Submitted:
10 July 2023
Posted:
12 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Human NSC Line
2.2. Ageing Animals and NSC Transplantation
2.3. Measurement of Physical Activity
2.3.1. Locomotor Activity
2.3.2. Rota-rod Performance
2.4. Measurement of Learning and Memory Functions
2.4.1. Passive Avoidance Performance
2.4.2. Water-maze Performance
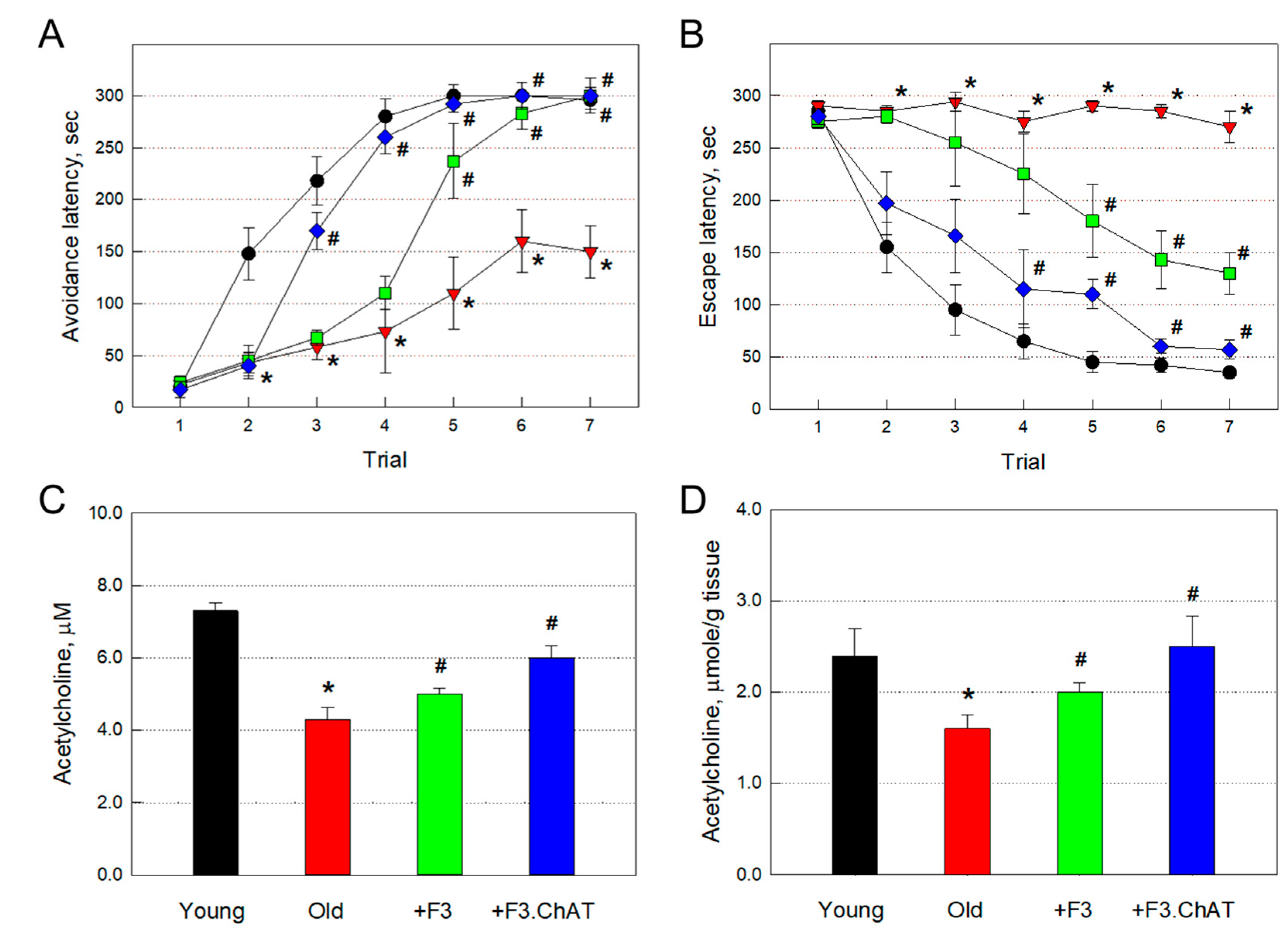
2.5. Analysis of ACh Concentration
2.6. Stem Cell Distribution
2.7. RT-PCR Analysis
2.8. Western Blot Analysis
2.9. Identification of Capillary
2.10. Antioxidative Activity of Stem Cells
2.11. Statistical Analysis
3. Results
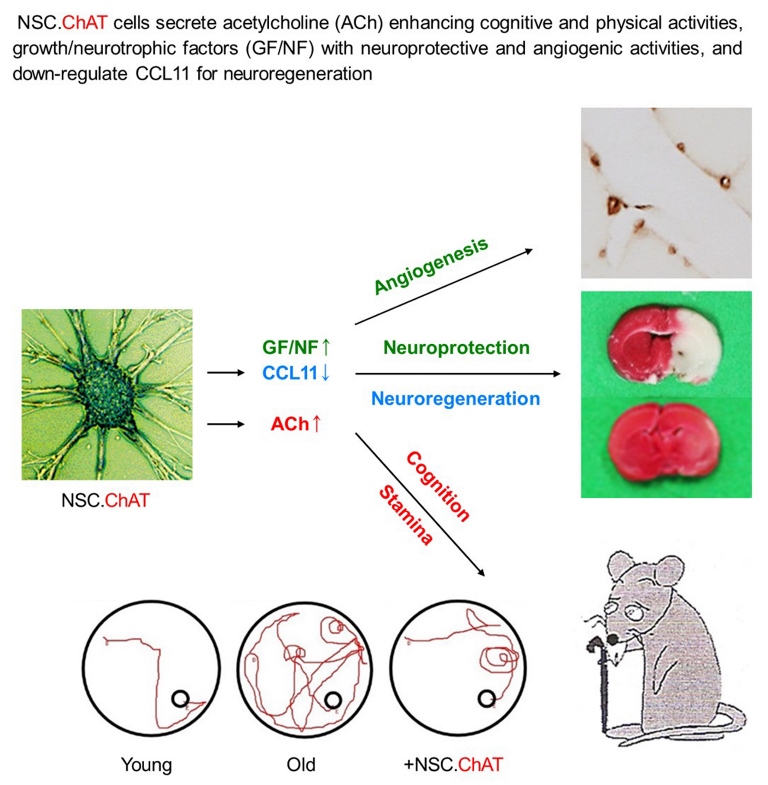
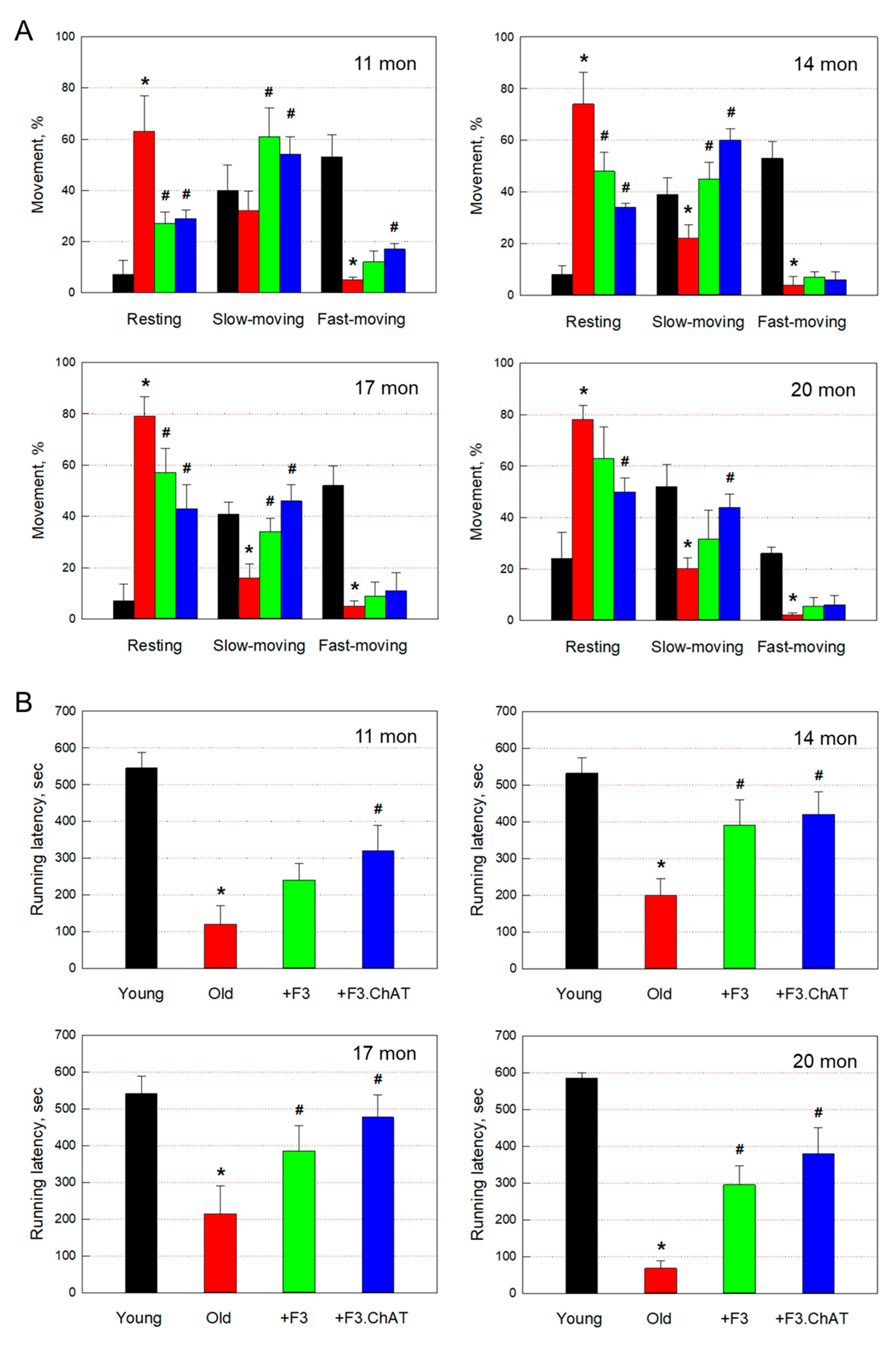
3.1. Improvement of Physical Activity
3.2. Recovery of Cognitive Function and ACh Concentration
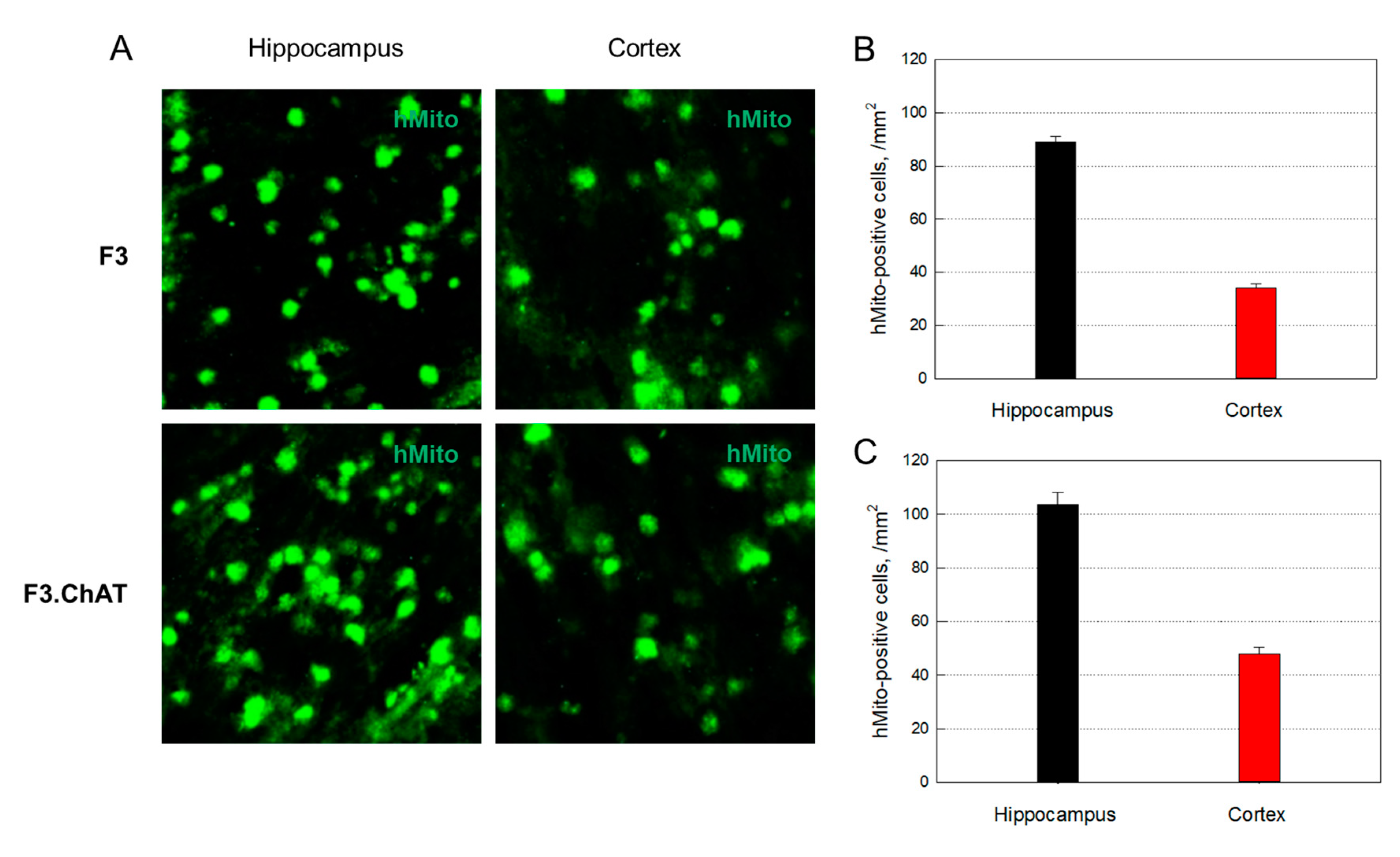
3.4. Distribution of Transplanted Cells
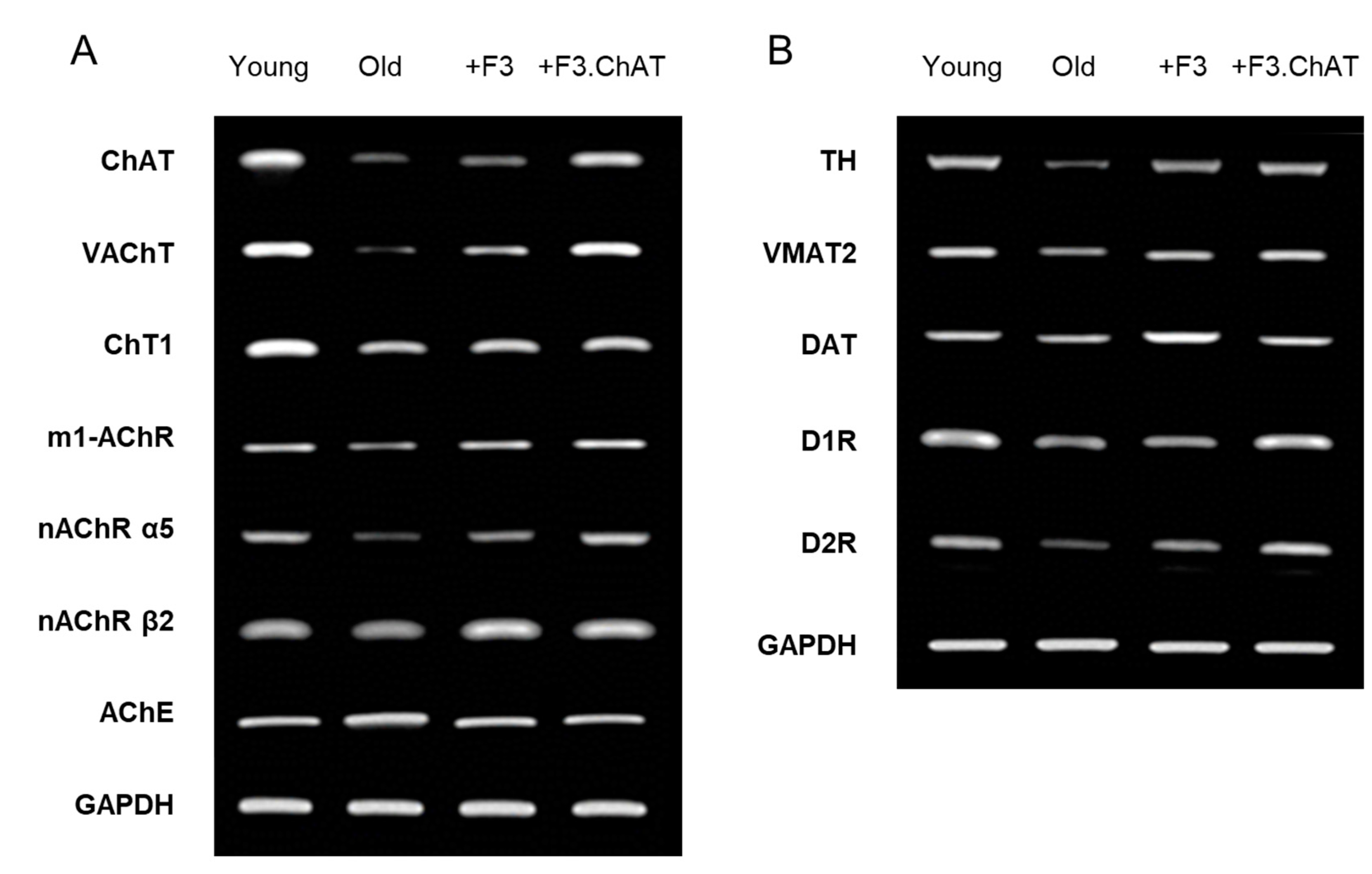
3.5. Cholinergic and Dopaminergic Activation in the Host Brain
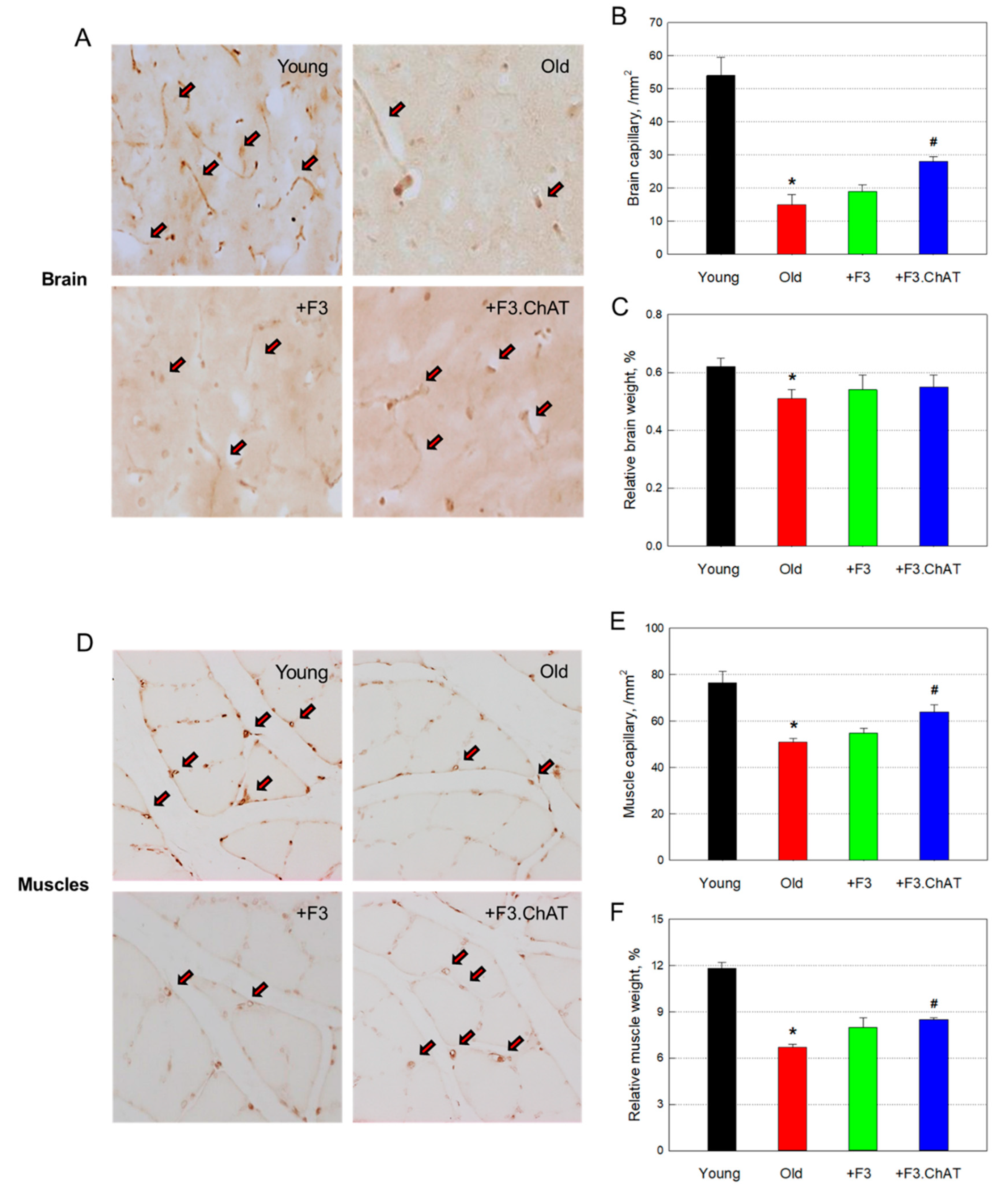
3.6. Angiogenic Effects
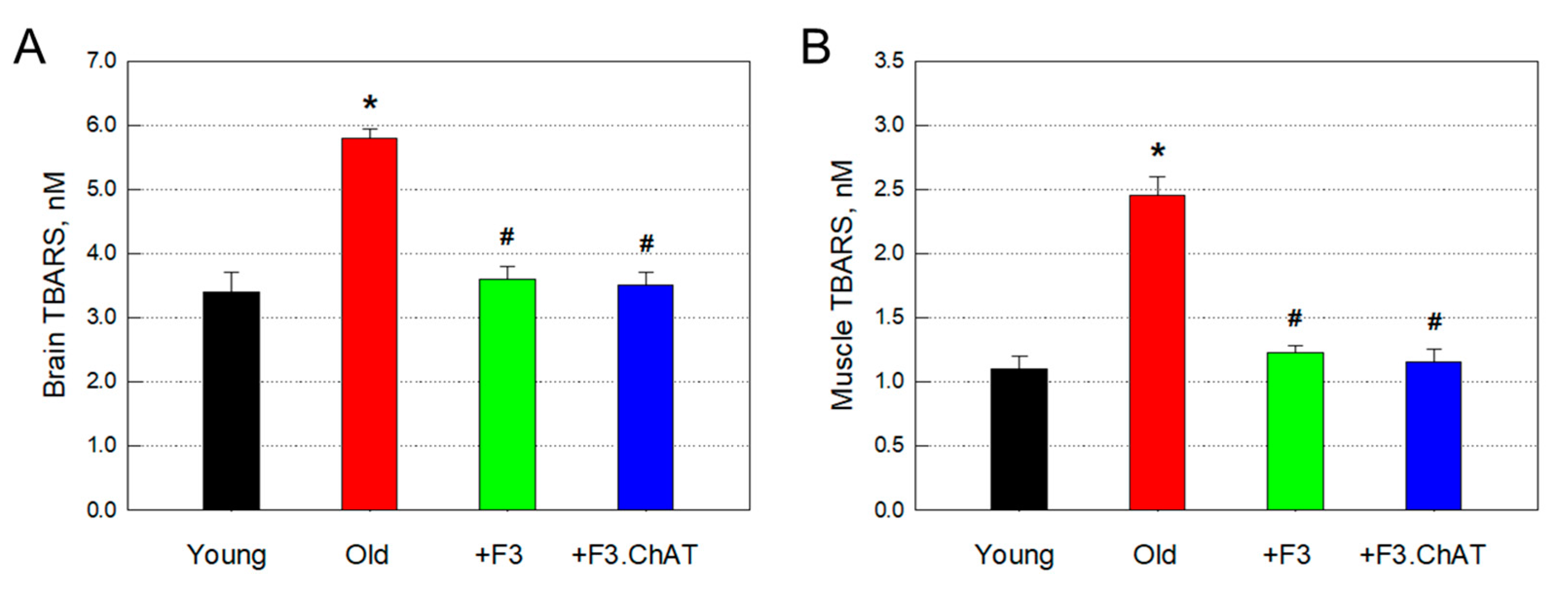
3.8. Antioxidative Effects
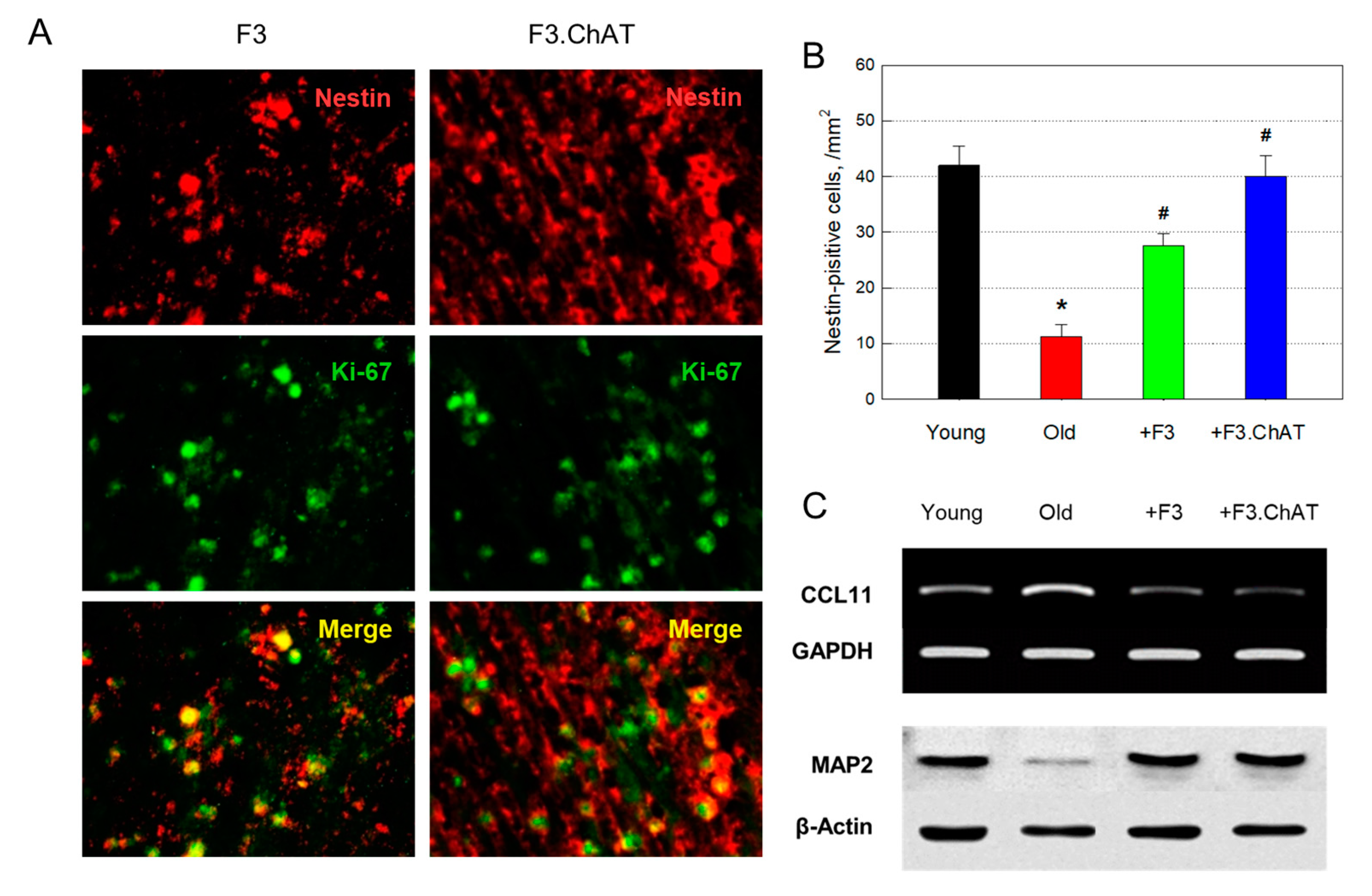
3.9. Neuroprotection and Regeneration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vanguilder, H.D.; Bixler, G.V.; Sonntag, W.E.; Freeman, W.M. Hippocampal expression of myelin-associated inhibitors is induced with age-related cognitive decline and correlates with deficits of spatial learning and memory. J. Neurochem. 2012, 121, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Sahay, A.; Scobie, K.N.; Hill, A.S.; O'Carroll, C.M.; Kheirbek, M.A.; Burghardt, N.S.; Fenton, A.A.; Dranovsky, A.; Hen, R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 2011, 472, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Artegiani, B.; Calegari, F. Age-related cognitive decline: Can neural stem cells help us? Aging 2012, 4, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Itou, Y.; Nochi, R.; Kuribayashi, H.; Saito, Y.; Hisatsune, T. Cholinergic activation of hippocampal neural stem cells in aged dentate gyrus. Hippocampus 2011, 21, 446–459. [Google Scholar] [CrossRef]
- Abdulla, F.A.; Abu-Bakra, M.A.; Calaminici, M.R.; Stephenson, J.D.; Sinden, J.D. Importance of forebrain cholinergic and GABAergic systems to the age-related deficits in water maze performance of rats. Neurobiol. Aging 1995, 16, 41–52. [Google Scholar] [CrossRef]
- Watanabe, T.; Yamagata, N.; Takasaki, K.; Sano, K.; Hayakawa, K.; Katsurabayashi, S.; Egashira, N.; Mishima, K.; Iwasaki, K.; Fujiwara, M. Decreased acetylcholine release is correlated to memory impairment in the Tg2576 transgenic mouse model of Alzheimer's disease. Brain Res. 2009, 1249, 222–228. [Google Scholar] [CrossRef]
- Li, J.Y.; Dahlström, A.M.; Hersh, L.B.; Dahlström, A. Fast axonal transport of the vesicular acetylcholine transporter (VAChT) in cholinergic neurons in the rat sciatic nerve. Neurochem. Int. 1998, 32, 457–467. [Google Scholar] [CrossRef]
- Hernandez, C. M/; Høifødt, H.; Terry, AV., Jr. Spontaneously hypertensive rats: further evaluation of age-related memory performance and cholinergic marker expression. J. Psychiatry Neurosci. 2003, 28, 197–209. [Google Scholar]
- Zurkovsky, L.; Bychkov, E.; Tsakem, E.L; Siedlecki, C.; Blakely, R.D.; Gurevich, E.V. Cognitive effects of dopamine depletion in the context of diminished acetylcholine signaling capacity in mice. Dis. Model Mech. 2013, 6, 171–183. [Google Scholar] [CrossRef]
- Pirondi, S.; D'Intino, G.; Gusciglio, M.; Massella, A.; Giardino, L.; Kuteeva, E.; Ogren, S.O.; Hökfelt, T.; Calzà, L. Changes in brain cholinergic markers and spatial learning in old galanin-overexpressing mice. Brain Res. 2007, 1138, 10–20. [Google Scholar] [CrossRef]
- Katoh-Semba, R.; Semba, R.; Takeuchi, I.K.; Kato, K. Age-related changes in levels of brain-derived neurotrophic factor in selected brain regions of rats, normal mice and senescence-accelerated mice: a comparison to those of nerve growth factor and neurotrophin-3. Neurosci. Res. 1998, 31, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.Q.; Zheng, J.L.; Karihaloo, M. Neurotrophin-4/5 (NT-4/5) and brain-derived neurotrophic factor (BDNF) act at Later stages of cerebellar granule cell Differentiation. J. Neurosci. 1995, 15, 2656–2667. [Google Scholar] [CrossRef] [PubMed]
- Bartkowska, K.; Turlejski, K.; Djavadian, RL. Neurotrophins and their receptors in early development of the mammalian nervous system. Acta Neurobiol. Exp. 2010, 70, 454–467. [Google Scholar]
- Aguiar, A.S., Jr.; Castro, A.A.; Moreira, E.L.; Glaser, V.; Santos, A.R.; Tasca, C.I.; Latini, A.; Prediger, R.D. Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: Involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech. Ageing Dev. 2011, 132, 560–567. [Google Scholar] [CrossRef]
- Chae, C.H.; Lee, H.C.; Jung, S.L.; Kim, T.W.; Kim, J.H.; Kim, N.J.; Kim, H.T. Swimming exercise increases the level of nerve growth factor and stimulates neurogenesis in adult rat hippocampus. Neuroscience 2012, 212, 30–37. [Google Scholar] [CrossRef]
- Campenot, R.B. Local control of neurite development by nerve growth factor. Proc. Natl. Acad. Sci. USA 1977, 74, 4516–4519. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, R.; Zweifel, L.S.; Glebova, N.O.; Lonze, B.E.; Valdez, G.; Ye, H.; Ginty, D.D. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell 2004, 118, 243–255. [Google Scholar] [CrossRef]
- Gao, P.; Shen, F.; Gabriel, R.A.; Law, D.; Yang, E.Y.; Yang, G.Y.; Young, W.L.; Su, H. Attenuation of brain response to VEGF-mediated angiogenesis and neurogenesis in aged mice. Stroke 2009, 40, 3596–3600. [Google Scholar] [CrossRef]
- Rachamimov, A.; Darland, T.; D'Amore, P.A. Vascular endothelial growth factor (VEGF) isoform regulation of early forebrain development. Dev. Biol. 2011, 358, 9–22. [Google Scholar]
- Sanchez, H.L.; Silva, L.B.; Portiansky, E.L.; Herenu, C.B.; Goya, R.G.; Zuccolilli, G.O. Dopaminergic mesencephalic systems and behavioral performance in very old rats. Neuroscience 2008, 154, 1598–1606. [Google Scholar] [CrossRef]
- Del Arco, A.; Segovia, G.; de Blas, M.; Garrido, P.; Acuña-Castroviejo, D.; Pamplona, R.; Mora, F. Prefrontal cortex, caloric restriction and stress during aging: Studies on dopamine and acetylcholine release, BDNF and working memory. Behav. Brain Res. 2011, 216, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Parr-Brownlie, L.C.; Hyland, B.I. Bradykinesia induced by dopamine D2 receptor blockade is associated with reduced motor cortex activity in the rat. J. Neurosci. Res. 2005, 25, 5700–5709. [Google Scholar] [CrossRef] [PubMed]
- Lapchak, P.A.; Miller, P.J.; Jiao, S. Glial cell line-derived neurotrophic factor induces the dopaminergic and cholinergic phenotype and increases locomotor activity in aged Fischer 344 rats. Neuroscience 1997, 77, 745–752. [Google Scholar] [CrossRef]
- Love, S.; Plaha, P.; Patel, N.K.; Hotton, G.R.; Brooks, D.J.; Gill, S.S. Glial cell line–derived neurotrophic factor induces neuronal sprouting in human brain. Nat. Med. 2005, 11, 703–704. [Google Scholar] [CrossRef] [PubMed]
- Horner, A.M.; Russ, D.W.; Biknevicius, A.R. Effects of early-stage aging on locomotor dynamics and hind limb muscle force production in the rat. J. Exp. Biol. 2011, 214, 3588–3595. [Google Scholar] [CrossRef] [PubMed]
- Audet, G.N.; Meek, T.H.; Garland, T., Jr.; Olfert, I.M. Expression of angiogenic regulators and skeletal muscle capillarity in selectively bred high aerobic capacity mice. Exp. Physiol. 1996, 11, 1138–1150. [Google Scholar] [CrossRef]
- Ming, Y.; Bergman, E.; Edström, E.; Ulfhake, B. Reciprocal changes in the expression of neurotrophin mRNAs in target tissues and peripheral nerves of aged rats. Neurosci. Lett. 1999, 273, 187–190. [Google Scholar] [CrossRef]
- Andreassen, C.S.; Jacobsen, J.; Flyvbjerg, A.; Andersen, H. Expression of neurotrophic factors in diabetic muscle—relation to neuropathy and muscle strength. Brain 2009, 132, 2724–2733. [Google Scholar] [CrossRef]
- McCullough, M.J.; Peplinski, N.G.; Kinnell, K.R.; Spitsbergen, J.M. Glial cell line-derived neurotrophic factor (GDNF) protein content in rat skeletal muscle is altered by increased physical activity in vivo and in vitro. Neuroscience 2011, 174, 234–244. [Google Scholar] [CrossRef]
- Wang, W.; Esbensen, Y.; Kunke, D.; Suganthan, R.; Rachek, L.; Bjørås, M.; Eide, L. Mitochondrial DNA damage level determines neural stem cell differentiation fate. J. Neurosci. 2011, 31, 9746–9751. [Google Scholar] [CrossRef]
- Bimonte-Nelson, H.A.; Granholm, A.C.; Nelson, M.E.; Moore, A.B. Patterns of neurotrophin protein levels in male and female Fischer 344 rats from adulthood to senescence: how young is “young” and how old is "old”? Exp. Aging Res. 2008, 34, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.H.; Mattson, M.P. Neurotrophic factors protect cortical synaptic terminals against amyloid- and oxidative stress-induced impairment of glucose transport, glutamate transport and mitochondrial function. Cereb. Cortex 2000, 10, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Joo, S.S.; Kim, T.K.; Lee, S.H.; Kang, H.; Lee, H.J.; Lim, I.; Matsuo, A.; Tooyama, I.; Kim, Y.-B.; Kim, S.U. Human neural stem cells overexpressing choline acetyltransferase restore cognitive function of kainic acid-induced learning and memory deficit animals. Cell Transplant. 2012, 21, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Lee, H.J.; Joo, S.S.; Bae, D.-K.; Yang, G.; Yang, Y.-H.; Lim, I.; Matuo, A.; Tooyama, I.; Kim, Y.-B.; Kim, S.U. Human neural stem cells over-expressing choline acetyltransferase restore cognition in rat model of cognitive dysfunction. Exp. Neurol. 2012, 234, 521–526. [Google Scholar] [CrossRef]
- Park, D.; Yang, Y.-H.; Bae, D.K.; Lee, S.H.; Yang, G.; Kyung, J.; Kim, D.; Choi, E.-K.; Lee, S.W.; Kim, G.H.; Hong, J.T.; Choi, K.-C.; Kim, S.U.; Kim, Y.-B. Improvement of cognitive function and physical activity of ageing mice by human neural stem cells over-expressing choline acetyltransferase. Neurobiol. Aging 2013, 34, 2639–2646. [Google Scholar] [CrossRef]
- Lee HJ; Kim KS; Kim EJ; Choi HB; Lee KH; Park IH; Ko Y; Jeong SW; Kim SU. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells 2007, 25, 1204–1212. [Google Scholar] [CrossRef]
- Kim, S.U.; Nagai, A.; Nakagawa, E.; Choi, H.B.; Bang, J.H.; Lee, H.J.; Lee, M.A.; Lee, Y.B.; Park, I.H. Production and characterization of immortal human neural stem cell line with multipotent differentiation property. Methods Mol. Biol. 2008, 438, 103–121. [Google Scholar]
- Schliebs, R.; Arendt, T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 2011, 221, 555–563. [Google Scholar] [CrossRef]
- Popescu, B.O.; Toescu, E.C.; Popescu, L.M.; Bajenaru, O.; Muresanu, D.F.; Schultzberg, M.; Bogdanovic, N. Blood-brain barrier alterations in ageing and dementia. J. Neurol. Sci. 2009, 283, 99–106. [Google Scholar] [CrossRef]
- Langmead, C.J.; Watson, J.; Reavill, C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol. Ther. 2008, 117, 232–243. [Google Scholar] [CrossRef]
- Wess, J. Muscarinic acetylcholine receptor knockout mice: Novel phenotypes and clinical implications. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 423–450. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chigurupati, S.; Holloway, H.W.; Mughal, M.; Tweedie, D.; Bruestle, D.A.; Mattson, M.P.; Wang, Y.; Harvey, B.K.; Ray, B.; Lahiri, D.K.; Greig, N.H. Exendin-4 ameliorates motor neuron degeneration in cellular and animal models of amyotrophic lateral sclerosis. PLoS One 2012, 7, e32008. [Google Scholar] [CrossRef] [PubMed]
- Couillard-Despres, S.; Iglseder, B.; Aigner, L. Neurogenesis, cellular plasticity and cognition: The impact of stem cells in the adult and aging brain—a mini-review. Gerontology 2011, 57, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Encinas, J.M.; Sierra, A. Neural stem cell deforestation as the main force driving the age-related decline in adult hippocampal neurogenesis. Behav. Brain Res. 2012, 227, 433–439. [Google Scholar] [CrossRef]
- Waldau, B. Stem cell transplantation for enhancement of learning and memory in adult neurocognitive disorders. Aging Dis. 2010, 1, 60–71. [Google Scholar] [PubMed]
- Blurton-Jones, M.; Kitazawa, M.; Martinez-Coria, H.; Castello, N.A.; Müller, F.J.; Loring, J.F.; Yamasaki, T.R.; Poon, W.W.; Green, K.N.; LaFerla, F.M. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc. Natl. Acad. Sci. USA 2009, 106, 13594–13599. [Google Scholar] [CrossRef]
- Lee, H.J.; Lim, I.; Park, S.W.; Kim, Y.-B.; Ko, Y.; Kim, S.U. Human neural stem cells genetically modified to express human nerve growth factor (NGF) gene restore cognition in the mouse with ibotenic acid-induced cognitive dysfunction. Cell Transplant. 2012, 21, 2487–2496. [Google Scholar] [CrossRef]
- Kim, J.; Shin, K.; Cha, Y.; Ban, Y.-H.; Park, S.K.; Jeong, H.S.; Park, D.; Choi, E.-K.; Kim, Y.-B. Neuroprotective effects of human neural stem cells over-expressing choline acetyltransferase in a middle cerebral artery occlusion model. J. Chem. Neuroanat. 2020, 103, 101730. [Google Scholar] [CrossRef]
- Park, D.; Choi, E.-K.; Cho, T.-H.; Joo, S.S.; Kim, Y.-B. Human neural stem cells encoding ChAT gene restore cognitive function via acetylcholine synthesis, Aβ elimination, and neuroregeneration in APPswe/PS1dE9 mice. Int. J. Mol. Sci. 2020, 21, 3958. [Google Scholar] [CrossRef]
- Uemura, M.; Kasahara, Y.; Nagatsuka, K.; Taguchi, A. Cell-based therapy to promote angiogenesis in the brain following ischemic damage. Curr. Vasc. Pharmacol. 2012, 10, 285–288. [Google Scholar] [CrossRef]
- Wei, L.; Fraser, J.L.; Lu, Z.Y.; Hu, X.; Yu, SP. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol. Dis. 2012, 46, 635–645. [Google Scholar] [CrossRef]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, TM.; Fainberg, N.; Ding, Z.; Eggel, A.; Lucin, K.M.; Czirr, E.; Park, J.S.; Couillard-Després, S.; Aigner, L.; Li, G.; Peskind, E.R.; Kaye, J.A.; Quinn, J.F.; Galasko, D.R.; Xie, X.S.; Rando, T.A.; Wyss-Coray, T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.-H.; Park, D.; Choi, E.-K.; Kim, T.M.; Joo, S.S.; Kim, Y.-B. Effectiveness of a combinational treatment for Alzheimer’s disease with human neural stem cells and microglial cells over-expressing functional genes. Int. J. Mol. Sci. 2023, 24, 9561. [Google Scholar] [CrossRef] [PubMed]
- Pertusa, M.; García-Matas, S.; Mammeri, H.; Adell, A.; Rodrigo, T.; Mallet, J.; Cristòfol, R.; Sarkis, C.; Sanfeliu, C. Expression of GDNF transgene in astrocytes improves cognitive function deficits in aged rats. Neurobiol. Aging 2008, 29, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Lester, D.B.; Rogers, T.D.; Blaha, C.D. Acetylcholine–dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci. Ther. 2010, 16, 137–162. [Google Scholar]
- Jiao, S.; Ren, H.; Li, Y.; Zhou, J.; Duan, C.; Lu, L. Differential regulation of IGF-I and IGF-II gene expression in skeletal muscle cells. Mol. Cell. Biochem. 2013, 373, 107–113. [Google Scholar] [CrossRef]
- Aguiar, A.F.; Vechetti-Júnior, I.J.; Alves de Souza, R.W.; Castan, E.P.; Milanezi-Aguiar, R.C.; Padovani, C.R.; Carvalho, R.F.; Silva, M.D. Myogenin, MyoD and IGF-I Regulate muscle mass but not fiber-type conversion during resistance training in rats. Int. J. Sports Med. 2013, 34, 293–301. [Google Scholar] [CrossRef]
- Yang, L.X.; Nelson, P.G. Glia cell line-derived neurotrophic factor regulates the distribution of acetylcholine receptors in mouse primary skeletal muscle cells. Neuroscience 2004, 128, 497–509. [Google Scholar] [CrossRef]
- Miura, P.; Amirouche, A.; Clow, C.; Bélanger, G.; Jasmin, B.J. Brain-derived neurotrophic factor expression is repressed during myogenic differentiation by miR-206. J. Neurochem. 2012, 120, 230–238. [Google Scholar] [CrossRef]
- Yoon, E.-J.; Seong, H.R.; Kyung, J.; Kim, D.; Park, S.; Choi, E.-K.; Kim, Y.-B.; Park, D. Stamina-enhancing effect of human adipose-derived stem cells. Cell Transplant. 2021, 30, 1–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




