Submitted:
11 July 2023
Posted:
12 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
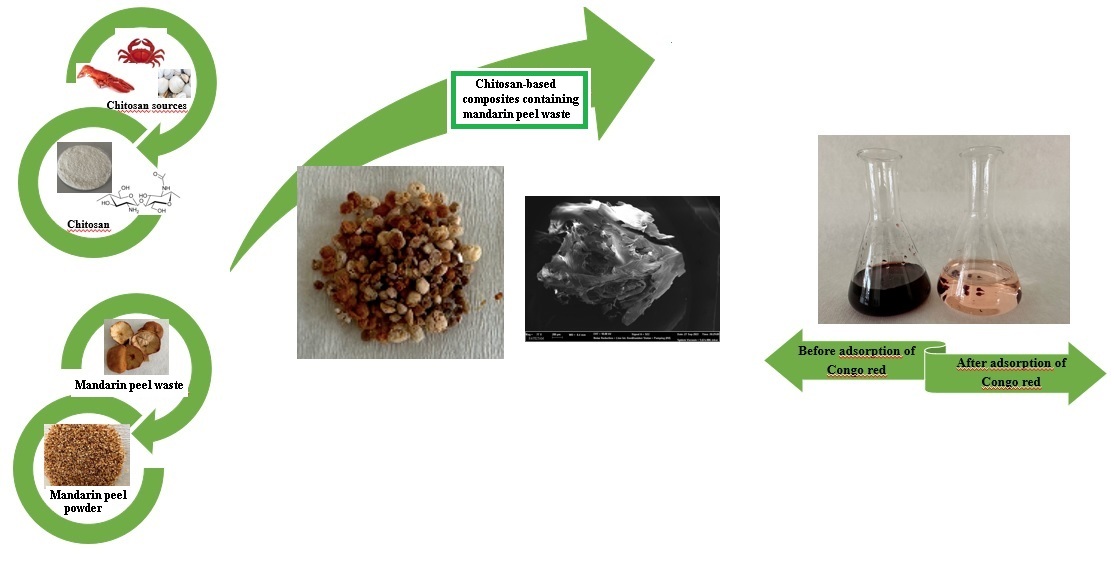
2.2. Preparation of the cross-linked C/CRPW composites
2.3. Characterization of the cross-linked C/CRPW composites
2.4. Batch adsorption studies
3. Results
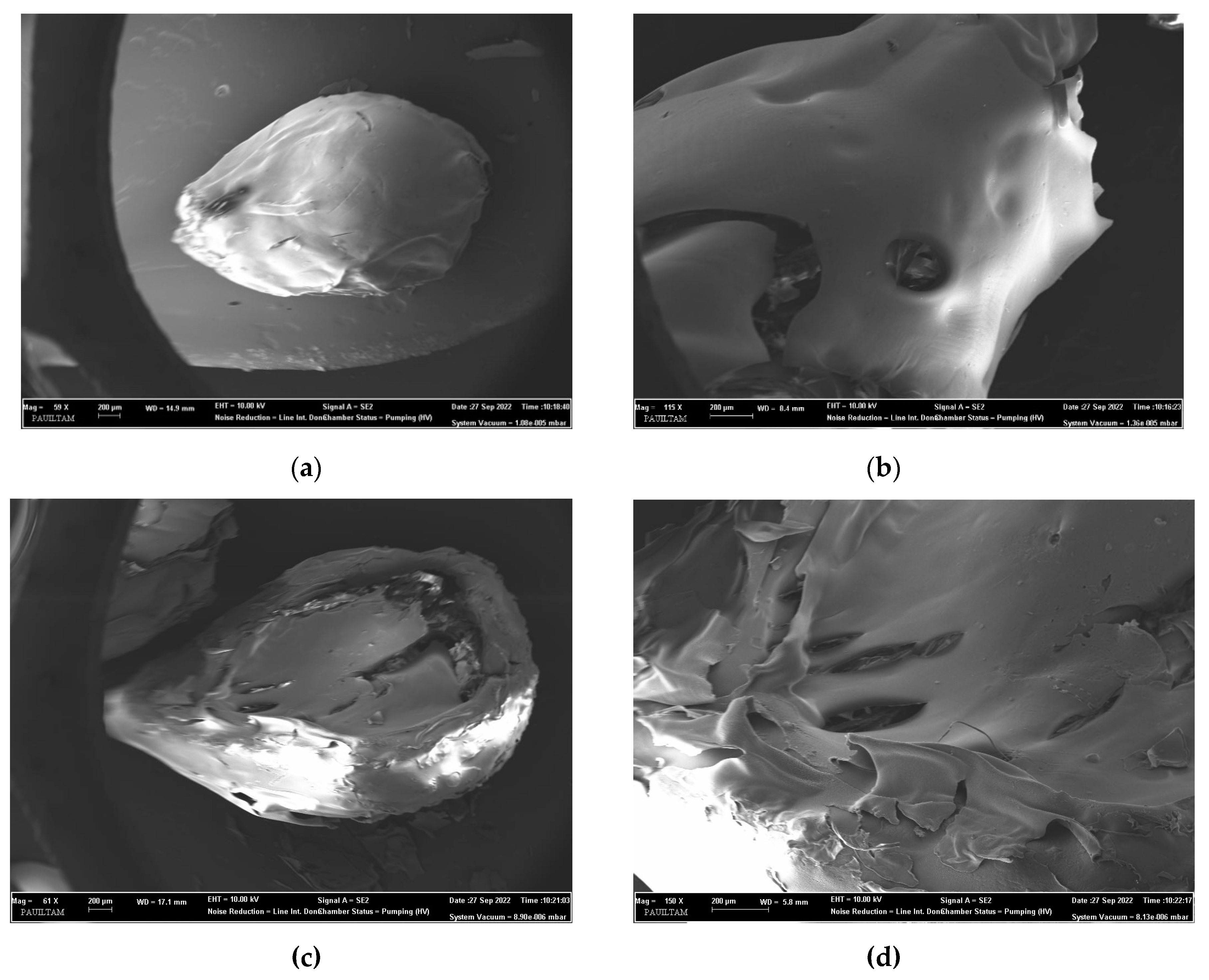
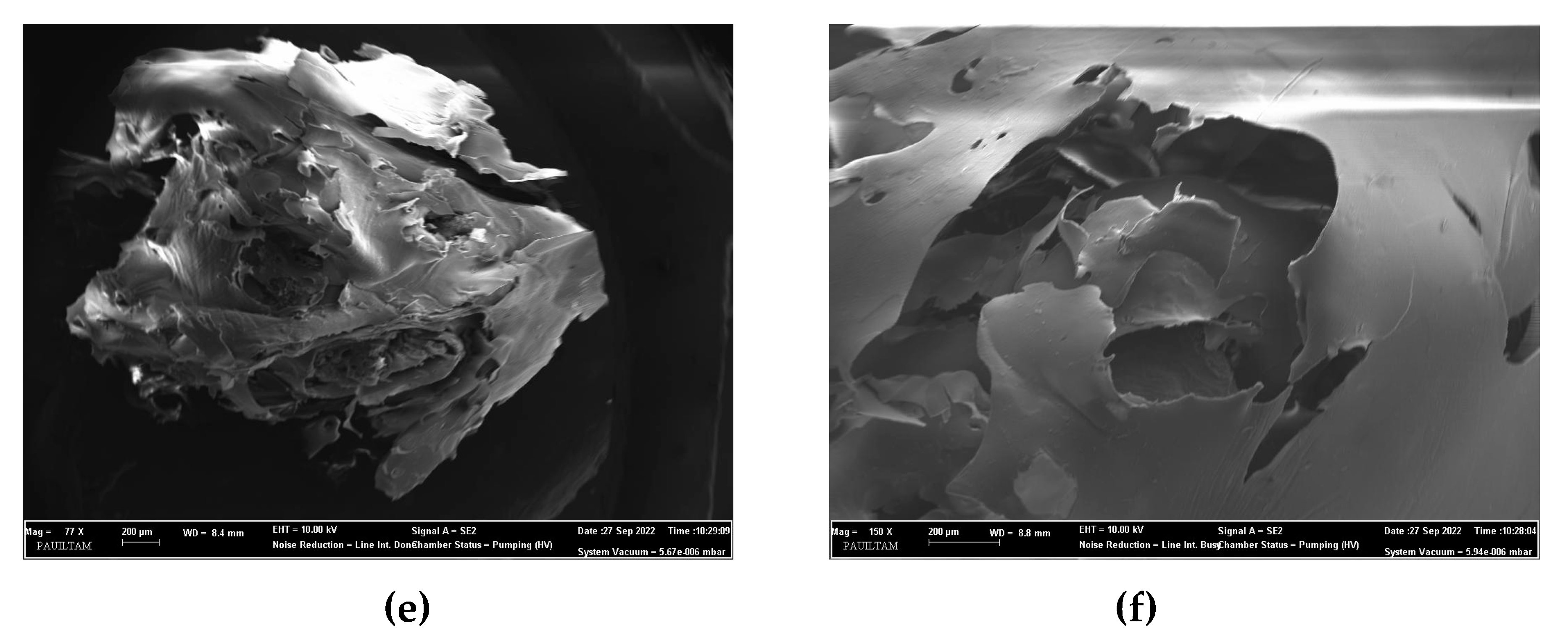
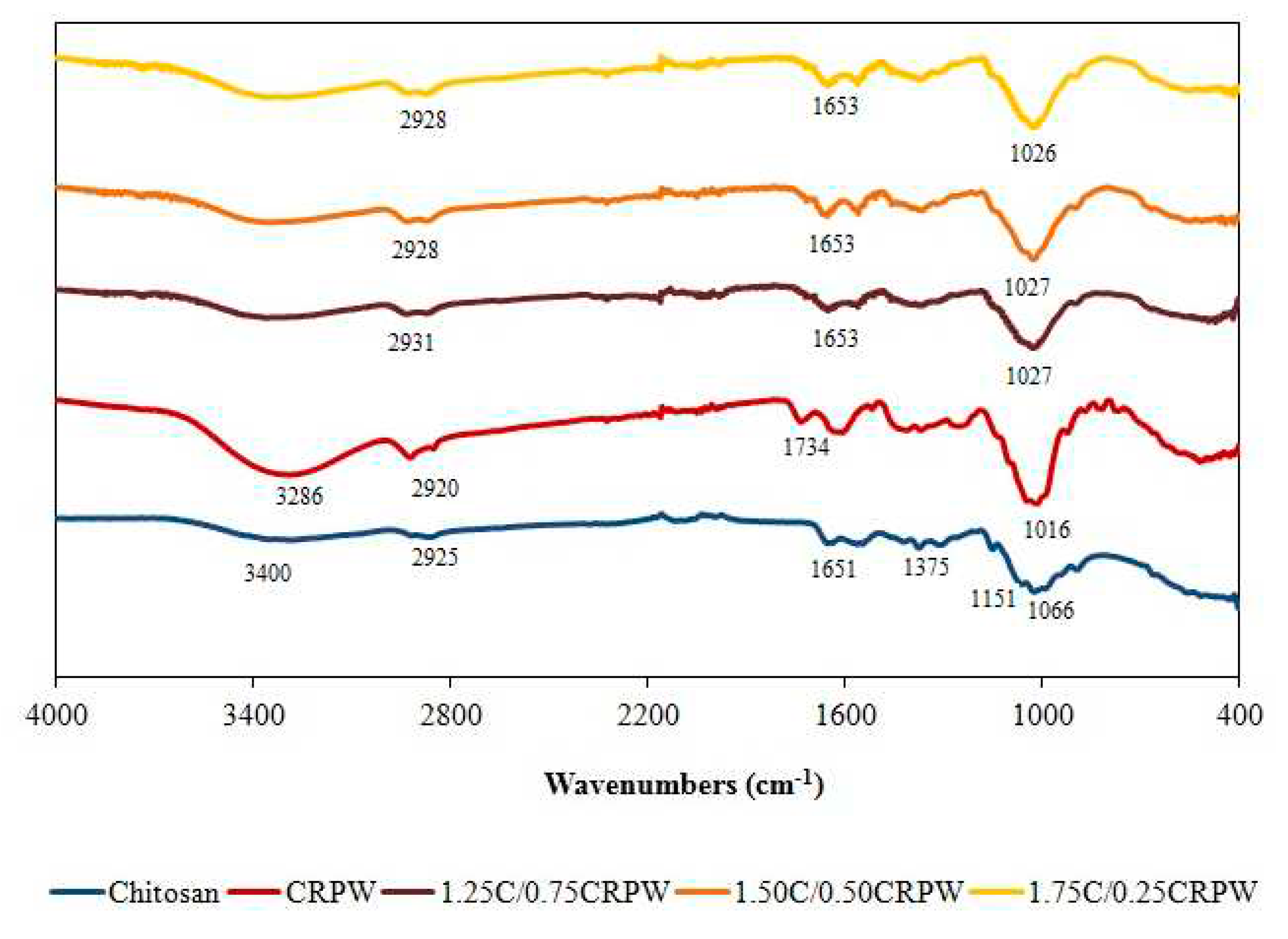
3.1. Characterization of the cross-linked C/CRPW composites
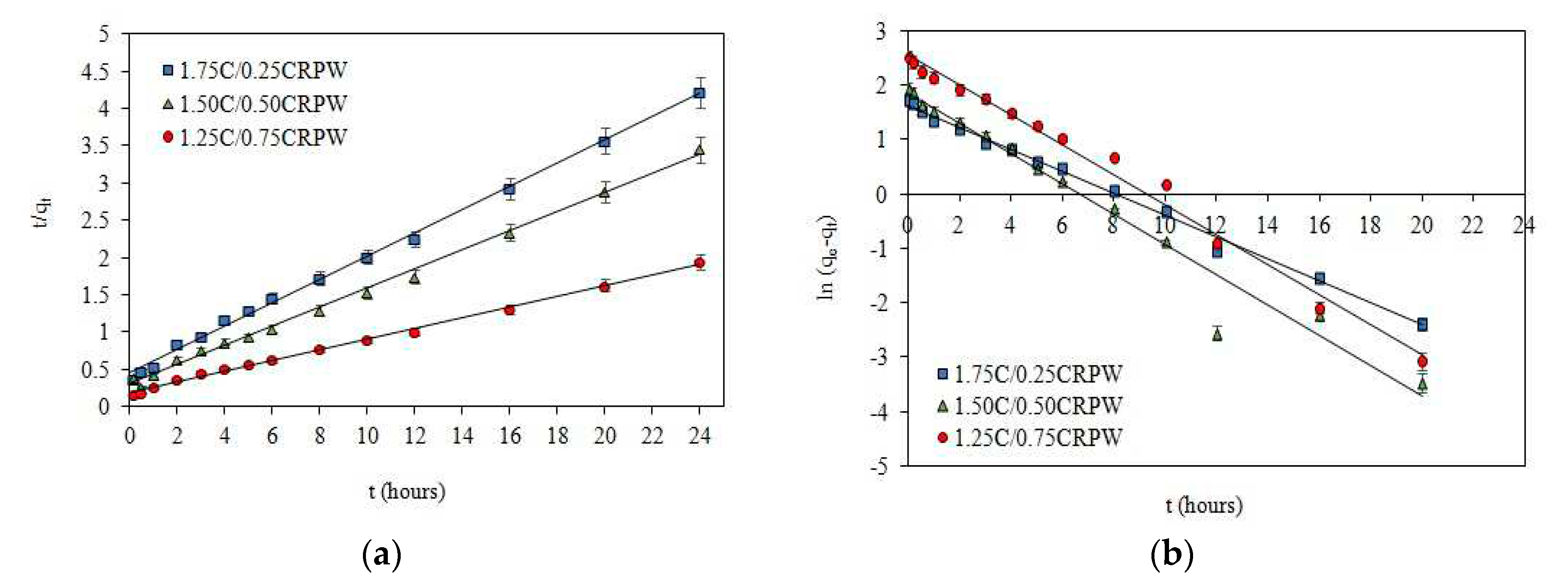
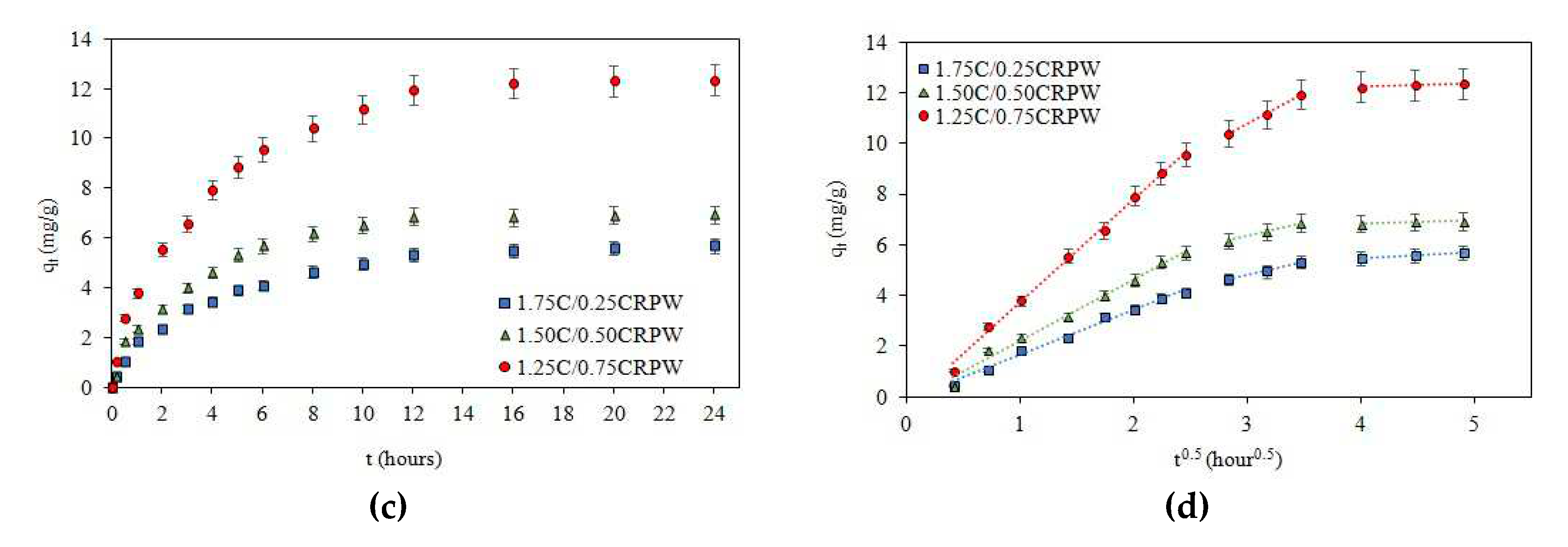
3.2. Effect of contact time and adsorption kinetic models
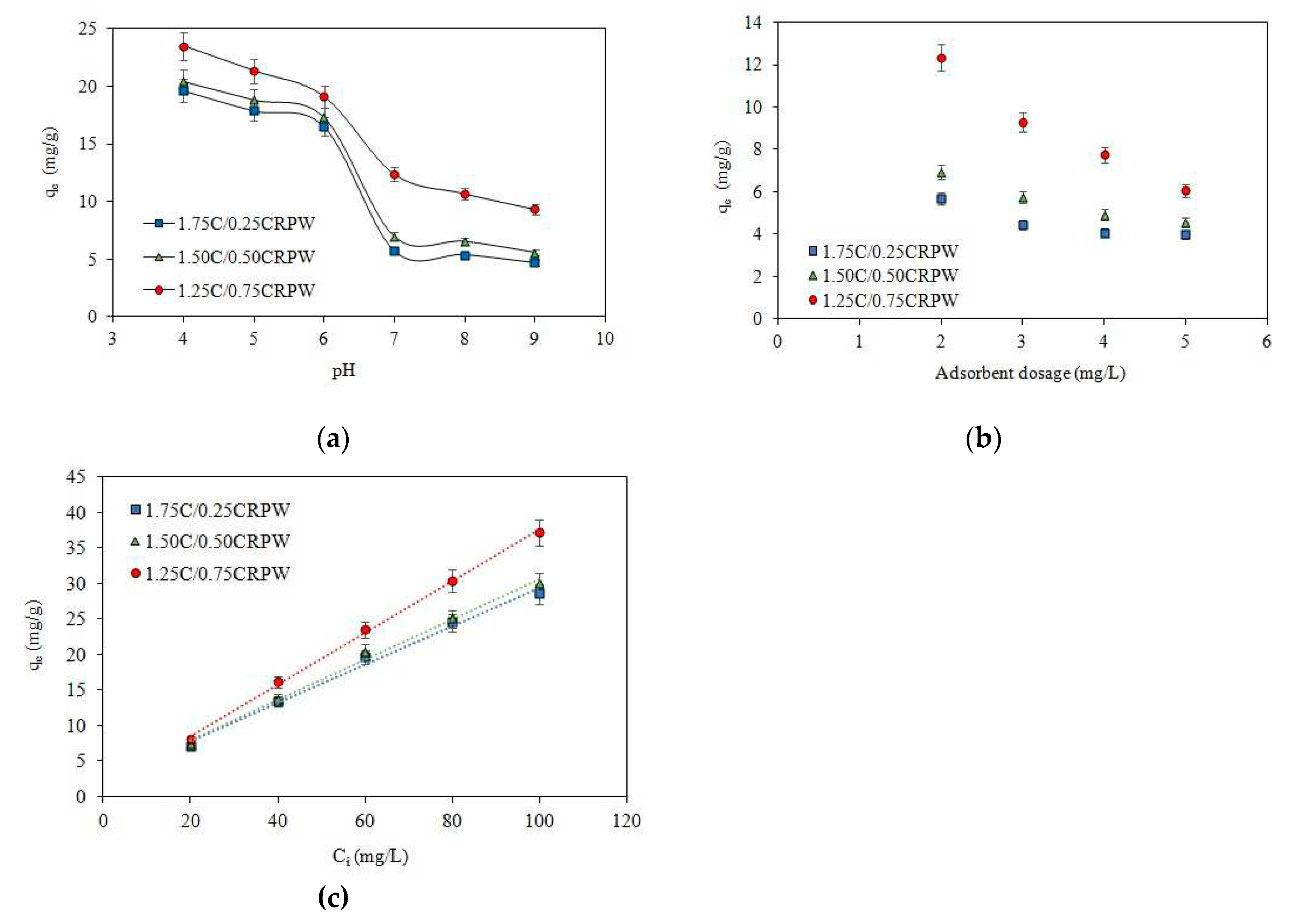
3.3. Effect of initial solution pH
3.4. Effect of adsorbent dosage
3.5. Effect of initial CR concentration
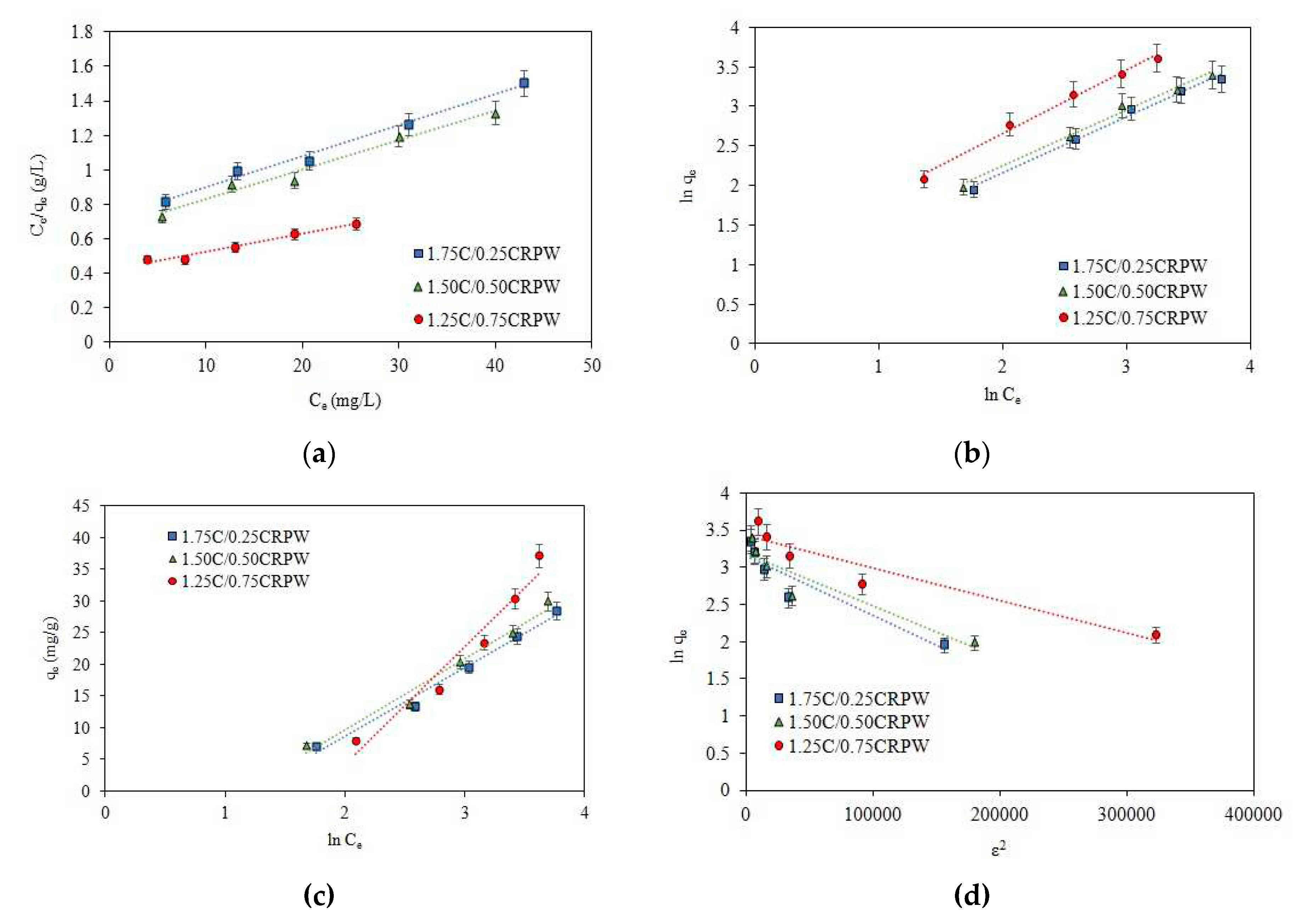
3.6. Adsorption isotherms
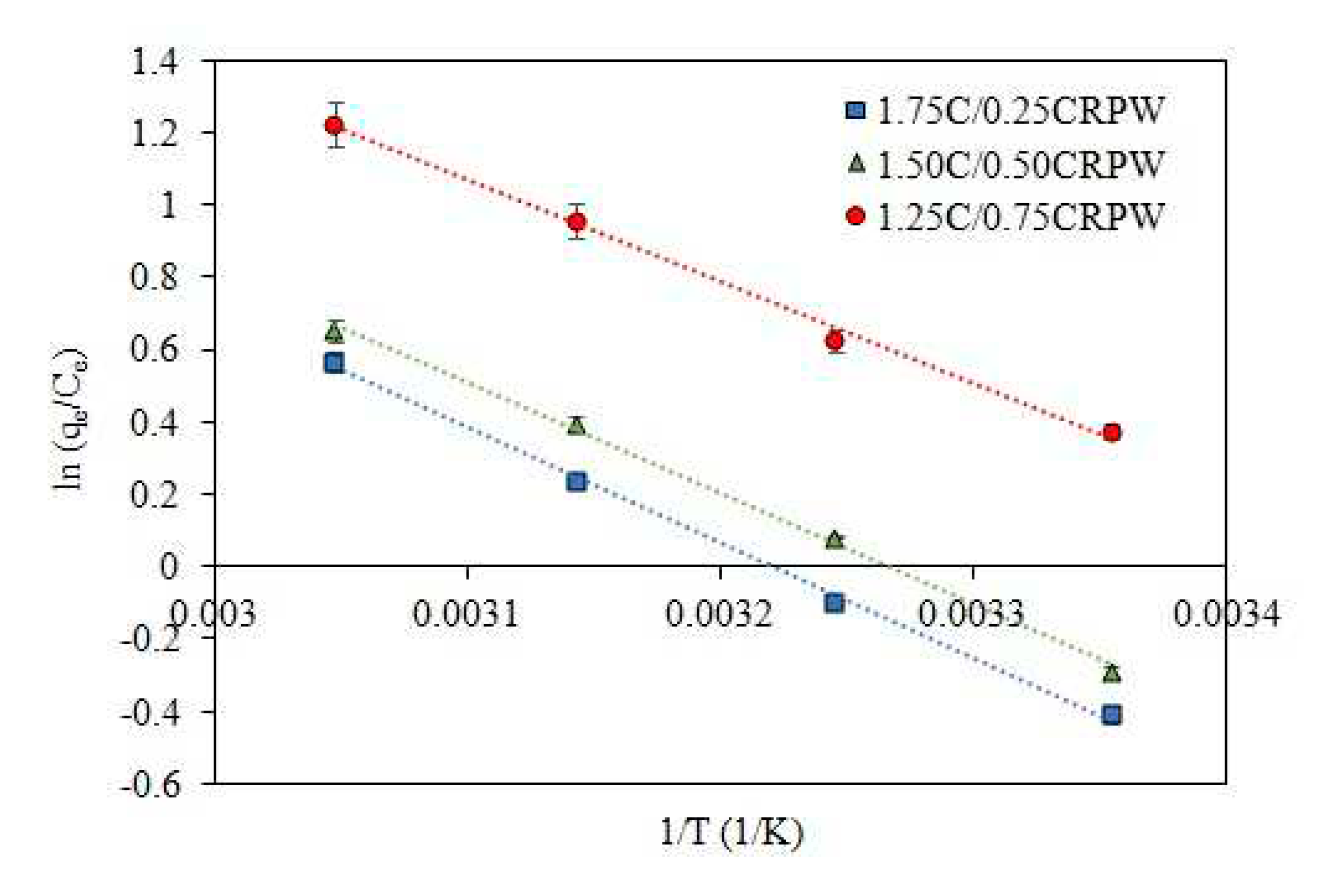
3.7. Effect of temperature and adsorption thermodynamics
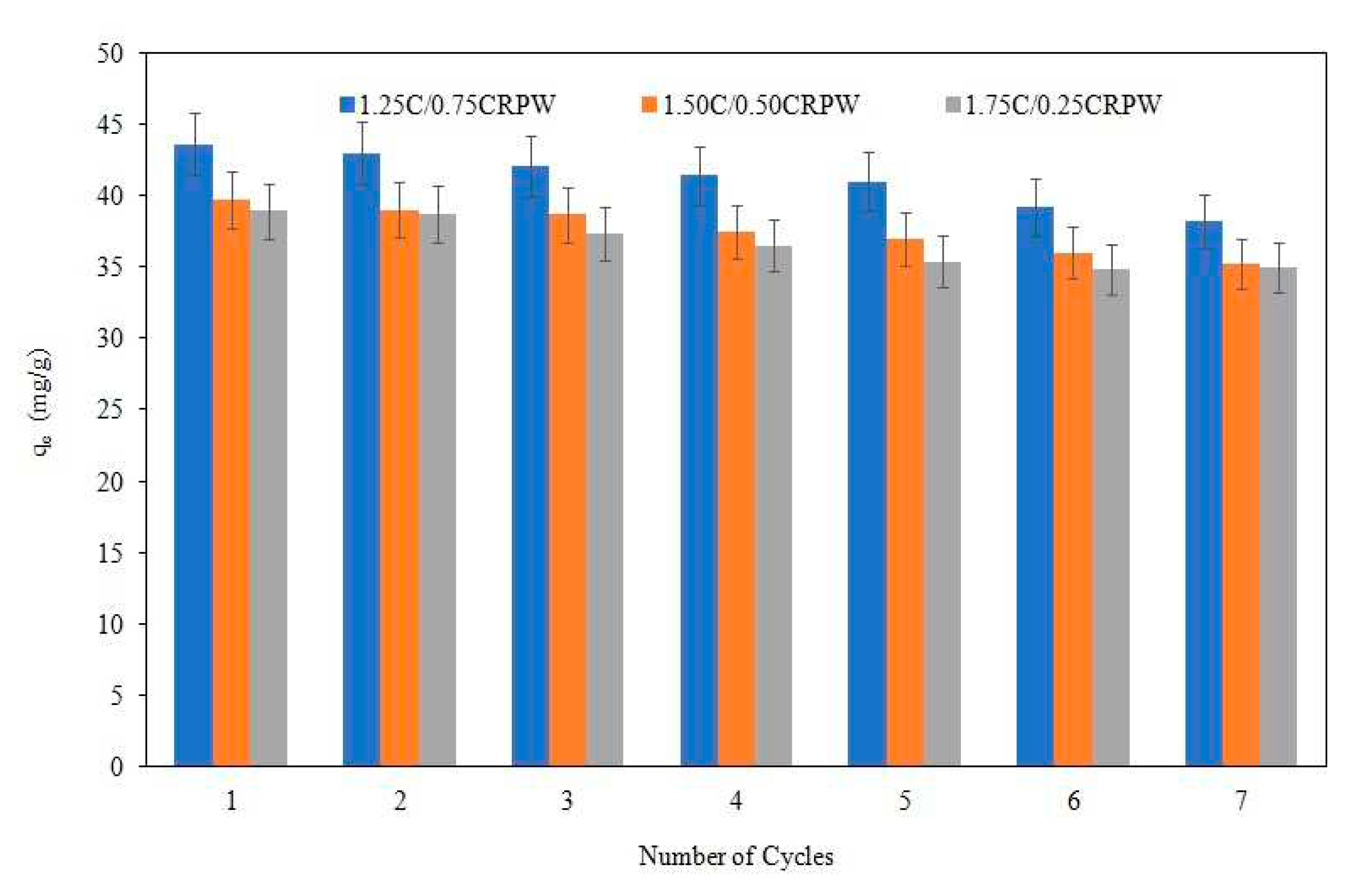
3.8. Reusability studies
3.9. Comparative study
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M. D.; Singh, A.; Khan, M. Z.; Tabraiz, S.; Sheikh, J. Current perspectives, recent advancements, and efficiencies of various dye-containing wastewater treatment technologies. J. Water Process. Eng. 2023, 53, 103579. [Google Scholar] [CrossRef]
- Moradihamedani, P. Recent advances in dye removal from wastewater by membrane technology: a review. Polym. Bull. 2022, 79, 2603–2631. [Google Scholar] [CrossRef]
- Gadekar, M. R.; Ahammed, M. M. Coagulation/flocculation process for dye removal using water treatment residuals: modelling through artificial neural networks. Desalination Water Treat. 2016, 57, 26392–26400. [Google Scholar] [CrossRef]
- Constantin, M.; Asmarandei, I.; Harabagiu, V.; Ghimici, L.; Ascenzi, P.; Fundueanu, G. Removal of anionic dyes from aqueous solutions by an ion-exchanger based on pullulan microspheres. Carbohydr. Polym. 2013, 91, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Ikhlaq, A.; Zafar, M.; Javed, F.; Yasar, A.; Akram, A.; Shabbir, S.; Qi, F. Catalytic ozonation for the removal of reactive black 5 (RB-5) dye using zeolites modified with CuMn2O4/gC3N4 in a synergic electro flocculation-catalytic ozonation process. Water Sci. Technol. 2021, 84, 1943–1953. [Google Scholar] [CrossRef]
- Vimonses, V.; Lei, S.; Jin, B.; Chow, C. W.; Saint, C. Adsorption of congo red by three Australian kaolins. Appl. Clay Sci. 2009, 43, 465–472. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, B.; Fan, J.; Yu, J.; Ho, W. Review on nickel-based adsorption materials for Congo red. J. Hazard. Mater. 2021, 403, 123559. [Google Scholar] [CrossRef]
- Ausavasukhi, A.; Kampoosaen, C.; Kengnok, O. Adsorption characteristics of Congo red on carbonized leonardite. J. Clean. Prod. 2016, 134, 506–514. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Wang, M.; Chen, B.; Zhang, Y.; Sun, Y.; Chen, K.; Du, Q.; Jing, Z.; Jin, Y. Preparation of MIL-88A micro/nanocrystals with different morphologies in different solvents for efficient removal of Congo red from water: Synthesis, characterization, and adsorption mechanisms. Microporous Mesoporous Mater. 2022, 345, 112241. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Q.; Zhang, L.; Pei, L.; Xue, H.; Li, Z. Adsorption of methylene blue and Congo red from aqueous solution on 3D MXene/carbon foam hybrid aerogels: A study by experimental and statistical physics modeling. J. Environ. Chem. Eng. 2023, 11, 109206. [Google Scholar] [CrossRef]
- Onizuka, T.; Iwasaki, T. Low-temperature solvent-free synthesis of polycrystalline hematite nanoparticles via mechanochemical activation and their adsorption properties for Congo red. Solid State Sci. 2022, 129, 106917. [Google Scholar] [CrossRef]
- Kavitha, D.; Namasivayam, C. Recycling coir pith, an agricultural solid waste, for the removal of procion orange from wastewater. Dyes Pigm. 2007, 74, 237–248. [Google Scholar] [CrossRef]
- Gupta, N.; Kushwaha, A. K.; Chattopadhyaya, M. C. Application of potato (Solanum tuberosum) plant wastes for the removal of methylene blue and malachite green dye from aqueous solution. Arab. J. Chem. 2016, 9, S707–S716. [Google Scholar] [CrossRef]
- Sudrajat, H.; Susanti, A.; Putri, D. K. Y.; Hartuti, S. Mechanistic insights into the adsorption of methylene blue by particulate durian peel waste in water. Water Sci. Technol. 2021, 84, 1774–1792. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, X.; Wang, R.; Zhang, Y.; Wang, X. Biosorption of Cd2+ from aqueous solution by Ca2+/Mg2+ type Citrus paradisi Macf. peel biosorbents. Water Sci. Technol. 2019, 80, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Eldeeb, T. M.; Aigbe, U. O.; Ukhurebor, K. E.; Onyancha, R. B.; El-Nemr, M. A.; Hassaan, M. A.; Osibote, O. A.; Ragab, S.; Okundaye, B.; Balogun, V. A.; El Nemr, A. Biosorption of acid brown 14 dye to mandarin-CO-TETA derived from mandarin peels. Biomass Convers. Biorefin. 2022, 1–21. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Removal of methylene blue dye from artificially contaminated water using citrus limetta peel waste as a very low cost adsorbent. J. Taiwan Inst. Chem. Eng. 2016, 66, 154–163. [Google Scholar] [CrossRef]
- Altunkaynak, Y.; Canpolat, M.; Yavuz, Ö. Adsorption of cobalt (II) ions from aqueous solution using orange peel waste: equilibrium, kinetic and thermodynamic studies. J. Iran. Chem. Soc. 2022, 19, 2437–2448. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Cignolo, D.; Fini, P.; Fanelli, F.; Cosma, P. From agricultural wastes to a resource: Kiwi Peels, as long-lasting, recyclable adsorbent, to remove emerging pollutants from water. The case of Ciprofloxacin removal. Sustain. Chem. Pharm. 2022, 29, 100749. [Google Scholar] [CrossRef]
- Cho, E. J.; Lee, Y. G.; Chang, J.; Bae, H. J. A high-yield process for production of biosugars and hesperidin from mandarin peel wastes. Molecules 2020, 25, 4286. [Google Scholar] [CrossRef]
- Jang, S. K.; Jung, C. D.; Seong, H.; Myung, S.; Kim, H. An integrated biorefinery process for mandarin peel waste elimination. J. Clean. Prod. 2022, 371, 133594. [Google Scholar] [CrossRef]
- Bhatti, H. N.; Zaman, Q.; Kausar, A.; Noreen, S.; Iqbal, M. Efficient remediation of Zr (IV) using citrus peel waste biomass: Kinetic, equilibrium and thermodynamic studies. Ecol. Eng. 2016, 95, 216–228. [Google Scholar] [CrossRef]
- Sadiq, A. C.; Olasupo, A.; Ngah, W. S. W.; Rahim, N. Y.; Suah, F. B. M. A decade development in the application of chitosan-based materials for dye adsorption: A short review. Int. J. Biol. Macromol. 2021, 191, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Barus, D. A.; Humaidi, S.; Ginting, R. T.; Sitepu, J. Enhanced adsorption performance of chitosan/cellulose nanofiber isolated from durian peel waste/graphene oxide nanocomposite hydrogels. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100650. [Google Scholar] [CrossRef]
- Habiba, U.; Siddique, T. A.; Joo, T. C.; Salleh, A.; Ang, B. C.; Afifi, A. M. Synthesis of chitosan/polyvinyl alcohol/zeolite composite for removal of methyl orange, Congo red and chromium (VI) by flocculation/adsorption. Carbohydr. Polym. 2017, 157, 1568–1576. [Google Scholar] [CrossRef]
- Abdić, Š.; Memić, M.; Šabanović, E.; Sulejmanović, J.; Begić, S. Adsorptive removal of eight heavy metals from aqueous solution by unmodified and modified agricultural waste: tangerine peel. Int. J. Environ. Sci. Technol. 2018, 15, 2511–2518. [Google Scholar] [CrossRef]
- Pavithra, S.; Thandapani, G.; Sugashini, S.; Sudha, P. N.; Alkhamis, H. H.; Alrefaei, A. F.; Almutairi, M. H. Batch adsorption studies on surface tailored chitosan/orange peel hydrogel composite for the removal of Cr (VI) and Cu (II) ions from synthetic wastewater. Chemosphere 2021, 271, 129415. [Google Scholar] [CrossRef]
- Li, B.; Shan, C. L.; Zhou, Q.; Fang, Y.; Wang, Y. L.; Xu, F.; Han, L. R.; Ibrahim, M.; Guo, L. B.; Xie, G. L.; Sun, G. C. Synthesis, characterization, and antibacterial activity of cross-linked chitosan-glutaraldehyde. Mar. Drugs 2013, 11, 1534–1552. [Google Scholar] [CrossRef]
- Ghorai, S.; Sarkar, A. K.; Panda, A. B.; Pal, S. Effective removal of Congo red dye from aqueous solution using modified xanthan gum/silica hybrid nanocomposite as adsorbent. Bioresour. Technol. 2013, 144, 485–491. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y. S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W. J.; Morris, J. C. Advances in water pollution research: removal of biologically resistant pollutant from wastewater by adsorption. Proceedings of the Intern. Conf. on Water Pollution Symposium, 2, Pergamon Press, Oxford, UK, 1962; 231-266.
- Xie, B.; Qin, J.; Wang, S.; Li, X.; Sun, H.; Chen, W. Adsorption of phenol on commercial activated carbons: modelling and interpretation. Int. J. Environ. Res. Public Health 2020, 17, 789. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. E.; Hu, Y. Y.; Xie, L.; Peng, K. Biosorption behavior of azo dye by inactive CMC immobilized Aspergillus fumigatus beads. Bioresour. Technol. 2008, 99, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, J.; Wang, F.; Chu, Y.; Yang, L.; Xia, M. Mechanism of carboxymethyl chitosan hybrid montmorillonite and adsorption of Pb (II) and Congo red by CMC-MMT organic-inorganic hybrid composite. Int. J. Biol. Macromol. 2020, 149, 1161–1169. [Google Scholar] [CrossRef]
- Ohemeng-Boahen, G.; Sewu, D. D.; Tran, H. N.; Woo, S. H. Enhanced adsorption of congo red from aqueous solution using chitosan/hematite nanocomposite hydrogel capsule fabricated via anionic surfactant gelation. Colloids Surf. A: Physicochem. Eng. Asp. 2021, 625, 126911. [Google Scholar] [CrossRef]
- Alardhi, S. M.; Fiyadh, S. S.; Salman, A. D.; Adelikhah, M. Prediction of methyl orange dye (MO) adsorption using activated carbon with an artificial neural network optimization modeling. Heliyon 2023, 9, e12888. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. M. F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Freundlich, H.; Heller, W. The adsorption of cis- and trans-azobenzene. J. Am. Chem. Soc. 1939, 61, 2228–2230. [Google Scholar] [CrossRef]
- Pursell, C. J.; Hartshorn, H.; Ward, T.; Chandler, B. D.; Boccuzzi, F. Application of the Temkin model to the adsorption of CO on gold. J. Phys. Chem. C 2011, 115, 23880–23892. [Google Scholar] [CrossRef]
- Dubinin, M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Mate, C. J.; Mishra, S. Synthesis of borax cross-linked Jhingan gum hydrogel for remediation of Remazol Brilliant Blue R (RBBR) dye from water: Adsorption isotherm, kinetic, thermodynamic and biodegradation studies. Int. J. Biol. Macromol. 2020, 151, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Atkins, P.; de Paula, J.; Keeler, J. Atkins’ Physical Chemistry; OUP Oxford: Oxford, 2017. [Google Scholar]
- Al-Harby, N. F.; Albahly, E. F.; Mohamed, N. A. Kinetics, isotherm and thermodynamic studies for efficient adsorption of Congo Red dye from aqueous solution onto novel cyanoguanidine-modified chitosan adsorbent. Polymers 2021, 13, 4446. [Google Scholar] [CrossRef] [PubMed]
- Wekoye, J. N.; Wanyonyi, W. C.; Wangila, P. T.; Tonui, M. K. Kinetic and equilibrium studies of Congo red dye adsorption on cabbage waste powder. Environ. Toxicol. Chem. 2020, 2, 24–31. [Google Scholar] [CrossRef]
- Namasivayam, C.; Kavitha, D. Removal of Congo Red from water by adsorption onto activated carbon prepared from coir pith, an agricultural solid waste. Dyes Pigm. 2002, 54, 47–58. [Google Scholar] [CrossRef]
- Sivarama Krishna, L.; Sreenath Reddy, A.; Muralikrishna, A.; Wan Zuhairi, W. Y.; Osman, H.; Varada Reddy, A. Utilization of the agricultural waste (Cicer arientinum Linn fruit shell biomass) as biosorbent for decolorization of Congo red. Desalination Water Treat. 2015, 56, 2181–2192. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, L. Y.; Liang, Y.; Chen, Q.; Li, Z. S.; Li, C. H.; Wang, Z.H.; Li, W. Coconut-based activated carbon fibers for efficient adsorption of various organic dyes. RSC Adv. 2018, 8, 42280–42291. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Dai, Z.; Yu, Q.; Miao, Y.; Xu, R. Facile preparation of a polypyrrole modified Chinese yam peel-based adsorbent: characterization, performance, and application in removal of Congo red dye. RSC Adv. 2022, 12, 9424–9434. [Google Scholar] [CrossRef]
- Dbik, A.; Bentahar, S.; El Khomri, M.; El Messaoudi, N.; Lacherai, A. Adsorption of Congo red dye from aqueous solutions using tunics of the corm of the saffron. Mater. Today: Proc. 2020, 22, 134–139. [Google Scholar] [CrossRef]
| Samples | BET surface area (m2/g) | BJH pore volume (cm3/g) | BJH pore size (A°) |
|---|---|---|---|
| 1.75C/0.25CRPW | 10.04 | 0.06225 | 29.83 |
| 1.50C/0.50CRPW | 13.86 | 0.1014 | 16.17 |
| 1.25C/0.75CRPW | 20.42 | 0.1500 | 16.39 |
| CRPW | 17.61 | 0.02269 | 18.85 |
| Models | Parameters | Values | ||
|---|---|---|---|---|
| 1.75C/0.25CRPW | 1.50C/0.50CRPW | 1.25C/0.75CRPW | ||
| Experimental result | qe,exp (mg/g) | 5.69 | 6.94 | 12.35 |
| Lagergren’s pseudo-first-order | qe,cal (mg/g) | 5.09 | 6.34 | 12.81 |
| k1 (1/hour) | 0.2021 | 0.2775 | 0.2763 | |
| R2 | 0.9950 | 0.9652 | 0.9893 | |
| Pseudo-second-order | qe,cal (mg/g) | 6.39 | 7.78 | 13.91 |
| k2 (g/mg hour) | 0.0545 | 0.0548 | 0.0275 | |
| R2 | 0.9971 | 0.9963 | 0.9968 | |
| Intra-particle diffusion | kid,1 (mg/g hour0.5) | 1.7985 | 2.4436 | 4.0972 |
| C1 | -0.1430 | -0.2177 | -0.3548 | |
| R2 | 0.9901 | 0.9876 | 0.9968 | |
| kid,2 (mg/g hour0.5) | 1.0920 | 1.0936 | 2.4286 | |
| C2 | 1.5399 | 3.0752 | 3.5203 | |
| R2 | 0.9954 | 0.9997 | 0.9984 | |
| kid,3 (mg/g hour0.5) | 0.2411 | 0.1211 | 0.1380 | |
| C3 | 4.5149 | 6.3582 | 11.682 | |
| R2 | 0.9972 | 0.9551 | 0.9870 | |
| Isotherm | Constants | 1.75C/0.25CRPW | 1.50C/0.50CRPW | 1.25C/0.75CRPW |
|---|---|---|---|---|
| Langmuir | qmax,L (mg/g) | 55.56 | 58.48 | 97.09 |
| KL (L/mg) | 0.0251 | 0.0260 | 0.0243 | |
| RL | 0.6659-0.2851 | 0.6581-0.2779 | 0.6729-0.2915 | |
| R2 | 0.9895 | 0.9764 | 0.9714 | |
| Freundlich | n | 1.4102 | 1.4098 | 1.2472 |
| KF ((mg/g)(mg/L)−1/n) | 2.115 | 2.284 | 2.886 | |
| R2 | 0.9904 | 0.9907 | 0.9908 | |
| Temkin | BT (J/mol) | 10.88 | 11.28 | 18.56 |
| KT (L/mg) | 0.3003 | 0.3193 | 0.1706 | |
| R2 | 0.9844 | 0.9771 | 0.9520 | |
| Dubinin-Radushkevich | qmax,D-R (mg/g) | 23.50 | 24.23 | 30.66 |
| KD-R (mol2/J2) | 8 x 10-6 | 7 x 10-6 | 4 x 10-6 | |
| E (kJ/mol) | 0.250 | 0.267 | 0.353 | |
| R2 | 0.8736 | 0.8631 | 0.9049 |
| Thermodynamic parameters | T (K) | 1.75C/0.25CRPW | 1.50C/0.50CRPW | 1.25C/0.75CRPW |
|---|---|---|---|---|
| ΔG° (J/mol) | 298.15 | 1052.27 | 668.89 | -878.22 |
| 308.15 | 199.92 | -164.92 | -1691.12 | |
| 318.15 | -652.43 | -998.73 | -2504.01 | |
| 328.15 | -1504.78 | -1832.54 | -3316.90 | |
| ΔH° (kJ/mol) | 26.465 | 25.528 | 23.358 | |
| ΔS° (J/mol.K) | 85.235 | 83.38 | 81.29 |
| Adsorbent | Adsorption capacity (mg/g) | References |
|---|---|---|
| Cabbage waste powder | 2.313 | [46] |
| Activated carbon prepared from coir pith | 6.72 | [47] |
| Bengal gram fruit shell | 22.22 | [48] |
| Coconut-based activated carbon fibers | 22.1 | [49] |
| Chinese yam peel–polypyrrole composites | 86.66 | [50] |
| Tunics of the corm of the saffron | 6.2 | [51] |
| 1.75C/0.25CRPW composites | 38.92 | Present work |
| 1.50C/0.50CRPW composites | 39.66 | Present work |
| 1.25C/0.75CRPW composites | 43.57 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





