Submitted:
10 July 2023
Posted:
12 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
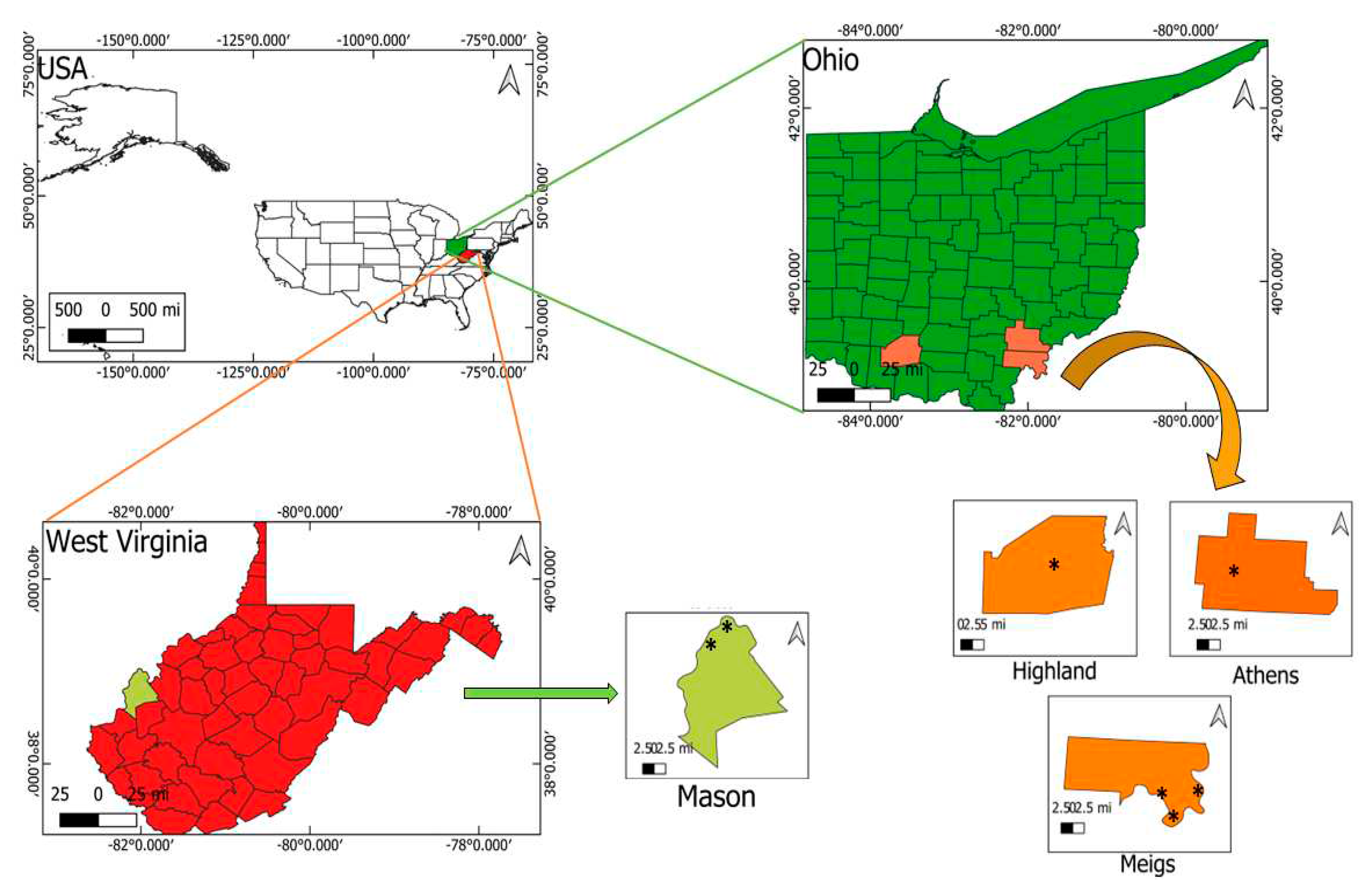
2.1. Site Description and Samples Collection
2.2. Sample Preparation and ICP – MS Analysis
2.3. Pollution Indicator Assessment
2.3.1. Enrichment Factor (EF)
2.3.2. Geo-Accumulation Index (Igeo)
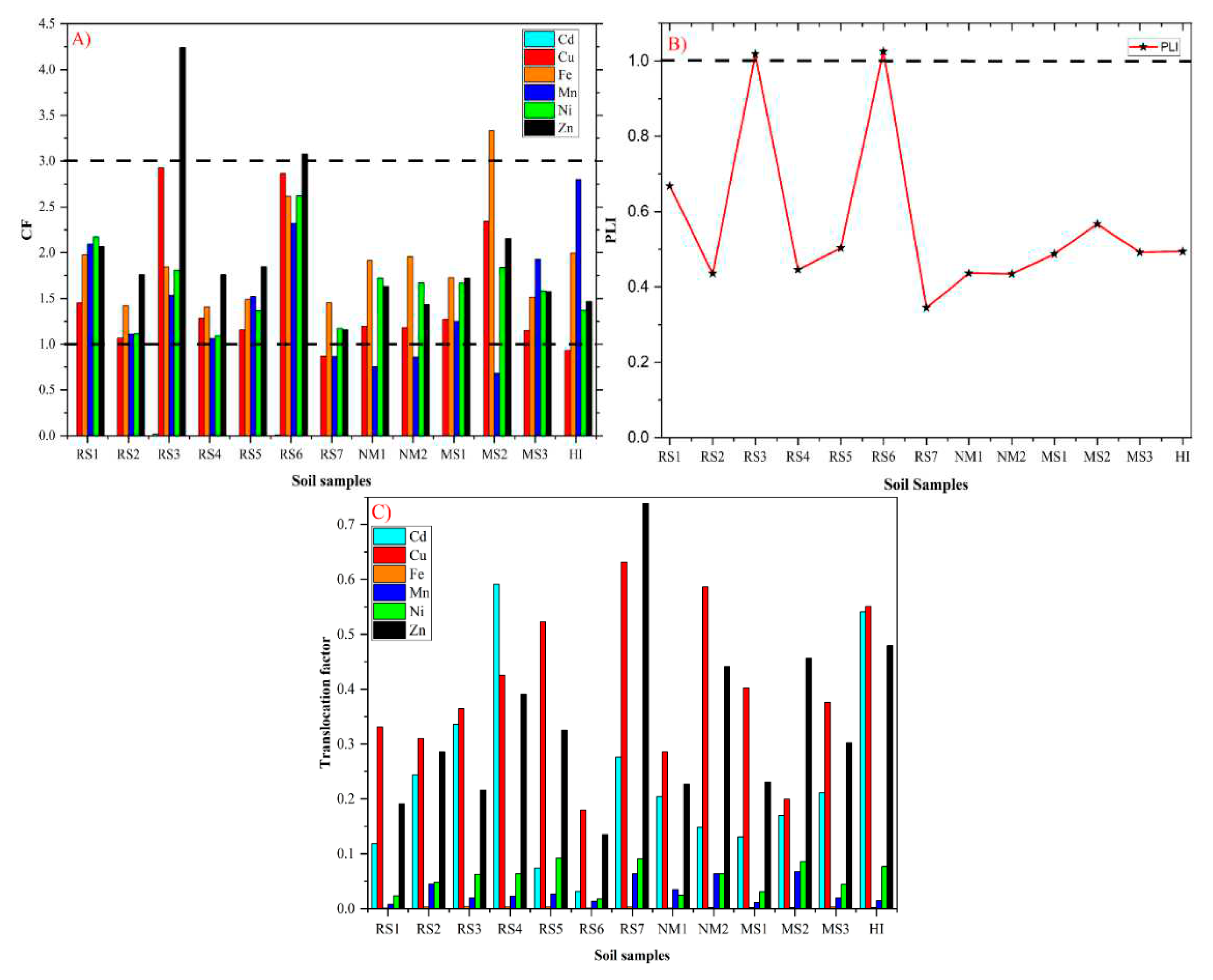
2.3.3. Contamination Factor (CF)
2.3.4. Pollution Load Index (PLI)
2.4. Translocation Factor (TF)
2.5. Calculation of Human Health Risk Assessment
2.5.1. EDI Assessment
2.5.2. THQ and HI Assessment
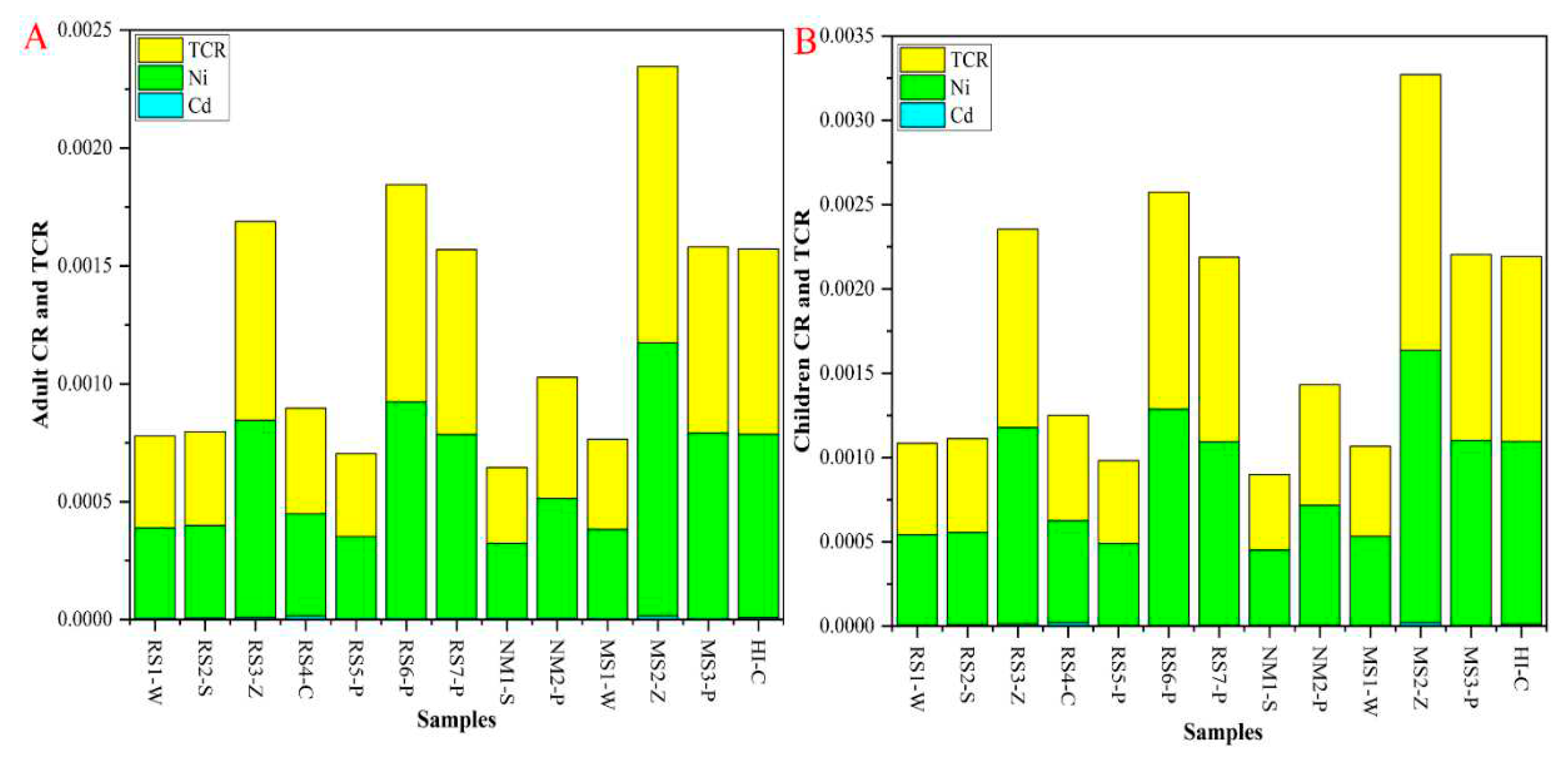
2.5.3. CR and TCR Assessment
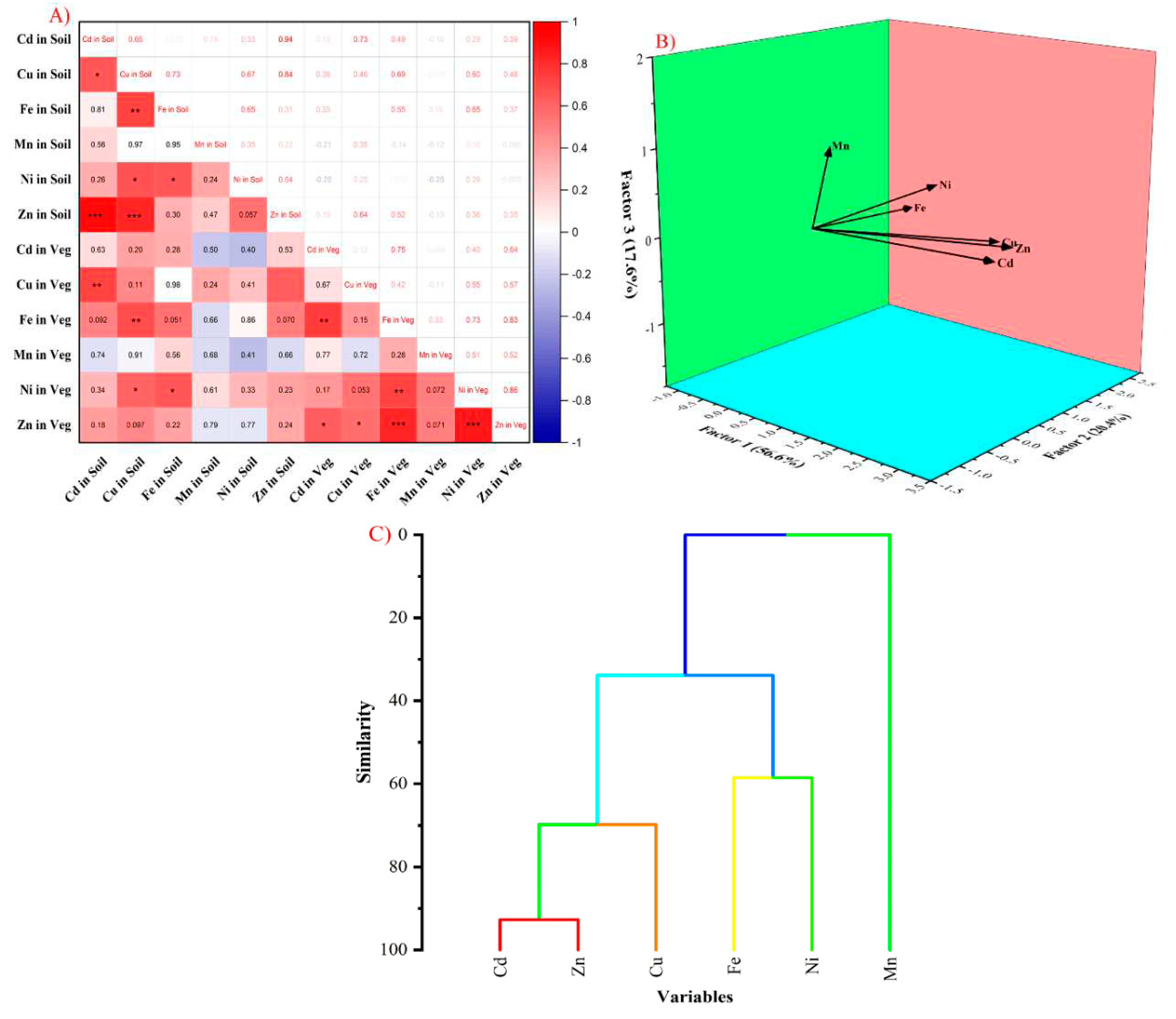
2.6. Statistical Analysis
3. Results and Discussion
3.1. HM Analysis in Agricultural Soil
3.2. Pollution Indicators Analysis
3.3. Traces of HMs in Vegetables
3.4. Identification of HM Contamination Source
3.5. Noncarcinogenic Health Risk Assessment
3.6. Carcinogenic Risk
4. Conclusion
Author contributions
Acknowledgments and Funding
Institutional Review Board Statement
Informed Consent Statement
Conflict of interest
Compliance with Ethics requirements
References
- Ahmed, S.; Fatema Tuj, Z.; Mahdi, M.M.; Nurnabi, M.; Alam, M.Z.; Choudhury, T.R. Health risk assessment for heavy metal accumulation in leafy vegetables grown on tannery effluent contaminated soil. Toxicol Rep 2022, 9, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J Environ Manage 2020, 254, 109779. [Google Scholar] [CrossRef] [PubMed]
- Aftab, K.; Iqbal, S.; Khan, M.R.; Busquets, R.; Noreen, R.; Ahmad, N.; Kazimi, S.G.T.; Karami, A.M.; Al Suliman, N.M.S.; Ouladsmane, M. Wastewater-Irrigated Vegetables Are a Significant Source of Heavy Metal Contaminants: Toxicity and Health Risks. Molecules 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bao, J.; Wang, T.; Moryani, H.T.; Kang, W.; Zheng, J.; Zhan, C.; Xiao, W. Hazardous Heavy Metals Accumulation and Health Risk Assessment of Different Vegetable Species in Contaminated Soils from a Typical Mining City, Central China. Int J Environ Res Public Health 2021, 18. [Google Scholar] [CrossRef]
- Parvez, M.S.; Nawshin, S.; Sultana, S.; Hossain, M.S.; Rashid Khan, M.H.; Habib, M.A.; Nijhum, Z.T.; Khan, R. Evaluation of Heavy Metal Contamination in Soil Samples around Rampal, Bangladesh. ACS omega 2023. [Google Scholar] [CrossRef]
- Mawari, G.; Kumar, N.; Sarkar, S.; Daga, M.K.; Singh, M.M.; Joshi, T.K.; Khan, N.A. Heavy Metal Accumulation in Fruits and Vegetables and Human Health Risk Assessment: Findings From Maharashtra, India. Environ Health Insights 2022, 16, 11786302221119151. [Google Scholar] [CrossRef]
- Lemessa, F.; Simane, B.; Seyoum, A.; Gebresenbet, G. Analysis of the concentration of heavy metals in soil, vegetables and water around the bole Lemi industry park, Ethiopia. Heliyon 2022, 8, e12429. [Google Scholar] [CrossRef]
- Ali, F.; Israr, M.; Ur Rehman, S.; Azizullah, A.; Gulab, H.; Idrees, M.; Iqbal, R.; Khattak, A.; Hussain, M.; Al-Zuaibr, F.M. Health risk assessment of heavy metals via consumption of dietary vegetables using wastewater for irrigation in Swabi, Khyber Pakhtunkhwa, Pakistan. PLoS One 2021, 16, e0255853. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Habibullah-Al-Mamun, M.; Raknuzzaman, M.; Ali, M.M.; Eaton, D.W. Health risk assessment due to heavy metal exposure from commonly consumed fish and vegetables. Environment systems and decisions 2016, 36, 253–265. [Google Scholar] [CrossRef]
- Crimmins, A.; Kolian, M.; Bacanskas, L.; Rosseel, K. Climate Change Indicators in the United States 2016. 2016.
- Kędzierska-Kapuza, K.; Szczuko, U.; Stolińska, H.; Bakaloudi, D.R.; Wierzba, W.; Szczuko, M. Demand for Water-Soluble Vitamins in a Group of Patients with CKD versus Interventions and Supplementation-A Systematic Review. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Ofoedu, C.E.; Iwouno, J.O.; Ofoedu, E.O.; Ogueke, C.C.; Igwe, V.S.; Agunwah, I.M.; Ofoedum, A.F.; Chacha, J.S.; Muobike, O.P.; Agunbiade, A.O.; et al. Revisiting food-sourced vitamins for consumer diet and health needs: a perspective review, from vitamin classification, metabolic functions, absorption, utilization, to balancing nutritional requirements. PeerJ 2021, 9, e11940. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Singha, S.; Kar, A.; Chanda, J.; Banerjee, S.; Dasgupta, B.; Haldar, P.K.; Sharma, N. Therapeutic importance of Cucurbitaceae: A medicinally important family. J Ethnopharmacol 2022, 282, 114599. [Google Scholar] [CrossRef] [PubMed]
- Canton, H. Food and agriculture organization of the United Nations—FAO. In The Europa directory of international organizations 2021, Routledge, 2021; pp 297-305. [CrossRef]
- Gao, J.; Zhang, D.; Proshad, R.; Uwiringiyimana, E.; Wang, Z. Assessment of the pollution levels of potential toxic elements in urban vegetable gardens in southwest China. Sci Rep 2021, 11, 22824. [Google Scholar] [CrossRef]
- Lego, B.; Deskins, J.; Bowen, E.; Mullett, D.; Farnum, A.; Gilyard, S.; Kaminski, D.; Wagstaff, W. West Virginia Economic Outlook: 2020-2024. 2019.
- Murphy, B.P. Identification of Mined Areas that may Contribute to Water Quality Degradation at Hobet Coal Mine, West Virginia. IdeaFest: Interdisciplinary Journal of Creative Works and Research from Cal Poly Humboldt 2019, 3, 10. [Google Scholar]
- Aken, B.V.; Quaranta, J.D.; Mack, B.; Yu, H.; Ducatman, A.M.; Ziemkiewicz, P.F. Environmental contaminants in coal slurry intended for underground injection in the state of West Virginia. Journal of Environmental Engineering 2015, 141, 05014004. [Google Scholar] [CrossRef]
- Holmgren, G.; Meyer, M.; Chaney, R.; Daniels, R. Cadmium, lead, zinc, copper, and nickel in agricultural soils of the United States of America; Wiley Online Library, 1993.
- Ergin, M.; Saydam, C.; Baştürk, Ö.; Erdem, E.; Yörük, R. Heavy metal concentrations in surface sediments from the two coastal inlets (Golden Horn Estuary and Izmit Bay) of the northeastern Sea of Marmara. Chemical geology 1991, 91, 269–285. [Google Scholar] [CrossRef]
- Rezapour, S.; Siavash Moghaddam, S.; Nouri, A.; Khosravi Aqdam, K. Urbanization influences the distribution, enrichment, and ecological health risk of heavy metals in croplands. Scientific Reports 2022, 12, 3868. [Google Scholar] [CrossRef]
- Muller, G. Index of geoaccumulation in sediments of the Rhine River. Geojournal 1969, 2, 108–118. [Google Scholar]
- Antoniadis, V.; Golia, E.E.; Liu, Y.T.; Wang, S.L.; Shaheen, S.M.; Rinklebe, J. Soil and maize contamination by trace elements and associated health risk assessment in the industrial area of Volos, Greece. Environ Int 2019, 124, 79–88. [Google Scholar] [CrossRef]
- Rinklebe, J.; Antoniadis, V.; Shaheen, S.M.; Rosche, O.; Altermann, M. Health risk assessment of potentially toxic elements in soils along the Central Elbe River, Germany. Environ Int 2019, 126, 76–88. [Google Scholar] [CrossRef]
- Tomlinson, D.; Wilson, J.; Harris, C.; Jeffrey, D. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer meeresuntersuchungen 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Gebeyehu, H.R.; Bayissa, L.D. Levels of heavy metals in soil and vegetables and associated health risks in Mojo area, Ethiopia. PLoS One 2020, 15, e0227883. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.; Ahmed, M.K.; Islam, M.S.; Habibullah-Al-Mamun, M.; Tukun, A.B.; Islam, S.; AT, M.A.R. Health risk assessment of trace elements via dietary intake of 'non-piscine protein source' foodstuffs (meat, milk and egg) in Bangladesh. Environ Sci Pollut Res Int 2016, 23, 7794–7806. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Nagpal, A.K.; Kaur, I. Heavy metal contamination in soil, food crops and associated health risks for residents of Ropar wetland, Punjab, India and its environs. Food Chem 2018, 255, 15–22. [Google Scholar] [CrossRef]
- Kamunda, C.; Mathuthu, M.; Madhuku, M. Health Risk Assessment of Heavy Metals in Soils from Witwatersrand Gold Mining Basin, South Africa. Int J Environ Res Public Health 2016, 13. [Google Scholar] [CrossRef]
- Li, G.; Kronzucker, H.J.; Shi, W. Root developmental adaptation to Fe toxicity: mechanisms and management. Plant signaling & behavior 2016, 11, e1117722. [Google Scholar] [CrossRef]
- Hansen, C.T.; Kleint, C.; Böhnke, S.; Klose, L.; Adam-Beyer, N.; Sass, K.; Zitoun, R.; Sander, S.G.; Indenbirken, D.; Dittmar, T. Impact of high Fe-concentrations on microbial community structure and dissolved organics in hydrothermal plumes: an experimental study. Scientific Reports 2022, 12, 20723. [Google Scholar] [CrossRef]
- Akhtar, S.; Luqman, M.; Farooq Awan, M.U.; Saba, I.; Khan, Z.I.; Ahmad, K.; Muneeb, A.; Nadeem, M.; Batool, A.I.; Shahzadi, M. Health risk implications of iron in wastewater soil-food crops grown in the vicinity of peri urban areas of the District Sargodha. Plos one 2022, 17, e0275497. [Google Scholar] [CrossRef]
- Ljung, K.; Otabbong, E.; Selinus, O. Natural and anthropogenic metal inputs to soils in urban Uppsala, Sweden. Environmental Geochemistry & Health 2006, 28. [Google Scholar] [CrossRef]
- Uddin, M.M.; Zakeel, M.C.M.; Zavahir, J.S.; Marikar, F.M.; Jahan, I. Heavy metal accumulation in rice and aquatic plants used as human food: A general review. Toxics 2021, 9, 360. [Google Scholar] [CrossRef]
- Toth, T.; Tomáš, J.; Lazor, P.; Bajčan, D.; Jomová, K. The transfer of heavy metals from contaminated soils into agricultural plants in High Tatras region. Czech J. Food Sci 2009, 27, 390–393. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: a promising approach for revegetation of heavy metal-polluted land. Frontiers in Plant Science 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Chaoua, S.; Boussaa, S.; El Gharmali, A.; Boumezzough, A. Impact of irrigation with wastewater on accumulation of heavy metals in soil and crops in the region of Marrakech in Morocco. Journal of the Saudi Society of Agricultural Sciences 2019, 18, 429–436. [Google Scholar] [CrossRef]
- Štofejová, L.; Fazekaš, J.; Fazekašová, D. Analysis of heavy metal content in soil and plants in the dumping ground of Magnesite Mining Factory Jelšava-Lubeník (Slovakia). Sustainability 2021, 13, 4508. [Google Scholar] [CrossRef]
- El Behairy, R.A.; El Baroudy, A.A.; Ibrahim, M.M.; Mohamed, E.S.; Rebouh, N.Y.; Shokr, M.S. Combination of GIS and Multivariate Analysis to Assess the Soil Heavy Metal Contamination in Some Arid Zones. Agronomy 2022, 12, 2871. [Google Scholar] [CrossRef]
- Proshad, R.; Islam, S.; Tusher, T.R.; Zhang, D.; Khadka, S.; Gao, J.; Kundu, S. Appraisal of heavy metal toxicity in surface water with human health risk by a novel approach: a study on an urban river in vicinity to industrial areas of Bangladesh. Toxin reviews 2021, 40, 803–819. [Google Scholar] [CrossRef]
- Iyama, W.A.; Okpara, K.; Techato, K. Assessment of heavy metals in agricultural soils and plant (Vernonia amygdalina Delile) in port harcourt metropolis, Nigeria. Agriculture 2021, 12, 27. [Google Scholar] [CrossRef]
- Röllin, H.B.; Nogueira, C. Manganese: Environmental pollution and health effects. Encyclopedia of environmental health 2011, 2, 229–242. [Google Scholar]
- Sharafi, K.; Mansouri, B.; Omer, A.K.; Bashardoust, P.; Ebrahimzadeh, G.; Sharifi, S.; Massahi, T.; Soleimani, H. Investigation of health risk assessment and the effect of various irrigation water on the accumulation of toxic metals in the most widely consumed vegetables in Iran. Scientific Reports 2022, 12, 20806. [Google Scholar] [CrossRef]
| Sampling location | HM concentrations (g/kg) | ||||||
|---|---|---|---|---|---|---|---|
| Cd (µg/g) | Cu | Fe | Mn | Ni | Zn | ||
| RS1 | 0.397c | 0.027b | 39.96c | 1.39c | 0.036ab | 0.114c | |
| RS2 | 0.250d | 0.02b | 28.41d | 0.737d | 0.019c | 0.097cd | |
| RS3 | 2.91a | 0.043a | 37.29c | 1.02d | 0.03b | 0.234a | |
| RS4 | 0.257d | 0.024b | 28.41d | 0.7d | 0.018b | 0.097cd | |
| RS5 | 0.295d | 0.021b | 30.13d | 1.01d | 0.022bc | 0.102c | |
| RS6 | 0.992b | 0.04a | 52.89b | 1.54b | 0.044a | 0.17b | |
| RS7 | 0.134f | 0.016bc | 29.34d | 0.57f | 0.019c | 0.064e | |
| NM1 | 0.17e | 0.022b | 38.74c | 0.5f | 0.029b | 0.09cd | |
| NM2 | 0.17e | 0.022b | 39.57c | 0.57f | 0.028b | 0.079d | |
| MS1 | 0.205de | 0.023b | 34.88cd | 0.83de | 0.028b | 0.095cd | |
| MS2 | 0.189d | 0.043a | 67.36a | 0.42f | 0.03b | 0.119c | |
| MS3 | 0.203de | 0.021b | 30.66d | 1.28cd | 0.026b | 0.087d | |
| HI | 0.166e | 0.017bc | 40.31c | 1.86a | 0.023bc | 0.081d | |
| Mean | 0.487 | 0.026 | 38.32 | 0.959 | 0.027 | 0.11 | |
| Median | 0.205 | 0.022 | 37.29 | 0.83 | 0.028 | 0.097 | |
| Mode | 0.17 | 0.022 | 28.41 | 0.57 | 0.028 | 0.097 | |
| SD | 0.761 | 0.009 | 11.08 | 0.44 | 0.007 | 0.045 | |
| Sampling location | EF | Igeo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd | Cu | Fe | Mn | Ni | Zn | Cd | Cu | Fe | Mn | Ni | Zn | |
| RS1 | 0.257 | 0.533 | 4.125 | 1.641 | 0.431 | 0.748 | -10.88 | -0.146 | 1.413 | 0.890 | -1.038 | -0.395 |
| RS2 | 0.151 | 0.365 | 2.766 | 0.812 | 0.206 | 0.594 | -11.55 | -0.592 | 0.936 | -0.0252 | -2.003 | -0.628 |
| RS3 | 0.481 | 0.453 | 2.041 | 0.638 | 0.190 | 0.814 | -8.480 | 0.536 | 1.313 | 0.443 | -1.302 | 0.641 |
| RS4 | 0.141 | 0.400 | 2.489 | 0.706 | 0.183 | 0.539 | -11.51 | -0.321 | 0.922 | -0.089 | -2.035 | -0.628 |
| RS5 | 0.127 | 0.283 | 2.076 | 0.796 | 0.156 | 0.446 | -11.31 | -0.473 | 1.006 | 0.429 | -1.711 | -0.556 |
| RS6 | 0.372 | 0.461 | 3.160 | 1.052 | 0.300 | 0.645 | -9.562 | 0.432 | 1.818 | 1.037 | -0.769 | 0.180 |
| RS7 | 0.051 | 0.189 | 1.785 | 0.400 | 0.137 | 0.247 | -12.45 | -0.880 | 0.967 | -0.380 | -1.928 | -1.228 |
| NM1 | 0.053 | 0.210 | 1.910 | 0.282 | 0.163 | 0.282 | -12.10 | -0.421 | 1.368 | -0.584 | -1.375 | -0.736 |
| NM2 | 0.056 | 0.224 | 2.106 | 0.347 | 0.171 | 0.267 | -12.10 | -0.440 | 1.399 | -0.395 | -1.416 | -0.925 |
| MS1 | 0.090 | 0.317 | 2.439 | 0.664 | 0.224 | 0.422 | -11.83 | -0.334 | 1.217 | 0.146 | -1.421 | -0.658 |
| MS2 | 0.057 | 0.403 | 3.253 | 0.249 | 0.170 | 0.365 | -11.95 | 0.545 | 2.166 | -0.730 | -1.279 | -0.334 |
| MS3 | 0.080 | 0.258 | 1.930 | 0.922 | 0.191 | 0.348 | -11.85 | -0.480 | 1.031 | 0.771 | -1.495 | -0.785 |
| HI | 0.057 | 0.183 | 2.216 | 1.170 | 0.144 | 0.283 | -12.14 | -0.777 | 1.426 | 1.310 | -1.705 | -0.888 |
| Sampling location | HM concentrations (g/kg) | |||||
|---|---|---|---|---|---|---|
| Cd (µg/g) | Cu | Fe | Mn | Ni | Zn | |
| RS1-W | 0.047d | 8.5d | 80.19de | 11.39d | 1.05d | 22.46cd |
| RS2-S | 0.061c | 6e | 101.26c | 34.31a | 1.12d | 28.92c |
| RS3-Z | 0.098b | 16.04a | 160.07b | 20.4c | 1.4cd | 50.21a |
| RS4-C | 0.152a | 10.14c | 104.02c | 16.28cd | 1.65c | 38.11b |
| RS5-P | 0.031e | 7.1e | 73.07e | 22.4c | 0.8e | 22.97cd |
| RS6-P | 0.022ef | 11.12bc | 90.19d | 28.0b | 2.1b | 33.2bc |
| RS7-P | 0.037de | 10.18c | 90.02d | 37.3a | 1.8bc | 47.3ab |
| NM1-S | 0.035de | 6.14e | 61.11ef | 17.62c | 1.12d | 20.49d |
| NM2-P | 0.043d | 8.33d | 100.19c | 26.45b | 1.4cd | 25.82c |
| MS1-W | 0.027e | 10.21c | 77.8e | 11.05d | 1.06d | 21.88cd |
| MS2-Z | 0.152a | 9.07cd | 176a | 31.09ab | 3a | 53.16a |
| MS3-P | 0.025e | 13.05b | 88.14d | 20.01c | 1.8bc | 35.7b |
| HI-C | 0.09b | 10c | 108.09c | 28.31b | 1.4cd | 38.3b |
| Mean | 0.063 | 9.684 | 100.7 | 23.43 | 1.515 | 33.73 |
| Median | 0.043 | 10 | 90.6 | 22.4 | 1 | 33.2 |
| Mode | 0.152 | 10 | - | 11 | 1 | 22 |
| SD | 0.045 | 2.75 | 32.78 | 8.207 | 0.673 | 11.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





