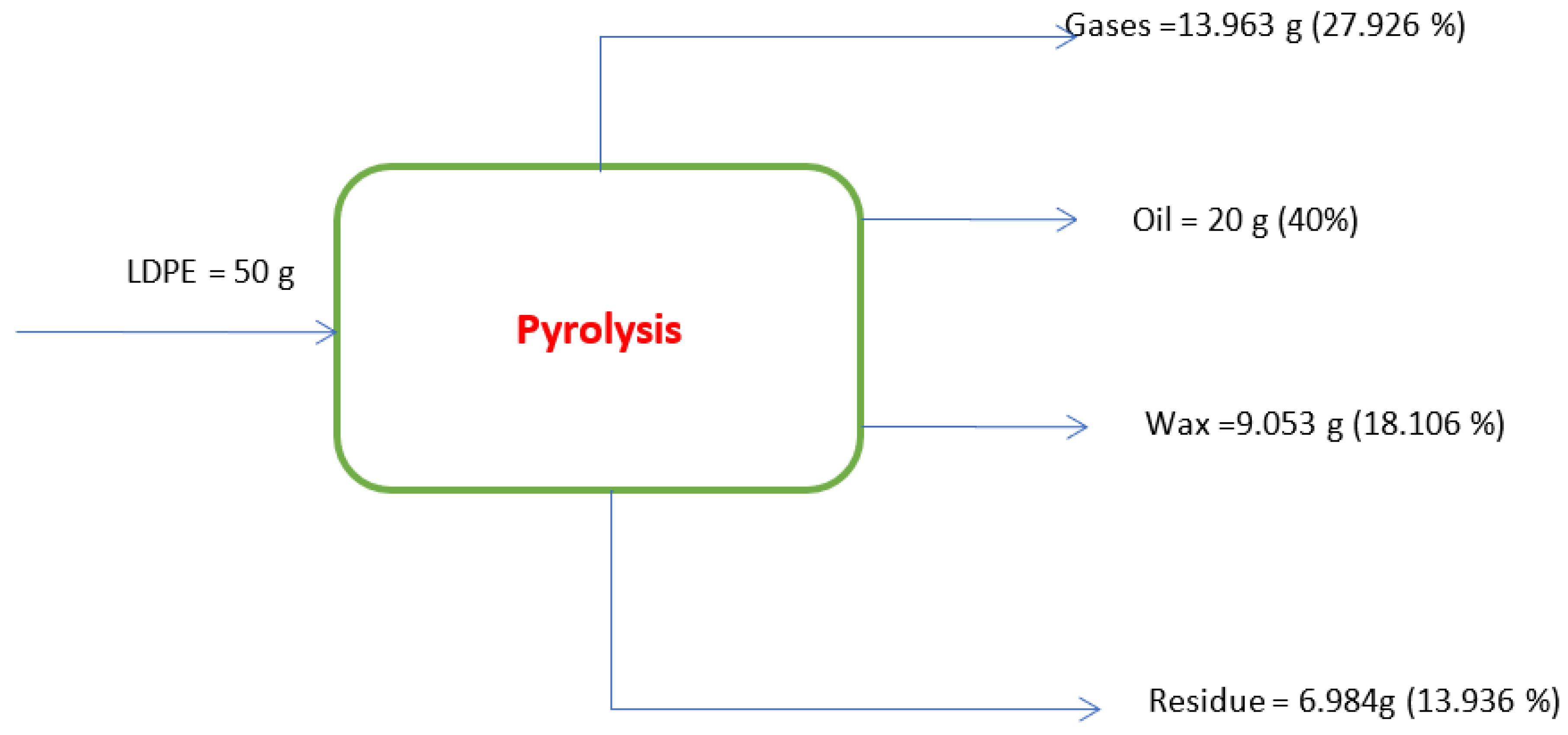
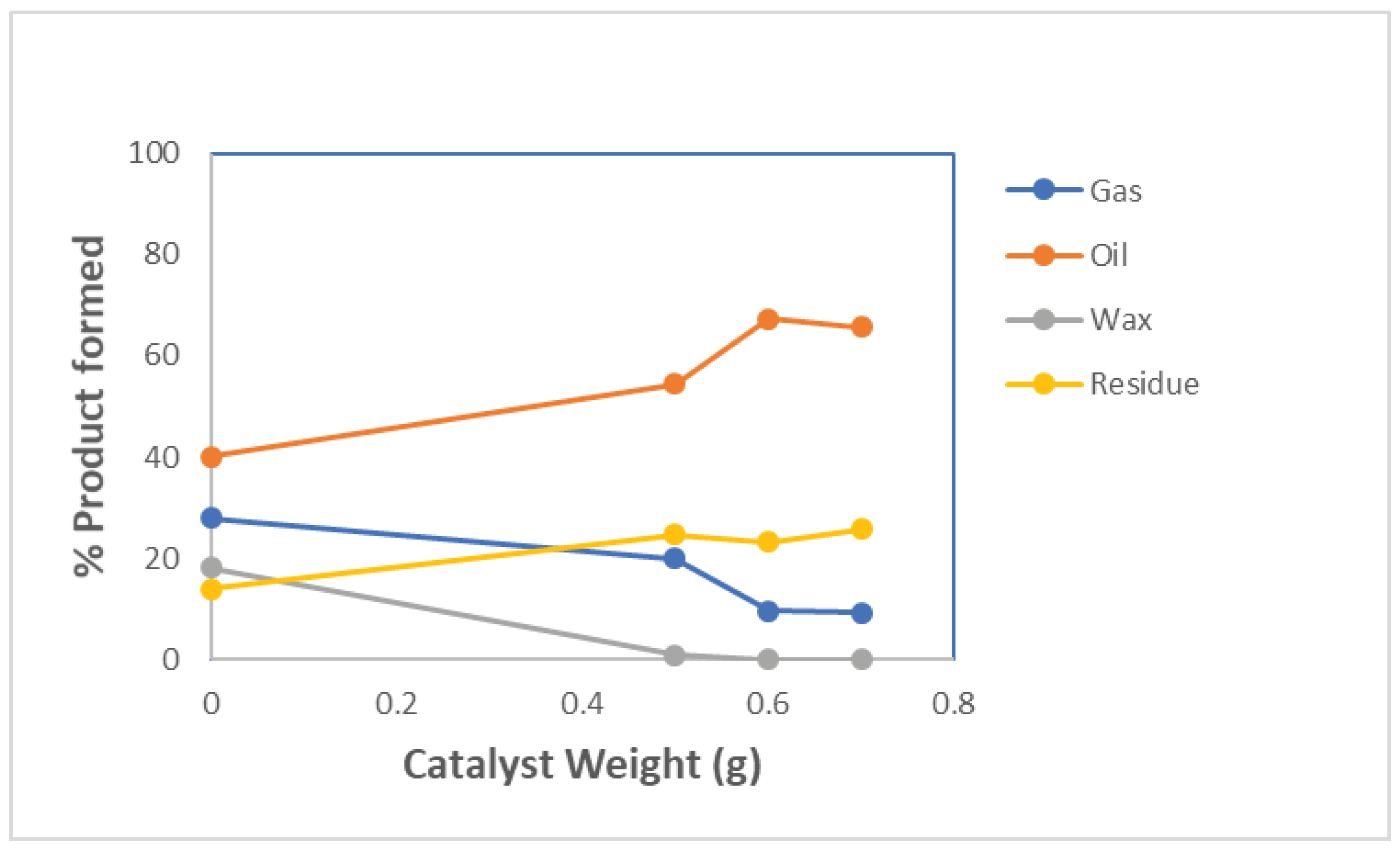
| Weight of Catalyst(g) | Gas (%) | Oil (%) | Wax (%) | Residue (%) |
|---|---|---|---|---|
| 0 | 27.926 | 40 | 18.106 | 13.936 |
| 0.5 | 20.008 | 54.434 | 0.858 | 24.7 |
| 0.6 | 9.546 | 67.31 | 0 | 23.144 |
| 0.7 | 9.322 | 65 | 0 | 25.678 |
| Wave number (cm-1) | Type of vibration | Nature of functional group |
| 3076 | =C-H stretching | Aromatics |
| 2956, 2954 | -C-H stretching | Alkane |
| 2922 | -C-H stretching | Alkane |
| 2852 | -C-H stretching | Alkane |
| 1641 | -C=C stretching | Alkene/ fingerprint region for phenyl ring substitution overtone |
| 1465 | -C=C stretching | Alkanes with methyl groups with C-H bending vibration |
| 1377 | -C-H scissoring and bending | Alkane |
| 1236 | -CH2 group bending | Alkane |
| 1088 | In-plane deformation | Aromatics |
| 991 | -C-H out-of-plane vibration | Alkane |
| 908 | =C-H bending | Alkene |
| 887 | -C-H out of plane bending | Alkene |
| 721 | -C-H rock | Alkene |
| 630 | -CH=CH2 twisting vibration | Alkene |
| Component | % (w/w) | % (v/v) |
| Benzene | 0.06 | 0.06 |
| Saturates | 41.36 | 43.54 |
| Olefins | 18.66 | 20.30 |
| Aromatics | 39.98 | 36.16 |
| Physical exergy (kJ) | Chemical exergy (kJ) | Total exergy (kJ) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catalyst loading (g) | Catalyst loading (g) | Catalyst loading (g) | ||||||||||
| 0 | 0.5 | 0.6 | 0.7 | 0 | 0.5 | 0.6 | 0.7 | 0 | 0.5 | 0.6 | 0.7 | |
| gas | 2.744 | 1.960 | 0.931 | 0.912 | 605.150 | 432.25 | 205.319 | 200.996 | 607.894 | 434.210 | 206.25 | 201.908 |
| oil | 6.098 | 8.309 | 10.260 | 10.016 | 846.300 | 1153.084 | 1423.9 | 1390.048 | 852.398 | 1161.0 | 1434.16 | 1400.064 |
| wax | 1.952 | 0.093 | 0 | 0 | 302.922 | 14.393 | 0 | 0 | 304.874 | 14.486 | 0 | 0 |
| carbon | 5.310 | 9.414 | 8.864 | 9.819 | 197.380 | 349.888 | 329.44 | 364.94 | 202.690 | 359.302 | 338.304 | 374.759 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





