1. Introduction
p53WT is a tumor suppressor protein encoded by the TP53 gene located on chromosome 17p13.1. In response to cellular stress, p53 activates the expression of several genes associated with cell cycle arrest, apoptosis, and DNA repair; however, p53 is mutated in more than 50% of human cancer [
1] TP53 missense mutations occur mainly in the DNA-binding domain and can be classified into two main categories. These two categories of mutations are commonly referred to as conformational or DNA contact mutations, or class I and II, respectively [
2]. Class I involves the substitution of an amino acid residue that causes loss of contact with DNA affecting its transcriptional capacity; within this category are mutations at positions R248Q and R273C. Mutated class II proteins present structural changes (R175H) that arise from their affinity for DNA. Class I mutants of p53 usually have a native conformation, whereas class II are unable to acquire the native conformation and therefore misfold [
3]. The effects of TP53 mutations can be classified into three. First, p53 mutations attenuate binding to its DNA response elements and block transcriptional activation of p53 target genes; so a partial or total loss function can define these mutations; Second, p53 mutant proteins exert a dominant-negative effect on the function of wild-type (WT) p53 protein, encoded by the second allele, through the formation of a heterotetramer deficient in its binding to specific DNA sequences, also known as dominant-negative mutations. Finally, some p53 mutants also acquire new functions independent of p53WT; this event is known as a gain of function (GOF) GOF p53 mutations are involved in critical oncogenic processes, such as increased cell migration and invasiveness [
4,
5]. The effects of TP53 mutations on function and cellular behavior depend on the cell type and environmental conditions [
6]. Thus, mutant p53 proteins are able to interact with specific intracellular proteins and induce gene expression changes [
7,
8]. Moreover, GOF activities of p53 missense mutations vary depending on the mutation type, giving rise to phenotypic differences in vivo associated with developing different cancer types [
9]. In summary, understanding how p53 mutants induce phenotypic differences may help cancer prevention and therapy strategies.
In the present study, we explored the possibility that p53 mutant proteins exert their gain of function activity by modulating the expression of miRNAs. The miRNAs are 20-24 nucleotides in length and are involved in the post-transcriptional control of genes [
10]. Recently, it has been reported that some miRNAs also play an important role in the gain of function of mutant p53; it has been demonstrated how p53 mutants regulate gene expression and exert oncogenic effects by unbalancing specific microRNAs (miRNAs) levels, even disrupting its biogenesis which provokes epithelial-mesenchymal transition, chemoresistance, and cell survival, among others [
11,
12]. However, the details of how and which miRNAs are regulated by p53 mutants promoting tumorigenesis are not yet fully understood.
In this study, we observed that p53R273C, p53R248Q, and p53R175H mutants have an overall negative effect on the expression of miRNAs in cancer. Moreover, in Saos-2 cells transfected with the p53R175H mutant, we found 93 decreased and 10 increased miRNAs. On the other hand, in the p53R273C profile, we observed a decrease of 72 miRNAs and an increase of 35. Finally, in the expression profile regulated by the p53R248Q mutant, we found a decrease of 167 miRNAs and an increase of 6, compared to the control (p53WT). Furthermore, within the miRNAs over-expressed in the presence of p53 mutants, we found the miR-182-5p, which is associated with cell invasion and migration, and its expression was significantly higher in p53R248Q. Interestingly, we demonstrate that the p53R248Q mutant induces over-expression of miR-182-5p and that this miRNA is required for the p53R248Q mutant to induce cell invasion and migration capacity in Saos-2 and OVCAR-3 cell lines.
2. Materials and Methods
2.1. Cell Culture and Treatments.
Saos-2/Osteosarcoma (null-p53), SKBR3/Breast carcinoma (p53R175H), C33a/Cervical cancer (p53R273C), and OVCAR-3/Ovarian cancer (p53R248Q) cell lines were cultured in DMEM medium (Gibco BRL) supplemented with 10% Fetal Bovine Serum (SFB). The non-tumorigenic breast epithelial cell line MCF10a (p53WT) was cultured in DMEM-F12 medium supplemented with 10% SFB, 10 μg/ml insulin, 0.5 μg/ml hydrocortisone, and 20 ng/ml recombinant human epidermal growth factor (EGF). All cells were obtained from ATCC (American Type Cell Culture Collection) and were maintained at 37°C and 5% CO2.
For treatments with pifithrin-α hydrobromide (CAS 63208-82-2, Santa Cruz, cat # sc-126 HRP), OVCAR-3 cells were treated with or without the drug (30, 50, 75, and 100 μM) for 24 h. The vehicle was Dimethyl sulfoxide (DMSO). Subsequently, the cells were collected and used in the corresponding experiments.
2.2. Plasmids and transfections.
Saos-2 cells at 70% confluency were transfected with p53R273C [
13], p53R175H [
14], p53R248Q [
13], p53WT [
15] or empty vector. Transfections corresponding to p53 (R273C, R175H, or R248Q) and transient (p53WT) were performed using Lipofectamine 3000 Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Clone selection for transfections was performed using 800 µg/ml G418 (Sigma-Aldrich). The activity of miR-182-5p was inhibited using the Anti-miR™ miRNA Inhibitor (MH12369) Ambion®; for this purpose, Saos-2 cells were transfected with 10 µM negative control #1 (AM17010), and Saos-2 (p53R248Q) with 10 µM of the negative control and/or anti-miR, this was done using Lipofectamine 3000 following the manufacturing instructions (Invitrogen). Samples were collected within 24 hours for further experiments.
2.3. RNA extraction.
Total RNA was extracted with the Trizol method (Invitrogen, USA) according to the protocol provided by the manufacturer. RNA concentration was measured using NanoDrop ND-2000 (Thermo Scientific), and RNA quality was evaluated by Tape Station 2200 bioanalyzer (Agilent Technologies), with minimum quality requirements: A260/280 ≥ 1.8; RNA integrity number- RIN≥ 7.
2.4. Reverse transcription
Using the Applied Biosystems miScript RT II kit, cDNA was synthesized following the manufacturer’s recommendations. The RT reaction was performed on the GeneAmp System 7500 thermal cycler (Applied Biosystems).
Human miRNome PCR Array
Expression profiling was performed with miScript miRNA PCRNA Arrays Human miRNome (384-well plate) (MIHS-3216Z) QIAGEN, which is based on 1066 miRNAs reported in miRbase Release 16 (
www.miRBase.org), plus controls. SYBR Green qPCR was performed as follows: 2.5 µL of the cDNA (p53WT, p53R275H, p53R248Q, or p53R273C) were mixed with 5 µL of RNAse-free water plus 12.5 µL of 2X QuantiTect SYBR Green PCR Master Mix and 2.5 µL of 10X miScript Universal primer. The reaction was carried out by programming one step at 95°C for 15 min, followed by 40 cycles of three temperatures 94°C for 15 s, 55°C for 30 s, and 70°C for 30 s. The amplification reactions were completed using the QuantStudio 6 Flex Real-Time PCR System (Life Technologies).
2.6. PCR Array Analysis
The data were analyzed through the "Web-based miScript miRNA PCR Array data analysis tool" software, which allowed us to generate the list of differentially expressed miRNAs using the comparative CT (2-∆∆ct) method. The expression resulting from the cell line transfected with p53WT was considered as a calibrator, and the expression of the small nucleolar RNAs RNU6 and RNU48 was considered as a normalizer. Differentially expressed miRNAs were considered to be those with a rate of change value of ≥2 or ≤-2 concerning the control and presenting statistical confidence or p-value < 0.05.
2.7. Bioinformatic Analysis and Visualization
All differentially expressed miRNAs from the three comparison analyses performed (p53R273C vs. p53WT, p53R248Q vs. p53WT, and p53R175H vs. p53WT) were used. Using a heatmap with an unsupervised hierarchical clustering approach with the gplots 2.14.1 library [
16] of the Bioconductor library [
17] of the R 3.1.1 statistical software [
18].
Differential expression visualization was performed using circos plots with the Circos tool [
19]. To identify similarities of differentially expressed miRNAs between comparisons, the online tool Venny 2.1 was used [
20].
Enrichment results of differentially expressed miRNAs were processed through the online software miRPath 3.0 [
21]. The microT-CDS 5.0 algorithm was used to determine hypothetical target genes and DIANA-TarBase 7.0 for experimentally validated targets. To identify signaling pathways altered by miRNAs, information from the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways was used [
22]. Pathways were considered altered when p <0.05 and visualized by dotplot performed in R 3.1.1 statistical software.
2.8. Validation of miRNA expression by RT-qPCR
Taqman probes specific for hsa-miR-509-5p (ID: 002235), hsa-miR-3151 (ID: 243597 MAT), has-miR-27b (ID 002174), has-miR-200c* (ID: 002286), has-miR-517a (ID: 001151), has-miR-101 (ID: 002253), miR-885-3p (ID 002372) and hsa-miR-182 (ID: 002334), as well as the internal control RNU6 (ID: 001093) were purchased from Ambion (Applied Biosystems, Foster City, CA, USA) (P/N: 4427975). First, miRNA RT was performed using stem-loop primers (Applied Biosystems). For this, 5 µL (100 ng/µL) of total RNA were added to the mixture containing: 0.15 µL of dNTPs with dTTP (100 mM); 3 µL of miRNA RT primers;1. 0 µL of MultiScribe reverse transcriptase enzyme (50 U/µL); 1.5 µL of 10X RT buffer; 0.19 µL of RNase inhibitor (20 U/ µL), and 4.16 µL of RNase-free water, totaling 15 µL per reaction. The RT reaction was performed on a GeneAmp System 9700 thermal cycler (Applied Biosystems), programming three temperatures: at 16°C for 30 min, 42°C for 30 min, and 85°C for 5 min, plus a final step at 4°C. The real-time PCR reaction required for each miRNA 1.33 µL of the RT reaction product, which was mixed with 10 µL of TaqMan master mix (Universal PCR Master Mix, No 4 AmpErase UNG, 2X), plus 7.67 µL of RNAse-free water and 1.0 µL of PCR probe (specific for each miRNA), giving a total of 20 µL. The analysis was performed on an Applied Biosystems QuantStudio 3 Real-Time PCR. The reaction was carried out by programming a step at 95°C for 10 min; followed by 45 cycles of two temperatures: 95°C for 15 s and 60°C for 1 min. The results were analyzed by the 2-∆∆ct method, as described above in the PCR Array Analysis section.
2.9. Expression analysis of miR-182-5p target genes via RT-qPCR.
"High Capacity cDNA Reverse Transcription Kit" (Thermo Fisher Scientific, UK) was used for the retrotranscription reaction according to the manufacturer’s instructions. FOXF2 and MTSS1 expression was determined by QRT-PCR using SYBR Green/ROX Master Mix (Thermo Fisher Scientific, UK) following the manufacturer’s instructions. Quantitative data were normalized relative to HPRT. The sequences of the primers were: FOXF2-For: CAA GGT AGC GTT CCC CAA TC; FOXF2-Rev: GTC TGC TTT TTT CAC ACC CTG AT; MTSS1-For: TGA CCC GCT CTG TTG; MTSS1-Rev: GGT GCC CAC TAC GGA AAC G and for HPRT-For AGG GTG TTT ATT CCT CAT GG; HPRT-Rev CAC AGA GGG CTA CAA TGT. The reactions were performed in QuantStudio 3 (Thermo Fisher Scientific, UK). The cycling conditions were: 50°C for 2 min to activate the UNG, initial denaturation at 95°C for 10 min and 45 cycles at 95°C for 15 s, and finally 60°C for 1 min. Standard curves were analyzed to verify the amplification efficiency of each gene. The 2-ΔΔCT equation was applied to calculate the relative expression of the samples.
2.10. Western blot
Cells were collected and subsequently lysed using RIPA buffer (Beyotime Biotechnology Co., Ltd., Shanghai, China). Protein concentration was then determined via the Lowry method, and 50 µg were loaded onto 10% SDS-PAGE gels. After blocking, we used the specific antibodies against p53 (DO-1, Santa Cruz, cat # sc-126 HRP) (1:500) and β-actin (AC-15, Sigma Aldrich, cat # A3854) (1:50,000). Protein expression was detected by chemoluminescence using Supersignal West Pico (Thermo Scientific, Waltham MA).
2.11. Cell migration and invasion assays
For all cell migration assays, the cell lines (2x105) were resuspended in 300 µL of serum-free MEM medium and seeded on the top surface of Transwell® inserts (8 µm; Corning, USA) in 24-well box, to which 500 µL of 10% SFB (Chemoattractant) DMEM medium was previously added. Cell invasion was assessed using QCM ECMatrix Cell Invasion Assay, 24-well (8 µm) (ECM554) Chemicon (Millipore, Billerica, MA, USA); cells were seeded in ECMatrix Cell Invasion Assay QCM chambers (1x105) in the absence of Fetal Bovine Serum; the inserts were placed in 24-well boxes to which 500 µL of 10% SFB (Chemoattractant) DMEM medium was previously added. Subsequently, cells were incubated at 37°C for 24 h for the cell migration and invasion assay, cells on top of the insert were gently removed, and cells that migrated to the bottom of the insert were fixed with 4% paraformaldehyde for 15 min, stained with 0.1 % crystal violet (Sigma-Aldrich, St. Louis, MO, USA). The absorbance of stained cells was read on an ELISA plate reader (SofMaxPro USA) at 560 nm.
2.12. Statistical Analysis
The statistics used were ANOVA to observe the differences between groups of different treatments and incubation times, and the unpaired Student’s t-test to compare the differences between two groups, using the statistical program GraphPad Prism software 5.0 (GraphPad Software, Inc., San Diego, CA, USA). Each experiment was performed in triplicate and repeated at least three times; differences were considered significant when p<0.05.
4. Discussion
In this study, we observed most of the miRNAs differentially expressed downregulated in the presence of p53R175H, p53R273C, and p53R248Q. Our results agree with a previous study in which a miRNA profile was performed in the presence of the p53R282W mutant; they also observed an overall negative effect on miRNA expression [
47]. In addition, Garibaldi et al. demonstrated that mutants bind and sequester the p72/82 RNA helicase of the microprocessor complex, interfering with the association between Drosha and pri-miRNA, inhibiting post-transcriptional maturation, which contributes to the negative regulation of miRNAs [
33].
The association between the differentially expressed miRNAs in the three miRNAs profiles and the signaling pathways in which they might participate (target mRNAs hipotetic/mRNAs validates) led us to conclude that they have the "Adherens junction" pathway in common. This coincides with reports that p53 mutants can promote mesenchymal phenotype, inducing transcription factors such as TWIST-1 and SLUG, which promotes the loss of adherens junction, favoring cell motility [
48]. In addition, up-regulation of some miRNAs involved in the regulation of EMT, metastasis, cell migration, and invasion (miR-130b, let-7i, miR-218, and miR-519a) has been previously reported in the presence of p53 mutants [
33,
49,
50]. In another study, the exogenous expression of p53R248Q and p53R282W mutants in H1299 cell line (null p53) induce miR-155 overexpression in breast cancer. Moreover, knockdown of the R249S endogenous p53 mutant in BT-549 cells resulted in a significantly reduced level of miR-155, confirming a role for mutant p53 in the aberrant activation of miR-155 [
51] In agreement with these reports, we also observed overexpression of miR-155 in the presence of the p53R248Q mutant. However, we also observed different pathways altered depending on which p53 mutant was expressed; This is in agreement with studies of GOF activities of p53 mutants, for example, in mice harboring a novel germline Trp53R245W allele (contact mutation), and compared them to existing with Trp53R172H (structural mutation) and Trp53R270H (contact mutation) alleles it was observed that Trp53R245W/+ and Trp53R270H/+ mice develop more frequently osteosarcomas and had a poor overall survival in contrast with Trp53R172H/+ mice [
9].
On the other hand, previous research showed that p53R248Q and p53R248W mutants induce invasion and migration binding Stat3 and enhance activating Stat3 phosphorylation in colorectal and pancreatic cancer [
52,
53]. Interestingly, a positive correlation has been reported between miR-182-5p and Stat3 pathway in gliomas and breast cancer [
41,
54]. Recently, it has also been demonstrated that miR-182-5p target de PIAS1 (protein inhibitor of activated STAT) mRNA in endometrial cancer, and the overexpression of PIAS1 inhibited the Stat3 activity [
42]. These reports suggest that p53 mutants could induce the activation of Stat3 pathway through miR-182-5p in addition to binding the protein directly; however, more experiments are necessary to prove this hypothesis.
Other signaling pathways shared in the three profiles based on target hypothetic mRNAs were the Hippo signaling pathway and the pluripotent stem cell regulation pathway, which in turn share several miRNAs (miR-101-3p , miR-3125, miR-3681-5p, miR-508-5p, miR-517a-3p, miR-888-3p, miR-200b-3p, miR-2052, miR-3122, miR-3140-3p, miR- 3151-5p, miR-3168, miR-3618, miR-3660, miR-3908, miR-3913-5p, miR-4267, miR-4275, miR-4280, miR-4288, miR-4311, miR-4323, miR-509-5p and miR-520c-3p). The Hippo signaling pathway is a key regulator of physiological processes such as: cell proliferation, differentiation, polarity and death [
55,
56]. Previously, it has been reported that the p53R280K and p53R175H mutants can physically interact with a modulator of this pathway known as YAP (YES-associated protein) and form a complex with NF-Y, increasing the transcription of genes involved in cell proliferation [
57]. In addition, YAP/TAZ overexpression has been reported to induce cell proliferation and the acquisition of cancer stem cell characteristics [
58]. It could be possible that p53R248Q, p53R175H, and p53R273C regulate the expression of some miRNAs through the Hippo pathway or induce this pathway by regulating some of these miRNAs; it would be interesting to analyze this possibility.
Moreover, analysis through DIANA-TarBase (validated mRNAs) shows that the Cell cycle is a shared pathway between the three miRNAs expression profiles from the three p53 mutants, with the following miRNAs in common: miR-200b-3p, miR-431-3p, miR-508-5p, miR-509-5p, miR-520c-3p, miR-888-3p, miR-3140-3p, miR-3913-5p. It is well known that activation of p53WT by DNA damage induces the expression of p21. However, the above does not occur in cells expressing p53 mutants, as p21 expression decreases [
59]. Furthermore, it has been reported that p53 mutants can regulate the cell cycle through miR-128-2, miR-223 and miR-517a [
33]. Interestingly, in our work, we found downregulation of miR-517a-3p, miR-509-5p, and miR-101-3p in the presence of all 3 mutants. In this sense, previous studies have shown that downregulation of miR-517a and miR-517c contribute to the development of hepatocellular carcinoma through post-transcriptional regulation of Pyk2 (protein tyrosine kinase 2 beta), which is associated with blockade of the G2/M transition [
32]. In our study, we found decreased miR-517a and miR-517c in the profile of the p53R248Q mutant. miR-509-5p can also delay the G1/S transition in the cell cycle, as well as facilitate apoptosis in cervical cancer cells; because miR-509-5p negatively regulates MDM2, which increases p53WT levels, resulting in p21 overexpression [
23]. Additionally, upregulation of miR-101-3p has been reported to suppress EZH2 and HDAC9 expression, thereby inhibiting cell cycle progression in Retinoblastoma cells [
36]. HDAC9 expression is associated with cell proliferation in vitro, and its inhibition with cell arrest in the G1 phase is consistent with the reduction in Cyclin E2 and CDK2 expression in retinoblastoma cells [
60]. On its part, EZH2 transcriptionally represses the cell cycle suppressor INK-ARF, driving cell cycle progression of cancer stem cells [
61], so p53 mutants could induce cell cycle progression by inducing Cyclin E2, CDK2 expression and silencing INK-ARF expression through negative regulation of miR-101-3p.
On the other hand, it has been reported that miR-3151 is silenced by methylation of its promoter in chronic lymphocytic leukemia (CLL), favoring cell proliferation [
27], although inactivation of this miRNA has not been associated with the presence of p53 mutations, it is known that mutations of this protein are frequent in patients with CLL and have been associated with resistance to chemotherapy and a poor prognosis [
62], so it would be interesting to demonstrate whether there is an association between the presence of p53 mutants and miR-3151 expression, as well as its possible relationship with chemotherapy resistance and/or prognosis in patients with CLL.
The miRNAs are known to repress gene expression, but WT-induced overexpression of ΔNp63α (a dominant-negative isoform of p63) in cisplatin-treated cells has been reported to activate the MDM4 (MDM4 regulator of p53) expression [
29]. Upregulation of miR-885-3p promotes p53-dependent cisplatin-induced mitochondrial pathway apoptosis in WT ΔNp63α-expressing head and neck squamous carcinoma cells through overexpression of MDM4 and downregulation of BCL2 (B-cell lymphoma 2). Interestingly, the decrease of MDM4 is related to resistance to cisplatin [
29]. It has also been shown that mutant p53R273H and p53WT can interact with ΔNp63α, mediating its degradation [
63]. Thus, it would be interesting to demonstrate whether there is an association with cisplatin resistance by p53 mutants dependent on inhibition of the ΔNp63α protein. On the other hand, miR-200c-5p suppresses proliferation and metastasis, inhibiting MAD2L1 (mitotic arrest deficient 2 like 1) in hepatocellular carcinoma [
64]. It should be noted that several studies have shown that p53 is frequently mutated in this type of cancer [
65,
66]. Furthermore, the presence of p53 mutations correlates with tumor progression and survival in hepatocellular carcinoma [
67], suggesting an important role of p53 mutations in hepatocellular carcinoma.
On the other hand, in our study, we observed overexpression of miR-27b-5p in the presence of p53 mutants. The miR-27b has been reported as an oncomiR and a tumor suppressor, which has also been observed for other miRNAs. It has been suggested that the cellular context is important to determine the expression and function of miRNAs, the balance between the targets of each miRNA present in a particular situation, and specific tissue. For example, miR-27b is overexpressed in breast, gastric, ovarian, and glioma cancers, where it has been associated with the induction of processes such as cell proliferation, metabolism, migration, and invasion [
46,
68]. In addition, miR-27b is overexpressed in the MDA-MB-231 breast cancer cell line. These levels increase in a subline selected for its high capacity to induce metastasis to the lung, called 4175. It was also demonstrated that inhibiting miR-27b expression decreases these cells’ migration and invasion capacity. Interestingly, the MDA-MB-231 cell line has a mutation in the p53 gene, so it would be interesting to analyze whether miR-27b expression is associated with the presence of p53 mutations in breast cancer [
68].
Finally, miR-182-5p is also considered an oncomir because it is closely related to migration, invasion, and metastasis [
38,
39,
40]. The active participation of adhesion molecules in the metastatic capacity of tumor cells is crucial since alteration in their expression generates a loss of function of the adhesion complex and gives rise to processes such as cell migration and invasion. This is consistent with the alteration of several miRNAs related to the signaling pathway "Adherens junction" in the expression profiles of p53 mutants, especially in the presence of the p53R248Q mutant. Among the differentially expressed miRNAs involved in regulating processes such as "Adherens junction" is the miR-182-5p. Some of the target genes of this miRNA have already been experimentally validated, such as FOXF2 and MTSS1. FOXF2 is a negative regulator of TWIST1 (Transcriptional repressor of E-cadherin) [
38,
69] and also negatively regulates the expression of matrix metalloproteinases such as MMP1 [
70]. On the other hand, MTSS1 suppresses the formation of F-actin fibers, which is an important event in the rearrangement of the cytoskeleton in cell migration and invasion processes. Besides, MTSS1 accelerates the kinetics of Adherens junction assembly and makes cells more resistant to cell-cell junction disassembly [
69,
71]. In this study, we demonstrated that the three most frequent p53 mutants in cancer induce overexpression of miR-182-5p. In addition, we also observed miR-182-5p overexpression in the OVCAR-3/p53R248Q cell line, which coincides with low expression levels of the miRNA target genes FOXF2 and MTSS1. In agreement with our results, in high-grade serous ovarian carcinoma (HG-SOC), it has been reported that p53 mutations are frequent, and overexpression of miR-182 is common in the early stages [
43]. Likewise, Xu et al., in 2014, observed that the OVCAR-3 cell line (p53R248Q) over-expressed miR-182-5p compared to the SKOV3 cell line (p53 null) [
69]. Of note, these studies did not associate the overexpression of miR-182-5p with the presence of p53R248Q.
Additionally, we demonstrated that inhibiting miR-182-5p in the presence of mutant p53R248Q reestablished FOXF2 and MTSS1 expression, which correlated with decreased cell migration and invasion. Interestingly, the therapeutic potential of anti-miR-182 has been suggested in an orthotopic animal model to mimic human ovarian cancer, using the cell lines SKOV3 with transfection of miR-182 (intrabursal injection) and OVCAR-3 (intraperitoneal injection). In these models, they demonstrated that treatment with anti-miR-182-5p decreased tumor size, invasion, and distant metastasis compared to control [
69]. On the other hand, Wang et al., in 2017, observed that miR-182 overexpression could promote the proliferation and migration of cancer cell lines from Head and Neck Squamous Cell Carcinoma (HNSCC), presenting TP53 mutations [
72]. Notably, in this study, they only observed an association between miR-182-5p overexpression and the presence of p53 mutations in patients and cell lines from HNSCC but did not demonstrate whether p53 mutants induce miR-182-5p overexpression.
Figure 1.
Representation of the p53R273C-regulated miRNome and Signaling Pathway Enrichment (KEGG) analysis. a) Saos-2 cells were transfected with the p53R273C mutant, and p53WT was used as a control. To determine which miRNAs were differentially expressed, a p-value <0.05 and Fold Change ≥2 or ≤-2 were used. The Circos map distributes the differentially expressed miRNAs according to their chromosomal locations, within which "bar graphs" correspond to the Fold Change value of miRNAs that increased (red) or decreased (green) in the presence of the p53R273C vs. p53WT mutant are shown. Additionally, the names of those miRNAs that were selected for validation through TaqMan probe assays are highlighted in red-augmented (miR-27b-5p) and green-decreased (miR-509-5p, miR-101-3p, miR-517a-3p and miR-200c-5p). b) "KEGG" pathway enrichment analysis of hypothetical target genes, based on miRPath (DIANA-microT-CDS algorithm). c) "KEGG" pathway enrichment analysis of experimentally validated genes, based on miRPath (DIANA-microT-CDS algorithm). The scatter plot in b) and c) shows the significance level of each signaling Pathway on the "X" axis (p <0.05) and the pathway name on "Y". The color of the circles represents the number of miRNAs involved in the signaling pathways, and the size of the circles is the number of hypothetical and/or validated genes.
Figure 1.
Representation of the p53R273C-regulated miRNome and Signaling Pathway Enrichment (KEGG) analysis. a) Saos-2 cells were transfected with the p53R273C mutant, and p53WT was used as a control. To determine which miRNAs were differentially expressed, a p-value <0.05 and Fold Change ≥2 or ≤-2 were used. The Circos map distributes the differentially expressed miRNAs according to their chromosomal locations, within which "bar graphs" correspond to the Fold Change value of miRNAs that increased (red) or decreased (green) in the presence of the p53R273C vs. p53WT mutant are shown. Additionally, the names of those miRNAs that were selected for validation through TaqMan probe assays are highlighted in red-augmented (miR-27b-5p) and green-decreased (miR-509-5p, miR-101-3p, miR-517a-3p and miR-200c-5p). b) "KEGG" pathway enrichment analysis of hypothetical target genes, based on miRPath (DIANA-microT-CDS algorithm). c) "KEGG" pathway enrichment analysis of experimentally validated genes, based on miRPath (DIANA-microT-CDS algorithm). The scatter plot in b) and c) shows the significance level of each signaling Pathway on the "X" axis (p <0.05) and the pathway name on "Y". The color of the circles represents the number of miRNAs involved in the signaling pathways, and the size of the circles is the number of hypothetical and/or validated genes.
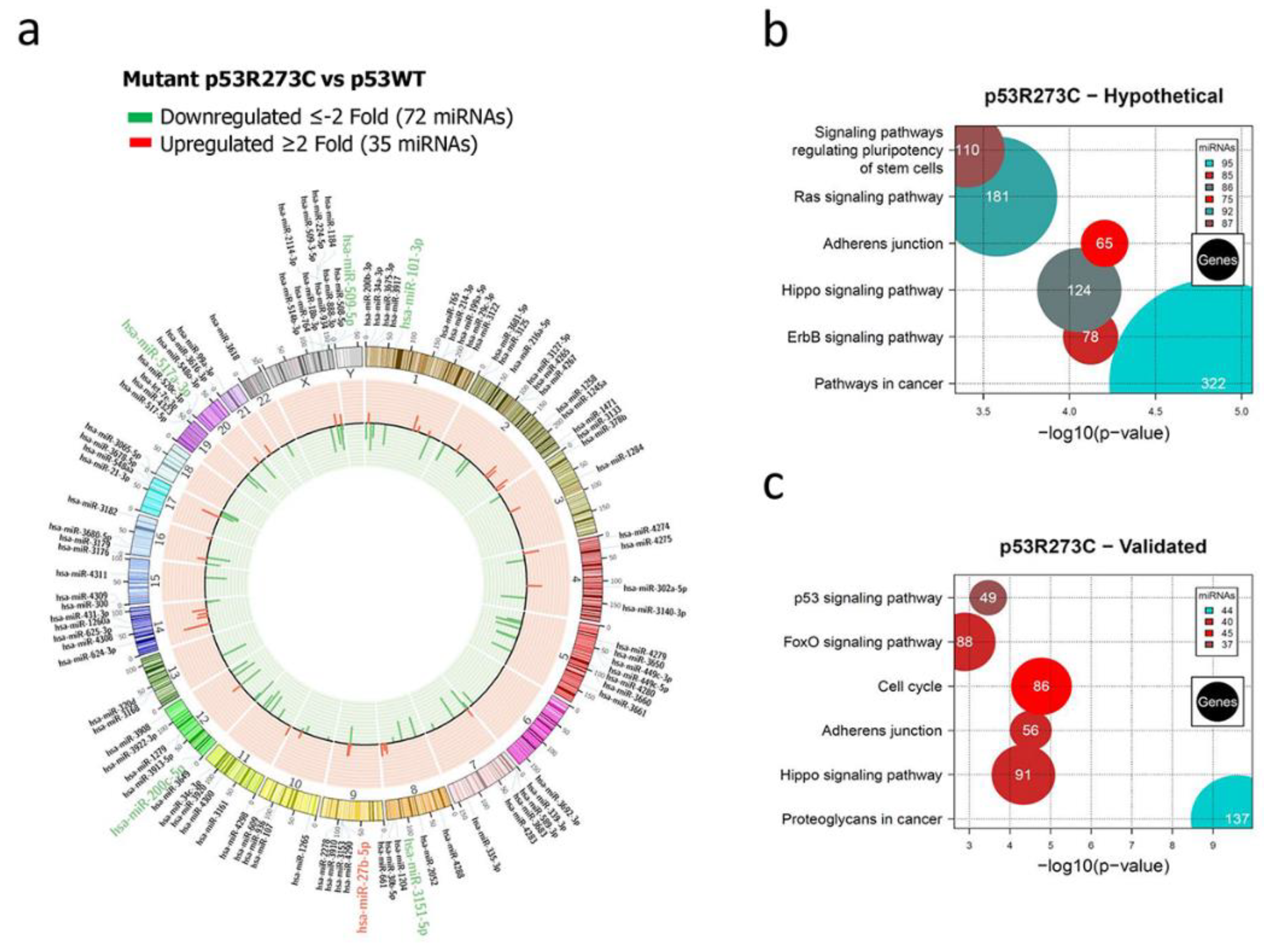
Figure 2.
Representation of the p53R175H-regulated miRNome and Signaling Pathway Enrichment (KEGG) analysis. a) Saos-2 cells were transfected with p53R175H, and p53WT was used as a control. To determine which miRNAs were differentially expressed, a value of p <0.05 and (Fold Change ≥2 or ≤-2) was used. The Circos map distributes the differentially expressed miRNAs according to their chromosomal locations, within which "bar graphs" correspond to the Fold Change value of miRNAs that increased (red) or decreased (green) in the presence of the p53R175H vs. p53WT mutant are shown. Additionally, the names of those miRNAs that were selected for validation through TaqMan probe assays are highlighted in red-increased (miR-182-5p) and green-decreased (miR-509-5p, miR-101-3p, miR-517a-3p, miR-885-3p, miR-3151-5p). (b) "KEGG" pathway enrichment analysis of hypothetical target genes, based on miRPath (DIANA-microT-CDS algorithm). C) "KEGG" pathway enrichment analysis of experimentally validated genes, based on miRPath (DIANA-microT-CDS algorithm). The scatter plot in b) and c) shows the significance level of each signaling Pathway on the "X" axis (p <0.05) and the pathway name on "Y". The color of the circles represents the number of miRNAs involved in signaling pathways, and the size of the circles the number of hypothetical and/or validated genes.
Figure 2.
Representation of the p53R175H-regulated miRNome and Signaling Pathway Enrichment (KEGG) analysis. a) Saos-2 cells were transfected with p53R175H, and p53WT was used as a control. To determine which miRNAs were differentially expressed, a value of p <0.05 and (Fold Change ≥2 or ≤-2) was used. The Circos map distributes the differentially expressed miRNAs according to their chromosomal locations, within which "bar graphs" correspond to the Fold Change value of miRNAs that increased (red) or decreased (green) in the presence of the p53R175H vs. p53WT mutant are shown. Additionally, the names of those miRNAs that were selected for validation through TaqMan probe assays are highlighted in red-increased (miR-182-5p) and green-decreased (miR-509-5p, miR-101-3p, miR-517a-3p, miR-885-3p, miR-3151-5p). (b) "KEGG" pathway enrichment analysis of hypothetical target genes, based on miRPath (DIANA-microT-CDS algorithm). C) "KEGG" pathway enrichment analysis of experimentally validated genes, based on miRPath (DIANA-microT-CDS algorithm). The scatter plot in b) and c) shows the significance level of each signaling Pathway on the "X" axis (p <0.05) and the pathway name on "Y". The color of the circles represents the number of miRNAs involved in signaling pathways, and the size of the circles the number of hypothetical and/or validated genes.
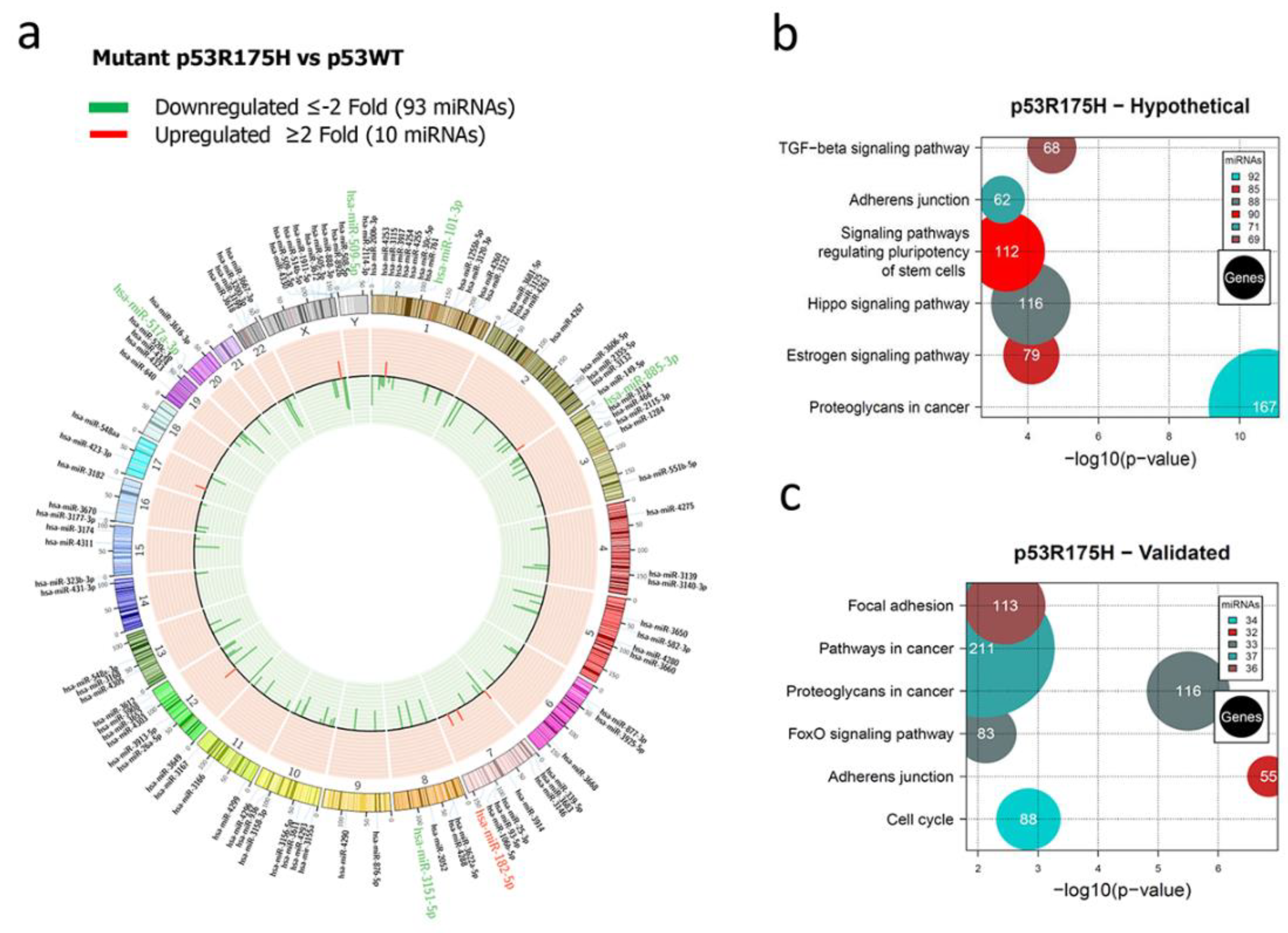
Figure 3.
Representation of the p53R248Q-regulated miRNome and Signaling Pathway Enrichment (KEGG) analysis. Saos-2 cells were transfected with p53R248Q, and p53WT was used as a control. To determine which miRNAs were differentially expressed, a value of p <0.05 and (Fold Change ≥2 or ≤-2 was used. The Circos map distributes the differentially expressed miRNAs according to their chromosomal locations, within which "bar graphs" correspond to the Fold Change value of miRNAs that increased (red) or decreased (green) in the presence of the p53R248Q vs. p53WT mutant are shown. Additionally, the names of those miRNAs that were selected for validation through TaqMan probe assays are highlighted in red-increased (miR-182-5p and miR-27b-5p) and green-decreased (miR-509-5p, miR-101-3p, miR-517a-3p, miR-885-3p, miR-3151-5p, miR-200c-5p) b). "KEGG" pathway enrichment analysis of hypothetical target genes, based on miRPath (DIANA-microT-CDS algorithm).c) "KEGG" pathway enrichment analysis of experimentally validated genes, based on miRPath (DIANA-microT-CDS algorithm). The scatter plot in b) and c) shows the significance level of each signaling Pathway on the "X" axis (p <0.05) and the pathway name on "Y". The color of the circles represents the number of miRNAs involved in signaling pathways, and the size of the circles the number of hypothetical and/or validated genes.
Figure 3.
Representation of the p53R248Q-regulated miRNome and Signaling Pathway Enrichment (KEGG) analysis. Saos-2 cells were transfected with p53R248Q, and p53WT was used as a control. To determine which miRNAs were differentially expressed, a value of p <0.05 and (Fold Change ≥2 or ≤-2 was used. The Circos map distributes the differentially expressed miRNAs according to their chromosomal locations, within which "bar graphs" correspond to the Fold Change value of miRNAs that increased (red) or decreased (green) in the presence of the p53R248Q vs. p53WT mutant are shown. Additionally, the names of those miRNAs that were selected for validation through TaqMan probe assays are highlighted in red-increased (miR-182-5p and miR-27b-5p) and green-decreased (miR-509-5p, miR-101-3p, miR-517a-3p, miR-885-3p, miR-3151-5p, miR-200c-5p) b). "KEGG" pathway enrichment analysis of hypothetical target genes, based on miRPath (DIANA-microT-CDS algorithm).c) "KEGG" pathway enrichment analysis of experimentally validated genes, based on miRPath (DIANA-microT-CDS algorithm). The scatter plot in b) and c) shows the significance level of each signaling Pathway on the "X" axis (p <0.05) and the pathway name on "Y". The color of the circles represents the number of miRNAs involved in signaling pathways, and the size of the circles the number of hypothetical and/or validated genes.
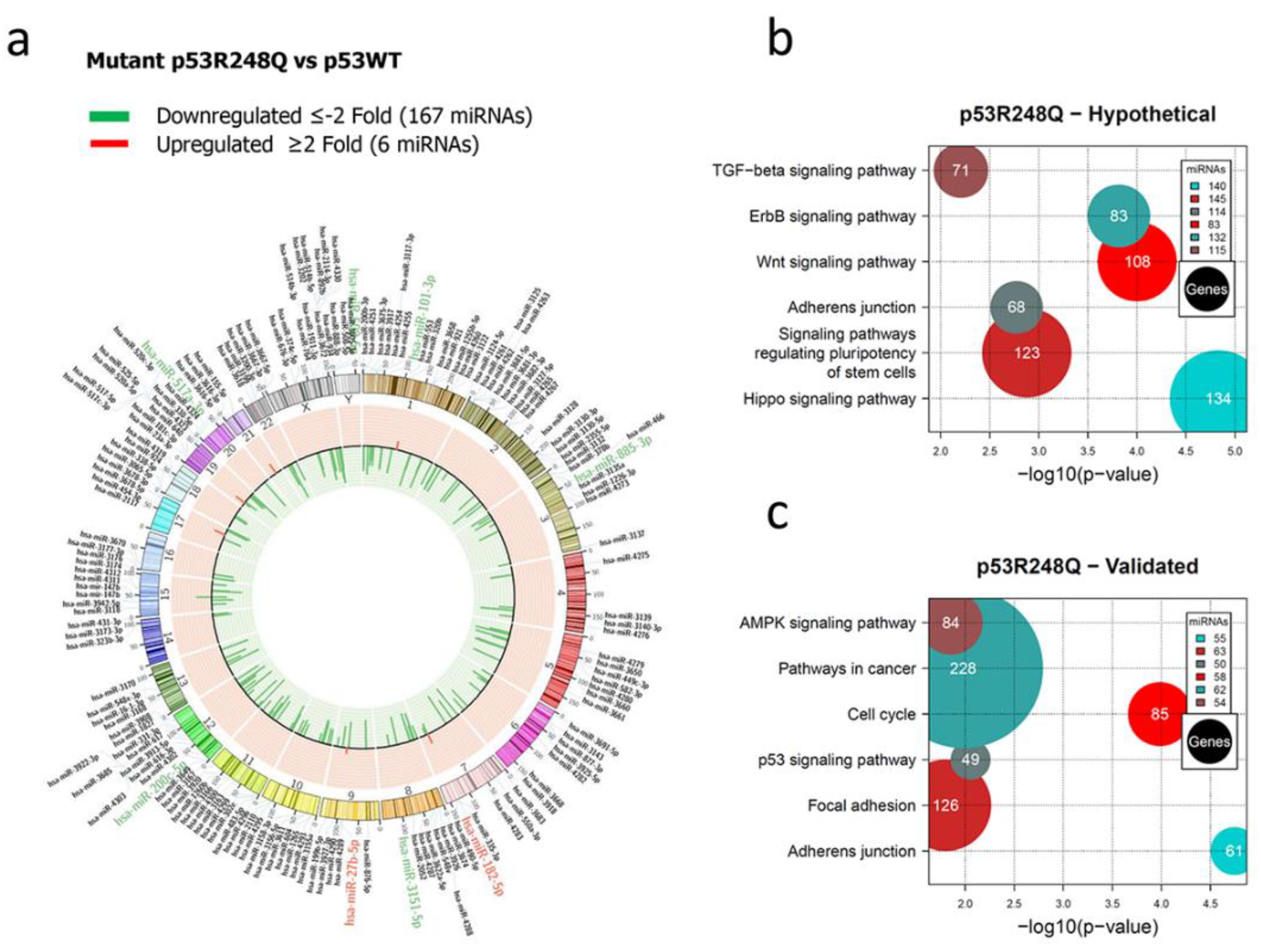
Figure 4.
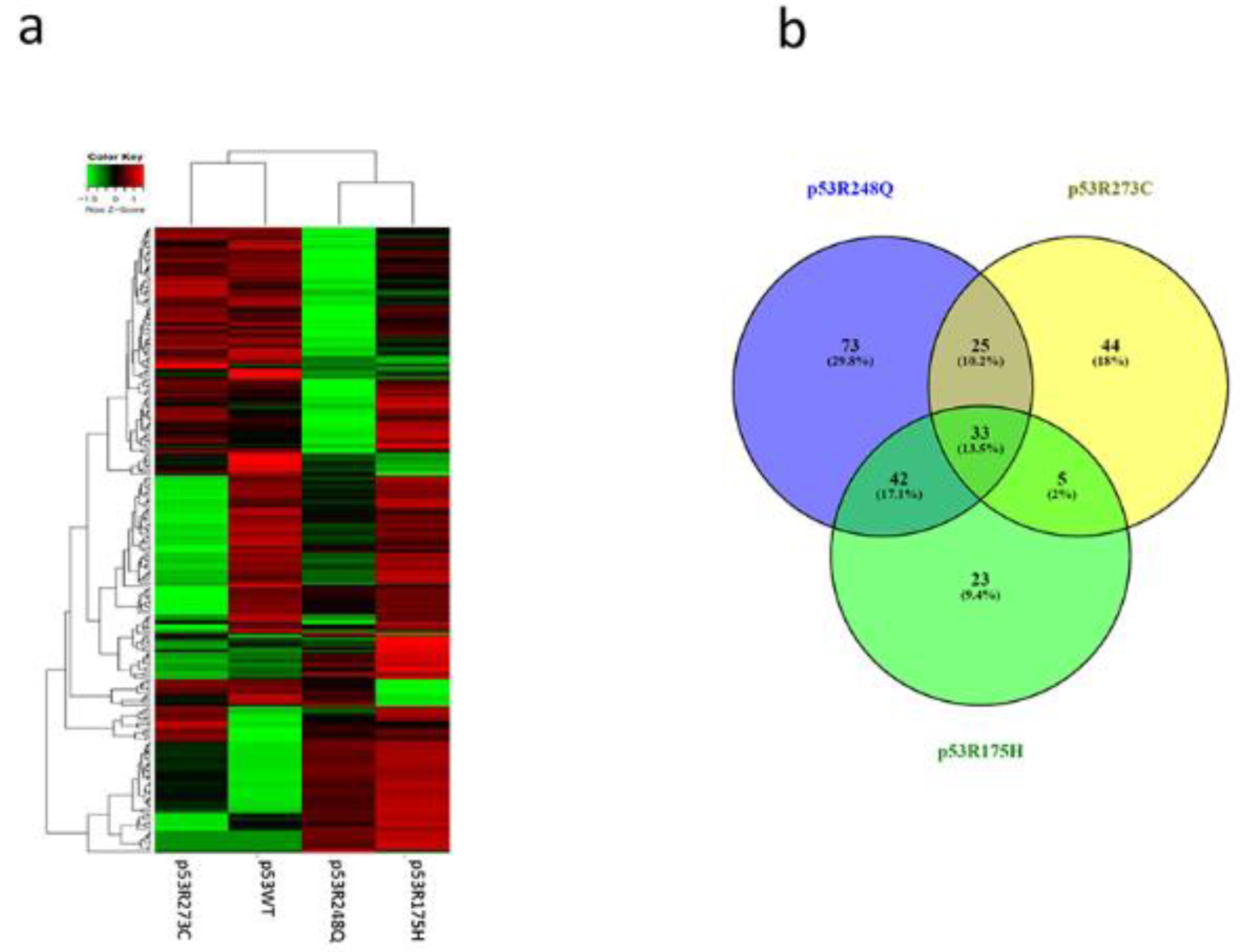
Heat map and Venn diagram of differentially expressed miRNAs in Saos-2 cells transfected with p53R273C, p53R248Q, and p53R175H mutants. a) The heat map represents the color-coded expression levels; red indicates overexpression, and green, indicates underexpression. The expression of each miRNA is hierarchically grouped on the "Y" axis; furthermore, the p53 mutants are represented on the "X" axis. b) The Venn Diagram shows the overlap of miRNAs among the three p53 mutants (33 miRNAs). The p53R273C mutant shares 25 miRNAs with p53R248Q and only 5 with p53R175H. In addition, the p53R248Q mutant exclusively regulates 73 miRNAs, and 42 are shared with the p53R175H mutant.
Figure 4.
Heat map and Venn diagram of differentially expressed miRNAs in Saos-2 cells transfected with p53R273C, p53R248Q, and p53R175H mutants. a) The heat map represents the color-coded expression levels; red indicates overexpression, and green, indicates underexpression. The expression of each miRNA is hierarchically grouped on the "Y" axis; furthermore, the p53 mutants are represented on the "X" axis. b) The Venn Diagram shows the overlap of miRNAs among the three p53 mutants (33 miRNAs). The p53R273C mutant shares 25 miRNAs with p53R248Q and only 5 with p53R175H. In addition, the p53R248Q mutant exclusively regulates 73 miRNAs, and 42 are shared with the p53R175H mutant.
Figure 5.
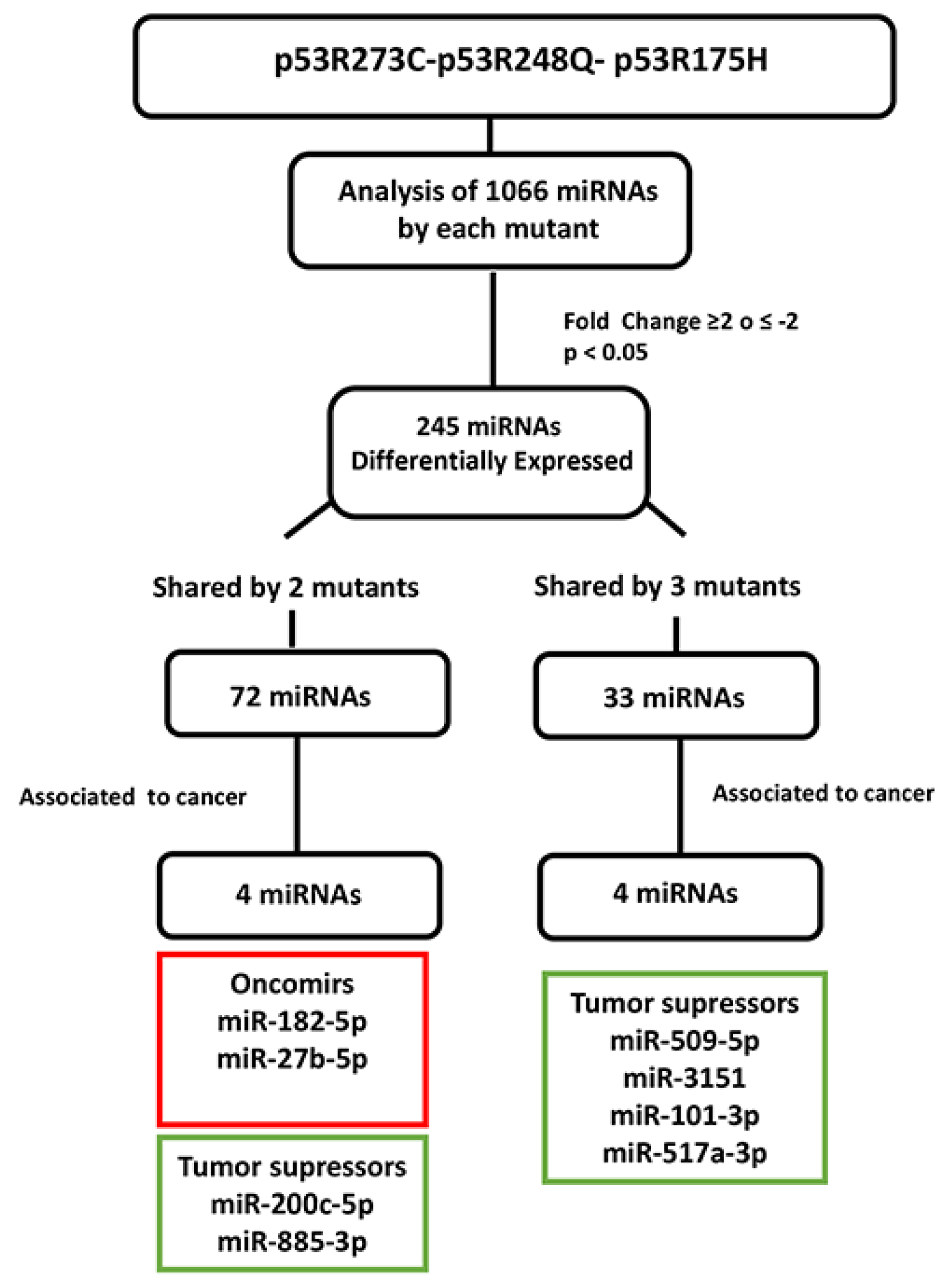
Flowchart showing the selection mechanism of miRNAs validated by Taqman Probes assays. PCR arrays (miRBase version 16, 1066 miRNAs; Qiagen) of the mutants (p53R175H, p53R273C, and p53R248Q) were performed. 254 differentially expressed miRNAs were identified according to cutoff points of ≥2 or ≤-2 (Fold Change) and a value of p<0.05. Subsequently, we found that 72 miRNAs were shared by at least 2 p53 mutants and 33 by all three. We also identified these miRNAs’ association with cancer through a literature survey. With this strategy, we selected and validated the downregulation of 6 tumor suppressor miRNAs: miR-509-5p , miR-200c-5p, miR-3151-5p, miR-885-3p, miR-517a-3p , miR-101-3p , as well as 2 oncomiRs: miR-182-5p and miR-27b-5p.
Figure 5.
Flowchart showing the selection mechanism of miRNAs validated by Taqman Probes assays. PCR arrays (miRBase version 16, 1066 miRNAs; Qiagen) of the mutants (p53R175H, p53R273C, and p53R248Q) were performed. 254 differentially expressed miRNAs were identified according to cutoff points of ≥2 or ≤-2 (Fold Change) and a value of p<0.05. Subsequently, we found that 72 miRNAs were shared by at least 2 p53 mutants and 33 by all three. We also identified these miRNAs’ association with cancer through a literature survey. With this strategy, we selected and validated the downregulation of 6 tumor suppressor miRNAs: miR-509-5p , miR-200c-5p, miR-3151-5p, miR-885-3p, miR-517a-3p , miR-101-3p , as well as 2 oncomiRs: miR-182-5p and miR-27b-5p.
Figure 6.
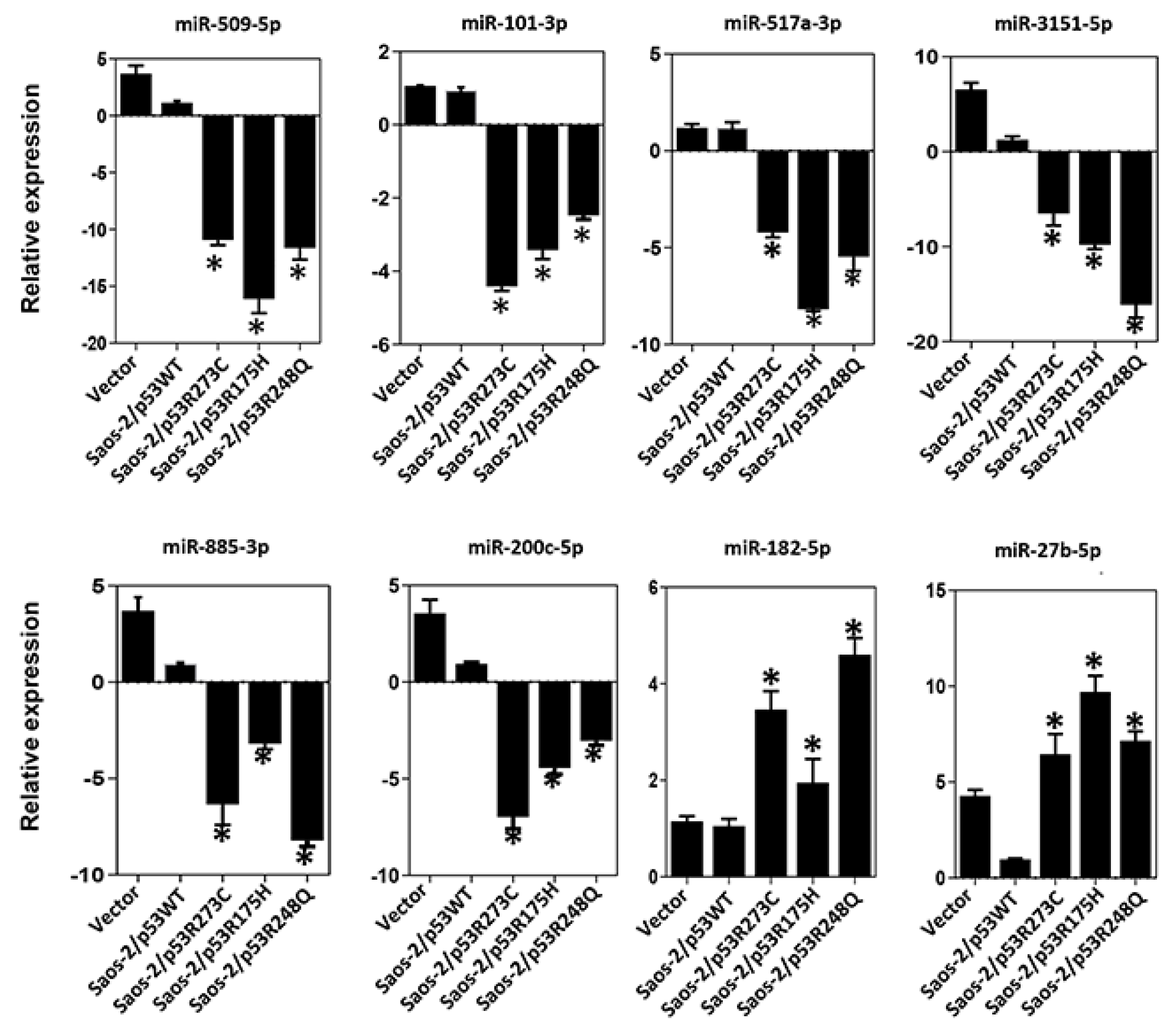
Validation of miRNAs by TaqMan probe assays. Relative expression of miR-509-5p, miR-101-3p, miR-517a-3p, miR-3151-5p, miR-885-3p, miR-200c-5p, miR-182-5p and miR-27b-5p in cell line Saos-2 transfected with empty vector (Control) or with mutant p53 (Saos-2/p53R248Q, Saos-2/p53R273C and Saos-2/p53R175H) vs Saos-2/p53WT. Data are presented as the two-fold change in miRNA level normalized for U6 (endogenous control). Data represent mean ± SD (n = 3) and (*) Refers to expression changes was significant compared to cells transfected with p53WT (Saos-2/p53WT) (p<0.05).
Figure 6.
Validation of miRNAs by TaqMan probe assays. Relative expression of miR-509-5p, miR-101-3p, miR-517a-3p, miR-3151-5p, miR-885-3p, miR-200c-5p, miR-182-5p and miR-27b-5p in cell line Saos-2 transfected with empty vector (Control) or with mutant p53 (Saos-2/p53R248Q, Saos-2/p53R273C and Saos-2/p53R175H) vs Saos-2/p53WT. Data are presented as the two-fold change in miRNA level normalized for U6 (endogenous control). Data represent mean ± SD (n = 3) and (*) Refers to expression changes was significant compared to cells transfected with p53WT (Saos-2/p53WT) (p<0.05).
Figure 7.
Mutant p53R248Q stimulates invasion and migration through miR-182 upregulation in Saos-2 cells. a) Relative expression of miR-182-5p in Saos-2 cell line (Null p53) and cell lines with endogenous mutant p53 (OVCAR-3/p53R248Q, C33a/p53R273C and SKBR3/p53R175H) vs MCF10a/p53WT. b) Relative expression of FOXF2 and MTSS1 in Saos-2 (null p53) vs OVCAR-3/p53R248Q (endogenous p53R248Q) and in Saos-2 transfected with empty vector (Empty vector) vs Saos-2/p53R248Q (transfected with p53R248Q). c) Relative expression of miR-182-5p in Saos-2 cells transfected with Control-anti-miR/Vector; Saos-2 transfected with p53R248Q and control anti-miR (p53R248Q/control-anti-miR) or transfected with antimiR-182 (p53R248Q/antimiR-182). RT-PCRs were normalized with GAPDH and/or U6, respectively. d) Cell invasion and migration assays in Saos-2 cells transfected with Control-anti-miR/Vector; Saos-2 transfected with p53R248Q and control anti-miR (p53R248Q/control-anti-miR) or transfected with antimiR-182 (p53R248Q/antimiR-182). e) Relative expression of FOXF2 and MTSS1 in Saos-2 cells transfected with empty vector (Empty vector), transfected with p53WT (Saos-2/p53WT), transfected with p53R248Q and control anti-miR (Saos-2/p53R248Q) or transfected with antimiR-182 (antimiR-182). Error bars represent (mean ± SD) from three independent experiments (n = 3), * p<0.05.
Figure 7.
Mutant p53R248Q stimulates invasion and migration through miR-182 upregulation in Saos-2 cells. a) Relative expression of miR-182-5p in Saos-2 cell line (Null p53) and cell lines with endogenous mutant p53 (OVCAR-3/p53R248Q, C33a/p53R273C and SKBR3/p53R175H) vs MCF10a/p53WT. b) Relative expression of FOXF2 and MTSS1 in Saos-2 (null p53) vs OVCAR-3/p53R248Q (endogenous p53R248Q) and in Saos-2 transfected with empty vector (Empty vector) vs Saos-2/p53R248Q (transfected with p53R248Q). c) Relative expression of miR-182-5p in Saos-2 cells transfected with Control-anti-miR/Vector; Saos-2 transfected with p53R248Q and control anti-miR (p53R248Q/control-anti-miR) or transfected with antimiR-182 (p53R248Q/antimiR-182). RT-PCRs were normalized with GAPDH and/or U6, respectively. d) Cell invasion and migration assays in Saos-2 cells transfected with Control-anti-miR/Vector; Saos-2 transfected with p53R248Q and control anti-miR (p53R248Q/control-anti-miR) or transfected with antimiR-182 (p53R248Q/antimiR-182). e) Relative expression of FOXF2 and MTSS1 in Saos-2 cells transfected with empty vector (Empty vector), transfected with p53WT (Saos-2/p53WT), transfected with p53R248Q and control anti-miR (Saos-2/p53R248Q) or transfected with antimiR-182 (antimiR-182). Error bars represent (mean ± SD) from three independent experiments (n = 3), * p<0.05.
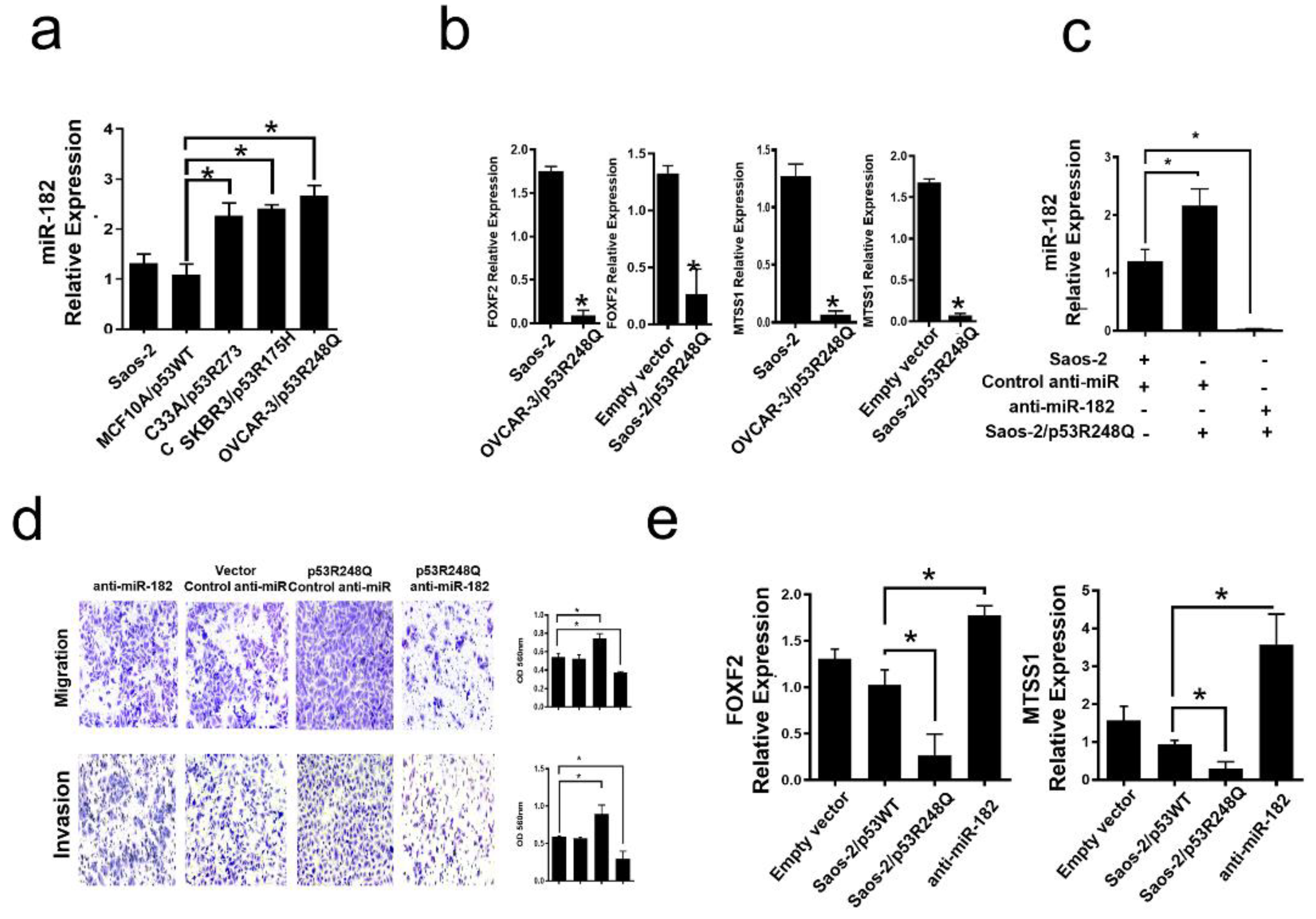
Figure 8.
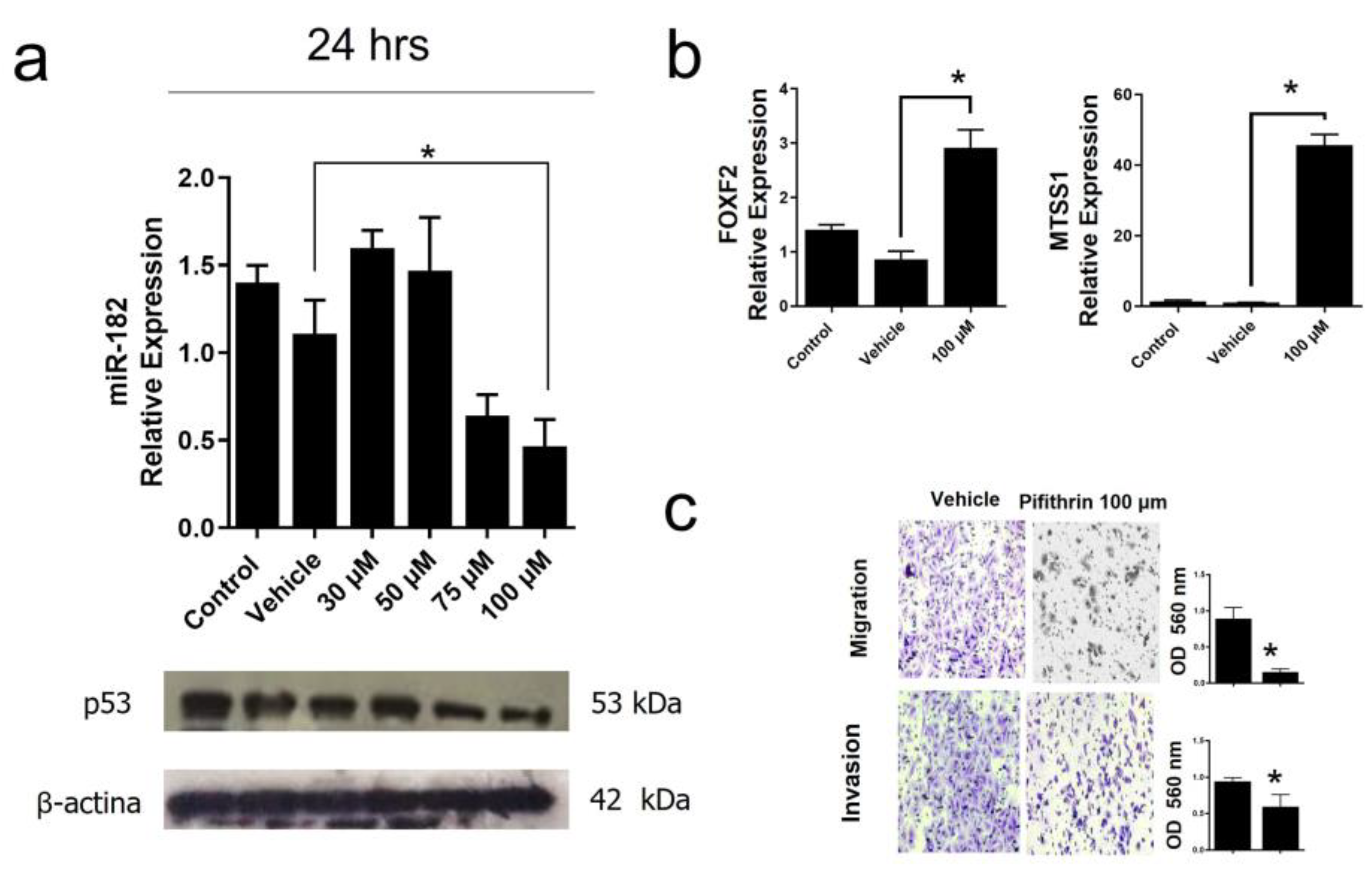
Inhibition of mutant p53R248Q in OVCAR-3 cells promotes decreased cell invasion and migration through miR-182. a) Relative expression of miR-182-5p and Western Blot of p53R248Q in the cell line OVCAR-3 (endogenous p53R248Q) treated with pifithrin-α (30 μM, 50 μM, 75 μM, 100 μM) for 24 h. using as control cells without treatment (Control) and only vehicle (Vehicle). b) Relative expression levels of FOXF2 and MTSS1 in cells OVCAR-3 (endogenous p53R248Q) treated with pifithrin-α for 24 h compared with cells without treatment (Control) and only vehicle (Vehicle). c) Invasion and migration assays of OVCAR-3 cells with vehicle (Vehicle) or treated with pifithrin-α (Pifithrin 100 μM). Error bars represent (mean ± SD) of three independent experiments (n = 3), * p <0.05.
Figure 8.
Inhibition of mutant p53R248Q in OVCAR-3 cells promotes decreased cell invasion and migration through miR-182. a) Relative expression of miR-182-5p and Western Blot of p53R248Q in the cell line OVCAR-3 (endogenous p53R248Q) treated with pifithrin-α (30 μM, 50 μM, 75 μM, 100 μM) for 24 h. using as control cells without treatment (Control) and only vehicle (Vehicle). b) Relative expression levels of FOXF2 and MTSS1 in cells OVCAR-3 (endogenous p53R248Q) treated with pifithrin-α for 24 h compared with cells without treatment (Control) and only vehicle (Vehicle). c) Invasion and migration assays of OVCAR-3 cells with vehicle (Vehicle) or treated with pifithrin-α (Pifithrin 100 μM). Error bars represent (mean ± SD) of three independent experiments (n = 3), * p <0.05.
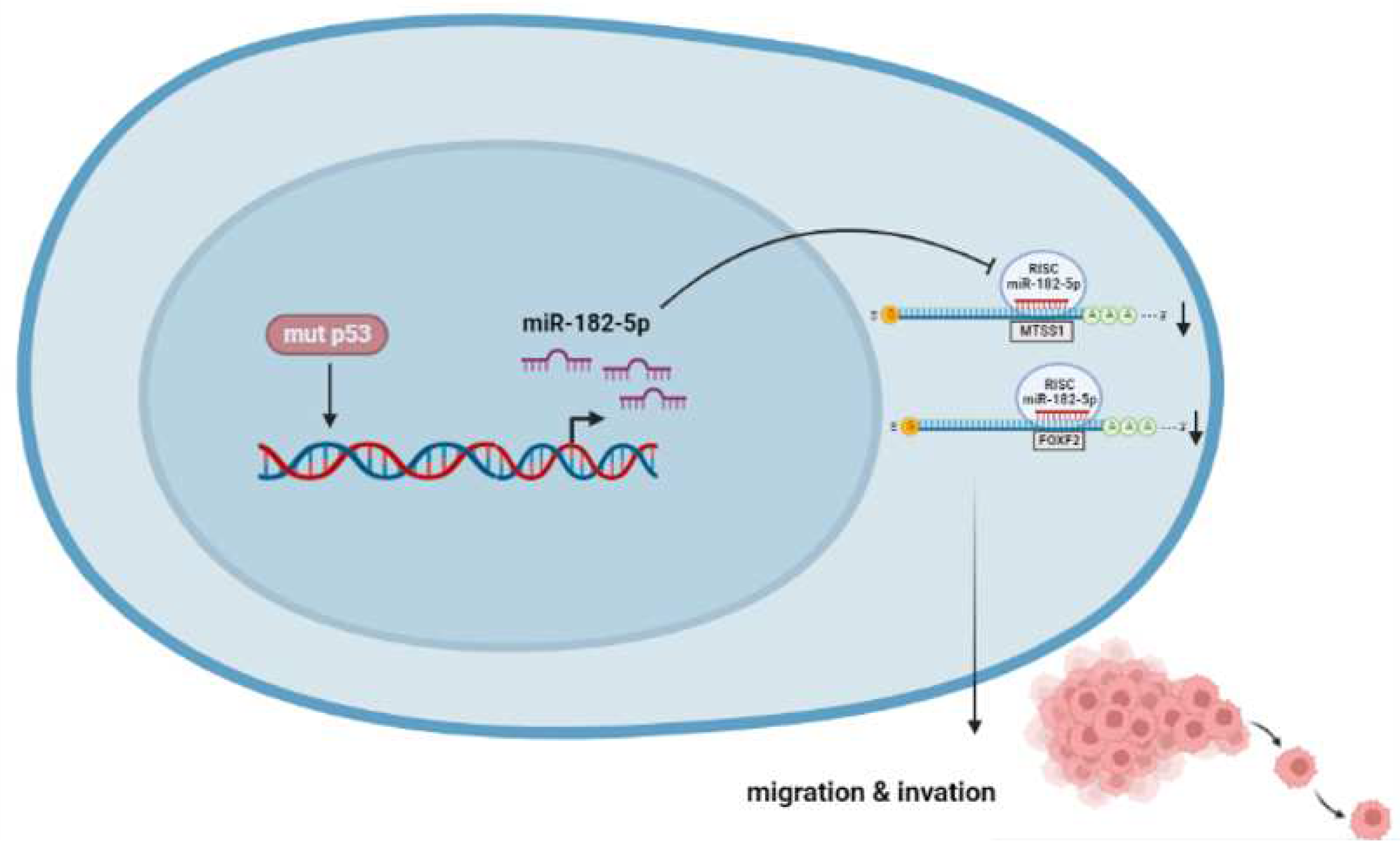
Figure 9.
p53R248Q induces cell migration and invasion through overexpression of miR-182-5p in cancer. The p53R248Q mutant induces overexpression of miR-182-5p, which promotes downregulation of MTSS1 and FOXF2 and increases cell migration and invasion. Mutant p53R248Q is required for the upregulation of miR-182-5p because inhibition of mutant p53 by pifithrin-α has a negative effect on the expression of miR-182-5p and its targets, leading to decreased cell migration and invasion.
Figure 9.
p53R248Q induces cell migration and invasion through overexpression of miR-182-5p in cancer. The p53R248Q mutant induces overexpression of miR-182-5p, which promotes downregulation of MTSS1 and FOXF2 and increases cell migration and invasion. Mutant p53R248Q is required for the upregulation of miR-182-5p because inhibition of mutant p53 by pifithrin-α has a negative effect on the expression of miR-182-5p and its targets, leading to decreased cell migration and invasion.