Submitted:
25 August 2023
Posted:
29 August 2023
You are already at the latest version
Abstract
Keywords:
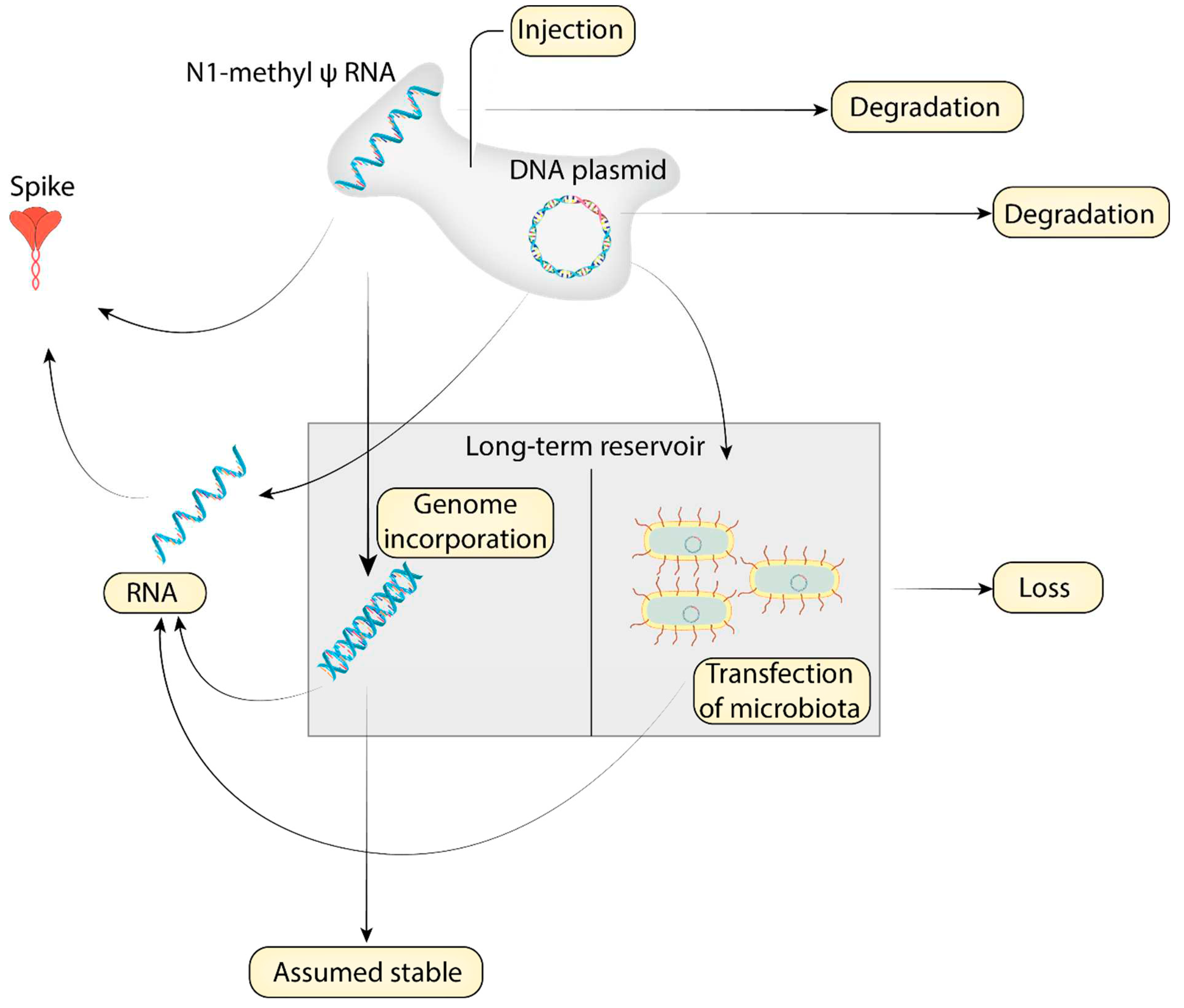
Introduction
Diagnostics
General
| Biomarker | Upper limit of normal |
| Peak cardiac troponin (T) | 14 ng/L [3] |
| Brain natriuretic Peptide (BNP) |
100pg/mL [3] |
| N-terminal prohormone of brain natriuretic peptide (NT-proBNP) | 450pg/mL [3] |
| C-reactive protein (CRP) | 8mg/L [3] |
| D-dimer | (patient’s age in years x 10mcg/L)* [13] |
Specific
- Causes of interpersonal variation
- Administration and dosing
- Individual variation
- Variation in cell uptake and biodistribution
Causation
Conclusion
References
- Kim, M.S.; Jung, S.Y.; Ahn, J.G.; Park, S.J.; Shoenfeld, Y.; Kronbichler, A.; Koyanagi, A.; Dragioti, E.; Tizaoui, K.; Hong, S.H.; et al. Comparative Safety of MRNA COVID-19 Vaccines to Influenza Vaccines: A Pharmacovigilance Analysis Using WHO International Database. Journal of Medical Virology 2022, 94, 1085–1095. https://doi.org/10.1002/jmv.27424. [CrossRef]
- Halma, M.T.J.; Rose, J.; Lawrie, T. The Novelty of MRNA Viral Vaccines and Potential Harms: A Scoping Review. J 2023, 6, 220–235. https://doi.org/10.3390/j6020017. [CrossRef]
- Yonker, L.M.; Swank, Z.; Bartsch, Y.C.; Burns, M.D.; Kane, A.; Boribong, B.P.; Davis, J.P.; Loiselle, M.; Novak, T.; Senussi, Y.; et al. Circulating Spike Protein Detected in Post–COVID-19 MRNA Vaccine Myocarditis. Circulation 2023, 147, 867–876. https://doi.org/10.1161/CIRCULATIONAHA.122.061025. [CrossRef]
- Theoharides, T.C.; Conti, P. Be Aware of SARS-CoV-2 Spike Protein: There Is More than Meets the Eye. J Biol Regul Homeost Agents 2021, 35, 833–838. https://doi.org/10.23812/THEO_EDIT_3_21. [CrossRef]
- Mörz, M. A Case Report: Multifocal Necrotizing Encephalitis and Myocarditis after BNT162b2 MRNA Vaccination against COVID-19. Vaccines 2022, 10, 1651. https://doi.org/10.3390/vaccines10101651. [CrossRef]
- Riley, T.P.; Chou, H.-T.; Hu, R.; Bzymek, K.P.; Correia, A.R.; Partin, A.C.; Li, D.; Gong, D.; Wang, Z.; Yu, X.; et al. Enhancing the Prefusion Conformational Stability of SARS-CoV-2 Spike Protein Through Structure-Guided Design. Frontiers in Immunology 2021, 12. [CrossRef]
- Mahajan, V.S.; Jarolim, P. How to Interpret Elevated Cardiac Troponin Levels. Circulation 2011, 124, 2350–2354. https://doi.org/10.1161/CIRCULATIONAHA.111.023697. [CrossRef]
- Melanson, S.E.F.; Morrow, D.A.; Jarolim, P. Earlier Detection of Myocardial Injury in a Preliminary Evaluation Using a New Troponin I Assay with Improved Sensitivity. Am J Clin Pathol 2007, 128, 282–286. https://doi.org/10.1309/Q9W5HJTT24GQCXXX. [CrossRef]
- Apple, F.S.; Sandoval, Y.; Jaffe, A.S.; Ordonez-Llanos, J.; IFCC Task Force on Clinical Applications of Cardiac Bio-Markers Cardiac Troponin Assays: Guide to Understanding Analytical Characteristics and Their Impact on Clinical Care. Clin Chem 2017, 63, 73–81. https://doi.org/10.1373/clinchem.2016.255109. [CrossRef]
- Thachil, J.; Lippi, G.; Favaloro, E.J. D-Dimer Testing: Laboratory Aspects and Current Issues. In Hemostasis and Thrombosis: Methods and Protocols; Favaloro, E.J., Lippi, G., Eds.; Methods in Molecular Biology; Springer: New York, NY, 2017; pp. 91–104 ISBN 978-1-4939-7196-1.
- Linkins, L.-A.; Takach Lapner, S. Review of D-Dimer Testing: Good, Bad, and Ugly. International Journal of Laboratory Hematology 2017, 39, 98–103. https://doi.org/10.1111/ijlh.12665. [CrossRef]
- Ridker, P.M. A Test in Context. Journal of the American College of Cardiology 2016, 67, 712–723. https://doi.org/10.1016/j.jacc.2015.11.037. [CrossRef]
- Urban, K.; Kirley, K.; Stevermer, J.J. PURLs: It’s Time to Use an Age-Based Approach to D-Dimer. J Fam Pract 2014, 63, 155–158.
- Bilotta, C.; Perrone, G.; Adelfio, V.; Spatola, G.F.; Uzzo, M.L.; Argo, A.; Zerbo, S. COVID-19 Vaccine-Related Thrombosis: A Systematic Review and Exploratory Analysis. Front Immunol 2021, 12, 729251. https://doi.org/10.3389/fimmu.2021.729251. [CrossRef]
- Mani, A.; Ojha, V. Thromboembolism after COVID-19 Vaccination: A Systematic Review of Such Events in 286 Patients. Annals of Vascular Surgery 2022, 84, 12-20.e1. https://doi.org/10.1016/j.avsg.2022.05.001. [CrossRef]
- Ogata, A.F.; Cheng, C.-A.; Desjardins, M.; Senussi, Y.; Sherman, A.C.; Powell, M.; Novack, L.; Von, S.; Li, X.; Baden, L.R.; et al. Circulating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Antigen Detected in the Plasma of MRNA-1273 Vaccine Recipients. Clinical Infectious Diseases 2022, 74, 715–718. https://doi.org/10.1093/cid/ciab465. [CrossRef]
- Bansal, S.; Perincheri, S.; Fleming, T.; Poulson, C.; Tiffany, B.; Bremner, R.M.; Mohanakumar, T. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer–BioNTech) Vaccination Prior to Development of Antibodies: A Novel Mechanism for Immune Activation by MRNA Vaccines. The Journal of Immunology 2021, 207, 2405–2410. https://doi.org/10.4049/jimmunol.2100637. [CrossRef]
- Aldén, M.; Olofsson Falla, F.; Yang, D.; Barghouth, M.; Luan, C.; Rasmussen, M.; De Marinis, Y. Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 MRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. Curr Issues Mol Biol 2022, 44, 1115–1126. https://doi.org/10.3390/cimb44030073. [CrossRef]
- McKernan, K.; Helbert, Y.; Kane, L.T.; McLaughlin, S. Sequencing of Bivalent Moderna and Pfizer MRNA Vaccines Reveals Nanogram to Microgram Quantities of Expression Vector DsDNA per Dose. OSF Preprints. April 2023, 10. [CrossRef]
- Brito, I.L. Examining Horizontal Gene Transfer in Microbial Communities. Nat Rev Microbiol 2021, 19, 442–453. https://doi.org/10.1038/s41579-021-00534-7. [CrossRef]
- Lugowski, A.; Nicholson, B.; Rissland, O.S. Determining MRNA Half-Lives on a Transcriptome-Wide Scale. Methods 2018, 137, 90–98. https://doi.org/10.1016/j.ymeth.2017.12.006. [CrossRef]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of Pseudouridine Into MRNA Yields Superior Nonimmunogenic Vector With Increased Translational Capacity and Biological Stability. Molecular Therapy 2008, 16, 1833–1840. https://doi.org/10.1038/mt.2008.200. [CrossRef]
- Leppek, K.; Byeon, G.W.; Kladwang, W.; Wayment-Steele, H.K.; Kerr, C.H.; Xu, A.F.; Kim, D.S.; Topkar, V.V.; Choe, C.; Rothschild, D.; et al. Combinatorial Optimization of MRNA Structure, Stability, and Translation for RNA-Based Therapeutics. Nat Commun 2022, 13, 1536. https://doi.org/10.1038/s41467-022-28776-w. [CrossRef]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 MRNA Vaccine Development. Sig Transduct Target Ther 2022, 7, 1–31. https://doi.org/10.1038/s41392-022-00950-y. [CrossRef]
- Patel, S.; Kim, J.; Herrera, M.; Mukherjee, A.; Kabanov, A.V.; Sahay, G. Brief Update on Endocytosis of Nanomedicines. Advanced Drug Delivery Reviews 2019, 144, 90–111. https://doi.org/10.1016/j.addr.2019.08.004. [CrossRef]
- Doherty, G.J.; McMahon, H.T. Mechanisms of Endocytosis. Annual Review of Biochemistry 2009, 78, 857–902. https://doi.org/10.1146/annurev.biochem.78.081307.110540. [CrossRef]
- Beyer, A.; Koch, T.; Schröder, H.; Schulz, S.; Höllt, V. Effect of the A118G Polymorphism on Binding Affinity, Potency and Agonist-Mediated Endocytosis, Desensitization, and Resensitization of the Human Mu-Opioid Receptor. Journal of Neurochemistry 2004, 89, 553–560. https://doi.org/10.1111/j.1471-4159.2004.02340.x. [CrossRef]
- Zhan, L.; Li, J.; Jew, B.; Sul, J.H. Rare Variants in the Endocytic Pathway Are Associated with Alzheimer’s Disease, Its Related Phenotypes, and Functional Consequences. PLOS Genetics 2021, 17, e1009772. https://doi.org/10.1371/journal.pgen.1009772. [CrossRef]
- Aladdin, Y.; Algahtani, H.; Shirah, B. Vaccine-Induced Immune Thrombotic Thrombocytopenia with Disseminated Intravascular Coagulation and Death Following the ChAdOx1 NCoV-19 Vaccine. Journal of Stroke and Cerebrovascular Diseases 2021, 30, 105938. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.105938. [CrossRef]
- Turner, S.; Khan, M.A.; Putrino, D.; Woodcock, A.; Kell, D.B.; Pretorius, E. Long COVID: Pathophysiological Factors and Abnormalities of Coagulation. Trends in Endocrinology & Metabolism 2023, 34, 321–344. https://doi.org/10.1016/j.tem.2023.03.002. [CrossRef]
- Pairo-Castineira, E.; Rawlik, K.; Bretherick, A.D.; Qi, T.; Wu, Y.; Nassiri, I.; McConkey, G.A.; Zechner, M.; Klaric, L.; Griffiths, F.; et al. GWAS and Meta-Analysis Identifies 49 Genetic Variants Underlying Critical COVID-19. Nature 2023, 617, 764–768. https://doi.org/10.1038/s41586-023-06034-3. [CrossRef]
- Hultström, M.; Frithiof, R.; Grip, J.; Lindelöf, L.; Rooijackers, O.; Pigazzini, S.; Niemi, M.; Cordioli, M.; Nkambule, L.; Maricic, T.; et al. Genetic Determinants of Mannose-Binding Lectin Activity Predispose to Thromboembolic Complications in Critical COVID-19. Nat Immunol 2022, 23, 861–864. https://doi.org/10.1038/s41590-022-01227-w. [CrossRef]
- Mancini, I.; Baronciani, L.; Artoni, A.; Colpani, P.; Biganzoli, M.; Cozzi, G.; Novembrino, C.; Boscolo Anzoletti, M.; De Zan, V.; Pagliari, M.T.; et al. The ADAMTS13-von Willebrand Factor Axis in COVID-19 Patients. J Thromb Haemost 2021, 19, 513–521. https://doi.org/10.1111/jth.15191. [CrossRef]
- Bordon, Y. A New Set of Genes Linked to Critical COVID-19. Nat Rev Immunol 2022, 22, 208–208. https://doi.org/10.1038/s41577-022-00709-0. [CrossRef]
- Vilma Lammi; Tomoko Nakanishi; Samuel E. Jones; Shea J. Andrews; Juha Karjalainen; Beatriz Cortés; Heath E. O’Brien; Brian E. Fulton-Howard; Hele H. Haapaniemi; Axel Schmidt; et al. Genome-Wide Association Study of Long COVID. medRxiv 2023, 2023.06.29.23292056. https://doi.org/10.1101/2023.06.29.23292056. [CrossRef]
- Ledford, H. Gene Linked to Long COVID Found in Analysis of Thousands of Patients. Nature 2023, 619, 445–445. https://doi.org/10.1038/d41586-023-02269-2. [CrossRef]
- Shun Nogawa; Hajime Kanamori; Koichi Tokuda; Kaoru Kawafune; Miyuki Chijiiwa; Kenji Saito; Shoko Takahashi Identification of Susceptibility Loci for Adverse Events Following COVID-19 Vaccination in the Japanese Population: A Web-Based Genome-Wide Association Study. medRxiv 2021, 2021.11.30.21267043. https://doi.org/10.1101/2021.11.30.21267043. [CrossRef]
- Li, P.; Shi, D.; Shen, W.; Shi, S.; Guo, X.; Li, J.; Xu, S.; Zhang, Y.; Zhao, Z. Pilot Genome-Wide Association Study of Antibody Response to Inactivated SARS-CoV-2 Vaccines. Frontiers in Immunology 2022, 13. [CrossRef]
- Delshad, M.; Sanaei, M.-J.; Pourbagheri-Sigaroodi, A.; Bashash, D. Host Genetic Diversity and Genetic Variations of SARS-CoV-2 in COVID-19 Pathogenesis and the Effectiveness of Vaccination. International Immunopharmacology 2022, 111, 109128. https://doi.org/10.1016/j.intimp.2022.109128. [CrossRef]
- Falahi, S.; Kenarkoohi, A. Host Factors and Vaccine Efficacy: Implications for COVID-19 Vaccines. Journal of Medical Virology 2022, 94, 1330–1335. https://doi.org/10.1002/jmv.27485. [CrossRef]
- Dudley, M.Z.; Gerber, J.E.; Budigan Ni, H.; Blunt, M.; Holroyd, T.A.; Carleton, B.C.; Poland, G.A.; Salmon, D.A. Vaccinomics: A Scoping Review. Vaccine 2023, 41, 2357–2367. https://doi.org/10.1016/j.vaccine.2023.02.009. [CrossRef]
- Filippatos, F.; Tatsi, E.-B.; Efthymiou, V.; Syriopoulou, V.; Michos, A. Time of Day of BNT162b2 COVID-19 Immunization Affects Total SARS-CoV-2 Antibody Levels but Not Neutralizing Activity. J Biol Rhythms 2022, 37, 562–566. https://doi.org/10.1177/07487304221100951. [CrossRef]
- Zhang, H.; Liu, Y.; Liu, D.; Zeng, Q.; Li, L.; Zhou, Q.; Li, M.; Mei, J.; Yang, N.; Mo, S.; et al. Time of Day Influences Immune Response to an Inactivated Vaccine against SARS-CoV-2. Cell Res 2021, 31, 1215–1217. https://doi.org/10.1038/s41422-021-00541-6. [CrossRef]
- Lammers-van der Holst, H.M.; Lammers, G.J.; van der Horst, G.T.J.; Chaves, I.; de Vries, R.D.; GeurtsvanKessel, C.H.; Koch, B.; van der Kuy, H.M. Understanding the Association between Sleep, Shift Work and COVID-19 Vaccine Immune Response Efficacy: Protocol of the S-CORE Study. Journal of Sleep Research 2022, 31, e13496. https://doi.org/10.1111/jsr.13496. [CrossRef]
- Schmitz, N.C.M.; van der Werf, Y.D.; Lammers-van der Holst, H.M. The Importance of Sleep and Circadian Rhythms for Vaccination Success and Susceptibility to Viral Infections. Clocks & Sleep 2022, 4, 66–79. https://doi.org/10.3390/clockssleep4010008. [CrossRef]
- Mason, A.E.; Kasl, P.; Hartogensis, W.; Natale, J.L.; Dilchert, S.; Dasgupta, S.; Purawat, S.; Chowdhary, A.; Anglo, C.; Veasna, D.; et al. Metrics from Wearable Devices as Candidate Predictors of Antibody Response Following Vaccination against COVID-19: Data from the Second TemPredict Study. Vaccines 2022, 10, 264. https://doi.org/10.3390/vaccines10020264. [CrossRef]
- Wagenhäuser, I.; Reusch, J.; Gabel, A.; Mees, J.; Nyawale, H.; Frey, A.; Lâm, T.-T.; Schubert-Unkmeir, A.; Dölken, L.; Kurzai, O.; et al. The Relationship between Mental Health, Sleep Quality and the Immunogenicity of COVID-19 Vaccinations. Journal of Sleep Research n/a, e13929. https://doi.org/10.1111/jsr.13929. [CrossRef]
- Bongiovanni, M.; Liuzzi, G.; Schiavon, L.; Gianturco, L.; Giuliani, G. Evaluation of the Immune Response to COVID-19 Vaccine MRNA BNT162b2 and Correlation with Previous COVID-19 Infection. Journal of Clinical Virology 2021, 143, 104962. https://doi.org/10.1016/j.jcv.2021.104962. [CrossRef]
- Sidler, D.; Born, A.; Schietzel, S.; Horn, M.P.; Aeberli, D.; Amsler, J.; Möller, B.; Njue, L.M.; Medri, C.; Angelillo-Scherrer, A.; et al. Trajectories of Humoral and Cellular Immunity and Responses to a Third Dose of MRNA Vaccines against SARS-CoV-2 in Patients with a History of Anti-CD20 Therapy. RMD Open 2022, 8, e002166. https://doi.org/10.1136/rmdopen-2021-002166. [CrossRef]
- Rockman, M.V.; Kruglyak, L. Genetics of Global Gene Expression. Nat Rev Genet 2006, 7, 862–872. https://doi.org/10.1038/nrg1964. [CrossRef]
- Zhang, W.; Duan, S.; Kistner, E.O.; Bleibel, W.K.; Huang, R.S.; Clark, T.A.; Chen, T.X.; Schweitzer, A.C.; Blume, J.E.; Cox, N.J.; et al. Evaluation of Genetic Variation Contributing to Differences in Gene Expression between Populations. The American Journal of Human Genetics 2008, 82, 631–640. https://doi.org/10.1016/j.ajhg.2007.12.015. [CrossRef]
- Svitkin, Y.V.; Cheng, Y.M.; Chakraborty, T.; Presnyak, V.; John, M.; Sonenberg, N. N1-Methyl-Pseudouridine in MRNA Enhances Translation through EIF2α-Dependent and Independent Mechanisms by Increasing Ribosome Density. Nucleic Acids Res 2017, 45, 6023–6036. https://doi.org/10.1093/nar/gkx135. [CrossRef]
- Lim, J.K.; Lisco, A.; McDermott, D.H.; Huynh, L.; Ward, J.M.; Johnson, B.; Johnson, H.; Pape, J.; Foster, G.A.; Krysztof, D.; et al. Genetic Variation in OAS1 Is a Risk Factor for Initial Infection with West Nile Virus in Man. PLOS Pathogens 2009, 5, e1000321. https://doi.org/10.1371/journal.ppat.1000321. [CrossRef]
- Liu, L.; Chen, X.; Skogerbø, G.; Zhang, P.; Chen, R.; He, S.; Huang, D.-W. The Human Microbiome: A Hot Spot of Microbial Horizontal Gene Transfer. Genomics 2012, 100, 265–270. https://doi.org/10.1016/j.ygeno.2012.07.012. [CrossRef]
- van Reenen, C.A.; Dicks, L.M.T. Horizontal Gene Transfer amongst Probiotic Lactic Acid Bacteria and Other Intestinal Microbiota: What Are the Possibilities? A Review. Arch Microbiol 2011, 193, 157–168. https://doi.org/10.1007/s00203-010-0668-3. [CrossRef]
- Groussin, M.; Poyet, M.; Sistiaga, A.; Kearney, S.M.; Moniz, K.; Noel, M.; Hooker, J.; Gibbons, S.M.; Segurel, L.; Froment, A.; et al. Elevated Rates of Horizontal Gene Transfer in the Industrialized Human Microbiome. Cell 2021, 184, 2053-2067.e18. https://doi.org/10.1016/j.cell.2021.02.052. [CrossRef]
- Sfera, A.; Osorio, C.; Hazan, S.; Kozlakidis, Z.; Maldonado, J.C.; Zapata-Martín del Campo, C.M.; Anton, J.J.; Rahman, L.; Andronescu, C.V.; Nicolson, G.L. Long COVID and the Neuroendocrinology of Microbial Translocation Outside the GI Tract: Some Treatment Strategies. Endocrines 2022, 3, 703–725. https://doi.org/10.3390/endocrines3040058. [CrossRef]
- Hazan, S.; Stollman, N.; Bozkurt, H.S.; Dave, S.; Papoutsis, A.J.; Daniels, J.; Barrows, B.D.; Quigley, E.M.; Borody, T.J. Lost Microbes of COVID-19: Bifidobacterium, Faecalibacterium Depletion and Decreased Microbiome Diversity Associated with SARS-CoV-2 Infection Severity. BMJ Open Gastroenterol 2022, 9, e000871. https://doi.org/10.1136/bmjgast-2022-000871. [CrossRef]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.-Y.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.-C.; et al. Gut Microbiota Dynamics in a Prospective Cohort of Patients with Post-Acute COVID-19 Syndrome. Gut 2022, 71, 544–552. https://doi.org/10.1136/gutjnl-2021-325989. [CrossRef]
- Fairgrieve, D.; Rizzi, M.; Kirchhelle, C.; Halabi, S.; Howells, G.; Witzleb, N. No-Fault Compensation Schemes for COVID-19 Vaccines: Best Practice Hallmarks. Public Health Rev 2023, 44, 1605973. https://doi.org/10.3389/phrs.2023.1605973. [CrossRef]
- Fairgrieve, D.; Borghetti, J.-S.; Dahan, S.; Goldberg, R.; Halabi, S.; Holm, S.; Howells, G.; Kirchelle, C.; Pillay, A.; Rajneri, E. Comparing No-Fault Compensation Systems for Vaccine Injury. Tul. J. Int’l & Comp. L. 2023, 31, 75.
- Crum, T.; Mooney, K.; Tiwari, B.R. Current Situation of Vaccine Injury Compensation Program and a Future Perspective in Light of COVID-19 and Emerging Viral Diseases. F1000Res 2021, 10, 652. https://doi.org/10.12688/f1000research.51160.2. [CrossRef]
- Demasi, M. Covid-19: Is the US Compensation Scheme for Vaccine Injuries Fit for Purpose? BMJ 2022, 377, o919. https://doi.org/10.1136/bmj.o919. [CrossRef]
- Long Covid Is a ‘National Crisis.’ So Why Are Grants Taking so Long to Get? Available online: https://www.science.org/content/article/long-covid-national-crisis-so-why-are-grants-taking-so-long-get (accessed on 11 July 2023).
- Halma, M.T.J.; Plothe, C.; Marik, P.; Lawrie, T.A. Strategies for the Management of Spike Protein-Related Pathology. Microorganisms 2023, 11, 1308. https://doi.org/10.3390/microorganisms11051308. [CrossRef]
- Post-Vac Syndrome — the Forgotten COVID Victims – DW – 03/21/2023 Available online: https://www.dw.com/en/post-vac-syndrome-the-forgotten-covid-victims/a-65051748 (accessed on 11 July 2023).
| Vaccine Spike | Viral Spike |
| No N protein present | N protein present |
| Sequence identical to vaccine sequence | Sequence much less constrained, reflects currently circulating variants |
| Locked into prefusion conformation | Conformationally flexible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




