1. Introduction
HER2 (human epidermal growth factor receptor 2) also called ERBB2, is a member of the ERBB family tyrosine kinase receptors for epidermal growth factor (EGF) family. Unlike other HER families, HER2 does not possess ligands and cannot form ligand-induced homodimers. To activate the downstream signaling of HER2, the heterodimer formation with other HER members in the presence of their specific ligands or ligand-independent homodimer formation under the overexpressed condition are required [
1]. After the homo- and heterodimer formation, HER2 activates the downstream signaling pathways such as the mitogen-activated protein kinases, the phosphoinositide 3-kinase/Akt, and the phospholipase C-γ/protein kinase C pathways [
2].
HER2 is overexpressed in ~20% of breast cancers and is associated with poor prognosis including a higher recurrence rate and shorter overall survival [
3]. Trastuzumab, an anti-HER2 monoclonal antibody (mAb), exhibited a growth inhibitory effect
in vitro and a potent antitumor effect
in vivo [
4]. The combination of trastuzumab with chemotherapy improves the response rates, progression-free survival, and overall survival in HER2-overexpressing breast cancer patients with metastasis [
5]. Trastuzumab has been a standard therapy for HER2-overexpressing breast cancers for more than 20 years [
6]. In the clinic, the HER2-overexpressing tumors are diagnosed by strong and complete immunohistochemistry (IHC) membranous staining of more than 10% of cells (IHC 3+) and/or
in situ hybridization (ISH)-amplified.
Trastuzumab-deruxtecan (T-DXd) is a trastuzumab-based antibody-drug conjugate (ADC). The cytotoxicity is achieved through the released topoisomerase I inhibitor, DXd following the endocytosis and enzymatic cleavage of T-DXd [
7]. T-DXd first showed beneficial results in metastatic breast cancer patients who had received multiple anti-HER2-targeting treatments [
8]. Currently, various clinical trials have evaluated the efficacy of T-DXd. Based on the studies, T-DXd has been approved in not only HER2-overexpressing breast cancer [
9,
10,
11] but also HER2-low (IHC 1+ or IHC 2+ / ISH-non-amplified) advanced breast cancer [
12].
Since about half of all breast cancers are classifiable as HER2-low [
13], a larger number of patients will reap the benefits of T-DXd treatment. The future clinical diagnostics of HER2-low breast cancer is thought to be important to maximize the patient's benefit from treatment. Furthermore, novel modalities against HER2 will be developed and evaluated in preclinical and clinical studies [
14]. Therefore, preclinical mouse models are important for the evaluation of novel modalities and the prediction of adverse effects.
Although the therapeutic modalities for tumors have been revolutionary developed, the number of newly approved cancer therapies is limited. Most failure is due to the lack of efficacy [
15], suggesting that the current preclinical methods are not sufficient to predict successful outcomes. Preclinical mouse models are essential for the development of cancer therapy. The first models were established through the transplantation of murine tumors into immunocompetent host mice [
16]. These models were successful preclinical ones for the evaluation of a chemotherapeutic agent before clinical trials [
17]. Furthermore, tumors derived from genetically modified mice can be maintained in fully immunocompetent syngeneic hosts [
15]. These syngeneic models were used in preclinical studies to evaluate not only molecular target therapies but also immunotherapies including immune checkpoint inhibitors [
15]. However, a preclinical model using anti-mouse HER2 (mHER2) mAb has not been reported.
We have developed the Cell-Based Immunization and Screening (CBIS) method which includes antigen-overexpressing cell immunization and high-throughput screening of hybridoma supernatants using flow cytometry. Using the CBIS method, we have developed mAbs against HER families, including human EGFR [
18], HER2 [
19], HER3 [
20], and mouse EGFR [
21]. In this study, we developed novel anti-mHER2 mAbs using the CBIS method and evaluated its application to flow cytometry.
2. Materials and Methods
2.1. Preparation of cell lines
LN229, Chinese hamster ovary (CHO)-K1, NMuMG (a mouse mammary gland epithelial cell), and P3X63Ag8U.1 (P3U1) were obtained from the American Type Culture Collection (Manassas, VA, USA).
The cDNA encoding mHER2 (Accession No.: NM_001003817) with C-terminal mycDDK in a pCMV6 vector (cat# MR227307) was purchased from OriGene Technologies, Inc. (Rockville, MD, USA). The mHER2 plasmid was transfected into CHO-K1 and LN229 cells, using a Neon transfection system (Thermo Fisher Scientific Inc., Waltham, MA, USA). Stable transfectants were established through cell sorting using a cell sorter, SH800 (Sony Corp., Tokyo, Japan) using an anti-mouse ErbB2/Her2 mAb (clone 666521, R&D systems, Minneapolis, MN, USA), after which cultivation in a medium, containing 0.5 mg/mL of G418 (Nacalai Tesque, Inc., Kyoto, Japan) was conducted. The mHER2 knockout NMuMG (BINDS-51) was generated by transfecting 10 μg of CRISPR/Cas9 plasmids for mHER2 (Guide RNA: AGAGGTTGCGCATCGTGAGA, Thermo Fisher Scientific, Inc.) into NMuMG cells using the Neon transfection system and established by cell sorting as described above.
CHO-K1, mHER2-overexpressed CHO-K1 (CHO/mHER2), and P3U1 cells were cultured in a Roswell Park Memorial Institute (RPMI)-1640 medium (Nacalai Tesque, Inc.), with 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific Inc.), 100 units/mL of penicillin, 100 μg/mL of streptomycin, and 0.25 μg/mL of amphotericin B (Nacalai Tesque, Inc.). LN229 and mHER2-overexpressed LN229 (LN229/mHER2), NMuMG, and BINDS-51 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Nacalai Tesque, Inc.), supplemented with 10% FBS, 100 U/mL of penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B.
All cells were grown in a humidified incubator at 37°C, at an atmosphere of 5% CO2 and 95% air.
2.2. Production of hybridomas
A five-week-old Sprague–Dawley rat was purchased from CLEA Japan (Tokyo, Japan). The animal was housed under specific pathogen-free conditions. All animal experiments were approved by the Animal Care and Use Committee of Tohoku University (Permit number: 2022MdA-001).
To develop mAbs against mHER2, we intraperitoneally immunized one rat with LN229/mHER2 (1 × 10
9 cells) plus Imject Alum (Thermo Fisher Scientific Inc.). After three additional injections every week (1 × 10
9 cells/rat), a final booster injection (1 × 10
9 cells/rat) was performed two days before harvesting spleen cells. The hybridomas were produced, as described previously [
22]. The hybridoma supernatants were screened using flow cytometry using CHO/mHER2, CHO-K1, and NMuMG.
2.3. Purification of mAbs
The cultured supernatants of H2Mab-300 and H2Mab-304-producing hybridomas were collected and filtered using Steritop (0.22 μm, Merck KGaA, Darmstadt, Germany). The supernatants were applied to 1 mL of Ab-Capcher (ProteNova, Kagawa, Japan). After washing with phosphate-buffered saline (PBS), the antibodies were eluted with an IgG elution buffer (Thermo Fisher Scientific Inc.). Finally, the eluates were concentrated, and the elution buffer was replaced with PBS using Amicon Ultra (Merck KGaA, Darmstadt, Germany).
2.4. Flow cytometric analysis
CHO-K1, CHO/mHER2, and NMuMG cells were harvested after a brief exposure to 0.25% trypsin and 1 mM ethylenediaminetetraacetic acid (EDTA, Nacalai Tesque, Inc.). The cells were subsequently washed with 0.1% bovine serum albumin (BSA) in PBS and treated with 0.001, 0.01, 0.1, and 1 μg/mL of primary mAbs for 30 min at 4°C. The cells were treated with Alexa Fluor 488-conjugated anti-rat IgG or Alexa Fluor 488-conjugated anti-mouse IgG (1:2000). The fluorescence data were collected using the SA3800 Cell Analyzer (Sony Corp.).
2.5. Determination of dissociation constant (KD) through flow cytometry
CHO/mHER2 and NMuMG cells were suspended in 100 μL serially-diluted H2Mab-300, H2Mab-304, and clone 666521 for 30 min at 4°C. The cells were treated with 50 μL of Alexa Fluor 488-conjugated anti-rat IgG (1:200). The GeoMean of each histogram including primary mAb + secondary Ab (Alexa Fluor 488-conjugated anti-rat IgG) and only secondary Ab (for background) was determined. We further withdrew the background from each data and determined the apparent KD by the fitting binding isotherms to built-in one-site binding models of GraphPad Prism 8 (GraphPad Software, Inc., La Jolla, CA, USA).
3. Results
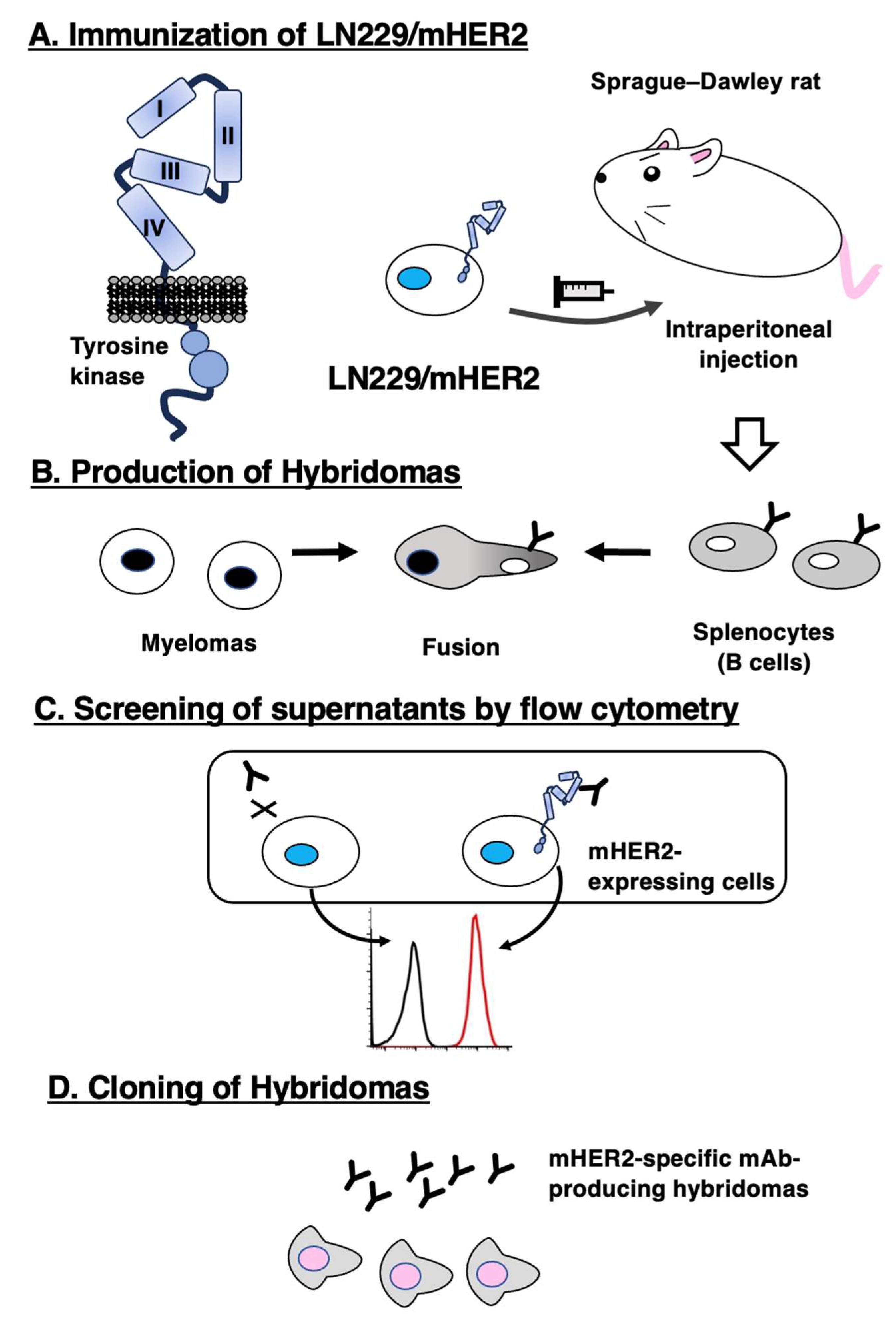
3.1. Development of anti-mHER2 mAbs by the CBIS method
To develop anti-mHER2 mAbs, one rat was immunized with LN229/mHER2 cells. (
Figure 1A). The spleen was then excised from the rat, and splenocytes were fused with myeloma P3U1 cells (
Figure 1B). The developed hybridomas were subsequently seeded into ten 96-well plates and cultivated for six days. The positive wells were screened by the selection of mHER2-expressing cell-reactive and CHO-K1-non-reactive supernatants, using flow cytometry (
Figure 1C). After the limiting dilution and several additional screenings, anti-mHER2 mAbs, such as H
2Mab-300 (rat IgG
2b, kappa) and H
2Mab-304 (rat IgG
1, kappa) were finally established (
Figure 1D).
3.2. Flow cytometric analyses
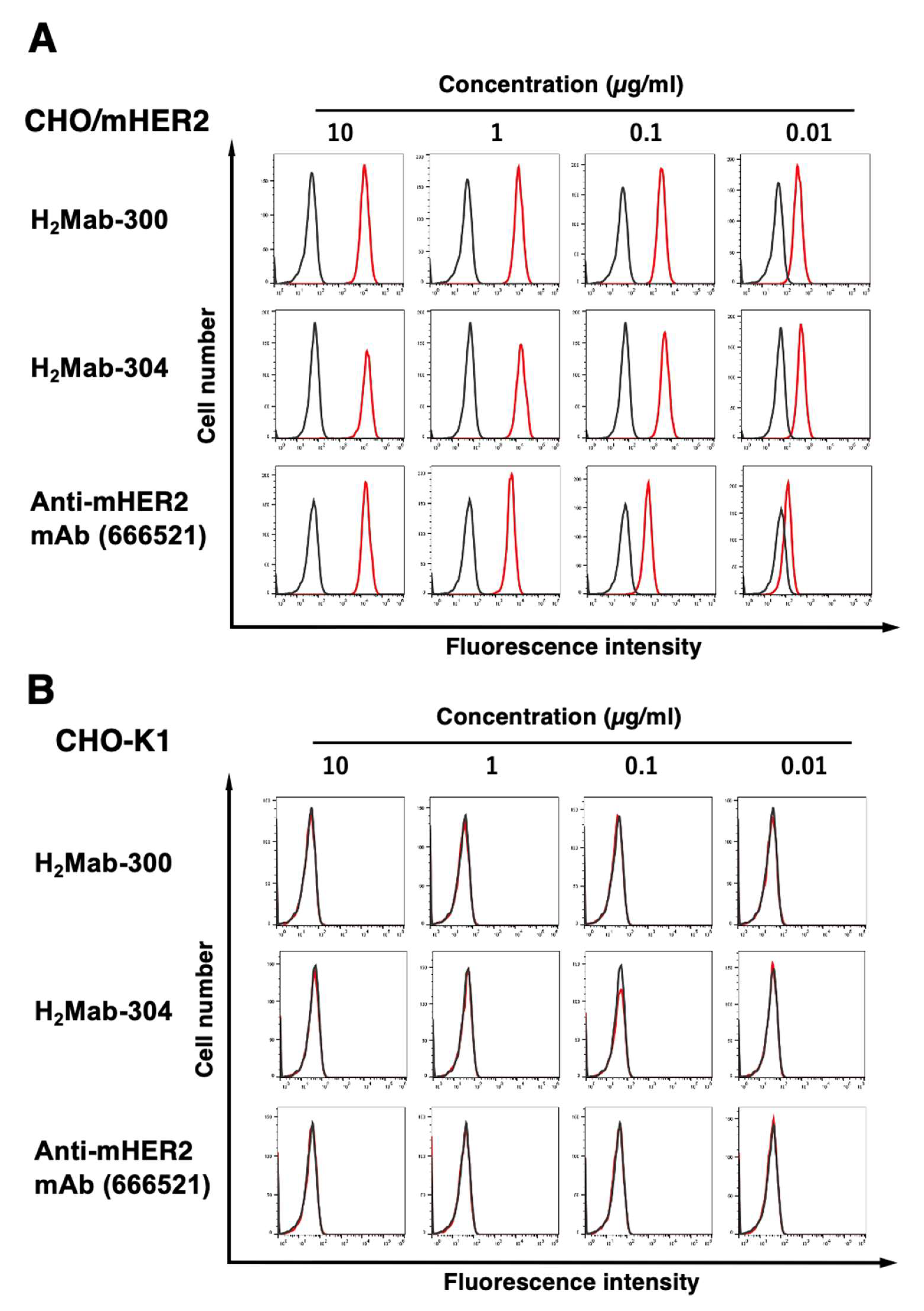
We conducted flow cytometry using three anti-mHER2 mAbs, H
2Mab-300, H
2Mab-304, and a commercially available clone (666521) against CHO/mHER2. As shown in
Figure 2A, both H
2Mab-300 and H
2Mab-304 recognized CHO/mHER2 cells dose-dependently at 10, 1, 0.1, and 0.01 μg/mL. In contrast, 666521 exhibited low reactivity against CHO/mHER2 compared to H
2Mab-300, H
2Mab-304. Parental CHO-K1 cells were not recognized even at 10 μg/mL of all mAbs (
Figure 2B).
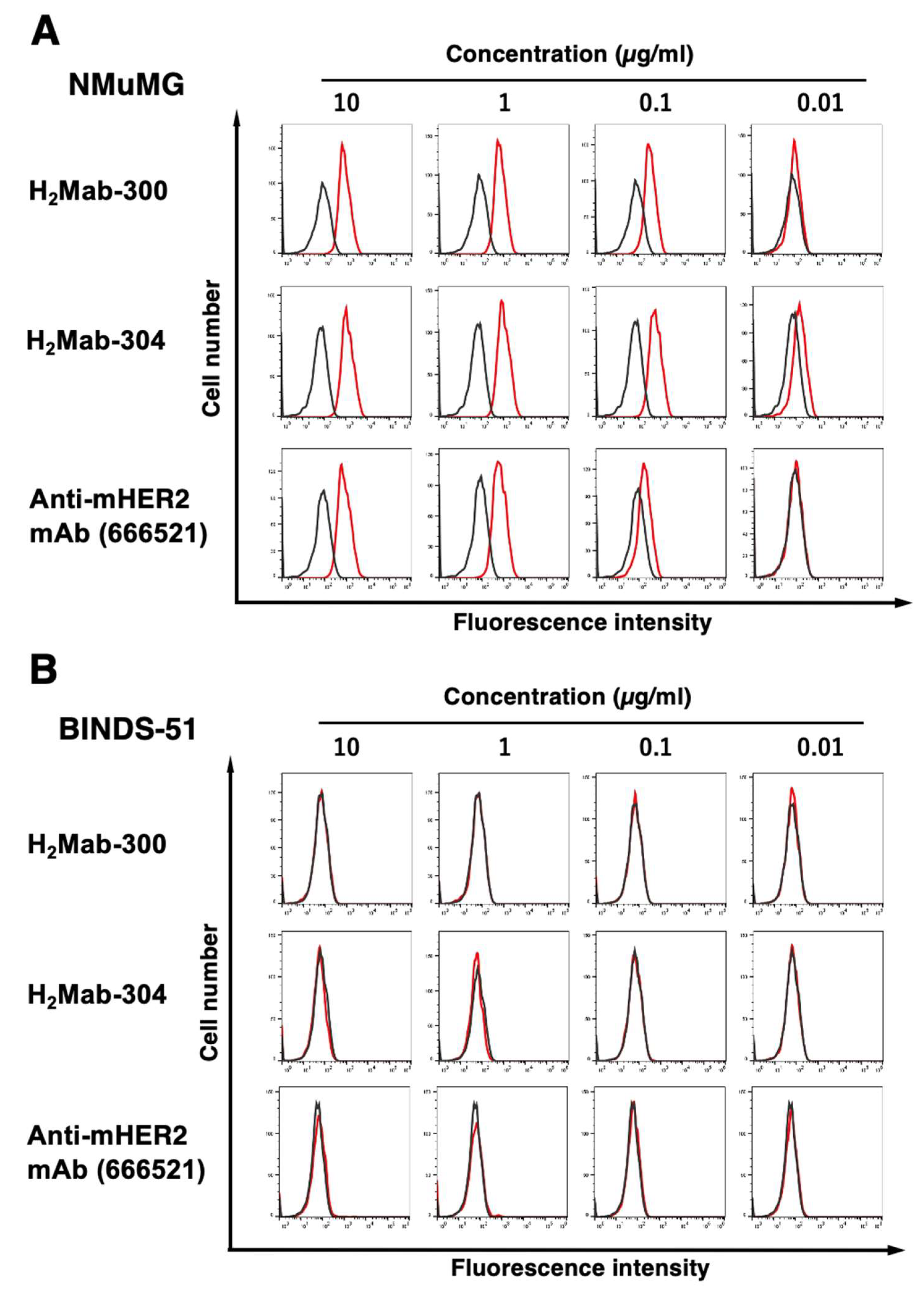
We next investigated the reactivity of H
2Mab-300, H
2Mab-304, and 666521 against an endogenously mHER2-expressed cell line, NMuMG (a mouse mammary gland epithelial cell). The three mAbs reacted with NMuMG at more than 0.1 μg/mL (
Figure 3A). H
2Mab-300 and 666521 could not react with NMuMG at 0.01 μg/mL. In contrast, H
2Mab-304 could react with NMuMG at 0.01 μg/mL. Furthermore, we could not detect the reactivity against mHER2 knockout NMuMG (BINDS-51,
Figure 3B) in the three mAbs. These results indicated that H
2Mab-300 and H
2Mab-304 specifically recognize mHER2, and are also useful for detecting endogenous mHER2 by flow cytometry.
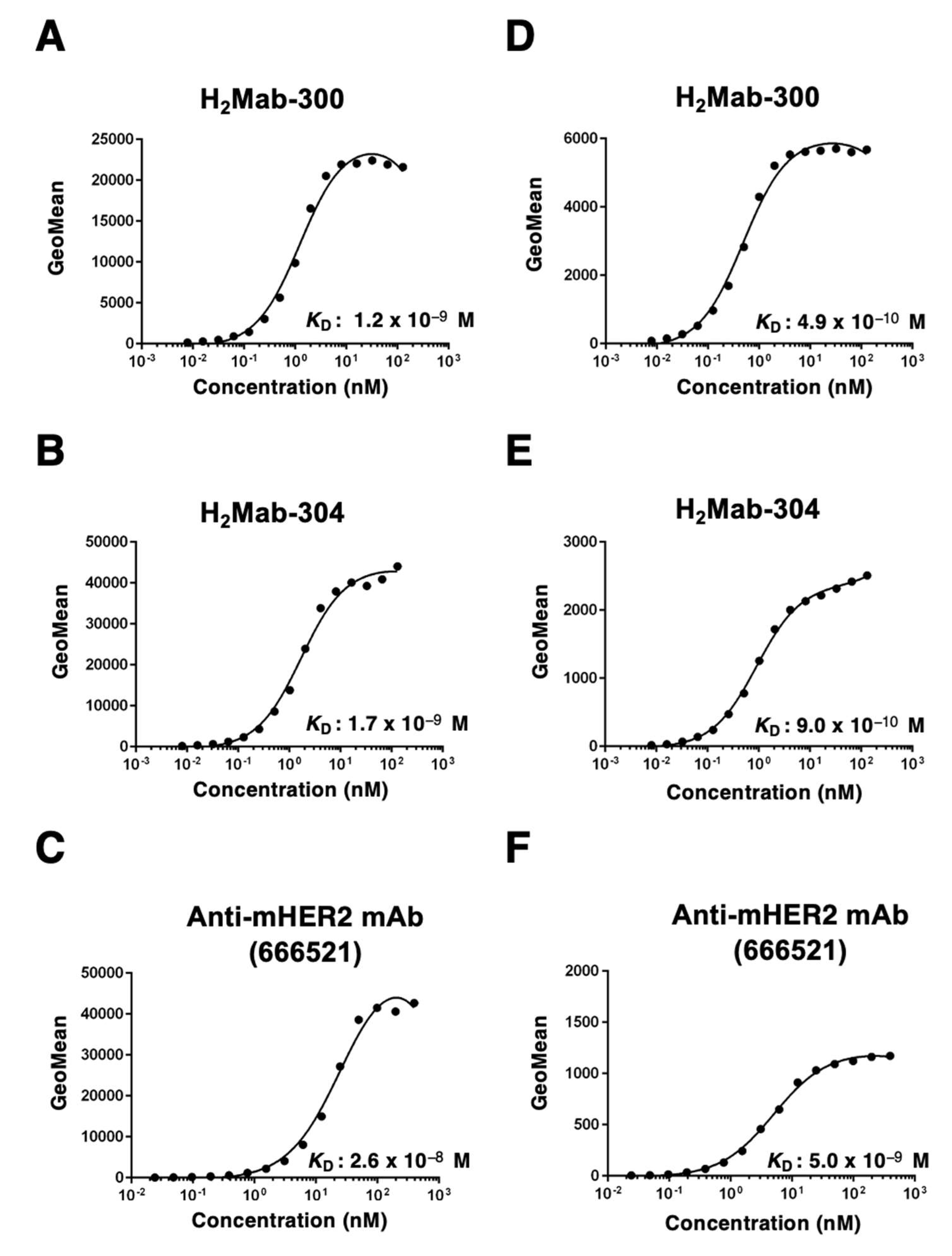
3.3. Kinetic analyses against mHER2-expressing cells using flow cytometry
To determine the KD of H2Mab-300 and H2Mab-304 with mHER2-expressing cells, we conducted kinetic analysis by flow cytometry using CHO/mHER2 and NMuMG cells. The geometric mean of the fluorescence intensity was plotted versus the concentration of H2Mab-300 and H2Mab-304. The KD values of H2Mab-300, H2Mab-304, and 666521 for CHO/mHER2 were determined as 1.2 × 10−9 M, 1.7 × 10−9 M, and 2.6 × 10−8 M, respectively (Fig. 4A-C). Furthermore, the KD values of H2Mab-300, H2Mab-304, and 666521 for NMuMG were determined as 4.9 × 10−10 M, 9.0 × 10−10 M and 5.0 × 10−9 M, respectively (Fig. 4D-F). These results indicated that H2Mab-300 and H2Mab-304 possess the high affinity to CHO/mHER2 and NMuMG cells compared to 666521, which was established using recombinant mHER2 as an antigen.
4. Discussion
The HER2 extracellular domain is composed of four domains (I–IV) (
Figure 1A). The domain II is essential for the formation of heterodimers with other HER members including EGFR, HER3, and HER4 in the presence of their ligands, such as EGF [
23] and neuregulin 1 (NRG1, a HER3 ligand) [
24]. The clinically approved anti-HER2 mAbs, trastuzumab and pertuzumab, recognize domains IV and II, respectively [
25,
26]. Pertuzumab was shown to inhibit the NRG1-induced heterodimerization with HER3 and intracellular signaling [
25]. In this study, we developed a novel anti-mHER2 mAb (H
2Mab-300 and 304) using the CBIS method and showed the application to flow cytometry (
Figure 2,
Figure 3 and
Figure 4). We should identify these epitopes and determine the property of mAbs including the effect on the homodimer and heterodimer formation of mHER2. We established the RIEDL insertion for epitope mapping (REMAP) method [
27,
28,
29,
30] to identify the conformational epitope because most of the mAbs developed by the CBIS method recognized the conformational epitopes. We determined the conformational epitopes of anti-human EGFR mAbs (EMab-51 and EMab-134) [
28,
30] using the REMAP method.
In a preclinical study, the antitumor activity of anti-HER2 mAbs have been evaluated by the HER2-positive tumor-bearing immunodeficient mice [
31] or HER2-overexpressed murine breast cancer-transplanted syngeneic models [
32]. Furthermore, mouse mammary tumor virus (MMTV)-driven overexpression of HER2 was generated to investigate the roles of HER2 in mammary adenocarcinoma development and therapeutic effects of anti-HER2 mAb such as 4D5 (murine version of trastuzumab) [
33]. The incidence of mammary tumors and tumor growth were significantly reduced in 4D5-treated mice [
34].
Currently, targetable HER2-positive tumors are expanded from IHC3+ to HER2-low in breast cancer, suggesting that other tumor types could apply to HER2-targeting therapy. Pancreatic cancer is a difficult-to-treat disease with critical
unmet needs. HER2 expression is also observed in pancreatic cancers and is associated with poor prognosis [
35]. Recently, new drugs that target KRAS
G12D, a common mutation in PDAC, have been developed [
36]. One of the drugs, MRTX1133 exhibited specific efficacy in tumor cells harboring KRAS
G12D mutations [
37,
38]. However, MRTX1133 promoted the expression and activation of ERBB receptors including HER2, which is expected as a mechanism of resistance to MRTX1133 [
39]. Similar ErbB receptors upregulation was also observed in Kras
+/LSL-G12D; Trp53
+/LSL-R172H; Pdx1-Cre (KPC) mice, a murine model of pancreatic cancer [
40], treated with MEK and AKT inhibitors [
41]. Therefore, specific kinase inhibitors or mAbs against ErbB receptors could potentiate the efficacy of the treatment. H
2Mab-300 and H
2Mab-304 may be applied to obtain the proof of concept in the above mouse model system. For the preclinical study, the conversion from H
2Mab-300 and H
2Mab-304 (rat IgG) to mouse IgG is essential to evaluate the efficacy in mice.
A major adverse effect of anti-HER2 therapeutic mAbs is cardiotoxicity, which is potentiated by co-treatment with chemotherapeutic agents. The cardiotoxic surveillance by routine cardiac monitoring is essential in clinic [
42]. Furthermore,
ErbB2-deficient mice exhibited embryonic lethal due to cardiac dysfunctions [
43]. Mice lacking
ErbB2 in ventricular displayed the characteristics of dilated cardiomyopathy [
44]. These results indicated that HER2 is essential in normal heart development and homeostasis. However, the detailed mechanism of cardiotoxicity has not been investigated. Therefore, H
2Mab-300 and H
2Mab-304 could be used to analyze the cardiotoxicity in various combination with chemotherapeutic agents in mice. Furthermore, clinical trials combining immunotherapies with HER2-targeted therapies have been conducted [
14]. H
2Mab-300 and H
2Mab-304 are also important to predict the side effect of the treatment.
5. Conclusion
H2Mab-300 and 304 could contribute to the preclinical study to obtain the proof of concept of antitumor effects and predict the side effects of HER2-targeting mAb therapy.
Author Contributions
T.O., and T.T. performed the experiments. M.K.K. and Y.K. designed the experiments. T.O., H.S. and Y.K. analyzed the data. H.S. and Y.K. wrote the manuscript. All authors read and approved the final manuscript and agreed to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work is appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by the Japan Agency for Medical Research and Development (AMED) under grant numbers: JP23ama121008 (to Y.K.), JP23am0401013 (to Y.K.), and JP23ck0106730 (to Y.K.); and by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) grant numbers 22K06995 (to H.S.), 21K07168 (to M.K.K.), and 22K07224 (to Y.K.).
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care and Use Committee of Tohoku University (Permit number: 2022MdA-001) for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All related data and methods are presented in this paper. Additional inquiries should be addressed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest involving this article.
References
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Cooper, A.J.; Sequist, L.V.; Lin, J.J. Third-generation EGFR and ALK inhibitors: mechanisms of resistance and management. Nat Rev Clin Oncol 2022, 19, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Tsao, L.C.; Force, J.; Hartman, Z.C. Mechanisms of Therapeutic Antitumor Monoclonal Antibodies. Cancer Res 2021, 81, 4641–4651. [Google Scholar] [CrossRef]
- Essadi, I.; Benbrahim, Z.; Kaakoua, M.; Reverdy, T.; Corbaux, P.; Freyer, G. HER2-Positive Metastatic Breast Cancer: Available Treatments and Current Developments. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Takegawa, N.; Nonagase, Y.; Yonesaka, K.; Sakai, K.; Maenishi, O.; Ogitani, Y.; Tamura, T.; Nishio, K.; Nakagawa, K.; Tsurutani, J. DS-8201a, a new HER2-targeting antibody-drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int J Cancer 2017, 141, 1682–1689. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N Engl J Med 2021. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.J.; Iwasa, S.; Sugimoto, N.; Ryu, M.H.; Sakai, D.; Chung, H.C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Rüschoff, J.; Friedrich, M.; Nagelmeier, I.; Kirchner, M.; Andresen, L.M.; Salomon, K.; Portier, B.; Sredni, S.T.; Schildhaus, H.U.; Jasani, B.; et al. Comparison of HercepTest™ mAb pharmDx (Dako Omnis, GE001) with Ventana PATHWAY anti-HER-2/neu (4B5) in breast cancer: correlation with HER2 amplification and HER2 low status. Virchows Arch 2022, 481, 685–694. [Google Scholar] [CrossRef]
- Mercogliano, M.F.; Bruni, S.; Mauro, F.L.; Schillaci, R. Emerging Targeted Therapies for HER2-Positive Breast Cancer. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Day, C.P.; Merlino, G.; Van Dyke, T. Preclinical mouse cancer models: a maze of opportunities and challenges. Cell 2015, 163, 39–53. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T., Jr.; Chu, E. A history of cancer chemotherapy. Cancer Res 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [PubMed]
- Kellar, A.; Egan, C.; Morris, D. Preclinical Murine Models for Lung Cancer: Clinical Trial Applications. Biomed Res Int 2015, 2015, 621324. [Google Scholar] [CrossRef]
- Itai, S.; Yamada, S.; Kaneko, M.K.; Chang, Y.W.; Harada, H.; Kato, Y. Establishment of EMab-134, a Sensitive and Specific Anti-Epidermal Growth Factor Receptor Monoclonal Antibody for Detecting Squamous Cell Carcinoma Cells of the Oral Cavity. Monoclon Antib Immunodiagn Immunother 2017, 36, 272–281. [Google Scholar] [CrossRef]
- Itai, S.; Fujii, Y.; Kaneko, M.K.; Yamada, S.; Nakamura, T.; Yanaka, M.; Saidoh, N.; Chang, Y.W.; Handa, S.; Takahashi, M.; et al. H(2)Mab-77 is a Sensitive and Specific Anti-HER2 Monoclonal Antibody Against Breast Cancer. Monoclon Antib Immunodiagn Immunother 2017, 36, 143–148. [Google Scholar] [CrossRef]
- Asano, T.; Ohishi, T.; Takei, J.; Nakamura, T.; Nanamiya, R.; Hosono, H.; Tanaka, T.; Sano, M.; Harada, H.; Kawada, M.; et al. Anti-HER3 monoclonal antibody exerts antitumor activity in a mouse model of colorectal adenocarcinoma. Oncol Rep 2021, 46. [Google Scholar] [CrossRef]
- Goto, N.; Suzuki, H.; Tanaka, T.; Ishikawa, K.; Ouchida, T.; Kaneko, M.K.; Kato, Y. EMab-300 Detects Mouse Epidermal Growth Factor Receptor-Expressing Cancer Cell Lines in Flow Cytometry. Antibodies (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Asano, T.; Nanamiya, R.; Takei, J.; Nakamura, T.; Yanaka, M.; Hosono, H.; Tanaka, T.; Sano, M.; Kaneko, M.K.; Kato, Y. Development of Anti-Mouse CC Chemokine Receptor 3 Monoclonal Antibodies for Flow Cytometry. Monoclon Antib Immunodiagn Immunother 2021, 40, 107–112. [Google Scholar] [CrossRef]
- Stern, D.F.; Kamps, M.P. EGF-stimulated tyrosine phosphorylation of p185neu: a potential model for receptor interactions. Embo j 1988, 7, 995–1001. [Google Scholar] [CrossRef]
- Wallasch, C.; Weiss, F.U.; Niederfellner, G.; Jallal, B.; Issing, W.; Ullrich, A. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. Embo j 1995, 14, 4267–4275. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.C.; Carey, K.D.; Vajdos, F.F.; Leahy, D.J.; de Vos, A.M.; Sliwkowski, M.X. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 2004, 5, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Mason, K.; Ramyar, K.X.; Stanley, A.M.; Gabelli, S.B.; Denney, D.W., Jr.; Leahy, D.J. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 2003, 421, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Kaneko, M.K.; Takei, J.; Tateyama, N.; Kato, Y. Epitope Mapping of the Anti-CD44 Monoclonal Antibody (C44Mab-46) Using the REMAP Method. Monoclon Antib Immunodiagn Immunother 2021, 40, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Kaneko, M.K.; Aasano, T.; Kato, Y. Epitope Mapping of an Antihuman EGFR Monoclonal Antibody (EMab-134) Using the REMAP Method. Monoclon Antib Immunodiagn Immunother 2021, 40, 191–195. [Google Scholar] [CrossRef]
- Asano, T.; Kaneko, M.K.; Kato, Y. Development of a Novel Epitope Mapping System: RIEDL Insertion for Epitope Mapping Method. Monoclon Antib Immunodiagn Immunother 2021, 40, 162–167. [Google Scholar] [CrossRef]
- Nanamiya, R.; Sano, M.; Asano, T.; Yanaka, M.; Nakamura, T.; Saito, M.; Tanaka, T.; Hosono, H.; Tateyama, N.; Kaneko, M.K.; et al. Epitope Mapping of an Anti-Human Epidermal Growth Factor Receptor Monoclonal Antibody (EMab-51) Using the RIEDL Insertion for Epitope Mapping Method. Monoclon Antib Immunodiagn Immunother 2021, 40, 149–155. [Google Scholar] [CrossRef]
- Pietras, R.J.; Pegram, M.D.; Finn, R.S.; Maneval, D.A.; Slamon, D.J. Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene 1998, 17, 2235–2249. [Google Scholar] [CrossRef]
- Tao, K.; Fang, M.; Alroy, J.; Sahagian, G.G. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer 2008, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Fry, E.A.; Taneja, P.; Inoue, K. Clinical applications of mouse models for breast cancer engaging HER2/neu. Integr Cancer Sci Ther 2016, 3, 593–603. [Google Scholar] [CrossRef]
- Finkle, D.; Quan, Z.R.; Asghari, V.; Kloss, J.; Ghaboosi, N.; Mai, E.; Wong, W.L.; Hollingshead, P.; Schwall, R.; Koeppen, H.; et al. HER2-targeted therapy reduces incidence and progression of midlife mammary tumors in female murine mammary tumor virus huHER2-transgenic mice. Clin Cancer Res 2004, 10, 2499–2511. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Ryu, K.H.; Kwon, A.Y. The Prognostic Impact of HER2 Genetic and Protein Expression in Pancreatic Carcinoma-HER2 Protein and Gene in Pancreatic Cancer. Diagnostics (Basel) 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Allen, S.; Blake, J.F.; Bowcut, V.; Briere, D.M.; Calinisan, A.; Dahlke, J.R.; Fell, J.B.; Fischer, J.P.; Gunn, R.J.; et al. Identification of MRTX1133, a Noncovalent, Potent, and Selective KRAS(G12D) Inhibitor. J Med Chem 2022, 65, 3123–3133. [Google Scholar] [CrossRef]
- Kemp, S.B.; Cheng, N.; Markosyan, N.; Sor, R.; Kim, I.K.; Hallin, J.; Shoush, J.; Quinones, L.; Brown, N.V.; Bassett, J.B.; et al. Efficacy of a Small-Molecule Inhibitor of KrasG12D in Immunocompetent Models of Pancreatic Cancer. Cancer Discov 2023, 13, 298–311. [Google Scholar] [CrossRef]
- Hallin, J.; Bowcut, V.; Calinisan, A.; Briere, D.M.; Hargis, L.; Engstrom, L.D.; Laguer, J.; Medwid, J.; Vanderpool, D.; Lifset, E.; et al. Anti-tumor efficacy of a potent and selective non-covalent KRAS(G12D) inhibitor. Nat Med 2022, 28, 2171–2182. [Google Scholar] [CrossRef]
- Gulay, K.C.M.; Zhang, X.; Pantazopoulou, V.; Patel, J.; Esparza, E.; Pran Babu, D.S.; Ogawa, S.; Weitz, J.; Ng, I.; Mose, E.S.; et al. Dual inhibition of KRASG12D and pan-ERBB is synergistic in pancreatic ductal adenocarcinoma. Cancer Res 2023. [Google Scholar] [CrossRef]
- Mallya, K.; Gautam, S.K.; Aithal, A.; Batra, S.K.; Jain, M. Modeling pancreatic cancer in mice for experimental therapeutics. Biochim Biophys Acta Rev Cancer 2021, 1876, 188554. [Google Scholar] [CrossRef]
- Ponz-Sarvise, M.; Corbo, V.; Tiriac, H.; Engle, D.D.; Frese, K.K.; Oni, T.E.; Hwang, C.I.; Öhlund, D.; Chio, I.I.C.; Baker, L.A.; et al. Identification of Resistance Pathways Specific to Malignancy Using Organoid Models of Pancreatic Cancer. Clin Cancer Res 2019, 25, 6742–6755. [Google Scholar] [CrossRef]
- Copeland-Halperin, R.S.; Liu, J.E.; Yu, A.F. Cardiotoxicity of HER2-targeted therapies. Curr Opin Cardiol 2019, 34, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.F.; Simon, H.; Chen, H.; Bates, B.; Hung, M.C.; Hauser, C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 1995, 378, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Crone, S.A.; Zhao, Y.Y.; Fan, L.; Gu, Y.; Minamisawa, S.; Liu, Y.; Peterson, K.L.; Chen, J.; Kahn, R.; Condorelli, G.; et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med 2002, 8, 459–465. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).