1. Introduction
Pentaclethra is a small genus of the Fabaceae family, comprising approximately three species of trees, distributed throughout tropical and subtropical regions of the world [
1,
2,
3,
4]. The species of the genus Pentaclethra, such as
Pentaclethra macroloba and
P. eetveldeana have been described as medicinal plants with their biological activities assessed [
3,
4].
P. macrophylla is the sole member of the genus occurring naturally in the lowlands of West Africa. It is abundantly found in the southern, Middle Belt and south eastern Nigeria [
5,
6,
7], with no varietal characterization [
8].
Nearly all parts of the
P. macrophylla plant are used for various human and animal ailments. The fruits, seeds, stem bark, leaves and roots have been reportedly used in traditional herbal practices in different countries. The stem bark has been credited in Nigeria as an antiulcer [
9], antidiabetic [
10], anti-inflammatory [
11], antigonorrhea [
12], antimicrobial [
13], antinociceptive [
14], anticonvulsant and antitussive [
12]. In Nigeria, the seeds have been reported as anticancer [
15] and abortificient [
10] whereas in Cameroon, it is used for infertility [
16]. The root bark is a laxative, enema against dysentery, liniment against itching and abortificient as an infusion in both Nigeria and Cameroon [
14]. The leaf is used to treat diarrhoeal, fever, stomach ache and convulsion [
8,
16,
17]. The plant has also many nutritional benefits [
18,
19]. In Ghana, it has been reported as an anthelmintic for leprosy sores [
13], antiepileptic [
20], antidiarrhoeal [
17], antipruritus and for wound management [
16]. Bergenin is an active constituent of the plant and research indicates that bergenin has multiple biological activities, including anti-inflammatory and immunomodulatory properties [
5]. A large body of ethnobotanical evidence indicates that the traditional use of
Pentaclethral macrophylla in Nigeria are most likely related to the plant’s anti-nociceptive and anti-inflammatory actions. However, since the pharmacological properties of
P. macrophylla have rarely been reported, the use of this plant have continued without solid scientific evidence to support them. The present study was carried out to evaluate the anti-nociceptive and anti-inflammatory activities of lipospheres prepared with the methanol extract of
P. macrophylla and administered orally in animal models of pain and inflammation.
2. Materials and Methods
2.1. Collection and Identification of Pentaclethra macrophylla Stem Bark
The stem barkof Pentaclethra macrophylla tree was collected from Ejuona Obukpa Nsukka, Enugu State, Nigeria in March 2022 and authenticated by a plant taxonomist Mr. Alfred Ozioko of the International Centre for Ethno medicine Development (InterCEED) Nsukka, Enugu State, Nigeria. The Voucher specimen (PCG/UNN/ 014/507) was assigned to the sample which was deposited in the Herbarium of the Department of Pharmacognosy and Environmental Medicines, University of Nigeria, Nsukka, Nigeria. The plant material was collected in line with the ethical guide of the University of Nigeria, Nsukka, on herbal research.
2.2. Maceration and Extraction of Plant Material
The fleshy stem bark of
P. macrophylla was harvested, thoroughly rinsed with distilled water, and air-dried at room temperature (28 ± 2˚C). Dried plant portion (2 kg) was mechanically pulverized using a hammer mill (Henan Always Machinery, Zhengzhou, China). Methanol (MeOH, 98%) extraction was carried out according to an earlier method [
9], at room temperature for 72 h, with vigorous shaking at intervals. The mixture was filtered using a muslin cloth, and the resulting filtrate was further filtered through multiple flutted filter-papers (Whatman No. 1 filter paper (Sigma-Aldrich,Johannesburg, South Africa) and the final filtrate concentrated
in vacuo at 40°C to obtain dry crude methanol extract. Extracts were stored in sterile containers until further use.
2.3. Extraction of goat fat from Capra hircus
The adipose tissue of freshly slaughtered
Capra hircus (goat) was obtained from the abattoir in Ikpa market Nsukka and freed of extraneous materials. The fat was extracted from the adipose tissue according to an earlier method with modification [
21]. Briefly, the adipose tissue was grated and boiled with half its weight of water on a water bath for 45 min. The molten fat was separated from the aqueous phase using a muslin cloth. The fat was further purified by heating the lipid with a 2% w/w suspension of Activated charcoal and bentonite (1:9) ratio blend at 100°C for 1 h. Thereafter the suspension was vacuum filtered using a Buchner funnel. The fat was stored in the refrigerator at 4 ̊C.
2.4. Preparation of Lipospheres
The lipid matrices were first prepared according to
Table 1. Briefly, Phospholipon 90H
® (GmbH, Köln, Germany) and goat fat corresponded to 1.25 % (0.75:0.5) respectively. A 0.5g quantity of goat fat was carefully weighed into a beaker and melted at 80°C using a hot water bath. A 0.75g of Phospholipon 90H
® was transferred into the melted goat fat and both were stirred continuously with a glass stirrer to ensure adequate mixing until a homogenous transparent liquid was obtained. Consequently, the aqueous phase consisting of Tween 80, sorbitol, sorbic acid and graded concentration of PM stem bark extract (0, 2.5, 5.0, 7.5 and 10 g) were equally heated to the same temperature and later added to the lipid phase. Lipospheres were formulated by high shear homogenization (Ultra-Turrax, T18 basic, IKA, Staufen, Germany) at 18,000 rpm for 5 min. The resultant lipospheres were cooled at room temperature and stored in amber-coloured bottles firmly secured for further analysis.
2.5. Characterization of Lipospheres
2.5.1. Determination of Particle size and morphology of lipospheres
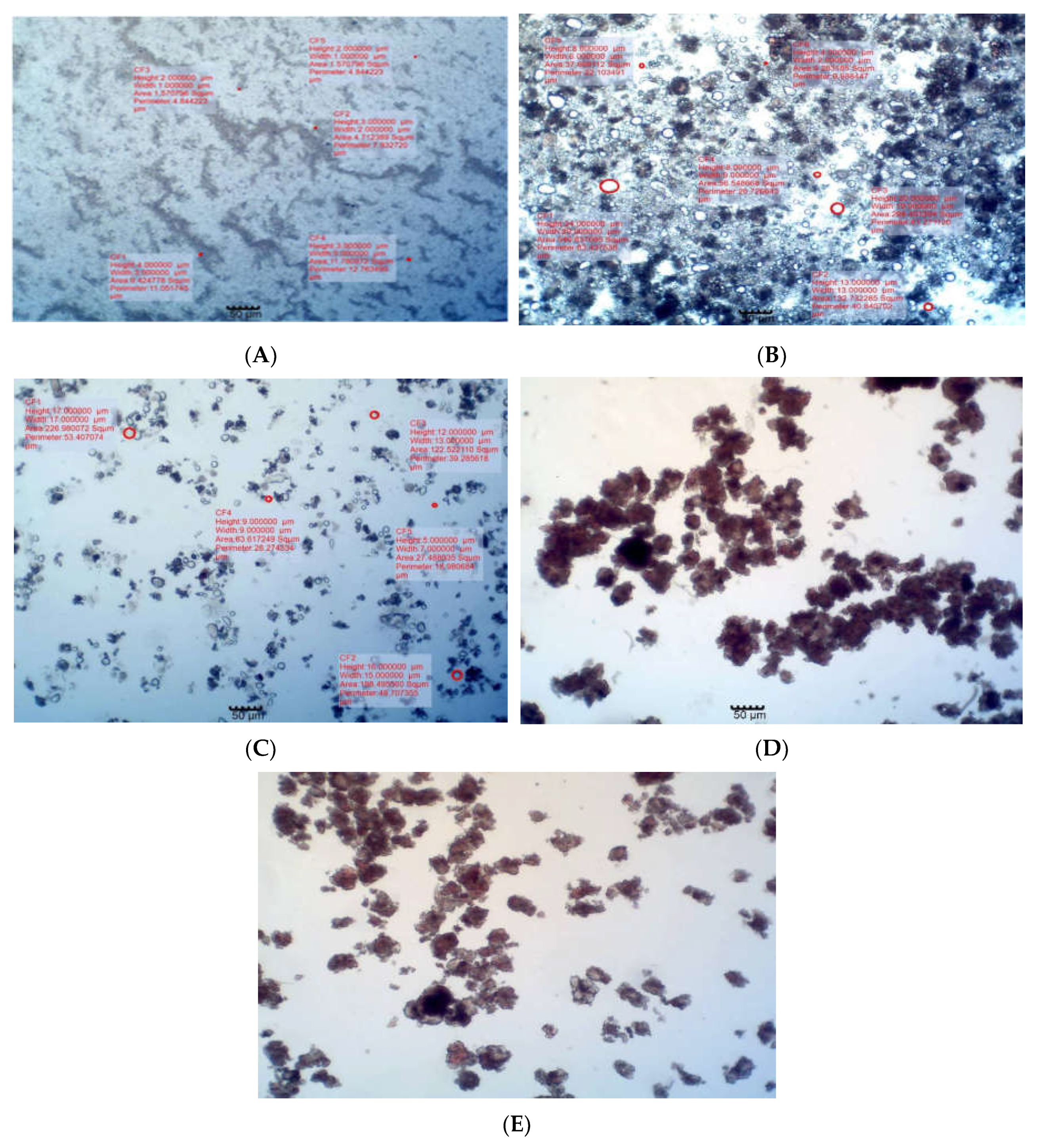
Microscopic evaluation and particle size analysis of the lipospheres were performed at ×100 magnification. A 5 mg of liposphere from each batch was placed on a slide, covered with a cover slip and observed using a compound phase-contrast microscope (Motic B3; Motic, Carlsbad, CA, USA). All digital micrographs were captured using Moticam 2.0 image system (Motic, Carlsbad, CA, USA). Each sample was assessed five times and representative measurements were made for each sample. The particle dimensions (height, width, area and perimeter) were measured in micrometer (µm).
2.5.2. pH – time dependent analysis
After the lipospheres preparation the pH of the batches were determined using a pH meter (Hanna, H198108). The pH analysis and stability of the loaded and unloaded lipospheres were determined in a time dependent manner (24 h, 7 and 30 days).
2.5.3. Encapsulation efficiency (EE %)
The entrapment efficiency of the liposphere formulations was calculated by using the indirect method of ultrafiltration using Amicon filter tubes (Germany), which consisted of a filter membrane with a molecular weight cut-off of 10,000 at the base of the sample donor chamber. For this, the liposphere (1 ml aliquot) of undiluted sample was placed in the upper chamber and the sample recovery chamber was fitted below the membrane in the lower compartment. The unit was closed and centrifuged at 2,500 rpm for 5 min using a centrifuge (Model 420R Rotina Hettich, Germany) at 25 ◦C in order to separate microparticles from the unentrapped drug and the free drug in the supernatant was quantified afterwards using HPLC. The encapsulation efficiency was calculated through the formula;
The loading capacity was determined in relation to the total weight of the lipids using the formula:
Wa is the weight of the P. macrophylla added to the formulation and Ws is the actual amount of P. macrophylla encapsulated in the lipospheres. Wl is the weight of the lipid added in the formulation.
2.5.4. HPLC method
HPLC determination of P. macrophylla in methanol was performed using a (ASI-100 automated sample injector) equipped with UV–vis detectors operating at 274 nm. Samples were chromatographed on a stainless steel C18 reverse phase column (Luna (2) 150x4.6 mm) packed with 5 mm particles (Lichrospher1 100 RP-18). For the separations, a gradient of mobile phase A (0.1 v/v % TFA in acetonitrile) and mobile phase B (0.1 v/v % TFA in water) was used. TFA was chosen to provide pH 2 in order to achieve narrow peak shapes. The flow rate was 1.0 mL min−1, the column temperature was 40 °C and the injection volume was 10 μL. UV detection wavelength was 274 nm. The detector cell temperature was 40 °C. A calibration curve was plotted for the extract in the concentration range of 2–10 mg/ml. A good linear relationship was observed between the concentration of extract and its peak area with a percentage correlation coefficient of 99.37%. The required studies were carried out to estimate the precision and accuracy of the HPLC method.
2.6. FTIR analysis
The unloaded and loaded liposphere formulations (0, 2.5, 5,7.5 and 10g) were analyzed for FTIR at spectra range of 4000-500cm . A 0.4g of KBr was weighed and ground to powder. 0.001g of the samples containing Pentaclethra macrophylla (2.5, 5,7.5 and 10 g) was separately weighed into the ground KBr and both were thoroughly mixed and moulded into a disc. The disc was inserted into the sample compartment of the instrument, and the IR spectrum was generated (Shimadzu FTIR. Model IR Affinity-1. Japan). The same procedure was repeated for the unloaded (placebo) liposphere.
2.7. In vitro Anti-nociceptive and Anti-inflammatory study
2.7.1. Assay of Membrane Stabilization by Hypotonicity induced hemolysis
The sample batches of the
P. macrophylla loaded lipospheres, unloaded lipospheres and acetyl salicylic acid (Aspirin) were dissolved in distilled water (hypotonic solution). A 5ml hypotonic solution containing graded doses of the lipospheres (100, 200, 400, 600 and 800 µg/ml) were made into duplicate pairs (per dose) of the centrifuge tubes. 5ml of the isotonic solution containing graded doses of the unloaded and loaded lipospheres (100-800 µg/ml) were prepared and put into duplicate pairs (per dose) of the centrifuge tubes. The control tubes contained 5ml of the vehicle (distilled water) and 5 ml of 200 µg/ml of Aspirin respectively. Erythrocyte suspension (0.1 ml) was added to each of the tubes and mixed gently [
22]. The mixtures were incubated for 1h at room temperature (37 ˚C), and then, centrifuged for 5 min at 1300 rpm. Absorbance (OD) of the hemoglobin content of the supernatant was estimated at 540 nm using a UV spectrophotometer (Milton Roy). The percentage haemolysis was calculated by assuming the haemolysis produced in the presence of distilled water was 100%.
The percentage inhibition of hemolysis was calculated using the following relation:
where OD
1 = absorbance of test sample in isotonic solution
OD2 =absorbance of test sample in hypotonic solution
OD3 = absorbance of control sample in hypotonic solutions
2.7.2. Determination of Anti-platelet aggregatory activity
This method of antiplatelet activity determination is a modification of Born and Cross [
23]. Briefly, a 5ml of fresh blood sample was withdrawn intravenously from healthy volunteers using 5ml plastic syringes and put into plastic tube containing 0.01ml of 1% EDTA as the anticoagulant. The EDTA plastic tubes were centrifuged at 3000 rpm for 10 min. The blood sample separated and the supernatant was collected, diluted twice using normal saline and then used as platelet-rich plasma [
24]. Absorption changes of the platelet rich plasma (PRP) was determined. A 0.2 ml of the platelet rich plasma, 0.4 ml of the 2M CaCl
2, and varying concentrations of the
Pentaclethra macrophylla loaded lipospheres (50, 100, 150, 200 mg representing 2.5, 5, 7.5 and 10 g formulations respectively), unloaded lipospheres and normal saline were incubated. The absorbances of the solutions were measured at 520 nm using UV spectrophotometer (Milton Roy). The changes in absorbances of the solutions at 520 nm were taken at time intervals; (0, 30, 60, 90 and 120 s).
2.8. In-vivo antinociceptive and anti-inflammatory study
The animal experimental protocols were in accordance with the guidelines for conducting animal experiments stipulated by our Institution’s Animal Ethics Committee and in compliance with the Federation of European Laboratory Animal Science Association and the European Community Council Directive of November 24, 1986 (86/609/EEC). Briefly, the rats were divided into groups and acclimatized to the laboratory environment a week before the study.
2.8.1. Egg albumin induced rat paw edema inflammatory model
Fresh egg albumen induced rat hind paw oedema was used as model for acute inflammation. Some 28 adult Wistar albino rats of either sexes were weighed and divided into 7 groups of 4 rats each. The animals were allowed to acclimatize to the environment for 7 days before the experiment. The animals were then fasted and deprived of water for 18 h before the experiment. Deprivation of water was to ensure uniform hydration and to minimize variability in edematous response [
24]. Immediately after the deprivation, their right hind paw volumes were taken at time zero (t = 0) volume (V = Vo) using a Vernier caliper. The first group of four animals were induced with edema but didn’t receive any treatment, the second group received 20 mg/kg of Diclofenac (positive control group). The third group received 50 mg/kg of blank liposphere containing no drug while the fourth to seventh groups received different doses (50, 100,150 and 200 mg/kg body weight corresponding to 2.5, 5, 7.5 and 10 g) of the
P. macrophylla loaded lipospheres respectively. One hour after the administration of the test substances, 0.1 ml of undiluted fresh egg albumin was injected into the sub-plantar region of the right hind paw of the rats to induce acute inflammation. After the egg albumin administration, the paw volumes were measured using a vernier caliper at 0, 1, 2, 3, 4 and 5 h. Formation of oedema was then assessed in terms of the difference in the zero-time paw edema volume of the injected paw and its paw edema volume at different times after egg albumin injection of the right hind paw. Average inflammation, % inflammation and % inhibition of inflammation were calculated for each dose of the loaded liposphere containing
P. macrophylla using the following relationships:
- (i)
where V
0 =Volume of oedema at time zero (initial time); V
T = Volume of oedema at time T (0.5, 1, 2, 3, 4 and 5h)
- (ii)
-
Average inflammation of control
2.8.2. 2% Formaldehyde induced arthritis model
This experiment was carried out using adult Wistar albino rats weighing 60-100 g. The animals were placed at random and allocated to treatment groups. They were housed in clean cages and allowed free access to food and water while they acclimatized to the laboratory environment one week prior to the experiment. A total of 32 animals were used and were divided into 8 groups of 4 rats each (n=4). Group 1 received the vehicle water and served as the control group. Group II were induced with 0.1ml of 2 % formaldehyde but received no treatment. Group III received the standard diclofenac (20 mg/kg/body weight), group IV received blank liposphere while groups V, VI, VII, VIII received doses of
Pentaclethra macrophylla lipospheres (50, 100, 150, 200 mg/kg bodyweight) respectively representing PM liposphere batches 2.5, 5, 7.5 and 10 g. One hour after the administration of oral doses of the vehicle and drugs, 0.1 ml of formaldehyde (2 %v/v) was used to induce arthritis in the sub-plantar region of the right hind paw of all the rats [
25].
After the intravenous 0.1ml formaldehyde (2 % v/v) administration, the volume of the paws were measured using a vernier caliper at 0, 1, 2, 3, 4, 5 and 48 h. Formation of oedema was then assessed in terms of the difference in the zero-time paw edema volume of the injected paw and its paw edema volume at different times after 0.1ml formaldehyde injection of the right hind paw. % inhibition of the paw edema was calculated using the following formula:
where ΔV treated= mean change in paw volume edema of treated rat
ΔV untreated = mean change in paw volume of untreated rat
After 48 h, 2ml of blood was collected by retro-orbital cavity under the influence of anesthesia. Aliquot of the collected blood was used to perform hematological studies to estimate total blood count, differential blood count including (eosinophils, basophils, lymphocytes, monocytes).
2.8.2.1. Total White blood cell count
This was carried out following standard hematological procedures [
26]. Briefly, well mixed anticoagulated blood was diluted 1:20 with Truk’s solution (2% glacial acetic acid) in a test tube. This was loaded into an improved Neubauer counting chamber. Appropriate squares were counted and added up to determine the total red cell count.
2.8.2.2. Differential blood count
Drops of blood (2 drops) were placed on a dry slide and spread on the surface using another slide (blood film method) [
26]. The Leishman’s stain was carefully dropped onto the blood film and was covered using a smaller slide. The stain was allowed to act on the blood for 2-3 min. After that distilled water was used to wash off the stain until a pink color was obtained. Excess water was shaken off, under the slide cleaned and the slide allowed to dry upright. The slide (smear side up) was placed on the microscope stage. The blood smear was examined under the microscope with x10 lens resolution. The area with plenty WBCs was selected and an immersion oil was placed on that area. The resolution was changed to the oil immersion objective (100x). The differential cell count was then carried out and the morphology of the WBC noted.
Interventionary studies involving animals or humans, and other studies that require ethical approval, must list the authority that provided approval and the corresponding ethical approval code.
3. Results
3.1. pH time dependent analysis
Time dependent pH analysis of lipospheres is presented in Table 2. There was no change in pH for the PM unloaded lipospheres and 2.5g of PM-loaded lipospheres from Day 1 to Day 30. However, in higher PM loadings, there were only slight changes in pH from Day 1 to Day 7. This change was highest in the 7.5g PM lipospheres.
Table 2.
Properties of the formulated lipospheres.
Table 2.
Properties of the formulated lipospheres.
| Liposphere batches (g) |
Particle size (µm) |
Encapsulation efficiency (EE %) |
Loading capacity (LC) |
pH time dependence (days) |
| 1 |
7 |
30 |
| 0 |
12.56 ± 2.18 |
- |
- |
3.5 |
3.5 |
3.5 |
| 2.5 |
26.87 ± 4.21 |
35.2 |
4.4 |
3.8 |
3.8 |
3.8 |
| 5.0 |
42.42 ± 3.64 |
75.6 |
1.89 |
3.8 |
3.9 |
3.9 |
| 7.5 |
82.27 ± 6.24 |
89.4 |
3.4 |
3.7 |
3.9 |
3.9 |
| 10.0 |
98.67 ± 10.23 |
94.1 |
4.7 |
3.7 |
3.8 |
3.8 |
3.2. Encapsulation Efficiency (EE) and Loading capacity (LC)
Results of EE and LC are also presented in Table 2. EE ranged from 35 % to 94 %. It increased consistently and progressively from 35.2 % for 2.5 g PM-loaded lipospheres to 94% in 10 g PM lipospheres. This trend however, was not obtainable in the LC. An increase in PM-loading from 2.5 g to 5.0 g led to a sharp fall in LC from 4.4 to 1.89; on further increase of PM-loading to 7.5 g, LC quickly rose back to 3.4 and finally to 4.7 at 10 g PM-loading.
3.3. Particle size and morphology of lipospheres
The photomicrographs of the lipospheres in
Figure 1 show that the particles were spherical in shape. The particle sizes were within the acceptable range for lipospheres. The particle size of unloaded lipospheres was lower than those of the PM-loaded lipospheres. The result also shows that particle size increased with increase in the amount of PM loaded in the lipospheres.
3.4. FTIR analysis
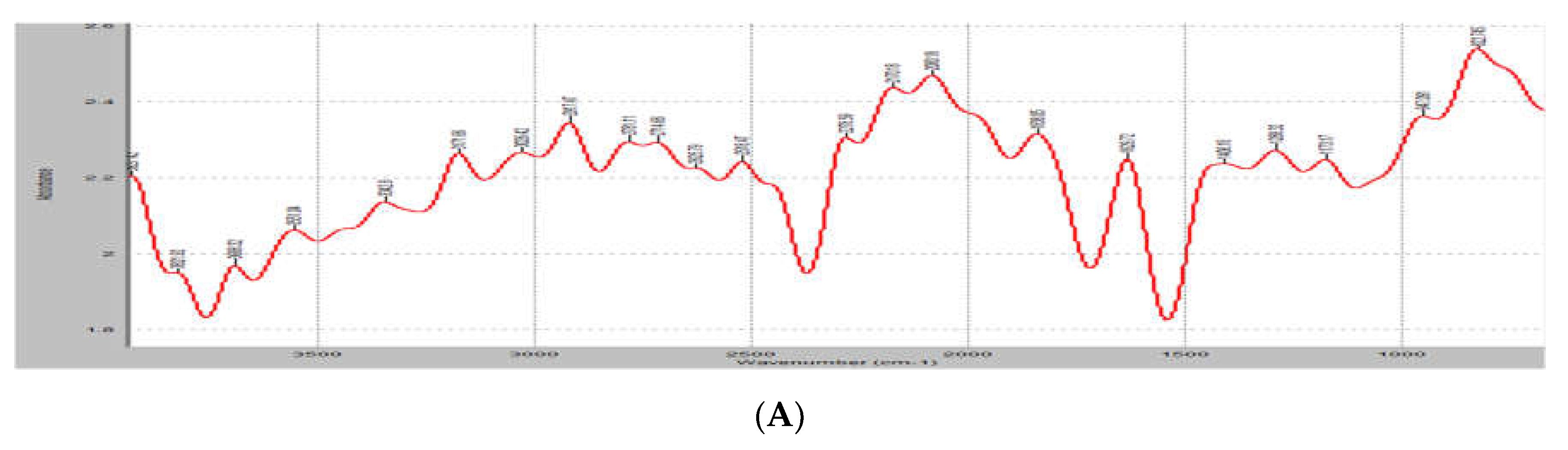
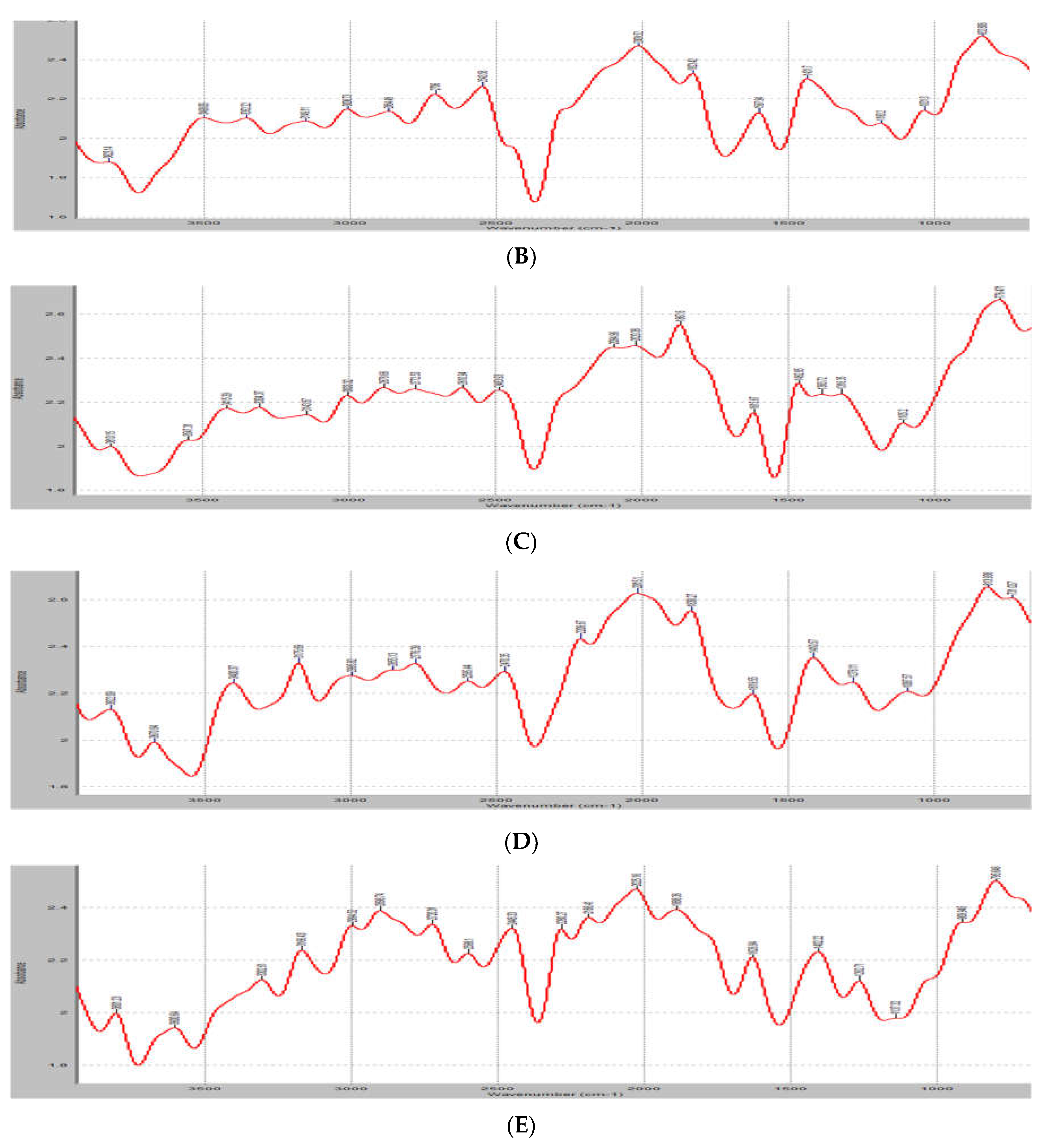
FTIR measures a sample’s absorbance of infrared light at various wavelengths to determine the material’s molecular composition and structure. The results of the FTIR of the unloaded and loaded samples of the lipospheres are shown in
Figure 2. The unloaded sample maintained peaks at 3623.14cm
-1 (N-H groups), 27706cm
-1 (C=O groups), 2009.92cm
-1 (C-O groups) and 832.9cm
-1 (C-H groups). The fingerprint region of the extract, PM-unloaded showed spectra with absorbance at 1457.4 cm
-1 and 1379.1 cm
-1. These peaks at this region corresponded to the asymmetric and symmetric bending of the C-H bond of methylene. Asymmetric and symmetric stretching of the C-H bond of methylene also occurred between 2935–2915 cm
-1 and 2865–2845 cm
-1 in all the formulated lipospheres samples. The loaded lipospheres had more sharp peaks at 2609cm
-1 (C=O
3.5. In-vitro anti-inflammatory studies
3.5.1. Membrane stabilization
In Table 2, the percentage (%) inhibition of membrane stabilization by the samples and the standard drug were shown. Inhibition of membrane increased steadily with an increase in concentration of PM in the lipospheres and the standard drug. However, after 400 µg/ml of sample, percentage inhibition fell drastically. The higher the percentage inhibition the more potent the concentration to stop the lysing of the red blood cells. The integrity of the cells is needed to maintain proper cellular function. In inflammation, lysis of some cellular component leads to release of inflammatory mediators. Stabilizing the red blood cell is a model to demonstrate the ability of the sample to stabilize those membranes of the stores of inflammatory mediators. Antioxidants play good role in this. The sample contains antioxidant activity which could account for the anti-inflammatory activity [
27]. Membrane stabilization assay of erythrocytes is a very popular tool to investigate the anti-inflammatory potential of a plant extract. When erythrocytes are exposed to hypotonic medium, heat, methyl salicylate, phenylhydrazine, among others, the lysis of their membrane occurs. It results in the leakage of serum protein and fluids into the tissues instigating inflammation [
27]. Membrane stabilization leads to prevention of leakage of serum protein and fluids into the tissues during a period of increased permeability caused by inflammatory mediators [
27]. So, the compounds capable of membrane stabilization might be very suitable as anti-inflammatory agents [
22,
28].
Table 2.
Membrane stabilization of the Samples and the standard drug.
Table 2.
Membrane stabilization of the Samples and the standard drug.
| Sample (µg/ml) |
% inhibition of membrane |
| 50 |
24.60 |
| 100 |
51.70 |
| 200 |
100.0 |
| 400 |
300.0 |
| 800 |
200.0 |
| Aspirin (µg/ml ) |
|
| 50 |
89.00 |
| 100 |
5.90 |
| 200 |
100.00 |
| 400 |
100.00 |
| 800 |
106.25 |
In other words, the crude methanol extract of
Pentaclethra macrophylla which contains copious amounts of bergenin demonstrated noticeable membrane stabilizing property [
5]. Several flavonoids and triterpenes have been reported earlier to have anti-inflammatory activity [
29]. As flavonoids and triterpenes are also present in
Pentaclethra macrophylla methanol extract [
30], it might be a reason for its membrane stabilizing anti-inflammatory potential.
3.5.2. Anti-platelet aggregation activity
In
Table 3, the percentage anti-platelet aggregatory activity of platelets by the samples and the standard drug were shown. The platelet aggregatory inhibition decreased steadily with an increase in concentration of the PM in the lipospheres as well as the standard. However, after 800 µg/ml of sample, percentage inhibition fell drastically without any effect on platelet. Antiplatelets prevent them from coming together as seen in inflammation (there is aggregation of platelets in inflammation).
The aim is to enable platelet generate some inflammatory mediators that increases inflammation. Then the mechanism of anti-platelet aggregation could be by indirectly inhibiting cyclooxygenase-2 or by blockade of calcium influx through membrane calcium channels [
31,
32,
33]. The model used showed that PM blocked calcium influx at acute time as shown by the result in
Table 3.
3.6. In-vivo anti-inflammatory studies
3.6.1. 2% Formaldehyde induced arthritis model
The effect of
Pentaclethra macrophylla herbal lipospheres on arthritis induced model of inflammation is as shown in
Table 4. The PM-loaded lipospheres inhibited edema consistently throughout the duration of observation. Among the extracts, the 150 mg/kg (corresponding to the 7.5 g PM-loaded liposphere) had the highest inhibition of edema. There were significant differences between the test and positive controls. Arthritis which is marked by pains around the joints could be as a result of auto-immunity, which could be genetic or by excessive deposition of metabolite leading to gout.
Pentaclethra macrophylla has been reported to have anti-inflammatory property. Farrukh et al [
34] have implicated down-regulation of the expression of genes for COX-2, PGE2, IL-1ᵝ, IL-6, TNF-α and NF-kᵝ and up-regulation of the expression of IL-4 and IL-10.
3.6.2. Egg albumin induced rat paw edema inflammatory model
Table 5 shows the effect of
Pentaclethra macrophylla herbal lipospheres on egg albumin induced inflammation in mice. The test substances at 50, and 100mg/kg did not inhibit the inflammation throughout the observation time. This is clearly shown by a negative percentage inhibition across all the time intervals. At dose of 150mg/kg, there was slight inhibition initially, then after 2h, the inhibition disappeared as shown by the negative percentage inhibition at 3h. This same trend was seen in the negative control which shows no justifiable and significant differences in activity when compared to the test samples at the different doses. Pentaclathra has been reported to have anti-inflammatory property [
33,
34], and Farrukh et al [
34] has implicated down-regulation of the expression of genes for COX-2, PGE2, IL-1ᵝ ,IL-6, TNF-α and NF-kᵝ and up-regulation of the expression of IL-4 and IL-10.
3.6.3. Blood Cells Counts of infected mice
The effect of
Pentaclethra macrophylla-loaded lipospheres on the blood cells profile of infected mice is a shown in
Table 6. The T50 dose of PM had the highest percentage of WBC and it decreased as the treatment doses increased from T100 to T200. There were no significant differences among the Neutrophil counts of the different groups. Similar trends was observed in the lymphocytes and Eosinophil counts. The monocytes and the basophils were undetected.
4. Conclusions
There are associated side effects with delivery system and anti-inflammatory agents. There was therefore a need to investigate the potency of herbal liposphere from Pentaclethra macrophylla. The excipients used were compatible with the extract and there was no chemical interaction thereby improving the stability of the formulations. The characterization of the herbal lipospheres formed presented good qualities and hence a promising delivery system for the herbal formulation of Pentaclethra macrophylla which is a potent drug that ameliorates different ailments. The study showed that Pentaclethra macrophylla was able to produce efficient herbal lipospheres with antinociceptive and anti-inflammatory activities.
Author Contributions
Petra Nnamani: Fund acquisition, Conceptualization, Methodology, Original draft preparation, Edith Diovu: Plant collection, identification and extraction, Peter Ikechukwu: Plant extraction, Onwuka Akachukwu: Methodology, Chiamaka Victory Ogbuanwu: Methodology, Abonyi Obiora Emmanuel: Animal study and analysis of data, Birgitta Loretz: Conceptualization and supervision, Claus-Michael Lehr: Conceptualization, supervision and proof reading.
Funding
Prof. P. O. Nnamani is grateful to the Georg Forster Research Award (Ref 3.4 - 1139093 - NGA - GFPR) of the Alexander von Humboldt Foundation Germany.
Acknowledgments
All authors read the manuscript and approved it for submission. No portion or part of the manuscript has been published earlier, nor is any part of it under consideration for publication at another journal.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Viana, F.A.; Pouliquen, Y.B.M.; Andrade-Neto, M.; Santiago, G.M.P.; Pessoa, O.D.L.; Rodrigues-Filho, E.; Braz-Filho, R. Complete1H and13C NMR assignments for two new monodesmoside saponins fromPentaclethra macroloba(Willd.) Kuntze. Org. Magn. Reson. 2004, 42, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Pertuit, D.; Kapundu, M.; Mitaine-Offer, A.-C.; Miyamoto, T.; Tanaka, C.; Delaude, C.; Lacaille-Dubois, M.-A. Triterpenoid Saponins From the Stem Bark of Pentaclethra eetveldeana. Nat. Prod. Commun. 2019, 14, 1–5. [Google Scholar] [CrossRef]
- Babady-Byla, Herz W. Triterpenes and 1-(ω-hydroxyceratyl) glycerols from Pentaclethra eetveldeana root bark. Phytochemistry. 1996; 42(2):501-504. [CrossRef]
- Viana, F.A.; Braz-Filho, R.; Pouliquen, Y.B.M.; Net, M.A.; Santiago, G.M.P.; Rodrigues-Filho, E. Triterpenoid saponins from stem bark of Pentaclethra macroloba. J. Braz. Chem. Soc. 2004, 15, 595–602. [Google Scholar] [CrossRef]
- Chinaka C. Nnennaya, Garuba A. Sunday and Augustine A. Ahmadu. Chemical Constituents from the Stem Bark of Pentaclethra macrophylla Benth (Fabaceae). Nig.J.Pharm. Res. 2017; 13 (1):37-44.
- Folefoc GN, Bisseck JP, Fomum ZT and Bodo B. A new Secokaurane diterpenoid and O-glucoside from seeds of Pentaclethera macrophylla. J Cameroon Academy of Science 2004; 4(3): 227-231.
- Akindahunsi, A. A. (2004). Physicochemical studies on African oil bean (Pentaclethra macrophylla Benth.) seed. Journal of Food Agriculture and Environment, 2: 14 - 17.
- Asoegwu S, Ohanyere S., Kanu O.and Iwueke C. “Physical Properties of African Oil Bean Seed (Pentaclethra macrophylla)” Agricultural Engineering International: the CIGR Ejournal. Manuscript FP 05 006. Vol. VIII. August, 2006.
- Novel Anti-Ulcer Phytosomal Formulation of Ethanol Extract of Pentaclethra macrophylla Stem-Bark. Trop. J. Nat. Prod. Res. 2020, 4, 385–391. [CrossRef]
- Igbe, I.; Osigwe, C. Hypoglycaemic activity of aqueous extract of Pentaclethra macrophylla Benth. (Fabaceae) stem bark in streptozotocin-induced diabetic rats. J of Pharmacy and Bioresources 201, 9, 39–44. [Google Scholar]
- Ukoro, B.; Chike, C.P.R.; Olorunfemi, O.J. Effects of Aqueous Extract of Pentaclethra macrophylla Leaves on Blood Pressure and Oxidative Stress in Albino Wistar Rats. International Journal of Scientific Research and Engineering Development 2020, 3. [Google Scholar]
- Iwu, I.C.; Uchegbu, R. Isolation and characterization of 4, 15- Cholestene-3-benzoate from the stem bark of Pentaclethra macrophylla (P. Benth). International Journal of Emerging Knowledge 2014, 1, 185–192. [Google Scholar]
- Tico Etnobotanical Dictionary. 2005. Pentaclethra macrophylla (Benth). Available online: http://www.ars-grin.gov/duke/dictionary/tico/p.html.
- Okunrobo, L. O.; Ching, F. P.; Ifijeh, F. Antinociceptive activity of methanol extract and aqueous fraction of the stem bark of Pentaclethra macrophylla Benth (Mimosaceae). Journal of Medicinal Plants Research 2009, 3, 101–104. [Google Scholar]
- Chidozie JI. African oil bean seed as an essential food supplement in the control of cancer and tobacco related diseases. Proceedings from the U1CC World Cancer Congress, July 8-12, Washington DC, 2006. 8 July.
- Balogun, B.I. Evaluation of the Nutritional Potentials of Fermented Oil Beans Seed Pentaclethra macrophyllah Benth. PAT 2013, 9, 73–87. [Google Scholar]
- Akah, P.A.; Aguwa, C.N.; Agu, R.U. Studies on the antidiarrhoeal properties ofPentaclethra macrophylla leaf extracts. Phytotherapy Res. 1999, 13, 292–295. [Google Scholar] [CrossRef]
- Alinnor, I.; Oze, R. Chemical Evaluation of the Nutritive Value of Pentaclethra macrophylla benth (African Oil Bean) Seeds. Pak. J. Nutr. 2011, 10, 355–359. [Google Scholar] [CrossRef]
- Osabor, VN, Pkpnkwo, PC and Ikeuba AI. Chemical profile of leaves and seeds of Pentaclethra macrophylla Benth. J Medinal Plant and Herbal Therapy Research 2017, 5, 11–17.
- Githens, TS. African Handbooks – Drug Plants of Africa. University of Pennsylvania Press. 1948: P. 64.
- Nnamani, P.; Ibezim, E.; Attama, A.; Adikwu, M. Surface modified solid lipid microparticles based on homolipids and Softisan® 142: preliminary characterization. Asian Pac. J. Trop. Med. 2010, 3, 205–210. [Google Scholar] [CrossRef]
- Yesmin, S.; Paul, A.; Naz, T.; Rahman, A.B.M.A.; Akhter, S.F.; Wahed, M.I.I.; Bin Emran, T.; Siddiqui, S.A. Membrane stabilization as a mechanism of the anti-inflammatory activity of ethanolic root extract of Choi (Piper chaba). Clin. Phytoscience 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Sur T. K, Biswas T. K., Ali L., Mukherjee B. Anti-inflammatory and anti-platelet aggregation activity of human placental extract. Acta Pharmacol Sin 2003, 24, 187–192.
- Zheng, Y.-Y.; Wu, T.-T.; Hou, X.-G.; Gao, Y.; Chen, Y.; Yang, Y.-N.; Li, X.-M.; Ma, X.; Ma, Y.-T.; Xie, X. Personalized antiplatelet therapy guided by a novel detection of platelet aggregation function in stable coronary artery disease patients undergoing percutaneous coronary intervention: a randomized controlled clinical trial. Eur. Hear. J. - Cardiovasc. Pharmacother. 2019, 6, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Nnamani, P.O.; Attama, A.A.; Kenechukwu, F.C.; Ibezim, E.C.; Adikwu, M.U. Pharmacodynamics of Piroxicam from Novel Solid Lipid Microparticles Formulated with Homolipids from Bos indicus. Curr. Drug Deliv. 2013, 10, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Ochei,J.and Kolhatkar,A. Medical LaboratoryScience Theory and Practice. Tata McGraw Hill Publishing Company Limited.New Dell.2008.
- Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–55. [CrossRef]
- Chaitanya R, Sandhya S, Banji D, Ravindran V and Murali S. “HRBC Membrane Stabilizing Property of Root, Stem and Leaf of Glochidion elutinum”. International Journal of Research in Pharmaceutical and Biomedical Sciences 2.1 (2011): 256-259.
- Shinde UA, Phadke AS, Nair AM, Mungantiwar AA, Dikshit VJ, Saraf MN. Membrane stabilizing activity — a possible mechanism of action for the anti-inflammatory activity of Cedrus deodarawood oil.Fitoterapia.2008;70:251–7.
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef]
- Beknal AK, Korwar PG, Halkai M, Kulkarni U, Patil BS, Soodam SR. Phytochemical investigation and antioxidant activity study of Drynaria quercifolia Linn. rhizome. Int J Curr Pharmaceut Res. 2010;4:36–9.
- Shah, B. H., Safdar, B., Virani, S. S., Nawaz, Z., Saeed, S. A. & Gilani, A. H. The anti-platelet aggregatory activity of Acacia nilotica is due to blockade of calcium influx through membrane calcium channels. Gen Pharmacol. 1997;29(2): 251-5. [CrossRef]
- Masafumi, Z., Yuhei, H., Shinji, N., Muneaki, M., Ichiro, F. & Eiichi, I. Thromboxane synthesis is increased by upregulating of cytosolic phospholipase A2 and cyclooxygenase-2 in peripheral polymorphonuclear leucocytes during bacterial infection in childhood. Am J Haematol 2003; 72 (2): 115-20. [CrossRef]
- Farrukh, M.; Saleem, U.; Qasim, M.; Manan, M.; Shah, M.A. Sarcococca saligna extract attenuates formaldehyde-induced arthritis in Wistar rats via modulation of pro-inflammatory and inflammatory biomarkers. Inflammopharmacology 2022, 30, 579–597. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).