Submitted:
10 July 2023
Posted:
12 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Backgrounds
3. Study Background Theoretical Foundations
3.1. Degree of Ionization
3.2. Electric Double Layer Development in Oil in Water Emulsion Systems
3.3. Zeta Potential Model (define zeta potential)
4. Relevance of Theoretical Models to Oil Water Interfacial Chemistry and Emulsion Stability
4.1. Salinity and pH Depended Surface Charge Density
4.2. pH Depended Degree of Ionization of Surface Ionisable Groups on Oil
5. Methodologies
5.1. Theoretical Calculations
5. Results and Discussion
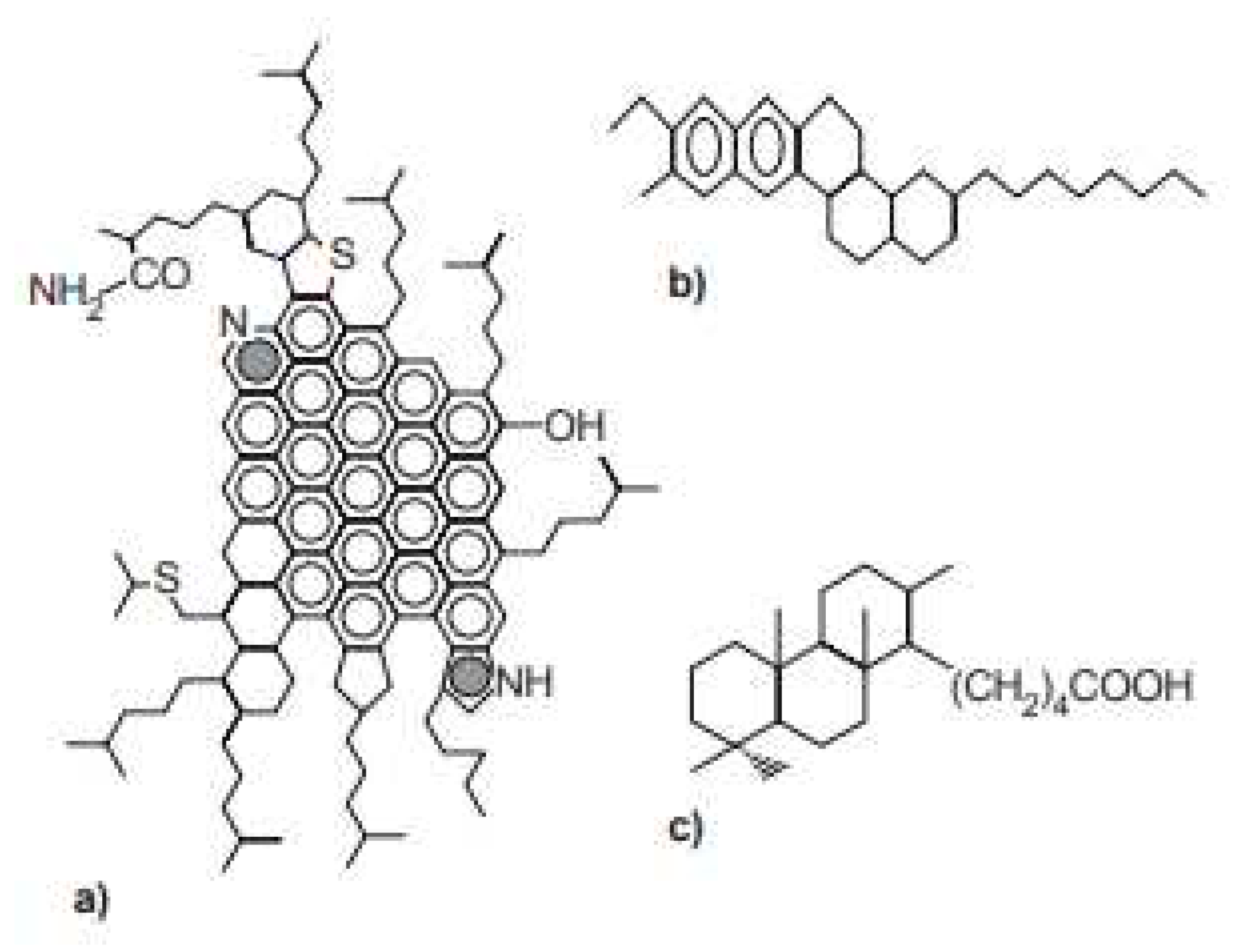
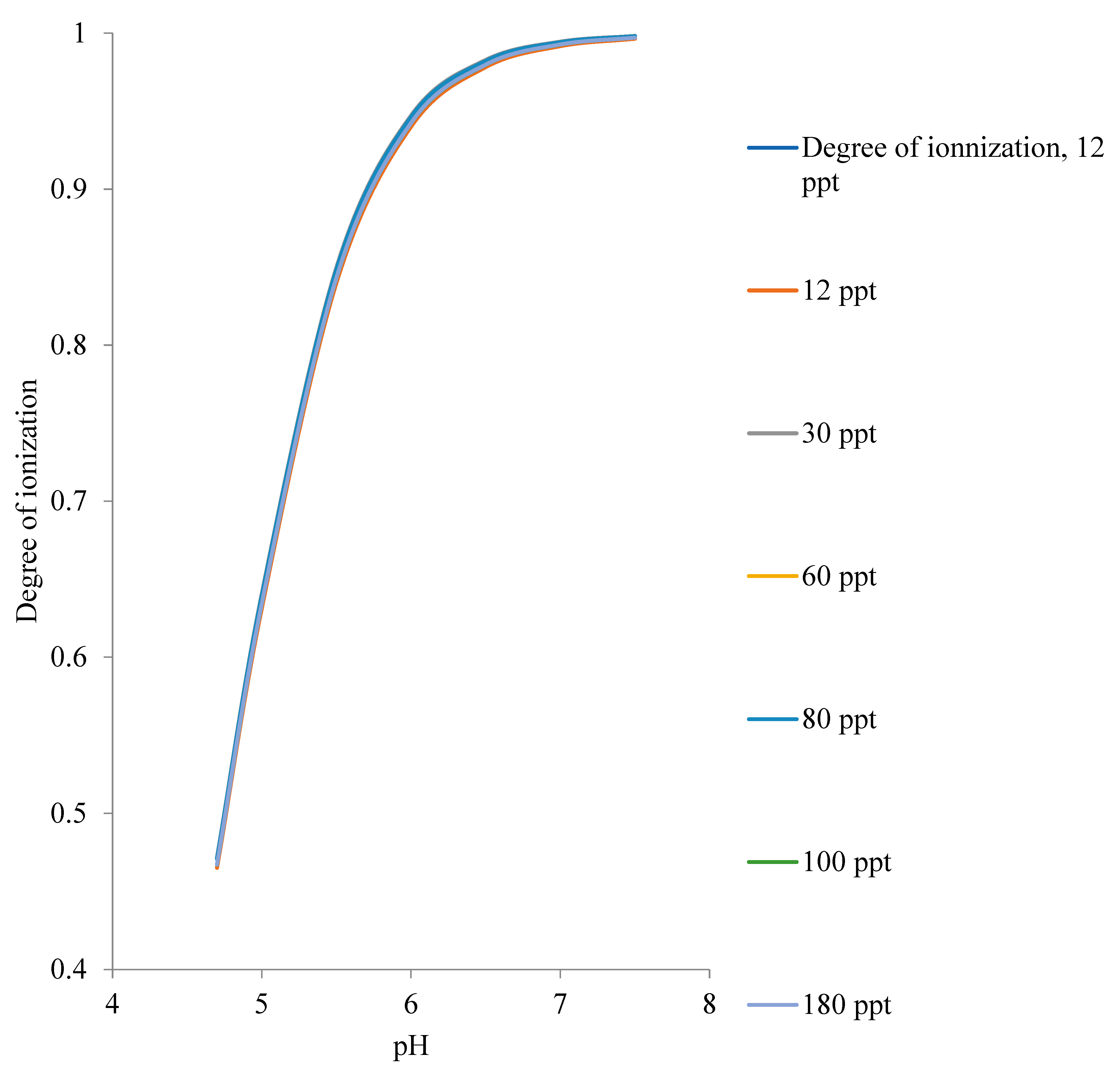
5.1. Degree of Ionization of carboxyl Acidic Group of Oils
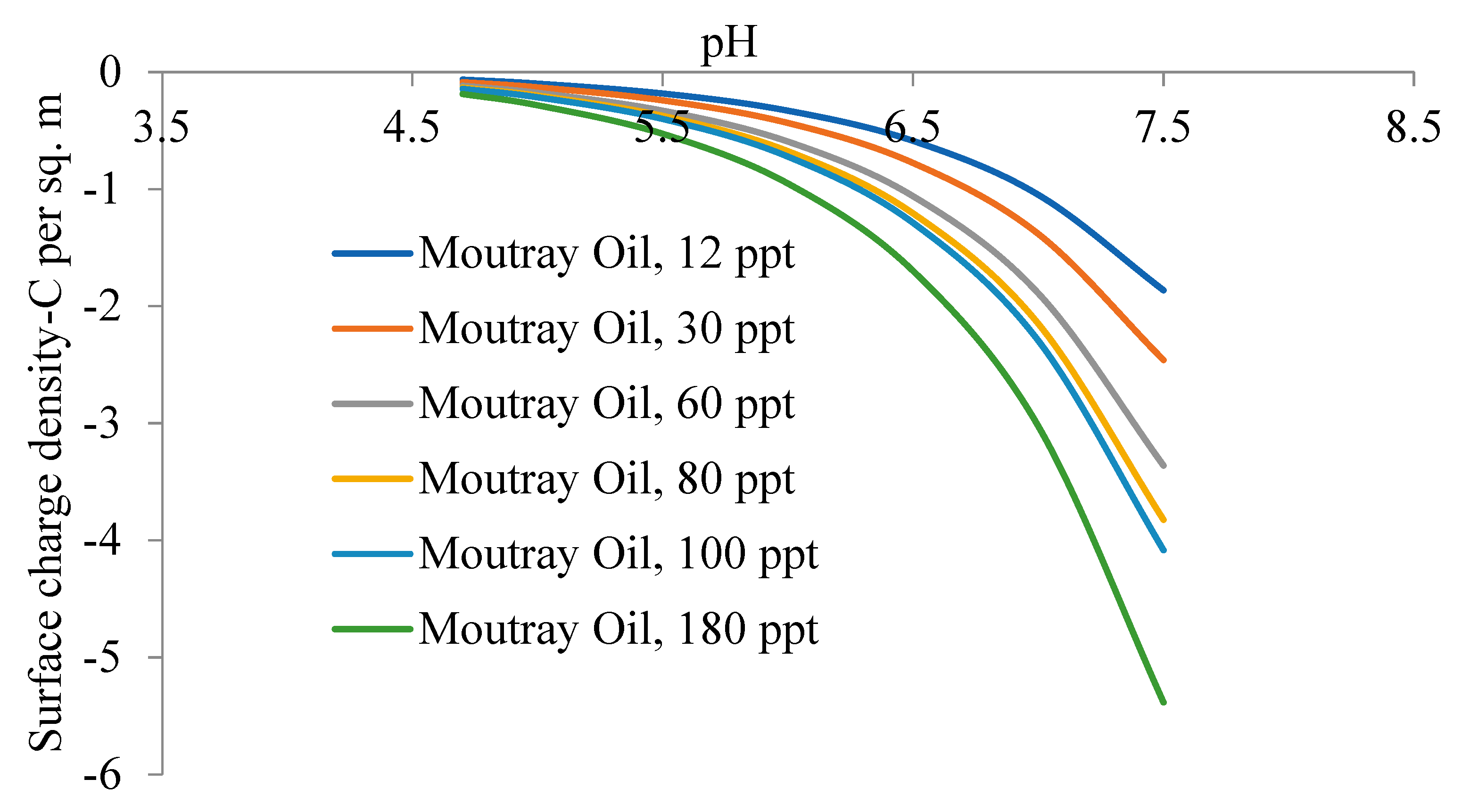
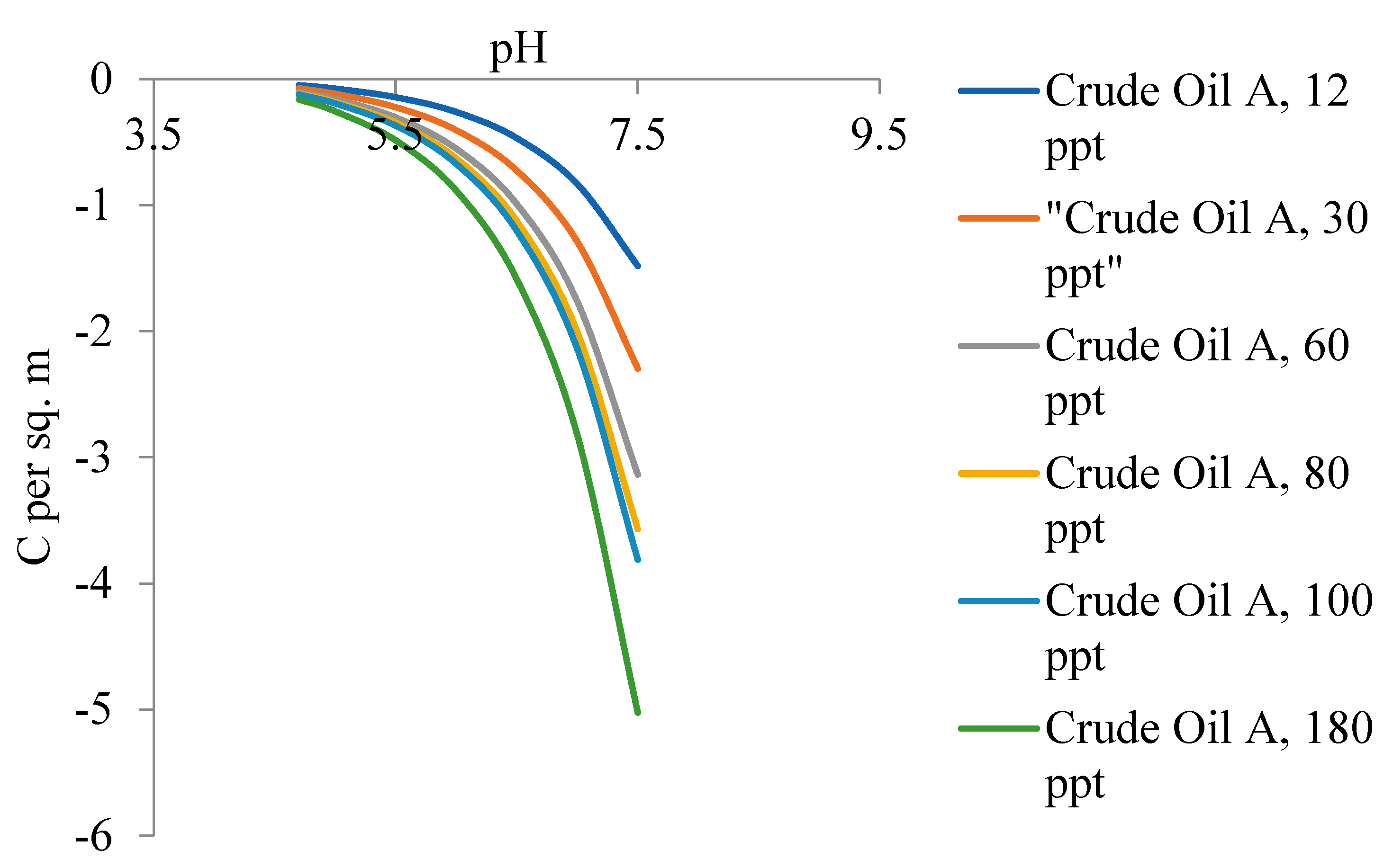
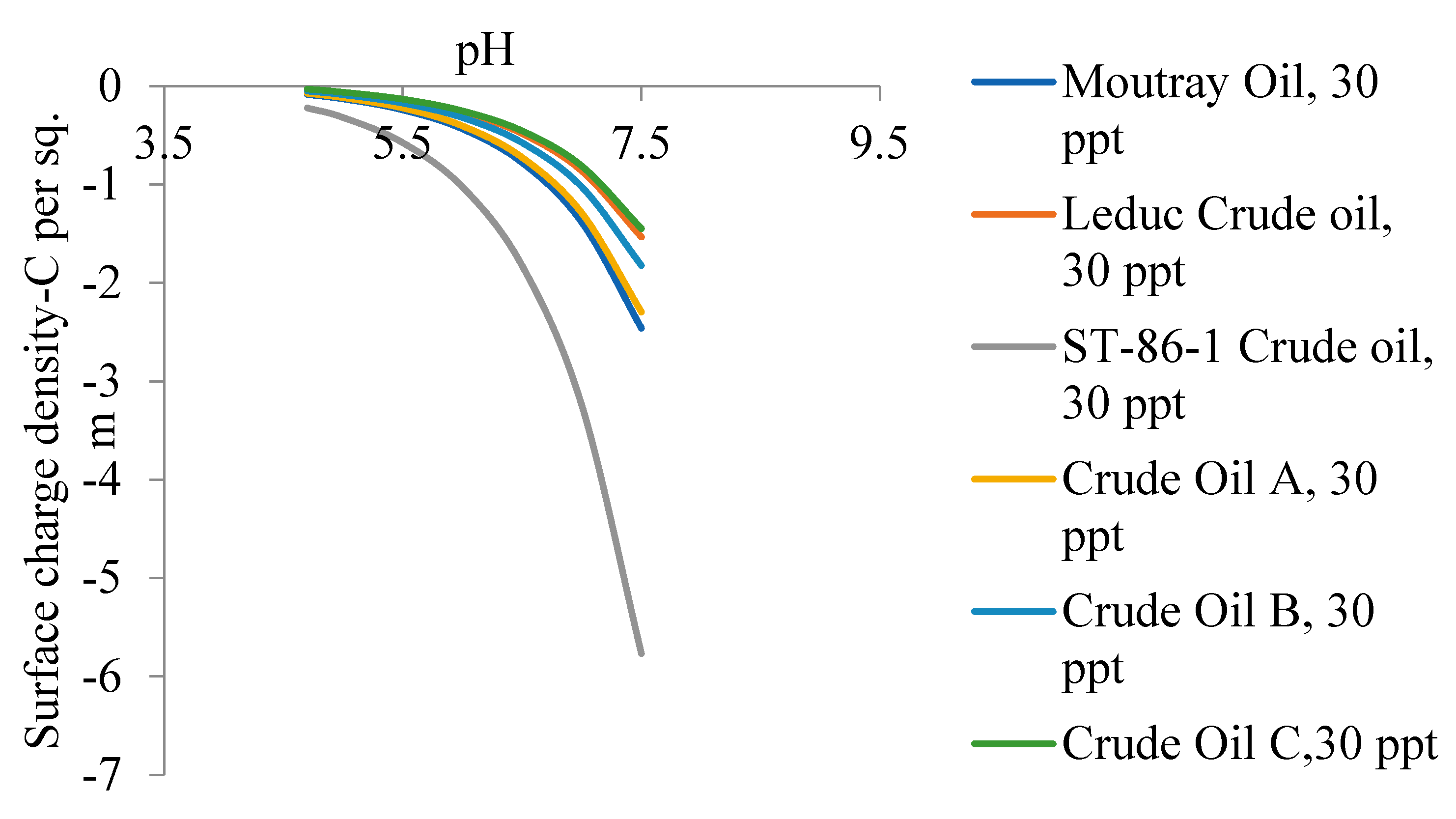
5.2. Zeta Potential of Crude Oils Samples
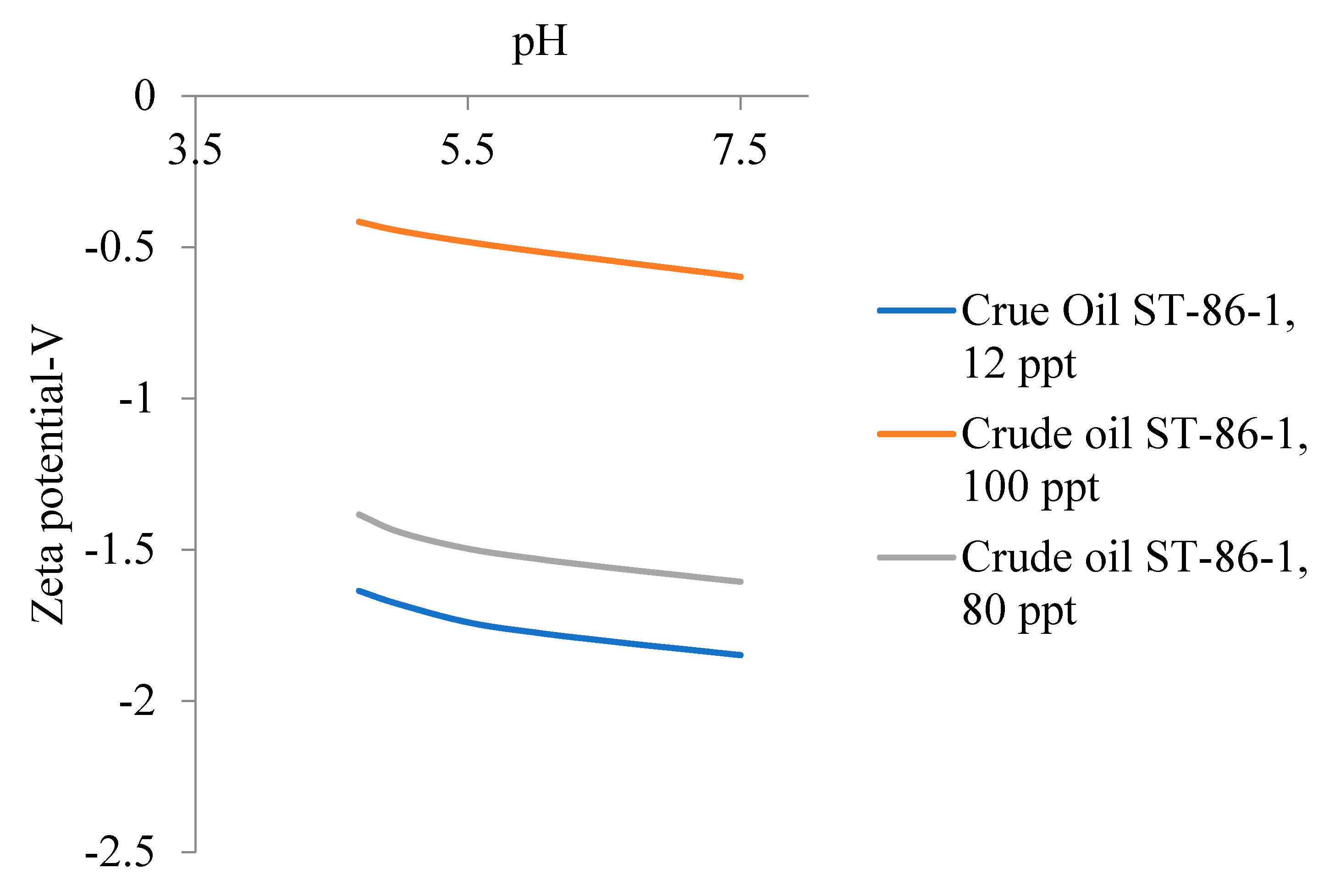
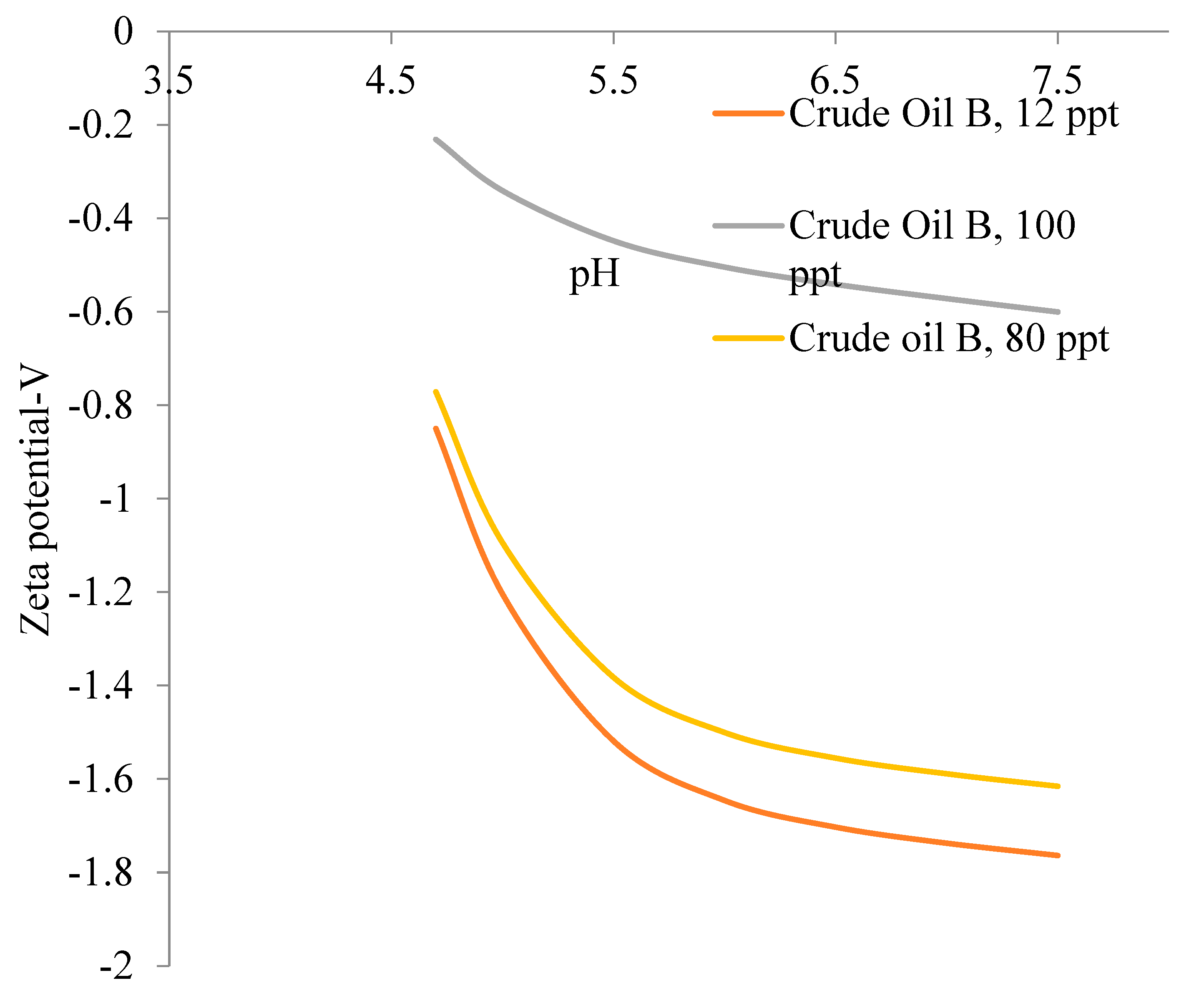
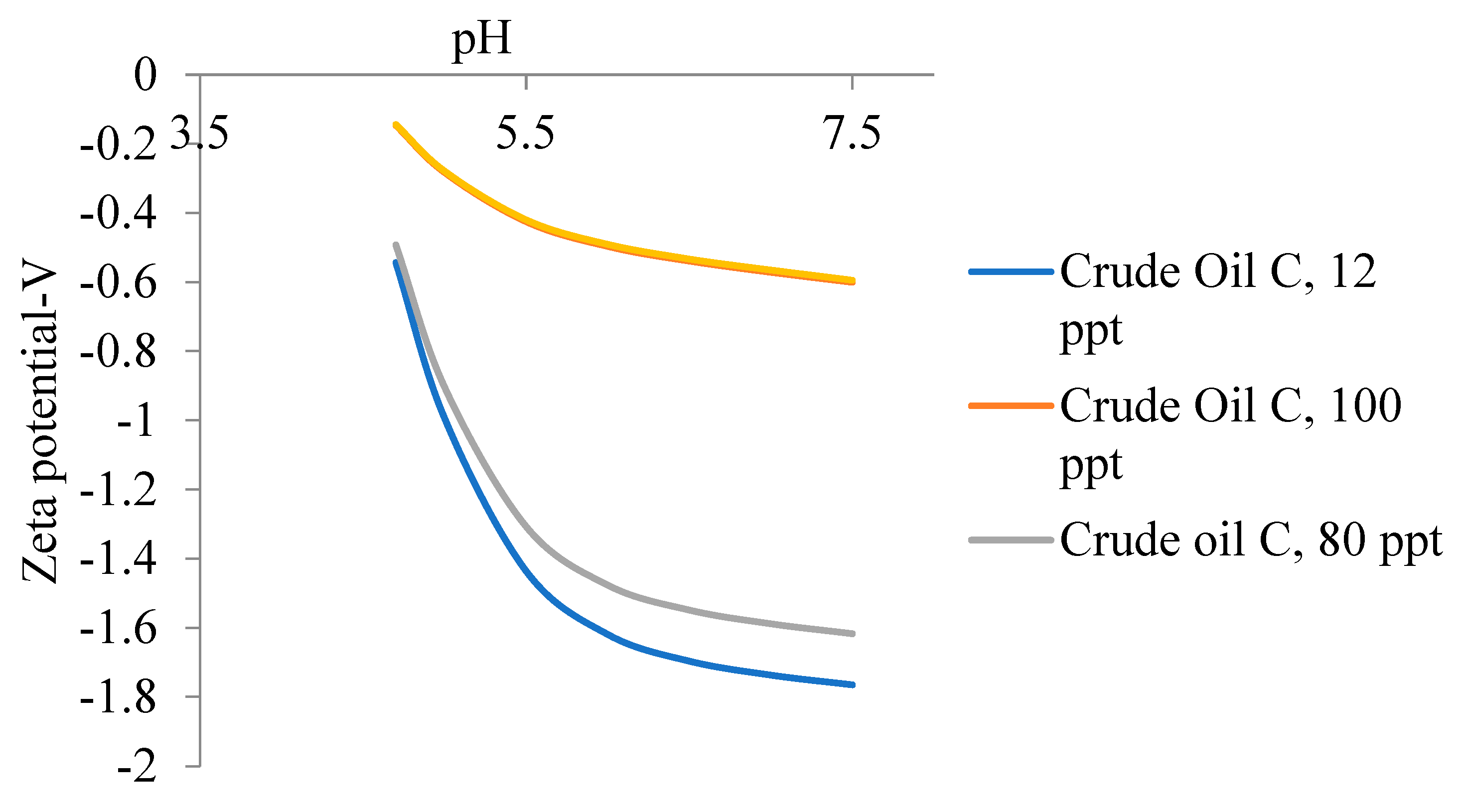
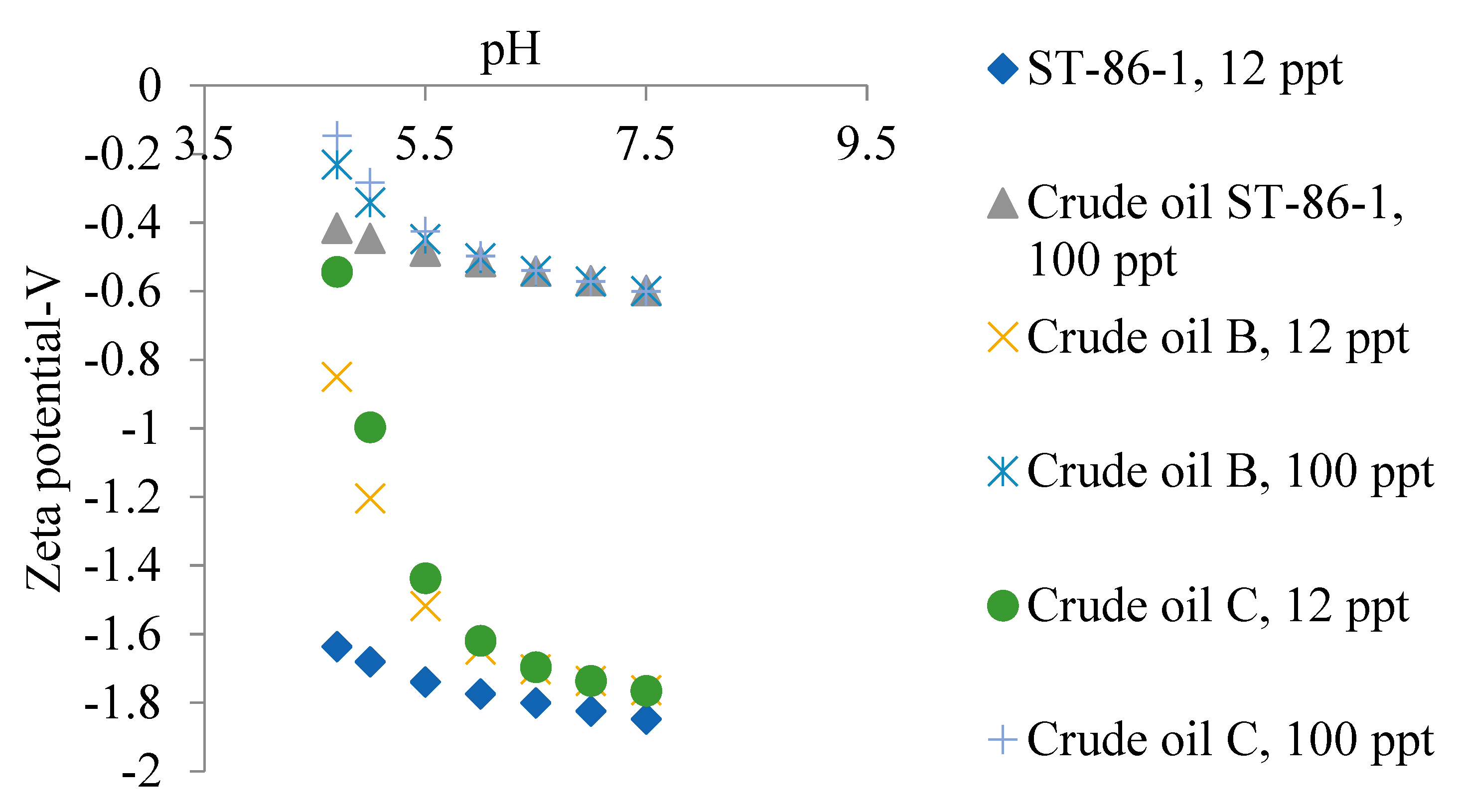
5.3. Emulsion Stability of Oil in Water Emulsion for Different Crude Oil Samples
5.4. Implications for Produced Oilfield Water Demulsification with Different Demulsifiers
5.5. Effect of Interfacial Energy on Demulsification
6. Conclusions
- Calculated trends in Surface charge densities obtained in this study conform to those found in the colloids literature, where there is a positive correlation with increasing salinity,
- Calculated trends in zeta potential obtained in this study conform to those found in the colloids literature, where there is a negative correlation with increasing salinity,
- The closeness of the isoelectric points of the oil samples studied in this paper causes a slight distinction between the degrees of ionization of the oils at a given pH for the range of produced oilfield water salinity,
- The zeta potential is the most significant determinant for emulsion stability. Based on our theoretical calculations, oil sample ST-86-1, has the most stabilized emulsion systems under room temperature conditions, and it will require a more intensive chemical demulsification procedures,
- Given the acidic nature of the isoelectric points of the oil samples, and the near neutral pH of produced oilfield waters, decreasing pH to approximately 4 will constitute a near zero surface charge/zeta potential, which is a cheap and efficient means of coalescence and coagulation leading to efficient demulsification.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ACS. "Development of the Pennsylvania Oil Industry," 2009. [Online]. Available online: https://www.acs.org/education/whatischemistry/landmarks/pennsylvaniaoilindustry.html#:~:text=On%20this%20site%20Edwin%20Drake,to%20be%20burned%20in%20lanterns.
- A. Brandt, "Oil substitution and the decline in conventional oil," 2023. [Online]. Available online: https://eao.stanford.edu/research-project/oil-substitution-decline-conventional-oil.
- Bentley, R. Global oil & gas depletion: an overview. Energy Policy 2002, 30, 189–205. [Google Scholar] [CrossRef]
- Höök, M.; Davidsson, S.; Johansson, S.; Tang, X. Decline and depletion rates of oil production: a comprehensive investigation. Philos. Trans. R. Soc. London. Ser. A: Math. Phys. Eng. Sci. 2014, 372. [Google Scholar] [CrossRef] [PubMed]
- R. Weyler, "The decline of oil has already begun," 2020. [Online]. Available online: https://www.greenpeace.org/international/story/29458/peak-oil-decline-coronavirus-economy/.
- Stratas-Advisors, "Heavy Crude Oil Supply Outlook Quarterly Q2 2019," 2019. [Online]. Available online: https://stratasadvisors.com/Insights/2019/061719-Upstream-Heavy-Crude-Outlook-Quarterly-Q2#:~:text=Global%20Heavy%20Crude%20Oil%20production%20Overview&text=Global%20heavy%20oil%20supply%20is,HSFO)%20yield%20in%20the%20world.
- L. Paszkiewicz, "EXTRA HEAVY OILS IN THE WORLD ENERGY SUPPLY," 2012. [Online]. Available online: http://www.oilproduction.net/files/extra-heavy-oils-in-the-world-energy-supply.pdf.
- Hedar, Y. ; Budiyono Pollution Impact and Alternative Treatment for Produced Water. E3S Web Conf. 2018, 31, 03004. [Google Scholar] [CrossRef]
- Konkel, L. Salting the Earth: The Environmental Impact of Oil and Gas Wastewater Spills. Environ. Heal. Perspect. 2016, 124, A230–A235. [Google Scholar] [CrossRef]
- Shaikh, S.S.; Abu-Dieyeh, M.H.; Al Naemi, F.A.; Ahmed, T.; Al-Ghouti, M.A. Environmental impact of utilization of “produced water” from oil and gas operations in turfgrass systems. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- EPA, "Detailed Study of the Centralized Waste Treatment Point Source Category for Facilities Managing Oil and Gas Extraction Wastes EPA-821-R-18-004," 2018. [Online]. Available: (2018) Detailed Study of the Centralized Waste Treatment Point Source Category for Facilities Managing Oil and Gas Extraction Wastes EPA-821-R-18-004.
- EPA, "Summary of Input on Oil and Gas Extraction Wastewater Management Practices Under the Clean Water Act," EPA, Washington, 2020.
- J. Pichte, "Oil and Gas Production Wastewater: Soil Contamination and Pollution Prevention," Soil Pollution Prevention and Remediation, pp. 1–14, 2016. [CrossRef]
- Jiang, W.; Lin, L.; Xu, X.; Wang, H.; Xu, P. Analysis of Regulatory Framework for Produced Water Management and Reuse in Major Oil- and Gas-Producing Regions in the United States. Water 2022, 14, 2162. [Google Scholar] [CrossRef]
- Maphosa, Y.; Jideani, V.A. Factors Affecting the Stability of Emulsions Stabilised by Biopolymers, in Science and Technology Behind Nanoemulsions, IntechOpen, 2018, pp. 65–81. [CrossRef]
- F. Ishii and T. Nii, "Chapter 22 - Lipid emulsions and lipid vesicles prepared from various phospholipids as drug carriers," in Colloid and Interface Science in Pharmaceutical Research and Development, 2014, pp. 469–501. [CrossRef]
- C. Costa, B. C. Costa, B. Medronho, A. Filipe, I. Mira, B. Lindman, H. Edlund and M. Norgren, "Emulsion Formation and Stabilization by Biomolecules: The Leading Role of Cellulose," Polymers (Basel). 2019, vol. 11, no. 10, pp. 1–18, 2019. [CrossRef]
- Håkansson, A. Emulsion Formation by Homogenization: Current Understanding and Future Perspectives. Annu. Rev. Food Sci. Technol. 2019, 10, 239–258. [Google Scholar] [CrossRef]
- Abdulredha, M.M.; Hussain, S.A.; Abdullah, L.C. Overview on petroleum emulsions, formation, influence and demulsification treatment techniques. Arab. Jounal Chem. 2020, 13, 3403–3428. [Google Scholar] [CrossRef]
- Ahmed, N.S.; Nassar, A.M.; Zaki, N.N.; Gharieb, H.K. Formation of fluid heavy oil-in-water emulsions for pipeline transportation. Fuel 1999, 78, 593–600. [Google Scholar] [CrossRef]
- S. S. Dol, "The Effect of Flow-Induced Oil-Water Emulsions on Pressure Drop," Journal of Theoretical and Applied Mechanics, vol. 2, pp. 73–78, 2019.
- Fajun, Z.; Zhexi, T.; Zhongqi, Y.; Hongzhi, S.; Yanping, W.; Yufei, Z. Research status and analysis of stabilization mechanisms and demulsification methods of heavy oil emulsions. Energy Sci. Eng. 2020, 8, 4158–4177. [Google Scholar] [CrossRef]
- Sams, G.W.; Zaouk, M. Emulsion Resolution in Electrostatic Processes. Energy Fuels 1999, 14, 31–37. [Google Scholar] [CrossRef]
- Cerbelaud, M.; Aimable, A.; Videcoq, A. Role of Electrostatic Interactions in Oil-in-Water Emulsions Stabilized by Heteroaggregation: An Experimental and Simulation Study. Langmuir 2018, 34, 15795–15803. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, J.; He, C.; He, L.; Li, X.; Sui, H. The Formation, Stabilization and Separation of Oil–Water Emulsions: A Review. Processes 2022, 10, 1–34. [Google Scholar] [CrossRef]
- Leunissen, M.E.; Van Blaaderen, A.; Hollingsworth, A.D.; Sullivan, M.T.; Chaikin, P. M. Electrostatics at the oil–water interface, stability, and order in emulsions and colloids. Proc. Natl. Acad. Sci. USA 2007, 104, 2585–2590. [Google Scholar] [CrossRef]
- Ghanavati, M.; Shojaei, M.-J.; S.A., A.R. Effects of Asphaltene Content and Temperature on Viscosity of Iranian Heavy Crude Oil: Experimental and Modeling Study. Energy Fuels 2013, 27, 7217–7232. [Google Scholar] [CrossRef]
- Shi, Q.; Wu, J. Review on Sulfur Compounds in Petroleum and Its Products: State-of-the-Art and Perspectives. Energy Fuels 2021, 35, 14445–14461. [Google Scholar] [CrossRef]
- Das, S.; Thundat, T.; Mitra, S.K. Analytical model for zeta potential of asphaltene. Fuel 2013, 108, 543–549. [Google Scholar] [CrossRef]
- Acevedo, S.; Castillo, J. Asphaltenes: Aggregates in Terms of A1 and A2 or Island and Archipielago Structures. ACS Omega 2023, 8, 4453–4471. [Google Scholar] [CrossRef]
- Salmón-Vega, S.; Herrera-Urbina, R.; Lira-Galeana, C.; Valdez, M.A. The Effect of Ionic Surfactants on the Electrokinetic Behavior of Asphaltene from a Maya Mexican Oil. Pet. Sci. Technol. 2012, 30, 986–992. [Google Scholar] [CrossRef]
- Li1, Y.; Xiang, D. Stability of oil-in-water emulsions performed by ultrasound power or high-pressure homogenization. PLoS ONE 2019, 14, 1–14. [Google Scholar]
- Bera, B.; Khazal, R.; Schroën, K. Coalescence dynamics in oil-in-water emulsions at elevated temperatures. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Macritchie, F. Barrier to Coalescence in Stabilized Emulsions. Nature 1967, 215, 1159–1160. [Google Scholar] [CrossRef]
- Huang, B.; Nan, X.; Fu, C.; Liu, W.; Guo, W.; Wang, S.; Zhang, L. Probing the Coalescence Mechanism of Oil Droplets in Fluids Produced by Oil Wells and the Microscopic Interaction between Molecules in Oil Films. Energies 2022, 15, 4274. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, J.; Lin, Y.-J.; Wang, X.; Biswal, S.L. Comparing the Coalescence Rate of Water-in-Oil Emulsions Stabilized with Asphaltenes and Asphaltene-like Molecules. Langmuir 2020, 36, 7894–7900. [Google Scholar] [CrossRef]
- Mehta, S.; Kaur, G. Microemulsions: Thermodynamic and Dynamic Properties. Thermodynamics 2011, 381–406. [Google Scholar] [CrossRef]
- Zore, A.; Geng, P.; Van De Mark, M.R. Equilibrium and Dynamic Surface Tension Behavior in Colloidal Unimolecular Polymers (CUP). Polymers 2022, 14, 2302. [Google Scholar] [CrossRef]
- Bonto, M.; Eftekhari, A.A.; Nick, H.M. An overview of the oil-brine interfacial behavior and a new surface complexation model. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Nenningsland, A.L.; Simon, S.; Sjöblom, J. Surface Properties of Basic Components Extracted from Petroleum Crude Oil. Energy Fuels 2010, 24, 6501–6505. [Google Scholar] [CrossRef]
- Bertheussen, A.; Simon, S.; Sjöblom, J. Equilibrium partitioning of naphtenic acids and bases and their consequences on interfacial properties. Colloids Surf. A-Physicochem. Eng. Asp. 2017, 529, 45–56. [Google Scholar] [CrossRef]
- Ameri, A.; Esmaeilzadeh, F.; Mowla, D. Effect of Brine on Asphaltene Precipitation at High Pressures in Oil Reservoirs. Pet. Chem. 2018, 58, 1076–1084. [Google Scholar] [CrossRef]
- R. Mokhtari, A. R. Mokhtari, A. Hosseini, M. Fatemi, S. I. Andersen and S. Ayatollahi, "Asphaltene destabilization in the presence of an aqueous phase: The effects of salinity, ion type, and contact time(2022," Journal of Petroleum Science and Engineering, vol. 208, pp. 1–11, 2008.
- Das, S.; Thundat, T.; Mitra, S.K. Analytical model for zeta potential of asphaltene. Fuel 2013, 108, 543–549. [Google Scholar] [CrossRef]
- Biesheuvel, P. Electrostatic free energy of interacting ionizable double layers. J. Colloid Interface Sci. 2004, 275, 514–522. [Google Scholar] [CrossRef]
- J. W. P. Chan and L. R. White, "Regulation of surface potential at amphoteric surfaces during particle–particle interaction," Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases, vol. 71, pp. Chan, D., et al. "Regulation of surface potential at amphoteric surfaces during particle–particle interaction. (1975): 1046-1057., 1975.
- Mullins, O.C.; Sabbah, H.; Eyssautier, J.; Pomerantz, A.E.; Barré, L.; Andrews, A.B.; Ruiz-Morales, Y.; Mostowfi, F.; McFarlane, R.; Goual, L.; et al. Advances in Asphaltene Science and the Yen–Mullins Model. Energy Fuels 2012, 26, 3986–4003. [Google Scholar] [CrossRef]
- Dolomatov, M.Y.; Shutkova, S.A.; Bakhtizin, R.Z.; Dolomatova, M.M.; Latypov, K.F.; Gilmanshina, K.A.; Badretdinov, B.R. Structure of Asphaltene Molecules and Nanoclusters Based on Them. Pet. Chem. 2020, 60, 16–21. [Google Scholar] [CrossRef]
- Alshareef, A.H. Asphaltenes: Definition, Properties, and Reactions of Model Compounds. Energy Fuels 2022, 34, 16–30. [Google Scholar] [CrossRef]
- Gharbi, K.; Benamara, C.; Benyounes, K.; Kelland, M.A. Toward Separation and Characterization of Asphaltene Acid and Base Fractions. Energy Fuels 2021, 35, 14610–14617. [Google Scholar] [CrossRef]
- Zheng, F.; Shi, Q.; Vallverdu, G.S.; Giusti, P.; Bouyssiere, B. Fractionation and Characterization of Petroleum Asphaltene: Focus on Metalopetroleomics. Processes 2020, 8, 1504. [Google Scholar] [CrossRef]
- Pillay, A.E.; Bassioni, G.; Stephen, S.; Kühn, F.E. Depth Profiling (ICP-MS) Study of Trace Metal ‘Grains’ in Solid Asphaltenes. J. Am. Soc. Mass Spectrom. 2011, 22, 1403–1408. [Google Scholar] [CrossRef]
- Erdman, J.G.; Harju, P.H. Capacity of Petroleum Asphaltenes to Complex Heavy Metals. J. Chem. Eng. Data 1963, 8, 252–258. [Google Scholar] [CrossRef]
- Thorn, K.A.; Cox, L.G. Probing the Carbonyl Functionality of a Petroleum Resin and Asphaltene through Oximation and Schiff Base Formation in Conjunction with N-15 NMR. PLoS ONE 2015, 1–25. [Google Scholar] [CrossRef]
- Hosseinpour, N.; Khodadadi, A.A.; Bahramian, A.; Mortazavi, Y. Asphaltene Adsorption onto Acidic/Basic Metal Oxide Nanoparticles toward in Situ Upgrading of Reservoir Oils by Nanotechnology. Langmuir 2013, 29, 14135–14146. [Google Scholar] [CrossRef]
- Langevin, D.; Poteau, S.; Hénaut, I.; Argillier, J.F. Crude Oil Emulsion Properties and Their Application to Heavy Oil Transportation. Oil Gas Sci. Technol. – Rev. d’IFP Energies Nouv. 2004, 59, 511–521. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, G.; Serhan, M.; Koivu, G.; Yang, Z.; Hollebone, B.; Lambert, P.; Brown, C.E. Characterization of naphthenic acids in crude oils and refined petroleum products. Fuel 2019, 255, 1–10. [Google Scholar] [CrossRef]
- Nordgård, E.L.; Sjöblom, J. Model Compounds for Asphaltenes and C80 Isoprenoid Tetraacids. Part I: Synthesis and Interfacial Activities. J. Dispers. Sci. Technol. 2008, 29, 1114–1122. [Google Scholar] [CrossRef]
- J. Sjöblom, S. Simon and Z. Xu, "Model molecules mimicking asphaltenes," Advances in Colloid and Interface Science, vol. 218, pp. 1–16, 2015.
- Bolintineanu, D.S.; Lane, J.M.D.; Grest, G.S. Effects of Functional Groups and Ionization on the Structure of Alkanethiol-Coated Gold Nanoparticles. Langmuir 2014, 30, 11075–11085. [Google Scholar] [CrossRef]
- Porus, M.; Labbez, C.; Maroni, P.; Borkovec, M. Adsorption of monovalent and divalent cations on planar water-silica interfaces studied by optical reflectivity and Monte Carlo simulations. J. Chem. Phys. 2011, 135, 064701. [Google Scholar] [CrossRef]
- Murray, B.S.; Dickinson, E.; Wang, Y. Bubble stability in the presence of oil-in-water emulsion droplets: Influence of surface shear versus dilatational rheology. Food Hydrocoll. 2009, 23, 1198–1208. [Google Scholar] [CrossRef]
- Lien, T.R.; Phillips1, C.R. Determination of Particle Size Distribution of Oil-in-Water Emulsions by Electronic Counting. Environ. Sci. Technol. 1974, 8, 558–561. [Google Scholar] [CrossRef]
- Eftekhari, A.A.; Thomsen, K.; Stenby, E.H.; Nick, H.M. Thermodynamic analysis of chalk-brine-oil interactions. Energy Fuels 2017, 31, 11773–11782. [Google Scholar] [CrossRef]
- Jang, W.; Nikolov, A.; Wasan, D.T.; Chen, K.; Campbell, B. Prediction of the Bubble Size Distribution during Aeration of Food Products. Ind. Eng. Chem. Res. 2005, 44, 1296–1308. [Google Scholar] [CrossRef]
- Adams, J.J. Asphaltene Adsorption, a Literature Review. Energy Fuels 2014, 28, 2831–2856. [Google Scholar] [CrossRef]
- Ok, S.; Mal, T.K. NMR Spectroscopy Analysis of Asphaltenes. Energy Fuels 2019, 33, 10391–10414. [Google Scholar] [CrossRef]
- Ok, S.; Mahmoodinia, M.; Rajasekaran, N.; Sabti, M.A.; Lervik, A.; van Erp, T.S.; Cabriolu, R. Molecular Structure and Solubility Determination of Asphaltenes. Energy Fuels 2019, 33, 8259–8270. [Google Scholar] [CrossRef]
- Bonto, M.; Eftekhari, A.A.; Nick, H.M. An overview of the oil-brine interfacial behavior and a new surface complexation model. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nagy, l.M.; Kónya, J. Study of pH-dependent charges of soils by surface acid–base properties. J. Colloid Interface Sci. 2007, 305, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.I.; Chandra, M.S.; Chen, J.; Zeng, B.Y.; Zou, F.; Mapolelo, M.; Abdallah, W.; Buiting, J.J. Detection and Impact of Carboxylic Acids at the Crude Oil–Water Interface. Energy Fuels 2016, 30, 4475–4485. [Google Scholar] [CrossRef]
- Miadonye, A.; Irwin, D.J.G.; Amadu, M. Effect of Polar Hydrocarbon Contents on Oil–Water Interfacial Tension and Implications for Recent Observations in Smart Water Flooding Oil Recovery Schemes. ACS Omega 2023, 8, 9086–9100. [Google Scholar] [CrossRef]
- Intertek, "Quantification of Naphthenic Acids in Produced Water," Worley, 2021.
- N. S. b. Shafiee, "CARBOXYLIC ACID COMPOSITION AND ACIDITY IN CRUDE OILS AND BITUMENS," 2014. [Online]. Available: https://theses.ncl.ac.uk/jspui/bitstream/10443/2763/1/Binti%20Shafiee%2C%20N%202014.pdf.
- Virga, E.; Spruijt, E.; de Vos, W.M.; Biesheuvel, P.M. Wettability of Amphoteric Surfaces: The Effect of pH and Ionic Strength on Surface Ionization and Wetting. Langmuir 2018, 34, 15174–15180. [Google Scholar] [CrossRef]
- Saboorian-Jooybari, H.; Chen, Z. Calculation of re-defined electrical double layer thickness in symmetricalelectrolyte solutions. Results Phys. 2019, 15, 1–13. [Google Scholar] [CrossRef]
- Chan, D.Y.; Mitchell, D. The free energy of an electrical double layer. J. Colloid Interface Sci. 1983, 95, 193–197. [Google Scholar] [CrossRef]
- Kallay, N.; Kovačević, D.; Žalac, S. Chapter 6 - Thermodynamics of the solid/liquid interface - its application to adsorption and colloid stability. Interface Sci. Technol. 2006, 11, 133–170. [Google Scholar]
- Tetteh, J.T.; Barimah, R.; Korsah, P.K. Ionic Interactions at the Crude Oil–Brine–Rock Interfaces Using Different Surface Complexation Models and DLVO Theory: Application to Carbonate Wettability. ACS Omega 2022, 7, 7199–7212. [Google Scholar] [CrossRef] [PubMed]
- Hanor, J.S. Origin and Migration of Subsurface Sedimentary Brines. SEPM 1987, 21. [Google Scholar]
- Milonjić, S.K. Determination of surface ionization and complexation constants at colloidal silica/electrolyte interface. Colloids Surfaces 1987, 23, 301–312. [Google Scholar] [CrossRef]
- Kallay, N.; Kovačević, D.; Žalac, S. Chapter 6 - Thermodynamics of the solid/liquid interface - its application to adsorption and colloid stability. Interface Sci. Technol. 2006, 133–170. [Google Scholar]
- Driver, H.; Elliott, H.; Linder, P. Application of the Gouy—Chapman equation in metal speciation modelling. Chem. Speciat. Bioavailab. 1991, 3, 61–62. [Google Scholar] [CrossRef]
- S. H. Behrens and D. G. Grier, J. S. H. Behrens and D. G. Grier, J. Chem. Phys., 115, pp. 6716–6721, 2001.
- Saboorian-Jooybari, H.; Chen, Z. Calculation of re-defined electrical double layer thickness in symmetricalelectrolyte solutions. Results Phys. 2019, 15, 1–13. [Google Scholar] [CrossRef]
- P. Bajpai, "Chapter 10 - Papermaking Chemistry," in Biermann's Handbook of Pulp and Paper (Third Edition), 2, 2018, pp. 207–236.
- Qing, L.; Zhao, S.; Wang, Z.-G. Surface Charge Density in Electrical Double Layer Capacitors with. J. Phys. Chem. B 2021, 125, 625–636. [Google Scholar] [CrossRef]
- D. K. Schroder, Semiconductor material and device characterization, John Wiley & Sons, 2015.
- R. M. Torresi, "Double layer capacitance," 2023. [Online]. Available: https://edisciplinas.usp.br/pluginfile.php/4283018/mod_resource/content/1/Double_layer.pdf.
- K. Divan, "IMPROVED SEPARATION AND DETECTION OF INORGANIC IONS BY CAPILLARY ELECTROPHORESIS," in Progress in Ion Exchange-Advances and Applications, Elsevier, 1977, pp. 176–186.
- R. J. Hunter, Foundations of Colloid Science, 1, Oxford University Press: Oxford, 1989.
- Bowden, J.; Posner, A.; Quirk, J. Ionic adsorption on variable charge mineral surfaces. Teoretical-charge development and, titration curves. Aust. J. Soil Res. 1997, 15, 121–136. [Google Scholar]
- Atkinson, R.J.; Posner, A.M.; Quirk, J.P. Adsorption of potential-determining ions at the ferric oxide-aqueous electrolyte interface. J. Phys. Chem. 1967, 71, 550–558. [Google Scholar] [CrossRef]
- R. W. Roach, R. S. R. W. Roach, R. S. Can and C. L. Howard, "An Assessment of Produced Water Impacts at Two Sites in the Galveston Bay System," 2023. [Online]. Available online: https://www.tceq.texas.gov/assets/public/comm_exec/pubs/gbnep/gbnep-23/gbnep_23_133-152.pdf.
- N. Gavish and K. Promislow, "Dependence of the dielectric constant of electrolyte solutions on," Phy Chem, pp. 1–14, 2016.
- G. Thyne and P. Brady, "Evaluation of Formation Water Chemistry: Bakken Shale," SAND2015-9055J, 2015.
- S. P. Clark Jr., Handbook of Physical Constants, vol. 97, Geological Society of America, 1966.
- T. J. Ahrens, Global Earth Physics: A Handbook of Physical Constants (AGU Reference Shelf), OH: Published by American Geophysical Union, 1995.
- Buckley, J.S.; Takamura, K.; Morrow, N.R. Infuence of electrical surface charges on the wetting properties of crude oils. SPE Reserv. Eng. 1989, 4, 332–340. [Google Scholar] [CrossRef]
- S. C. Moldoveanu and V. David, "Chapter 5 - Properties of Analytes and Matrices Determining HPLC Selection," 189-230, 2017.
- A. Adamson and A. Gast, "Physical Chemistry of Surfaces, John Wiley and Sons, 1997, 1997.
- Menéndez, J.A.; Illán-Gómez, M.; Leon, C.A.L.Y.; Radovic, L. On the difference between the isoelectric point. Carbon 1995, 33, 165–1659. [Google Scholar] [CrossRef]
- Holmes-Farley, S.R.; Reame, R.H.; McCarthy, T.J. Acid-Base Behavior of Carboxylic Acid Groups Covalently Attached at the Surface of Polyethylene: The Usefulness of Contact Angle in Following the Ionization of Surface Functionality. Langmuir 1985, 1, 725–740. [Google Scholar] [CrossRef]
- Lunkad, R.; Murmiliuk, A.; Tošner, Z.; Štěpánek, M.; Košovan, P. Role of pKA in Charge Regulation and Conformation of Various Peptide Sequences. Polymers 2021, 13, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Delhorme, M.; Labbez, C.; Caillet, C.; Thomas, F. Acid-Base Properties of 2:1 Clays. I. Modeling the Role of Electrostatics. Langmuir 2010, 26, 9240–9249. [Google Scholar] [CrossRef]
- M. Kosmulski, "The pH dependent surface charging and points of zero charge. IX. Update," Advances in Colloid and Interface Science, vol. 296, 2021.
- Yildiz, H.O.; Morrow, N.R. Effect of brine composition on recovery of Moutray crude oil by waterflooding. J. Pet. Sci. Eng. 1996, 14, 159–168. [Google Scholar] [CrossRef]
- Sugimoto, T.; Adachi, Y.; Kobayashi, M. Heteroaggregation rate coefficients between oppositely charged particles in a mixing flow: Effect of surface charge density and salt concentration. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 632, 1–8. [Google Scholar] [CrossRef]
- Stenberg, S.; Forsman, J. Overcharging and Free Energy Barriers for Equally Charged Surfaces Immersed in Salt Solutions. Langmuir 2021, 37, 14360–14368. [Google Scholar] [CrossRef]
- Chakibi, H.; Hénaut, A.S.I.; Langevin, D.; Argillier, J.-F. Role of Bubble–Drop Interactions and Salt Addition in Flotation Performance. Energy & Fuels 2018, 4049–4056. [Google Scholar]
- Jackson, M.D.; Al-Mahrouqi, D.; Vinogradov, J. Zeta potential in oil-water-carbonate systems and its impact on oil recovery during controlled salinity water-flooding. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Ma, Q.; Li, H.; Li, Y. The Study to Improve Oil Recovery through the Clay State Change during Low Salinity Water Flooding in Sandstones. ACS Omega 2020, 5, 29816–29829. [Google Scholar] [CrossRef] [PubMed]
- Kataya, A.; Khamehchi, E.; Bijani, M. The impact of salinity, alkalinity and nanoparticle concentration on zeta-potential of sand minerals and their implication on sand production. Energy Geosci. 2022, 3, 314–322. [Google Scholar] [CrossRef]
- D. A. Haydon, "A Study of the Relation Between Electrokinetic Potential and Surface Charge Density," in Proceedings of the Royal Society of London. Series A, Mathematical and Physical Sciences, 1960.
- Ge, Z.; Wang, Y. Estimation of Nanodiamond Surface Charge Density from Zeta Potential and Molecular Dynamics Simulations. J. Phys. Chem. B 2017, 121, 3394–3402. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, H. Approximate expressions for the surface charge density/surface potential relationship and double-layer potential distribution for a spherical or cylindrical colloidal particle based on the modified Poisson-Boltzmann equation. Colloid Polym. Sci. 2018, 296, 647–652. [Google Scholar] [CrossRef]
- S. Mohapatra, S. Ranjan, N. Dasgupta, R. K. Mishra and S. Thomas, Characterization and Biology of Nanomaterials for Drug Delivery, Elsevier, 2019.
- Brown, M.A.; Abbas, Z.; Kleibert, A.; Green, R.G.; Goel, A.; May, S.; Squires, T.M. Determination of Surface Potential and Electrical Double-Layer Structure at the Aqueous. Phys. Rev. X 2016, X, 1–12. [Google Scholar]
- Ohshima, H. Surface Charge Density/Surface Potential Relationship for a Spherical Colloidal Particle in a Salt-Free Medium. J. Colloid Interface Sci. 2002, 247, 18–23. [Google Scholar] [CrossRef]
- E. DeCola, A. E. DeCola, A. Hall, M. Popovich and H. P. Dahlslett, "Assessment of Demulsification and Separation Technologies for Use in Offshore Oil Recovery Operations," Bureau of Safety and Environmental Enforcement, Plymouth, 2018.
- Alao, K.T.; Alara, O.R.; Abdurahman, N.H. Trending approaches on demulsification of crude oil in the petroleum industry. Appl. Petrochem. Res. 2021, 11, 281–293. [Google Scholar] [CrossRef]
- Yonguep, E.; Kapiamba, K.F.; Kabamba, K.J.; Chowdhury, M. Formation, stabilization and chemical demulsification of crude oil-in-water emulsions: A review. Pet. Res. 2022, 7, 459–472. [Google Scholar] [CrossRef]
- Zolfaghari, R.; Fakhrul-Razi, L.; Abdullah, A.; Elnashaie SSEH, P.A. Demulsifcation techniques of water-in-oil and oil-in-water emulsions in petroleum industry. Sep. Purif. Technol. 2016, 170, 377–407. [Google Scholar] [CrossRef]
- Lv, P.; Liu, Y.; Zhang, Y.; Sun, L.; Meng, X.; Meng, X.; Zou, J. Optimization of non-ionic surfactants for removing emulsified oil from gas condensate oil–water emulsion in N oilfield. J. Pet. Explor. Prod. Technol. 2020, 10, 3025–3030. [Google Scholar] [CrossRef]
- Moritz, R.; Zardalidis, G.; Butt, H.-J.; Wagner, M.; Müllen, K.; George, F. Ion Size Approaching the Bjerrum Length in Solvents of Low Polarity by Dendritic Encapsulation. Macromolecules 2014, 47, 191–196. [Google Scholar] [CrossRef]
- Seal, S.; Doblhoff-Dier, K.; Meyer, J. Dielectric Decrement for Aqueous NaCl Solutions: Effect of Ionic Charge Scaling in Nonpolarizable Water Force Fields. J. Phys. Chem. B 2019, 123, 9912–9921. [Google Scholar] [CrossRef] [PubMed]
- S. M. Abed, N. H. Abdurahman, R. M. Yunus, H. A. Abdulbari and S. Akbari, "Oil emulsions and the different recent demulsification techniques in the petroleum industry - A review," in 1st ProSES Symposium, 2019.
- Miadonye, A.; Amadu, M. How pH induced surface charge modification explains the effect of petrophysical and hydrological factors on recovery trends of water drive gas reservoirs. J. Nat. Gas Sci. Eng. 2022, 98, 1–10. [Google Scholar] [CrossRef]
- Kobayashi, J.; Hinoue, T.; Watarai, H. Study of Adsorption of Water-Soluble Porphyrin at Glass–Solution Interface in the Presence of Cationic Surfactant Admicelles by Means of Total Internal Reflection Spectroscopy. Bull. Chem. Soc. Jpn. 1990, 71. [Google Scholar] [CrossRef]
- Vatanparast, H.; Shahabi, F.; Bahramian, A.; Javadi, A.; Miller, R. The Role of Electrostatic Repulsion on Increasing Surface Activity of Anionic Surfactants in the Presence of Hydrophilic Silica Nanoparticles. Sci Rep. 2018, 8, 8–1. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.P.; Bennetzen, M.V.; Klamt, A.; Stipp, S.S. First-Principles Prediction of Liquid/Liquid Interfacial Tension. J. Chem. Theory Comput. 2014, 10, 3401–3408. [Google Scholar] [CrossRef]
- Chen, Y.; Narayan, S.; Dutcher, C.S. Phase-Dependent Surfactant Transport on the Microscale: Interfacial Tension and Droplet Coalescence. Langmuir 2020, 36, 14904–14923. [Google Scholar] [CrossRef]
- Vis, M.; Peters, V.F.; Blokhuis, E.M.; Lekkerkerker, H.N.; Erné, B.H.; Tromp, R.H. Effects of Electric Charge on the Interfacial Tension between Coexisting Aqueous Mixtures of Polyelectrolyte and Neutral Polymer. Macromolecules 2015, 48, 7335–7345. [Google Scholar] [CrossRef]
- Zha, F.; Dong, S.; Rao, J.; Chen, B. Pea protein isolate-gum Arabic Maillard conjugates improves physical and oxidative stability of oil-in-water emulsions. Food Chem. 2019, 285, 130–138. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Aluthgun Hewage, S.; Batagoda, J.H. Stability of Nanobubbles. Environ. Eng. Sci. 2018, 35. [Google Scholar] [CrossRef]
- Schaefer, M.; Karplus, M. A Comprehensive Analytical Treatment of Continuum Electrostatics. J. Phys. Chem. 1996, 100, 1578–1599. [Google Scholar] [CrossRef]
| Crude Oil | Isoelectric point |
|---|---|
| Moutray Crude | 3.80 |
| Leduc Crude | 4.35 |
| ST-86—1 Crude | 3.20 |
| Crude Oil A | 4.00 |
| Crude Oil B | 4.20 |
| Crude Oil C | 4.40 |
| Sodium chloride dominated salinity[ppt] | Molar concentration of ions [M] | Ionic strength [M] | Number density[m3] | Permittivity [Fm-1] |
|---|---|---|---|---|
| 12 | 0.21 | 0.205339 | 1.27721 | 78.00 |
| 30 | 0.51 | 0.513347 | 3.19302 | 75.00 |
| 60 | 1.03 | 1.026694 | 6.38604 | 70.00 |
| 80 | 1.37 | 1.368925 | 8.51472 | 68.00 |
| 100 | 1.71 | 1.711157 | 1.06434 | 62.00 |
| 180 | 3.08 | 3.080082 | 1.91581 | 60.00 |
| Crude oil A | Crude oil B | Crude oil C | |
|---|---|---|---|
| -4.75 | -4.62 | -4.65 | -4.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




