Submitted:
12 July 2023
Posted:
13 July 2023
You are already at the latest version
Abstract
Keywords:
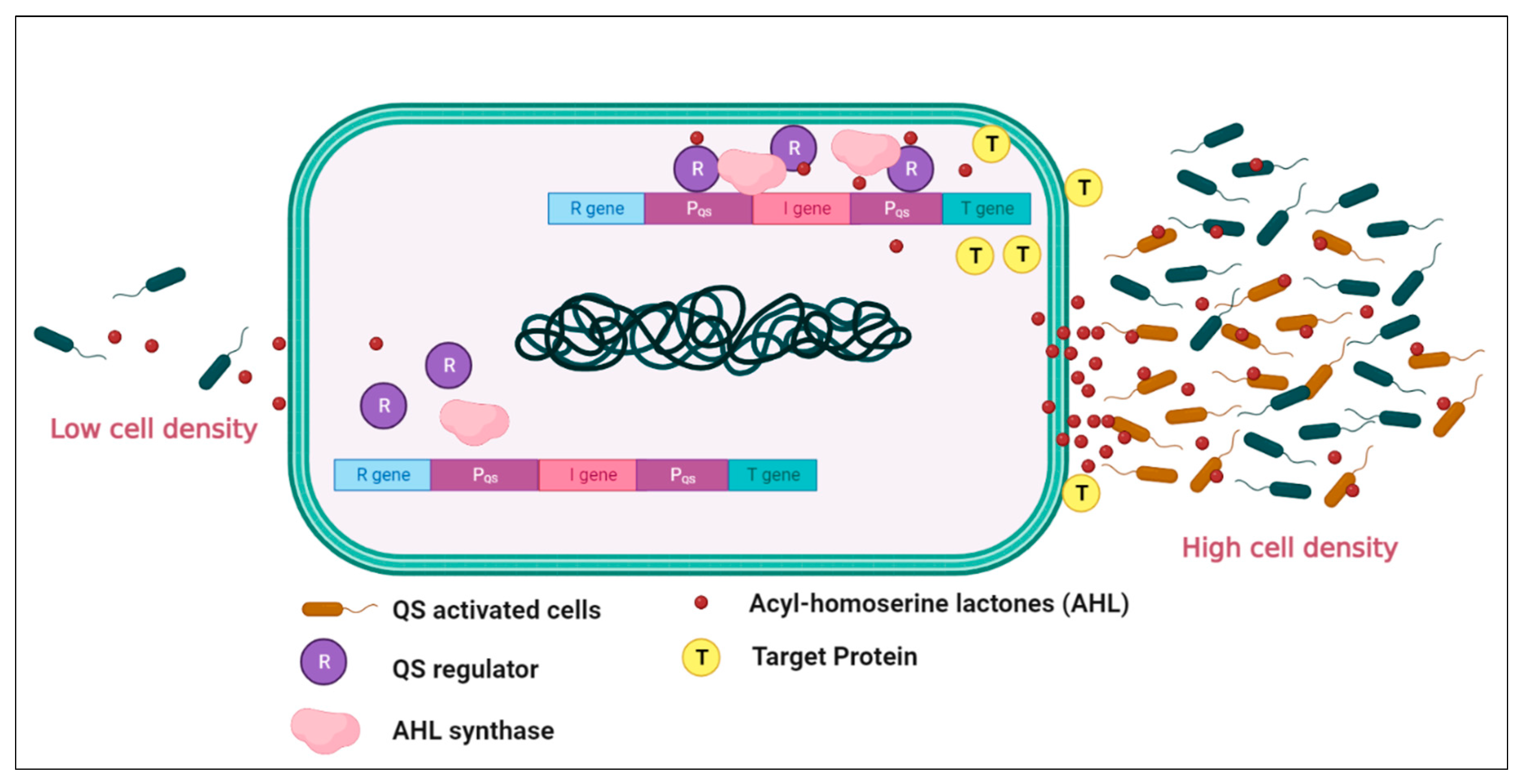
1. Introduction
2. Bacterial Quorum Sensing in the Deep Sea
| Bacteria | Activity mediated by QS | Location of occurrence | Signaling Molecules | QS System | Reference |
|---|---|---|---|---|---|
| Staphylococcus aureus | Virulence factor production control Biofilm formation control through the agr system |
Human body | Autoinducing peptides (AIPs) | Agr system | [31,32] |
| Bacillus cereus | Virulence factor control through the PlcR system | Various habitats including soil and food | Cyclic peptide | PlcR system | |
| Pseudomonas aeruginosa | Virulence factor control through LuxI/LuxR-type system | Various habitats including soil, water, and plants | N-acyl homoserine lactones (AHLs) | LasR/RhlR system | [33] |
| Vibrio fischerii | Light emission gene control through the LuxI system | Bobtail squid (Euprymna scolopes) in marine waters | N-acyl homoserine lactone (AHL) | LuxI/LuxR system | [25] |
| Vibrio diabolicus | Biofilm formation control | Polychaete annelid Alvinella pompejana deep-sea hydrothermal vent | Autoinducing peptides (AIPs) | Unknown QS system | [34,35] |
| Escherichia coli | Regulation of biofilm formation, motility, and virulence | Various habitats including the human gastrointestinal tract | Autoinducer-2 (AI-2) | LuxS/AI-2 system | [36,37] |
| Vibrio cholerae | Toxin production regulation and colonization of the human intestine | Hadel zones and human gastrointestinal tract | N-acyl homoserine lactones (AHLs) | LuxO/LuxR system | [38,39] |
| Streptococcus pneumoniae | Competence development and genetic transformation | Human respiratory tract | Peptides | ComDE system | [40] |
| Acinetobacter baumannii | Biofilm formation and antibiotic resistance | Various environments including hospitals and soil | Unknown | Unknown QS system | [41,42] |
| Aliivibrio fischeri | Symbiotic colonization of the Hawaiian bobtail squid | Bobtail squid (Euprymna scolopes) in marine waters | N-acyl homoserine lactone (AHL) | LuxI/LuxR system | [43] |
| Photobacterium phosphoreum | Bioluminescence control | Deep-sea waters | Autoinducer-2 | LuxS/AI-2 system | [23] |
| Sulfitobacter sp. | Production of extracellular enzymes and biofilm formation | Marine environments including surface water and sediments | Unknown | Unknown QS system | [44] |
| Ruegeria sp. | Biofilm formation and production of extracellular enzymes | Marine environments including coastal seawater and sediment | N-acyl homoserine lactone (AHL) | LuxI/LuxR system | [45] |
| Shewanella oneidensis | Regulation of biofilm formation and metal oxide reduction | Marine and freshwater environments | Unknown | Unknown QS system | [46,47] |
| Colwellia psychrerythraea | Cold adaptation and biofilm formation | Cold marine environments | Unknown | Unknown QS system | [48,49] |
| Psychrobacter sp. | Production of extracellular enzymes and biofilm formation | marine sediments | N-acylhomoserine lactones (AHL) | LuxI/LuxR system QS system | [50] |
| Marinobacter sp. | Biofilm formation and quorum quenching | Marine environments including deep-sea sediments | Unknown | Unknown QS system | [51] |
| Sulfurovum lithotrophicum | Quorum sensing in deep-sea vent bacteria | Deep-sea hydrothermal vents | Autoinducer-2 (AI-2) | LuxS/AI-2 system | [26,52] |
| Caminibacter mediatlanticus | Quorum sensing in deep-sea vent bacteria | Deep-sea hydrothermal vents | Autoinducer-2 (AI-2) | LuxS/AI-2 system | [26,52] |
| Thiomicrospira sp. | Symbiotic interactions and biofilm formation | Deep-sea hydrothermal vents | Unknown | Unknown QS system | [53] |
3. Biofilm Formation
| Location | Bacteria | Biofilm composition | Functions | Reference | Quorum Sensing Involvement |
|---|---|---|---|---|---|
| Antarctic Waters | Marine bacteria | Charged uronic acid moieties and sulfate groups | Plays a role in cold adaptation | [55] | QS system(s) involved |
| Korean yellow sea | Bacillus sp. I450 | Neutral sugars and uronic acids | Exhibits antimicrobial activity | [55,61] | QS system(s) involved |
| Solar Saltern and Spanish Mediterranean Seaboard | Halomonas maura & Salipiger mucosus | Sulfated polysaccharide with high uronic acid content and fucose-rich polysaccharides | Adapted to high salinity environments and high capacity for binding cations | [62,63] | QS system(s) involved |
| Deep-sea hydrothermal vent | Caminibacter mediatlanticus | Sulfated polysaccharide high in glucosamine | Thrives in high-temperature hydrothermal vents | [26,64] | QS system(s) involved |
| Deep-sea hydrothermal vent | Alteromonas infernus | Lacking lipopolysaccharides (LPS) | Exhibits unique adaptations to extreme conditions, including high-pressure and high-temperature environments | [65] | QS system(s) involved |
| Host in deep-sea hydrothermal vent | Vibrio diabolcus | Large amounts of uronic acid and no sulfate groups | Forms symbiotic relationship with host organism, the polychaete annelid Alvinella pompejana, in deep-sea hydrothermal vent ecosystems | [34] | QS system(s) involved |
| Antarctic Ocean | Psychrobacter sp. | Extracellular polysaccharides | Exhibits cold-adapted enzymatic activity and plays a role in biofilm formation under low temperatures | [66] | Not specified |
| Deep-sea sediments | Marinobacter sp. | Exopolysaccharides | Involved in sediment stabilization and biogeochemical cycling in deep-sea sediments | [67,68] | Not specified |
| Gulf of Mexico | Vibrio vulnificus | Alginate and extracellular DNA | Forms biofilms on oyster shells and contributes to oyster pathogenesis | [69] | QS system(s) involved |
| Deep-sea hydrothermal vent | Thiomicrospira sp. | Extracellular sulfur and polysaccharides | Capable of sulfur oxidation and plays a role in ecosystem functioning in deep-sea hydrothermal vents | [70] | Not specified |
| Mariana Trench | Pseudomonas sp. | Exopolysaccharides | Exhibits unique adaptations to extreme pressures and low nutrient availability in the Mariana Trench | [71,72] | QS system(s) involved |
4. Biogeochemical Cycling and Quorum Sensing
5. Methods of Monitoring Quorum Sensing
6. Biotechnological Applications
7. Conclusion
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sood, U.; Dhingra, G.G.; Anand, S.; Hira, P.; Kumar, R.; Kaur, J.; Verma, M.; Singhvi, N.; Lal, S.; Rawat, C.D.; et al. Microbial Journey: Mount Everest to Mars. Indian J. Microbiol. 2022, 62, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Nealson, K.H.; Platt, T.; Hastings, J.W. Cellular control of the synthesis and activity of the bacterial luminescent system. J. Bacteriol. 1970, 104, 313–322. [Google Scholar] [PubMed]
- Nealson, K.H. Autoinduction of bacterial luciferase: occurrence, mechanism and significance. Arch. Microbiol. 1977, 112, 73–79. [Google Scholar] [CrossRef]
- Engebrecht, J.; Nealson, K.; Silverman, M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell 1983, 32, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bai, L.; Li, S.; Yan, W. Similarities and Differences in Quorum Sensing-Controlled Bioluminescence between Photobacterium phosphoreum T3 and Vibrio qinghaiensis sp.-Q67. Appl. Sci. 2022, 12. [Google Scholar] [CrossRef]
- Preda, V.G.; Săndulescu, O. Communication is the key: biofilms, quorum sensing, formation and prevention. Discoveries 2019, 7, e10. [Google Scholar] [CrossRef]
- Shanker, E.; Federle, M.J. Quorum sensing regulation of competence and bacteriocins in Streptococcus pneumoniae and mutans. Genes (Basel). 2017, 8. [Google Scholar]
- van Gestel, J.; Bareia, T.; Tenennbaum, B.; Dal Co, A.; Guler, P.; Aframian, N.; Puyesky, S.; Grinberg, I.; D’Souza, G.G.; Erez, Z.; et al. Short-range quorum sensing controls horizontal gene transfer at micron scale in bacterial communities. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- İnat, G.; Sırıken, B.; Başkan, C.; Erol, İ.; Yıldırım, T.; Çiftci, A. Quorum sensing systems and related virulence factors in Pseudomonas aeruginosa isolated from chicken meat and ground beef. Sci. Rep. 2021, 11, 1–9. [Google Scholar]
- Kai, K. Bacterial quorum sensing in symbiotic and pathogenic relationships with hosts. Biosci. Biotechnol. Biochem. 2018, 82, 363–371. [Google Scholar] [CrossRef]
- Albuquerque, P.; Casadevall, A. Quorum sensing in fungi – a review. Med. Mycol. 2012, 50, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Hull, C.M.; Genet Author manuscript, C. Sporulation: How to survive on planet Earth (and beyond) HHS Public Access Author manuscript. Curr. Genet. 2017, 63, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Joshi, C.; Kothari, V.; Patel, P. Importance of selecting appropriate wavelength, while quantifying growth and production of quorum sensing regulated pigments in bacteria. Recent Pat. Biotechnol. 2016, 10, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Bramhachari, P.V.; Yugandhar, N.M.; Prathyusha, A.; Mohana Sheela, G.; Naravula, J.; Venkateswarlu, N. Quorum sensing regulated swarming motility and migratory behavior in bacteria. In Implication of quorum sensing system in biofilm formation and virulence; Springer, 2018; pp. 49–66. [Google Scholar]
- Butrico, C.E.; Cassat, J.E. Quorum sensing and toxin production in Staphylococcus aureus osteomyelitis: Pathogenesis and paradox. Toxins (Basel). 2020, 12, 516. [Google Scholar] [CrossRef]
- Armes, A.C.; Walton, J.L.; Buchan, A. Quorum Sensing and Antimicrobial Production Orchestrate Biofilm Dynamics in Multispecies Bacterial Communities. Microbiol. Spectr. 2022, 10, e02615-22. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bimerew, M.; Ma, Y.; Müller, H.; Ovadis, M.; Eberl, L.; Berg, G.; Chernin, L. Quorum-sensing signaling is required for production of the antibiotic pyrrolnitrin in a rhizospheric biocontrol strain of Serratia plymuthica. FEMS Microbiol. Lett. 2007, 270, 299–305. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Mannathan, S.; Lee, C.-K.; Merlo, M.E.; Meng, X.-F.; Nihira, T.; Takano, E.; Minnaard, A.J.; Dijkhuizen, L.; Petrusma, M. Identification and characterisation of enzymes involved in γ-butyrolactone biosynthesis in Streptomyces coelicolor. In Quorum sensing in Streptomyces coelicolor; 2016; pp. 92–137. [Google Scholar]
- Pan, J.; Ren, D. Quorum sensing inhibitors: a patent overview. Expert Opin. Ther. Pat. 2009, 19, 1581–1601. [Google Scholar] [CrossRef]
- Carradori, S.; Di Giacomo, N.; Lobefalo, M.; Luisi, G.; Campestre, C.; Sisto, F. Biofilm and quorum sensing inhibitors: The road so far. Expert Opin. Ther. Pat. 2020, 30, 917–930. [Google Scholar] [CrossRef]
- Bassler, B.L. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 1999, 2, 582–587. [Google Scholar] [CrossRef]
- Whiteley, M.; Diggle, S.P.; Greenberg, E.P. Progress in and promise of bacterial quorum sensing research. Nature 2017, 551, 313–320. [Google Scholar] [PubMed]
- Tanet, L.; Tamburini, C.; Baumas, C.; Garel, M.; Simon, G.; Casalot, L. Bacterial bioluminescence: Light Emission in Photobacterium phosphoreum Is Not under Quorum-Sensing Control. Front. Microbiol. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Muras, A.; López-Pérez, M.; Mayer, C.; Parga, A.; Amaro-Blanco, J.; Otero, A. High prevalence of quorum-sensing and quorum-quenching activity among cultivable bacteria and metagenomic sequences in the mediterranean sea. Genes (Basel). 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Turovskiy, Y.; Kashtanov, D.; Paskhover, B.; Chikindas, M.L. Quorum sensing: fact, fiction, and everything in between. Adv. Appl. Microbiol. 2007, 62, 191–234. [Google Scholar] [PubMed]
- Pérez-Rodríguez, I.; Bolognini, M.; Ricci, J.; Bini, E.; Vetriani, C. From deep-sea volcanoes to human pathogens: A conserved quorum-sensing signal in Epsilonproteobacteria. ISME J. 2015, 9, 1222–1234. [Google Scholar] [CrossRef]
- Keller, L.; Surette, M.G. Communication in bacteria: an ecological and evolutionary perspective. Nat. Rev. Microbiol. 2006, 4, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Schauder, S.; Potier, N.; Van Dorsselaer, A.; Pelczer, I.; Bassler, B.L.; Hughson, F.M. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 2002, 415, 545–549. [Google Scholar] [CrossRef]
- del Mar Cendra, M.; Torrents, E. Pseudomonas aeruginosa biofilms and their partners in crime. Biotechnol. Adv. 2021, 49, 107734. [Google Scholar] [CrossRef]
- Alain, K.; Zbinden, M.; Le Bris, N.; Lesongeur, F.; Quérellou, J.; Gaill, F.; Cambon-Bonavita, M.A. Early steps in microbial colonization processes at deep-sea hydrothermal vents. Environ. Microbiol. 2004, 6, 227–241. [Google Scholar] [CrossRef]
- Derakhshan, S.; Navidinia, M.; Haghi, F. Antibiotic susceptibility of human-associated Staphylococcus aureus and its relation to agr typing, virulence genes, and biofilm formation. BMC Infect. Dis. 2021, 21, 1–10. [Google Scholar] [CrossRef]
- Kong, K.-F.; Vuong, C.; Otto, M. Staphylococcus quorum sensing in biofilm formation and infection. Int. J. Med. Microbiol. 2006, 296, 133–139. [Google Scholar] [CrossRef]
- Wu, W.; Jin, Y.; Bai, F.; Jin, S. Pseudomonas aeruginosa. In Molecular medical microbiology; Elsevier, 2015; pp. 753–767. [Google Scholar]
- Raguénès, G.; Christen, R.; Guezennec, J.; Pignet, P.; Barbier, G. Vibrio diabolicus sp. nov., a new polysaccharide-secreting organism isolated from a deep-sea hydrothermal vent polychaete annelid, Alvinella pompejana. Int. J. Syst. Evol. Microbiol. 1997, 47, 989–995. [Google Scholar]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Reading, N.C.; Rasko, D.A.; Torres, A.G.; Sperandio, V. The two-component system QseEF and the membrane protein QseG link adrenergic and stress sensing to bacterial pathogenesis. Proc. Natl. Acad. Sci. 2009, 106, 5889–5894. [Google Scholar] [CrossRef]
- Bassler, B.L.; Losick, R. Bacterially Speaking. Cell 2006, 125, 237–246. [Google Scholar] [CrossRef]
- Zhu, J.; Miller, M.B.; Vance, R.E.; Dziejman, M.; Bassler, B.L.; Mekalanos, J.J. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 3129–3134. [Google Scholar] [CrossRef]
- Liang, J.; Liu, J.; Wang, X.; Sun, H.; Zhang, Y.; Ju, F.; Thompson, F.; Zhang, X.H. Genomic Analysis Reveals Adaptation of Vibrio campbellii to the Hadal Ocean. Appl. Environ. Microbiol. 2022, 88. [Google Scholar] [CrossRef]
- Peterson, S.N.; Sung, C.K.; Cline, R.; Desai, B. V; Snesrud, E.C.; Luo, P.; Walling, J.; Li, H.; Mintz, M.; Tsegaye, G. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol. Microbiol. 2004, 51, 1051–1070. [Google Scholar] [CrossRef]
- Niu, C.; Clemmer, K.M.; Bonomo, R.A.; Rather, P.N. Isolation and characterization of an autoinducer synthase from Acinetobacter baumannii. J. Bacteriol. 2008, 190, 3386–3392. [Google Scholar] [CrossRef]
- Antunes, L.C.M.; Ferreira, R.B.R.; Buckner, M.M.C.; Finlay, B.B. Quorum sensing in bacterial virulence. Microbiology 2010, 156, 2271–2282. [Google Scholar] [CrossRef]
- Visick, K.L.; Ruby, E.G. Vibrio fischeri and its host: it takes two to tango. Curr. Opin. Microbiol. 2006, 9, 632–638. [Google Scholar] [CrossRef]
- Caruso, G. Microbial colonization in marine environments: Overview of current knowledge and emerging research topics. J. Mar. Sci. Eng. 2020, 8, 1–22. [Google Scholar] [CrossRef]
- Su, Y.; Tang, K.; Liu, J.; Wang, Y.; Zheng, Y.; Zhang, X.-H. Quorum sensing system of Ruegeria mobilis Rm01 controls lipase and biofilm formation. Front. Microbiol. 2019, 9, 3304. [Google Scholar] [CrossRef]
- El-Naggar, M.Y.; Wanger, G.; Leung, K.M.; Yuzvinsky, T.D.; Southam, G.; Yang, J.; Lau, W.M.; Nealson, K.H.; Gorby, Y.A. Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. Proc. Natl. Acad. Sci. 2010, 107, 18127–18131. [Google Scholar] [CrossRef]
- Gorby, Y.A.; Yanina, S.; McLean, J.S.; Rosso, K.M.; Moyles, D.; Dohnalkova, A.; Beveridge, T.J.; Chang, I.S.; Kim, B.H.; Kim, K.S. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. 2006, 103, 11358–11363. [Google Scholar] [CrossRef]
- Marx, J.G.; Carpenter, S.D.; Deming, J.W. Production of cryoprotectant extracellular polysaccharide substances (EPS) by the marine psychrophilic bacterium Colwellia psychrerythraea strain 34H under extreme conditions. Can. J. Microbiol. 2009, 55, 63–72. [Google Scholar] [CrossRef]
- Casillo, A.; D’Angelo, C.; Parrilli, E.; Tutino, M.L.; Corsaro, M.M. Membrane and Extracellular Matrix Glycopolymers of Colwellia psychrerythraea 34H: Structural Changes at Different Growth Temperatures. Front. Microbiol. 2022, 13, 820714. [Google Scholar] [CrossRef] [PubMed]
- Packiavathy, I.A.S.V.; Kannappan, A.; Thiyagarajan, S.; Srinivasan, R.; Jeyapragash, D.; Paul, J.B.J.; Velmurugan, P.; Ravi, A.V. AHL-Lactonase producing Psychrobacter sp. from Palk Bay sediment mitigates quorum sensing-mediated virulence production in Gram negative bacterial pathogens. Front. Microbiol. 2021, 12, 634593. [Google Scholar] [CrossRef] [PubMed]
- Rughöft, S.; Jehmlich, N.; Gutierrez, T.; Kleindienst, S. Comparative proteomics of Marinobacter sp. Tt1 reveals corexit impacts on hydrocarbon metabolism, chemotactic motility, and biofilm formation. Microorganisms 2020, 9, 3. [Google Scholar] [CrossRef]
- Sievert, S.M.; Vetriani, C. Chemoautotrophy at deep-sea vents, past, present, and future. Oceanography 2012, 25, 218–233. [Google Scholar] [CrossRef]
- Nunoura, T.; Takaki, Y.; Kazama, H.; Kakuta, J.; Shimamura, S.; Makita, H.; Hirai, M.; Miyazaki, M.; Takai, K. Physiological and genomic features of a novel sulfur-oxidizing gammaproteobacterium belonging to a previously uncultivated symbiotic lineage isolated from a hydrothermal vent. PLoS One 2014, 9, e104959. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef]
- Di Donato, P.; Poli, A.; Taurisano, V.; Abbamondi, G.R.; Nicolaus, B.; Tommonaro, G. Recent advances in the study of marine microbial biofilm: From the involvement of quorum sensing in its production up to biotechnological application of the polysaccharide fractions. J. Mar. Sci. Eng. 2016, 4. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Mekalanos, J.J. Quorum Sensing-Dependent Biofilms Enhance Colonization in Vibrio cholerae), the phenomenon by which bacteria monitor their cell population density through the extracellular accumulation of signaling molecules called autoinduc. Dev. Cell 2003, 5, 647–656. [Google Scholar] [CrossRef]
- Seyedsayamdost, M.R.; Carr, G.; Kolter, R.; Clardy, J. Roseobacticides: small molecule modulators of an algal-bacterial symbiosis. J. Am. Chem. Soc. 2011, 133, 18343–18349. [Google Scholar] [CrossRef]
- Cude, W.N.; Mooney, J.; Tavanaei, A.A.; Hadden, M.K.; Frank, A.M.; Gulvik, C.A.; May, A.L.; Buchan, A. Production of the antimicrobial secondary metabolite indigoidine contributes to competitive surface colonization by the marine roseobacter Phaeobacter sp. strain Y4I. Appl. Environ. Microbiol. 2012, 78, 4771–4780. [Google Scholar] [CrossRef]
- Krupke, A.; Hmelo, L.R.; Ossolinski, J.E.; Mincer, T.J.; Van Mooy, B.A.S. Quorum sensing plays a complex role in regulating the enzyme hydrolysis activity of microbes associated with sinking particles in the ocean. Front. Mar. Sci. 2016, 3, 55. [Google Scholar] [CrossRef]
- Kumar, C.G.; Joo, H.-S.; Choi, J.-W.; Koo, Y.-M.; Chang, C.-S. Purification and characterization of an extracellular polysaccharide from haloalkalophilic Bacillus sp. I-450. Enzyme Microb. Technol. 2004, 34, 673–681. [Google Scholar] [CrossRef]
- Llamas, I.; Quesada, E.; Martínez-Cánovas, M.J.; Gronquist, M.; Eberhard, A.; Gonzalez, J.E. Quorum sensing in halophilic bacteria: detection of N-acyl-homoserine lactones in the exopolysaccharide-producing species of Halomonas. Extremophiles 2005, 9, 333–341. [Google Scholar] [CrossRef]
- Llamas, I.; Mata, J.A.; Tallon, R.; Bressollier, P.; Urdaci, M.C.; Quesada, E.; Béjar, V. Characterization of the exopolysaccharide produced by Salipiger mucosus A3T, a halophilic species belonging to the Alphaproteobacteria, isolated on the Spanish mediterranean seaboard. Mar. Drugs 2010, 8, 2240–2251. [Google Scholar] [CrossRef]
- Voordeckers, J.W.; Starovoytov, V.; Vetriani, C. Caminibacter mediatlanticus sp. nov., a thermophilic, chemolithoautotrophic, nitrate-ammonifying bacterium isolated from a deep-sea hydrothermal vent on the Mid-Atlantic Ridge. Int. J. Syst. Evol. Microbiol. 2005, 55, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Raguénès, G.H.C.; Peres, A.; Ruimy, R.; Pignet, P.; Christen, R.; Loaec, M.; Rougeaux, H.; Barbier, G.; Guezennec, J.G. Alteromonas infernus sp. nov., a new polysaccharide-producing bacterium isolated from a deep-sea hydrothermal vent. J. Appl. Microbiol. 1997, 82, 422–430. [Google Scholar] [CrossRef]
- Floris, R.; Rizzo, C.; Lo Giudice, A. Biosurfactants from Marine Microorganisms. Metabolomics - New Insights into Biol. Med. 2020. [Google Scholar]
- Gutiérrez-Arnillas, E.; Rodríguez, A.; Sanromán, M.A.; Deive, F.J. New sources of halophilic lipases: Isolation of bacteria from Spanish and Turkish saltworks. Biochem. Eng. J. 2016, 109, 170–177. [Google Scholar] [CrossRef]
- Gutierrez, T.; Singleton, D.R.; Berry, D.; Yang, T.; Aitken, M.D.; Teske, A. Hydrocarbon-degrading bacteria enriched by the Deepwater Horizon oil spill identified by cultivation and DNA-SIP. ISME J. 2013, 7, 2091–2104. [Google Scholar] [CrossRef]
- Heng, S.-P.; Letchumanan, V.; Deng, C.-Y.; Ab Mutalib, N.-S.; Khan, T.M.; Chuah, L.-H.; Chan, K.-G.; Goh, B.-H.; Pusparajah, P.; Lee, L.-H. Vibrio vulnificus: an environmental and clinical burden. Front. Microbiol. 2017, 8, 997. [Google Scholar] [CrossRef]
- Zhou, Z.; St John, E.; Anantharaman, K.; Reysenbach, A.-L. Global patterns of diversity and metabolism of microbial communities in deep-sea hydrothermal vent deposits. Microbiome 2022, 10, 1–22. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, Y.; Liu, Y.; Liu, B.; Wang, D.; Xu, Y.; Wei, Y. Pseudomonas marianensis sp. nov., a marine bacterium isolated from deep-sea sediments of the Mariana Trench. Arch. Microbiol. 2022, 204, 1–7. [Google Scholar] [CrossRef]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from marine and marine extremophilic bacteria: Structures, properties, ecological roles and applications. Mar. Drugs 2018, 16. [Google Scholar] [CrossRef]
- Weiland-Bräuer, N. Friends or foes—microbial interactions in nature. Biology (Basel). 2021, 10, 496. [Google Scholar] [CrossRef]
- Dang, H.; Lovell, C.R. Microbial surface colonization and biofilm development in marine environments. Microbiol. Mol. Biol. Rev. 2016, 80, 91–138. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.Z.; Sasidharan, R.S.; Alghamdi, H.A.; Dang, H. Understanding Interaction Patterns within Deep-Sea Microbial Communities and Their Potential Applications. Mar. Drugs 2022, 20, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Keleştemur, S.; Avci, E.; Çulha, M. Raman and surface-enhanced Raman scattering for biofilm characterization. Chemosensors 2018, 6, 5. [Google Scholar] [CrossRef]
- Bodelón, G.; Montes-García, V.; López-Puente, V.; Hill, E.H.; Hamon, C.; Sanz-Ortiz, M.N.; Rodal-Cedeira, S.; Costas, C.; Celiksoy, S.; Pérez-Juste, I. Detection and imaging of quorum sensing in Pseudomonas aeruginosa biofilm communities by surface-enhanced resonance Raman scattering. Nat. Mater. 2016, 15, 1203–1211. [Google Scholar] [CrossRef]
- Prescott, R.D.; Decho, A.W. Flexibility and adaptability of quorum sensing in nature. Trends Microbiol. 2020, 28, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Qu, Y.; Wu, W.; Li, S.; Chen, Z.; Lian, S.; Jing, J. QSP: An open sequence database for quorum sensing related gene analysis with an automatic annotation pipeline. Water Res. 2023, 235, 119814. [Google Scholar] [CrossRef]
- May, A.L.; Eisenhauer, M.E.; Coulston, K.S.; Campagna, S.R. Detection and quantitation of bacterial acylhomoserine lactone quorum sensing molecules via liquid chromatography–isotope dilution tandem mass spectrometry. Anal. Chem. 2012, 84, 1243–1252. [Google Scholar] [CrossRef]
- Senni, K.; Gueniche, F.; Changotade, S.; Septier, D.; Sinquin, C.; Ratiskol, J.; Lutomski, D.; Godeau, G.; Guezennec, J.; Colliec-Jouault, S. Unusual glycosaminoglycans from a deep sea hydrothermal bacterium improve fibrillar collagen structuring and fibroblast activities in engineered connective tissues. Mar. Drugs 2013, 11, 1351–1369. [Google Scholar] [CrossRef]
- Montgomery, K.; Charlesworth, J.C.; LeBard, R.; Visscher, P.T.; Burns, B.P. Quorum sensing in extreme environments. Life 2013, 3, 131–148. [Google Scholar] [CrossRef]
- Bzdrenga, J.; Daude, D.; Remy, B.; Jacquet, P.; Plener, L.; Elias, M.; Chabriere, E. Biotechnological applications of quorum quenching enzymes. Chem. Biol. Interact. 2017, 267, 104–115. [Google Scholar] [CrossRef]
- Liao, N.Q.; Li, H.M. Conceivable bioremediation techniques based on quorum sensing. Appl. Mech. Mater. 2013, 295, 39–44. [Google Scholar] [CrossRef]
- Sivasankar, P.; Poongodi, S.; Seedevi, P.; Sivakumar, M.; Murugan, T.; Loganathan, S. Bioremediation of wastewater through a quorum sensing triggered MFC: A sustainable measure for waste to energy concept. J. Environ. Manage. 2019, 237, 84–93. [Google Scholar] [CrossRef]
- Smith, J.L.; Fratamico, P.M.; Novak, J.S. Quorum sensing: a primer for food microbiologists. J. Food Prot. 2004, 67, 1053–1070. [Google Scholar] [CrossRef] [PubMed]
- Tonkin, M.; Khan, S.; Wani, M.Y.; Ahmad, A. Quorum sensing-a stratagem for conquering multi-drug resistant pathogens. Curr. Pharm. Des. 2021, 27, 2835–2847. [Google Scholar] [CrossRef]
- Kameswaran, S.; Ramesh, B. Quenching and Quorum Sensing in Bacterial Bio-films. Res. Microbiol. 2023, 104085. [Google Scholar] [CrossRef] [PubMed]
- Abbamondi, G.R.; Tommonaro, G. Research progress and hopeful strategies of application of quorum sensing in food, agriculture and nanomedicine. Microorganisms 2022, 10, 1192. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Gerchman, Y.; Collins, C.H.; Arnold, F.H.; Weiss, R. A synthetic multicellular system for programmed pattern formation. Nature 2005, 434, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Patel, S.K.S.; Kang, Y.C.; Lee, J.-K. Quorum sensing inhibitors as antipathogens: biotechnological applications. Biotechnol. Adv. 2019, 37, 68–90. [Google Scholar] [CrossRef]
- Shaaban, M.; Elgaml, A.; Habib, E.-S.E. Biotechnological applications of quorum sensing inhibition as novel therapeutic strategies for multidrug resistant pathogens. Microb. Pathog. 2019, 127, 138–143. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





