Submitted:
10 July 2023
Posted:
11 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Mite Sampling
Morphological Analysis
Terminology
Abbreviations for Museums and Collections
Phylogeny Reconstructions Methods
Cladistic Analysis
3. Results
3.1. Systematics
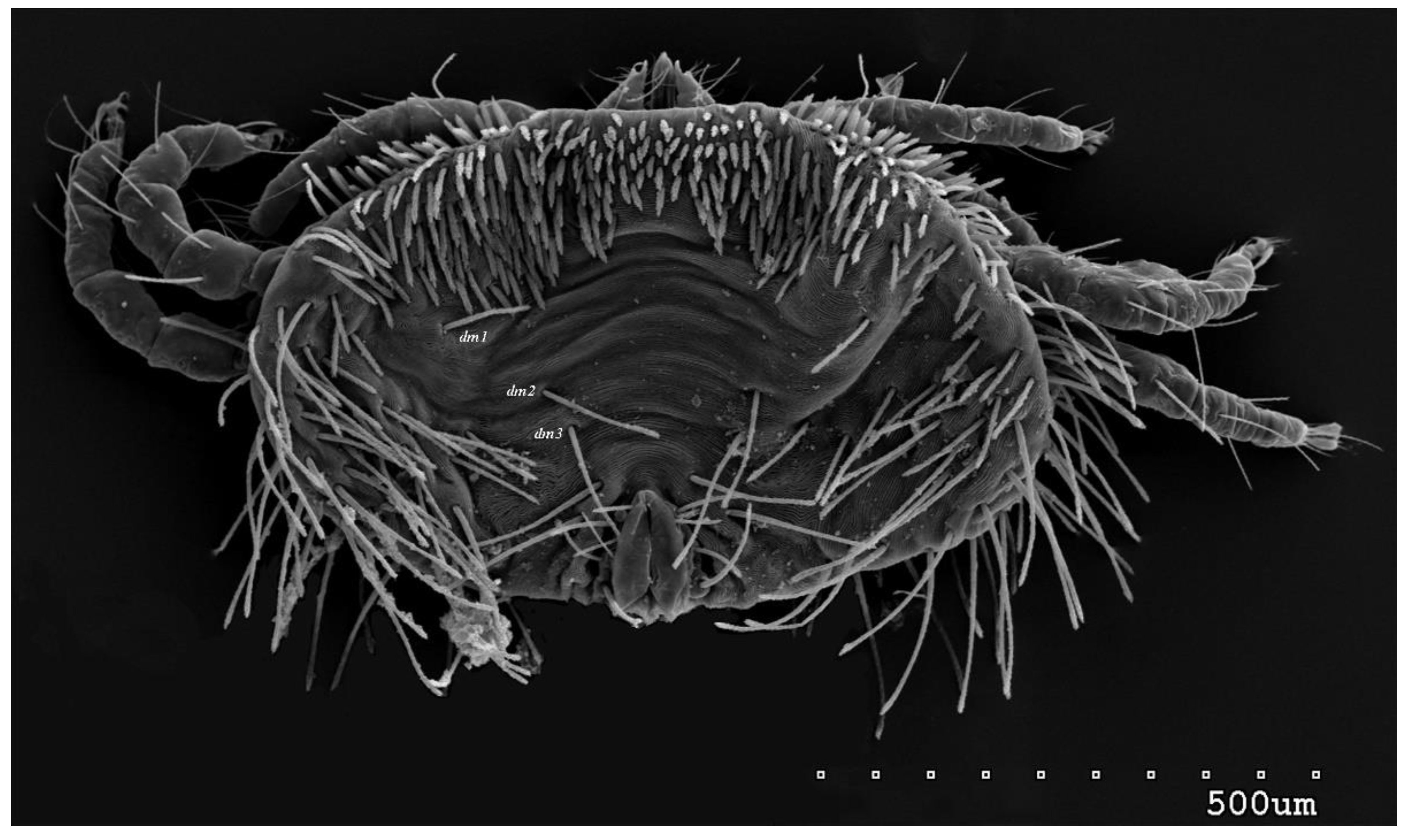
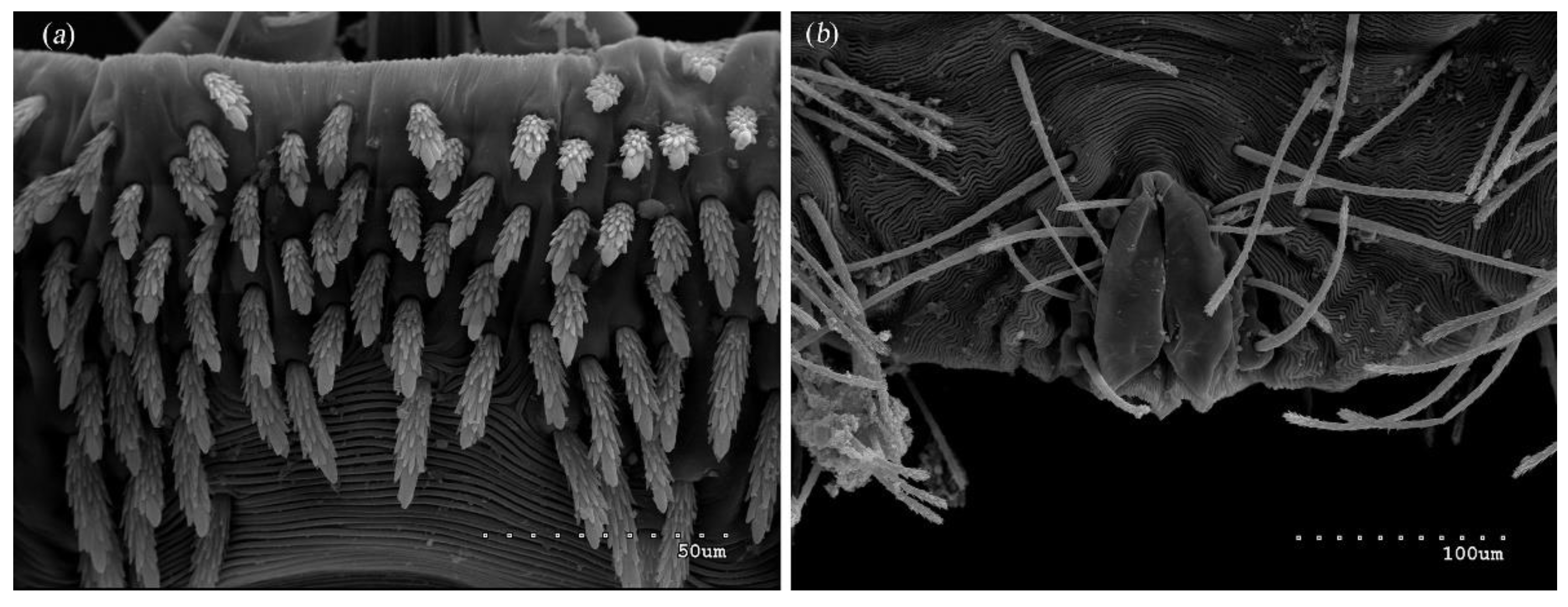
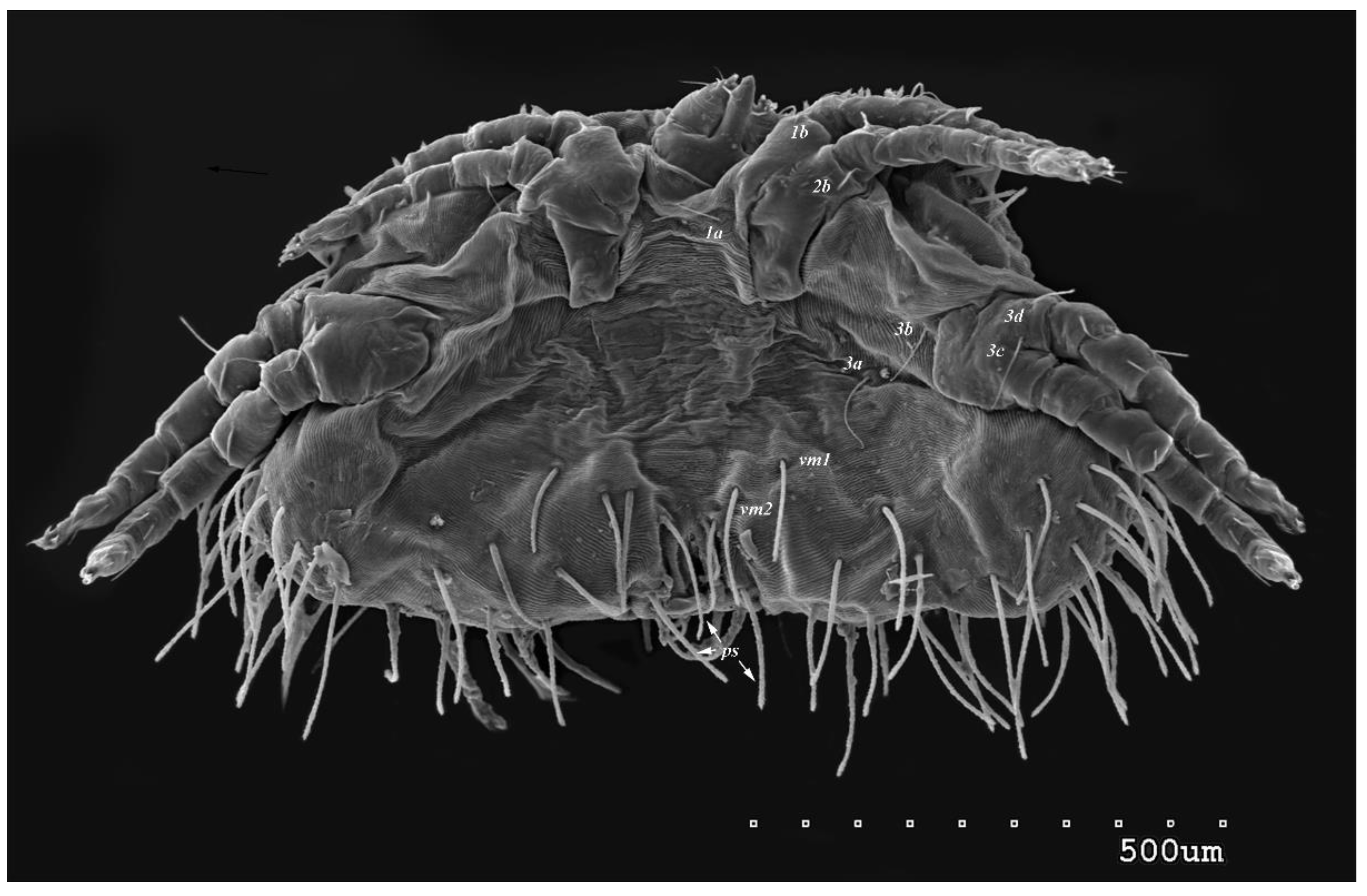
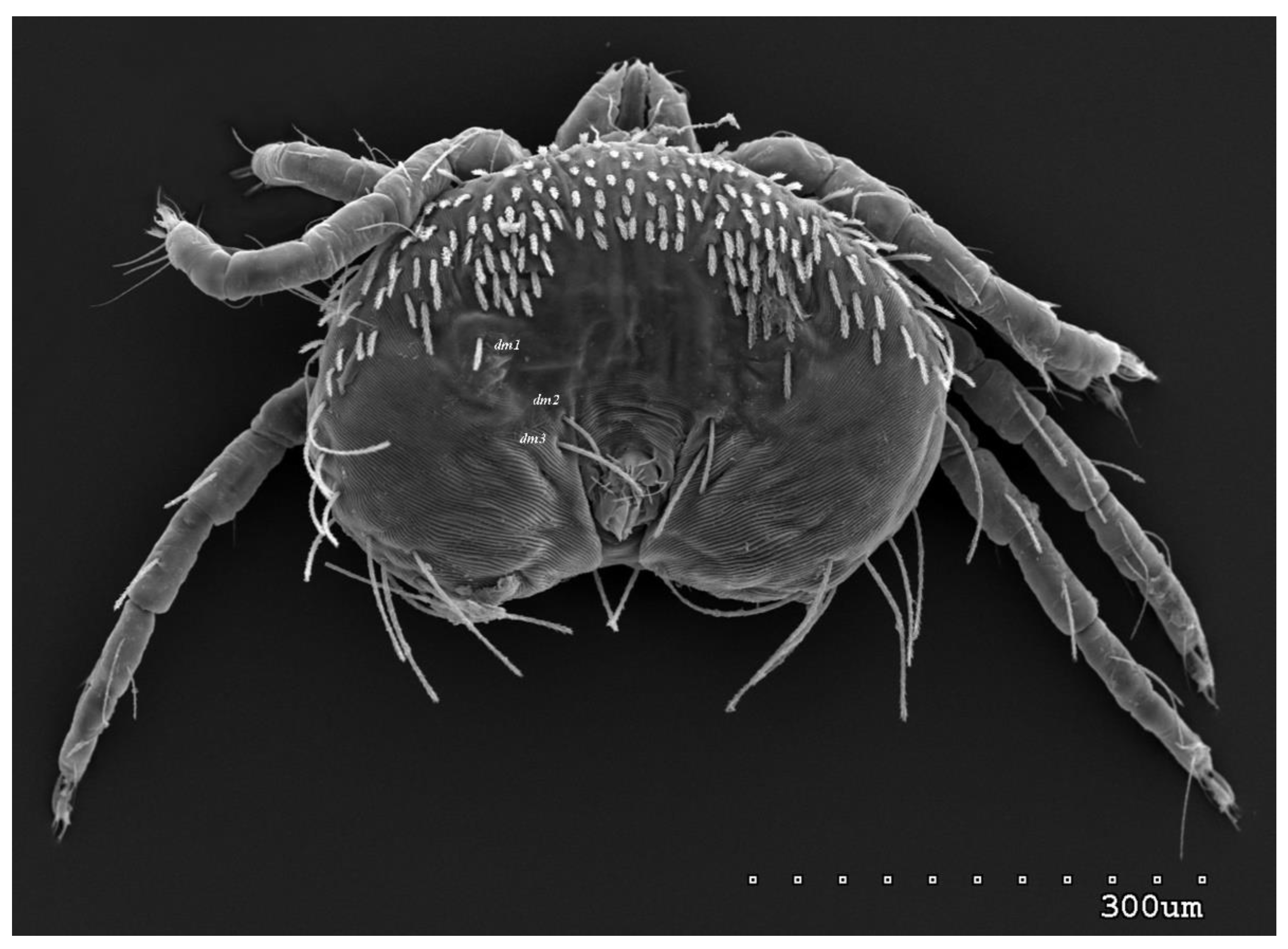
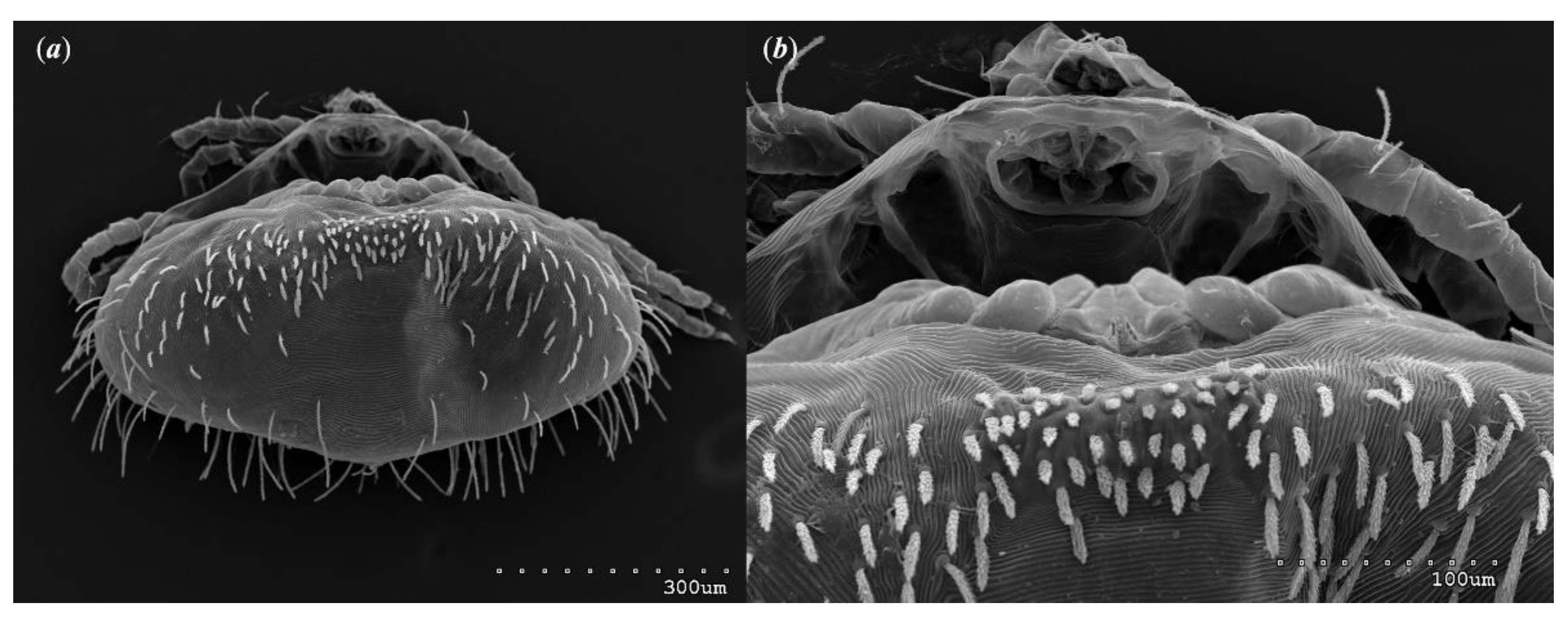
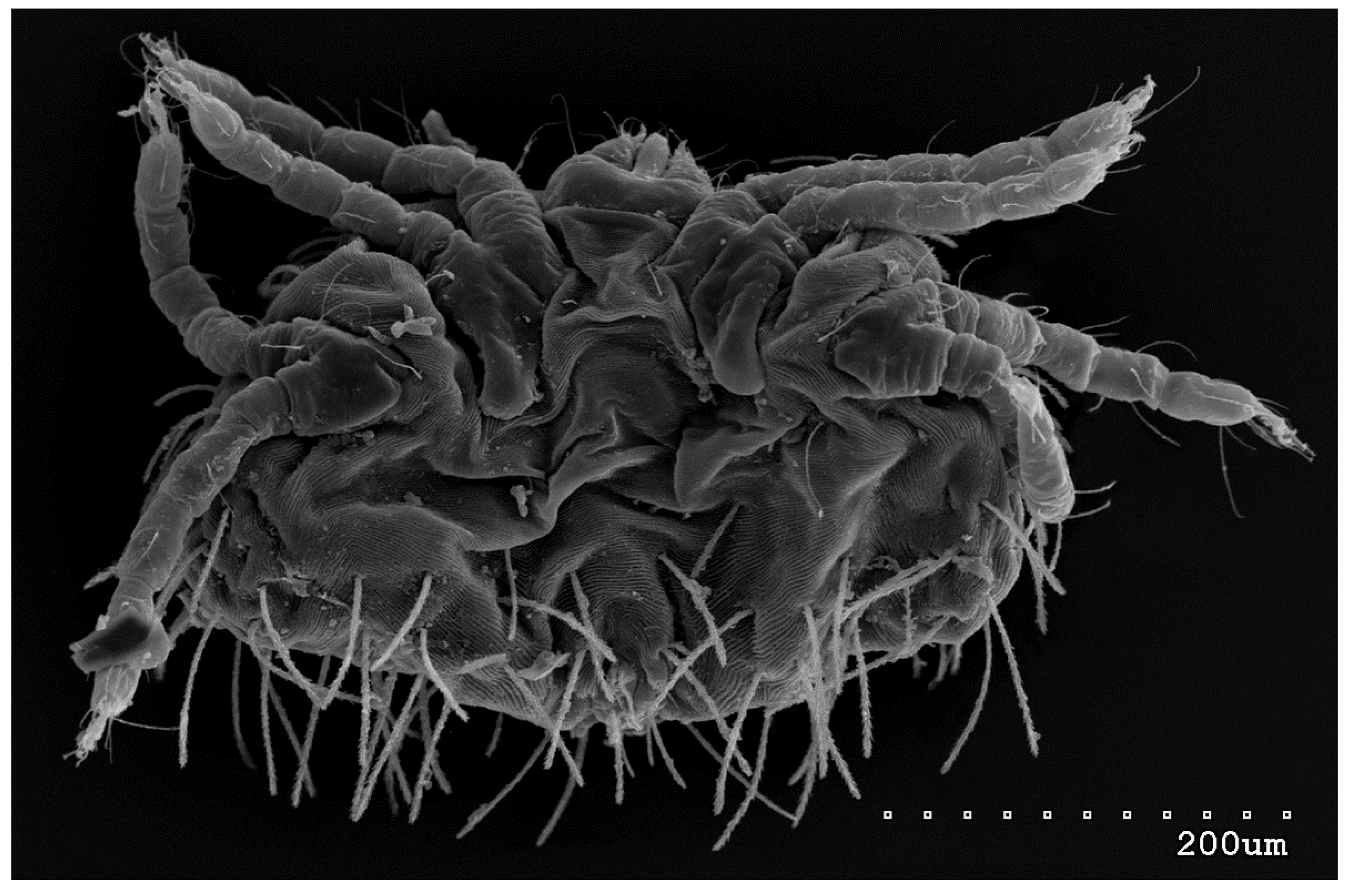
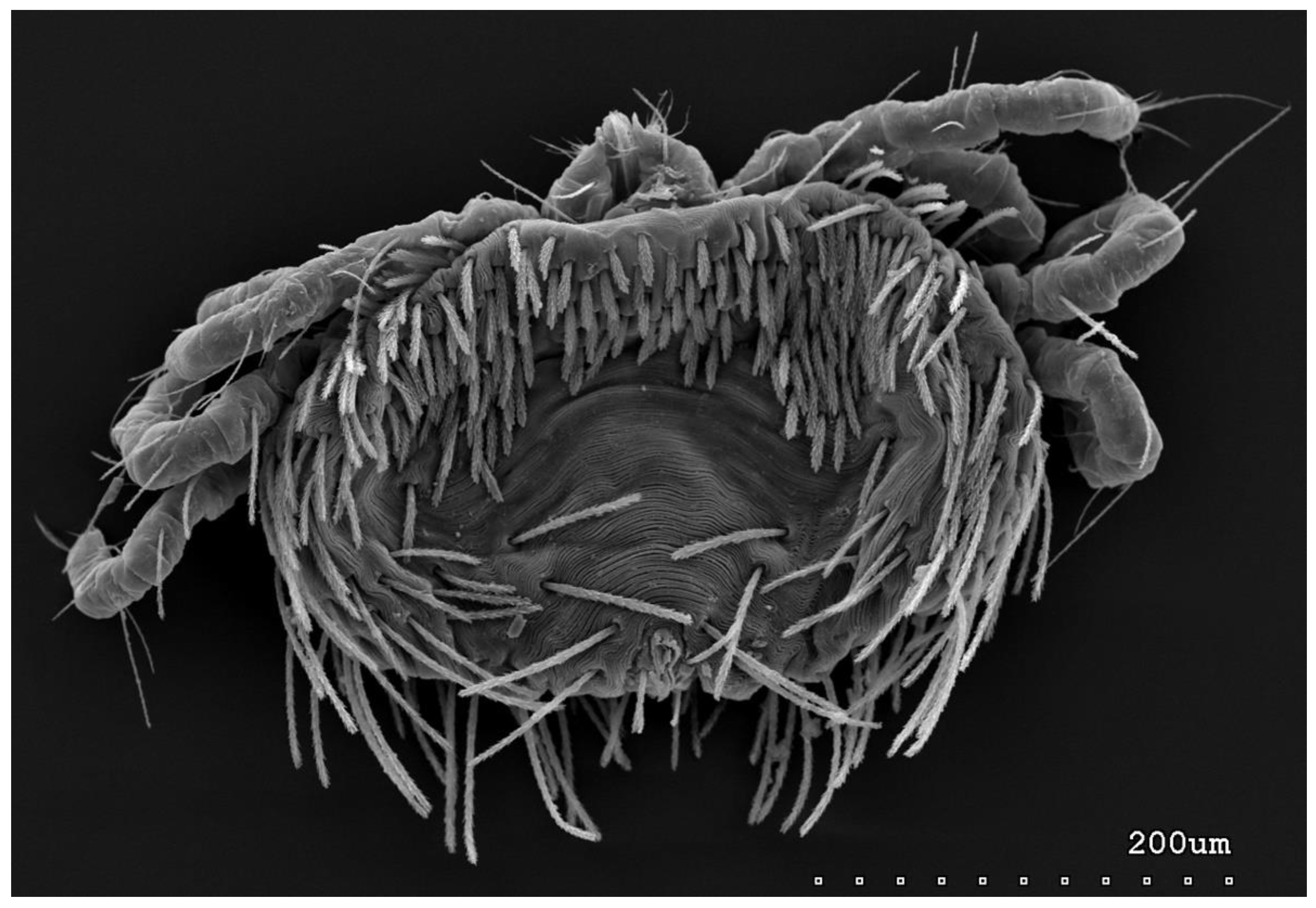
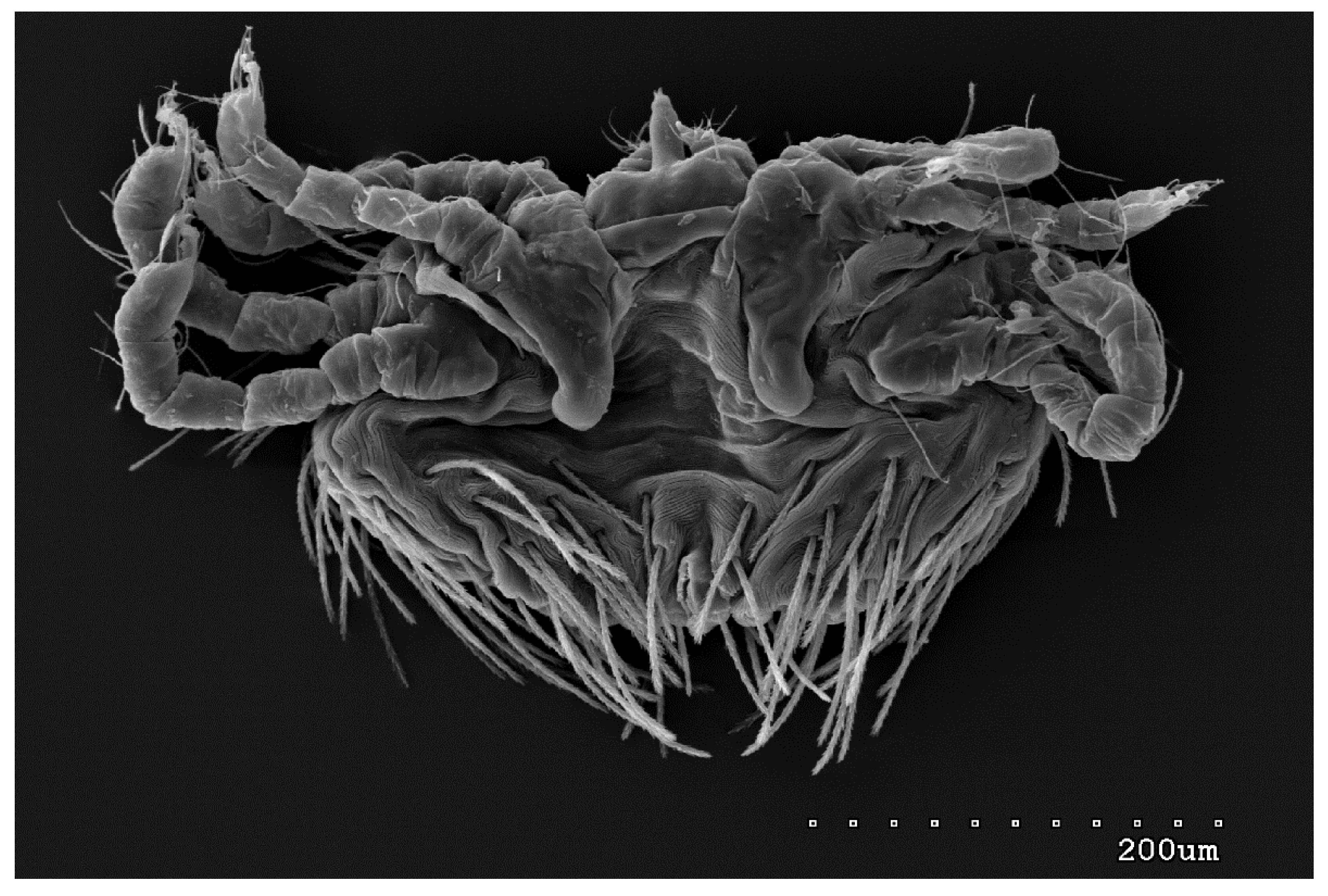
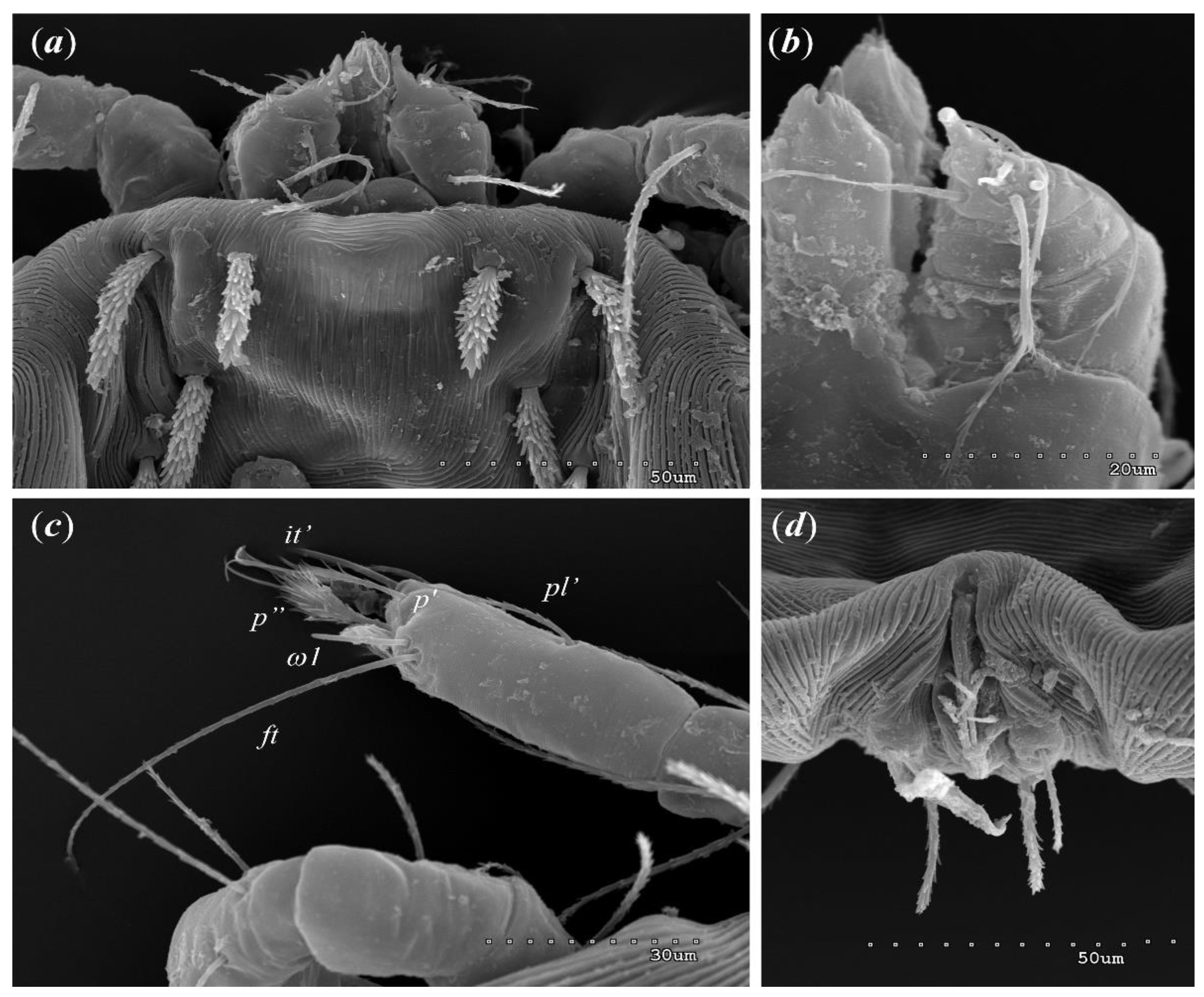
3.1.1. Description
3.1.2. Key to Species of Neopterygosoma (Females) (Based on the Key of FAJFER [4])
- 1.
- Body much wider than long (1.5–1.8 times). Setae tc’ and tc” of legs II–IV serrate. Peripheral setae much longer than dorsal and ventral setae situated anteriorly, medially and laterally … chilensis group 2
- -
- Body circular, only slightly wider than long (1.1–1.3 times). Setae tc’ and tc” of legs II–IV smooth. Peripheral setae subequal with anterior, medial and lateral setae on idiosomal dorsum and venter … patagonica group ... N. patagonica (Dittmar de la Cruz, Morando & Avila, 2004)
- 2.
- Five setae on genu I and 5 pseudanal setae ps… 3
- -
- Four setae on genu I and 3 pseudanal setae ps … N. formosus (Fajfer & González–Acuña, 2013)
- 3.
- Four setae on femur II … 4
- -
- Five setae on femur II … 5
- 4.
- Five pseudanal setae present. Setae vTrI–IV densely serrate. Swollen cheliceral part of chelicerae shorter than slender distal part. Subcapitular setae n short (45–65 long) ... N. chilensis (Fajfer & González–Acuña, 2013)
- -
- Four pseudanal setae present. Setae vTrI–IV smooth. Swollen cheliceral part of chelicerae longer than slender distal part. Subcapitular setae n long (about 125 long) ... N. schroederi Fajfer, 2019
- 5.
- Three setae on femur IV. One pair of genital setae g1. Dorsomedial setae dm represented by 15–21 pairs of setae. Ventro–medial setae vm represented by 10–18 pairs ...6
- -
- Two setae on femur IV. Four or five pairs of genital setae. Dorsomedial setae dm represented by 3–5 pairs of setae. Ventromedial setae vm represented by 1–3 pairs … N. robertmertensi sp. n.
- 6.
- Genital setae smooth. Fixed cheliceral digit spinous, palp setae dF serrate only distally, subcapitular setae n serrate … N. cyanogasteri (Fajfer & González-Acuña, 2013)
- -
- Genital setae serrate. Fixed cheliceral digit reduced to rounded structure, palp setae dF serrate on all length, subcapitular setae n smooth … 7
- 7.
- Coxal fields I with 2 setae. Gnathosoma situated apically. Free peritremal branch present. Setae dG serrate on all length … 8
- -
- Coxal fields I with 3 setae. Gnathosoma displaced on dorsal side. Free peritremal branch absent. Setae dG serrate only at distal tip … N. ovata (Fajfer & González-Acuña, 2013)
- 8.
- Antero-medial setae increase in length from anterior to posterior part of setal cluster. Setae a’ and a” of tarsi I slightly serrate. Setae v’TrI–IV serrate. Setae 3a smooth and situated outside coxal plates … N. levissima (Fajfer & González–Acuña, 2013)
- -
- Antero-medial setae subequal in length. Setae a’ and a” of tarsi I smooth. Setae v’TrI–IV with barely discernible serration. Setae 3a slightly serrate and situated on coxal plates … N. ligare (Fajfer & González-Acuña, 2013).
3.2. Phylogeny
3.2.1. Unweighted Parsimony Analysis
Discussion
Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fajfer, M.; González-Acuña, D. Pterygosomatid mites of a new species group ligare (Acariformes: Pterygosomatidae: Pterygosoma) parasitizing tree iguanas (Squamata: Liolaemidae: Liolaemus). Zootaxa 2013, 3693, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Dittmar de la Cruz, K.; Morando, M.; Avila, L. Description of a new pterygosomatid mite (Acari: Actinedida: Pterygosomatidae) parasitic on Liolaemus spp. (Iguania: Liolaemini) from Argentina. Zootaxa 2004, 521, 1–6. [Google Scholar] [CrossRef]
- Fajfer, M. Systematics of reptile-associated scale mites of the genus Pterygosoma (Acariformes: Pterygosomatidae) derived from external morphology. Zootaxa 2019, 4603. [Google Scholar] [CrossRef] [PubMed]
- Fajfer, M. A systematic revision of the genus Neopterygosoma Fajfer, 2019 (Acariformes: Pterygosomatidae) with the description of a new species. Syst. Parasitol. 2020, 97, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Fajfer, M. Redescription of Pterygosoma patagonica (Acariformes: Pterygosomatidae) with new host and distribution data. Int. J. Acarol. 2014, 40, 160–164. [Google Scholar] [CrossRef]
- Norton, R.A. A. A review of F. Grandjean's system of leg chaetotaxy in the Oribatei and its application to the Damaeidae. In Biology of Oribatid Mites, 1st ed.; Dindal, D.L., Ed.; S.U.N.Y College of Environmental Science and Forestry: Syracuse, NY, USA, 1977; pp. 33–61. [Google Scholar]
- Bochkov, A.V.; OConnor, B.M. A review of the external morphology of the family Pterygosomatidae and its systematic position within the Prostigmata (Acari: Acariformes). Parasitologiya 2006, 40, 201–214. [Google Scholar]
- Krantz, G.W.; Walter, D.E. A Manual of Acarology; Texas Tech University Press: Lubbock, TX, USA, 2009. [Google Scholar]
- Grandjean, F. Les Segments Post-Larvaires de L’hystérosoma Chez Les Oribates (Acariens). Bull. Soc. Zool. Fr. 1939, 64, 273–284. [Google Scholar]
- Grandjean, F. Observations sur les Acariens de la famille des Stigmaeidae. Arch. Sci. Phys. Nat. 1944, 26, 103–1131. [Google Scholar]
- Grandjean, F. Au sujet de l'organe de Claparede, des eupathides multiples et des taenidies mandiubulaires chez les Acariens actinochitineux. Arch. Sci. Phys. Nat. 1946, 28, 63–87. [Google Scholar]
- The Reptile Database. Available online: http://www.reptile-database.org (accessed on 1 25 June 2023).
- Page, R.D.M. NDE, NEXUS Data Editor 0.5.0; University of Glasgow: Glasgow, Scotland, 2001. [Google Scholar]
- Swofford, D.L. PAUP*. In Phylogenetic Analysis Using Parsimony (*and Other Methods); Version 4; Sinauer Associates: Sunderland, MA, USA, 2002; p. 144. [Google Scholar]
- Müller, K. PRAP—Computation of Bremer support for large data sets. Mol. Phylogenet. Evol. 2004, 31, 780–782. [Google Scholar] [CrossRef]
- Rambaut, A.; Institute of Evolutionary Biology, University of Edinburgh, Edinburgh. FigTree v1.3.1. 2010. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 10 June 2023).
- Pincheira-Donoso, D.; Scolaro, J.; Sura, P. A monographic catalogue on the systematics and phylogeny of the South American iguanian lizard family Liolaemidae (Squamata, Iguania). Zootaxa 2008, 1800, 1–85. [Google Scholar] [CrossRef]
- Pincheira-Donoso, D.; Tregenza, T.; Hodgson, D.J. Body size evolution in South American Liolaemus lizards of the boulengeri clade: A contrasting reassessment. J. Evol. Biol. 2007, 20, 2067–2071. [Google Scholar] [CrossRef] [PubMed]
- Schulte, J.A.; Macey, J.R.; Espinoza, R.E.; Larson, A. Phylogenetic relationships in the iguanid lizard genus Liolaemus: Multiple origins of viviparous reproduction and evidence for recurring Andean vicariance and dispersal. Zool. J. Linn. Soc. 2000, 69, 75–102. [Google Scholar] [CrossRef]
- Fontanella, F.M.; Olave, M.; Avila, L.J.; Morando, M. Molecular dating and diversification of the South American lizard genus Liolaemus (subgenus Eulaemus) based on nuclear and mitochondrial DNA sequences. Zool. J. Linn. Soc. 2012, 164, 825–835. [Google Scholar] [CrossRef]
- Avila, L.J.; Perez, C.H.F.; Minoli, I.; Medina, C.D.; Sites, J.W., Jr.; Morando, M. New species of Liolaemus (Reptilia, Squamata, Liolaemini) of the Liolaemus donosobarrosi clade from northwestern Patagonia, Neuquén province, Argentina. Zootaxa, 2017, 4362, 535–563. [Google Scholar] [CrossRef]
- Sánchez, K.I.; Morando, M.; Avila, L.J. A new lizard species of the Liolaemus kingii group (Squamata: Liolaemidae) from northwestern Chubut province (Argentina). Zootaxa 2023, 235–255. [Google Scholar] [CrossRef]
- Laurent, R.F. Contribución al conocimiento de la estructura taxonómica del género Liolaemus Wiegmann (Iguanidae). Bol. Asoc. Herp. Arg. 1983, 1, 16–18. [Google Scholar]
- Fernández, M.G.; Abdala, C.S.; Ruiz-Monachesi, M.R.; Semham, R.V.; Quinteros, A.S. Redescription of Liolaemus robertmertensi, Hellmich 1964 (Iguania: Liolaemidae) with description of a new species. Cuad. herpetol. 2021, 35, 65–78. [Google Scholar] [CrossRef]
- Espinoza, R.E.; Wiens, J.J.; Tracy, C.R. Recurrent evolution of herbivory in small, cold-climate lizards: Breaking the ecophysiological rules or reptilian herbivory. Proc. Natl. Acad. Sci. USA 2004, 101, 16819–16824. [Google Scholar] [CrossRef]
- Troncoso-Palacios, J.; Schulte, J.A.; Marambio-Alfaro, Y.; Hiriart, D. Phenotypic variation, phylogenetic position and new distributional records for the poorly known Liolaemus silvai Ortiz, 1989 (Iguania: Iguanidae: Liolaemini). S. Am. J. Herpetol. 2015, 10, 71–81. [Google Scholar] [CrossRef]
- Schulte, J.A.; Losos, J.B.; Cruz, F.B.; Núñez, H. The relationship between morphology, escape behaviour and microhabitat occupation in the lizard clade Liolaemus (Iguanidae: Tropidurinae: Liolaemini). J. Evol. Biol. 2004, 17, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Fajfer, M. Mites of the new species group nitidus (Acariformes: Pterygosomatidae: Geckobia), parasites of lizards in South America. Syst. Parasitol. 2015, 90, 213–222. [Google Scholar] [CrossRef]
- Panzera, A.; Leaché, A.D.; D’Elia, G.; Victoriano, P.F. Phylogenomic analysis of the Chilean clade of Liolaemus lizards (Squamata: Liolaemidae) based on sequence capture data. PeerJ 2017, 5, e3941. [Google Scholar] [CrossRef]
- Pincheira-Donoso, D.; Núñez, H. Las especies chilenas del género Liolaemus Wiegmann, 1834 (Iguania Tropiduridae: Liolaeminae). Taxonomía, sistemática y evolución. Mus. Nac. Hist. Nat. Chile, Publ. Occ. 2005, 59, 7–486. [Google Scholar]
- Ruiz de Gamboa, M. Lista actualizada de los reptiles de Chile. Bol. Chil. Herp. 2016, 3, 7–12. [Google Scholar]
- Lobo, F.; Espinoza, R.E.; Quinteros, S. A critical review and systematic discussion of recent classification proposals for liolaemid lizards. Zootaxa 2010, 2549, 1–30. [Google Scholar] [CrossRef]
- Fajfer, M.; Karanth, P. New morphological and molecular data reveal an underestimation of species diversity of mites of the genus Geckobia (Acariformes: Pterygosomatidae) in India. Diversity 2022, 14, 1064. [Google Scholar] [CrossRef]
- Fajfer, M. Three new species of scale mites (Acari: Pterygosomatidae) parasitizing Agama sankaranica (Sauria: Agamidae). Zootaxa 2013, 3700, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Fajfer, M. Two new species of the genus Pterygosoma (Acariformes: Pterygosomatidae) parasitizing agamid lizards (Sauria: Agamidae) from the Indian subcontinent. Acta Parasitol. 2016, 61, 343–354. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




